Abstract
Despite numerous reports on the beneficial effects of catechin or epicatechin contained in tea and cacao extract on human health, a conclusive and precise molecular mechanism has not been elucidated. Metabolism of chemical compounds in gut microbiota recently gained significant attention, and extensive studies have been devoted in this field. In conjunction with these results, our group focused on the anti-inflammatory effects of both enantiomers of DHPV (5-(3′,4′-dihydroxyphenyl)-γ-valerolactone), produced in the intestine by microbiota metabolism, on IEC-6 cells. Divergent and efficient enantioselective synthesis of (S)- and (R)-DHPV was efficiently achieved by cross-metathesis and Sharpless asymmetric dihydroxylation as a key reaction for four steps in 16% and 14% overall yields, respectively. The anti-inflammatory effects of two enantiomers were tested on IEC-6 cells, and we found that (S)-DHPV was more active than (R)-DHPV. This result implicates that the metabolite produced in the gut has beneficial effects on IEC-6 cells of rat intestines, and the chirality of the metabolite is important for its anti-inflammatory activity. This also provided information for the future discovery of novel small molecular therapeutics for the treatment of inflammatory bowel disease.
Keywords: divergent synthesis, asymmetric dihydroxylation, DHPV, anti-inflammatory effect, NF-κB, IκBα
1. Introduction
Inflammatory bowel disease (IBD) is a chronic and multifactorial inflammatory condition of the gastrointestinal tract, related to an infectious and immunological imbalance of the intestinal mucosa [1,2]. The incidence and prevalence of IBD has relentlessly climbed for past several decades [3], and it is known to be one of the most important risk factors for developing colorectal cancer [4]. IBD is most commonly classified into two principal types: Crohn’s disease (CD) and ulcerative colitis (UC). CD can involve any segment of the gastrointestinal tract and causes non-continuous whole layer inflammation, while UC occurs in the inner lining of the large intestine or rectum and causes continuous inflammation of the colonic mucosa [5,6]. This involves immunity, damage, and patient’s symptoms such as diarrhea and hematochezia [7]. These responses are controlled by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which are master regulators of the inflammatory reaction. Dysregulation of NF-κB signaling is well known to patients with IBD and are associated with the rapid and acute production of diverse pro-inflammatory mediators [8]. Therefore, pharmacological attempts to block the activation of NF-κB signaling are needed to develop new therapeutic strategies in IBD.
Metabolism in the body plays an important role in the elimination of xenobiotics absorbed by diverse route. Among them, the liver is the major organ contributing to these transformations of phase I and phase II metabolisms [9]. Sometimes, metabolites produced in these biological processes can be newly discovered therapeutics, for example cetirizine or oxazepam. Recently, growing interests in metabolism on the intestine and its effects on brain have also been reported.
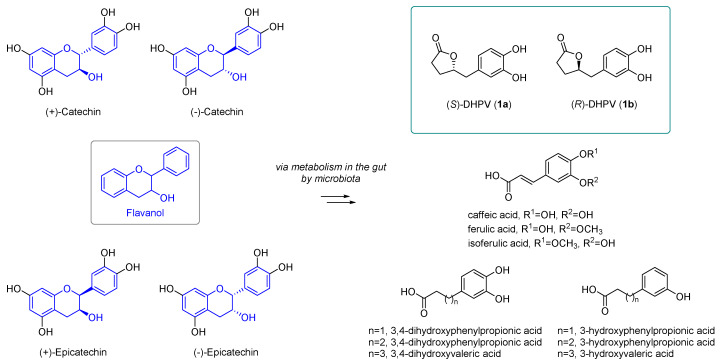
Flavanols, such as (−)-catechin, (−)-epicatechin, and each enantiomer, are widely found in red wine, green tea, and cocoa extract (Figure 1). Catechins exist in four isomers, and the enantiomers can be separated by chiral HPLC chromatography [10]. Many research groups have investigated the molecular mechanism, signaling pathway, and metabolisms of polyphenols due to their well-known antioxidant and free radical scavenging activities. However, beneficial effects of these compounds on human health were not conclusive, because these compounds are not readily absorbed into the body as their original structures. Rather, these flavanols undergo metabolism into smaller structures in the gut by microbiota. These metabolites, produced in the intestine, were smoothly absorbed into the bloodstream, even though their potency was decreased compared to the parent molecule.
Figure 1.
Structures of catechin, epicatechin, and metabolites, including 5-(3′,4′-dihydroxyphenyl)-γ-valerolactones (DHPVs).
Catechin and epicatechin undergo intestinal metabolism, producing diverse metabolites including caffeic acid, ferulic acid, isoferulic acid, and so on (Figure 1) [11]. Among the various microbial metabolites from polyphenols in the human body, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (DHPV) is the representative metabolite of flavanols containing the intact catechin and epicatechin moiety. DHPV, well-known for its antioxidant activities, such as its protective effect against UVB induced wrinkle formation and neuroprotective effect in the brain, can exist as two enantiomers by the stereocenter in γ-position of butyrolactone moiety. However, their structures are represented in ambiguous or racemic forms in numerous literatures, and we became interested in confirming which DHPV enantiomer exhibited more potent biological activities, because there are numerous reports on the completely different biological activities of enantiomers [12,13].
Herein, we reported the enantioselective synthesis of (S)- and (R)-DHPV by the divergent and efficient approach. We also evaluated the effects of both enantiomers on lipopolysaccharide (LPS) induced phosphorylation and degradation of inhibitor of κB alpha (IκBα), the major key regulators of NF-κB signaling pathway, to test the mechanism of anti-inflammatory effects on IEC-6 cells.
2. Results and Discussion
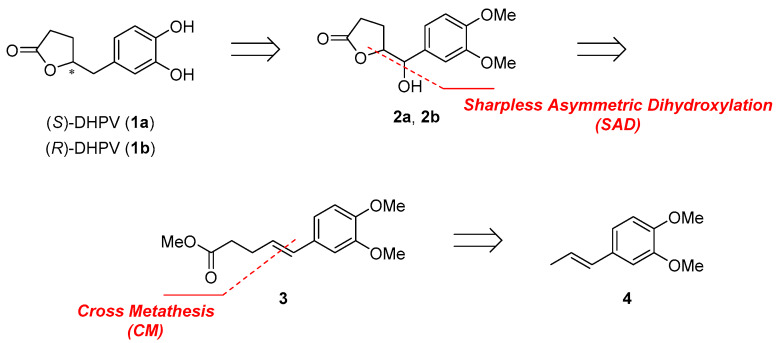
2.1. Retrosynthetic Analysis of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone (DHPV)
Until now, four groups completed the racemic or enantioselective total synthesis of DHPV. The Watanabe [14] group reported the first racemic synthesis of DHPV from benzyl epoxide as a key intermediate in a four step synthesis, and the Lambert [15] group also reported the racemic synthesis of DHPV via Heck reaction and dehydrative lactonization for a total of eight synthetic steps. The first enantioselective synthesis of DHPV was completed by the Nakajima [16,17] group from optically pure epoxide in seven steps, and the Curti [18] group achieved the relatively recent asymmetric synthesis of DHPV via the concise and catalytic enantioselective Mukaiyama aldol reaction in six steps. All of the previous reports of DHPV were accomplished in four to eight steps, but the only racemic DHPV was obtained through the minimal synthetic pathway.
Our group planned the divergent synthesis and biological evaluation of both enantiomers. Representative retrosynthetic analysis of DHPV was summarized in Scheme 1. (S) or (R)-DHPV could be obtained by deoxygenation of benzylic alcohol and demethylation from 2a or 2b [19,20]. δ-Hydroxy-γ-valerolactone (2a, 2b) could be formed by Sharpless Asymmetric Dihydroxylation (SAD) [21] and spontaneous lactonization from common intermediate alkene 3. Alkene 3 can be obtained from 4 by cross metathesis, and styrene 4 was used to lower homo-dimerization of terminal olefin [22]. Although we planned the synthesis of γ,δ-unsaturated ester 3 by Johnson–Claisen rearrangement [15] instead of cross metathesis at the beginning of this project, we couldn’t obtain meaningful result.
Scheme 1.
Retrosynthetic analysis of DHPV.
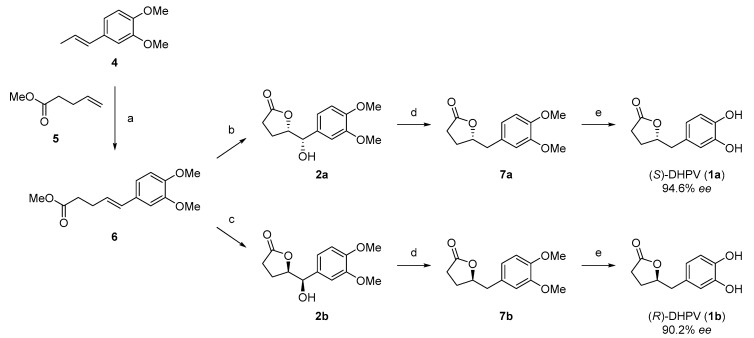
2.2. Divergent Synthesis of DHPV
Synthesis of (S)- and (R)-DHPV began with construction of the phenylpentanoate scaffold (Scheme 2). Cross metathesis of commercially available (E)-3,4-dimethoxyphenylpropene (4) and methyl 4-pentenoate (5) by Grubbs 2nd generation catalyst afforded the desired product 6 in 59% yield. Interestingly, only (E)-olefinic isomer was obtained, which was identified through 1H-NMR analysis (Supplementary Materials). One pot Sharpless asymmetric dihydroxylation (AD-mix-α and AD-mix-β, respectively) and concomitant lactonization of alkene 6 provided 2a and 2b in 62% and 55% for each isomer, respectively. Then, alcohols 2a and 2b were subjected to hydrogenolysis, providing 7a and 7b by Pearlman’s catalyst in good yields of 72% and 68%, which were transformed to 1a and 1b by demethylation with BBr3 in 60% and 62%, respectively. The 1H- and 13C-NMR spectral data of both enantiomers were compared and matched well. A detailed summary of the spectrum was represented in Supplementary Materials.
Scheme 2.
Divergent and enantioselective synthesis of DHPV. (a) 4, 5, Grubbs 2nd generation catalyst, CH2Cl2, reflux, 59% (70% BORSM); (b) AD-mix-α, MeSO2NH2, t-BuOH/H2O (1/1), 62%; (c) AD-mix-β, MeSO2NH2, t-BuOH/H2O (1/1), 55%; (d) Pd(OH)2, H2, MeOH, r.t. 72% (7a) and 68% (7b); and (e) BBr3, CH2Cl2, 0 °C to r.t., 60% (1a) and 62% (1b).
The enantiomeric purities of asymmetric dihydroxylation products, 2a and 2b, and final (S)- and (R)-DHPV, 1a and 1b, were confirmed by the analytical chiral HPLC experiment (Supplementary Materials), according to previous report of Suh group [23]. The synthetic final enantiomers were pure enough for further biological study, with the enantioselectivity of 97.3:2.7 for 1a and 95.1:4.9 for 1b.
2.3. Anti-Inflammatory Activity of (R)- and (S)-DHPV on IEC-6 Cells
NF-κB is a major regulator of the immune system, and the activation of NF-κB induces inflammation and carcinogenesis [6,8,24]. Dysregulated immune responses were implicated in the pathogenesis of IBD, and the transcription factor, NF-κB, was found to be one of the key regulatory components in this complex scenario [25]. It is well known that the expression and activation of NF-κB was strongly induced in the inflamed gut of IBD patients. In particular, macrophages and epithelial cells isolated from inflamed gut specimens from IBD patients showed increased levels of the NF-κB subunit [26]. Interestingly, the amount of activated NF-κB significantly correlated with the severity of intestinal inflammation [27]. Classic activation of NF-κB can be initiated by various stimuli, including bacterial cell wall components like lipopolysaccharide (LPS), pro-inflammatory cytokines, viruses, and DNA damaging agents. These triggering substances stimulate a phosphorylation of Iκβs, and the translocation of NF-κB into the nucleus promotes the transcription of genes that encode inflammation-related proteins [28,29]. Therefore, the measurement of phosphorylation and degradation of IκBα could be the simple but valid assay for the anti-inflammatory ability of synthesized compounds through the suppression of NF-κB in IBD.
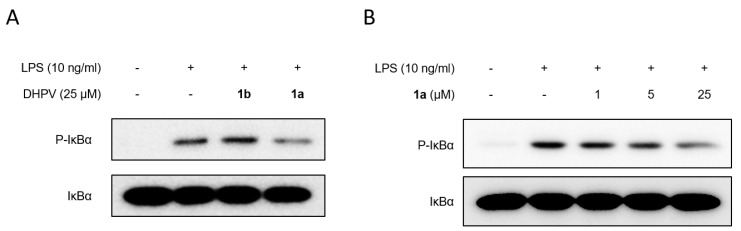
To evaluate the preventive effects of DHPVs against inflammatory responses, we pretreated rat intestinal epithelial IEC-6 cells with each DHPV (25 μM) for 1 h prior to the LPS stimulation (Figure 2A). The inhibitory effect of (R)-DHPV (1b) and (S)-DHPV (1a) on phosphorylation and degradation of IκBα expression were tested under the same conditions for comparison. We observed that the inhibitory effect of (S)-DHPV on the phosphorylation and degradation of IκBα was more significant than that of (R)-DHPV in LPS-treated IEC-6 cells (Figure 2A). Comparing the anti-inflammatory effects of (S)-DHPV with (R)-DHPV, in terms of suppression of phosphorylation and degradation of IκBα, (S)-DHPV was found to be more effective in suppressing NF-κB signaling than (R)-DHPV. We also observed that (S)-DHPV (1a) downregulated LPS-induced phosphorylation and degradation of IκBα in a dose dependent manner (Figure 2B). These results indicated that (S)-DHPV can protect against LPS-induced inflammation by inhibiting NF-κB activation through repression of IκBα degradation.
Figure 2.
Biological activity of synthesized (R)- and (S)-DHPV. (A) The effects of 1b (R)-DHPV (25 μM) and 1a (S)-DHPV (25 μM) on the phosphorylation and degradation of IκBα were determined by Western blot analysis in IEC-6 cells exposed to lipopolysaccharides (LPS; 10 ng/mL) for 1 h. (B) The effect of 1a (S)-DHPV on the expression of phosphorylation and degradation of IκBα was analyzed dose dependently by Western blot analysis in LPS (10 ng/mL)-treated IEC-6 cells.
3. Materials and Methods
3.1. General Information
3.1.1. General Methods and Materials
Unless otherwise noted, all the reactions were run under nitrogen (N2) atmosphere in anhydrous condition. DHPVs were dissolved in dimethyl sulfoxide (DMSO) as stock solution and stored at −20 °C. DMSO was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Lipopolysaccharide (LPS) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies for Phosphorylated-IkBα and total IkBα were purchased from Cell Signaling Technology (Danvers, MA, USA).
3.1.2. Materials
All reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) or TCI (Tokyo, Japan), and solvents were purchased from Daejung (Siheung, Korea) and used without further purification. Dichloromethane (CH2Cl2) was distilled from calcium hydride (CaH2). Methanol (MeOH) was distilled from magnesium sulfate (MgSO4).
3.1.3. Instrumentation
1H and 13C-spectra were recorded on a 500 MHz JNM-ECZ 500R (JEOL, Tokyo, Japan). Chemical shifts are recorded as a δ value (δ 7.26 for 1H and δ 77.0 for 13C). Data were represented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br = broad), integration, coupling constant in Hz, and assignment. Infrared (IR) spectra were measured on a 1600 FT–IR spectrometer (Perkin-Elmer, Waltham, MA, USA). High resolution mass spectrometry data were recorded by JMS-700 (JEOL, Tokyo, Japan) (FAB or ESI), and values were calculated to four decimal places from the molecular formula. Melting point (m.p.) was obtained using MEL-TEMP® (Cole-Parmer, Staffordshire, UK).
3.2. Experimental Part
(E)-Ethyl-5-(3,4-dimethoxyphenyl)pent-4-enoate (6). To a stirred solution of ethyl pent-4-enoate (967mg, 7.54 mmol) and 4-(1-propenyl)-1,2-dimethoxybenzene (2.67 g, 15.1 mmol, 2.0 equiv.) in CH2Cl2 (50 mL) was added Grubbs 2nd generation catalyst (310 mg, 0.38 mmol, 0.05 equiv.) and then heated to reflux for 6 h. Then, the reaction mixture was cooled to room temperature, and solvent was removed under reduced pressure. EtOAc/n-hexane (1:10, 30 mL) was added, and the solid was filtered to provide the 1.76 g dimer of styrene. The filtrate was purified by silica gel column chromatography to afford 6 (1.17 g, 4.44 mmol), a colorless oil with recovered ethyl pent-4-enoate (160 mg); Yield 59% (70% BORSM); 1H-NMR (500 MHz, CDCl3) δ 6.88 (d, J = 1.7 Hz, 1H), 6.85 (dd, J = 8.0, 1.7 Hz, 1H), 6.78 (d, J = 8.5 Hz, 1H), 6.35 (d, J = 15.5 Hz, 1H), 6.06 (dt, J = 16.0, 6.6 Hz, 1H), 4.13 (q, J = 7.3 Hz, 2H), 3.88 (s, 3H), 3.86 (s, 3H), 2.52–2.44 (m, 4H), and 1.24 (t, J = 7.2 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.1, 149.0, 148.5, 130.6, 130.6, 126.6, 119.1, 111.1, 108.6, 60.4, 55.9, 55.8, 34.2, 28.3, and 14.3; FT-IR (thin film, neat) νmax 2980, 2358, 2341, 2040, 1732, 1514, 1263, 1024, and 966 cm−1; and HRMS (ESI+) found 265.1444 (calculated for C15H21O4 ([M + H]+): 265.1434).
(S)-5-((S)-(3,4-dimethoxyphenyl)(hydroxy)methyl)dihydrofuran-2(3H)-one (2a). To a stirred solution of AD-mix-α (1.47 g, 1.40g/mmol) and methane sulfonamide (120.9 mg, 1.27 mmol, 1.21 equiv.) in t-BuOH/H2O (1:1, 30 mL) was added 6 (280 mg, 1.05 mmol) in t-BuOH (10 mL) and stirred at 0 °C for 72 h. Then, EtOAc (100 mL) was added to the reaction mixture and the organic layer was separated. The aqueous layer was extracted again with EtOAc (two times, 50 mL) and the combined organic layer was washed with H2O (two times, 10 mL). Then, the organic layer was dried over MgSO4 and the solvent was removed under reduced pressure. The lactone was purified by silica gel column chromatography (EtOAc/n-hexane, 2:1) to afford 2a (164 mg, 0.65 mmol) as a white solid; Yield 62%; m.p. 138–140 °C; 1H-NMR (500 MHz, CDCl3) δ 6.91 (d, J = 1.7 Hz, 1H), 6.89 (dd, J = 8.0, 1.7 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 4.63–4.59 (m, 2H), 3.87 (s, 3H), 3.86 (s, 3H), 2.50–2.43 (m, 3H), and 2.03–1.95 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ 177.0, 149.3, 149.2, 130.8, 119.4, 111.0, 109.7, 83.5, 76.3, 56.0, 55.9, 28.5, and 24.0; FT–IR (thin film, neat) νmax 3477, 2939, 1770, 1514, 1263, and 1095 cm−1; and HRMS (ESI+) found 252.0979 (calculated for C13H16O5 ([M]+): 252.0992).
(R)-5-((R)-(3,4-dimethoxyphenyl)(hydroxy)methyl)dihydrofuran-2(3H)-one (2b). This reaction was conducted with AD-mix-β, instead of AD-mix-α. Yield 55%, white solid; m.p. 129–131 °C; 1H-NMR (500 MHz, CDCl3) δ 6.93 (d, J = 2.3 Hz, 1H), 6.91 (dd, J = 8.6, 1.7 Hz, 1H), 6.85 (d, J = 8.6 Hz, 1H), 4.70–4.62 (m, 2H), 3.88 (s, 3H), 3.87 (s, 3H), 2.75 (bs, 1H), 2.49–2.43 (m, 2H), and 2.03–1.99 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ 177.2, 149.2, 149.2, 130.9, 119.4, 111.0, 109.8, 83.6, 76.2, 56.0, 55.9, 28.5, and 24.0; FT–IR (thin film, neat) νmax 3477, 2939, 1770, 1516, 1263, 1184, and 1141 cm−1; HRMS (ESI+) found 252.1003 (calculated for C13H16O5 ([M]+): 252.0992).
(S)-5-(3,4-dimethoxybenzyl)dihydrofuran-2(3H)-one (7a). To a stirred solution of 2a (150 mg, 0.603 mmol) in MeOH (20 mL) and N2 atmosphere was added Pd(OH)2 on activated charcoal (20 mg). Then, nitrogen gas was removed under reduced pressure, and the reaction mixture was charged with a hydrogen gas balloon. After 6 h, the reaction mixture was filtered through a Celite pad, and solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography (EtOAc/n-hexane, 1:1) to afford 2a (102 mg, 0.43 mmol), a colorless oil; Yield 72%; 1H-NMR (500 MHz, CDCl3) δ 6.79 (d, J = 8.1 Hz, 1H), 6.75–6.71 (m, 2H), 4.71 (quintet, J = 6.4 Hz, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 2.96 (dd, J = 14.4, 5.8 Hz, 1H), 2.89 (dd, J = 14.3, 6.3 Hz, 1H), 2.50–2.22 (m, 3H), and 1.96–1.89 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ 177.2, 149.0, 148.1, 128.3, 121.6, 112.6, 111.4, 111.3, 80.9, 55.9, 55.9, 28.7, and 27.0; FT-IR (thin film, neat) νmax 3522, 2937, 1770, 1734, 1514, 1261, 1178, 1157, 1141, and 1026 cm−1; HRMS (ESI+) found 237.1129 (calculated for C13H17O4 ([M + H]+): 237.1121).
(R)-5-(3,4-dimethoxybenzyl)dihydrofuran-2(3H)-one (7b). Yield 68%, colorless oil; 1H-NMR (500 MHz, CDCl3) δ 6.80 (d, J = 8.0 Hz, 1H), 6.76–6.73 (m, 2H), 4.71 (quintet, J = 6.9 Hz, 1H), 3.86 (s, 3H), 3.85 (s, 3H), 2.97 (dd, J = 14.3, 5.8 Hz, 1H), 2.89 (dd, J = 14.4, 5.8 Hz, 1H), 2.47–2.23 (m, 3H), and 1.97–1.89 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ 177.2, 149.0, 148.2, 128.4, 121.6, 112.7, 113.4, 80.9, 56.0, 55.9, 40.9, 28.7, and 27.0; FT–IR (thin film, neat) νmax 3477, 2939, 1772, 1516, 1263, 1184, 1143, and 1026 cm−1; HRMS (ESI+) found 237.1125 (calculated for C13H17O4 ([M + H]+): 237.1121).
(S)-5-(3,4-dihydroxybenzyl)dihydrofuran-2(3H)-one (1a). To a stirred solution of 7a (50 mg, 0.212 mmol) in CH2Cl2 (10 mL) was added to BBr3 (100 µL, 1.06 mmol, 5.0 equiv.) at 0 °C, and the reaction mixture was warmed to room temperature. Then, the reaction mixture was quenched with H2O (10 mL) at 0 °C, and the organic layer was separated. The aqueous layer was extracted again with CH2Cl2 (two times, 50 mL), and the combined organic layer was washed with H2O (two times, 10 mL). Then, the organic layer was dried over MgSO4, and the solvent was removed under reduced pressure. The lactone was purified by silica gel column chromatography (CH2Cl2/MeOH = 10:1) to afford 1a (26 mg, 0.127 mmol), a white solid. Yield 60%; m.p. 163–165 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.74 (bs, 2H), 6.60 (d, J = 8.0 Hz, 1H), 6.58 (d, J = 2.3 Hz, 1H), 6.44 (dd, J = 8.0, 2.3 Hz, 1H), 4.58 (quintet, J = 6.7 Hz, 1H), 2.73 (dd, J = 13.8, 6.9 Hz, 1H), 2.66 (dd, J = 13.7, 5.8 Hz, 1H), 2.41 (dd, J = 17.8, 8.6 Hz, 1H), 2.31 (ddd, J = 17.8, 9.8, 5.2 Hz, 1H), 2.14–2.07 (m, 1H), and 1.83–1.76 (m, 1H); 13C-NMR (125 MHz, DMSO-d6) δ 177.6, 145.5, 144.4, 128.0, 120.5, 117.2, 115.9, 81.3, 40.2, 28.7, and 27.1; FT–IR (thin film, neat) νmax 3404, 1575, 1517, 1273, and 1186 cm−1; HRMS (ESI+) found 209.0811 [calculated for C11H13O4 ([M + H]+): 209.0808].
(R)-5-(3,4-dihydroxybenzyl)dihydrofuran-2(3H)-one (1b). Yield 62%, white solid; m.p. 154−156 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.78 (bs, 2H), 6.60 (d, J = 7.5 Hz, 1H), 6.57 (d, J = 1.7 Hz, 1H), 6.44 (dd, J = 8.0, 2.3 Hz, 1H), 4.58 (quintet, J = 6.7 Hz, 1H), 2.73 (dd, J = 14.3, 6.8 Hz, 1H), 2.65 (dd, J = 14.3, 6.5 Hz, 1H), 2.41 (dd, J = 17.8, 9.2 Hz, 1H), 2.31 (ddd, J = 17.2, 9.2, 4.6 Hz, 1H), 2.13–2.08 (m, 1H), and 1.83–1.75 (m, 1H); 13C-NMR (125 MHz, DMSO-d6) δ 177.6, 145.6, 144.4, 127.9, 120.5, 117.2, 115.9, 81.3, 40.2, 28.7, and 27.1; FT-IR (thin film, neat) νmax 3331, 3055, 1762, 1517, 1444, 1265, 1180, and 736 cm−1; HRMS (ESI+) found 209.0808 (calculated for C11H13O4 ([M + H]+): 209.0808).
3.3. Cell Culture and Treatments
Rat intestinal epithelial IEC-6 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained according to the ATCC’s instructions. IEC-6 cells were grown as a monolayer in Dulbecco’s modified Eagle’s medium (HyClone, GE Healthcare, Logan, UT, USA), containing 10% (v/v) fetal bovine serum (ATCC), 100 U/mL penicillin, and 100 μg/mL streptomycin and maintained at 37 °C in a humidified atmosphere containing 5% CO2. The cells were treated with or without LPS for 1 h, in the presence or absence of DHPV.
3.4. Cell Lysates and Western Blot Analysis
Cell monolayers were washed twice with PBS, scraped on ice into cold cell lysis buffer containing protease inhibitor (Roche Applied Science, Mannheim, Germany), and centrifuged to remove the pellet and debris. Western blot analysis was performed as previously described [1]. Proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes, which were incubated with the primary antibodies, washed, incubated with peroxidase conjugated secondary antibodies, rewashed, and then visualized using an enhanced chemiluminescence system (Thermo Fisher Scientific, Waltham, MA, USA).
4. Conclusions
Our results, focused on the metabolite produced by metabolism of the catechins in the intestine and subsequent effects on the intestine, has been disclosed here. In this context, we synthesized both enantiomers of DHPV via the efficient and divergent approach and confirmed their biological activity. The synthesis was satisfactorily achieved with overall yield of 14% to 16% and the enantioselectivity of 97.3:2.7 (1a) and 95.1:4.9 (1b), respectively. The main cause of the IBD was related to inflammation on the intestine. Therefore, the anti-inflammatory effects of these compounds on rat intestinal epithelial IEC-6 cells were examined. (S)-DHPV proved to more efficiently inhibit inflammation induced by LPS by inhibiting the phosphorylation of IκBα, dose-dependently.
Our findings indicate that there was a new rationale to use (S)-DHPV as a new therapeutic agent for the prevention and treatment of IBD. Due to the complex mucosal immune system and very broad panel of genes controlled by NF-κB, which contribute to the pathogenesis of IBD, it is necessary to carefully explore the further effects of (S)-DHPV on NF-κB signaling.
Supplementary Materials
The following are available online: 1H and 13C-NMR spectra and the chiral HPLC analysis.
Author Contributions
Conceptualization and methodology, S.-H.K., D.S., and E.-H.K.; investigation, H.S.K., S.C., M.-Y.S., H.S., and H.K.; formal analysis, H.S.K., C.L., J.H., H.S., H.K., S.-H.K., and E.-H.K.; writing—original draft preparation, S.-H.K., D.S., and E.-H.K.; writing—review and editing, Y.-G.S., S.-H.K., D.S., and E.-H.K.; supervision, Y.-G.S., S.-H.K., and E.-H.K.; funding acquisition, S.-H.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by S.-H. Kim, National Research Foundation of Korea grant NRF-2017R1D1A1B03034612, and the GRRC program of Gyeonggi province GRRC-CHA2018-B02, Development of Effective Substance from Region-specific Resources.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 6, 2a, 2b, 7a, 7b, 1a and 1b are available from the authors.
References
- 1.Kaser A., Lee A.-H., Franke A., Glickman J.N., Zeissig S., Tilg H., Nieuwenhuis E.E., Higgins D.E., Schreiber S., Glimcher L.H. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpin S.J., Ford A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky N.A., Soon S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Kim E.R., Chang D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014;20:9872–9881. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgart D.C., Sandborn W.J. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 6.Roda G., Narula N., Pinotti R., Skamnelos A., Katsanos K., Ungaro R., Burisch J., Torres J., Colombel J.F. Systematic review with meta-analysis: Proximal disease extension in limited ulcerative colitis. Aliment. Pharm. Ther. 2017;45:1481–1492. doi: 10.1111/apt.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neish A.S. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDaniel D.K., Eden K., Ringel V.M., Allen I.C. Emerging roles for noncanonical NF-κB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm. Bowel Dis. 2016;22:2265–2279. doi: 10.1097/MIB.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Wang H., Liang X., Roberts M. Liver Pathophysiology. Elsevier; Amsterdam, The Netherlands: 2017. Hepatic Metabolism in Liver Health and Disease; pp. 391–400. [Google Scholar]
- 10.Rinaldo D., Batista Jr J.M., Rodrigues J., Benfatti A.C., Rodrigues C.M., Dos Santos L.C., Furlan M., Vilegas W. Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD. Chirality. 2010;22:726–733. doi: 10.1002/chir.20824. [DOI] [PubMed] [Google Scholar]
- 11.Monagas M., Urpi-Sarda M., Sanchez-Patan F., Llorach R., Garrido I., Gomez-Cordoves C., Andres-Lacueva C., Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 12.H Brooks W., C Guida W., G Daniel K. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011;11:760–770. doi: 10.2174/156802611795165098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha D., Kharbanda A., Yan W., Lakkaniga N.R., Frett B., Li H.-Y. The Exploration of Chirality for Improved Druggability within the Human Kinome. J. Med. Chem. 2020;63:441–469. doi: 10.1021/acs.jmedchem.9b00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe H. The Chemical Structure of the Intermediate Metabolites of Catechin I–IV: Chemical Properties of the Intermediate Metabolites (G and H) and their Derivatives Oxidative Decomposition of the Intermediate Metabolites (G and H) Synthesis of the Intermediate Metabolites (G and H) Structure of the Intermediate Metabolite (F) J. Agric. Chem. Soc. Jpn. 1959;23:257–271. [Google Scholar]
- 15.Lambert J.D., Rice J.E., Hong J., Hou Z., Yang C.S. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy-derivatives. Bioorg. Med. Chem. Lett. 2005;15:873–876. doi: 10.1016/j.bmcl.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 16.Nakano S., Hamada M., Kishimoto T., Nakajima N. Synthesis of γ-valerolactones as the tea catechin metabolites. Heterocycles. 2008;76:1001–1005. [Google Scholar]
- 17.Hamada M., Furuno A., Nakano S., Kishimoto T., Nakajima N. Synthesis of optically pure lactone metabolites of tea catechins. Synthesis. 2010:1512–1520. [Google Scholar]
- 18.Curti C., Brindani N., Battistini L., Sartori A., Pelosi G., Mena P., Brighenti F., Zanardi F., Del Rio D. Catalytic, Enantioselective Vinylogous Mukaiyama Aldol Reaction of Furan-Based Dienoxy Silanes: A Chemodivergent Approach to γ-Valerolactone Flavan-3-ol Metabolites and δ-Lactone Analogues. Adv. Synth. Catal. 2015;357:4082–4092. doi: 10.1002/adsc.201500705. [DOI] [Google Scholar]
- 19.Wu B., Gao X., Yan Z., Chen M.-W., Zhou Y.-G. C–H Oxidation/Michael Addition/Cyclization Cascade for Enantioselective Synthesis of Functionalized 2-Amino-4H-chromenes. Org. Lett. 2015;17:6134–6137. doi: 10.1021/acs.orglett.5b03148. [DOI] [PubMed] [Google Scholar]
- 20.Shopsowitz K.E., Edwards D., Gallant A.J., MacLachlan M.J. Highly substituted Schiff base macrocycles via hexasubstituted benzene: A convenient double Duff formylation of catechol derivatives. Tetrahedron. 2009;65:8113–8119. doi: 10.1016/j.tet.2009.07.094. [DOI] [Google Scholar]
- 21.Kolb H.C., VanNieuwenhze M.S., Sharpless K.B. Catalytic asymmetric dihydroxylation. Chem. Rev. 1994;94:2483–2547. doi: 10.1021/cr00032a009. [DOI] [Google Scholar]
- 22.Chatterjee A.K., Choi T.-L., Sanders D.P., Grubbs R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 23.Hur J., Kim A.-R., Kim H.S., Lim C., Kim T., Kim T.-A., Sim J., Suh Y.-G. Concise Synthesis of Catechin Metabolites 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactones (DHPV) in Optically Pure Form and Their Stereochemical Effects on Skin Wrinkle-Reducing Activities. Molecules. 2020;25:1970. doi: 10.3390/molecules25081970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neish A.S. The gut microflora and intestinal epithelial cells: A continuing dialogue. Microb. Infect. 2002;4:309–317. doi: 10.1016/S1286-4579(02)01543-5. [DOI] [PubMed] [Google Scholar]
- 25.Atreya I., Atreya R., Neurath M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 26.Neurath M.F., Pettersson S., Meyer zum Büschenfelde K.H., Strober W. Local administration of antisense phosphorothiate olignucleotides to the p65 subunit of NF–κB abrogates. Nat. Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 27.Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T., Knuechel R., Baeuerle P.A., Schölmerich J., Gross V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/S0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 28.Harikumar K.B., Kunnumakkara A.B., Ahn K.S., Anand P., Krishnan S., Guha S., Aggarwal B.B. Modification of the cysteine residues in IκBα kinase and NF-κB (p65) by xanthohumol leads to suppression of NF-κB–regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobar J., Pereda J., Arduini A., Sandoval J., Sabater L., Aparisi L., López-Rodas G., Sastre J. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: A key role for protein phosphatases. Curr. Pharm. Des. 2009;15:3027–3042. doi: 10.2174/138161209789058075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.