Abstract
Aging increases autonomic support of blood pressure; however the impact of aerobic fitness on autonomic support of blood pressure has not been addressed in women. As such, we hypothesized that aerobic fitness would be related to the change in blood pressure during ganglionic blockade such that women with greater aerobic fitness would have a blunted fall in blood pressure during ganglionic blockade due to increased vagal tone. 13 young premenopausal and 13 older postmenopausal women completed a screening visit where aerobic fitness (maximal oxygen consumption, ) was measured. On a separate study day, participants were instrumented for assessment of muscle sympathetic nerve activity (MSNA), heart rate (HR, electrocardiography), and beat by beat blood pressure (arterial catheter and pressure transducer) and underwent pharmacological blockade of the autonomic ganglia using trimethaphan camyslate. HR, blood pressure, and MSNA were analyzed before and during ganglionic blockade. In young women, there was a significant relationship between aerobic fitness and the change in blood pressure during ganglionic blockade (r=0.761, p=0.003). In older women, there was no relationship between aerobic fitness and the change in blood pressure during ganglionic blockade (r=−0.106, p=0.73). Measures of heart rate variability were related to fitness in young women, but not older women (RMSSD: r=0.713, p=0.006 vs. r=−0.172, p=0.575). Our data suggest that in young women, autonomic support of blood pressure is attenuated in those that are highly fit; however, this relationship is not significant in older women.
Keywords: aging, blood pressure, humans, physiology, women, autonomic nervous system
Graphical Abstract

Introduction
Aging increases autonomic support of blood pressure (the change in blood pressure with pharmacological blockade of the autonomic nervous system)1–3. For example, older men exhibit greater autonomic support of blood pressure when compared to younger men with the level of support being related to baseline muscle sympathetic nerve activity (MSNA)3. A relationship between MSNA and the fall in blood pressure with ganglionic blockade is indicative of an important role for the sympathetic branch of the autonomic nervous system. In contrast, an increase in heart rate during ganglionic blockade is indicative of a role for the parasympathetic branch of the autonomic nervous system. Increased MSNA, resting heart rate and by extension autonomic support of blood pressure is indicative of increased sympathetic tone4–6. Evidence of increased sympathetic tone is linked to many health complications including hypertension, sleep apnea, and heart failure. However, aerobic fitness is known to have a preventative role against these disorders, and has been associated with reductions in MSNA when looking at exercise training effects within patient populations7, 8. Additionally, aerobic fitness is also associated with a lower resting heart rate and greater parasympathetic tone9. Increased parasympathetic tone and heart rate reserve may increase the ability to increase heart rate through parasympathetic mechanisms, thus defending against the fall in blood pressure during ganglionic blockade. Therefore, it is reasonable that aerobic fitness may modulate autonomic support of blood pressure.
However, Jones and colleagues demonstrated that fitness does not reduce autonomic support of blood pressure in young men as shown by similar falls in blood pressure in trained and untrained men during ganglionic blockade10. Nevertheless, sex differences exist in blood pressure regulation and autonomic support of blood pressure11–13. In both older and younger men, autonomic support of blood pressure is tightly linked to baseline indices of peripheral sympathetic nervous system activity, including MSNA11. Similar to men, older women exhibit greater autonomic support than younger women1. In young women, autonomic support of blood pressure is not related to indices of baseline sympathetic activity. These data suggest the importance of the parasympathetic nervous system. In contrast, older women have a relationship between autonomic support of blood pressure and MSNA, supporting greater sympathetic support of blood pressure in this population. This suggests that it is possible to glean insight into the relative contributions of the sympathetic and parasympathetic nervous systems to blood pressure control using ganglionic blockade. Data suggest that aerobic fitness is important in women as it contributes to improvements in quality of life, blood pressure, and other cardiovascular risk factors14, 15.
In this context, the effects of aerobic fitness on autonomic support of blood pressure in women are unknown. Additionally, the combined effect of aerobic fitness and aging on autonomic support of blood pressure in women is unknown. Given this lack of information, we investigated the role of aerobic fitness on autonomic support of blood pressure. We also investigated the relative contributions of the sympathetic and parasympathetic nervous systems in young premenopausal and older postmenopausal women of varying fitness levels. In contrast to data in men, we hypothesize that aerobic fitness is associated with the change in blood pressure during ganglionic blockade, such that women with higher fitness would have a smaller decrease in blood pressure. Further, we hypothesize that aerobic fitness is associated with an increase in heart rate with ganglionic blockade, such that women with greater aerobic fitness will have a larger increase in heart rate than less aerobically fit women.
Methods
Participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. We completed a retrospective analysis of a subset of women who had completed previous ganglionic blockade studies in our laboratory1, 2, and who had also completed a maximal exercise test within 6 months of their ganglionic blockade visit. Thirteen premenopausal and thirteen postmenopausal women were included in this study. Participants were non-obese (BMI < 30), non-smokers, and not currently taking any medications with the exception of oral contraceptives (n=7) and transdermal estradiol (n=2). All young women on contraceptives were taking combination medications with ethinyl estradiol and a 3rd generation progestin. Women were studied on days 2–5 of the placebo phase (greater than >48 hrs from the last active pill). Estradiol was 25±15 and 39±20 pg/mL in naturally cycling vs. young women on OCs. Average estradiol was 4.6±4.6 pg/mL for the older women not on MHT and 39 pg/mL and 40 pg/mL for the 2 older women on MHT. All participants were free of hypertension, cardiovascular disease, and diabetes or prediabetes mellitus as reported in their medical record. All premenopausal participants provided a negative urine pregnancy test prior to the study. The older women were ≥50 years old and postmenopausal (>12 months from last menstruation) based on self-report. Informed consent was obtained from all participants. This study was approved by the Mayo Clinic Institutional Review Board. All procedures followed were in accordance with institutional guidelines and adhered to the principles of the declaration of Helsinki and Title 45, US Code of Federal Regulation, part 46, Protection of Human Subjects.
Screening Visit
Prior to the study visit, participants reported to the Clinical Research and Trials Unit at Mayo Clinic after refraining from exercise, caffeine and alcohol consumption for 24 hours. Dual energy X-ray absorptiometry was used to assess body composition (Lunar iDXA, GE Healthcare, Chicago Illinois). Maximal oxygen consumption () was measured using a metabolic cart (Ultima, Medical Graphics, St. Paul Minnesota) and heart rate was recorded using a 12-lead electrocardiogram (CASE™ System, GE Medical Systems, Milwaukee, WI) during progressive exercise (starting workload 15–40W and increased by an amount equivalent to the starting workload each minute) to exhaustion on an upright cycle ergometer (Corival, Lode, Groningen Netherlands) in a temperature controlled room (20–22° C). Criteria for test end were 2 or more of the following: plateau in oxygen consumption with increasing workload, respiratory exchange ratio ≥1.15, heart rate ≥95% of age-adjusted estimate of maximum heart rate, and/or inability to maintain 60 revolutions per minute on the cycle ergometer.
Study Visit
Study visits were completed a minimum of 48 hours following the screening visit. We separated testing by at least 2 days based on the known impact of maximal intensity exercise on cardiovascular and autonomic function. There are data to suggest BP may remain low for several hours after exercise from reductions in vascular resistance due to combined mechanisms of baroreceptor resetting and local vasodilatory mechanisms including Histamine H1 and H2 receptor activation16, 17. Participants were again asked to refrain from strenuous exercise, caffeine and alcohol consumption for 24 hours. Participants arrived at the CRTU at 0800 hrs and were positioned supine for instrumentation. Heart rate was measured using a three-lead electrocardiogram (Cardiocap/5, Datex-Ohmeda, Louisville Colorado). A 20-gauge catheter was then placed into a brachial artery under local anesthesia (2% lidocaine) and connected to a high resolution pressure transducer (FloTrac, Edwards Lifesciences, Irvine California) positioned at heart level to measure beat by beat blood pressure waveforms. A baseline arterial blood draw was completed through the catheter for assessment of estradiol levels. An intravenous catheter was placed for infusion access. Stroke volume, total peripheral resistance, cardiac output were calculated using model flow analysis software (WinCPRS, Absolute Aliens Oy, Turku Finland). Multiunit MSNA was measured using techniques described previously (Data collection: Nerve Traffic analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA; Data analysis: WinCPRS, Absolute Aliens Oy, Turku, Finland)2. The recorded signal was amplified 100,000 fold, band-pass filtered (700 to 2000 Hz), rectified and integrated (resistance-capacitance integrator circuit, time constant 0.1 sec). Time domain heart rate variability, frequency domain heart rate variability, and spontaneous cardiac baroreflex sensitivity were also analyzed using WinCPRS software. Main outcome measures for time domain heart rate variability were root mean square of successive differences between normal heartbeats (RMSSD) and percentage of adjacent normal R-R intervals that differ from each other by more than 50 ms (pNN50), which are considered markers of parasympathetic activity18, 19. For measures of frequency domain heart rate variability, a Fourier transformation with a Hanning window was used. The magnitude of oscillations in R-R interval was quantified by calculating the power spectral density (total power 0.04–0.4Hz; high frequency power 0.15–0.4; low frequency power 0.04–0.15Hz)20. High-frequency power of R-R interval (RRIHF) was used as another marker of parasympathetic activity18, 19. Spontaneous cardiovagal baroreflex sensitivity was determined using the sequence method from beat to beat changes in R-R interval and systolic blood pressure21, 22. Three or more beats of progressive changes in systolic blood pressure with corresponding changes in R-R interval were identified. Rising (Up-up; increases in systolic pressure followed by a lengthening of R-R interval) and falling (down-down; progressive decreases of systolic blood pressure followed by a shortening of the R-R interval) sequences were recorded and the minimum criteria for accepting a sequence was set at 1mmHg for systolic pressure and 4ms for R-R interval. An r-value of 0.7 was used as acceptance criteria for sequences. Rising or falling sequences within the 5min baseline period were averaged for each participant.
Experimental Protocol
Following successful instrumentation, 10 minutes of baseline data were recorded during quiet rest. Intravenous infusion of trimethaphan was then started to initiate ganglionic blockade. With a physician present, the infusion began with 0.5 or 2 mg/min and increased by 0.5–1 mg/min every 6 minutes up to 7 mg/min or when successful ganglionic blockade was achieve. Ganglionic blockade was considered successful if participants demonstrated ≥2 of the following criteria: an absence of bursts of sympathetic activity, no change in blood pressure in response to increasing trimethaphan dose, or <5 bpm increase in HR during phase II of the Valsalva maneuver. Once blockade was established and a steady state HR and blood pressure had been reached, data were recorded during an additional 10 minutes of quiet rest. After discontinuation of the trimethaphan infusion and de-instrumentation, participants remained in the Clinical Research and Trial Unit for ≥2 hours for observation.
Data Analysis and Statistics
Steady-state physiological variables (HR, blood pressure, MSNA, and BRS) were assessed as an average during 5 minutes of baseline and 5 minutes of ganglionic blockade. Data were analyzed statistically using commercially available software (Sigma Plot 12 [San Jose, CA]; IBM SPSS Statistics, version 24 [Chicago, IL]). Baseline age group differences were assessed using independent sample t-tests, and data are expressed as mean±SEM. To measure whether there was a relationship between aerobic fitness and autonomic/hemodynamic variables, regression analysis was performed, and Pearson correlation coefficients were calculated. The α-level was set at 0.05.
Results
Participant Characteristics
Twenty-six women successfully completed this study, 13 older and 13 young women. Time since last menstrual period in the older women was 8.0±3.9 years (n=12). Exact data were unavailable from one older participant, however she was able to verify last menstrual period was >1 year. Estradiol was 5.2 pg/mL in this individual, supporting postmenopausal status. There were no significant group differences between height, weight, and BMI (Table 1). Baseline resting blood pressure did not differ between age groups. Max workload was significantly lower in the older women (p=0.027). Percent predicted (p=0.269) and aerobic fitness (, p=0.055) were not different between age groups (p=0.269). Body fat percentage did not differ by age group (p=0.408). As we have previously reported1, 2, a larger dose of trimethaphan was required to achieve ganglionic blockade in younger women as compared to older women (2.2±0.2 vs 4.3±0.3mg/min, p=0.039). Even with twice the dose of trimethaphan, the younger women still had a smaller change in mean arterial pressure than the older women during ganglionic blockade (−11±7 vs. −29±7mmHg)2.
Table 1.
Participant Characteristics
| Variable | Young | Older | ||
|---|---|---|---|---|
| n | 13 | 13 | ||
| Age (years) | 26±1 | 58±1* | ||
| Weight (kg) | 64±3 | 61±1 | ||
| Height (cm) | 167±2 | 163±2 | ||
| BMI (kg/m2) | 23±1 | 23±1 | ||
| SBP (mmHg) | 117±3 | 121±4 | ||
| DBP (mmHg) | 68±2 | 71±3 | ||
| MAP (mmHg) | 84±2 | 88±3 | ||
| HR (bpm) | 71±4 | 67±3 | ||
| (mL/kg/min) | 35±2 | 29±2 | ||
| Max workload (W) | 196±10 | 158±13* | ||
| Max Heart Rate (bpm) | 182±12 | 164±14* | ||
| % Predicted (%) | 114±6 | 104±7 | ||
| Body Fat (%) | 28±2 | 31±2 |
p<0.05 vs. Young;
BMI= Body Mass index; MAP= Mean Arterial Pressure; Mean±SE
Role of aerobic fitness in sympathetic support of blood pressure
Autonomic support of blood pressure, as determined by the change in MAP with ganglionic blockade, was directly related to in young (r=0.761, p=0.003), but not older (r=−0.106, p=0.73) women (Figure 1, panel A). Change in total peripheral resistance was related to in young (r=−0.688, p=0.009), but not older (r=−0.408, p=0.167; Figure 1, panel C) women. Baseline MSNA burst incidence was significantly related to in young (r=.820, p=0.001), but not older (r=−.156, p=0.611; Figure 1, panel B) women. Norepinephrine however, was not significantly related to in young (r=−0.438, p=0.134) or older women (r=−0.300, p=0.319).
Figure 1.
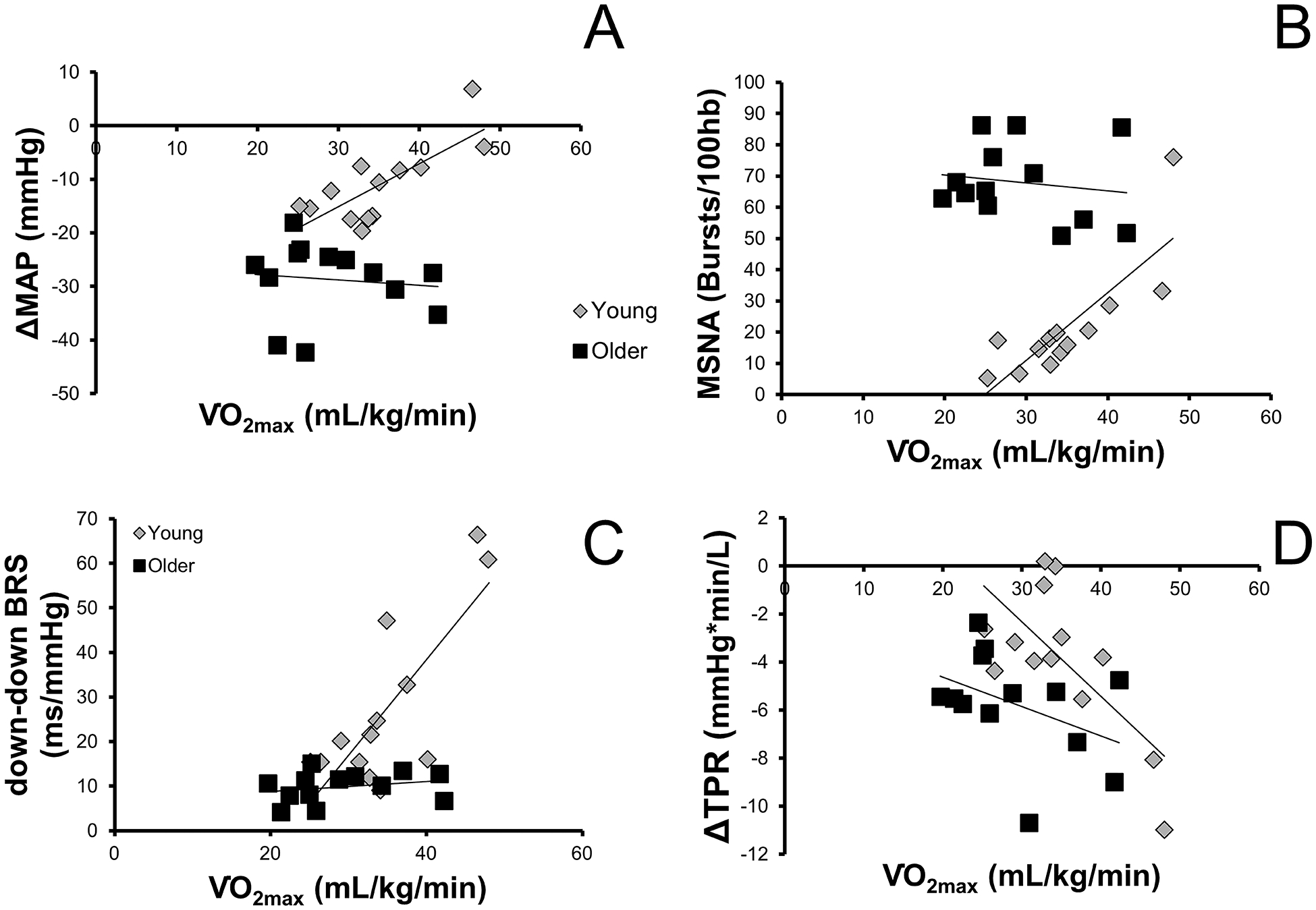
Depicts the relationship between aerobic fitness () and A) the change in mean arterial blood pressure (MAP) during ganglionic blockade B) muscle sympathetic nerve activity (MSNA), C) cardiac baroreflex sensitivity to falling blood pressures (down-down BRS), and D) the change in total peripheral resistance (TPR) during ganglionic blockade in young and older women.
Effects of aerobic fitness and age on parasympathetic function
Measures of heart rate variability were significantly associated with in young, but not older women (RMSSD: r=0.713, p=0.006 vs. r=−0.172, p=0.575; RRIHF: r=0.675, p=0.011 vs. r=0.275, p=0.197; young vs. old, respectively). The change in heart rate with ganglionic blockade tended to be related to in young (r=0.521, p=0.068), but not older (r=0.300, p=0.319) women. Finally, spontaneous cardiac baroreflex sensitivity (down-down sequences) followed the same pattern where there was a relationship between down-down cardiac baroreflex sensitivity and aerobic fitness in young (r=0.778, p=0.002), but not older (r=0.248, p=0.414) women. Furthermore, there was a significant relationship between cardiac output and in young women (r=0.634, p=0.02) where women with higher aerobic fitness were able to increase cardiac output in response to ganglionic blockade. There was little change in cardiac output in women with lower aerobic fitness (Figure 2 panel C).
Figure 2.
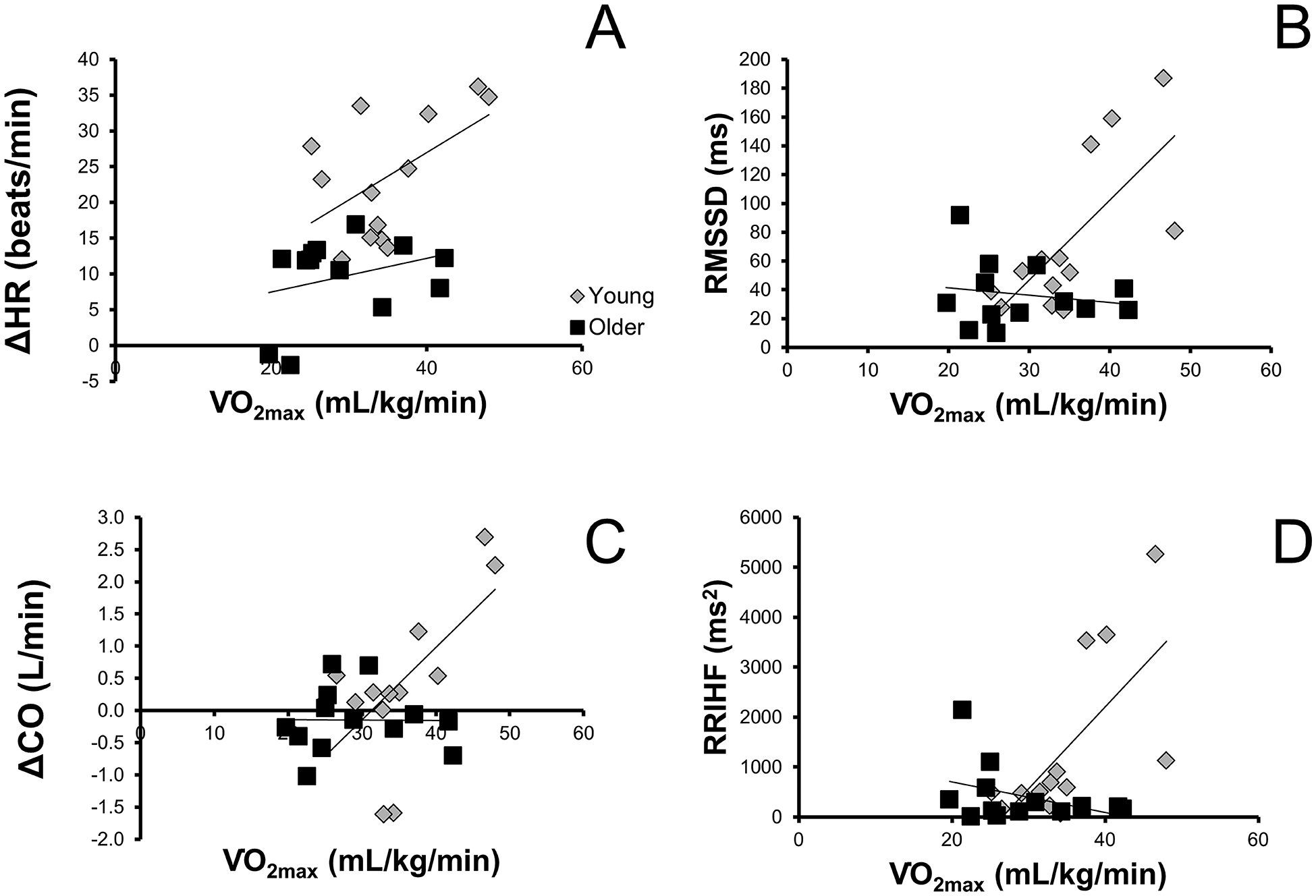
Depicts the relationship between aerobic fitness () and A) the change in heart rate (HR) during ganglionic blockade, B) root mean square of the successive differences (RMSSD) in heart rate (time domain assessment of heart rate variability), C) the change in cardiac output during ganglionic blockade, and D) high frequency power in the R-R interval (RRIHF) in young and older women.
Discussion
Main findings from the present study are two-fold. First, we observed a significant relationship between aerobic fitness and the decrease in blood pressure with ganglionic blockade in young, but not older women. Second, we observed a significant relationship between aerobic fitness and the rise in heart rate with ganglionic blockade in young, but not older women. Together, these data suggest greater parasympathetic tone in young, fit women contributes to a smaller decrease in blood pressure during ganglionic blockade. In contrast, older women (regardless of fitness level) have low parasympathetic tone and thus are unable to elicit a significant rise in heart rate. As a result, blood pressure decreases more significantly in older women, compared to younger women.
MSNA: Aging, Fitness and Blood Pressure
We hypothesized that aerobic fitness would be related to a decline in blood pressure during ganglionic blockade, such that women with greater aerobic fitness would have a smaller change in blood pressure. We only found a relationship between aerobic fitness and the fall in blood pressure with ganglionic blockade in young women. One possible contributor to this finding could be MSNA. Our data suggest that aerobic fitness influences baseline MSNA in younger but not older women. Other work has reported no differences in sympathetic function with aerobic fitness; however most of this work has been done primarily in young men23–25. Our observations also differ from work suggesting endurance training increases sympathetic activity in older women26. The combined group difference (males and females) between endurance trained runners compared to sedentary individuals in Ng et al was driven by a doubling of MSNA in the female athletes relative to the sedentary women in the study26. One possible explanation of these discrepancies is that our participants were less fit than the women in this paper. This is evidenced by lower in our more aerobically fit older participants (n=7) as compared to the Ng group ( = 34 vs. 39 mL/kg/min). Furthermore, exercise training studies7, 8 demonstrate a reduction in MSNA with improved aerobic fitness. However, cross sectional data suggests that individuals who are aerobically fit likely have greater MSNA than unfit individuals4, 26, 27. The training studies in this area are generally in already at risk populations with heightened MSNA. With exercise training, these at risk populations are able to lower their MSNA, whereas MSNA is increased when taking a cross sectional look at healthy populations comparing aerobically trained individuals to non-aerobically trained individuals25. Similarly, total plasma norepinephrine spill over rates are increased in endurance trained middle-aged and older individuals as compared to sedentary individuals in cross sectional and training studies5, 6. Our data support the pool of knowledge suggesting that aerobic fitness increases MSNA and influences sympathetic control of blood pressure, at least in younger women.
Heart Rate: Aging, Fitness and Blood Pressure
Aerobic fitness increases cardiac vagal modulation of heart rate in healthy individuals28. We found that the change in heart rate and measures of heart rate variability were related to aerobic fitness in our younger, but not older female participants. Our data diverge from the results of previous studies and conflict with the assertion that exercise training increases parasympathetic tone in older women9. However a meta-analysis demonstrated an attenuated training response in high-frequency heart rate variability in older participants, which may help explain discrepancies between our data and others29. Furthermore, our participants were active and met physical activity guidelines, but were not actively training for endurance events. The training dose may have been insufficient to increase vagal tone in our older women. In young men, the change in heart rate with trimethaphan was not different between sedentary and exercise trained individuals [9]. Our data in younger women showed that the change in cardiac output during ganglionic blockade was related to aerobic fitness. Increased cardiac output in young women was primarily driven by elevated heart rate, while older women demonstrated little change in cardiac output. Previous work in younger men showed no influence of aerobic fitness on the decrease in blood pressure during autonomic blockade10. It is well established that blood pressure control differs with age and between men and women12, 13, 30. As such, it was not surprising that our findings were divergent from previous work in young men.
Role of Body Composition
Body composition is known to have an effect on autonomic function and should be considered in the context of fitness. and body fat percentage were closely related in this study (r=−0.823, p<0.001). As such, it is difficult to distinguish between fitness and body composition in our data. Most of the hemodynamic indices had a tighter relationship to fitness than body fat percentage, although this was not consistent for all variables. The relationships between hemodynamic indices and fitness or body fat percentage appeared similar in magnitude and in opposite directions. Data in young men suggest that there are effects of fitness on sympathetic neural regulation outside of differences in adiposity27. Recent meta-analysis suggests that unfit individuals have twice the risk of all-cause mortality as compared to fit individuals, regardless of whether they are overweight or obese31.
Role of Female Sex Hormones
Importantly, several studies have suggested that aerobic fitness (relative and absolute ) is minimally affected by menstrual cycle phase32–34. MSNA, however, fluctuates across the menstrual cycle where it is greatest in the low-hormone early follicular phase and highest in the high-hormone midluteal phase of the menstrual cycle35, 36. These changes are thought to be related to changes in circulating hormone levels, particularly estrogen36. Sympathetic baroreflex sensitivity is greater during the midluteal phase than the early follicular phase of the menstrual cycle35. Finally, vascular transduction does not seem to change across the menstrual cycle35. We studied women in the early follicular phase of the menstrual cycle, when estrogen is low. Given MSNA can fluctuate during the cycle, if women were studied in a later phase, this may reduce the slope of the relationship between MSNA and in our data. However, the changes are generally small (~3 bursts/min increase in MSNA from early follicular to midluteal phase of the menstrual cycle)36 and as such any effect on the slope of the relationship would likely be small.
About half of the young women in this study were taking oral contraceptives. The data from the naturally cycling young women was not qualitatively different from the women on oral contraceptives (ΔMAP with trimethaphan= −12±3 vs −9±2 mmHg; ΔHR with trimethaphan = 22±3 vs 26±4 bpm oral contraceptives vs. naturally cycling). Oral contraceptives do not influence MSNA during the active or placebo pill phases, however resting blood pressure is higher in oral contraceptive users (~5 mmHg)37. Whether autonomic mechanisms are involved in this increase in blood pressure is unclear. Exogenous estrogen can increase vascular transduction whereas exogenous progesterone can reduce vascular transduction38. β-adrenergic mediated vasodilation can also be augmented by oral contraceptives39. There is conflicting data as to whether baroreflex sensitivity is impacted by oral contraceptive use40, 41. The data that shows a difference suggests that the differences in baroreflex function are smallest during the placebo phase of oral contraceptive use40. As such, since the differences were quite small between naturally cycling young women and oral contraceptive users and our population of young women generally reflects the North American population where many young women report a history of oral contraceptive use42, we feel that our use of pooled data from young women who are naturally cycling and on oral contraceptives is justified.
Older women have low endogenous estrogen. If hormones are added back in (n=2 on transdermal estrogen), it could lead to reduced MSNA or reduced fall in blood pressure with ganglionic blockade, leading us to potentially underestimate the strength of these relationships with . We chose to keep these participants in as their data were remarkable similar to the other older women and removing these participants from the analysis did not change our conclusions.
Limitations
This study had several limitations. First, blood volume was not assessed. Differences in blood volume likely exist between higher and lower aerobic fitness groups and may contribute to the cardiac output differences seen between fit and unfit participants. However, the blood volume of postmenopausal women increases with training, and blood volume is related to 43. Second, the vasopressin response (or more accurately, copeptin) to the trimethaphan infusion is unknown, and is likely different between age groups due to the magnitude of blood pressure changes seen in these groups. Furthermore, no blood samples were collected during ganglionic blockade that could have provided ideas of compensatory mechanisms (e.g. vasopressin, copeptin, and/or angiotensin II). Finally, we used a cross-sectional approach to assess differences in sympathetic and parasympathetic support in blood pressure by fitness. Aerobic fitness did not improve parasympathetic tone in the older women in our study, though there may be some selection bias with our relatively healthy older population. Despite this being a very healthy older group, we still had a wide range of aerobic fitness within the older group ( range: 19.7 – 42.3 mL/kg/min). We would presume that a more typical older group may start with higher blood pressure and have even less heart rate reserve than our participants that could result in a larger reduction in blood pressure with ganglionic blockade.
Perspectives
In summary, our data suggest that aerobic fitness influences autonomic support of blood pressure in younger, but not older women. The change in mean arterial pressure during ganglionic blockade is related to aerobic fitness in young women. This change is largely due to an increased heart rate response to ganglionic blockade. Conversely, the heart rates of older women do not significantly change during ganglionic blockade, regardless of fitness level. This results in a greater decline in blood pressure. Taken together, enhanced aerobic fitness contributes to greater vagal tone in younger women, but not older women. Elevated vagal tone attenuates sympathetic nervous system response in cardiovascular regulation. These data have important implications for the effect of aerobic fitness on blood pressure regulation.
Table 2.
Pearson correlation coefficients describing the relationship between autonomic and hemodynamic indices with or body fat percentage. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; HR, heart rate; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance; MSNA, muscle sympathetic nerve activity; RMSSD, root mean square of the successive differences in heart rate (time domain assessment of heart rate variability); pNN50, percentage of successive normal sinus RR intervals >50 ms (time domain assessment of heart rate variability). Bolded values are p<0.05.
| Variable | Relationship with | Relationship with Body Fat Percentage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Participants | Young Women | Older Women | All Participants | Young Women | Older Women | ||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | ||||||
| ΔSBP | .397 | .045 | .659 | .014 | −.186 | .543 | −.343 | .118 | −.566 | .055 | .133 | .714 | |||||
| ΔDBP | .555 | .003 | .807 | .001 | .068 | .824 | −.533 |
.011 |
−.757 | .004 | −.331 | .350 | |||||
| ΔMAP | .478 | .014 | .761 | .003 | −.106 | .730 | −.432 | .045 | −.668 | .018 | −.070 | .848 | |||||
| ΔHR | .540 | .004 | .521 | .068 | .300 | .319 | −.485 | 0.022 | −.537 | .072 | −.466 | .175 | |||||
| ΔSV | −.169 | .410 | .231 | .448 | −.267 | .379 | .133 | .557 | .046 | .887 | .203 | .573 | |||||
| ΔCO | .459 | .018 | .634 | .020 | −.007 | .982 | −.475 | .025 | −.498 | .099 | −.231 | .521 | |||||
| ΔTPR | −.348 | .081 | −.688 | .009 | −.409 | .165 | .334 | .129 | .526 | .079 | .192 | .595 | |||||
| MSNA Burst Frequency | −.292 | .147 | .773 | .002 | −.375 | .207 | .083 | .713 | −.575 | .050 | .537 | .109 | |||||
| MSNA Burst Incidence | −.125 | .542 | .820 | .001 | −.156 | .611 | −.099 | .660 | −.687 | .014 | .285 | .425 | |||||
| RMSSD | .492 | .011 | .713 | .006 | −.172 | .575 | −.462 | .030 | −.582 | .047 | .246 | .493 | |||||
| pNN50 | .519 | .007 | .631 | .021 | −.015 | .960 | −.448 | .037 | −.520 | .083 | .025 | .944 | |||||
| Down-down Baroreflex Sensitivity | .633 | .001 | .778 | .002 | .248 | .414 | −.575 | .005 | −.649 | .022 | −.350 | .322 | |||||
Novelty and Significance.
- What Is New?- with a few bullet points highlighting the novelty;
- The role of aerobic fitness on autonomic support of blood pressure has not been previously explored in women.
- What Is Relevant?” - with a few bullet points indicating how the study relates to hypertension; and
- Aerobic fitness clearly improves blood pressure; however the mechanisms behind these improvements are not always clear.
- Summary - of the conclusions of the study.
- Aerobic fitness influences autonomic support of blood pressure in young women, but not older women.
- Parasympathetic tone was related to aerobic fitness in young, but not older women and played an important role in buffering blood pressure changes during ganglionic blockade in young women.
Acknowledgements
Many thanks to the participants, the Human Integrative Physiology Laboratory and the Clinical Research and Trials Unit at Mayo Clinic.
Sources of Funding
Funding: HL083947 (MJJ), HL131151 (SEB), T32 DK 7352-37 (SEB), HL118154 (JNB); NIH RR024150 (CCaTS), U54 AG44170-09 (CCaTS).
Footnotes
Disclosures
None.
References
- 1.Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N and Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker SE, Limberg JK, Dillon GA, Curry TB, Joyner MJ and Nicholson WT. Aging Alters the Relative Contributions of the Sympathetic and Parasympathetic Nervous System to Blood Pressure Control in Women. Hypertension. 2018;72:1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PP, Christou DD, Jordan J and Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–4. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S and Tanaka H. Systemic alpha-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol. 2007;293:H1466–72. [DOI] [PubMed] [Google Scholar]
- 5.Poehlman ET and Danforth E, Jr. Endurance training increases metabolic rate and norepinephrine appearance rate in older individuals. Am J Physiol. 1991;261:E233–9. [DOI] [PubMed] [Google Scholar]
- 6.Poehlman ET, McAuliffe T and Danforth E, Jr. Effects of age and level of physical activity on plasma norepinephrine kinetics. Am J Physiol. 1990;258:E256–62. [DOI] [PubMed] [Google Scholar]
- 7.Carter JR and Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci. 2015;188:36–43. [DOI] [PubMed] [Google Scholar]
- 8.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR and Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–6. [DOI] [PubMed] [Google Scholar]
- 9.Davy KP, Miniclier NL, Taylor JA, Stevenson ET and Seals DR. Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? Am J Physiol. 1996;271:H455–60. [DOI] [PubMed] [Google Scholar]
- 10.Jones PP, Shapiro LF, Keisling GA, Quaife RA and Seals DR. Is autonomic support of arterial blood pressure related to habitual exercise status in healthy men? J Physiol. 2002;540:701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D and Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–8. [DOI] [PubMed] [Google Scholar]
- 12.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH and Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart EC, Joyner MJ, Wallin BG and Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawton BA, Rose SB, Raina Elley C, Dowell AC, Fenton A and Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. Br J Sports Med. 2009;43:120–3. [PubMed] [Google Scholar]
- 15.Uritani D, Matsumoto D, Asano Y, Yoshizaki K, Nishida Y and Shima M. Effects of regular exercise and nutritional guidance on body composition, blood pressure, muscle strength and health-related quality of life in community-dwelling Japanese women. Obes Res Clin Pract. 2013;7:e155–e163. [DOI] [PubMed] [Google Scholar]
- 16.Halliwill JR, Buck TM, Lacewell AN and Romero SA. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol. 2013;98:7–18. [DOI] [PubMed] [Google Scholar]
- 17.Isea JE, Piepoli M, Adamopoulos S, Pannarale G, Sleight P and Coats AJ. Time course of haemodynamic changes after maximal exercise. Eur J Clin Invest. 1994;24:824–9. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer F and Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017;5:258–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer F, McCraty R and Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 21.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A and Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254:H377–83. [DOI] [PubMed] [Google Scholar]
- 22.Blaber AP, Yamamoto Y and Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol. 1995;268:H1682–7. [DOI] [PubMed] [Google Scholar]
- 23.Seals DR. Sympathetic neural adjustments to stress in physically trained and untrained humans. Hypertension. 1991;17:36–43. [DOI] [PubMed] [Google Scholar]
- 24.Svedenhag J, Wallin BG, Sundlof G and Henriksson J. Skeletal muscle sympathetic activity at rest in trained and untrained subjects. Acta Physiol Scand. 1984;120:499–504. [DOI] [PubMed] [Google Scholar]
- 25.Ray CA and Carter JR. Effects of aerobic exercise training on sympathetic and renal responses to mental stress in humans. Am J Physiol Heart Circ Physiol. 2010;298:H229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng AV, Callister R, Johnson DG and Seals DR. Endurance exercise training is associated with elevated basal sympathetic nerve activity in healthy older humans. J Appl Physiol. 1994;77:1366–74. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez GE, Halliwill JR, Ballard TP, Beske SD and Davy KP. Sympathetic neural regulation in endurance-trained humans: fitness vs. fatness. Journal of applied physiology (Bethesda, Md : 1985). 2005;98:498–502. [DOI] [PubMed] [Google Scholar]
- 28.Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Slavov I, Fang Y and Myers MM. The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health. 2009;99:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandercock GR, Bromley PD and Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–9. [DOI] [PubMed] [Google Scholar]
- 30.Briant LJ, Burchell AE, Ratcliffe LE, Charkoudian N, Nightingale AK, Paton JF, Joyner MJ and Hart EC. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol. 2016;594:4753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry VW, Baruth M, Beets MW, Durstine JL, Liu J and Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56:382–90. [DOI] [PubMed] [Google Scholar]
- 32.Gordon D, Scruton A, Barnes R, Baker J, Prado L and Merzbach V. The effects of menstrual cycle phase on the incidence of plateau at V O2max and associated cardiorespiratory dynamics. Clin Physiol Funct Imaging. 2018;38:689–698. [DOI] [PubMed] [Google Scholar]
- 33.Lebrun CM, McKenzie DC, Prior JC and Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27:437–44. [PubMed] [Google Scholar]
- 34.De Souza MJ, Maguire MS, Rubin KR and Maresh CM. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc. 1990;22:575–80. [DOI] [PubMed] [Google Scholar]
- 35.Minson CT, Halliwill JR, Young TM and Joyner MJ. Influence of the Menstrual Cycle on Sympathetic Activity, Baroreflex Sensitivity, and Vascular Transduction in Young Women. Circulation. 2000;101:862–868. [DOI] [PubMed] [Google Scholar]
- 36.Carter JR, Fu Q, Minson CT and Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey RE, Hart EC, Charkoudian N, Curry TB, Carter JR, Fu Q, Minson CT, Joyner MJ and Barnes JN. Oral Contraceptive Use, Muscle Sympathetic Nerve Activity, and Systemic Hemodynamics in Young Women. Hypertension. 2015;66:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weitz G, Elam M, Born J, Fehm HL and Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab. 2001;86:344–8. [DOI] [PubMed] [Google Scholar]
- 39.Limberg JK, Peltonen GL, Johansson RE, Harrell JW, Kellawan JM, Eldridge MW, Sebranek JJ, Walker BJ and Schrage WG. Greater Beta-Adrenergic Receptor Mediated Vasodilation in Women Using Oral Contraceptives. Front Physiol. 2016;7:215–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilczak A, Marciniak K, Klapcinski M, Rydlewska A, Danel D and Jankowska EA. Relations between combined oral contraceptive therapy and indices of autonomic balance (baroreflex sensitivity and heart rate variability) in young healthy women. Ginekol Pol. 2013;84:915–21. [DOI] [PubMed] [Google Scholar]
- 41.Brunt VE, Miner JA, Kaplan PF, Halliwill JR, Strycker LA and Minson CT. Short-term administration of progesterone and estradiol independently alter carotid-vasomotor, but not carotid-cardiac, baroreflex function in young women. Am J Physiol Heart Circ Physiol. 2013;305:H1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boldo A and White WB. Blood pressure effects of the oral contraceptive and postmenopausal hormone therapies. Endocrinol Metab Clin North Am. 2011;40:419–32, ix. [DOI] [PubMed] [Google Scholar]
- 43.Parker Jones P, Davy KP, Desouza CA and Tanaka H. Total blood volume in endurance-trained postmenopausal females: relation to exercise mode and maximal aerobic capacity. Acta Physiol Scand. 1999;166:327–33. [DOI] [PubMed] [Google Scholar]


