Abstract
A dibenzylbutane-type lignan (16), along with eight furofuran-type (1–8), five furan-type (9–13), two dibenzylbutane-type (14 and 15), two bibenztetrahydronaphthalene-type lignans (17 and 18), two neolignans (19 and 20), and six phenolic derivatives (21–26) were isolated from an MeOH extract of the stem bark of Albizia julibrissin Durazz. The chemical structures of the obtained compounds were elucidated by nuclear magnetic resonance (NMR) and mass spectrometry (MS) analyses. Of the evaluated compounds, 14 were isolated from A. julibrissin and the Fabaceae family for the first time. Anti-inflammatory effects of the isolated analogs were investigated in terms of the inhibition of the nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophage cells. Ten compounds (10–12, 14, and 17–22) displayed significant dose-dependent inhibitory effects against the NO production, with IC50 values ranging from 5.4 to 19.2 µM. Moreover, eight compounds (1–4, 9, 13, 15, and 16) exhibited moderate inhibitory activities, with IC50 values ranging from 21.0 to 62.5 µM.
Keywords: Albizia julibrissin Durazz, Fabaceae, lignan, phenolic, nitric oxide
1. Introduction
Albizzia julibrissin Durazz, commonly known as the mimosa or silk tree, belongs to a traditional Chinese medicine practice called He Huan, and is widely distributed in Korea, China, India, and other Asian regions. The dried stem bark of A. julibrissin has been utilized in traditional Chinese medicine to treat insomnia, melancholia, diuresis, asthenia, ascariasis, depression, anxiety, and confusion [1,2,3]. Previous pharmacological studies of A. julibrissin revealed its sedative, antidepressant, antitumor, anti-infertility, immunomodulatory, and antioxidant properties [4,5,6]. Furthermore, the bark extract can be applied to bruises, ulcers, abscesses, burns, hemorrhoids, and fractures, and has been shown to exhibit cytotoxic activity [7]. A previous study involving phytochemical analyses of A. julibrissin revealed that oleanane-type triterpenoid saponins were the principle constituents of this species. Compounds of this type feature acacic acid, aglycone, and several saccharide moieties at the C-3, C-21, and C-28 positions, respectively [8]. The presence of flavonoids, alkaloids, ceramides, phenolic glycosides, and steroids in the bark and leaves of this tree has also been reported [9,10,11,12]. A. julibrissin is recorded in the Chinese Pharmacopoeia as a sedative and anti-inflammatory agent used to treat injuries resulting from falls, and to remove carbuncles [13]. Nevertheless, lignans, phenolic compounds, and their associated bioactivities have not been sufficiently evaluated. Little is known about the presence of phenolic glycosides and lignans in this plant, and their respective biological effects have not been studied. In this study, we aimed to isolate new anti-inflammatory compounds which would complement the panel of known active components of A. julibrissin. We successfully isolated and elucidated the structure of a novel dibenzylbutane-type lignan, along with eight furofuran-, five furan-, two dibenzylbutane-, and two bibenztetrahydronaphthalene-type lignans, as well as two neolignans and six phenolic derivatives from an MeOH extract of the stem bark of A. julibrissin. Moreover, the inhibitory activity of the isolated compounds against nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophage cells and the structure–activity relationship were evaluated through utilizing various analytical techniques, including nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS).
2. Results and Discussion
2.1. Isolation and Structural Elucidation
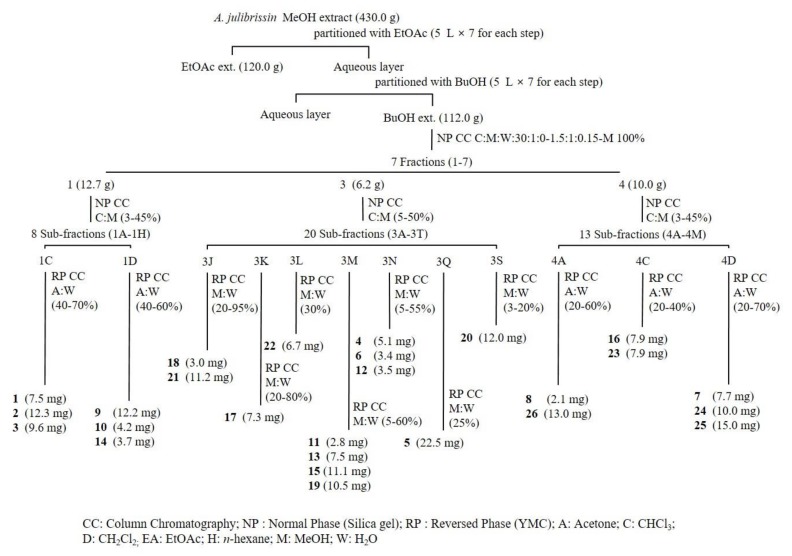
Using combined chromatographic separation techniques, 26 compounds (1–26), including eight furofuran-type (FF) lignans (1–8), five furan-type (FR) lignans (9–13), three dibenzylbutane-type (DB) lignans (14–16), two bibenztetrahydronaphthalene-type (BN) lignans (17 and 18), two neolignans (19 and 20), and six phenolic derivatives (21–26), were effectively isolated from an MeOH extract of A. julibrissin stem bark (Figure 1). The high-performance liquid chromatography (HPLC) evaluation showed that the purity of the isolated compounds was >90%. Based on the conducted analysis, the structures were determined as: (−)-lirioresinol B (1) [14], (−)-piperitol (2) [15], (−)-pinoresinol (3) [16], (−)-syringaresinol-4-O-β-d-glucopyranoside (4) [17], obtusifoside A (5) [18], (−)-syringaresinol-4-O-β-d-apiofuranosyl-(1→2)-β-d glucopyranoside (6) [14], simplexoside (7) [19], (−)-pinoresinol-β-d-glucopyranoside (8) [20], (+)-lariciresinol (9) [21], vladinol B (10) [21], (8R, 7’S, 8’R)-5,5’-dimethoxylariciresinol 4’-O-β-d-glucopyranoside (11) [22], manglieside E (12) [23], alangilignoside C (13) [19], (+)-(8S, 8’S)-bisdihydrosyringenin (14) [24], secoisolariciresinol (15) [25], julibrissinoside (16), (+)-(8S, 7′S, 8′S)-burselignan-9′-O-β-d-glucopyranoside (17) [26], (+)-(8R, 7′S, 8′R)-isolariciresinol-9′-O-β-d-fucopyranoside (18) [26], icariside E5 (19) [27], (7S,8R)-picraquassioside C (20) [28], albibrissinoside B (21) [29], khaephuoside B (22) [30], leonuriside A (23) [31], coniferin (24) [31], syringin (25) [31], and dihydrosyringin (26) [20]. The structures were confirmed by comparing the obtained spectroscopic data with the previously reported values (Figure 2). Among them, julibrissinoside (16) is a new compound. Moreover, 14 compounds (2, 5, 7, 11–18, and 20–22) were isolated from A. julibrissin and the Fabaceae family for the first time. To the best of our knowledge, the present study involved the first comprehensive chemical assessment of lignans and phenolic compounds from the A. julibrissin stem bark.
Figure 1.
Schematic diagram displaying the process of isolation of compounds 1–26 from Albizzia julibrissin.
Figure 2.
Structures of compounds 1–26 from A. julibrissin.
Compound 16 was obtained as a brown amorphous powder. The molecular formula was established as C34H50O18 by high-resolution electrospray ionization time-of-flight mass spectrometry (HR-ESI-TOF-MS; m/z 769.2890 ([M+Na]+)). The 1H-NMR spectrum of 16 (Table 1) exhibited four typical peaks corresponding to the 1,2,3,5-tetra-subsituted aromatic protons at δH 6.34 (s, H-5’/9’) and 6.40 ppm (s, H-5/9), and to four methoxy moieties at δH 3.72 (s, 6/8-OMe) and 6.40 ppm (s, 6’/8’-OMe). Furthermore, the presence of two peaks for anomeric protons at δH 4.28 (d, J = 8.0 Hz, H-1’’’) and 4.72 ppm (d, J = 7.8 Hz, H-1’’) indicated that 16 contains two sugar moieties. Notably, the larger coupling constants of the anomeric protons suggested the β-configuration of the two glucosyl scaffolds. Enzymatic hydrolysis of 16 yielded aglycone and glucose. The comparison with an authentic sample provided evidence for the identification of aglycone as (+)-(8S,8’S)-bisdihydrosyringenin (compound 14) [24]. The absolute configurations of the two D-glucosyl moieties were established by gas chromatography (GC) analysis.
Table 1.
1H and 13C NMR spectroscopic data for compound 16 in methanol-d4.
| Position | δHa (J/Hz) | δCb | Position | δHa (J/Hz) | δCb |
|---|---|---|---|---|---|
| 1 | 3.61 mc 4.06 dd (6.0, 9.4) |
70.8 | 1′′ | 4.72 d (7.8) | 105.4 |
| 2 | 2.35 mc | 41.3 | 2′′ | 3.69–3.75 m | 76.3 |
| 3 | 2.70 dd (14.0, 7.0) 2.82 dd (14.0, 7.0) |
36.1 | 3′′ | 3.69–3.75 m | 79.0 |
| 4 | 132.5 | 4′′ | 3.69–3.75 m | 72.1 | |
| 5 | 6.40 s | 107.8 | 5′′ | 3.79–3.90 m | 78.6 |
| 6 | 149.3 | 6′′ | 4.28 m 4.23 m |
70.8 | |
| 7 | 130.0 | 1′′′ | 4.28 d (8.0) | 105.0 | |
| 8 | 149.3 | 2′′′ | 3.79–3.90 m | 75.6 | |
| 9 | 6.40 s | 107.8 | 3′′′ | 3.79–3.90 m | 78.3 |
| 1′ | 3.69 m 3.90 dd (6.0, 11.0) |
63.1 | 4′′′ | 3.69–3.75 m | 71.8 |
| 2′ | 2.14 m | 44.0 | 5′′′ | 3.79–3.90 m | 78.8 |
| 3′ | 2.64 dd (11.0, 13.7) 2.81 dd (7.0, 13.7) |
36.3 | 6′′′ | 3.50 m | 62.0 |
| 4′ | 132.0 | 6-OMe | 3.72 s | 56.5 | |
| 5′ | 6.34 s | 107.7 | 8-OMe | 3.72 s | 56.5 |
| 6′ | 148.9 | 6′-OMe | 3.82 s | 56.7 | |
| 7′ | 130.1 | 8′-OMe | 3.82 s | 56.7 | |
| 8′ | 148.9 | ||||
| 9′ | 6.34 s | 107.7 |
Assignments were achieved by the analysis of the HMQC and HMBC experiments; J values (Hz) are given in parentheses. a 600 MHz. b 150 MHz. c Overlapped.
Accordingly, the obtained 13C-NMR spectrum (Table 1) contained eight signals corresponding to the quaternary carbon atoms of the aromatic rings at δC 130.0 (C-7), 130.1 (C-7′), 132.0 (C-4′), 132.5 (C-4), 148.9 (C-6′/8′), and 149.3 ppm (C-6/8), along with four aromatic carbon signals at δC 107.7 (C-5′/9′) and 107.8 ppm (C-5/9), and six carbon signals at δC 36.1 (C-3), 36.3 (C-3′), 41.3 (C-2), 44.0 (C-2′), 63.1 (C-1′), and 70.8 ppm (C-1), which were consistent with compound 14 [24]. The structure of the skeleton was identified by elucidation of the observed heteronuclear multiple bond correlations (HMBCs) at δH 2.14 (H-2′)/δC 132.0 (C-4′) and δH 2.35 (H-2)/δC 132.5 (C-4). The noted HMBC between C-1 (δH 3.61/4.06 and δC 70.8) and C-1′′ (δH 4.72 and δC 105.4) implied the presence of a glucosyl moiety attached at the C-1 position of the main skeleton. It is noteworthy that this observation was previously reported for compound 15 (secoisolariciresinol) [25]. The difference between the two compounds was that 16 contained a glucosyl group at the C-6′′ position. This finding was evidenced by the observation of a key HMBC between H-1′′′ (δH 4.28) and C-6′′ (δC 70.8). Based on these data, the structure of 16 was elucidated and named julibrissinoside.
2.2. Bioassays
To evaluate the anti-inflammatory activities of the isolated compounds 1–26, we assessed the LPS-induced production of inflammatory mediators such as NO by RAW 264.7 cells. Cell viability was measured utilizing the 3-(dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results demonstrated that none of the compounds affected cell viability (100 μM, data not shown). Furthermore, NO production was measured by employing Griess reaction assays. The obtained outcomes showed that 18 compounds exhibited significant inhibitory activities against NO production. Of them, 10 compounds (10–12, 14, and 17–22) showed strong dose-dependent inhibitory effects (IC50 < 20 µM), with IC50 values of 6.5 ± 0.1, 18.3 ± 0.3, 19.2 ± 0.3, 11.8 ± 0.2, 10.1 ± 0.2, 12.3 ± 0.7, 5.4 ± 0.1, 7.7 ± 0.2, 8.9 ± 0.3, and 8.3 ± 0.1 µM, respectively (Table 2). Compounds 1–4, 9, 13, 15, and 16 exhibited moderate inhibitory activities (20 µM < IC50 < 100 µM), with IC50 values ranging from 21.0 to 62.5 µM, while compounds 5–8 and 23–26 showed weaker inhibitory effects (IC50 > 100 µM).
Table 2.
NO inhibitory effects of isolated compounds 1–26.
| Compounds | IC50 (µM) | Compounds | IC50 (µM) |
|---|---|---|---|
| 1 | 28.1 ± 0.8 | 14 | 11.8 ± 0.2 |
| 2 | 42.6 ± 1.1 | 15 | 38.7 ± 1.9 |
| 3 | 31.1 ± 0.3 | 16 | 62.5 ± 1.6 |
| 4 | 52.7 ± 1.2 | 17 | 10.1 ± 0.2 |
| 5 | >100 | 18 | 12.3 ± 0.7 |
| 6 | >100 | 19 | 5.4 ± 0.1 |
| 7 | >100 | 20 | 7.7 ± 0.2 |
| 8 | >100 | 21 | 8.9 ± 0.3 |
| 9 | 21.0 ± 1.5 | 22 | 8.3 ± 0.1 |
| 10 | 6.5 ± 0.1 | 23 | >100 |
| 11 | 18.3 ± 0.3 | 24 | >100 |
| 12 | 19.2 ± 0.3 | 25 | >100 |
| 13 | 21.7 ± 1.8 | 26 | >100 |
| Quercetina | 15.6 ± 0.4 |
Values represent means ± SD (n = 3). a Quercetin was used as a positive control.
Upon investigating the structure–activity relationship of the isolated compounds, it was determined that, among the furofuran-type lignans (1–8), compounds 1 and 3 displayed the strongest inhibitory effects. This outcome indicated that the number of substituents on the aromatic rings and sugar units were important factors for the observed inhibitory activity. When the sugar units were linked to the aglycone, the effects weakened; when the methoxy group was linked to C-3 and C-5, the anti-inflammatory activity increased significantly. Moreover, all the furan-type lignans (9–13) displayed strong activities. Particularly strong activity was determined for compound 10, which contains a hydroxyl moiety at the C-2 position that appears to play a role in the NO inhibitory activity. On the other hand, the inhibitory effects of dibenzylbutane- and bibenztetrahydronaphthalene-type lignans (14–18), which exhibit similar structures, are believed to be dependent on the number of sugar moieties. Previous studies reported that some lignans inhibit NO production [32,33,34]. However, the structure–activity relationship did not provide elaborate detail, especially on the substituents of aromatic rings. These results imply that the numbers of sugar units, substituents on the aromatic rings, and hydroxyl groups are significant in the process of inhibiting NO production.
3. Conclusions
In this study, 26 compounds were successfully isolated from A. julibrissin. Julibrissinoside (16), a novel compound, as well as 25 additional known natural products were isolated. To the best of our knowledge, the current work comprises the first comprehensive report on lignans, the phenolic components of A. julibrissin, as well as their associated NO production inhibitory activity. These obtained results confirm that both lignans and phenolic compounds constitute new bioactive components of A. julibrissin. Overall, the outcomes of our study provide a valuable platform for the application of lignans and phenolic analogs isolated from A. julibrissin in the treatment of inflammatory diseases.
4. Materials and Methods
4.1. General Information
Optical rotations were determined using a Jasco DIP-370 automatic polarimeter. The NMR spectra were recorded using a JEOL ECA 600 spectrometer (1H, 600 MHz; 13C, 150 MHz). The LCQ advantage trap mass spectrometer (Thermo Finnigan, San Jose, CA, U.S.A.) was equipped with an electrospray ionization (ESI) source, and high-resolution electrospray ionization mass spectra (HR-ESI-MS) were obtained using an Agilent 6530 Accurate-Mass Q-TOF LC/MS system. Column chromatography was performed using a silica gel (Kieselgel 60, 70-230, and 230-400 mesh, Merck, Darmstadt, Germany), YMC RP-18 resins, and thin layer chromatography (TLC) was performed using pre-coated silica-gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck, Darmstadt, Germany).
4.2. Plant Material
Dried stem bark of A. julibrissin was provided by Bomyeong Herbal Market, Seoul in 2017, and taxonomically identified by Dr. Wei Li. A voucher specimen (KM-004101) was deposited at Korean Medicine (KM) Application Center, Korea Institute of Oriental Medicine, Republic of Korea.
4.3. Extraction and Isolation
The dried stem bark of A. julibrissin (3.0 kg) was extracted with MeOH (5 L × 3 times) under reflux condition. The solvents were evaporated using a rotary vacuum evaporator to provide MeOH extract (150.0 g). The MeOH extract was suspended in water and partitioned with EtOAc and n-BuOH. The BuOH fraction (112.0 g) was subjected to silica gel (10 × 30 cm) column chromatography with CHCl3-MeOH-H2O (10:1:0–1.5:1:0.15–MeOH 100%) to give seven fractions (Fr. 1–7). Fraction 1 (12.7 g) was subjected to silica gel (2.5 × 80 cm) column chromatography with CHCl3-MeOH (MeOH 3–45%) elution solvent to give 8 sub-fractions (Fr. 1A–1H). Fraction 1C was separated using YMC (1 × 80 cm) column chromatography with an acetone-H2O (acetone 40–70%) elution solvent to give (−)-lirioresinol B (1) (7.5 mg), (−)-piperitol (2) (12.3 mg), and (−)-pinoresinol (3) (9.6 mg). Fraction 1D was separated using YMC (1 × 80 cm) column chromatography with an acetone-H2O (acetone 40–60%) elution solvent to give (+)-lariciresinol (9) (12.2 mg), vladinol B (10) (4.2 mg), and (+)-(8S,8′S)-bisdihydrosyringenin (14) (3.7 mg).
Fraction 3 (6.2 g) was subjected to silica gel (2.0 × 80 cm) column chromatography with CHCl3-MeOH (MeOH 5–50%) elution solvent to give 20 sub-fractions (Fr. 3A–3T). Fraction 3J was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 20–95%) elution solvent to give (+)-(8R,7′S,8′R)-isolariciresinol-9′-O-β-d-fucopyranoside (18) (3.0 mg) and albibrissinoside B (21) (11.2 mg). Fraction 3K was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 20–80%) elution solvent to give (+)-(8S,7′S, 8′S)-burselignan-9′-O-β-D-glucopyranoside (17) (7.3 mg). Fraction 3L was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 30%) elution solvent to give khaephuoside B (22) (6.7 mg). Fraction 3M was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 5–60%) elution solvent to give (8R,7′S,8′R)-5,5′-dimethoxylariciresinol 4′-O-β-d-glucopyranoside (11) (2.8 mg), alangilignoside C (13) (7.5 mg), secoisolariciresinol (15) (11.1 mg), and icariside E5 (19) (10.5 mg). Fraction 3N was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 5–55%) elution solvent to give (−)-syringaresinol-4-O-β-d-glucopyranoside (4) (5.1 mg), (−)-syringaresinol-4-O-β-d-apiofuranosyl-(1→2)-β-d glucopyranoside (6) (7.5 mg), and manglieside E (12) (3.5 mg). Fraction 3Q was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 25%) elution solvent to give obtusifoside A (5) (22.5 mg). Fraction 3S was separated using YMC (1.0 × 80 cm) column chromatography with an MeOH-H2O (MeOH 3–20%) elution solvent to give (7S,8R)-picraquassioside C (20) (12.0 mg).
Fraction 4 (10.0 g) was subjected to silica gel (3.0 × 80 cm) column chromatography with CHCl3-MeOH (MeOH 3–45%) elution solvent to give 13 sub-fractions (Fr. 4A–4M). Fraction 4A was separated using YMC (1.0 × 80 cm) column chromatography with an acetone-H2O (acetone 20–60%) elution solvent to give (−)-pinoresinol β-D-glucopyranoside (8) (2.1 mg) and dihydrosyringin (26) (13.0 mg). Fraction 4C was separated using YMC (1.0 × 80 cm) column chromatography with an acetone-H2O (acetone 20–40%) elution solvent to give julibrissinoside (16) (7.9 mg) and leonuriside A (23) (7.9 mg). Fraction 4D was separated using YMC (1.0 × 80 cm) column chromatography with an acetone-H2O (acetone 20–70%) elution solvent to give simplexoside (7) (7.7 mg), coniferin (24) (10.0 mg), and syringin (25) (15.0 mg).
Julibrissinoside (16): brown amorphous powder; : –38.3 (c 0.1, MeOH); 1H NMR (methanol-d4, 600 MHz) and 13C NMR data (methanol-d4, 150 MHz), see Table 1; HR-ESI-MS: m/z 769.2890 [M + Na]+ (calcd. for 769.2890).
4.4. Enzymatic Hydrolysis
Compound 16 (3.0 mg) was mixed with β-glucosidase (3.0 mg) in water (1.0 mL) and the solution was shaken in a water bath at 37 °C for 12 h. Subsequently, the reaction mixture was concentrated and subjected to silica gel column chromatography (1.0 × 15.0 cm, 40–63 μm) using CHCl3-MeOH (15:1, 60 mL) and CHCl3-MeOH-H2O (7:3:0.5, 60 mL) as the solvent systems to afford aglycone 16a (1.2 mg) and a sugar fraction. The sugar fraction was concentrated until dried using N2 gas. The resulting residue was dissolved in anhydrous pyridine (0.1 mL), and L-cysteine methyl ester hydrochloride in pyridine (0.06 M, 0.1 mL) was then added to the solution. Following heating the reaction mixture at 60 °C for 2 h, 0.1 mL of trimethylsilylimidazole was added. Heating at 60 °C was continued for another 1.5 h. The dried product was partitioned with n-hexane and H2O (0.1 mL each), and the organic layer was analyzed using gas chromatography (GC): DB-5 capillary column (0.32 mm × 30 m); FID detector; column temp., 210 °C; injector temp., 270 °C; detector temp., 300 °C; carrier gas, He (2 mL/min). Under these conditions, the standard sugars gave peaks at tR (min) = 14.12 and 12.24 for L- and D-glucose, respectively. The peak of the hydrolysate of 16 was detected at tR (min) = 12.21, which was identified as D-glucose by comparison with the retention time of the authentic samples following treatment with trimethylsilylimidazole in pyridine.
4.5. Cell Culture and Stimulation
The RAW 264.7 cells were obtained from the Korean Cell Line Bank (KCLB, Chongno-gu, Seoul, Korea) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin at 37 °C in a humidified incubator containing 5% CO2. The cells were cultured at 2 × 105 cells/mL in RPMI 1640 medium containing 10% FBS in 96-well tissue culture plates for 18 h and were subsequently pretreated with 0.4, 2, 10, 50, and 100 μM of the analyzed compounds 1 h prior to stimulation with LPS (1 μg/mL) for 24 h in an incubator.
4.6. Cell Viability
The cell viability was evaluated using the MTT method. Briefly, MTT was added to the cell culture medium for 4 h. The supernatant was then removed and the formazan crystals were dissolved in dimethyl sulfoxide (DMSO). The absorbance was measured at 540 nm. The percentage of dead cells was determined relative to the control group.
4.7. Nitric Oxide Assay
Nitrite, which accumulated in the culture medium, was measured using the Griess reaction as an indicator for the NO production. Briefly, the cell culture medium (100 μL, without phenol red) was mixed with an equal volume of the Griess reagent containing equal volumes of 1% (w/v) sulfanilamide in 5% (v/v) H3PO4 and 0.1% (w/v) naphthylethylenediamine-HCl. The solution was incubated at room temperature for 20 min, after which the absorbance was measured at 520 nm using a microplate reader. Fresh culture medium was employed as the blank in all experiments. The amounts of nitrite in the samples were established using the NaNO2 serial dilution standard curve.
4.8. Statistical Analysis
All data are represented as means ± SD of at least three independent experiments performed in triplicate. Statistical significance is indicated as determined by one-way ANOVA, followed by Dunnett’s multiple comparison test (p < 0.05) utilizing GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA).
Author Contributions
W.L. performed the isolation, structure elucidation of the constituents, and prepared the manuscript. H.J.Y. conducted the isolation and bioassay experiments. The whole research was performed based on the planning of W.L. All authors approved the final version of the manuscript.
Funding
The present study was supported by Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education (Grant number NRF2020R1C1C1006749), Republic of Korea.
Conflicts of Interest
The authors declare no competing financial interests.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Kim T., Park C., Choi K., Jeong J., Kang I., Park S.-J., Chae C. Comparison of Two Commercial Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Modified Live Vaccines against Heterologous Type 1 and Type 2 PRRSV Challenge in Growing Pigs. Clin. Vaccine Immunol. 2015;22:631–640. doi: 10.1128/CVI.00001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu D.-H., Qiao S.-Y., Zhao Y.-M. Advances in study on bark of Albizzia julibrissin. China J. Chin. Mater. Med. 2004;29:619–624. [PubMed] [Google Scholar]
- 3.Youssef N.A., Rich C.L. Does Acute Treatment with Sedatives/Hypnotics for Anxiety in Depressed Patients Affect Suicide Risk? A Literature Review. Ann. Clin. Psychiatry. 2008;20:157–169. doi: 10.1080/10401230802177698. [DOI] [PubMed] [Google Scholar]
- 4.Karim A.A., Azlan A. Fruit Pod Extracts as a Source of Nutraceuticals and Pharmaceuticals. Molecules. 2012;17:11931–11946. doi: 10.3390/molecules171011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau C.S., Danielle J., Carrier D.J., Beitle R.R., Bransby D.I., Howard L.R., Lay J.O., Liyanage R., Clausen E.C. Identification and quantification of glycoside Xavonoidsin the energy crop Albizia julibrissin. Bioresour. Technol. 2007;98:429–443. doi: 10.1016/j.biortech.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi H., Kinjo J., Nohara T. An arrhythmic-inducing glycoside from Albizia julibrissin Durazz. IV. Chem. Pharm. Bull. 1992;40:829–831. doi: 10.1248/cpb.40.829. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T., Fujiwara S., Araki K., Kinjo J., Nohara T., Miyoshi T. Cytotoxic Glycosides from Albizia julibrissin. J. Nat. Prod. 1997;60:102–107. doi: 10.1021/np960556t. [DOI] [PubMed] [Google Scholar]
- 8.Chen S.P., Zhang R.Y. Studies on the triterpene sapogenins from Albizziae Cortex. Yao Xue Xue Bao (Acta Pharm. Sin.) 1997;32:144–147. [PubMed] [Google Scholar]
- 9.Chamsuksai P., Choi J.S., Woo W.W. 3′-4′-7-Trihydroxyflavone in Albizzia julibrissin. Arch. Pharm. Res. 1981;4:129–131. doi: 10.1007/BF02855756. [DOI] [Google Scholar]
- 10.Woo W.S., Kang S.S. Isolation of a new monoterpene conjugated tripenoid from the stem bark of Albizzia julibrissin. J. Nat. Prod. 1984;49:547–549. doi: 10.1021/np50033a029. [DOI] [PubMed] [Google Scholar]
- 11.Kang T.H., Jeong S.T., Kim N.Y., Higuchi R., Kim Y.C. Sedactive activity of two flavonol glycosides isolated from the flower of Albizzia julibrissin Durazz. J. Ethnopharmacol. 2000;71:321–323. doi: 10.1016/S0378-8741(99)00202-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim W.-K., Jung J.W., Ahn N.Y., Oh H.R., Lee B.K., Oh J.K., Cheong J.H., Chun H.S., Ryu J.H. Anxiolytic-like effects of extracts from Albizzia julibrissin bark in the elevated plus-maze in rats. Life Sci. 2004;75:2787–2795. doi: 10.1016/j.lfs.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 13.China Pharmacopoeia Committee . Pharmacopoeia of the People’s Republic of China, the First Division of 2005 Edition ed. China Chemical Industry Press; Beijing, China: 2005. [Google Scholar]
- 14.Tong W., Mi L., Liang H., Zhao Y. Isolation and identification of chemical constituents from Albizia julibrissin Durazz. Beijing Da Xue Xue Bao Yi Xue Ban (J. Peking Univ. Health Sci.) 2003;35:180–183. [PubMed] [Google Scholar]
- 15.Jing Y., Zhang Y.-F., Shang M.-Y., Liu G.-X., Li Y.-L., Wang X., Cai S.-Q. Chemical Constituents from the Roots and Rhizomes of Asarum heterotropoides var. mandshuricum and the In Vitro Anti-Inflammatory Activity. Molecules. 2017:125. doi: 10.3390/molecules22010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okunishi T., Umezawa T., Shimada M. Enantiomeric compositions and biosynthesis ofWikstroemia sikokiana lignans. J. Wood Sci. 2000;46:234–242. doi: 10.1007/BF00776455. [DOI] [Google Scholar]
- 17.Ding Y., Liang C., Choi E.M., Ra J.C., Kim Y.H. Chemical constituents from Artemisia iwayomogi increase the function of osteoblastic MC3T3-E1 cells. Nat. Prod. Sci. 2009;15:192–197. [Google Scholar]
- 18.Liu Z.-Z., Zhan Z.-L., Liu F., Yang Y., Feng Z.-M., Jiang J.-S., Zhang P. Acyl glycosides lignans, coumarins, and terpenes from the stems of Erycibe obtusifolia. Carbohydr. Res. 2013;372:47–54. doi: 10.1016/j.carres.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Li W., Sun Y.-N., Yan X.T., Yang S.Y., Kim E.-J., Kang H.K., Kim Y.-H. Coumarins and Lignans from Zanthoxylum schinifolium and Their Anticancer Activities. J. Agric. Food Chem. 2013;61:10730–10740. doi: 10.1021/jf403479c. [DOI] [PubMed] [Google Scholar]
- 20.Kim M.-R., Moon H.T., Lee D.G., Woo E.-R. A new lignan glycoside from the stem bark of styrax japonica S. et Z. Arch. Pharmacal Res. 2007;30:425–430. doi: 10.1007/BF02980215. [DOI] [PubMed] [Google Scholar]
- 21.Duan H., Takaishi Y., Momota H., Ohmoto Y., Taki T. Immunosuppressive constituents from Saussurea medusa. Phytochemistry. 2002;59:85–90. doi: 10.1016/S0031-9422(01)00429-0. [DOI] [PubMed] [Google Scholar]
- 22.Ida Y., Satoh Y., Ohtsuka M., Nagasao M., Shoji J. Phenolic constituents of phellodendron amurense bark. Phytochemistry. 1993;35:209–215. doi: 10.1016/S0031-9422(00)90536-3. [DOI] [Google Scholar]
- 23.Van Kiem P., Tri M.D., Tuong L.V.D., Tung N.H., Hanh N.N., Quang T.H., Cuong N.X., Van Minh C., Choi E.-M., Kim Y.-H. Chemical constituents from the leaves of Manglietia phuthoensis and their effects on osteoblastic MC3T3-E1 cells. Chem. Pharm. Bull. 2008;56:1270–1275. doi: 10.1248/cpb.56.1270. [DOI] [PubMed] [Google Scholar]
- 24.Fu L.-C., Huang X.-A., Lai Z.-Y., Hu Y.-J., Liu H.-J., Cai X.-L. A New 3-Benzylchroman Derivative from Sappan Lignum (Caesalpinia sappan) Molecules. 2008;13:1923–1930. doi: 10.3390/molecules13081923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achenbach H., Benirschke M., Torrenegra R. Alkaloids and other compounds from seeds of Tabernaemontana cymosa. Phytochemistry. 1997;45:325–335. doi: 10.1016/S0031-9422(96)00645-0. [DOI] [Google Scholar]
- 26.Zhang C.F., Zhou J., Yang J.Z., Li C.J., Ma J., Zhang D., Li L., Zhang D.M. Three new lignanosides from the aerial parts of Lespedeza cuneate. J. Asian Nat. Prod. Res. 2016;18:913–920. doi: 10.1080/10286020.2016.1187603. [DOI] [PubMed] [Google Scholar]
- 27.Li W., Zhou W., Shim S.H., Kim Y.-H. Chemical constituents of Zanthoxylum schinifolium (Rutaceae) Biochem. Syst. Ecol. 2014;55:60–65. doi: 10.1016/j.bse.2014.02.028. [DOI] [Google Scholar]
- 28.Sun J., Xun H., Yu J., Tang F., Yue Y.-D., Guo X.-F. Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”. Molecules. 2015;20:15686–15700. doi: 10.3390/molecules200915686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung M.J., Kang S.S., Jung Y.J., Choi J.S. Phenolic glycosides from the stem bark of Albizzia julibrissin. Chem. Pharm. Bull. 2004;52:1501–1503. doi: 10.1248/cpb.52.1501. [DOI] [PubMed] [Google Scholar]
- 30.Kanchanapoom T., Kasai R., Yamasaki K. Phenolic glycosides from Markhamia stipulate. Phytochemistry. 2002;59:557–563. doi: 10.1016/S0031-9422(01)00466-6. [DOI] [PubMed] [Google Scholar]
- 31.Lin S., Wang S., Liu M., Gan M., Li S., Yang Y., Wang Y., He W., Shi J. Glycosides from the Stem Bark ofFraxinussieboldiana. J. Nat. Prod. 2007;70:817–823. doi: 10.1021/np0700467. [DOI] [PubMed] [Google Scholar]
- 32.Jin Q., Lee J.W., Kim J.G., Lee D., Hong J.T., Kim Y., Lee M.K., Hwang B.Y. Lignans from Saururus chinensis with Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2019;82:3002–3009. doi: 10.1021/acs.jnatprod.9b00520. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y., Nasu M., Kano M., Kameoka H., Okuyama T., Nishizawa M., Ikeya Y. Lignans from guaiac resin decrease nitric oxide production in interleukin 1β-treated hepatocytes. J. Nat. Med. 2016;71:190–197. doi: 10.1007/s11418-016-1048-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D., Li J., Ruan D., Chen Z., Zhu W., Shi Y., Chen K., Li Y., Wang R. Lignans from Isatis indigotica roots and their inhibitory effects on nitric oxide production. Fitoterapia. 2019 doi: 10.1016/j.fitote.2019.104189. [DOI] [PubMed] [Google Scholar]