Abstract
The prokaryotic filamentous marine cyanobacteria are photosynthetic microbes that are found in diverse marine habitats, ranging from epiphytic to endolithic communities. Their successful colonization in nature is largely attributed to genetic diversity as well as the production of ecologically important natural products. These cyanobacterial natural products are also a source of potential drug leads for the development of therapeutic agents used in the treatment of diseases, such as cancer, parasitic infections and inflammation. Major sources of these biomedically important natural compounds are found predominately from marine cyanobacterial orders Oscillatoriales, Nostocales, Chroococcales and Synechococcales. Moreover, technological advances in genomic and metabolomics approaches, such as mass spectrometry and NMR spectroscopy, revealed that marine cyanobacteria are a treasure trove of structurally unique natural products. The high potency of a number of natural products are due to their specific interference with validated drug targets, such as proteasomes, proteases, histone deacetylases, microtubules, actin filaments and membrane receptors/channels. In this review, the chemistry and biology of selected potent cyanobacterial compounds as well as their synthetic analogues are presented based on their molecular targets. These molecules are discussed to reflect current research trends in drug discovery from marine cyanobacterial natural products.
Keywords: marine cyanobacteria, natural products, drug discovery, molecular targets
1. Introduction
The photosynthetic filamentous marine cyanobacteria are prokaryotic microorganisms found in diverse habitats, including epiphytic, epilithic, endolithic and microbial mats communities, in coral reef ecosystems [1]. Natural products research on marine cyanobacteria has revealed its impressive biosynthetic capacity in producing structurally novel bioactive secondary metabolites [2]. The high success rate of marine cyanobacteria in colonizing different aquatic habitats could be attributed to the ecological roles of these compounds, such as UV-radiation protection, feeding deterrence, allelopathy and signaling [3]. To date, more than 550 secondary metabolites have been reported from diverse marine cyanobacterial genera, including Lyngbya, Moorea, Symploca and Oscillatoria [4]. A majority of these biomolecules are nitrogen-containing and are products of the modular biosynthetic enzymes, such as the non-ribosomal peptide synthetases (NRPS), polyketide synthases (PKS) and hybrid NRPS-PKS [5]. Moreover, research on the biosynthetic machinery of these microbial systems revealed unusual mechanistic and enzymatic features, resulting in the production of diverse chemical structures [5].
Several pharmacological trends have been observed amongst the various marine cyanobacterial secondary metabolites. A significant number of molecules have been reported to possess either potent cytotoxic (e.g., largazole, coibamide A and curacin A), neuromodulating (e.g., antillatoxin, kalkitoxin and jamaicamides) or antiinfective (e.g., almiramides and gallinamide A) properties [6,7,8,9]. The high potency of these compounds is due to their specific disruption/interference with validated drug targets implicated in various human diseases, including cancer, inflammation and neurodegenerative disorders. These drug targets include enzymes, e.g., proteasomes, proteases, and histone deacetylases, cellular cytoskeletal structures, e.g., microtubules and actin filaments, as well as membrane channels, e.g., voltage-gated sodium channels and Sec61 protein translocation channels. As such, these natural products make excellent lead compounds for drug discovery and development. For instance, a number of marine cyanobacterial compounds and their synthetic analogues formulated as Antibody-Drug Conjugates (ADCs), including dolastatin 10, auristatin E and OKI-179, have undergone/undergoing clinical trials for the treatment of cancer diseases [10,11].
Integrated genomic and metabolomics approaches have been used to mine marine cyanobacteria for structurally unique natural products. In particular, the MS/MS-based metabolomics platform, Global Natural Product Social Molecular Networking (GNPS), is a powerful tool for rapid chemical profiling of natural products mixtures [12]. The use of GNPS, in combination with genomic or biological assays, has extended the accessible chemical space for the discovery of novel bioactive cyanobacterial compounds. For instance, a new class of acyl amides, columbamides, with cannabinomimetic activity was uncovered based on genomic and mass spectrometric profiling of three marine cyanobacterial strains of the genus Moorea [13]. In addition, the use of bioassay-guided fractionation and MS-based molecular networking resulted in the isolation of a cytotoxic cyclic octapeptide, samoamide A [14]. Moreover, recent advances in NMR spectroscopy have enabled new strategies for detection of new natural products from cyanobacterial extracts. One such tool is the recently developed Small Molecule Accurate Recognition Technology (SMART), which is based on a convolutional neural network to classify 2D NMR spectra, such as HSQC spectra, of natural products using pattern recognition principles [15]. Such innovative NMR-based method has led to the detection of several new cyclic depsipeptides belonging to the viequeamide class of molecules as well as new chimeric swinholide-like macrolide, symplocolide A [16,17].
Due to the sheer number of reported bioactive marine cyanobacterial compounds, it is probably impossible to provide a comprehensive coverage of all potent compounds from marine cyanobacteria in this review. There are a number of reviews on the diversity of marine cyanobacterial compounds as well as their pharmaceutical importance that readers can refer to [2,18,19,20,21,22,23,24,25]. Instead, this mini review will feature selected potent natural products and their clinically relevant molecular targets, including both enzyme and non-enzyme-based targets/pathways. The selection includes cyanobacterial molecules that have been identified as drug leads for further structural optimization as well as SAR studies. These natural products and their synthetic analogues are discussed based on their interference with molecular targets/pathways, such as histone deacetylases, proteasomes, proteases, actin and microtubule filaments, and membrane receptors/channels. Furthermore, this review serves to complement and provide an update on information of two reviews previously published by Tan as well as Salvador-Reyes and Luesch [21,22].
2. Marine Cyanobacterial Drug Leads and their Molecular Targets
2.1. Histone Deacetylases
2.1.1. Largazole
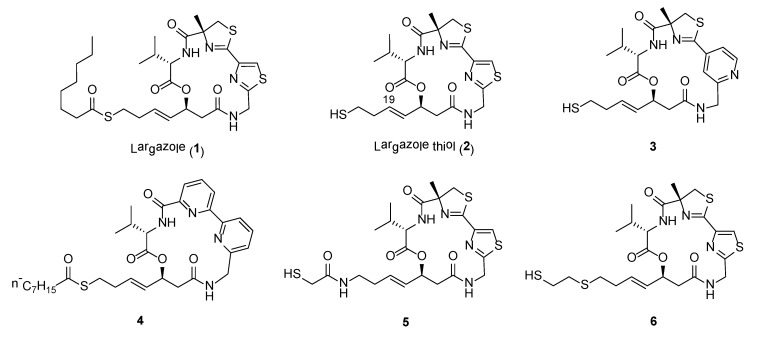
Largazole (1) (Figure 1), a cyclic depsipeptide, is a highly potent class I histone deacetylase (HDAC) inhibitor originally discovered from the marine cyanobacterium, Symploca sp., collected from Key Largo (FL, USA) [26]. Largazole consists of a number of unusual structural features, including a 3-hydroxy-7-mercaptohept-4-enoic acid unit and the linkage of a 4-methylthiazoline unit to a thiazole. The compound is found to be a potent inhibitor on the growth of transformed human mammary epithelial cells (MDA-MB-231) with GI50 of 7.7 nM. In addition, compound 1 showed exquisite antiproliferative activity against transformed fibroblastic osteosarcoma U2OS cells (GI50 55 nM) over non-transformed fibroblasts NIH3T3 (GI50 480 nM) when compared to paclitaxel, actinomycin D, and doxorubicin [26].
Figure 1.
Largazole and related compounds.
Largazole is a prodrug and upon hydrolysis of the thioester provides the highly active largazole thiol (2) (Figure 1) [27]. Due to the impressive anticancer property and unique structure of largazole, it has attracted interest from synthetic chemists on its total synthesis and generation of synthetic analogues. To date, more than a dozen reports on its total synthesis have been successfully accomplished [28]. A scale-up synthesis of largazole was also recently reported by Chen et al., where the syntheses of each fragment and final product were optimized to achieve an overall yield of 21% in eight steps [29]. In addition, various in vivo and in vitro biological as well as epigenetic studies performed on largazole revealed its potential use in broad spectrum therapy (Table 1) [30,31,32,33,34,35,36,37,38,39,40,41].
Table 1.
Summary of pharmacological studies on largazole (1).
| Disease/Target | Significant Biological Activity | Reference |
|---|---|---|
| Angiogenesis-associated diseases |
|
[31] |
| Lung cancer |
|
[32] |
| Rheumatoid arthritis |
|
[33] |
| Liver fibrosis |
|
[34] |
| Breast cancer |
|
[35] |
| Protein ubiquitination |
|
[36] |
| Epstein-Barr virus (EBV)-associated lymphomas |
|
[37] |
| Bone-related disorders |
|
[38] |
| Colon cancer |
|
[39] |
| Oncogenic super-enhancers |
|
[40] |
| Antiretroviral therapy |
|
[41] |
Extensive SAR studies on synthetic largazole-based analogues were carried out and the results of these studies were summarized recently by Poli et al. [42]. In general, the thiazole-thiazoline unit of largazole is the recommended moiety where structural modifications should be carried out to generate new analogues with selective inhibition on HDAC as well as anticancer activity. For instance, pyridine analogues (e.g., 3) and bipyridine analogue, 4, provided potent anticancer and HDAC inhibitory activities (Figure 1, Table 2) [43,44]. Modification to the L-valine unit in largazole is well tolerated and in some cases able to confer higher selectivity for cancer cells over normal cells. In addition, changes to the conformation and the flexibility of the macrocyclic core can impact HDAC activity as well as on the selectivity for HDACs 1-3 over HDAC6 [45]. Furthermore, largazole analogues with modified warheads generally showed decreased in activity. However, a number of analogues containing zinc binding groups, including α-thioacetamide (e.g., 5) or mercaptosulfide (e.g., 6), have altered selectivity profile, such as conferring selectivity for HDAC6 or HDAC10 (Figure 1, Table 2) [46,47]. Largazole analogues with selectivity for HDAC6 or HDAC10 have possible usage for the treatment of CNS disorders or ovarian cancer, respectively [48,49]. Recent study on the incorporation of monofluoro and gem-difluoro substitution at the Zn2+-binding thiolate side chain of largazole thiol 2 revealed that C19-position fluoro-substituted largazole analogue exhibited high selectivity against HDAC1 over HDAC6 [50]. Due to the exceptional activity of largazole, new studies on the design of synthetic analogues with enhanced potency and selectivity are expected to be pursued to expand the chemical space of this class of compounds.
Table 2.
HDAC inhibitory activities of largazole-based synthetic analogues 3 to 6 (IC50 nM).
| HDAC | Compound | |||
|---|---|---|---|---|
| 3 | 4 | 5 | 6 | |
| 1 | 2.20 | 21.0 | 1.0 | 40.0 |
| 2 | 4.42 | 28.0 | 1.9 | 90.0 |
| 3 | 2.31 | 27.0 | 1.5 | 43.0 |
| 5 | >10,000 | |||
| 6 | 35.16 | 13,000 | 0.24 | 2800 |
| 8 | 101.8 | 9600 | ||
| 10 | 3.6 | |||
Amongst the various largazole analogues, a synthetic compound, OKI-179, has recently been identified as a drug candidate and subsequently, proceeded to Phase I clinical trial for the treatment of advanced solid tumors [51,52]. OKI-179 was developed based on several chemical optimization steps involving largazole thiol and OKI-006. OKI-179 showed potent inhibition of the Class 1 HDACs, namely HDAC 1, 2 and 3, with IC50s’ values of 1.2, 2.4 and 2.0 nM, respectively. Moreover, it shows impressive antiproliferative and apoptotic properties against a wide range of cancer cell lines, activity in xenograft cancer models as well as good oral pharmacokinetic properties in mouse, rat and dog models. Results from the first dosing cohorts revealed that the drug was well-tolerated and achieved good exposure following oral administration [52].
2.1.2. Santacruzamate A
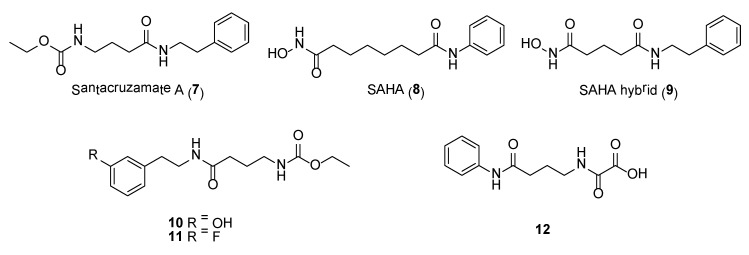
A picomolar-range histone deacetylase inhibitor, santacruzamate A (7) (Figure 2), was obtained from the organic extracts of a tuft-forming marine cyanobacterium collected from Coiba National Park, Panama [53]. Due to the structural similarities of santacruzamate A with the clinically approved HDAC inhibitor, suberoylanilide hydroxamic acid (= SAHA, 8) (Figure 2), the natural product was further evaluated in a series of anti-HDAC assays. This led to the discovery of santacruzamate A as a potent inhibitor of HDAC2, a Class I HDAC, with an IC50 of 119 pM. Compound 7 also showed cytotoxicity against HCT116 and Hut-78 cancer cell lines with GI50 of 28.3 µM and 1.4 µM, respectively. Total synthesis, achieved in two steps, of santacruzamate A as well as a synthetic hybrid compound, 9 (Figure 2), was reported [54].
Figure 2.
Santacruzamate A, SAHA and related compounds.
Synthetic compound 9 was found to be 30 times less potent compared to santacruzamate A when tested against HDAC2. In another SAR study by Randino et al., two classes of santacruzamate derivatives having several aromatic amines containing either the ethyl carbamate unit (class I) or oxamic acid moiety (class II) as zinc-binding groups were synthesized [55]. The study identified three synthetic molecules, including 10, 11 and 12 (Figure 2), having potent antiproliferative activity in HCT116 cancer cells, with IC50s’ of 0.476 µM, 0.825 µM and 0.814 µM, respectively [55]. However, HDAC inhibitory activity was not detected in these synthetic derivatives, which underscore the importance of the zinc binding group positioning into the HDAC catalytic site as a crucial step in the inhibition process.
Recent pharmacological study using santacruzamate A, in combination with other HDAC inhibitors, such as HDAC1 inhibitor, tacedinaline, HDAC1/2 common inhibitor, romidepsin (FK228) and global HDAC inhibitor, vorinostat (SAHA), to treat hepatocellular carcinoma (HCC) was reported [56]. The combined chemotherapy led to the inhibition of HDAC1/2 as well as changes in HCC cell morphology, growth inhibition, cell cycle blockage and apoptosis in vitro and growth suppression of subcutaneous HCC xenograft tumors in vivo [56]. Santacruzamate A has also been explored as potential candidate lead compound for the treatment of endoplasmic reticulum (ER) stress- and unfolded protein response (UPR)-related neurodegenerative disorders, such as Alzheimer’s disease [57].
2.2. Proteasome
Carmaphycins
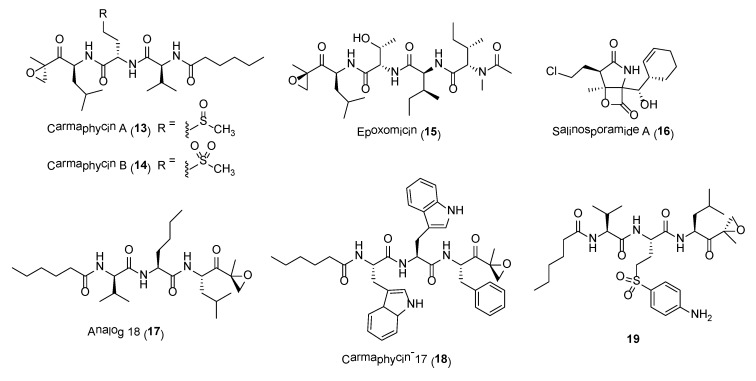
Carmaphycins A (13) and B (14) (Figure 3) are potent novel proteasome inhibitors isolated in low yield from organic extracts of Symploca sp. obtained from CARMABI beach, Curacao [50]. Structurally, the carmaphycins consist of a leucine-derived α,β-epoxyketone warhead directly attached to either a methionine sulfoxide in 13 or a methionine sulfone in 14, which is linked to a valine and an alkyl chain terminal tail. Their total synthesis was accomplished using an efficient and scalable convergent method [58]. The proteasome inhibitory properties of carmaphycins A and B were evaluated against Saccharomyces cerevisiae 20S proteasome and found to have comparable IC50 values of 2.5 nM and 2.6 nM, respectively. Such inhibitory activities are comparable with those reported for epoxomicin (15) and the marine-derived salinosporamide A (16), with IC50 values of 2.7 nM and 1.4 nM, respectively (Figure 3). In addition, cytotoxic assay showed carmaphycins to be particularly active against solid tumor cell lines, including human lung adenocarcinoma and colon cancer cell lines. Preliminary structural biology investigation of the carmaphycins suggested distinct binding site as compared to epoxomicin, salinosporamide A and bortezomib.
Figure 3.
Carmaphycins, epoxomicin, salinosporamide A and related compounds.
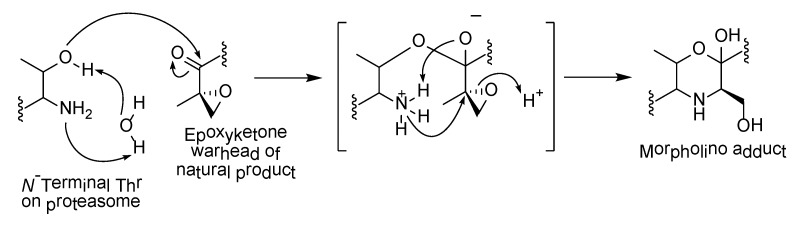
The α,β-epoxyketone warhead in carmaphycins is also a structural feature in epoxomicin (12), a known proteasome inhibitor, as well as the FDA-approved anticancer drug, carfilzomib. Their irreversible inhibition of proteasome is due to the formation of the morpholino adduct between the epoxyketone warheads with the Thr1 residues in the catalytic sites of the 20S proteasome core. The mechanism involves warhead carbonyl and epoxide undergoing two successive nucleophilic attacks by Thr1 Oγ and Thr1 N, respectively (Scheme 1) [59]. A novel mechanism of proteasome inhibition via hydroamination using alkene derivatives of the carmaphycin was recently reported by Trivella and co-workers [60]. The action of the carmaphycin enone electrophile was found to be partially reversible and this could provide further insights on the design of proteasome inhibitors for cancer treatment.
Scheme 1.
Mechanism of action of carmaphycins with α,β-epoxyketone warhead.
A number of synthetic analogues of carmaphycin B have been explored as lead molecules for the treatment of protozoan and metazoan parasitic infection as well as cancer. One such synthetic compound is analog 18 (17) (Figure 3), which was synthesized based on the structure of carmaphycin B [61]. Analog 18 was shown to possess potent in vitro antimalarial activity against asexual blood stages and gametocytes as well as strongly inhibits the activity of Plasmodium proteasome. Its potent activity was due to specific inhibition of the β5 subunit of the proteasome. Moreover, the study showed that incorporating a d-amino acid at the P3 position can significantly modulate host cytotoxicity without interfering significantly with anti-plasmodial activity [61]. In another study, a carmaphycin-related synthetic analogue, carmaphycin-17 (18, Figure 3), was found to have greater inhibitory activity against Trichomonas vaginalis than the reference drug metronidazole [62]. Trichomonas vaginalis is the causative agent of the sexually transmitted disease, trichomoniasis. In addition, 18 was found to be selective for T. vaginalis due to its increased potency against the β1 and β5 catalytic subunits of the T. vaginalis proteasome as compared to the human proteasome subunits [62]. Compound 18 is also shown to be selective for the Schistosoma mansoni proteasome (about 13.2-fold more potent) over the human proteasome [63]. Treatment of adult S. mansoni with 18 resulted in the same phenotypic changes as the FDA-approved drugs, bortezomib and carfilzomib.
In another development, a library of carmaphycin B analogues containing amine handles were synthesized and screened for their cytotoxic properties as Antibody-Drug Conjugates [64]. The screening effort resulted in identifying a highly potent analog of carmaphycin B, 19 (Figure 3), containing a 4-sulfonylaniline handle as an attachment point for the linker antibody. However, the study found that the free analogues, such as 19, gave better cytotoxic activity at sub-nanomolar levels as compared to their ADCs when tested against the SKBR3 and MDA-MB-231 cancer cell lines. In addition, it was revealed that the cytotoxicity of free analogues with linear amines was greatly reduced as compared to free analogues with aromatic amines. The reduced potency of the ADCs could be due to protein-mediated degradation of the conjugated carmaphycin backbone, lysosomal degradation of the analogues or inappropriate ADC trafficking [64].
2.3. Protease Enzymes
Proteases are ubiquitous proteolytic enzymes found in eukaryotic and prokaryotic cells. The classification of these enzymes is based on key catalytic group present in the active site, such as serine, threonine, cysteine, aspartate, glutamate or zinc in metalloproteases. They account for about 2% of the genes in human cells and are involved primarily in proteins activation, synthesis and turnover [65]. Due to their involvement in many signaling pathways, they represent potential drug targets in human diseases, including cardiovascular disorders, inflammation, cancers as well as parasitic and viral infections [66]. It has been reported that filamentous marine cyanobacteria are sources of potent protease inhibitors, particular molecules targeting serine and cysteine proteases.
2.3.1. Serine Protease Inhibitors
Serine proteases, including elastase, chymotrypsin, and trypsin, are a large class of enzymes having different roles related to human health, such as immune response, wound healing and blood coagulation. Studies have shown that an increase or decrease of protease activity can induce pathologies, including cancer, inflammation, heart attack, stroke and pancreatitis [67]. A number of potent serine protease inhibitors have been reported from marine cyanobacteria.
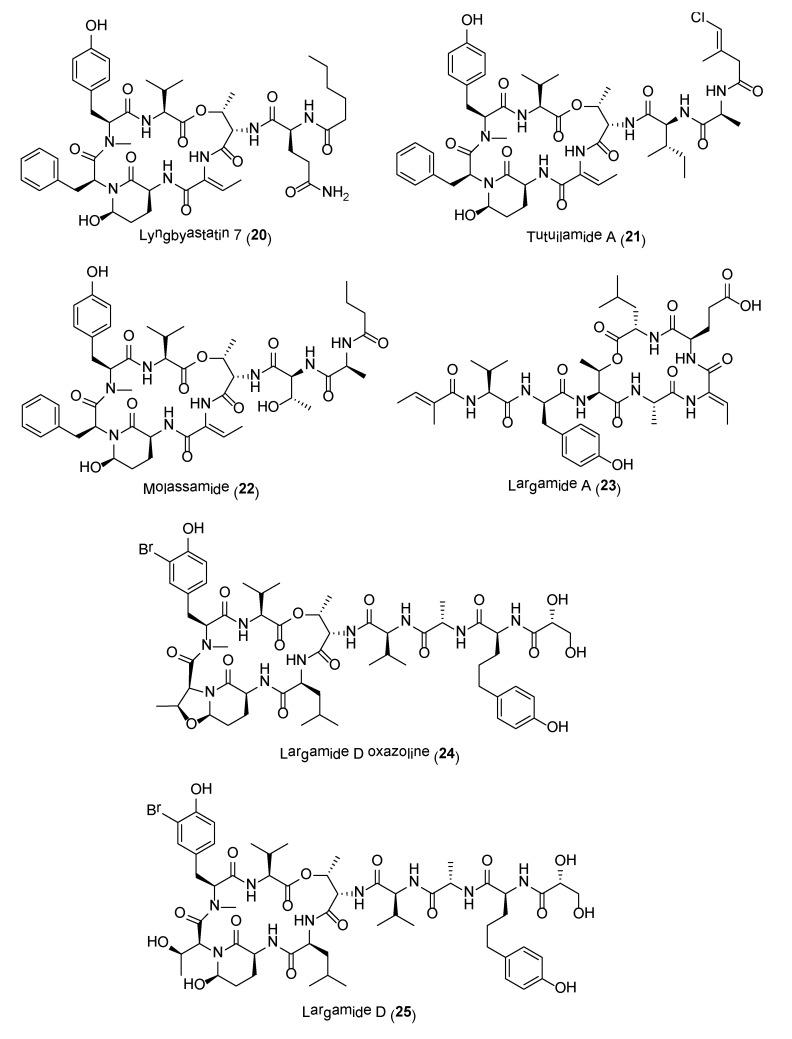
A majority of these serine protease inhibitors are 3-amino-6-hydroxypiperidone (Ahp)-containing cyclodepsipeptides, including lyngbyastatins 4–10 (e.g., 20) (Figure 4), pompanopeptin A, symplocamide A, kempopeptins, molassamide, bouillomides, somamide B as well as recently reported molecules, loggerpeptins, kyanamide and tutuilamides (e.g., 21) [68,69,70,71,72,73,74,75,76,77,78]. All Ahp-cyclodepsipeptides contain an N-methyl aromatic amino acid at conserved positions in their 19-membered ring structure, while other residues are less conserved. In addition to elastase selectivity, molassamide (22) was reported to inhibit the migration of the highly invasive MDA-MB-231 breast cancer cells [76]. Other non Ahp-containing cyclodepsipeptides, such as the largamides (e.g., 23) and tiglicamides, have also been reported to possess moderate to potent serine protease inhibitory activities (Figure 4) [79,80,81].
Figure 4.
Serine protease inhibitors from marine cyanobacteria.
It has been proposed that these cyanobacterial serine protease inhibitors may function as chemical defenses against marine predators [82]. Ecological studies by Matthew and co-workers led to the isolation of an Ahp-containing largamide D derivative 24 (Figure 4), formed via intramolecular condensation of largamide D (25) (Figure 4). This molecule, largamide D oxazolidine (24), exhibited 11-fold and 33-fold reduction in activity against chymotrypsin and elastase, respectively, when compared to largamide D [82]. The Ahp moiety is essential for serine protease inhibition and any structural or conformational changes to this unit will affect activity.
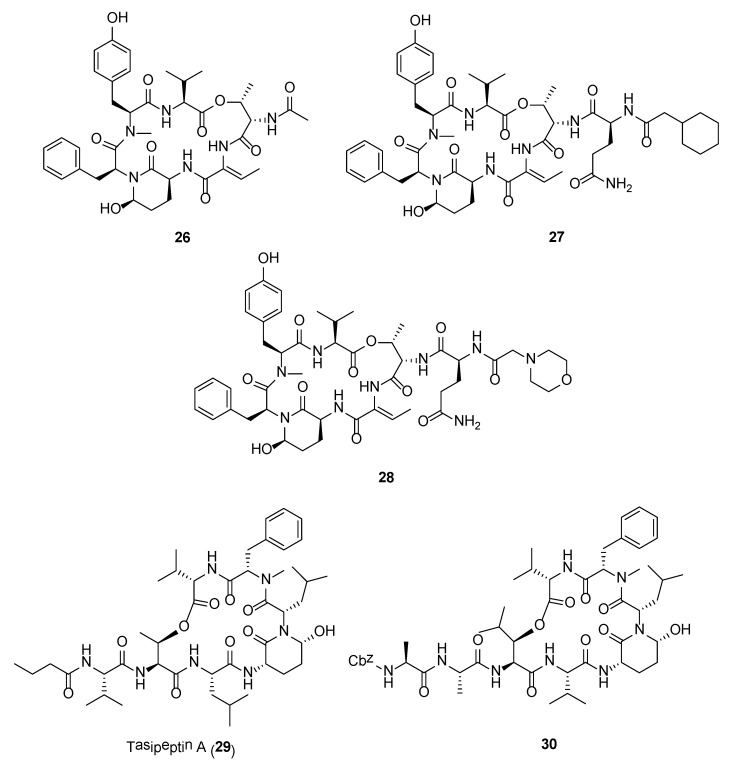
Crystal structures of Ahp-cyclodepsipeptides in complex with serine proteases revealed that the inhibition is based on a substrate-like binding mode where distinct amino acid residues sit in the S- and S′-pockets with no proteolytic cleavage. These cyanobacterial inhibitors act in a similar manner to proteinaceous canonical serine protease inhibitors, where the conserved residues stabilize the inhibitory fold, and the other less conserved residues define serine protease selectivity through optimal accommodation at the specific S and S′-sites [83]. Such finding suggests that Ahp-cyclodepsipeptides may represent a suitable scaffold for designing non-covalent S1 serine protease inhibitors. As such a number of potent Ahp-cyclodepsipeptides has been targeted for total synthesis as well as generating synthetic analogues with enhanced activity. For instance, the total synthesis of lyngbyastatin 7 (20) was carried out in 31 steps by Luo and co-workers [84]. The synthetic compound showed superior activity over the only approved elastase inhibitor drug, sivelestat. Specifically, compound 20 demonstrated strong ability in protecting bronchial epithelial cells against elastase-induced antiproliferation as well as negating the elastase-triggered induction of pro-inflammatory cytokine expression [84]. A scale-up synthesis of lyngbyastatin 7 was recently developed with improved reaction yields and simplified purification steps [85]. Based on this synthetic strategy, a pilot library of lyngbyastatin 7 analogues (e.g., 26–28) was constructed for the purpose of compound tailoring and modulation of lipophilicity/hydrophilicity via manipulation of the pendent side chain. More importantly, this study revealed the importance of the side chain for elastase inhibition in addition to the Ahp and 2-aminobutenoic acid moiety in the core cyclic structure of lyngbyastatin 7. This major development would path the way for the translation of the natural product into pharmacotherapeutics for the treatment of diseases with overactive human neutrophil elastase [85]. In another study, a practical mixed solid- and solution-phase synthesis were employed for the synthesis of tasipeptins (e.g., 29) and synthetic analogues [86]. More importantly, this synthetic strategy resulted in the generation of the human HTRA protease inhibitors (e.g., 30) with enhanced potency (Figure 5).
Figure 5.
Lyngbyastatin 7 synthetic analogues, tasipeptin A and related compound.
2.3.2. Falcipain Inhibitors
Gallinamide A
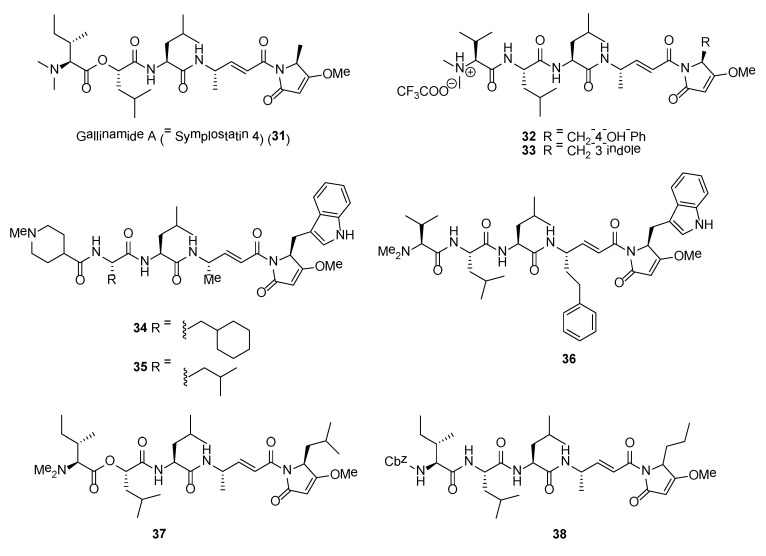
Gallinamide A (= symplostatin 4) (31) (Figure 6) is one of the most potent marine cyanobacterial antimalarial compounds reported to date, with EC50 of 74 nM when tested against Plasmodium falciparum strain 3D7 [87,88,89]. However, gallinamide A has moderate activity against mammalian Vero cells and no detectable cytotoxicity against the NCI-H460 lung tumor and neuro-2a mouse neuroblastoma cell lines [87]. Gallinamide A, a linear depsipeptide, has several unique structural features, including a dimethylated N-terminal amino acid moiety, a 4-amino-2-pentenoic acid unit, and a C-terminal N-acyl-pyrrolinone unit. Gallinamide A was initially reported by Linington and co-workers from an organic extract of a Schizothrix species collected from reef near Piedras Gallinas, Panama [87]. At the same time, symplostatin 4, having identical planar structure with gallinamide A, was also reported by Taori and co-workers from a different cyanobacterial species, Symploca sp., obtained from Florida Keys [88]. Based on the detailed 1D NMR analysis of the synthetic molecules, it was concluded that the structures of gallinamide A and symplostatin 4 are identical [89,90].
Figure 6.
Gallinamide A and related compounds.
The potent antimalarial activity of gallinamide A (31) led to investigations by Stolze and co-workers on its mode of action and SAR studies based on synthetic and biochemical methods [91]. It was observed that Plasmodium falciparum-infected red blood cells treated with gallinamide A, at nanomolar concentration, exhibited swollen food vacuole phenotype. Through the use of fluorescent probes based on rhodamine fluorophore-tagged molecules, it was revealed that the natural product is a specific inhibitor of the plasmodial cysteine proteases, falcipains 2, 2′ and 3. In addition, the methoxypyrrolinone unit in gallinamide A is essential for antimalarial activity and modifications to the N-terminal end of the molecule are tolerated [91].
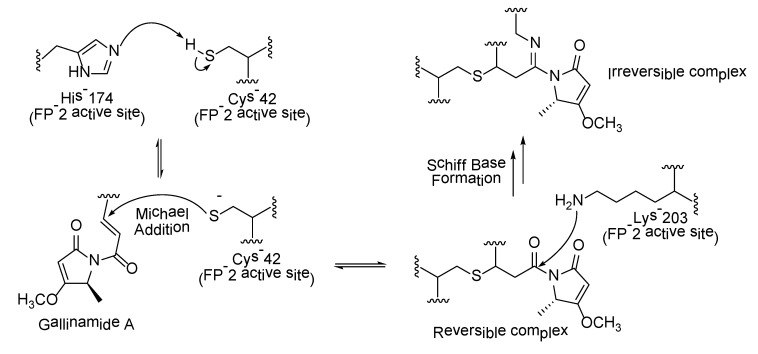
Falcipains are Plasmodium falciparum cysteine proteases involved in several key processes of the erythrocytic cycle of the malarial parasite, such as the hydrolysis of host hemoglobin, erythrocyte invasion, and rupture [92]. These cysteine proteases therefore constitute promising molecular targets in the search for novel antimalarial agents. The proposed mechanism of gallinamide A inhibition of falcipain 2 was deduced based on molecular dynamics simulation studies [93]. The simulation studies suggested that the methyl methoxylpyrrolinone moiety in gallinamide A inserts into the active site of falcipain 2, facilitating Michael addition and Schiff base formation with Cys-42 and Lys-203, respectively, leading to the irreversible inhibition of the enzyme (Scheme 2) [93].
Scheme 2.
Proposed mechanism of gallinamide A at active site of falcipain 2 (FP-2) (adapted from Ref. [93]).
A number of synthetic analogues, based on gallinamide A structural motif, having potent antiprotozoal or anticancer activity have been identified. In a study by Conroy and co-workers, analogues of gallinamide A were synthesized for SAR studies and tested for their inhibitory activity against falcipains 2 and 3 [94]. It was revealed that the α,β-unsaturated imide moiety of gallinamide A is important for inhibitory activity. In addition, several analogues, such as compounds 32 and 33 (Figure 6), showed potent inhibition of the chloroquine-sensitive 3D7 strain of P. falciparum as well as the chloroquine-resistant Dd2 strain of P. falciparum (Table 3) [94]. A recent study by Stoye et al. created a library of gallinamide A-based analogues using a highly efficient and convergent synthetic route [95]. Based on this library, several synthetic analogues (e.g., 34–36) possessed potent inhibitory activity against the P. falciparum falcipain 2 and falcipain 3 as well as cultured chloroquine-sensitive (3D7) and chloroquine-resistant (W2) strains of P. falciparum (Table 3). Three synthetic lead molecules, namely 34, 35 and 36, were subsequently evaluated for their in vivo efficacy against P. berghei infection in mice (Figure 6). Of the three compounds, compound 36 cured P. berghei-infected mice in the Peters 4 day-suppressive test when administered 25 mg kg−1 intraperitoneally daily over 4 days [95].
Table 3.
Inhibition of falcipains 2, 3 and various strains of P. falciparum by gallinamide A analogues 32–36 (IC50 nM).
| Compound | Falcipain | P. falciparum | |||
|---|---|---|---|---|---|
| 2 | 3 | 3D7 | Dd2 | W2 | |
| 32 | 5.25 | 81.4 | 20.0 | 67.0 | |
| 33 | 12.0 | 66.7 | 9.7 | 29.0 | |
| 34 | 31.0 | 117.0 | 26.0 | 28.0 | |
| 35 | 29.0 | 79.0 | 42.0 | 49.0 | |
| 36 | 33.0 | 112.0 | 1.0 | 4.0 | |
Synthetic analogues of gallinamide A were recently explored as potent inhibitor of cathepsin L for the treatment of Chagas disease [96]. The study, conducted by Boudreau and co-workers, showed that gallinamide A and its synthetic analogs potently inhibit cruzain, a Trypanosoma cruzi cysteine protease, and are exquisitely toxic toward T. cruzi in the intracellular amastigote stage. Compound 37 (Figure 6) was the most active cruzain inhibitor with an IC50 of 5.1 nM. This molecule was found to be inactive to the epimastigote and the host cell [96]. Synthetic analogues of gallinamide A were also explored for their anticancer activity. In a study by Liu et al., an anti-cancer stem cells lead molecule, 38, was found to significantly suppress tumor growth both in vitro and in vivo. Moreover, 38 was able to significantly reduce the number of melanoma tumor spheres and decrease the percentage of ALDH+ melanoma cells by blocking the Wnt/β-catenin signaling pathway [97].
2.3.3. Cathepsin Inhibitors
Cathepsins are protease enzymes, classified by various families, including serine protease, cysteine protease and aspartyl protease. There are currently about 15 classes of cathepsins in humans. These enzymes are essential for diverse range of normal physiological functions, including digestion, blood coagulation, bone resorption, ion channel activity, innate immunity, vesicular trafficking and autophagy. However, dysregulated cathepsins have been implicated in a number of pathologies, including arthritis, periodontitis, pancreatitis, macular degeneration, muscular dystrophy, atherosclerosis, obesity, stroke, Alzheimer’s disease, schizophrenia, tuberculosis, and carcinogenesis [98]. As such, cathepsins have a diagnostic value and are promising drug targets for a variety of human diseases through the inhibition of these proteases [99].
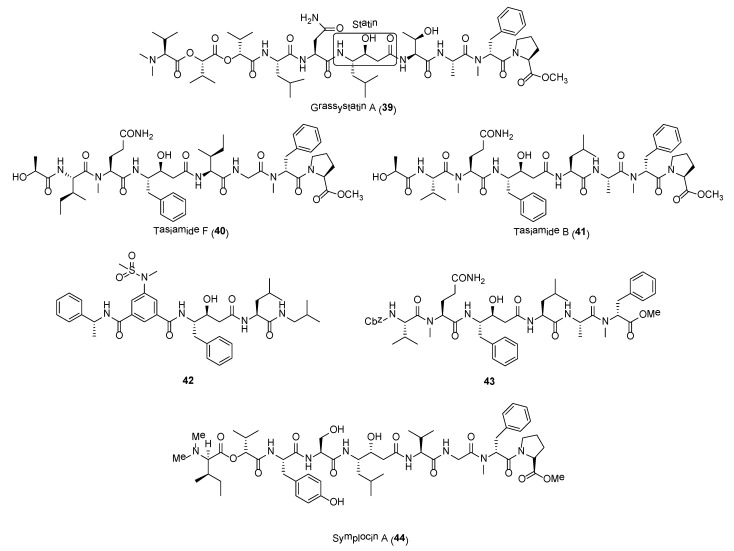
A series of statine-containing linear depsipeptides, including grassystatins (e.g., 39), tasiamides, and symplocin A, were found to be potent inhibitors of aspartyl proteases, cathepsins D and E (Figure 7). Cathepsins D and E are potential drug targets as their overexpressions have been observed in various cancer forms, such as pancreatic ductal adenoma, cervical adenocarcinoma, lung carcinoma, and gastric adenocarcinoma [100]. The linear decadepsipeptides, grassystatins A-F (e.g., 39) were isolated from Lyngbya confervoides and VPG 14-61 collected at Grassy Key, Florida and Cetti Bay, Guam, respectively [101,102]. These statine unit-containing molecules were initially isolated from a screening program by evaluating the inhibitory activities of natural products against 59 proteases. Grassystatins A (39) and B displayed potent inhibitory activity against cathepsins D and E with IC50 values averaging at 16.9 nM and 0.62 nM, respectively. In addition, grassystatin A was able to reduce antigen presentation by dendritic cells [101]. The total synthesis of grassystatin A was recently accomplished by Yang and co-workers and pharmacological studies revealed that the inhibition of cathepsin E by the molecule did not impact ovalbumin antigen processing and peptide presentation [103]. In addition, grassystatin F was shown to inhibit the cleavage of cystatin C and plasminogen activator inhibitor type-1 (PAI-1) via its inhibition on cathepsin D. Cystatin C and PAI-1 are involved in the downstream activation of cysteine cathepsins and tissue plasminogen activator (tPA), which would lead to the migration of highly aggressive triple negative breast cancer cells (MDA-MD-231) [102].
Figure 7.
Grassystatin A, tasiamides B and F, symplocin A and related compounds.
Tasiamides are grassystatin-related molecules found to possess potent cathepsins D and E inhibitory activity. In particular, tasiamides B (41) and F (40) (Figure 7) displayed potent activity against cathepsins D and E, with IC50 values of 50 nM and 9.0 nM for 41 and 57 nM and 23 nM for 40, respectively [104]. When tested against BACE1 (β-site Amyloid precursor protein Cleaving Enzyme type 1), an enzyme implicated in Alzheimer’s disease, compound 40 was about 12- to 30-fold less active as an BACE1 inhibitor compared to cathepsins D and E [104]. A number of synthetic tasiamide B analogues, such as compounds 42 and 43 (Figure 7), have been developed as selective inhibitors of these aspartic proteases [105,106,107]. Compound 42 was found to be highly selectivity for cathepsin D with 576-fold over cathepsin E and 554-fold over BACE1 [106]. In another study by Li et al., synthetic compound 43 (Figure 6) displayed significant selective inhibitory activity against cathepsin D with IC50 of 3.29 nM over cathepsin E (72-fold) and BACE1 (295-fold) [107]. These results could path the way for the development of highly selective cathepsin D inhibitors.
Symplocin A (44) (Figure 7) is another highly potent cathepsin E inhibitor isolated from the Bahamian cyanobacterium, Symploca sp. [108]. Symplocin A is structurally related to grassypeptins in having a statine-unit. In addition to Marfey’s method, a new strategy using 2-naphthacyl esters of N,N-dimethylamino and 2-hydroxy acids were employed for absolute stereochemistry determination of this molecule. Symplocin A is a potent inhibitor of cathepsin E with IC50 value of 300 pM, which is comparable to that of pepstatin, a known inhibitor of aspartyl proteases. Taken together, the biological data of these series of statine-containing compounds, including grassystatins, tasiamides, and symplocin A, showed that selectivity can be tuned and these structural scaffolds can serve as a starting point for development of selective aspartic protease inhibitors.
2.3.4. β-Secretase 1 (BACE1) Inhibitors
Tasiamide B
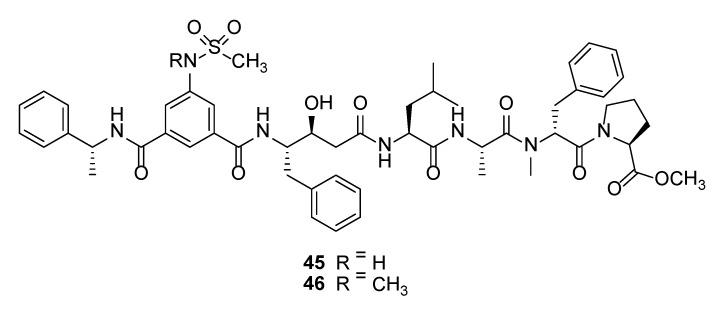
Tasiamide B (41) (Figure 7) is a statine-containing linear depsipeptide shown to inhibit the aspartic protease, BACE1, with an IC50 of 0.19 µM [105,109]. BACE1, also known as β-secretase 1, is a potential drug target for the treatment of Alzheimer’s disease (AD) due to its involvement in the abnormal production of β-amyloid plaques in AD patients [110,111]. The total synthesis of tasiamide B led to a revision in stereochemistry on the structure of the original reported molecule [112]. Several synthetic tasiamide B-analogues have been synthesized with superior inhibitory activities against BACE1. For instance, hybrid molecules containing structural features of tasiamide B and sulfonamide-containing isophthalic acid unit, such as compounds 45 and 46 (Figure 8) showed potent inhibition of BACE1 with IC50 of 128 nM and 57.2 nM, respectively [105]. Moreover, these synthetic molecules showed selectivity for BACE1 over γ-secretase and compound 38 exhibited in vivo activity by reducing levels of amyloid β-peptide in brain of rodent [105]. Recent SAR studies based on 19 synthetic analogues of tasiamide B revealed the importance of the hydrophobic substituents, valine, leucine, alanine, and phenylalanine, for inhibitory activity against BACE1 [113].
Figure 8.
Tasiamide B-related compounds.
2.4. Interference of the Actin and Microtubule Filaments
Dolastatins 10/15
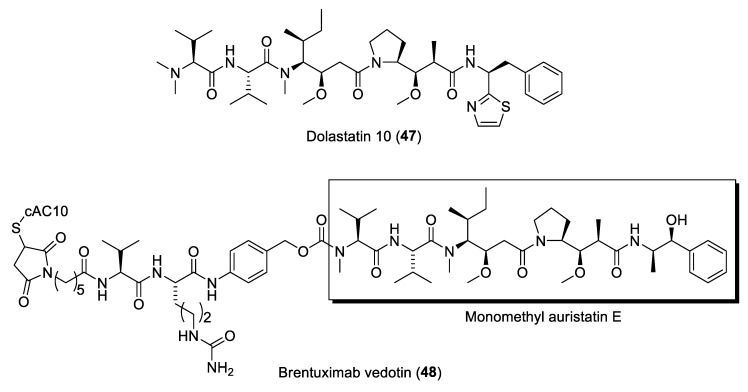
One of the earliest examples of potent microtubule inhibitors reported from marine cyanobacteria are the dolastatin class of molecules, including dolastatins 10 (47) and 15 (Figure 9) [114]. The dolastatins were originally isolated from the Indian Ocean sea hare, Dolabella auricularia. However, these cytotoxic compounds are now known to be produced by marine cyanobacteria due to the isolation of a number of dolastatin-related molecules from these microbes [115]. Dolastatins 10, 15 and their synthetic analogues, including the auristatins, have undergone a number of clinical trials as anticancer agents [11]. It was eventually realized that the auristatins are of great value as payloads in antibody drug conjugates (ADCs) formulation [116]. This discovery led to the FDA-approved ADC brentuximab vedotin (48) (Figure 9) in 2011 for the treatment of elapsed or refractory Hodgkin’s lymphoma as well as systemic anaplastic large cell lymphoma [117]. Brentuximab vedotin targets CD30 of tumor cells and selectively delivers monomethyl auristatin E (MMAE) into the cells and induces cancer cell apoptosis. Numerous modifications of the N- and C-terminal groups in auristatins have been synthesized. A review on the design of new auristatins and SAR analysis has been published [118]. Currently, more than 16 ADCs, incorporating auristatins as payloads, are currently in clinical trials for cancer therapy [119].
Figure 9.
Dolastatin 10 and brentuximab vedotin.
2.5. Sec61 Protein Translocation Channels
2.5.1. Apratoxin A
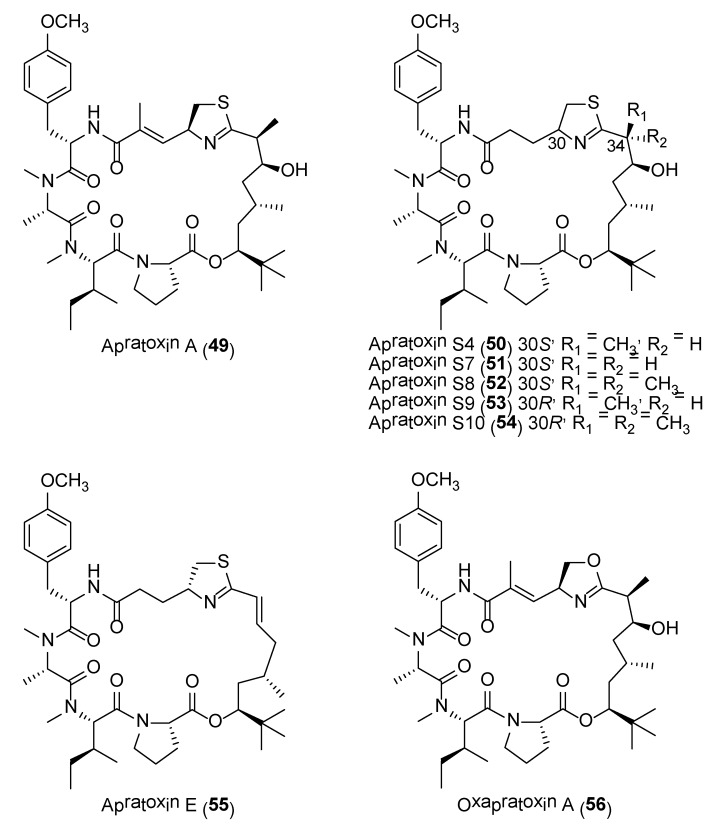
The apratoxins are a novel class of potent cytotoxic cyclodepsipeptides reported from several Lyngbya sp. strains collected from various locations. They possess significant biological activities in the nanomolar range when tested against a panel of cancer cell lines, including HT29, HeLa, and U2OS. To date, a total of nine apratoxin-related compounds have been reported with apratoxin A (49) (Figure 10) being the most cytotoxic [120]. The biosynthetic gene cluster of apratoxin A was identified via a single-cell genome-amplification approach and reveals a PKS-type loading module and nine extension modules, including four polyketide synthases (PKS) and five nonribosomal peptide synthetases (NRPS) [121]. It was recently reported that the tert-butyl group in apratoxin A is formed as a pivaloyl acyl carrier protein by AprA, the PKS loading module of the apratoxin A biosynthetic pathway [122]. Due to the significant potency of apratoxins, a number of synthetic efforts on the generation of potent synthetic analogues have been achieved. For instance, synthetic analogues, such as apratoxin S4 (50) and apratoxins S7 (51) to S9 (53), with improved antitumor activity, inhibitory secretion of the angiogenic vascular endothelial growth factor –A (VEGF-A) and tolerability in human HCT116 xenograft mouse model at subnanomolar IC50 concentrations have been reported(Table 4) (Figure 10) [123,124]. The structures of these synthetic molecules are based on the structural features of apratoxins A (49) and E (55), giving rise to the apratoxin A/E hybrid structure, differing in various degrees of methylation at C34 as well as epimeric configuration at C30. From the biological data, it was revealed that the configuration of C-34 is independent of the cytotoxic activity (Table 4).
Figure 10.
Apratoxin A and related compounds.
Table 4.
Activities of apratoxin-related analogues, apratoxins S4 (50), S7 (51)–S9 (53) on viability of HCT116 cell and secretion of VEGF-A (IC50 nM).
| Apratoxin | HCT116 | VEGF-A Secretion |
|---|---|---|
| S4 (50) | 1.43 | 0.32 |
| S7 (51) | 1.25 | 0.30 |
| S8 (52) | 1.99 | 0.47 |
| S9 (53) | 0.69 | 0.12 |
Further studies on apratoxin S4 (50), revealed the molecule to possess potent antiangiogenic activity by inhibiting the activation of retinal endothelial cells and pericytes through mediating multiple angiogenic pathways [125]. This finding could path the way for the development of apratoxin S4 as a potential cure for prevention or treatment of vision loss. A scalable synthesis of a stable analogue, apratoxin S10 (54) (Figure 10), was shown to potently inhibit angiogenesis in vitro and growth of cancer cells from vascularized tumors [126]. A recent study revealed another synthetic analogue, apratoxin S10, as a potential anti-pancreatic cancer agent [127]. The study showed that the molecule inhibited the growth of established and patient-derived primary pancreatic cancer cells. The growth inhibitory activity of apratoxin S10 on pancreatic cancer cells was due to the downregulation of multiple receptor tyrosine kinases as well as inhibition of growth factor and cytokine secretion [127].
A series of mechanistic studies on apratoxin A (49) revealed its ability to interfere with specific cellular signaling pathways as well as protein interactions involved with the formation and maintenance of cancer cells. Using functional genomics approach, apratoxin A was found to exert its antiproliferative property through induction of G1 cell cycle arrest and apoptosis by antagonism of the fibroblast growth factor (FGF) signaling via Signal Transducer and Activator of Transcription 3 (STAT3) [128]. In another study based on a synthetic oxazoline analog of apratoxin A, it was found that 56 (Figure 10) stabilizes the Hsp90 (heat shock protein 90) client proteins-Hsc70 /Hsp70 interaction, thereby inhibiting the function of Hsp90 [129]. The inhibition of Hsp90 resulted in the promotion of Hsp90 client proteins degradation via chaperone-mediated autophagy. Apratoxin A was also found to inhibit the secretory pathway by preventing cotranslational translocation of newly synthesized secretory and membrane proteins into the ER [130].
The specific mode of action of apratoxin A was eventually elucidated in two studies conducted by Paatero et al. and Huang et al. [131,132]. Apratoxin A kills cancer cells by directly blocking the Sec61 protein translocation channel [131,132]. Specifically, apratoxin A prevents protein translocation into the ER by direct binding with the central subunit of the protein translocation channel, Sec61α [131]. Binding of apratoxin A on the luminal end of the Sec61 lateral gate resulted in blocking the biogenesis of a range of Sec61 clients [131]. In addition, pathologic studies revealed apratoxin A to target pancreas, resulting in severe pancreatic atrophy in apratoxin A-treated animals [132]. The importance of Sec61 as a potential drug target is a promising concept for anticancer therapy. Recently, it was reported that the function of human epidermal growth factor receptor 3 (HER3), implicated in several cancer types, can be inhibited through direct binding of Sec61 with substrate-specific Sec61 inhibitor, cotransin [133].
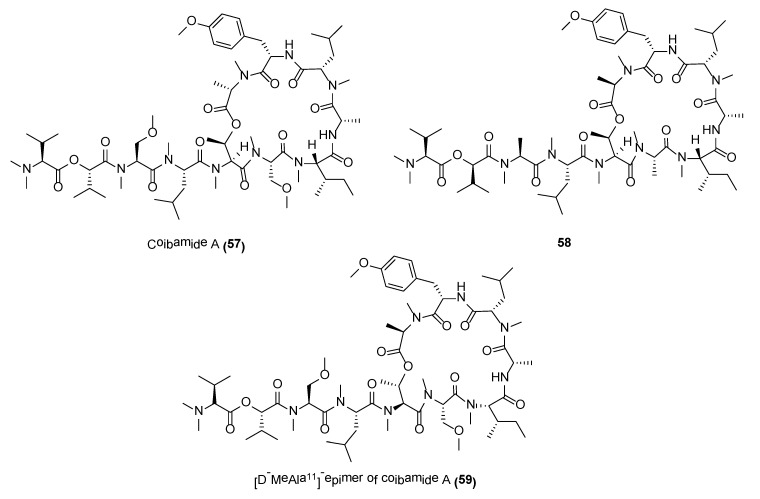
2.5.2. Coibamide A
Coibamide A (57) (Figure 11) is a structurally novel lariat-type cyclic depsipeptide, with potent antiproliferative properties, reported from the Panamanian marine cyanobacterium, Leptolyngbya sp. [134]. The complete structure of coibamide A (57) was deduced by various 2D NMR spectroscopic experiments, including COSY, TOCSY, multiplicity-edited HSQS, HSQC-TOCSY, HMBC, H2BC, 1H-15N gHMBC, and ROESY as well as mass spectroscopic data. This molecule possesses a high degree of N-methylation with eight out of 11 residues being N-methylated. More importantly, coibamide A displayed potent cytotoxicity against NCI-H460 lung cancer cells and mouse neuro-2a cells, with LC50s reported to be less than 23 nM. The compound was evaluated in the NCI’s panel of 60 cancer cell lines and it exhibited significant activities against MDA-MB-231, LOX IMVI, HL-60(TB), and SNB-75 at IC50 values of 2.8 nM, 7.4 nM, 7.4 nM and 7.6 nM, respectively [134]. Total synthesis of coibamide A was accomplished using solid-phase peptide strategy by several synthetic groups. These synthetic efforts led to the revision on the stereochemical assignment on the original reported molecule as well as generation of potent analogues for SAR studies [135,136,137]. Several synthetic analogues, such as 58 and 59 (Figure 11), showed either similar inhibitory activity or increased cytotoxicity as compared to the natural product [136,137]. A number of pharmacological studies have also been conducted on coibamide A [138,139,140]. A recent study, using a synthetic coibamide A photoaffinity probe, showd that coibamide A directly targets the Sec61α subunit of the trimeric Sec61translocon. The binding of the molecule to Sec61 resulted in broad substrate-nonselective inhibition of ER protein import and conferred potent cytotoxicity against specific cancer cell lines [141].
Figure 11.
Coibamide A and related compounds.
2.6. Interference of Inflammatory Pathways
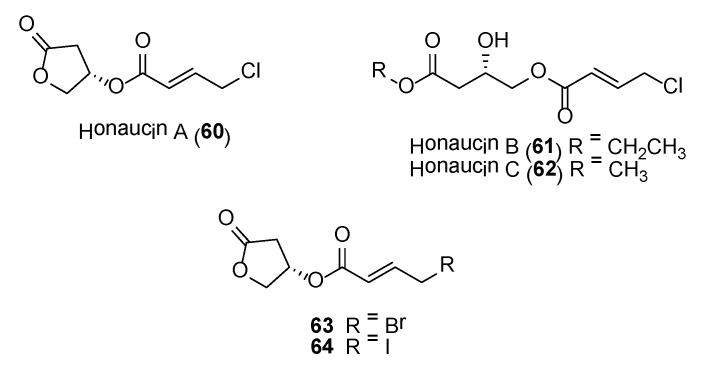
Honaucins
Honaucins A (60)–C (62) (Figure 12) are potent anti-inflammatory and bacterial quorum sensing (QS) inhibitory molecules isolated from a collection of Leptolyngbya crossbyana found overgrowing on corals in Hawaii [142]. The structure of the major compound, honaucin A, consists of a unit each of (S)-3-hydroxy-γ-butyrolactone and 4-chlorocrotonic acid, connected via an ester linkage. These molecules showed inhibition of bioluminescence in Vibrio harveyi BB120 and lipopolysaccharide-stimulated nitric oxide production in the murine macrophage cell line RAW264.7 [142]. Additional pharmacological studies performed on honaucin A showed that the molecule’s ability to attenuate inflammation via activation of the Nrf2-ARE pathway [143]. Synthetic analogues, based on honaucin A, revealed that the halogen atom at the 4-position of the crotonoic acid moiety was essential for both anti-inflammatory and QS inhibitory activities.
Figure 12.
Honaucins and related compounds.
Two synthetic analogues, 63 and 64 (Figure 12), were found to possess enhanced anti-inflammatory and QS inhibitory properties when compared to the natural products [142]. Subsequent pharmacological studies conducted on the bromo-analogue of honaucin A, compound 63, showed that it inhibited osteoclastogenesis from macrophage cell line in vitro and reduced the RANKL-induced expression of osteoclast-associated genes, such as MMP9, Cath K, GAB2, C-MYC, C-SRC, MITF, PU 1 and DC-STAMP [144]. These results showed that compound 63 could have potential as a therapeutic drug for treatment of disorders associated with bone loss.
3. Conclusions
This review covers a range of unique marine cyanobacterial natural products as well as their synthetic analogues having potent biological activities, including anticancer and antiinfective properties. A majority of these compounds are nitrogen-containing and products of the modular PKS-NRPS metabolic pathways. Due to their exquisite interference against clinically relevant drug targets, such as proteasomes, proteases and microtubule filaments, they represent important lead compounds for further development into therapeutic drugs.
A number of noteworthy drug leads include largazole, apratoxin A, carmaphycins, gallinamide A and the dolastatin class of molecules. Drug candidates, such as the largazole-based synthetic analogue, OKI-179, and apratoxin S4 have been identified from synthetic analogues of these natural drug leads and are currently undergoing or being considered for clinical testing. In addition, a high number of auristatin-based compounds, formulated as ADCs, are undergoing clinical testing as anticancer agents. Unique structural features attributed to marine cyanobacterial compounds could account for their high potency. These include high degree of N-methylation (e.g., coibamide A), macrocycles with incorporation of polyketide moiety (e.g., apratoxins), presence of heterocycles, such as thiazole, thiazoline, oxazole oxazoline and methoxypyrrolinone (e.g., apratoxins, gallinamide A, dolastatins 10 and 15), incorporation of D- and non-proteinogenic amino acids (e.g., 2-aminobutenoic acid unit in lyngbyastatin 7) as well as peptide acylation (e.g., largazole). These structural features contribute to compound permeability and adoption of desired conformation for interaction of cellular drug targets. Moreover, combination of these structural motifs expands the chemical space of marine cyanobacterial natural products.
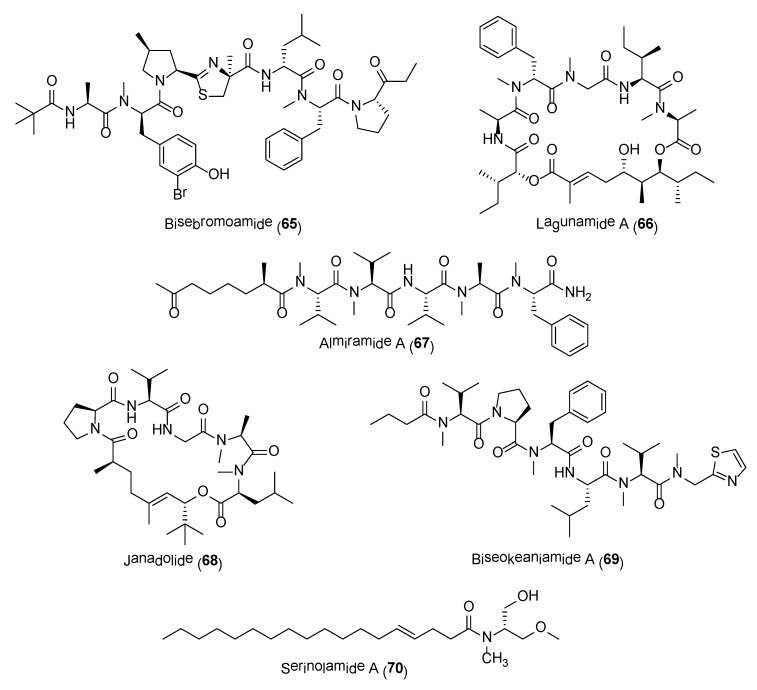
The range of molecules presented in this review is by no means exhaustive, as there are numerous potent cyanobacterial compounds reported in the literature and are beyond the scope of this review. These compounds include the highly cytotoxic compounds, bisebromoamide (65) and aurilide-class of compounds (e.g., 66), antiinfective molecules, almiramides (e.g., 67) and janadolide (68), anti-inflammatory molecule, biseokeaniamide A (69) as well as cannabimimetics/CNS modulatory agents, such as mooreamide A and serinolamides (e.g., 70) (Figure 13) [145,146,147,148,149,150,151,152,153]. Further biological evaluation and synthesis of analogues have also been initiated for some of these compounds [154,155,156,157,158,159]. The search for novel bioactive cyanobacterial compounds has also been expedited with recent advances in genomic and metabolomics techniques, e.g., the integrated use of mass spectrometric based molecular networking and genomic approaches [160,161]. It is without a doubt that these prokaryotic marine cyanobacteria will continue to provide essential drug leads in drug discovery and development efforts.
Figure 13.
Other potent marine cyanobacteria compounds.
Author Contributions
All authors discussed, commented on and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Charpy L., Casareto B.E., Langlade M.J., Suzuki Y. Cyanobacteria in coral reef ecosystems: A review. J. Mar. Biol. 2012;2012:1–9. doi: 10.1155/2012/259571. [DOI] [Google Scholar]
- 2.Tan L.T. Marine cyanobacteria: A treasure trove of bioactive secondary metabolites for drug discovery. In: Atta-ur-Rahman F.R.S., editor. Studies in Natural Products Chemistry. 1st ed. Volume 36. Elsevier; Amsterdam, The Netherlands: 2012. pp. 67–110. [Google Scholar]
- 3.Leão P.N., Engene N., Antunes A., Gerwick W.H., Vasconcelos V. The chemical ecology of cyanobacteria. Nat. Prod. Rep. 2012;29:372–391. doi: 10.1039/c2np00075j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demay J., Bernard C., Reinhardt A., Marie B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs. 2019;17:320. doi: 10.3390/md17060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleigrewe K., Gerwick L., Sherman D.H., Gerwick W.H. Unique marine derived cyanobacterial biosynthetic genes for chemical diversity. Nat. Prod. Rep. 2016;33:348–364. doi: 10.1039/C5NP00097A. [DOI] [PubMed] [Google Scholar]
- 6.Costa M., Costa-Rodrigues J., Fernandes M.H., Barros P., Vasconcelos V., Martins R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs. 2012;10:2181–2207. doi: 10.3390/md10102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aráoz R., Molgó J., Tandeau de Marsac N. Neurotoxic cyanobacterial toxins. Toxicon. 2010;56:813–828. doi: 10.1016/j.toxicon.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Rivas L., Rojas V. Cyanobacterial peptides as a tour de force in the chemical space of antiparasitic agents. Arch. Biochem. Biophys. 2019;664:24–39. doi: 10.1016/j.abb.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Niedermeyer T.H. Anti-infective natural products from cyanobacteria. Planta Med. 2015;81:1309–1325. doi: 10.1055/s-0035-1546055. [DOI] [PubMed] [Google Scholar]
- 10.Newman D.J., Cragg G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs. 2014;12:255–278. doi: 10.3390/md12010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman D.J., Cragg G.M. Current status of marine-derived compounds as warheads in anti-tumor drug candidates. Mar. Drugs. 2017;15:99. doi: 10.3390/md15040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watrous J., Roach P., Alexandrov T., Heath B.S., Yang J.Y., Kersten R.D., van der Voort M., Pogliano K., Gross H., Raaijmakers J.M., et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA. 2012;109:E1743–E1752. doi: 10.1073/pnas.1203689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleigrewe K., Almaliti J., Tian I.Y., Kinnel R.B., Korobeynikov A., Monroe E.A., Duggan B.M., Marzo V.D., Sherman D.H. Dorrestein, P.C.; et al. Combining mass spectrometric metabolic profiling with genomic analysis: A powerful approach for discovering natural products from cyanobacteria. J. Nat. Prod. 2015;78:1671–1682. doi: 10.1021/acs.jnatprod.5b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naman C.B., Rattan R., Nikoulina S.E., Lee J., Miller B.W., Moss N.A., Armstrong L., Boudreau P.D., Debonsi H.M., Valeriote F.A., et al. Integrating molecular networking and biological assays to target the isolation of a cytotoxic cyclic octapeptide, samoamide A, from an American Samoan marine cyanobacterium. J. Nat. Prod. 2017;80:625–633. doi: 10.1021/acs.jnatprod.6b00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerwick W.H. The face of a molecule. J. Nat. Prod. 2017;80:2583–2588. doi: 10.1021/acs.jnatprod.7b00624. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C., Idelbayev Y., Roberts N., Tao Y., Nannapaneni Y., Duggan B.M., Min J., Lin E.C., Gerwick E.C., Cottrell G.W., et al. Small molecule accurate recognition technology (SMART) to enhance natural products research. Sci. Rep. 2017;7:14243. doi: 10.1038/s41598-017-13923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reher R., Kim H.W., Zhang C., Mao H.H., Wang M., Nothias L.-E., Caraballo-Rodriguez A.M., Glukhov E., Teke B., Leao T., et al. A convolutional neural network-based approach for the rapid annotation of molecularly diverse natural products. J. Am. Chem. Soc. 2020;142:4114–4120. doi: 10.1021/jacs.9b13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Tan L.T. Filamentous tropical marine cyanobacteria: a rich source of natural products for anticancer drug discovery. J. Appl. Phycol. 2010;22:659–676. doi: 10.1007/s10811-010-9506-x. [DOI] [Google Scholar]
- 20.Nunnery J.K., Mevers E., Gerwick W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010;21:787–793. doi: 10.1016/j.copbio.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L.T. Pharmaceutical agents from filamentous marine cyanobacteria. Drug Discov. Today. 2013;18:863–871. doi: 10.1016/j.drudis.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Salvador-Reyes L.A., Luesch H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015;32:478–503. doi: 10.1039/C4NP00104D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi Y., Zhang J., He S., Yan X. New peptides isolated from marine cyanobacteria, an overview over the past decade. Mar. Drugs. 2017;15:132. doi: 10.3390/md15050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah S.A.A., Akhter N., Auckloo B.N., Khan I., Lu Y., Wang K., Wu B., Guo Y.W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Mar. Drugs. 2017;15:354. doi: 10.3390/md15110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M., Zhang J., He S., Yan X. A review study on macrolides isolated from cyanobacteria. Mar. Drugs. 2017;15:126. doi: 10.3390/md15050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taori K., Paul V.J., Luesch H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008;130:1806–1807. doi: 10.1021/ja7110064. [DOI] [PubMed] [Google Scholar]
- 27.Ying Y., Taori K., Kim H., Hong J., Luesch H. Total synthesis and molecular target of largazole, a histone deacetylase inhibitor. J. Am. Chem. Soc. 2008;130:8455–8459. doi: 10.1021/ja8013727. [DOI] [PubMed] [Google Scholar]
- 28.Li S., Yao H., Xu J., Jiang S. Synthetic routes and biological evaluation of largazole and its analogues as potent histone deacetylase inhibitors. Molecules. 2011;16:4681–4694. doi: 10.3390/molecules16064681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q.-Y., Chaturvedi P.R., Luesch H. Process development and scale-up total synthesis of largazole, a potent class I histone deacetylase inhibitor. Org. Process Res. Dev. 2018;22:190–199. doi: 10.1021/acs.oprd.7b00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong J., Luesch H. Largazole: From discovery to broad-spectrum therapy. Nat. Prod. Rep. 2012;29:449–456. doi: 10.1039/c2np00066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou H., Jiang S., Chen J., Ren X., Jin J., Su S.B. Largazole, an inhibitor of class I histone deacetylases, attenuates inflammatory corneal neovascularization. Eur. J. Pharmacol. 2014;740:619–626. doi: 10.1016/j.ejphar.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Wu L.C., Wen Z.S., Qiu Y.T., Chen X.Q., Chen H.B., Wei M.M., Liu Z., Jiang S., Zhou G.B. Largazole arrests cell cycle at G1 phase and triggers proteasomal degradation of E2F1 in lung cancer cells. ACS Med. Chem. Lett. 2013;4:921–926. doi: 10.1021/ml400093y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S., Riegsecker S., Beamer M., Rahman A., Bellini J.V., Bhansali P., Tillekeratne L.M. Largazole, a class I histone deacetylase inhibitor, enhances TNF-α-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmcol. 2013;270:87–96. doi: 10.1016/j.taap.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Wang Z., Wang J., Lam W., Kwong S., Li F., Friedman S.L., Zhou S., Ren Q., Xu Z., et al. A histone deacetylase inhibitor, largazole, decreases liver fibrosis and angiogenesis by inhibiting transforming growth factor-β and vascular endothelial growth factor signalling. Liver Int. 2013;33:504–515. doi: 10.1111/liv.12034. [DOI] [PubMed] [Google Scholar]
- 35.Law M.E., Corsino P.E., Jahn S.C., Davis B.J., Chen S., Patel B., Pham K., Lu J., Sheppard B., Nørgaard P., et al. Glucocorticoids and histone deacetylase inhibitors cooperate to block the invasiveness of basal-like breast cancer cells through novel mechanisms. Oncogene. 2013;32:1316–1329. doi: 10.1038/onc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungermannova D., Parker S.J., Nasveschuk C.G., Wang W., Quade B., Zhang G., Kuchta R.D., Phillips A.J., Liu X. Largazole and its derivatives selectively inhibit ubiquitin activating enzyme (E1) PLoS ONE. 2012;7:e29208. doi: 10.1371/journal.pone.0029208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh S.K., Perrine S.P., Williams R.M., Faller D.V. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood. 2012;119:1008–1017. doi: 10.1182/blood-2011-06-362434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S.U., Kwak H.B., Pi S.H., You H.K., Byeon S.R., Ying Y., Luesch H., Hong J., Kim S.H. In vitro and in vivo osteogenic activity of largazole. ACS Med. Chem. Lett. 2011;2:248–251. doi: 10.1021/ml1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Salvador L.A., Byeon S., Ying Y., Kwan J.C., Law B.K., Hong J., Luesch H. Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J. Pharmacol. Exp. Ther. 2010;335:351–361. doi: 10.1124/jpet.110.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez G.J., Richmond P.A., Bunker E.N., Karman S.S., Azofeifa J., Garnett A.T., Xu Q., Wheeler G.E., Toomey C.M., Zhang Q., et al. Genome-wide dose-dependent inhibition of histone deacetylases studies reveal their roles in enhancer remodeling and suppression of oncogenic super-enhancers. Nucleic Acids Res. 2018;46:1756–1776. doi: 10.1093/nar/gkx1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert B.J., Niu A., Ramani R., Marshall G.R., Wender P.A., Williams R.M., Ratner L., Barnes A.B., Kyei G.B. Combinations of isoform-targeted histone deacetylase inhibitors and bryostatin analogues display remarkable potency to activate latent HIV without global T-cell activation. Sci. Rep. 2017;7:7456. doi: 10.1038/s41598-017-07814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poli G., Di Fabio R., Ferrante L., Summa V., Botta M. Largazole analogues as histone deacetylase inhibitors and anticancer agents: an overview of structure-activity relationships. Chem. Med. Chem. 2017;12:1917–1926. doi: 10.1002/cmdc.201700563. [DOI] [PubMed] [Google Scholar]
- 43.Clausen D.J., Smith W.B., Haines B.E., Wiest O., Bradner J.E., Williams R.M. Modular synthesis and biological activity of pyridyl-based analogs of the potent class I histone deacetylase inhibitor largazole. Bioorg. Med. Chem. 2015;23:5061–5074. doi: 10.1016/j.bmc.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almaliti J., Al-Hamashi A.A., Negmeldin A.T., Hanigan C.L., Perera L., Pflum M.K.H., Casero R.A., Jr., Tillekeratne L.M.V. Largazole analogues embodying radical changes in the depsipeptide ring: Development of a more selective and highly potent analogue. J. Med. Chem. 2016;59:10642–10660. doi: 10.1021/acs.jmedchem.6b01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F., Chai H., Su M.B., Zhang Y.-M., Li J., Xie X., Nan F.-J. Potent and orally efficacious bisthiazole-based histone deacetylase inhibitors. ACS Med. Chem. Lett. 2014;5:628–633. doi: 10.1021/ml400470s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowers A.A., West N., Newkirk T.L., Troutman-Youngman A.E., Schreiber S.L., Wiest O., Bradner J.E., Williams R.M. Synthesis and histone deacetylase inhibitory activity of largazole analogs: Alteration of the zinc-binding domain and macrocyclic scaffold. Org. Lett. 2009;11:1301–1304. doi: 10.1021/ol900078k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim B., Ratnayake R., Lee H., Shi G., Zeller S.L., Li C., Luesch H., Hong J. Synthesis and biological evaluation of largazole zinc-binding group analogs. Bioorg. Med. Chem. 2017;25:3077–3086. doi: 10.1016/j.bmc.2017.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivieccio M.A., Brochier C., Willis D.E., Walker B.A., D’Annibale M.A., McLaughlin K., Siddiq A., Kozikowski A.P., Jaffrey S.R., Twiss J.L., et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc. Natl. Acad. Sci. USA. 2009;106:19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam M.M., Banerjee T., Packard C.Z., Kotian S., Selvendiran K., Cohn D.E., Parvin J.D. HDAC10 as a potential therapeutic target in ovarian cancer. Gynecol. Oncol. 2017;144:613–620. doi: 10.1016/j.ygyno.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B., Liu J., Gao D., Yu X., Wang J., Lei X. A fluorine scan on the Zn2+-binding thiolate side chain of HDAC inhibitor largazole: synthesis, biological evaluation, and molecular modeling. Eur. J. Med. Chem. 2019;182:111672. doi: 10.1016/j.ejmech.2019.111672. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Waschke B.C., Woolaver R.A., Chen Z., Zhang G., Piscopio A.D., Liu X., Wang J.H. Histone deacetylase inhibition sensitizes PD1 blockade-resistant B-cell lymphomas. Cancer Immunol. Res. 2019;7:1318–1331. doi: 10.1158/2326-6066.CIR-18-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diamond J.R., Kagihara J.A., Liu X., Gordon G., Heim A.M., Winkler J., DeMattei J.A., Piscopio A.D., Eckhardt S.G. OKI-179 is a novel, oral, class I specific histone deacetylase inhibitor in phase 1 clinical trials; Proceedings of the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; Boston, MA, USA. 26–30 October 2019. [Google Scholar]
- 53.Pavlik C.M., Wong C.Y., Ononye S., Lopez D.D., Engene N., McPhail K.L., Gerwick W.H., Balunus M.J. Santacruzamate A, A potent and selective histone deacetylase inhibitor from the Panamanian marine cyanobacterium cf. Symploca sp. J. Nat. Prod. 2013;76:2026–2033. doi: 10.1021/np400198r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gromek S.M., de Mayo J.A., Maxwell A.T., West A.M., Pavlik C.M., Zhao Z., Li J., Wiemer A.J., Zweifach A., Balunas M.J. Synthesis and biological evaluation of Santacruz amate A analogues for anti-proliferative and immunomodulatory activity. Bioorg. Med. Chem. 2016;24:5183–5196. doi: 10.1016/j.bmc.2016.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Randino R., Gazzerro P., Mazitschek R., Rodriquez M. Synthesis and biological evaluation of santacruzamate—A based analogues. Bioorg. Med. Chem. 2017;25:6486–6491. doi: 10.1016/j.bmc.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Zhou H., Cai Y., Liu D., Li M., Sha Y., Zhang W., Wang K., Gong J., Tang N., Huang A., et al. Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21Waf1/Cip1 and p19INK4d upregulation in hepatocellular carcinoma. Cell Prolif. 2018;51:e12447. doi: 10.1111/cpr.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L., Liu Y.C., Tan H., Zhang Y., Xu J., Liu W.L., Li Z.Y., Li W.P. Santacruzamate A ameliorates AD-like pathology by enhancing ER stress tolerance through regulating the functions of KDELR and Mia40-ALR in vivo and in vitro. Front. Cell. Neurosci. 2019;13:61. doi: 10.3389/fncel.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Pereira A.R., Kale A.J., Fenley A.T., Byrum T., Debonsi H.M., Gilson M.K., Valeriote F.A., Moore B.S., Gerwick W.H. The carmaphycins: New proteasome inhibitors exhibiting an α,β-epoxyketone warhead from a marine cyanobacterium. ChemBioChem. 2012;13:810–817. doi: 10.1002/cbic.201200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groll M., Kim K.B., Kairies N., Huber R., Crews C.M. Crystal structure of epoxomicin: 20S proteasome reveals a molecular basis for selectivity of α,β-epoxyketone proteasome inhibitors. J. Am. Chem. Soc. 2000;122:1237–1238. doi: 10.1021/ja993588m. [DOI] [Google Scholar]
- 60.Trivella D.B., Pereira A.R., Stein M.L., Kasai Y., Byrum T., Valeriote F.A., Tantillo D.J., Groll M., Gerwick W.H., Moore B.S. Enzyme inhibition by hydroamination: Design and mechanism of a hybrid carmaphycin-syringolinenone proteasome inhibitor. Chem. Biol. 2014;21:782–791. doi: 10.1016/j.chembiol.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LaMonte G.M., Almaliti J., Bibo-Verdugo B., Keller L., Zou B.Y., Yang J., Antonova-Koch Y., Orjuela-Sanchez P., Boyle C.A., Vigil E., et al. Development of a potent inhibitor of the Plasmodium proteasome with reduced mammalian toxicity. J. Med. Chem. 2017;60:6721–6732. doi: 10.1021/acs.jmedchem.7b00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Donoghue A.J., Bibo-Verdugo B., Miyamoto Y., Wang S.C., Yang J.Z., Zuill D.E., Matsuka S., Jiang Z., Almaliti J., Caffrey C.R., et al. 20S proteasome as a drug target in Trichomonas vaginalis. Antimicrob. Agents Chemother. 2019;63:e00448-19. doi: 10.1128/AAC.00448-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bibo-Verdugo B., Wang S.C., Almaliti J., Ta A.P., Jiang Z., Wong D.A., Lietz C.B., Suzuki B.M., El-Sakkary N., Hook V., et al. The proteasome as a drug target in the metazoan pathogen, Schistosoma mansoni. ACS Infect. Dis. 2019;5:1802–1812. doi: 10.1021/acsinfecdis.9b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almaliti J., Miller B., Pietraszkiewicz H., Glukhov E., Naman C.B., Kline T., Hanson J., Li X., Zhou S., Valeriote F.A., et al. Exploration of the carmaphycins as payloads in antibody drug conjugate anticancer agents. Eur. J. Med. Chem. 2019;161:416–432. doi: 10.1016/j.ejmech.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro H.C., Abreu P.A., Geraldo R.B., Martins R.C.A., dos Santos R., Loureiro N., Cabral L.M., Rodrigues C.R. Looking at the proteases from a simple perspective. J. Mol. Recognit. 2011;24:165–181. doi: 10.1002/jmr.1091. [DOI] [PubMed] [Google Scholar]
- 66.Docherty A.J.P., Crabbe T., O’Connell J.P., Groom C.R. Proteases as drug targets. Biochem. Soc. Symp. 2003;70:147–161. doi: 10.1042/bss0700147. [DOI] [PubMed] [Google Scholar]
- 67.Soualmia F., El Amri C. Serine protease inhibitors to treat inflammation: A patent review (2011–2016) Expert Opin. Ther. Pat. 2018;28:93–110. doi: 10.1080/13543776.2018.1406478. [DOI] [PubMed] [Google Scholar]
- 68.Matthew S., Ross C., Rocca J.R., Paul V.J., Luesch H. Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007;70:124–127. doi: 10.1021/np060471k. [DOI] [PubMed] [Google Scholar]
- 69.Taori K., Matthew S., Rocca J.R., Paul V.J., Luesch H. Lyngbyastatins 5-7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007;70:1593–1600. doi: 10.1021/np0702436. [DOI] [PubMed] [Google Scholar]
- 70.Kwan J.C., Taori K., Paul V.J., Luesch H. Lyngbyastatins 8-10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs. 2009;7:528–538. doi: 10.3390/md7040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matthew S., Ross C., Paul V.J., Luesch H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron. 2008;64:4081–4089. doi: 10.1016/j.tet.2008.02.035. [DOI] [Google Scholar]
- 72.Linington R.G., Edwards D.J., Shuman C.F., McPhail K.L., Matainaho T., Gerwick W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008;71:22–27. doi: 10.1021/np070280x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taori K., Paul V.J., Luesch H. Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2008;71:1625–1629. doi: 10.1021/np8002172. [DOI] [PubMed] [Google Scholar]
- 74.Gunasekera S.P., Miller M.W., Kwan J.C., Luesch H., Paul V.J. Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J. Nat. Prod. 2010;73:459–462. doi: 10.1021/np900603f. [DOI] [PubMed] [Google Scholar]
- 75.Rubio B.K., Parrish S.M., Yoshida W., Schupp P.J., Schils T., Williams P.G. Depsipeptides from a Guamanian marine cyanobacterium, Lyngbya bouillonii, with selective inhibition of serine proteases. Tetrahedron Lett. 2010;51:6718–6721. doi: 10.1016/j.tetlet.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Awadhi F.H., Paul V.J., Luesch H. Structural diversity and anticancer activity of marine-derived elastase inhibitors: key features and mechanisms mediating the antimetastatic effects in invasive breast cancer. ChemBioChem. 2018;19:815–825. doi: 10.1002/cbic.201700627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozakia K., Iwasaki A., Suenaga K., Teruya T. Kyanamide, a new Ahp-containing depsipeptide from marine cyanobacterium Caldora penicillata. Tetrahedron. 2019;75:3382–3386. doi: 10.1016/j.tet.2019.04.046. [DOI] [Google Scholar]
- 78.Keller L., Canuto K.M., Liu C., Suzuki B.M., Almaliti J., Sikandar A., Naman C.B., Glukhov E., Luo D., Duggan B.M., et al. Tutuilamides A–C: Vinyl-chloride-containing cyclodepsipeptides from marine cyanobacteria with potent elastase inhibitory properties. ACS Chem. Biol. 2020;15:751–757. doi: 10.1021/acschembio.9b00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matthew S., Paul V.J., Luesch H. Largamides A–C, tiglic acid-containing cyclodepsipeptides with elastase-inhibitory activity from the marine cyanobacterium Lyngbya confervoides. Planta Med. 2009;75:528–533. doi: 10.1055/s-0029-1185332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plaza A., Bewley C.A. Largamides A–H, unusual cyclic peptides from the marine cyanobacterium Oscillatoria sp. J. Org. Chem. 2006;71:6898–6907. doi: 10.1021/jo061044e. [DOI] [PubMed] [Google Scholar]
- 81.Matthew S., Paul V.J., Luesch H. Tiglicamides A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry. 2009;70:2058–2063. doi: 10.1016/j.phytochem.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthew S., Ratnayake R., Becerro M.A., Ritson-Williams R., Paul V.J., Luesch H. Intramolecular modulation of serine protease inhibitor activity in a marine cyanobacterium with antifeedant properties. Mar. Drugs. 2010;8:1803–1816. doi: 10.3390/md8061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salvador L.A., Taori K., Biggs J.S., Jakoncic J., Ostrov D.A., Paul V.J., Luesch H. Potent elastase inhibitors from cyanobacteria: Structural basis and mechanisms mediating cytoprotective and anti-inflammatory effects in bronchial epithelial cells. J. Med. Chem. 2013;56:1276–1290. doi: 10.1021/jm3017305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo D., Chen Q.Y., Luesch H. Total synthesis of the potent marine-derived elastase inhibitor lyngbyastatin 7 and in vitro biological evaluation in model systems for pulmonary diseases. J. Org. Chem. 2016;81:532–544. doi: 10.1021/acs.joc.5b02386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo D., Luesch H. Ahp-cyclodepsipeptide inhibitors of elastase: lyngbyastatin 7 stability, scalable synthesis, and focused library analysis. ACS Med. Chem. Lett. 2020;11:419–425. doi: 10.1021/acsmedchemlett.9b00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Köcher S., Rey J., Bongard J., Tiaden A.N., Meltzer M., Richards P.J., Ehrmann M., Kaiser M. Tailored Ahp-cyclodepsipeptides as potent non-covalent serine protease inhibitors. Angew. Chem. Int. Edit. Engl. 2017;56:8555–8558. doi: 10.1002/anie.201701771. [DOI] [PubMed] [Google Scholar]
- 87.Linington R.G., Clark B.R., Trimble E.E., Almanza A., Ureña L.D., Kyle D.E., Gerwick W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009;72:14–17. doi: 10.1021/np8003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taori K., Liu Y., Paul V.J., Luesch H. Combinatorial strategies by marine cyanobacteria: Symplostatin 4, an antimitotic natural dolastatin 10/15 hybrid that synergizes with the coproduced HDAC inhibitor largazole. Chembiochem. 2009;10:1634–1639. doi: 10.1002/cbic.200900192. [DOI] [PubMed] [Google Scholar]
- 89.Conroy T., Guo J.T., Hunt N.H., Payne R.J. Total synthesis and antimalarial activity of symplostatin 4. Org. Lett. 2010;12:5576–5579. doi: 10.1021/ol1024663. [DOI] [PubMed] [Google Scholar]
- 90.Conroy T., Guo J.T., Linington R.G., Hunt N.H., Payne R.J. Total synthesis, stereochemical assignment, and antimalarial activity of gallinamide A. Chemistry. 2011;17:13544–13552. doi: 10.1002/chem.201102538. [DOI] [PubMed] [Google Scholar]
- 91.Stolze S.C., Deu E., Kaschani F., Li N., Florea B.I., Richau K.H., Colby T., van der Hoorn R.A., Overkleeft H.S., Bogyo M., et al. The antimalarial natural product symplostatin 4 is a nanomolar inhibitor of the food vacuole falcipains. Chem. Biol. 2012;19:1546–1555. doi: 10.1016/j.chembiol.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mane U.R., Gupta R.C., Nadkarni S.S., Giridhar R.R., Naik P.P., Yadav M.R. Falcipain inhibitors as potential therapeutics for resistant strains of malaria: a patent review. Expert Opin. Ther. Pat. 2013;23:165–187. doi: 10.1517/13543776.2013.743992. [DOI] [PubMed] [Google Scholar]
- 93.Omotuyi O.I. Methyl-methoxylpyrrolinone and flavinium nucleus binding signatures on falcipain-2 active site. J. Mol. Model. 2014;20:2386. doi: 10.1007/s00894-014-2386-2. [DOI] [PubMed] [Google Scholar]
- 94.Conroy T., Guo J.T., Elias N., Cergol K.M., Gut J., Legac J., Khatoon L., Liu Y., McGowan S., Rosenthal P.J., et al. Synthesis of gallinamide A analogues as potent falcipain inhibitors and antimalarials. J. Med. Chem. 2014;57:10557–10563. doi: 10.1021/jm501439w. [DOI] [PubMed] [Google Scholar]
- 95.Stoye A., Juillard A., Tang A.H., Legac J., Gut J., White K.L., Charman S.A., Rosenthal P.J., Grau G.E.R., Hunt N.H., et al. Falcipain inhibitors based on the natural product gallinamide A are potent in vitro and in vivo antimalarials. J. Med. Chem. 2019;62:5562–5578. doi: 10.1021/acs.jmedchem.9b00504. [DOI] [PubMed] [Google Scholar]
- 96.Boudreau P.D., Miller B.W., McCall L.I., Almaliti J., Reher R., Hirata K., Le T., Siqueira-Neto J.L., Hook V., Gerwick W.H. Design of gallinamide A analogs as potent inhibitors of the cysteine proteases human cathepsin L and Trypanosoma cruzi cruzain. J. Med. Chem. 2019;62:9026–9044. doi: 10.1021/acs.jmedchem.9b00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu S., Gao X., Zhang L., Qin S., Wei M., Liu N., Zhao R., Li B., Meng Y., Lin G., et al. A novel anti-cancer stem cells compound optimized from the natural symplostatin 4 scaffold inhibits Wnt/β-catenin signaling pathway. Eur. J. Med. Chem. 2018;156:21–42. doi: 10.1016/j.ejmech.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 98.Patel S., Homaei A., El-Seedi H.R., Akhtar N. Cathepsins: Proteases that are vital for survival but can also be fatal. Biomed. Pharmacother. 2018;105:526–532. doi: 10.1016/j.biopha.2018.05.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turk B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 100.Tan G.J., Peng Z.K., Lu J.P., Tang F.Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 2013;4:91–101. doi: 10.4331/wjbc.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kwan J.C., Eksioglu E.A., Liu C., Paul V.J., Luesch H. Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009;52:5732–5747. doi: 10.1021/jm9009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Awadhi F.H., Law B.K., Paul V.J., Luesch H. Grassystatins D-F, potent aspartic protease inhibitors from marine cyanobacteria as potential antimetastatic agents targeting invasive breast cancer. J. Nat. Prod. 2017;80:2969–2986. doi: 10.1021/acs.jnatprod.7b00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang S., Zhang W., Ding N., Lo J., Liu Y., Clare-Salzler M.J., Luesch H., Li Y. Total synthesis of grassystatin A, a probe for cathepsin E function. Bioorg. Med. Chem. 2012;20:4774–4780. doi: 10.1016/j.bmc.2012.05.077. [DOI] [PubMed] [Google Scholar]
- 104.Al-Awadhi F.H., Ratnayake R., Paul V.J., Luesch H. Tasiamide F, a potent inhibitor of cathepsins D and E from a marine cyanobacterium. Bioorg. Med. Chem. 2016;24:3276–3282. doi: 10.1016/j.bmc.2016.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y., Zhang W., Li L., Salvador L.A., Chen T., Chen W., Felsenstein K.M., Ladd T.B., Price A.R., Golde T.E., et al. Cyanobacterial peptides as a prototype for the design of potent β-secretase inhibitors and the development of selective chemical probes for other aspartic proteases. J. Med. Chem. 2012;55:10749–10765. doi: 10.1021/jm301630s. [DOI] [PubMed] [Google Scholar]
- 106.Xu H., Bao K., Tang S., Ai J., Hu H., Zhang W. Cyanobacterial peptides as a prototype for the design of cathepsin D inhibitors. J. Pept. Sci. 2017;23:701–706. doi: 10.1002/psc.3014. [DOI] [PubMed] [Google Scholar]
- 107.Li Z., Bao K., Xu H., Wu P., Li W., Liu J., Zhang W. Design, synthesis, and bioactivities of tasiamide B derivatives as cathepsin D inhibitors. J. Pept. Sci. 2019;25:e3154. doi: 10.1002/psc.3154. [DOI] [PubMed] [Google Scholar]
- 108.Molinski T.F., Reynolds K.A., Morinaka B.I. Symplocin A, a linear peptide from the Bahamian cyanobacterium Symploca sp. configurational analysis of N,N-dimethylamino acids by chiral-phase HPLC of naphthacyl esters. J. Nat. Prod. 2012;75:425–431. doi: 10.1021/np200861n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams P.G., Yoshida W.Y., Moore R.E., Paul V.J. The isolation and structure elucidation of tasiamide B, a 4-amino-3-hydroxy-5-phenylpentanoic acid containing peptide from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2003;66:1006–1009. doi: 10.1021/np030114z. [DOI] [PubMed] [Google Scholar]
- 110.Iraji A., Khoshneviszadeh M., Firuzi O., Khoshneviszadeh M., Edraki N. Novel small molecule therapeutic agents for Alzheimer disease: focusing on BACE1 and multi-target directed ligands. Bioorg. Chem. 2020;97:103649. doi: 10.1016/j.bioorg.2020.103649. [DOI] [PubMed] [Google Scholar]
- 111.Naushad M., Durairajan S.S.K., Bera A.K., Senapati S., Li M. Natural compounds with anti-BACE1 activity as promising therapeutic drugs for treating Alzheimer’s Disease. Planta Med. 2019;85:1316–1325. doi: 10.1055/a-1019-9819. [DOI] [PubMed] [Google Scholar]
- 112.Sun T., Zhang W., Zong C., Wang P., Li Y. Total synthesis and stereochemical reassignment of tasiamide B. J. Pept. Sci. 2010;16:364–374. doi: 10.1002/psc.1254. [DOI] [PubMed] [Google Scholar]
- 113.Liu J., Chen W., Xu Y., Ren S., Zhang W., Li Y. Design, synthesis and biological evaluation of tasiamide B derivatives as BACE1 inhibitors. Bioorg. Med. Chem. 2015;23:1963–1974. doi: 10.1016/j.bmc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 114.Poncet J. The dolastatins, a family of promising antineoplastic agents. Curr. Pharm. Des. 1999;5:139–162. [PubMed] [Google Scholar]
- 115.Luesch H., Harrigan G.G., Goetz G., Horgen F.D. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr. Med. Chem. 2002;9:1791–1806. doi: 10.2174/0929867023369051. [DOI] [PubMed] [Google Scholar]
- 116.Maderna A., Leverett C.A. Recent advances in the development of new auristatins: Structural modifications and application in antibody drug conjugates. Mol. Pharm. 2015;12:1798–1812. doi: 10.1021/mp500762u. [DOI] [PubMed] [Google Scholar]
- 117.Diamantis N., Banerji U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer. 2016;114:362–367. doi: 10.1038/bjc.2015.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akaiwa M., Dugal-Tessier J., Mendelsohn B.A. Antibody-drug conjugate payloads; study of auristatin derivatives. Chem. Pharm. Bull. (Tokyo) 2020;68:201–211. doi: 10.1248/cpb.c19-00853. [DOI] [PubMed] [Google Scholar]
- 119.Doronina S.O., Senter P.D. Auristatin payloads for antibody–drug conjugates (ADCs) In: Thurston D.E., Jackson P.J.M., editors. Cytotoxic Payloads for Antibody-Drug Conjugates. 1st ed. Royal Society of Chemistry; Cambridge, UK: 2019. pp. 73–99. [Google Scholar]
- 120.Luesch H., Yoshida W.Y., Moore R.E., Paul V.J., Corbett T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001;123:5418–5423. doi: 10.1021/ja010453j. [DOI] [PubMed] [Google Scholar]
- 121.Grindberg R.V., Ishoey T., Brinza D., Esquenazi E., Coates R.C., Liu W.-T., Gerwick L., Dorrestein P.C., Pevzner P., Lasken R., et al. Single cell genome amplification accelerates identification of the apratoxin biosynthetic pathway from a complex microbial assemblage. PLoS ONE. 2011;6:e18565. doi: 10.1371/journal.pone.0018565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skiba M.A., Sikkema A.P., Moss N.A., Lowell A.N., Su M., Sturgis R.M., Gerwick L., Gerwick W.H., Sherman D.H., Smith J.L. Biosynthesis of t-butyl in apratoxin A: functional analysis and architecture of a PKS Loading Module. ACS Chem. Biol. 2018;13:1640–1650. doi: 10.1021/acschembio.8b00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Q.Y., Liu Y., Cai W., Luesch H. Improved total synthesis and biological evaluation of potent apratoxin S4 based anticancer agents with differential stability and further enhanced activity. J. Med. Chem. 2014;57:3011–3029. doi: 10.1021/jm4019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Q.Y., Liu Y., Luesch H. Systematic chemical mutagenesis identifies a potent novel apratoxin A/E hybrid with improved in vivo antitumor activity. ACS Med. Chem. Lett. 2011;2:861–865. doi: 10.1021/ml200176m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qiu B., Tan A., Veluchamy A.B., Li Y., Murray H., Cheng W., Liu C.-H., Busoy J.M., Chen Q.-Y., Sistla S., et al. Apratoxin S4 inspired by a marine natural product, a new treatment option for ocular angiogenic diseases. Invest. Ophthalmol. Vis. Sci. 2019;60:3254–3263. doi: 10.1167/iovs.19-26936. [DOI] [PubMed] [Google Scholar]
- 126.Cai W., Chen Q.Y., Dang L.H., Luesch H. Apratoxin S10, a dual inhibitor of angiogenesis and cancer cell growth to treat highly vascularized. ACS Med. Chem. Lett. 2017;8:1007–1012. doi: 10.1021/acsmedchemlett.7b00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cai W., Ratnayake R., Gerber M.H., Chen Q.-Y., Yu Y., Derendorf H., Trevino J.G., Luesch H. Development of apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent and its preliminary evaluation in an orthotopic patient-derived xenograft (PDX) model. Invest. New Drugs. 2019;37:364–374. doi: 10.1007/s10637-018-0647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luesch H., Chanda S.K., Raya R.M., DeJesus P.D., Orth A.P., Walker J.R., Izpisúa Belmonte J.C., Schultz P.G. A functional genomics approach to the mode of action of apratoxin A. Nat. Chem. Biol. 2006;2:158–167. doi: 10.1038/nchembio769. [DOI] [PubMed] [Google Scholar]
- 129.Shen S., Zhang P., Lovchik M.A., Li Y., Tang L., Chen Z., Zeng R., Ma D., Yuan J., Yu Q. Cyclodepsipeptide toxin promotes the degradation of Hsp90 client proteins through chaperone-mediated autophagy. J. Cell Biol. 2009;185:629–639. doi: 10.1083/jcb.200810183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y., Law B.K., Luesch H. Apratoxin a reversibly inhibits the secretory pathway by preventing cotranslational translocation. Mol. Pharmacol. 2009;76:91–104. doi: 10.1124/mol.109.056085. [DOI] [PubMed] [Google Scholar]
- 131.Paatero A.O., Kellosalo J., Dunyak B.M., Almaliti J., Gestwicki J.E., Gerwick W.H., Taunton J., Paavilainen V.O. Apratoxin kills cells by direct blockade of the Sec61 protein translocation channel. Cell Chem. Biol. 2016;23:561–566. doi: 10.1016/j.chembiol.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 132.Huang K.C., Chen Z., Jiang Y., Akare S., Kolber-Simonds D., Condon K., Agoulnik S., Tendyke K., Shen Y., Wu K.M., et al. Apratoxin A shows novel pancreas-targeting activity through the binding of Sec 61. Mol. Cancer Ther. 2016;15:1208–1216. doi: 10.1158/1535-7163.MCT-15-0648. [DOI] [PubMed] [Google Scholar]
- 133.Ruiz-Saenz A., Sandhu M., Carrasco Y., Maglathlin R.L., Taunton J., Moasser M.M. Targeting HER3 by interfering with its Sec61-mediated cotranslational insertion into the endoplasmic reticulum. Oncogene. 2015;34:5288–5294. doi: 10.1038/onc.2014.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Medina R.A., Goeger D.E., Hills P., Mooberry S.L., Huang N., Romero L.I., Ortega-Barría E., Gerwick W.H., McPhail K.L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008;130:6324–6325. doi: 10.1021/ja801383f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yao G., Pan Z., Wu C., Wang W., Fang L., Su W. Efficient synthesis and stereochemical revision of coibamide A. J. Am. Chem. Soc. 2015;137:13488–13491. doi: 10.1021/jacs.5b09286. [DOI] [PubMed] [Google Scholar]
- 136.Nabika R., Suyama T.L., Hau A.M., Misu R., Ohno H., Ishmael J.E., McPhail K.L., Oishi S., Fujii N. Synthesis and biological evaluation of the [d-MeAla(11)]-epimer of coibamide A. Bioorg. Med. Chem. Lett. 2015;25:302–306. doi: 10.1016/j.bmcl.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yao G., Wang W., Ao L., Cheng Z., Wu C., Pan Z., Liu K., Li H., Su W., Fang L. Improved total synthesis and biological evaluation of coibamide A analogues. J. Med. Chem. 2018;61:8908–8916. doi: 10.1021/acs.jmedchem.8b01141. [DOI] [PubMed] [Google Scholar]
- 138.Hau A.M., Greenwood J.A., Löhr C.V., Serrill J.D., Proteau P.J., Ganley I.G., McPhail K.L., Ishmael J.E. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS ONE. 2013;8:e65250. doi: 10.1371/journal.pone.0065250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Serrill J.D., Wan X., Hau A.M., Jang H.S., Coleman D.J., Indra A.K., Alani A.W.G., McPhail K.L., Ishmael J.E. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Invest. New Drugs. 2016;34:24–40. doi: 10.1007/s10637-015-0303-x. [DOI] [PubMed] [Google Scholar]
- 140.Wan X., Serrill J.D., Humphreys I.R., Tan M., McPhail K.L., Ganley I.G., Ishmael J.E. ATG5 promotes death signaling in response to the cyclic depsipeptides coibamide A and apratoxin A. Mar. Drugs. 2018;16:77. doi: 10.3390/md16030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tranter D., Paatero A.O., Kawaguchi S., Kazemi S., Serrill J.D., Kellosalo J., Vogel W.K., Richter U., Thornburg C.C., Oishi S., et al. Coibamide A targets Sec61 to prevent biogenesis of secretory and membrane proteins. ChemRxiv. 2019 doi: 10.26434/chemrxiv.10092182.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi H., Mascuch S.J., Villa F.A., Byrum T., Teasdale M.E., Smith J.E., Preskitt L.B., Rowley D.C., Gerwick L., Gerwick W.H. Honaucins A–C, potent inhibitors of inflammation and bacterial quorum sensing: synthetic derivatives and structure-activity relationships. Chem. Biol. 2012;19:589–598. doi: 10.1016/j.chembiol.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mascuch S.J., Boudreau P.D., Carland T.M., Pierce N.T., Olson J., Hensler M.E., Choi H., Campanale J., Hamdoun A., Nizet V., et al. Marine natural product honaucin A attenuates inflammation by activating the Nrf2-ARE pathway. J. Nat. Prod. 2018;81:506–514. doi: 10.1021/acs.jnatprod.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sapkota M., Li L., Choi H., Gerwick W.H., Soh Y. Bromo-honaucin A inhibits osteoclastogenic differentiation in RAW 264.7 cells via Akt and ERK signaling pathways. Eur. J. Pharmacol. 2015;769:100–109. doi: 10.1016/j.ejphar.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Teruya T., Sasaki H., Fukazawa H., Suenaga K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: Isolation, stereostructure, and biological activity. Org. Lett. 2009;11:5062–5065. doi: 10.1021/ol9020546. [DOI] [PubMed] [Google Scholar]
- 146.Tripathi A., Puddick J., Prinsep M.R., Rottmann M., Tan L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010;73:1810–1814. doi: 10.1021/np100442x. [DOI] [PubMed] [Google Scholar]
- 147.Sanchez L.M., Lopez D., Vesely B.A., Togna G.D., Gerwick W.H., Kyle D.E., Linington R.G. Almiramides A–C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010;53:4187–4197. doi: 10.1021/jm100265s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ogawa H., Iwasaki A., Sumimoto S., Kanamori Y., Ohno O., Iwatsuki M., Ishiyama A., Hokari R., Otoguro K., Omura S., et al. Janadolide, a cyclic polyketide-peptide hybrid possessing a tert-butyl group from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2016;79:1862–1866. doi: 10.1021/acs.jnatprod.6b00171. [DOI] [PubMed] [Google Scholar]
- 149.Iwasaki A., Tadenuma T., Sumimoto S., Ohshiro T., Ozaki K., Kobayashi K., Teruya T., Tomoda H., Suenaga K. Biseokeaniamides A, B, and C, sterol O-acyltransferase inhibitors from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2017;80:1161–1166. doi: 10.1021/acs.jnatprod.7b00137. [DOI] [PubMed] [Google Scholar]
- 150.Mevers E., Matainaho T., Allara’ M., Di Marzo V., Gerwick W.H. Mooreamide A: A cannabinomimetic lipid from the marine cyanobacterium Moorea bouillonii. Lipids. 2014;49:1127–1132. doi: 10.1007/s11745-014-3949-9. [DOI] [PubMed] [Google Scholar]
- 151.Gutiérrez M., Pereira A.R., Debonsi H.M., Ligresti A., Di Marzo V., Gerwick W.H. Cannabinomimetic lipid from a marine cyanobacterium. J. Nat. Prod. 2011;74:2313–2317. doi: 10.1021/np200610t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Montaser R., Paul V.J., Luesch H. Marine cyanobacterial fatty acid amides acting on cannabinoid receptors. ChemBioChem. 2012;13:2676–2681. doi: 10.1002/cbic.201200502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rague A.L., Parker S.J., Tidgewell K.J. Evaluating marine cyanobacteria as a source for CNS receptor ligands. Molecules. 2018;23:2665. doi: 10.3390/molecules23102665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnston H.J., Boys S.K., Makda A., Carragher N.O., Hulme A.N. Naturally inspired peptide leads: Alanine scanning reveals an actin-targeting thiazole analogue of bisebromoamide. Chembiochem. 2016;17:1621–1627. doi: 10.1002/cbic.201600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang X., Huang W., Li L., Sun X., Song S., Xu Q., Zhang L., Wei B.G., Deng X. Structure determinants of lagunamide A for anticancer activity and its molecular mechanism of mitochondrial apoptosis. Mol. Pharm. 2016;13:3756–3763. doi: 10.1021/acs.molpharmaceut.6b00564. [DOI] [PubMed] [Google Scholar]
- 156.Chung J.H., Tang A.H., Geraghty K., Corcilius L., Kaiser M., Payne R.J. Total synthesis and antitrypanosomal activity of janadolide and simplified analogues. Org. Lett. 2020;22:3089–3093. doi: 10.1021/acs.orglett.0c00840. [DOI] [PubMed] [Google Scholar]
- 157.Sanchez L.M., Knudsen G.M., Helbig C., De Muylder G., Mascuch S.M., Mackey Z.B., Gerwick L., Clayton C., McKerrow J.H., Linington R.G. Examination of the mode of action of the almiramide family of natural products against the kinetoplastid parasite Trypanosoma brucei. J. Nat. Prod. 2013;76:630–641. doi: 10.1021/np300834q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Das D., Khan H.P., Shivahare R., Gupta S., Sarkar J., Siddiqui M.I., Ampapathi R.S., Chakraborty T.K. Synthesis, SAR and biological studies of sugar amino acid-based almiramide analogues: N-methylation leads the way. Org. Biomol. Chem. 2017;15:3337–3352. doi: 10.1039/C6OB02610A. [DOI] [PubMed] [Google Scholar]
- 159.Ohno O., Terasaki T., Sano T., Hitomi Y., Miyamoto J., Matsuno K. Inhibitory effects of biseokeaniamide A against lipopolysaccharide-induced signal transduction. Bioorg. Med. Chem. Lett. 2020;30:127069. doi: 10.1016/j.bmcl.2020.127069. [DOI] [PubMed] [Google Scholar]
- 160.Boudreau P.D., Monroe E.A., Mehrotra S., Desfor S., Korobeynikov A., Sherman D.H., Murray T.F., Gerwick L., Dorrestein P.C., Gerwick W.H. Expanding the described metabolome of the marine cyanobacterium Moorea producens JHB through orthogonal natural products workflows. PLoS ONE. 2015;10:e0133297. doi: 10.1371/journal.pone.0133297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moss N.A., Bertin M.J., Kleigrewe K., Leão T.F., Gerwick L., Gerwick W.H. Integrating mass spectrometry and genomics for cyanobacterial metabolite discovery. J. Ind. Microbiol. Biotechnol. 2016;43:313–324. doi: 10.1007/s10295-015-1705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]