Abstract
Hypoxia-inducible factor-1 (HIF-1) has been implicated in the pathogenesis of choroidal neovascularization (NV) and is an appealing target because it increases multiple pro-angiogenic proteins and their receptors. Acriflavine (ACF) binds HIF-1α and HIF-2α preventing binding to HIF-1β and inhibiting transcriptional activity of HIF-1 and HIF-2. Delivery of ACF to the eye by multiple routes strongly, but transiently, suppresses choroidal NV. We overcame design challenges and loaded highly water soluble ACF into poly(lactic-co-glycolic acid) (PLGA) microparticles (PLGA-ACF MPs) that release ACF in vitro for up to 60 days. Intravitreous injection of PLGA-ACF MPs in mice suppressed choroidal NV for at least 9 weeks and suprachoroidal injection of PLGA-ACF in rats suppressed choroidal NV for at least 18 weeks. Intravitreous, but not suprachoroidal injection, of PLGA-ACF MPs containing 38 μg of ACF in rabbits resulted in modest reduction of full-field electroretinogram (ERG) function. Over the span of 28 days after suprachoroidal injection of PLGA-ACF MP, rabbits had normal appearing retinas on fundus photographs, normal electroretinogram scotopic a- and b-wave amplitudes, no increase in intraocular pressure, and normal retinal histology. The active components of ACF, trypaflavine, had steady-state levels in the low nM range in RPE/choroid > retina for at least 16 weeks with a gradient from the side of the eye where the injection was done to the opposite side. These data suggest that suprachoroidal injection of PLGA-ACF MPs has the potential to provide a durable new treatment for retinal and choroidal vascular diseases.
Keywords: Age-related macular degeneration, PLGA, microparticles
Graphical Abstract
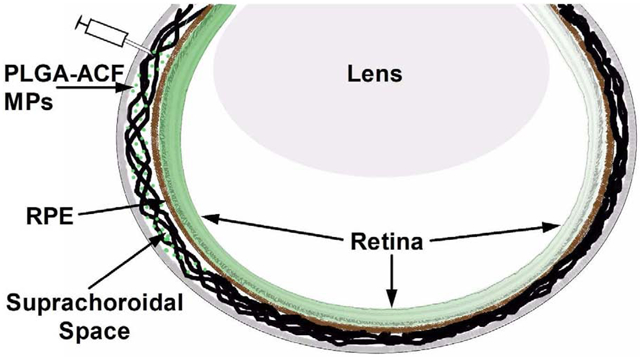
Suprachoriodal delivery of PLGA-ACF microparticles delivers pharmacologically relevant levels of ACF throughout the eye that significantly reduces development of laser-induced choroidal neovascularization in rats. The site of suprachoroidal injections is the same as that for intravitreous injections but the depth of penetration is different.
Introduction
Age-related macular degeneration (AMD) is a prevalent neurodegenerative disease in which deposits called drusen occur under the retinal pigmented epithelium (RPE). In a large percentage of patients, there is gradual death of RPE cells and photoreceptors resulting in patches of atrophy and central blind spots. Atrophic spots gradually increase and coalesce and when the fovea becomes involved, visual acuity declines. The loss of vision is usually slow occurring over many years or decades, but can be complicated at any time in the course by choroidal neovascularization (NV), growth of new vessels from the choroid or deep capillary bed of the retina into the subretinal space. Unlike normal retinal vessels that have tight junctions and barrier characteristics that prevent leakage of fluid into the retina, the new vessels are leaky resulting in collection of fluid under or within the retina causing rapid, initially reversible, loss of vision. If untreated, the new vessels recruit other cells causing scarring, photoreceptor damage, and permanent loss of central vision.
The molecular pathogenesis of choroidal NV in AMD is complex, but is gradually being unraveled. Progress was much faster elucidating mechanisms involved in retinal NV in ischemic retinopathies such as diabetic retinopathy and retinal vein occlusion. It has long been known that retinal hypoxia plays a central role in retinal NV (1–3) and macular edema (4) in ischemic retinopathies. HIF-1 is a master regulator of hypoxia- induced gene expression (5) that is increased in ischemic retina and upregulates multiple angiogenic proteins and their receptors, including vascular endothelial growth factor-A (VEGF) and its receptors (6, 7). It also appears that retinal hypoxia contributes to progression of background diabetic retinopathy, because wide angle fluorescein angiography shows correlation between progression of nonperfusion and worsening of diabetic retinopathy (8).
The first hint that HIF-1 might also be important in the molecular pathogenesis of choroidal NV came from the observation that mice in which the hypoxia response element of the Vegf promoter was deleted, develop significantly less choroidal NV at Bruch’s membrane rupture sites indicating that HIF-1 is involved in the upregulation of VEGF in this model (9). While it was not clear that tissue hypoxia was present in choroidal NV in patients with AMD as it clearly is in ischemic retinopathies, hypoxia-generated oxidative stress has been implicated in the stabilization of HIF-1 that occurs in hypoxic tissue (10, 11). Thus, oxidative stress, whether or not it is related to hypoxia, can stabilize HIF-1. Oxidative stress is present in AMD, because antioxidant vitamins decreased the progression to advanced stages of AMD including choroidal NV (12); therefore, oxidative stress unrelated to hypoxia was felt to be the mechanism of HIF-1 stabilization in AMD. In addition to stabilizing HIF-1, oxidative stress increases the severity of ocular neovascularization through upregulation of NF-κB-regulated genes that contribute (13, 14). But recent evidence suggests that hypoxia may play a larger role in AMD than previously thought. Histopathologic studies of eyes from patients with AMD have shown reduced vascular density in the choroid in early AMD and greater reduction in advanced AMD with geographic atrophy (15, 16). In addition, optical coherence tomography angiography, which makes it possible to visualize perfused vessels or the absence of perfused vessels within each layer of the retina and in the choroid (17, 18), shows flow voids in the choroid of patients with AMD (19). This indicates that there is reduced perfusion to the RPE and outer retina in AMD which has also been suggested by high resolution ultrasound (20). Thus, hypoxia of the RPE and outer retina may be present in AMD and along with other sources of oxidative stress, may contribute to HIF-1 stabilization and the observed upregulation of VEGF and other angiogenic factors.
The hypothesis that HIF-1 plays an important role in both ischemic retinopathies and AMD is further supported by demonstrations that HIF-1 inhibitors are strong suppressors of both choroidal NV at Bruch’s membrane rupture sites and ischemia-induced retinal NV (21–23). The HIF-1 inhibitors digoxin, doxorubicin, daunorubicin, and acriflavine (ACF) are strong inhibitors of retinal and choroidal NV, but at high doses they reduce full-field electroretinogram (ERG) function (22, 23). While off-target effects may contribute, at least part of this toxicity at high doses may be a class effect of HIF-1 inhibitors, because HIF-1 not only induces angiogenic factors, but also increases expression of glycolytic enzymes which help to compensate for the metabolic demands of hypoxia. This potential problem was largely overcome for doxorubicin by sustained delivery of low levels (22).
HIF-2 is a transcription factor that is highly homologous to HIF-1 and it also contributes to hypoxia-regulated gene expression (24, 25). Since HIF-2 contributes to upregulation of VEGF and other angiogenic/propermeability factors in some other ischemic tissues (26), it may also contribute in the retina. Therefore, treatments that inhibit both HIF-1 and HIF-2 may have greater benefits than those that target only HIF-1. ACF binds to HIF-1α and HIF-2α preventing dimerization to HIF-1β, and thereby inhibits both HIF-1 and HIF-2 (27). We have previously demonstrated that ACF strongly suppresses choroidal and retinal NV, (23) but small molecules are cleared rapidly from the eye so that more frequent administration of ACF would be required compared with the much larger antiVEGF proteins that are currently used in clinical practice. Sustained delivery of ACF is necessary for its utilization as a treatment for retina and choroidal vascular diseases. In this study, we incorporated ACF into poly lactic-co-glycolic acid (PLGA), formulated microparticles (MPs), and evaluated them in several ways. Efficacy was tested for 12 weeks after intravitreous injection in a mouse model choroidal NV from laser-induced rupture of Bruch’s membrane and for 16 weeks after suprachoroidal injection in a similar rat model of choroidal NV. Pharmacokinetics were tested after suprachoroidal injection in rats and safety was tested in rabbits.
Methods
Preparation of PLGA-ACF MPs
PLGA-ACF MPs were prepared using a single emulsion solvent evaporation method. Parameters that were varied include (i) the PLGA polymer end group (carboxylic vs. ester), (ii) polymer molecular weight (PLGA1A, 2A, 7A), (iii) polymer concentration (50–200 mg/ml), and (iv) the pH of the water phase (5.0, 6.8, 7.4, 9.0) (Supplemental Tables 1–4). The final microparticle formulation used in animal studies was formulated by dissolving 200 mg PLGA (2A, 50:50 LA:GA) (Evonik Corporation, Piscataway, NJ) in 2 mL of dichloromethane (DCM, Sigma-Aldrich), and mixing with 40 mg acriflavine hydrochloride (Sigma-Aldrich) dissolved in 0.5 ml dimethyl sulfoxide (DMSO, Sigma-Aldrich). The mixture was homogenized (L4RT, Silverson Machines) at 5000 RPM for 1 minute. The homogenized mixture was then poured into a solution containing 1% polyvinyl alcohol (25 kDa, Polysciences, Warrington, PA) in phosphate buffered saline (PBS, pH 7.4) under continuous stirring. Particles were hardened by allowing solvent to evaporate while stirring at room temperature for 2 h. Particles were collected via centrifugation (International Equipment Co) at 2,000 × g for 15 min, and washed with HyPure cell culture grade water (endotoxin-free, HyClone™, Logan, UT) and re-collected by centrifugation three times. The washed particles were then lyophilized and stored frozen until use. Microparticles were resuspended in a sodium hyaluronate solution (Healon®, Abbott Medical Optics, Santa Ana, CA diluted 5-fold with endotoxin-free water at the desired concentration prior to injection.
Characterization of PLGA-ACF MPs
Particle size distribution was determined using a Coulter Multisizer 4 (Beckman Coulter, Inc., Miami, FL). Particles were resuspended in double distilled water and added dropwise to 100 ml of ISOTON II solution until the coincidence of the particles was between 8% and 10%. At least 100,000 particles were sized for each batch of particles to determine the mean particle size and size distribution. Particle morphology was evaluated by LEO1530/Zeiss Field-emission scanning electron microscopy (SEM). Particles were lyophilized and mounted onto SEM stubs at room temperature before sputter coating with a thin layer of platinum (Denton Vacuum, LLC Technologies). To determine the drug loading, microparticles were dissolved in DMSO and the total drug content was calculated by measuring the UV absorbance at 420 nm in triplicate. Absorbance of blank particles dissolved in DMSO at the same polymer concentration was subtracted to account for polymer interference. The microparticle size was 7.3 ± 1.8 μm and the ACF loading was 6.7% (w/w).
ACF release from PLGA-ACF MPs
Release kinetics were obtained by resuspending microparticles in 1 ml phosphate buffered saline (PBS, pH 7.4) and incubating at 37°C on a platform shaker (140 RPM). Supernatant was collected at predetermined intervals by centrifugation at 2,000 × g for 5 min. Drug-containing supernatant was collected and particles were resuspended in 1 ml of fresh PBS. ACF concentration in the collected supernatant was assayed via absorbance at 420 nm in triplicate for each sample (n=3).
Ethics Statement
All experimental protocols for mice, rats, and rabbits were approved by the Johns Hopkins Animal Care and Use Committee. All rats, mice, and rabbits were cared for and treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) concerning the use of animals in ophthalmic research.
Mouse model of choroidal NV
Female C57BL/6 mice (Charles River Labs, Frederick, MD), 4–6 weeks of age, were sedated with ketamine/xylazine (Henry Schein Animal Health, Dublin, OH) and using pulled glass pipettes and a microinjector (Harvard Apparatus, Holliston, MA) were given a 1 μl intravitreous injection of 29.4 μg PLGA-ACF MPs containing 2 μg of ACF in one eye and 29.4 μg of empty PLGA MPs in the other eye. At 2, 4, 8 or 12 weeks after MP injection, Bruch’s membrane was ruptured at 3 locations in each eye as previously described (28). Briefly, 532 nm diode laser (75 μm spot size, 0.1 sec duration, 150 mW) was delivered to the retina using the slit lamp delivery system of an OcuLight GL Photocoagulator (Iridex, Mountain View, CA) and a hand held cover slide as a contact lens. In other experiments mice received a 1 μl intravitreous injection of 50 ng ACF, 50 ng of proflavine (PRF), or PBS immediately after rupture of Bruch’s membrane. Seven days after rupture of Bruch’s membrane, mice were euthanized, eyecups were fixed and stained with FITC-labeled Griffonia simplicifolia lectin (GSA, Vector Laboratories, Burlingame, CA), and flat mounted. The area of choroidal NV at each Bruch’s membrane rupture site was measured by image analysis by an observer masked with respect to treatment group. The area of choroidal NV at the 3 rupture sites in one eye was averaged to give one experimental value and n in each of the figures represents the number of eyes
Rat model of choroidal NV and histology
Male Norway Brown rats (Charles River Labs, Frederick, MD), 7–8 weeks of age, were sedated with ketamine/xylazine and given a 5 μl suprachoroidal injection of 147 μg of PLGA-ACF MPs containing 10 μg acriflavine in one eye and 147 μg of empty PLGA MPs as previously described (23). Briefly, a 27-gauge needle was used to partially penetrate the sclera at an oblique angle 2 mm posterior to the limbus. A blunt 33-gauge needle of a Hamilton syringe was inserted into the scleral opening and advanced to enter the suprachoroidal space. The plunger was slowly depressed and after 15 seconds pressure was applied to the opening with a cotton tip applicator as the needle was withdrawn. At 2, 4, 8, 12, or 16 weeks after injection, rats had laser-induced rupture of Bruch’s membrane in 4 locations in each eye (100-μm spot size, 100-ms duration, 150-mW power). Fourteen days later, rats were euthanized, eyes were removed, retinas were dissected out, and eyecups were fixed and stained with 1:500 Alexa-594 labeled-GSA (Vector Laboratories, Burlingame, CA). The area of choroidal NV at Bruch’s membrane rupture sites was measured by image analysis with the investigator masked with respect to treatment group. The mean of the 4 values in each eye was used as a single experimental value.
Localization of PLGA-ACF MPs was assessed 2 weeks after suprachoroidal injection of 3 μl containing 88 μg of PLGA-ACF MPs (6 μg ACF) 2 mm posterior to the limbus at superior pole of each eye. Eyes were removed and fixed in 4% formaldehyde for 2 hours. After cryopreservation with a sucrose gradient, the eyes were embedded in OCT compound (Sakura Finetek, Torrence, CA) and frozen. Ten μm sections were cut through the injection site and fluorescence was imaged using and Axioskop 2 microscope (Zeiss, Oberkochen, Germany).
Rabbit safety study
Intraocular pressure (IOP) measurements
Dutch belted rabbit IOP was measured under gentle restraint without topical anesthesia using a tonometer (Icare TONOVET, Vantaa, Finland). IOP was measured once every other day for 6 days to establish the baseline. for each eye. IOP was then measured on the specified days after injection (1, 7, 14, 28) and reported as the average of three measurements. IOP readings are reported as the change in IOP from the baseline value for each eye.
Intraocular Injections
Rabbits were anesthetized with ketamine/xylazine, and the conjunctiva was cleaned with 5% povidone-iodine. For intravitreal injection, a 30G needle was inserted through the pars plana. For suprachoroidal injection, a 30G Hamilton Neuro Syringe with an adjustable protective needle sleeve was used and inserted above the pars plana. For both intravitreal and suprachoroidal injections, 50 μL of sodium hyaluronate solution containing either drug loaded (567 μg microparticles, 38 μg ACF) or blank microparticles (10 mg) was injected using a sterile syringe.
Fundus photography
Rabbits were anesthetized with ketamine/xylazine, and the pupils were dilated with 2.5% phenylephrine. After the application of Gonioscopic prism solution (Alcon Labs, Fort Worth, TX), a macula lens (Haag-streit AG, Koeniz, Switzerland) was placed above the cornea and fundus photographs were taken with a Zeiss OPMI VISU 210 Ceiling Mounted Operating Microscope with S8 Control.
Electroretinography (ERG)
Rabbits were dark adapted overnight, anesthetized with ketamine/xylazine, and placed on a heating pad set to 39°C. The pupils wer e dilated with 2.5 % phenylephrine. Gonioscopic prism solution (Alcon Labs, Fort Worth, TX) was applied followed by placing platinum electrodes over both corneas. The reference electrode was attached to the scalp between the eyes and a ground electrode was inserted into the back. A ganzfeld bowl illuminator was placed above both eyes, and scotopic ERG responses were recorded (Espion ERG Diagnosys, Diagnosys, Littleton, MA). Recordings for both eyes were made simultaneously with the electrical impedance balanced. Four intensities (0.63, 0.4, 10, 25 (cd-s/m2)) of lights were tested with flash intervals for 15, 15, 30, and 50 s, respectively, and 1 ms flash stimuli duration. For each intensity, 15 readings were taken and averaged. All measurements were taken using the Diagnosis Epsion software, and extracted data was transferred to Prism for statistical analysis and plotting.
Histology
Rabbit eyes were enucleated and immediately placed in Davidson’s fixative (33% ethanol, 11.1% acetic acid, 22% neutral buffered formalin). The lens was removed from the fixed tissue before dehydrating and sending to the Johns Hopkins Reference Histology Laboratory for paraffin embedding, sectioning (5 μm thick), and H&E staining.
Pharmacokinetics in rats
Male Norway Brown rats, 7–8 weeks of age, received a suprachoroidal injection of 5 μl containing 147 μg of PLGA-ACF MPs (10 μg ACF) 2 mm posterior to the limbus at superior pole of each eye. The injection site was marked in each eye. Six rats were euthanized at each of the following time points after injection: 1 and 2 weeks, and 1, 2, 3, and 4 months. After removal of the anterior segment and lens of each eye, the retina and RPE/choroid/sclera were dissected and cut in half along the horizontal meridian through the optic nerve head and frozen separately. Samples were analyzed for acriflavine and proflavine by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Tissue samples were homogenized in 200 μl of methanol and homogenized with Next Advance Bullet Blender. The analytes were extracted from 25 μl of homogenized tissue with 250 μl of acetonitrile containing internal standard (5 ng/ml of acridine orange). Samples were centrifuged and the top layer was transferred to an auto sampler vial for LC/MS/MS analysis. Chromatographic separation was achieved with a Phenomenex Luna CN analytical column (3 × 50 mm, 3 m) with a gradient. Mobile phase A was water containing 0.1% formic acid and mobile phase B was acetonitrile containing 0.1% formic acid. The gradient started with mobile phase B held at 50% for 1 minute and increased to 100% over 0.5 minutes; 100% mobile phase B was held for 1.5 minutes and then returned back to 50% mobile phase B and allowed to equilibrate for 1 minute. Total run time was 4 minutes with a flow rate of 0.6 ml/minute. The column effluent was monitored using a Sciex 4500 triple quadrupole mass spectrometer with electrospray ionization operating in positive mode. The mass spectrometer was programmed to monitor the following Multiple Reaction Monitoring transitions: 224.0 > 182.1 for acriflavine, 209.9 > 166.1 for proflavine and 266.1 > 234.1 for the internal standard acridine orange. The calibration curve was computed using area ratio peak of the analyte to the internal standard by using a quadratic equation with a 1/x2 weighting function over the range of 0.5 – 1,000 ng/ml. Tissue samples from untreated animals were used to verify that there was no endogenous background interference with analyte detection.
Results
Development of microparticles that provide sustained release of acriflavine
Acriflavine (ACF) is highly water soluble, which makes achieving high drug loading and sustained release from hydrophobic, biodegradable polymeric particles challenging. Past reports of loading ACF into MPs, lipid vesicle creams, and wafers described rapid burst release in just minutes to hours in vitro (29). Here, formulation variables were modified to achieve the highest possible drug loading into MPs in the appropriate size range that can be easily administered through needle gauges that are standard for use in ophthalmic injections. Specifically, PLGA polymer end group, polymer molecular weight, polymer concentration, and the pH of the water phase were varied. Spherical PLGA-ACF MPs were obtained (Figure 1A) with an average size of 7.3 ± 1.8 μm, which could easily be injected through a needle as small as 30G. Contrary to prior reports, there was minimal ACF burst release from the PLGA-ACF MPs in vitro, and the release was sustained for at least 40 days (Figure 1B).
Figure 1. Characterization of ACF microparticles.
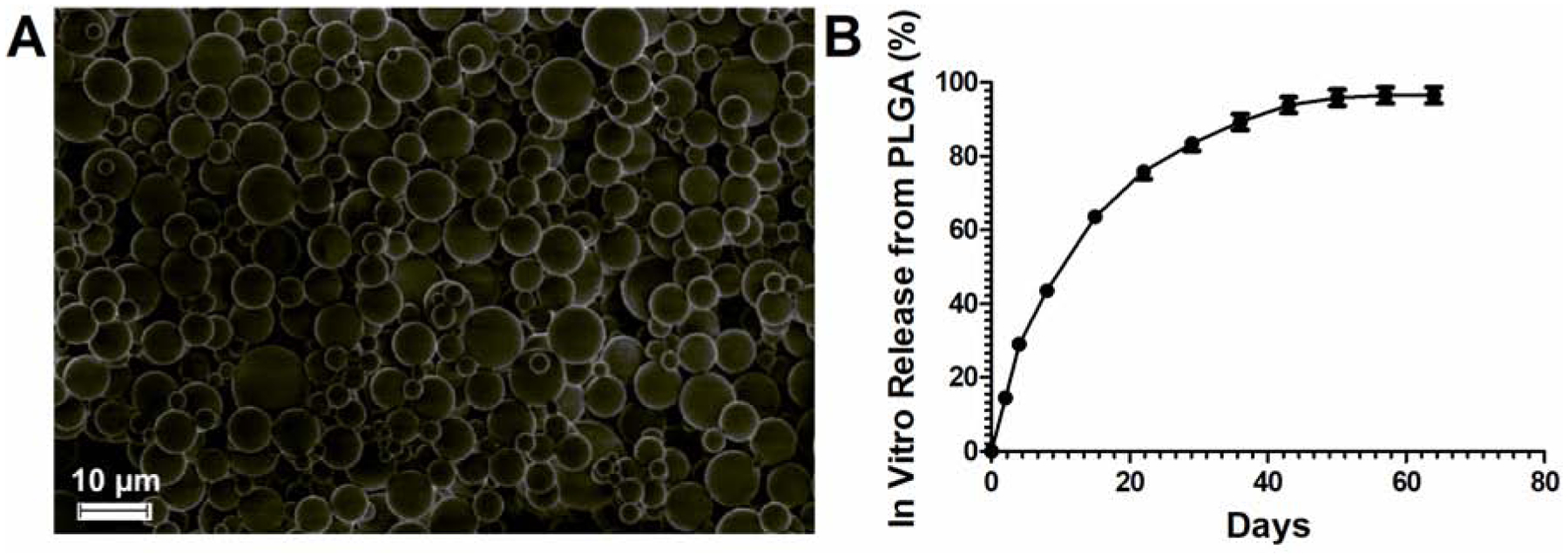
(A) Scanning electron microscopy (SEM) image of microparticles. Scale bar = 10 μm. (B) ACF release in vitro (37°C, pH 7.4 PBS) shown a s a percentage of the initial drug loading (mean ± SEM, n=3)
Intravitreous injection of PLGA-ACF MPs suppresses choroidal NV in mice for at least 9 weeks
C57BL/6 mice were given an intravitreous injection of PLGA-ACF MPs containing 2 μg ACF in one eye and empty MPs in the other eye. At 2, 4, 8, and 12 weeks after injection, Bruch’s membrane was ruptured by laser photocoagulation at 3 locations in each eye and after 1 week, the area of choroidal NV at Bruch’s membrane rupture sites was measured. Compared to empty MP injected eyes, areas of choroidal NV appeared smaller in eyes injected with PLGA-ACF MPs (Figure 2A). When choroidal NV was measured 3, 5, or 9 weeks after MP injection, the mean (±SEM) area of choroidal NV was significantly less in PLGA-ACF MP-injected eyes compared with fellow eye controls, but 13 weeks after injection, there was no significant difference (Figure 2B, Supplemental Figure 1A).
Figure 2. Suppression of Choroidal Neovascularization (CNV) at Bruch’s Membrane Rupture Sites for 9 Weeks after Intravitreous Injection of PLGA- Acriflavine (PLGA-ACF) Microparticles (MPs).

C57BL/6 mice were given an intravitreous injection of 2 μg of PLGA-ACF in one eye and 2 μg of empty PLGA MPs in the other eye. At 2, 4, 8, or 12 weeks after injection, Bruch’s membrane was ruptured by laser photocoagulation. After 1 week mice were euthanized and choroidal flat mounts were stained with FITC-Griffonia simplicifolia lectin which selectively stains vascular cells. (A) The area of CNV at Bruch’s membrane rupture sites appeared smaller in eyes injected with PLGA-ACF MP compared with those injected with empty PLGA MPs at all time points. (B) Image analysis showed a significant reduction in mean (± SEM) area of CNV in eyes injected with PLGA-ACF MPs compared with those injected with empty PLGA MPs at 2, 4, and 8 weeks after injection. N is the number of eyes evaluated in each group. (** p<0.01, * p<0.05 by unpaired t-test).
Suprachoroidal injection of PLGA-ACF MPs suppresses choroidal NV in rats for at least 18 weeks
Suprachoroidal injection provides a new route of delivery of therapeutic agents to the eye (30–32). Since MPs injected into the vitreous cavity have the potential to migrate throughout the eye, injection of MPs into the suprachoroidal space, where they are sequestered away from the retina and anterior structures of the eye, could have advantages. We are unable to perform suprachoroidal injections in mice due to the small size of the eye, but can reliably perform them in rats (23). Two weeks after suprachoroidal injection of PLGA-ACF MPs containing 6 μg ACF in in Brown Norway rats, fluorescence microscopy using an excitation wavelength of 470 nm and emission wavelength of 525 nm of an ocular section showed green fluorescence of ACF in the retina extending more than halfway around the circumference of the eye (Figure 3A, Supplemental Figure 2). High magnification showed MPs in the choroid (arrows, Figure 3B) and bright fluorescence from ACF that had entered the retina (Figure 3B). Bruch’s membrane was ruptured 2, 4, 8, 12, or 16 weeks after suprachoroidal injection of 6 μg ACF in PLGA-ACF MPs in one eye and empty PLGA MPs in the other eye and 2 weeks after Bruch’s membrane rupture, choroidal NV lesions appeared much smaller in ACF- PLGA MP-injected eyes (Figure 3C). The mean (±SEM) area of choroidal NV was significantly less in ACF-PLGA MP-injected eyes compared with contralateral controls at each time point through 18 weeks after injection (Figure 3D, Supplemental Figure 1B).
Figure 3. Suppression of Choroidal Neovascularization (CNV) at Bruch’s Membrane Rupture Sites for 18 Weeks after Suprachoroidal Injection of PLGA- Acriflavine (PLGA-ACF) Microparticles (MPs).

Brown Norway rats were given a suprachoroidal injection of 6 μg of PLGA-ACF MPs in one eye and empty PLGA MPs in the other eye. (A) Two weeks after injection, a frozen ocular section showed ACF fluorescence in the retina and choroid on the side of the eye that had been injected. (B) A high magnification fluorescence microscopy image (left side) and a light micrograph of the same section stained with Hoechst (right side) showed PLGA-ACF MPs in the choroid (arrows) and bright fluorescence from ACF in retinal pigmented epithelium (RPE), photoreceptor outer segments (OS) and inner segments (IS), outer nuclear layer (ONL) and inner nuclear layer (INL) of the retina. There was weak fluorescence from ACF in the sclera. At 2, 4, 8, 12, and 16 weeks after injection, Bruch’s membrane was ruptured by laser photocoagulation in each eye. Two weeks after rupture of Bruch’s membrane, choroidal flat mounts were stained with FITC- Griffonia simplicifolia lectin. At each time point (weeks between MP injection and measurement of CNV), the area of CNV at Bruch’s membrane rupture sites appeared smaller in eyes that had been injected with PLGA-ACF MPs (C). Image analysis showed a significant reduction in mean (± SEM) area of CNV in eyes injected with PLGA-ACF MPs compared with those injected with empty PLGA MPs at all time points. N is the number of eyes evaluated in each group (** p<0.01, * p<0.05 by unpaired t- test).
Reduced electroretinogram (ERG) amplitudes after intravitreous, but not suprachoroidal injections of PLGA-ACF MPs in rabbits
Safety studies were done in Dutch Belted rabbits. Mean ERG a- and b-wave amplitudes were significantly reduced at some stimulus intensities in eyes given an intravitreous injection of 38 μg of ACF in PLGA-ACF MPs compared with eyes given an injection of 38 μg of ACF in PLGA-ACF MPs into the suprachoroidal space or untreated eyes (Figure 4A). However, there were no differences in mean ERG a- and b-wave amplitudes in eyes given a suprachoroidal injection of either PLGA-ACF MPs or empty PLGA MPs, which were both similar to amplitudes in untreated eyes (Figure 4B). Because of the reduced ERG function after intravitreous injection of PLGA-ACF MPs, all additional safety studies were done after suprachoroidal injection of PLGA-ACF MPs. Fundus photographs showed normal appearing peripheral retina and optic nerve in eyes given a suprachoroidal injection of PLGA-ACF MPs similar to those in untreated eyes or those given a suprachoroidal injection of empty PLGA MPs (Figure 4C). There was very little change in mean intraocular pressure (IOP) in eyes given a suprachoroidal injection of empty PLGA or PLGA-ACF MP at 1, 7, 14, 21, and 28 days after baseline, but at 7 days after injection there was a small but significant reduction in mean IOP in eyes injected with PLGA-ACF MPs compared with those injected with empty PLGA MPs, and at day 28 there was a small but significant increase in mean IOP in the empty PLGA MP group (Figure 4D). However, such small changes are unlikely to be clinically significant. Ocular sections showed normal retina and choroid in eyes injected with empty PLGA MPs or PLGA-ACF MPs (Figure 4E), and no signs of inflammation or abnormalities were observed near the injection site.
Figure 4. Suprachoroidal Injection (SC), but not Intravitreous Injection (IVT) of PLGA-Acriflavine (PLGA-ACF) Microparticles (MPs) in Rabbits is Safe.
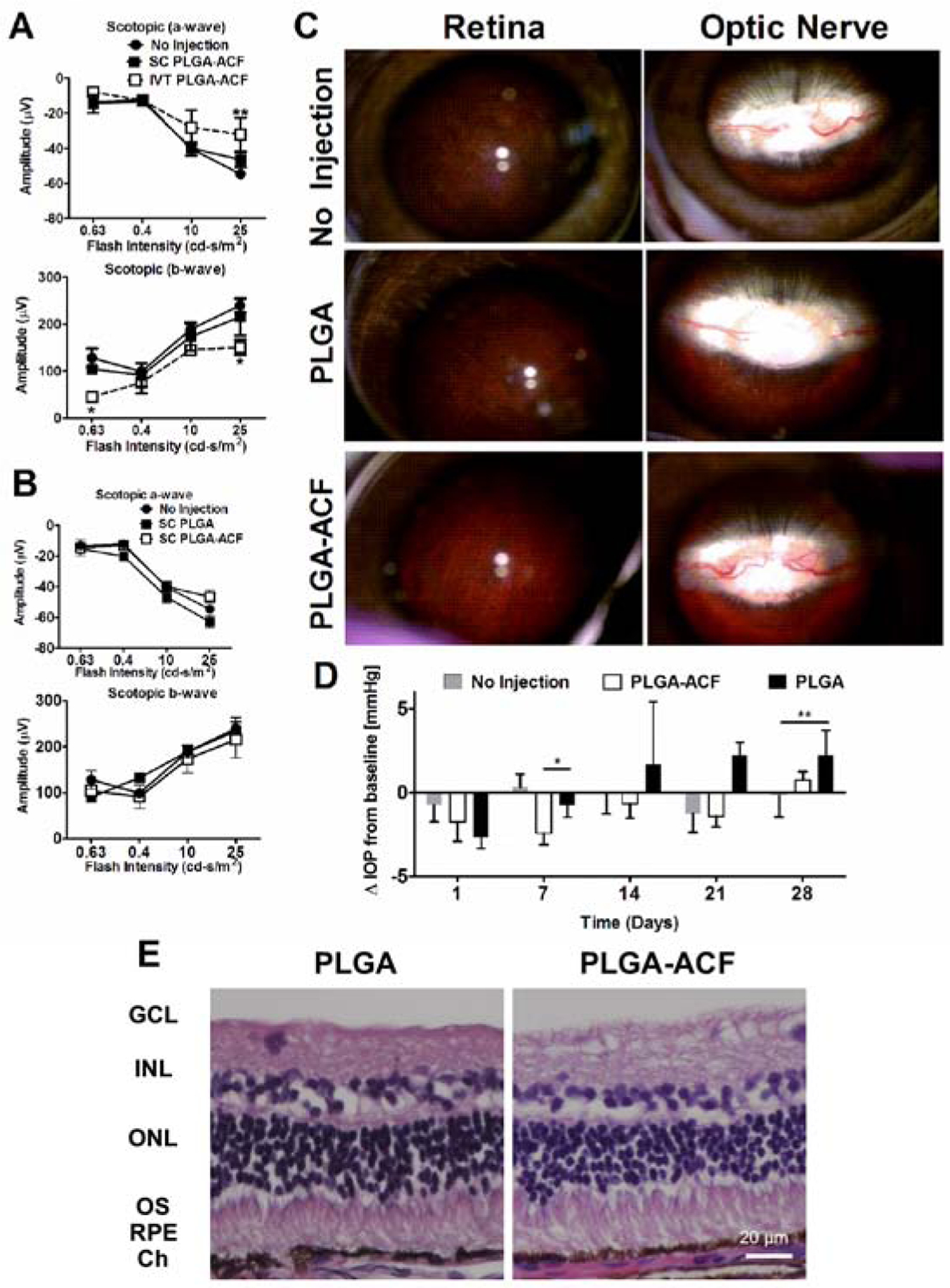
Dutch belted rabbits were given a suprachoroidal (SC) or intravitreous injection of 50 μl containing 10 mg of PLGA-ACF MPs (38 μg of ACF), 10 mg of empty PLGA MPs, or no injection (n=3–4). (A) Scotopic electroretinograms 28 days after injection showed that compared to eyes with no injection, there was no significant difference in mean (±SEM) a-wave or b-wave amplitude in eyes given a SC injection of PLGA-ACF, but there was a significant reduction in mean a-wave amplitude at the highest stimulus intensity and in b-wave amplitude at the highest and lowest stimulus intensity in eyes given a an IVT injection of PLGA-ACF (*p<0.05; **p<0.01 by ANOVA with Bonferroni correction for multiple comparisons). (B) Compared with eyes that received no injection, there was no significant reduction in a-wave or b-wave amplitudes 28 days after SC injection 50 μl containing 10 mg of PLGA-ACF MPs (38 μg of ACF) or 10 mg of empty PLGA MPs (n=3–4). (C) Fundus photographs showed normal appearing peripheral retina (first column) and optic nerve (second column) 28 days after SC injection of 50 μl containing 10 mg of PLGA-ACF MPs (38 μg of ACF) or 10 mg of empty PLGA MPs, indistinguishable from eyes that had no injection. (D) Mean (± SEM) changes from baseline intraocular pressure (ΔIOP) were small and not clinically significant through 28 days after SC injections of PLGA-ACF MPs or PLGA MPs, but there were some statistically significant differences between groups. At day 7, there was a significant reduction in the PLGA-ACF group compared with the others (*p<0.05 by ANOVA with Bonferroni correction for multiple comparisons) and at day 28, there was a significant increase in the PLGA group compared with no injection (**p<0.01). (E) Histopathology of the retina was normal 28 days after SC injection of PLGA-ACF or PLGA MPs (n=3).
Tissue Levels of the Components of ACF in Ocular Tissues after Suprachoroidal injection of PLGA-ACF MPs
Acriflavine is a mixture of trypaflavine (TRF) and proflavine (PRF) (27). PRF is the precursor for TRF and is difficult to separate from TRF, and therefore pure TRF is not available. In contrast to ACF, PRF does not suppress choroidal NV, indicating that TRF is the active component of ACF (Supplemental Figure 3). An LC-MS assay was developed to measure TRF and PRF in ocular tissues after suprachoroidal injection of PLGA-ACF MPs containing 10 μg ACF in Brown Norway rats. The suprachoroidal injections were done at the superior pole of the eye and to determine if levels were constant throughout the entire eye, retinas and eyecups (RPE/choroid consisting of the RPE, choroid, and sclera) were dissected and divided into superior and inferior halves. There was very little difference in TRF and PRF levels in either tissue at any time point; both were above 10 nM at 1 week after injection in the superior and inferior half of retina and RPE/choroid. There was ≥ 10-fold drop in levels between 1 and 2 weeks after which levels were fairly stable through 16 weeks (Figure 5). Steady-state levels were approximately 2–8 nM and 0.5–1 nM in superior and inferior RPE/choroid, respectively, and 0.5–1 nM and 0.1 nM in superior and inferior retina, respectively.
Figure 5. Measurement of the Components of Acriflavine in Retinal Pigmented Epithelium (RPE)/Choroid and Retina after Suprachoroidal injection of PLGA-ACF MPs.

Brown Norway rats were given a suprachoroidal injection of 5 μl containing 147 μg of PLGA-ACF MPs (10 μg of ACF) in each eye. At 1, 2, 4, 8, 12, and 16 weeks after injection, three rats were euthanized, eyes removed (n=6 for each time point) and the retina and RPE/choroid was dissected and cut into superior (side of the eye that received the SC injection) and inferior halves. The level of each component of ACF, Trypaflavine (TRF) and Proflavine (PRF), were measured by LC-MS. Each point represents the mean (±SEM).
Discussion
Rupture of Bruch’s membrane in mice (28) or rats (33) causes choroidal NV that mimics the characteristics of choroidal NV in patients with AMD, and vascular endothelial growth factor (VEGF) was found to be a critical stimulator in this model (34, 35). Clinical trials in patients with choroidal NV due to AMD demonstrated that monthly injections of ranibizumab, an antigen binding fragment that neutralizes all isoforms and fragments of VEGF-A, reduced fluid under or within the retina and improved vision (36, 37). However, in an extension study in which the frequency of visits and injections was reduced to every 3 months, visual benefits that were maintained during two years of monthly injections were lost within a year (38). This suggests that in most patients with choroidal NV due to AMD, the excess production of VEGF is chronic and sustained suppression of VEGF is required. Clinical trials have shown less frequent injections of aflibercept and brolucizumab may have similar outcomes as monthly injections of ranibizumab, but the treatment burden is still high (39, 40). Observational studies have shown that outcomes in clinical practice are far worse than those in clinical trials primarily due to high treatment burden which is difficult for many patients to sustain (41–43). Also, not all patients with choroidal NV due to AMD respond optimally to strong suppression of VEGF achieved with monthly injections of a VEGF antagonist suggesting that other pro-angiogenic/pro-permeability factors may contribute in those patients. Therefore, new treatments are needed to address these shortcomings of intravitreous injections of antiVEGF agents, our current treatment for choroidal NV due to AMD.
One strategy is to identify factors other than VEGF that contribute to choroidal NV, develop antagonists, and combine them with anti-VEGF agents. Leading candidates include platelet-derived growth factor-B (PDGF-B), angiopoietin-2 (angpt2), vascular endothelial protein-tyrosine phosphatase (VE-PTP), and VEGF-C, all of which, like VEGF-A, are hypoxia-regulated (44). While biologic plausibility and pre-clinical studies support a possible role for these factors, clinical trials in patients with choroidal NV due to AMD failed to demonstrate that combined suppression of VEGF and PDGF-B (ClinicalTrials.gov Identifier: NCT01944839) or combined suppression of VEGF and Angpt2 (ClinicalTrials.gov Identifier: NCT02713204) provided superior outcomes compared with suppression of VEGF alone. A possible reason is that VEGF plays the predominant role in growth and leakage of choroidal NV and a host of other hypoxia- regulated factors contribute only a small amount making it difficult to identify added benefit when only one of these factors is inhibited in combination with VEGF suppression.
We have been investigating an alternative strategy of targeting HIF-1 and thereby suppressing all hypoxia-induced factors which includes VEGF and all of the other factors that have been implicated as well as their receptors (7, 21–23, 44). Intraocular injection of the HIF-1 inhibitors doxorubicin and daunorubicin caused strong suppression of retinal and choroidal NV, but at high doses, also caused reduction in retinal ERG function (22). Intravitreous injection of nanoparticles (NPs) of doxorubicin (10 μg) conjugated to copolymers of branched polyethylene glycol and poly(sebacic acid) suppressed choroidal and retinal NV in mice for at least 35 days with no reduction in ERG function. After intravitreous injection of NPs containing 2.7 mg doxorubicin, there were detectable levels of doxorubicin in aqueous for 105 days. While formulation changes might have resulted in longer treatment effects, we decided to turn our attention to ACF because it provides the theoretical advantage of inhibiting both HIF-1 and HIF-2. In this study, we incorporated ACF into PLGA MPs and found that intravitreous injection of PLGA-ACF MPs containing 2 μg ACF suppressed choroidal NV at Bruch’s membrane rupture sites for at least 8 weeks. Intravitreous injection of MPs requires modifications that promote aggregation and prevent dispersion of MPs which can degrade vision. We tested a new route of delivery, suprachoroidal injection (30–32) that sequesters MPs away from the retina, but still in close proximity allowing diffusion of ACF into the retina. Due to their small size, we are unable to perform suprachoroidal injections in mouse eyes; therefore, a rat model of choroidal NV was used. Suprachoroidal injection PLGA-ACF MPs containing 10 μg ACF suppressed choroidal NV at Bruch’s membrane rupture sites for at least 16 weeks, far longer than the duration of in vitro ACF release. Intravitreous injection, but not suprachoroidal injection, of PLGA-ACF MPs containing 38 μg of ACF in rabbits resulted in modest reduction of ERG function, suggesting that sustained release of ACF in the suprachoroidal space may provide an added layer of safety. There was a mean increase from baseline IOP of 2 mm Hg 28 days after suprachoroidal injection of empty microparticles but such a small difference is not clinically significant. Future studies in the context of potential product development will include identifying approaches, such as freeze drying, for ensuring long-term stability of the formulation during storage. Additionally, characterization of the polymer degradation rate in the suprachoroidal space will be important in the context of repeated injection over time.
In patients with choroidal NV due to AMD treated with standard clinical care outside clinical trials, the frequency of injections is less and visual outcomes are far inferior (41, 45, 46) compared with that seen in several clinical trials (36, 37, 39, 47, 48). New treatments designed to provide sustained suppression of VEGF are being tested in clinical trials including a surgically implanted refillable reservoir that slowly releases ranibizumab into the eye (49) and subretinal injection of an AAV8 vector expressing an antigen binding fragment similar to ranibizumab (50) (ClinicalTrials.gov NCT03066258). Both of these approaches require a surgical procedure and suprachoroidal injections of PLGA-ACF MPs has two potential advantages. It will provide prolonged suppression of other hypoxia-induced factors in addition to VEGF which could provide added benefit in patients who have a suboptimal response to VEGF blockade and it is less invasive, safer, and can be done in an outpatient clinic rather than an operating room. Thus, suprachoroidal injection of PLGA-ACF MPs has potential to provide a new, less invasive, long duration treatment for retinal and choroidal vascular diseases.
Supplementary Material
Acknowledgments
This work was primarily supported by the National Eye Institute (R01EB916121) and a grant from the Altsheler-Durell Foundation with some additional support from the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30CA006973 and UL1TR001079, and the Shared Instrument Grant S10OD020091). The project described was also supported by grant number UL1 TR001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCATS or NIH.
PAC: AERPIO PHARMACEUTICALS: Advisory Board, Honoraria to JHU, Investigator, Grant; ALIMERA SCIENCES, INC: Investigator, Grant; ALLEGRO: Advisory Board, Investigator, Grant, Equity; ALLERGAN, INC: Consultant, Honoraria, Grant; APPLIED GENETIC TECHNOLOGIES CORPORATION: Advisory Board, Honoraria; ASCLIPIX: Consultant, Honoraria, Grant; ASTELLAS PHARMA, INC: Consultant, Honoraria; CLEARSIDE BIOMEDICAL INC: Investigator, Grant; EXONATE LTD: Advisory Board, Honoraria GENENTECH/ROCHE INC: Advisory Board, Honoraria to JHU, Investigator, Grants; SANOFI GENZYME: Investigator, Grant; GRAYBUG VISION: Consultant, Honoraria, Co-Founder, Equity, Grant; MERCK & CO, INC: Advisory Board, Honoraria; NOVARTIS PHARMACEUTICALS CORPORATION: Consultant, Honoraria; OXFORD BIOMEDICA: Investigator, Grant; REGENERON PHARMACEUTICALS, INC: Investigator, Grant; REGENXBIO, INC: Investigator, Grant; RXI PHARMACEUTICALS: Consultant, Honoraria, Investigator, Grant.
JH: KALA PHARMACEUTICALS: Co-founder, equity, royalty distributions; GRAYBUG VISION: Board of Directors, Co-Founder, Equity; SPIRAL THERAPEUTICS: Chief Scientific Officer, Equity; ORPHERIS: Co-founder, Equity; THERALY PHARMACEUTICALS: Co-Founder, Equity
JF: KALA PHARMACEUTICALS: equity, royalty distributions; GRAYBUG VISION: Co-Founder, Equity
LME: KALA PHARMACEUTICALS: royalty distributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- 1.Bresnick GH, deVenecia G, Myers FL, Harris JA, and Davis MD. Retinal ischemia in diabetic retinopathy. Arch Ophthalmol. 1975;93(12):1300–10. [DOI] [PubMed] [Google Scholar]
- 2.Merin C, Ber I, and Ivry M. Retinal ischemia (capillary nonperfusion) and retinal neovascularization in patients with diabetic retinopathy. Ophthalmologica. 1978;177(3):140–5. [DOI] [PubMed] [Google Scholar]
- 3.Magargal LE, Donoso LA, and Sanborn GE. Retinal ischemia and risk of neovascularization following central retinal vein obstruction. Ophthalmology. 1982;89(11):1241–5. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, and Campochiaro PA. Supplemental inspired oxygen improves diabetic macular edema; a pilot study. Invest Ophthalmol Vis Sci. 2003;45(2):617–24. [DOI] [PubMed] [Google Scholar]
- 5.Wang GL, Jiang B-H, Rue EA, and Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozaki H, Yu A, Della N, Ozaki K, Luna JD, Yamada H, et al. Hypoxia inducible factor-1a is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40(1):182–9. [PubMed] [Google Scholar]
- 7.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, et al. Cell type- specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia- inducible factor 1. Circ Res. 2003;93(11):1074–81. [DOI] [PubMed] [Google Scholar]
- 8.Silva PS, Dela Cruz AJ, Ledesma MG, van Hemert J, Radwan A, Cavallerano JD, et al. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology. 2015;122(12):2465–72. [DOI] [PubMed] [Google Scholar]
- 9.Vinores SA, Xiao WH, Aslam S, Shen J, Oshima Y, Nambu H, et al. Implication of the hypoxia response element of the VEGF promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206(3):749–58. [DOI] [PubMed] [Google Scholar]
- 10.Chandel NS, Maltepe E, Godwasser E, Mathieu CE, Simon MC, and Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95(20):11715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochodrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia. J Biol Chem. 2000;275:25130–1138. [DOI] [PubMed] [Google Scholar]
- 12.The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119(10):1417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong A, Xie B, Shen J, Yoshida T, Yokoi K, Hackett SF, et al. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong A, Shen J, Zeng M, and Campochiaro PA. Vascular cell adhesion molecule-1 plays a central role in the proangiogenic effects of oxidative stress. Proc Natl Acad Sci USA. 2011;108(35):14614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullins RF, Johnson MN, Faidley EA, Skeie JM, and Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn JH, Flamme-Wiese MJ, Witmore SS, Workalemahu G, Mameros AG, Boese EA, et al. Choriocapillaris degeneration in geographic atrophy. Am J Pathol. 2019;189(7):1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White B, Pierce M, Nassif N, Cense B, Park B, Tearney G, et al. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical coherence tomography. Opt Express. 2003;11(25):3490–7. [DOI] [PubMed] [Google Scholar]
- 18.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin J, Rinella N, Zhang Q, Zhou H, Wong J, Deiner M, et al. OCT Angiography and Cone Photoreceptor Imaging in Geographic Atrophy. Invest Ophthalmol Vis Sci. 2018;59(15):5985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman DJ, Silverman RH, Rondeau MJ, Lloyd HO, Khanifar AA, and Chan RV. Age-related macular degeneration: choroidal ischaemia? Br JOphthalmol. 2013;97(8):1020–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida T, Zhang H, Iwase T, Shen J, Semenza G, and Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24(6):1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwase T, Fu J, Yoshida T, Muramatusu D, Miki A, Hashida N, et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J Control Release. 2013;172(3):625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng M, Shen J, Liu Y, Lu LY, Ding K, Fortmann SD, et al. The HIF-1 antagonist acriflavine: visualization in retina and suppression of ocular neovascularization. J Mol Med (Berl). 2017;95(4):417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, and Risau W. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1-alpha and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. [DOI] [PubMed] [Google Scholar]
- 25.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, and Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1-alpha regulates VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94(9):4273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, et al. Hypoxia-inducible factor 2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27(40):5354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K, Zhang H, Qlan DZ, Rey S, Liu JO, and Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106(42):17910–5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153(5):1641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangraviti A, Raghavan T, Volpin F, Skuli N, Guillotti D, Zhou J, et al. Hif-1α- targeting acriflavine provides long term survival and radiological tumor response in brain cancer therapy. Sci Rep. 2017;7:14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR P Lin AS, Edelhauser HF, and Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow needles. Pharm Res. 2011;28(1):166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, and Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campochiaro PA, Wykoff CC, Brown DM, Boyer DS, Barakat M, Taraborelli D, et al. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the Tanzanite Study. Ophthalmology Retina. 2017;2(4):320–8. [DOI] [PubMed] [Google Scholar]
- 33.Dobi ET, Puliafito CA, and Destro M. A new model of choroidal neovascularization in the rat. Arch Ophthalmol. 1989;107(2):264–9. [DOI] [PubMed] [Google Scholar]
- 34.Kwak N, Okamoto N, Wood JM, and Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(10):3158–64. [PubMed] [Google Scholar]
- 35.Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge J, et al. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195(2):241–8. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355(14):1419–31. [DOI] [PubMed] [Google Scholar]
- 37.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355(14):1432–44. [DOI] [PubMed] [Google Scholar]
- 38.Singer MA, Awh CC, Sadda S, Freeeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175–83. [DOI] [PubMed] [Google Scholar]
- 39.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal Aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48. [DOI] [PubMed] [Google Scholar]
- 40.Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. [DOI] [PubMed] [Google Scholar]
- 41.Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao P, Lum F, Wood K, Salman C, Buruqapalli b, Hall R, et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS registry. Ophthalmology. 2018;125(4):522–8. [DOI] [PubMed] [Google Scholar]
- 43.Prenner JL, Halperin LS, Rycroft C, Hoguet A, Williams Liu Z, and Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725–31. [DOI] [PubMed] [Google Scholar]
- 44.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49(11):67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen SY, Mimoun G, Oubrham H, Zourdani A, Malbril C, Quere S, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33(3):474–81. [DOI] [PubMed] [Google Scholar]
- 46.Finger RP, Wiedemann P, Blumhagen F, Pohl K, and Holz FG. Treatment patterns, visual acuity and quality-of-life outcomes of the WAVE study- a noninterventional study of retnibizumab treatment for neovascular age-related macular degeneration in Germany. Acta Ophthalmol. 2013;91(6):540–6. [DOI] [PubMed] [Google Scholar]
- 47.CATT Research Group Martin DF, Maguire MG Ying GS, Grunwald JE Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Eng J Med. 2011;364(20):1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–56. [DOI] [PubMed] [Google Scholar]
- 49.Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 Ladder clinical trial. Ophthalmology. 2019;126(8):1141–54. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Fortmann SD, Shen J, Wielechowski E, Tretiakova A, Yoo S, et al. AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol Ther. 2017;26(2):542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


