Abstract
Increased activity of IDO, which catalyzes the degradation of Trp into kynurenine (Kyn), is observed during HIV/SIV infection, and it may contribute to the persistence of HIV/SIV by suppressing antiviral T cell responses. We administered the IDO inhibitor 1-methyl-D-tryptophan (D-1mT) for 13 days to SIV-infected rhesus macaques receiving antiretroviral therapy (ART). D-1mT treatment increased the plasma levels of Trp, without reducing the levels of Kyn, suggesting only a partial effect on IDO enzymatic activity. Surprisingly, D-1mT significantly reduced the virus levels in plasma and lymph nodes of ART-treated animals with incomplete responsiveness to ART. In SIV-infected animals that were not receiving ART, D-1mT was ineffective in reducing the plasma viral load and had only a marginal effect on the plasma Kyn/Trp ratio. Increased IDO and TGF-β mRNA expression in lymph nodes of ART-treated macaques after D-1mT treatment suggested that compensatory counterregulatory mechanisms were activated by D-1mT, which may account for the lack of effect on plasma Kyn. Finally, D-1mT did not interfere with the ART-induced T cell dynamics in lymph nodes (increased frequency of total CD4 T cells, increase of CD8 T cells expressing the antiapoptotic molecule Bcl2, and reduction of regulatory T cells). Thus, D-1mT appeared to synergize with ART in inhibiting viral replication and did not interfere with the beneficial immunologic effects of ART. Further studies are required to elucidate the immunologic or virologic mechanism by which D-1mT inhibited SIV replication in vivo.
The human and simian immunodeficiency viruses (HIV/SIV) establish chronic infections in humans and rhesus macaques, respectively (1). Progressive loss of immune function and depletion of CD4 T cells are hallmarks of HIV/SIV disease (2, 3). Despite the advances made in the management of HIV/SIV infection by antiretroviral therapy (ART),6 complete clearance of the virus from the host is not yet achievable, and incomplete responsiveness to ART is often observed (4). T cell responses are important in controlling acute infection and maintaining the viral set point during the chronic phase (5–8). In particular, the reduction in viremia during acute infection is temporally associated with the appearance of HIV-specific CD8 T cells (5, 6), and vigorous CD8 T cell responses are observed in subjects with long-term nonprogressive infection (8). However, in most HIV/SIV-infected individuals, the immune system fails to control viral replication (9). Dysregulated activation of immune regulatory mechanisms, including regulatory T cells (Treg) and IDO, may contribute to cripple efficient antiviral responses in chronically infected hosts (10, 11). Immunotherapeutic approaches are under investigation, with the goal of improving antiviral immune responses, thus allowing partial control of viral replication and preventing disease progression (12).
IDO is the enzyme catalizing the rate-limiting step of degradation of the essential amino acid Trp into the kynurenine (Kyn) pathway, exerting immune down-regulatory function on T cells (13). A tight functional relationship exists between IDO and Treg, in that Treg induce IDO expression in APCs through CTLA-4/B7 interactions (14) and IDO promotes the development of a Treg phenotype in CD4 T cells (15). The rate of IDO-mediated Trp degradation is elevated during HIV infection, which translates into altered plasma levels of Trp and Kyn (11, 16). We reported increased IDO expression in lymphoid tissues from HIV-infected humans and SIV-infected macaques, which directly correlated with phenotypic and functional markers of Treg (11, 17–19). Also, IDO can be induced in macrophages by HIV infection (20, 21) and in plasmacytoid dendritic cells by exposure to infectious or reverse transcription-deficient HIV particles (22). We recently reported that in vitro HIV-induced IDO differentially inhibits CD4 and CD8 T cell responses by arresting CD4 T cell cycle at the G1-S transition and by reducing CD28 expression on CD8 T cells (23).
1-Methyl-D-tryptophan (D-1mT) is experimentally used in vitro and in vivo as a competitive inhibitor of IDO to prevent or reverse its immunologic effect (10, 13, 24). The activity of HIV-specific CTL was increased by D-1mT in a murine model of HIV-induced encephalopathy, resulting in efficient elimination of infected macrophages (25). Furthermore, we reported that the in vitro proliferative response of CD4 T cells from HIV-infected patients is improved by D-1mT (22).
In two separate studies we recently assessed the effect of CTLA-4 blockade on immune function, viral replication, and IDO activity in SIV-infected macaques (26, 27). Single administration of anti-CTLA-4 Ab appeared to reduce IDO mRNA expression and viral RNA levels in lymph nodes of ART-treated animals, but failed to restore normal plasma levels of Trp and Kyn (27). Conversely, repeated administration of the same Ab significantly augmented viral replication during the acute phase and abolished the beneficial effect of therapeutic vaccination during the chronic phase, likely as a consequence of increased immune activation (26). In this second study, IDO activity increased in animals treated with anti-CTLA-4 Ab, probably due to the increased viral replication and direct induction of IDO in plasmacytoid dendritic cells or macrophages (20–22, 26, 28). These observations suggest that CTLA-4 may play only a marginal role in controlling IDO during HIV/SIV infection. Consistent with this hypothesis, we reported that HIV is more potent than CTLA-4 in inducing IDO in vitro (22). Collectively considered, these reports pose the rationale for attempting direct blockade of IDO in SIV-infected macaques by administration of D-1mT, with the goal of improving antiviral responses and limiting SIV replication. Furthermore, two phase I clinical trials are currently recruiting to begin evaluation of D-1mT for the treatment of metastatic or refractory solid tumors (clinical-trials.gov; identifiers NCT00567931 and NCT00617422).
In the present study, we investigated the effect of D-1mT in combination with ART on virologic and immunologic markers in SIV-infected animals. D-1mT transiently increased plasma levels of Trp but did not reduce Kyn. Increased IDO and TGF-β mRNA expression in lymph nodes suggest that D-1mT treatment induced immune compensatory mechanisms, which may account for the limited effect observed on IDO enzymatic activity. Surprisingly, D-1mT treatment reduced SIV RNA levels in both plasma and lymph nodes when used in combination with ART, but not in ART-free animals.
Materials and Methods
Animals and study design
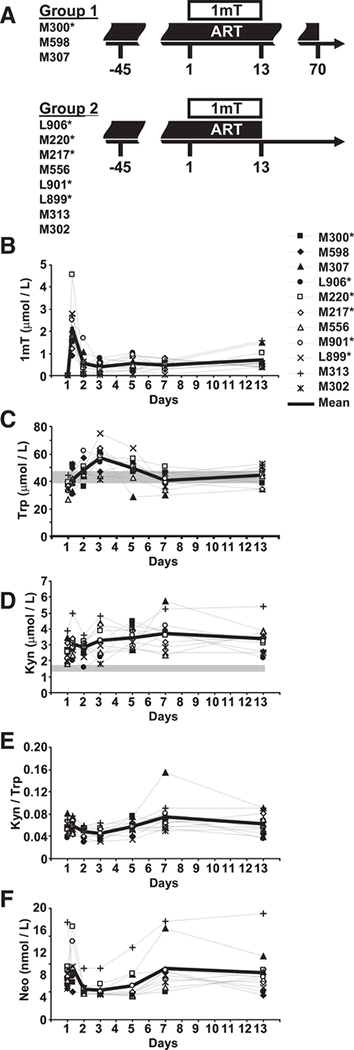
All animals were colony-bred rhesus macaques (Macaca mulatta) obtained from Covance Research Products. Animals were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, and the study was reviewed and approved by the animal care and use committees at Advanced BioScience Laboratories and Bioqual. The care and use of animals were in compliance with institutional (National Institutes of Health) guidelines. At the time of purchase, all animals were in good health, 2–4 years of age, and weighed 4–9 kg. Before study, the animals were seronegative for SIV, simian T cell lymphotropic virus type 1 (STLV-1), and herpesvirus B. A total of 19 macaques were included in the study and were infected by i.v. route, as previously described (26). Eleven SIV-infected macaques were initiated on ART, 4 mo before D-1mT administration. ART treatment consisted of i.v. administration of didanosine (10 mg/kg/day), oral administration of stavudine twice a day (1.2 mg/kg/dose), and s.c. administration of 9-(2-phosphonylmethoxypropyl)adenine (20 mg/kg/day). Eight of the 11 ART-treated animals showed only partial response to treatment at the time of D-1mT administration, in that plasma virus levels were not reduced below the detection limit of 50 copies of viral RNA/ml. D-1mT (Sigma-Aldrich), was administered daily, orally, at a dose of 45 mg/kg for 13 days (Fig. 1A). The daily dose of D-1mT was determined based on the maximum dose used in the ongoing escalation dose human trial (clinical-trials.gov; identifier NCT00617422). Thus, a maximum daily single dose of 3200 mg is used in the clinical trial, equivalent to ∼45 mg/kg (for individuals weighing 70 kg). Because no information is available on pharmacokinetic and biologic effects of D-1mT in primates, we arbitrarily set the duration of treatment to 13 days. ART-treated animals were divided in two groups (Fig. 1A). Group 1 (n = 3) was maintained on ART after D-1mT treatment until day 70. Group 2 (n = 8) received a single dose ALVAC-SIV vaccine on day 4 and both ART and D-1mT treatment were suspended on day 13 to evaluate the effect of treatment on the rebound of plasma virus levels. No differences were observed between groups 1 and 2 in any of the parameters measured, suggesting that the immunologic and virologic changes observed are not dependent on the single-dose ALVAC-SIV vaccination.
FIGURE 1.
D-1mT administration to ART-treated SIV-infected macaques and effect on plasma Kyn, Trp, and Neo. A, Schematic representation of study design and animal groups; details are given in the Materials and Methods. B–F, Plasma levels of D-1mT (B), Trp (C), Kyn (D), Kyn/Trp ratio (E), and Neo (F) measured on days 1 (before D-1mT administration and 8 h after D-1mT administration), 2, 3, 5, 7, and 13. Each animal is represented by a symbol, according to the legend displayed on the right of B. In all graphs the thick lines represent the mean values among all animals at each time point. The gray shaded bars indicate the 95% confidence interval of values measured in SIV-uninfected macaques according to historical data. Mamu*A-01+ animals are marked by asterisks in A and in the legend displayed on the right of B.
D-1mT, Trp, Kyn, and neopterin (Neo) concentration measurement
Plasma samples were collected from all animals at different times during D-1mT treatment and frozen at −20°C until analysis. Trp and Kyn concentrations were determined by HPLC as previously described (29). D-1mT concentration was determined by the same method but extending the analysis time to 30 min. Neo concentrations were measured by ELISA (BRAHMS Diagnostica).
SIV plasma virus levels measurement
SIVmac251 RNA in plasma was quantified by nucleic acid sequence-based amplification (NASBA), which has a detection limit of 50 RNA copies/ml (30).
Cell preparation
Mononuclear cells were isolated from lymph nodes by density-gradient centrifugation and resuspended in RPMI 1640 medium (Invitrogen) containing 10% FCS.
Flow cytometry
For FACS analysis, cells were washed with FACS buffer and stained with appropriately fluorochrome-labeled anti-CD3, anti-CD8, anti-CD25, and anti CD69 (all from BD Pharmingen). Cells were then fixed and permeabilized according to eBioscience’s instructions, incubated with normal rat serum for 15 min, and stained with appropriately fluorochrome-labeled anti-FoxP3 and anti-Bcl2 (both from eBioscience) for 30 min. The appropriate isotype-matched control Abs were used to define positivity. Marker expression was analyzed with a FACSCanto flow cytometer using FACSDiva software (BD Biosciences). FACS analysis was preformed using FlowJo software (Tree Star). A minimum of 10,000 cells per tube was analyzed.
RNA extraction and real-time PCR
Total RNA was extracted from isolated cells using the guanidium thiocyanate-phenol-chloroform method, modified for TRIzol (Invitrogen). RNA (1 μg) was reverse transcribed into first-strand cDNA using random hexanucleotide primers, oligo(dT), and Moloney murine leukemia virus reverse transcriptase (Promega). cDNA quantification was performed by real-time PCR, conducted with the ABI Prism 7900HT (Applied Biosystems). All reactions were performed using a SYBR Green PCR mix (Qiagen), according to the following thermal profile: denaturation at 95°C for 15 s, annealing at 60°C for 15 s, extension at 72°C for 15 s (data collection was performed during the extension step). Primer sequences for macaque mRNA were designed using the Primer3 software and are presented in Supplemental Table I.7 Primers for SIVgag have previously been described (31).
Statistical analysis
Statistical analysis was performed using the SPSS 13.0 software. Differences between groups 1 and 2 were assessed using a nonparametric Mann-Whitney U test. Differences before and after treatment within the same group were assessed using the Wilcoxon test. All p values shown in the text and figures are two-tailed. Values of p < 0.05 were considered statistically significant. All figures report p values before correction for multiple tests made by the Benjamini-Hochberg method; details on the results of the corrections are given in the text.
Results
Lack of effect of single-dose ALVAC-SIV
A single-dose ALVAC-SIV vaccine was administered to animals in group 2 during D-1mT treatment (day 4) to verify whether the immunostimulatory properties of D-1mT may affect antiviral responses elicited by the vaccine. Leukocytes were harvested from lymph nodes excised on day 13 (9 days after immunization for group 2). Antiviral responses were quantified as frequency of T cells producing cytokine (IFN-γ, TNF-α, or IL-2, measured using flow cytometry by intracellular staining) after in vitro stimulation with different SIV peptide pools (gag or pol). We did not observe any difference in antiviral immune response, as measured by cytokine-producing leukocytes, between group 1 and group 2 (supplemental Fig. 1). Additionally, no significant differences were observed in the frequency of CM9-tetramer+ CD8 T cells in Mamu*A-01+ animals (M300, L906, M220, M217, M901, L899) between the two groups (data not shown), although this analysis was limited by the fact that group 1 included only one Mamu*A-01+ animal (M300).
Thus, single-dose ALVAC-SIV did not induce detectable immune responses, suggesting that, even in the presence of D-1mT, such protocol delivers suboptimal immunization compared with previously described repeated administration (32). Additionally, none of the immunologic and virologic parameters measured during D-1mT treatment differed between group 1 and group 2.
Plasma levels of D-1mT
D-1mT was administered every morning before animals were fed. Plasma concentration of D-1mT was quantified by HPLC 8 h after the first administration and in the morning before administration on days 2, 3, 5, 7, and 13 (Fig. 1B). Eight hours after the first administration D-1mT plasma levels ranged between 0.91 and 4.58 μmol/L (median, 1.79 μmol/L). On day 2 (24 h after first administration) plasma levels of D-1mT decreased to 0.05–1.70 μmol/L (median, 0.40 μmol/L; p = 0.003 compared with 8 h) and reached 0.38–1.56 μmol/L (median, 0.54 μmol/L) after 13 days. Thus, D-1mT reached the highest levels in the plasma within the first few hours after administration and was rapidly cleared from the system within 24 h. This information may be vital to identify the adequate dose and time of administration required to reach biological effective levels for future studies and clinical trials.
Effect of D-1mT on IDO activity
D-1mT inhibits the enzymatic conversion of Trp into Kyn catalyzed by IDO (13). Therefore, we measured plasma levels of Kyn and Trp by HPLC in D-1mT-treated macaques throughout the 13 days of treatment (Fig. 1, C–E). Plasma levels of Trp increased within the first 3 days of D-1mT administration (median, 36.3, 46.8, and 58.7 μmol/L on days 1, 2, and 3 respectively; p = 0.004 and p = 0.003 for day 1 vs days 2 and 3, respectively) (Fig. 1C). On days 5, 7, and 13 Trp plasma levels decreased compared with the peak reached on day 3 (p = 0.050, 0.004, and 0.016 for day 3 vs days 5, 7, and 13, respectively) but remained higher than those measured before D-1mT-treatment was started (p = 0.008 for day 1 vs day 13) (Fig. 1C).
Surprisingly, plasma levels of Kyn were not significantly reduced at any time during treatment but reached levels higher than those measured before D-1mT treatment was started (median, 2.48 and 3.26 μmol/L on days 1 and 13, respectively; p = 0.003) (Fig. 1D). Consequently, the plasma Kyn/Trp ratio was reduced only on days 2 and 3 (p = 0.010 and 0.004, respectively) and then rebounded to levels comparable to those measured before D-1mTtreatment was started (68.21 and 69.53 mol/mmol on days 1 and 13, respectively) (Fig. 1E).
Production of the pteridine derivative Neo is induced in human/primate monocytes/macrophages by IFN-γ and correlates with immune activation-induced IDO enzymatic activity (16). We found that plasma Neo levels paralleled those of Kyn/Trp during the course of D-1mT treatment (median, 7.80, 4.72, and 7.65 nmol/L on days 1, 3, and 13, respectively) (Fig. 1F). These data collectively suggest that D-1mT had a partial, transient effect on Trp metabolism, decreasing its consumption within the first 3 days and throughout the entire treatment with D-1mT, without affecting Kyn production.
Effect of D-1mT administration on plasma virus levels in ART-treated animals
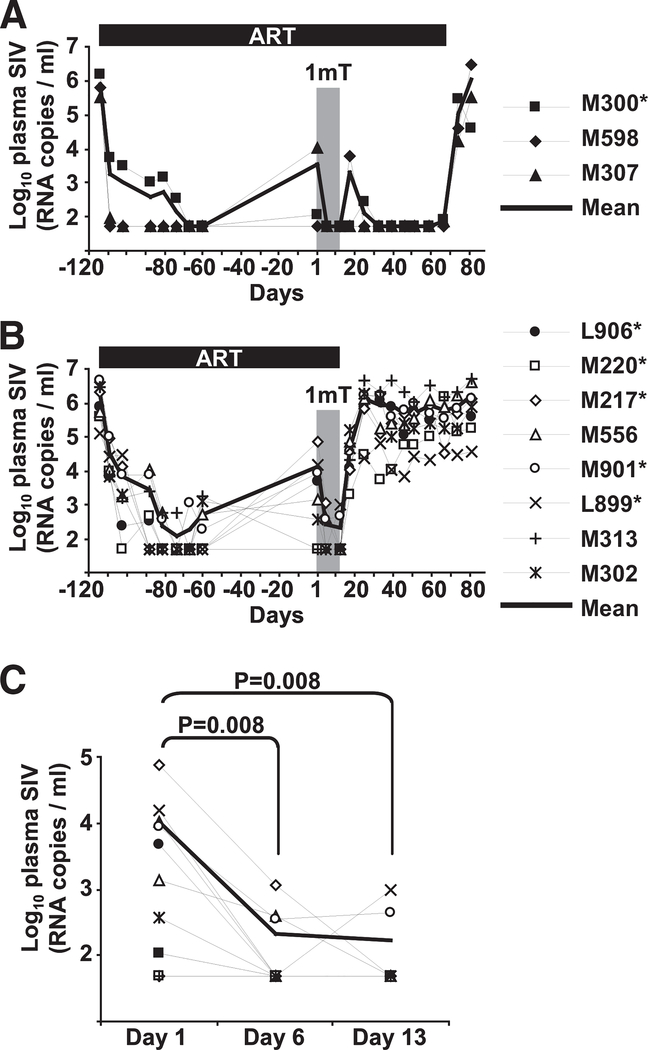
Plasma virus levels measured before ART initiation until after ART interruption are shown for groups 1 and 2 in Fig. 2, A and B, respectively. At the time of D-1mT administration, 8 of 11 (73%) animals showed incomplete response to therapy, in that plasma virus levels were not reduced below the detection threshold (50 copies/ml; Fig. 2C). Interestingly, plasma virus levels decreased significantly after D-1mT treatment and remained detectable in only 3 of 11 (27%) animals on day 6 and 2 of 11 (18%) animals on day 13 (Fig. 2C). These differences remained significant after correction for multiple tests (p < 0.05). Upon ART interruption (group 1 on day 70, group 2 on day 13) plasma virus levels rebounded in all animals to levels comparable to those measured before ART was initiated (Fig. 2, A and B).
FIGURE 2.
Plasma virus levels in ART-treated SIV-infected macaques receiving D-1mT. A and B, Plasma virus levels recorded from before initiation of ART until after ART interruption are shown for all animals in group 1 (A) and group 2 (B). Each animal is represented by a symbol according to the legends; the thick solid lines represent the arithmetic means. Bars on top of graphs indicate duration of ART treatment. Gray shaded boxes indicate the period of D-1mT administration. C, Detail of plasma virus levels changes in each animal during the period of D-1mT administration. Each animal is represented by a symbol according to the legends on the right of A and B; the thick line represents the mean values among all animals at each time point. Mamu*A-01+ animals are marked by asterisks in the legends.
These data suggest that not only D-1mT did not interfere with ART, but that it may also have improved the responsiveness to therapy in animals that showed only limited inhibition of viral replication.
Lack of effect of D-1mT administration on plasma virus levels in ART-free animals
We tested whether the observed beneficial effect of D-1mT on plasma virus level in ART-treated animals could be reproduced in animals that were not receiving ART. Eight SIV-infected ART-free macaques received D-1mT for 13 days in the same dosage, administration route, and conditions used for groups 1 and 2. No significant alteration of plasma virus levels was observed during or after D-1mT administration (Fig. 3A).
FIGURE 3.
Effect of D-1mT administration in ART-free SIV-infected macaques. A, Plasma virus levels measured before, during, and after D-1mT treatment in all ART-free SIV-infected animals. Each animal is represented by a symbol, according to the legend; the thick line represents the mean values among all animals at each time point. The gray shaded box indicates the period of D-1mT administration. B–E, Plasma levels of Trp (B), Kyn (C), Kyn/Trp ratio (D), and Neo (E) measured on days 1, 6, 13, and 32. Each animal is represented by a symbol, according to the legend; the thick lines represent the means among all animals at each time point. The gray shaded boxes indicate the period of D-1mT administration. Mamu*A-01+ animals are marked by asterisks in the legends.
We then tested whether the lack of effect on plasma virus levels in these animals was associated with significant differences in the pharmacokinetic or biologic effect of D-1mT. Plasma levels of D-1mT, Trp, Kyn, and Neo were measured on day 1 (before D-1mT administration was initiated), on day 6 and day 13 (during D-1mT administration; day 13 was the last day of treatment), and on day 32 (19 days after D-1mT administration was stopped). D-1mT could not be detected in plasma samples from any of the eight ART-free macaques (data not shown). This pattern was different from that observed in ART-treated animals in which plasma D-1mT levels were detectable, albeit extremely low, throughout the period of administration. No significant effects were observed on Trp (Fig. 3B) during D-1mT treatment in ART-free animals, whereas a trend to decreased Kyn was observed on day 6 and day 13 compared with day 1 (Fig. 3C), which approached statistical significance ( p = 0.070). No statistically significant change in the Kyn/Trp ratio was recorded (Fig. 3D). An increase of Kyn/Trp appeared to occur between day 13 and day 32 (Fig. 3D), but the correction for multiple tests revealed that the observed difference was not statistically significant. Surprisingly, on both day 6 and day 13, D-1mT treatment resulted in a statistically significant reduction of Neo, which returned to pretreatment levels after D-1mT administration ceased (Fig. 3E). These differences remained significant ( p < 0.05) after correction for multiple tests.
Thus, D-1mT appeared to have only marginal consequences, if any, on Trp catabolism in ART-free animals, which may explain the complete lack of effect on viral load in these animals. However, the alterations of Neo levels caused by D-1mT suggest that the compound exerted a biologic effect even in animals not receiving ART.
Effect of D-1mT administration on SIV RNA expression in lymph nodes in ART-treated animals
We quantified the expression of RNA for the SIV protein gag (SIVgag) in lymph node leukocytes collected from ART-treated macaques before (day −45, in both groups 1 and 2), at the end (day 13, in both groups 1 and 2), and after (day 70, only in group 1) D-1mT treatment. SIVgag RNA decreased significantly with D-1mT administration (day 13 compared with day −45) and rebounded after D-1mT treatment cessation (group 1, day 70) to levels comparable to those measured before D-1mT was administered (Fig. 4, A and B). Similar results were observed when SIVgag was normalized on GAPDH or CD4 RNA (compare Fig. 4, A and B). The differences in SIVgag between day −45 and day 13 remained significant after correction for multiple tests (corrected p values were 0.024 and 0.020 for normalization on GAPDH and CD4, respectively). Because only three animals (group 1) were still on ART at day 70, results for this time point are presented but they are not statistically analyzed. We found a significant increase in CD4 mRNA after D-1mT treatment (maintained after correction for multiple tests; corrected p = 0.024), which persisted for at least 2 mo in ART-treated animals (Fig. 4C), consistent with an ART-induced partial reconstitution of the CD4 T cell population.
FIGURE 4.
SIVgag, CD4, IDO, IFN-γ, and TGF-β RNA expression in lymph nodes of ART-treated SIV-infected macaques receiving D-1mT. A and B, SIVgag RNA levels, normalized on GAPDH (A) and CD4 (B) mRNA, in lymph node leukocytes of ART-treated SIV-infected macaques. C–F, mRNA expression for CD4 (C), IDO (D), IFN-γ (E), and TGF-β (F) normalized on GAPDH in lymph node leukocytes of ART-treated SIV-infected macaques. Mean values ± SD are shown in all bar graphs. Lymph nodes were excised 45 days before and at the end of D-1mT administration (day 13) from animals in both group 1 and 2, as well as before ART interruption (day 70) from animals in group 1.
Effect of D-1mT administration on IDO, IFN-γ, and TGF-β mRNA expression in lymph nodes in ART-treated animals
Because plasma levels of Kyn did not significantly decrease in ART-treated macaques during D-1mT treatment, we analyzed IDO expression in lymph node leukocytes. IDO mRNA expression was increased at day 13, compared with both day −45 and day 70, although these differences were not statistically significant (Fig. 4D). Similar changes were observed in the expression of the IDO-inducing cytokine IFN-γ (Fig. 4E), supporting the hypothesis that increased T cell activation following D-1mT treatment may have induced IDO expression and activity through IFN-γ, thus resulting in the observed rebound of plasma Kyn/Trp. Consistent with this hypothesis are the dynamics of plasma Neo (Fig. 1F), which is a well-accepted marker for type 1 T cell responses associated with IFN-γ production. Furthermore, mRNA expression for the immunosuppressive cytokine TGF-β increased with D-1mT treatment (Fig. 4F), suggesting that multiple immune regulatory mechanisms may have been affected by D-1mT (p value corrected for multiple tests was 0.020). No changes were observed in CTLA-4 mRNA expression (data not shown).
Effect of D-1mT plus ART on lymph node T cells in ART-treated animals
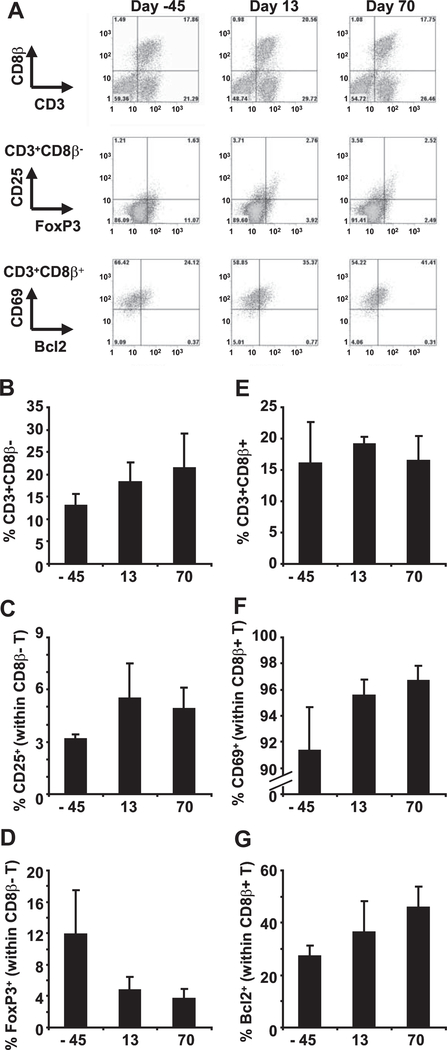
We analyzed functional and phenotypic markers of lymph node lymphocytes from a subset of ART-treated macaques before (M224, M300, M313; day −45), at the end (M300, M313, M307; day 13), and after (M300, M598, M307; day 70) D-1mT treatment. Because of the limited number of samples tested, these data were not statistically analyzed. Total frequency of CD4 T cells (CD3+CD8β−), but not CD8 T cells, was increased in lymph nodes after D-1mT treatment (day 13) and was maintained at increased levels during ART continuation (day 70, group 1) (Fig. 5, A, B, and E), consistent with an ART-induced reconstitution of the CD4 T cell population. We tested the expression of CD25, the high-affinity receptor for the mitogenic cytokine IL-2, and of the transcription factor FoxP3, a marker for Treg (33), in the CD4 T cell population. The frequency of CD25 cells within the CD4 T cells population was increased after D-1mT treatment (day 13) and was maintained at increased levels during ART continuation (day 70, group 1) (Fig. 5, A and C). Conversely, a decreased frequency of CD4 T cells expressing the Treg marker FoxP3 was observed after D-1mT treatment (Fig. 5, A and D). No changes were observed in the frequency of CD25+ and FoxP3+ CD8 T cells (data not shown).
FIGURE 5.
Analysis of lymph node CD4 and CD8 T lymphocytes from ART-treated SIV-infected macaques receiving D-1mT. A, Flow cytometry dot plots showing staining for CD3/CD8β (upper panels), FoxP3/CD25 on gated CD4 T cells (middle panels), and Bcl2/CD69 on gated CD8 T cells (lower panels) on lymph node leukocytes isolated 45 days before and at the end of D-1mT administration (day 13), as well as before ART interruption (day 70) from one example animal (M307) from group 1. B–G, Frequency of CD4 T cells (CD3+CD8β−) on total leukocytes (B), CD25+ among CD4 T cells (C), FoxP3+ among CD4 T cells (D), CD8 T cells (CD3+CD8β+) on total leukocytes (E), CD69+ among CD8 T cells (F), and Bcl2+ among CD8 T cells (G) in lymph node leukocytes of ART-treated SIV-infected macaques. Mean values ± SD are shown in B–G. Lymph nodes were excised 45 days before and at the end of D-1mT administration (day 13) from animals in both groups 1 and 2, as well as before ART interruption (day 70) from animals in group 1.
We tested whether D-1mT treatment would affect expression of the activation marker CD69 and of the antiapoptotic protein Bcl2 in T cells. No changes in the frequency of Bcl2+ and CD69+ cells were observed among the CD4 T cell population (data not shown). The vast majority of CD8 T cells expressed CD69, and the frequency of this subpopulation was only marginally affected by D-1mT treatment (Fig. 5, A and F). Additionally, the percentage of Bcl2+ CD8 T cells was increased after D-1mT treatment (day 13) and was maintained at increased levels during ART continuation (day 70, group 1) (Fig. 5, A and G).
These data suggest that D-1mT did not interfere with the beneficial effects of ART, allowing the expansion of CD4, but not CD8, T cells, the reduction of Treg, and the increase of CD8 T cells expressing the antiapoptotic molecule bcl2 in lymphoid tissues. However, because all the animals included in the study had already been receiving ART for 4 mo before D-1mT administration, we cannot exclude that at least part of the changes observed in T cell dynamics may have been driven by D-1mT and subsequently maintained in animals that were kept under ART regimen, even after D-1mT treatment ceased.
Discussion
The rate of HIV/SIV disease progression is directly dependent on viral replication, and plasma virus level is the best indicator of viral activity. ART limits viral replication by directly inhibiting crucial steps of the virus life cycle, but it fails to eliminate productively infected cells and eradicate the infection (34). In most cases, T cell responses are crippled and fail to control viral replication, possibly due to virus-mediated hyperactivation and induction of immune down-regulatory mechanisms (2, 3, 10, 35). Immune-based therapeutic interventions are undermined by the risk of enhancing chronic immune activation and favoring viral replication by boosting the proliferation of infected CD4 T cells (26). In the present study we attempted for the first time to directly inhibit the immunosuppressive enzyme IDO in SIV-infected macaques that showed incomplete responsiveness to ART. We found that not only the IDO inhibitor D-1mT did not interfere with the expected virologic and immunologic outcomes of ART, but that it may also synergize with ART in reducing viral replication.
The effect of D-1mT on Trp metabolism appeared to be only transient in ART-treated macaques, in that plasma Trp levels rapidly increased upon D-1mT administration, but decreased again within 7 days of treatment. The surprising lack of decrease in plasma Kyn, at any time during treatment, poses some doubts on the efficacy of D-1mT in inhibiting IDO activity at the dose and administration regimen used in this study. The discrepancy between the effect of D-1mT on Trp and Kyn levels may be a consequence of: 1) inefficient blocking of IDO activity, affecting Trp consumption, but only marginally interfering with Kyn production; 2) interference with cellular systems of Trp transport, as reported for placental transporters (36); 3) maintenance of constant Kyn levels by liver metabolic enzymes; and 4) induction of a compensatory increase in IDO expression and activity. Supporting the latter hypothesis is the trend to increased IDO and IFN-γ mRNA. The variations of plasma Neo levels also support a rebound in type 1 Th cell responses associated with IFN-γ production (16). It is well described that Neo production in humans correlates with T cell activation (37, 38), and the observed changes in Neo and Kyn/Trp may both reflect swings in immune activation. Thus, although we cannot exclude that D-1mT administration affected Trp plasma levels by mechanisms not directly related to IDO inhibition, the changes in Neo, IDO, and IFN-γ, as well as the observed increase in plasma Kyn, suggest that D-1mT may have favored the activation of compensating immunosuppressive mechanisms, probably triggered by T cell-derived cytokines. The observed increase in TGF-β mRNA expression also suggests a more general effect of D-1mT on the immune balance. It should also be considered that Kyn is only an intermediate metabolite of Trp degradation and may not represent an ideal marker for the biologic effects of D-1mT, since the final catabolites of the enzymatic pathway initiated by IDO may also be secreted. Additionally, plasma Kyn may not reflect the level of IDO activity and Kyn in specific tissues or in specific cell types (39, 40), which may respond differently to D-1mT. Inhibition of IDO in a small specific subset of cells may be sufficient to affect immunologic and virologic parameters without affecting the systemic levels of Kyn. An alternative, but not exclusive, possibility is that D-1mT may preferentially interfere with a recently identified isoform of IDO (IDO2), which is particularly sensitive to the D-isomer of 1mT used in this study (41). IDO2 is enzymatically less active than IDO, but it exerts similar immunosuppressive activity (41). It is possible that in our study D-1mT acted on IDO2, inhibiting its immunosuppressive function. A subsequent increase in T cell activation and IFN-γ production may have triggered IDO expression and caused the rebound of Kyn/Trp.
The main objective of this study was to test whether D-1mT may be considered as a potential immunotherapeutic agent to be used in combination with ART. We obtained encouraging results in ART-treated macaques, in that D-1mT reduced plasma and lymph node virus to undetectable levels in animals that showed incomplete suppression of viral replication. However, D-1mT treatment did not affect the rebound in viral replication once ART had been interrupted. We did not find any evidence of enhanced SIV-specific CD4 or CD8 T cell responses, rendering it arduous to interpret the observed virologic effect in light of the immunostimulatory effect of D-1mT. Note that anti-SIV T cell responses were tested on leukocytes from lymph nodes excised on day 13, at which point plasma Trp, Kyn, and Neo had returned to levels comparable to those measure before D-1mT treatment, suggesting that the transient effect of D-1mT may have already disappeared. Of interest, we observed some positive outcomes on the dynamics of T cells in lymph nodes during and after D-1mT administration to ART-treated macaques. Thus, ART is known to positively affect the recovery of CD4 Th cells (42) and to reduce the accumulation of Treg in lymphoid tissues (18), as well as to affect apoptotic pathways in CD8 T cells (43). These effects were observed in ART-treated animals that were administered D-1mT. Although we cannot exclude that D-1mT affected CD4 Th and Treg dynamics, as well as CD8 T cell survival, similar to that described in other studies (15, 22, 23), it is likely that the changes reported herein between day −45 and day 13 are the direct consequence of continued ART therapy rather than of short-term D-1mT treatment.
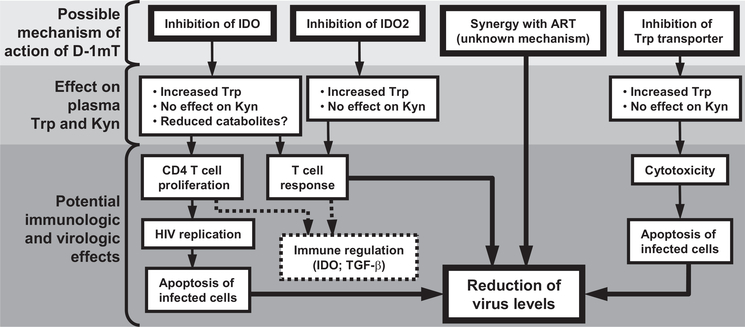
Different explanations for the beneficial virologic effect of D-1mT in the absence of detectable immunologic benefit must be considered. Although HIV-induced IDO inhibits both CD4 and CD8 T cell function (23), we reported that in vitro inhibition of IDO in peripheral leukocytes from HIV-infected patients improved the proliferative responses of CD4 T cells but did not exert significant effects on CD8 T cells (22). Thus, it is possible that D-1mT preferentially increased the proliferation of CD4 T cells, including those infected by SIV, potentially enhancing the cytopathic effect of SIV replication, resulting in the clearance of infected CD4 T cells. An alternative, nonexclusive possibility is that D-1mT synergized with ART at a cellular or molecular level by directly affecting the virus life cycle or the efficacy of the antiretroviral drugs, independent of its immunologic effect. Such possibility is supported by the observation that D-1mT had no effect on plasma virus levels in ART-free animals. This lack of effect was not limited to animals with elevated viral loads, but was evident also in ART-free animals that had viral loads <105 copies/ml at the time D-1mT was started (M642, M608, and M649), comparable to those measured in ART-treated animals. Furthermore, we were unable to measure detectable levels of D-1mT in ART-free animals, which may partially explain the lack of effect on viremia. The reasons why D-1mT was not detectable in ART-free animals are obscure. It is possible that uncontrolled viral replication affects the absorption, transport, or metabolism of D-1mT, thus altering its pharmacokinetics. Nevertheless, D-1mT exerted a beneficial effect on the virologic markers of disease progression only when used in combination with ART, but not as a single-drug regimen. Fig. 6 is a summary diagram of the potential effects of D-1mT administration.
FIGURE 6.
Possible effects of D-1mT administration. The potential biologic effects of D-1mT that may result in inhibition of SIV replication and alteration of plasma levels of Kyn and Trp are schematically represented.
Further investigation will be required to elucidate the outcome of in vivo D-1mT administration, to test its applicability for extended periods of time, and to verify whether such an approach may be beneficial in the treatment of HIV/SIV infection. Nevertheless, the data presented herein provide encouraging evidence on the potential application of D-1mT to HIV/SIV infection, particularly when this prospective drug is administered in combination with ART.
Supplementary Material
Acknowledgments
We thank Dr. David Venzon (Biostatistics and Data Management Section, National Cancer Institute, Bethesda, MD) for his assistance with the statistical analyses.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (to A.B., M.V., A.W.D., W.P.T., E.T., G.M.S., and G.F.), and by the government of the State of the Austrian Tyrol (to D.F.).
Abbreviations used in this paper
- ART
antiretroviral therapy
- D-1mT
1-methyl-D-tryptophan
- Kyn
kynurenine
- Neo
neopterin
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Desrosiers RC 1990. The simian immunodeficiency viruses. Annu. Rev. Immunol. 8: 557–578. [DOI] [PubMed] [Google Scholar]
- 2.Dickmeiss E 1990. Immunology of the human immunodeficiency virus infection. Tokai J. Exp. Clin. Med. 15: 263–267. [PubMed] [Google Scholar]
- 3.Douek DC, Picker LJ, and Koup RA 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21: 265–304. [DOI] [PubMed] [Google Scholar]
- 4.Volberding PA, and Deeks SG 1998. Antiretroviral therapy for HIV infection: promises and problems. J. Am. Med. Assoc. 279: 1343–1344. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Hahn BH, Shaw GM, and Oldstone MB 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68: 6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, and Ho DD 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68: 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Ho DD, Todd J, Kokka R, Urdea M, Lifson JD, Piatak M Jr., Chen S, Hahn BH, Saag MS, et al. 1995. Clinical evaluation of branched DNA signal amplification for quantifying HIV type 1 in human plasma. AIDS Res. Hum. Retroviruses 11: 353–361. [DOI] [PubMed] [Google Scholar]
- 8.Harrer T, Harrer E, Kalams SA, Elbeik T, Staprans SI, Feinberg MB, Cao Y, Ho DD, Yilma T, Caliendo AM, et al. 1996. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retroviruses 12: 585–592. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman J, Shankar P, Manjunath N, and Andersson J 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98: 1667–1677. [DOI] [PubMed] [Google Scholar]
- 10.Boasso A, and Shearer GM 2007. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Curr. Drug Metab. 8: 217–223. [DOI] [PubMed] [Google Scholar]
- 11.Boasso A, Vaccari M, Nilsson J, Shearer GM, Andersson J, Cecchinato V, Chougnet C, and Franchini G 2006. Do regulatory T-cells play a role in AIDS pathogenesis? AIDS Rev 8: 141–147. [PubMed] [Google Scholar]
- 12.Franchini G, Nacsa J, Hel Z, and Tryniszewska E 2002. Immune intervention strategies for HIV-1 infection of humans in the SIV macaque model. Vaccine 20(Suppl. 4): A52–A60. [DOI] [PubMed] [Google Scholar]
- 13.Mellor AL, and Munn DH 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4: 762–774. [DOI] [PubMed] [Google Scholar]
- 14.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, and Puccetti P 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4: 1206–1212. [DOI] [PubMed] [Google Scholar]
- 15.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. 2006. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 176: 6752–6761. [DOI] [PubMed] [Google Scholar]
- 16.Schroecksnadel K, Zangerle R, Bellmann-Weiler R, Garimorth K, Weiss G, and Fuchs D 2007. Indoleamine-2,3-dioxygenase and other interferon-γ-mediated pathways in patients with human immunodeficiency virus infection. Curr. Drug Metab. 8: 225–236. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, and Chougnet C 2006. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108: 3808–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, Shearer GM, and Chougnet CA 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174: 3143–3147. [DOI] [PubMed] [Google Scholar]
- 19.Boasso A, Vaccari M, Hryniewicz A, Fuchs D, Nacsa J, Cecchinato V, Andersson J, Franchini G, Shearer GM, and Chougnet C 2007. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J. Virol. 81: 11593–11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant RS, Naif H, Thuruthyil SJ, Nasr N, Littlejohn T, Takikawa O, and Kapoor V 2000. Induction of indolamine 2,3-dioxygenase in primary human macrophages by human immunodeficiency virus type 1 is strain dependent. J. Virol. 74: 4110–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant RS, Naif H, Thuruthyil SJ, Nasr N, Littlejohn T, Takikawa O, and Kapoor V 2000. Induction of indoleamine 2,3-dioxygenase in primary human macrophages by HIV-1. Redox Rep. 5: 105–107. [DOI] [PubMed] [Google Scholar]
- 22.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, and Shearer GM 2007. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 109: 3351–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boasso A, Hardy AW, Anderson SA, Dolan MJ, and Shearer GM 2008. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS ONE 3: e2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munn DH, and Mellor AL 2007. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117: 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, and Persidsky Y 2005. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood 106: 2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, et al. 2008. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J. Immunol. 180: 5439–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, et al. 2006. CTLA-4 blockade decreases TGF-, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 108: 3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, and Brew BJ 2001. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J. Neurovirol. 7: 56–60. [DOI] [PubMed] [Google Scholar]
- 29.Widner B, Werner ER, Schennach H, Wachter H, and Fuchs D 1997. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 43: 2424–2426. [PubMed] [Google Scholar]
- 30.Romano JW, Williams KG, Shurtliff RN, Ginocchio C, and Kaplan M 1997. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol. Invest. 26: 15–28. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, and Ruprecht RM 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retroviruses 16: 1247–1257. [DOI] [PubMed] [Google Scholar]
- 32.Clerici M, Barassi C, Devito C, Pastori C, Piconi S, Trabattoni D, Longhi R, Hinkula J, Broliden K, and Lopalco L 2002. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the α-helix of gp41. AIDS 16: 1731–1741. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Gavin MA, and Rudensky AY 2003. Foxp3 programs the development and function of CD4CD25 regulatory T cells. Nat. Immunol. 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 34.Geeraert L, Kraus G, and Pomerantz RJ 2008. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu. Rev. Med. 59: 487–501. [DOI] [PubMed] [Google Scholar]
- 35.Boasso A, and Shearer GM 2008. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin. Immunol. 126: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo Y, and Boyd CA 2001. Characterisation of L -tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J. Physiol. 531: 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Look MP, Altfeld M, Kreuzer KA, Riezler R, Stabler SP, Allen RH, Sauerbruch T, and Rockstroh JK 2000. Parallel decrease in neurotoxin quinolinic acid and soluble tumor necrosis factor receptor p75 in serum during highly active antiretroviral therapy of HIV type 1 disease. AIDS Res. Hum. Retroviruses 16: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 38.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, and Fuchs D 2002. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin. Immunol. 104: 242–247. [DOI] [PubMed] [Google Scholar]
- 39.Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, and Seishima M 2002. L -tryptophan-L -kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect. Immun. 70: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, and Gazzinelli RT 2002. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect. Immun. 70: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, and Prendergast GC 2007. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 67: 7082–7087. [DOI] [PubMed] [Google Scholar]
- 42.Autran B 1999. Effects of antiretroviral therapy on immune reconstitution. Antiviral Ther. 4(Suppl. 3): 3–6. [PubMed] [Google Scholar]
- 43.Miro O, Villarroya J, Garrabou G, Lopez S, Rodriguez de la Concepcion M, Pedrol E, Martinez E, Giralt M, Gatell JM, Cardellach F, et al. 2005. In vivo effects of highly active antiretroviral therapies containing the protease inhibitor nelfinavir on mitochondrially driven apoptosis. Antiviral Ther. 10: 945–951. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.