Abstract
Mucosal-associated invariant T (MAIT) cells participate in both protective immunity and pathogenesis of diseases. Most murine MAIT cells express an invariant TCRVα19-Jα33 (iVα19) TCR, which triggers signals crucial for their development. However, signal pathways downstream of the iVα19TCR and their regulation in MAIT cells are unknown. Diacylglycerol (DAG) is a critical second messenger that relays the TCR signal to multiple downstream signal cascades. DAG is terminated by DAG kinase (DGK)-mediated phosphorylation and conversion to phosphatidic acid. We have demonstrated here that downregulation of DAG caused by enhanced DGK activity impairs late stage MAIT cell maturation in both thymus and spleen. Moreover, deficiency of DGKζ but not DGKα by itself causes modest decreases in MAIT cells, and deficiency of both DGKα and ζ results in severe reductions of MAIT cells in an autonomous manner. Our studies have revealed that DAG signaling is not only critical but also must be tightly regulated by DGKs for MAIT cell development and that both DGKα and, more prominently, DGKζ contribute to the overall DGK activity for MAIT cell development.
Keywords: Mucosal-associated invariant T cells, MAIT cells, Diacylglycerol kinases, DGKα, DGKζ, Signal transduction
INTRODUCTION
Mucosal-associated invariant T (MAIT) cells are innate like T cells that participate in both protective immunity and disease pathogenesis. Most MAIT cells express an invariant TCRα chain with a restricted TCR repertoire. MAIT cells express the iVα19-Jα33 (iVα19) TCR in mice and the iVα7.2-Jα33 TCR in humans [1, 2]. Unlike conventional αβT (cαβT) cells, MAIT cells do not respond to peptides presented by classic MHC molecules but instead recognize microbe-derived riboflavin (vitamin B2) metabolites presented by the MHC-I-related molecule MR1 [3, 4]. MAIT cells mature and differentiate into effector lineages in the thymus and can rapidly respond to agonists and produce a variety of cytokines and other effector molecules that shape both innate and adaptive immunity [5–8]. Recent evidence has revealed that MAIT cells are not limited to mucosal tissues but also exist in peripheral lymphoid organs such as the spleen, lymph nodes (LNs), and liver, suggesting broad roles for these cells in immune responses. Abnormal MAIT cell numbers and/or functions are associated with many diseases such as multiple sclerosis, cancer, chronic infections, and autoimmune diseases [9–11].
In humans, MAIT cells are abundant, accounting for up to 30% of T cells depending on the organ [8, 12]. In mice, MAIT cells are rare and account for a very small portion of the overall T cell population. Very little is known about the mechanisms that regulate MAIT cell development and function because of an historical inability to clearly detect them in mice. Recently, MR1 tetramers for specific detection of MAIT cells in both mice and humans have been developed [5, 13]. In mice, MAIT cell development in the thymus takes place in three stages: CD24+CD44− stage 1, CD24−CD44− stage 2, and CD24−CD44+ stage 3 [14]. An absence of MAIT cells in MR1-deficient mice and an increase in MAIT cells in iVα19TCR transgenic mice suggest that the TCR signal is crucial for MAIT cell development [4, 6, 15, 16]. However, the signal pathways downstream of the iVα19TCR and the regulation of these pathways important for MAIT cell development remain unknown.
Following TCR engagement, diacylglycerol (DAG) is produced through the activation of phospholipase C γ1. DAG recruits downstream effector molecules to the membrane and allosterically activates effectors such as PKCθ, RasGRP1, PKDs, Munc13s, and chimaerins in T cells [17, 18]. PKCθ leads to TCR-mediated NF-κB and mTORC1 activation [19, 20], which affects key processes, including T cell activation and survival, TH2 and TH17, and iNKT cell and Treg development [21–24]. RasGRP1 activates the Ras-RAF1-MEK1/2-ERK1/2 pathway, leading to mTORC1, mTORC2, and PI3K as well as AP1 activation [25–27]. RasGRP1 plays essential roles in cαβT cell development, early iNKT cell development, IL-17 expressing γδT17 differentiation, and γδT cell activation [26, 28–31]. However, whether the DAG-mediated signal also participates in MAIT cell development is unknown.
The diverse and important functions of DAG-mediated signaling suggest that its levels require tight control. In mammals, ten DGK isoforms encoded by distinct genes catalyze the phosphorylation of DAG to produce phosphatidic acid (PA). DGKα and DGKζ, along with DGKδ, are the major isoforms expressed in T cells [32–35]. Both DGKα and ζ regulate multiple signaling pathways such as the RasGRP1-Ras-Erk1/2 pathway, the PKCθ-IKK-NFκB pathway, and mTOR signaling [27, 32, 36–38]. DGKα and/or ζ control T cell development, activation and anergy, survival, effector function, antimicrobial and antitumor immunity, and iNKT cell development, as well as Treg generation [33, 34, 37–47].
In this report, we demonstrate that expression of a gain of mutant DGKζ in thymocytes causes severe decreases of terminally differentiated MAIT cells in mice, suggesting that DAG plays a critical role in MAIT cell development. Using mice deficient in either DGKα, ζ, or both, we have further shown that DGKζ but not DGKα deficiency causes modest decreases in MAIT cells in mice and that DGKα and ζ double deficiency greatly decreases MAIT cells via cell autonomous mechanisms. Our data suggest that DAG signaling is not only essential but also must be tightly controlled by DGKs for proper MAIT cell development and that DGKα and ζ function synergistically or redundantly to promote MAIT cell development, with DGKζ playing a more prominent role than DGKα.
RESULTS
Enhanced DGK activity inhibits MAIT cell development
Following TCR engagement, PLCγ1 is activated and hydrolyzes phosphor inositol-4,5-bis-phosphates to produce DAG and IP3; both are important second messengers in T cells. To investigate whether DAG-mediated signals play a role in MAIT cell development, we analyzed mice carrying either a wild-type (DDKζWT), nuclear localization mutant (DGKζΔNLS), or kinase dead (DGKζKD) mutant DGKζ transgene that had been knocked into the Rosa26 locus. We fused DGKζ transgenes to the carboxyl-terminus of EGFP, which allowed us to examine their expression by fluorescence. The transgenes can be induced to express starting at CD4+CD8+ double positive (DP) thymocytes after CD4Cre-mediated deletion of a floxed transcription STOP cassette located between the promoter and the GFP-Dgkz transgene (Figure 1A). GFP levels were further upregulated in CD4+ SP and CD8+ SP thymocytes. Interestingly, MAIT cells expressed higher levels of GFP-DGKζ compared with CD4+ SP and CD8+ SP thymocytes. Within MAIT cells, stage 1 expressed lower levels of the transgene than stages 2 and 3 (Figure 1B). In a separate study, we have found that DGKζΔNLS is a gain-of-function mutation with enhanced ability to inhibit TCR-induced DAG-mediated signaling in comparison with DGKζWT (manuscript submitted).
Figure 1. Severe decreases of thymic MAIT cells in DgkzΔNLS-Cd4Cre mice.
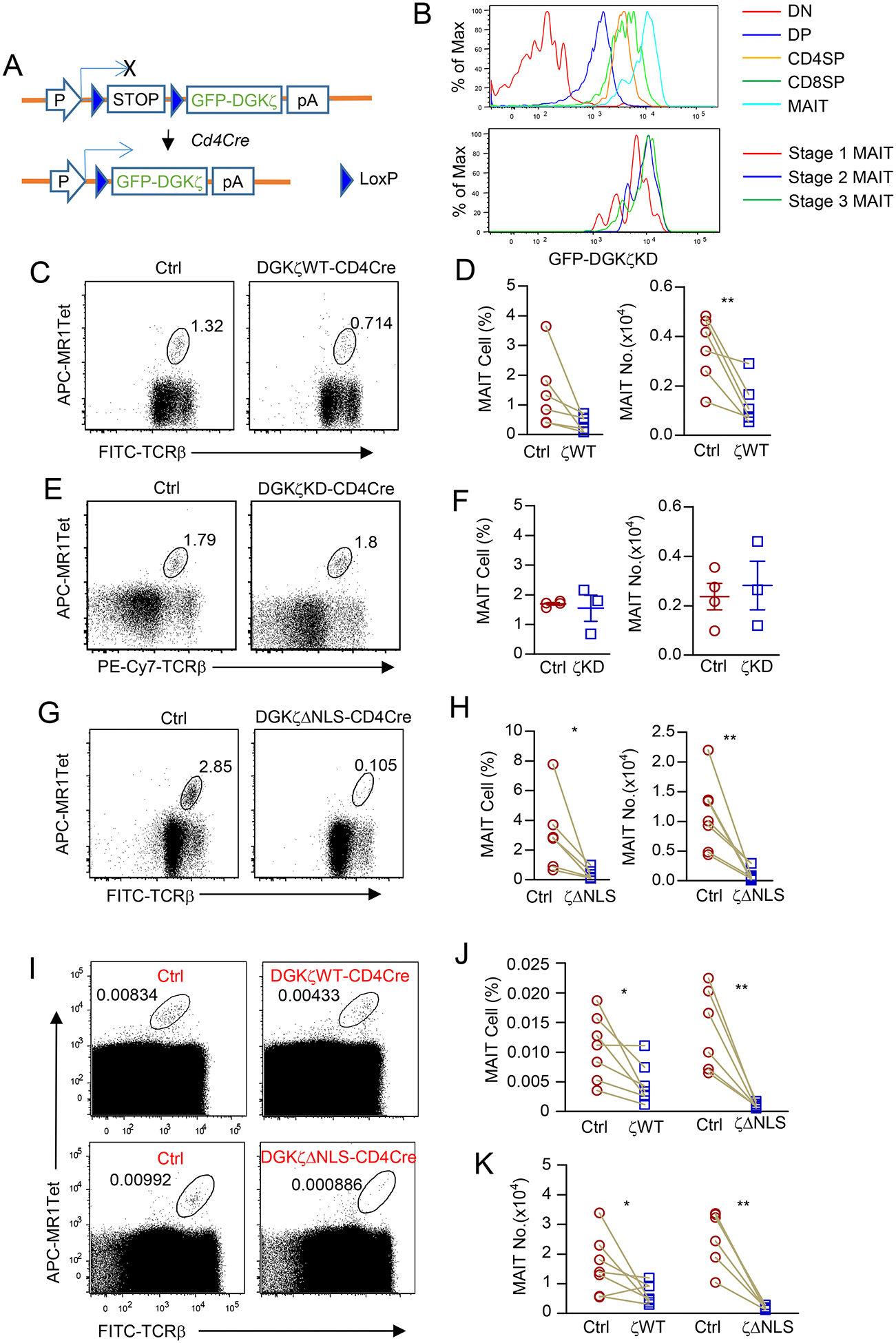
A. Schematic structure of GFP-Dgkz knock-in (DgkzKI) mice. B. GFP-DGKζKD levels in CD4−CD8− double negative (DN), DP, CD4 SP, and CD8 SP thymocytes and thymic MAIT cells (top panel). GFP-DGKζKD levels in stage 1 – 3 thymic MAIT cells (bottom panel). Data shown are representative of three experiments. C-K. Assessment of MAIT cells in GFP-DGKζKI-CD4Cre and control mice. Thymocytes from 8–10-week-old mice were either enriched for MAIT cells with 5-OP-RU loaded MR1-Tet and then stained with anti-TCRβ and lineage antibodies (C-H) or directly stained with MR1-Tet and antibodies without enrichment (I-K). C, D. DGKζWT-CD4Cre and control mice. Dot plots showing TCRβ and MR1-Tet straining in live gated Lin− cells (C). Scatter plots represent mean ± SEM of MAIT cell percentages and numbers (D). Data shown are representative of (C) and pooled from (D) six experiments. N = 6 for both control and DGKζWT-CD4Cre mice. E, F. DGKζKD-CD4Cre and control mice. Dot plots showing TCRβ and MR1-Tet straining in live gated Lin− cells (E). Scatter plots represent mean ± SEM of MAIT cell percentages and numbers (F). Data shown are representative of (E) and pooled from (F) three experiments. N = 4 for WT and N = 3 for DGKζKD-CD4Cre mice. G, H. DGKζΔNLS-CD4Cre and control mice. Dot plots showing TCRβ and MR1-Tet straining in live gated Lin− cells (G). Scatter plots represent mean ± SEM of MAIT cell percentages and numbers (H). Data shown are representative of (G) and pooled from (H) seven experiments. N = 7 for both control and DGKζΔNLS-CD4Cre mice. I. Dot plots show TCRβ and MR1-Tet staining in live gated Lin− cells from DGKζKI-CD4Cre and control mice without pre-enrichment of MAIT cells. Data shown are representative of seven (top panels) and six (low panels) experiments. J, K. MAIT cell percentages (J) and numbers (K) in DgkzKI-Cd4Cre and control mice without pre-enrichment of MAIT cells. Data shown are pooled from seven (J) and six (K) experiments. Each circle or square represents one mouse of the indicated genotypes. Each connection line represents one pair of sex and age matched test and control mice examined in one experiment. *, p<0.05; **, p<0.01 determined by pairwise Student t-test except F. F was analyzed by unpaired Student t-test.
Because of the extremely low percentages of thymic MAIT cells in mice, we examined these cells both before and after enrichment with 5-OP-RU loaded MR1-tetramers (MR1-Tet) from total thymocytes. In addition to MR1-Tet and anti-TCRβ, CD24, and CD44 antibodies, we included LIVE/DEAD® Fixable Dead Cell Stain and anti-CD11b, Gr1, B220, CD11c, Ter119, F4/80, and TCRγδ antibodies to dump dead cells and non-αβT cells lineages (Lin). Due to their scarcity, MAIT cell numbers are also influenced by age and environmental factors. We performed many experiments in a way that individual experiment examined a pair of age- and sex-matched test and control mice with most pairs being littermates and housed in the same cage. Each pair of mice in individual experiment was marked by a connecting line between test and control mice. The gating of Lin−MR1-Tet+TCRβ+ cells as MAIT cells was validated using TCRJα18−/− mice, which lack both iNKT cells and MAIT cells [48].
Compared with littermate controls (GFP-Dgkzwt or Cd4Cre), GFP-Dgkzwt-Cd4Cre (ζWT) thymus showed 60% decreases in Lin−TCRβ+MR1-Tet+ MAIT cells (Figures 1C,1D). We did not observe such decreases in GFP-Dgkzkd-Cd4Cre (ζKD) mice (Figure 1E,1F), which indicated that DGK kinase activity was responsible for the decreases in thymic MAIT cells in GFP-Dgkzwt-Cd4Cre mice. MAIT cells in GFP-DgkzΔNLS-Cd4Cre (ζΔNLS) mice were further reduced to 10% of those in WT mice (Figure 1G,1H). We confirmed such graded decreases of MAIT cell percentages and numbers in GFP-Dgkzwt-Cd4Cre and GFP-DgkzΔNLS-Cd4Cre mice by directly staining thymocytes without pre-enriching MAIT cells (Figure 1I–1K). Together, these results revealed that increased DGKζ activity inhibits MAIT cell generation, suggesting that DAG-mediated signaling plays a critical role during MAIT cell development.
Enhanced DGK activity causes reduced MAIT cells in the peripheral organs
MAIT cells are localized in both mucosal tissues and peripheral lymphoid organs [14]. We could detect very low percentages of MAIT cells in the spleen and peripheral lymph nodes (pLNs) but relatively high percentages in the lung and liver in WT mice. In all these organs, MAIT cell percentages and numbers were slightly or moderately decreased in GFP-Dgkzwt-Cd4Cre (Figures 2A, 2C, 2D) and greatly decreased in GFP-DgkzΔNLS-Cd4Cre mice (Figures 2B–2D) in comparison with control mice. Thus, enhanced DGKζ function causes severe decreases of MAIT cells in peripheral organs at least because generation of these cells in the thymus is impaired.
Figure 2. Severe reduction of MAIT cells in the peripheral organs in DGKζΔNLS-CD4Cre mice.
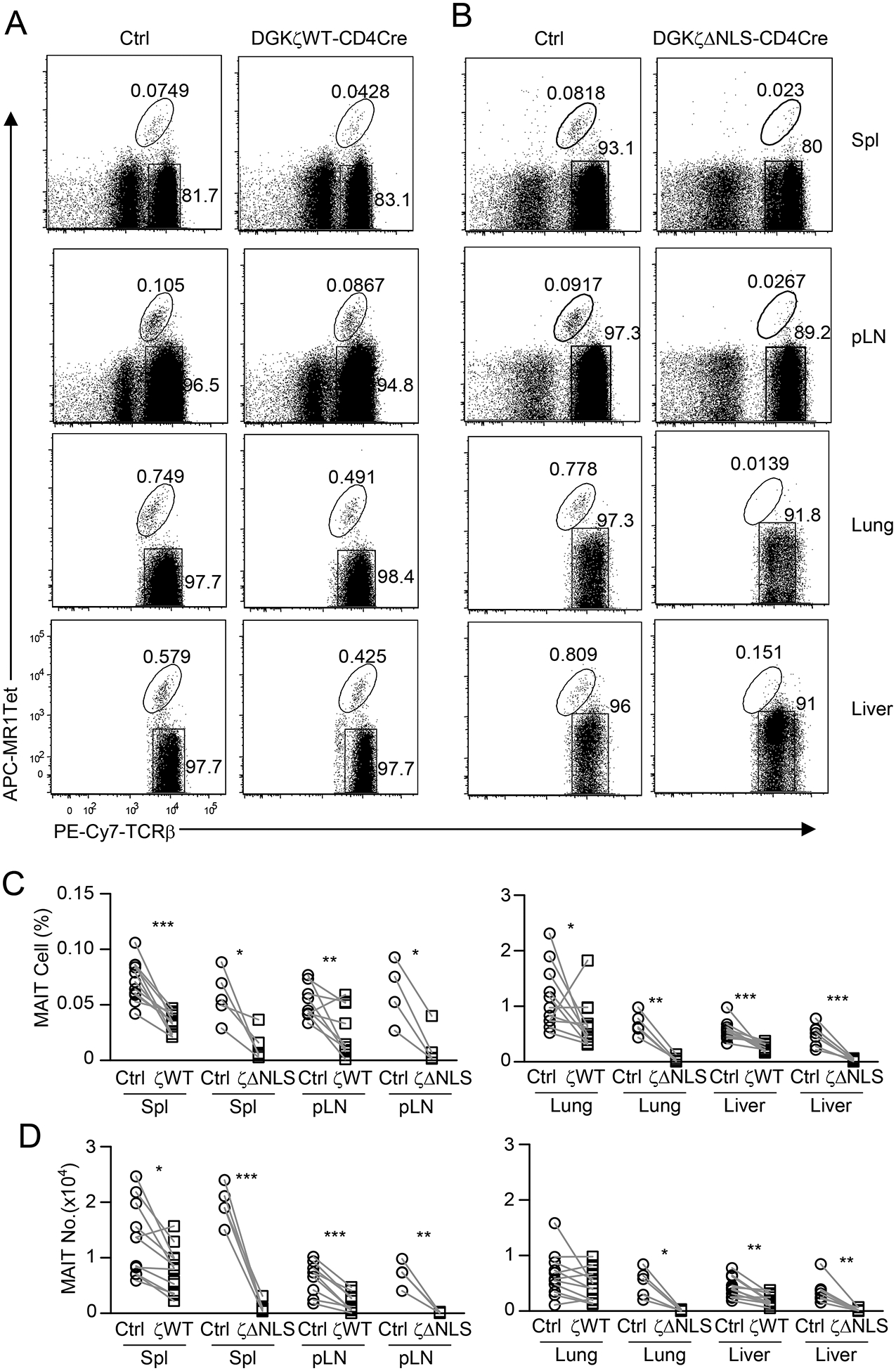
Single cell suspensions of the spleen, pLNs, lung, and mononuclear cells (MNCs) of the liver from 8–10 week-old GFP-Dgkzwt-Cd4Cre (A) or GFP-DgkzΔNLS-Cd4Cre mice, and their control mice were directly stained with MR1-Tet, anti-TCRβ, CD44, CD24, and lineage antibodies. A, B. Dot plots show TCRβ and MR1-Tet straining in live gated Lin− cells. Data shown are representative of at least nine experiments (A) and at least four experiments (B). C, D. MAIT cell percentages (C) and numbers (D). For both GFP-Dgkzwt-Cd4Cre and control mice shown in C and D, data are pooled from 9 – 11 experiments (N = 11 (spleen, Lung, liver); N = 9 (LN)); For both GFP-DgkzΔNLS-Cd4Cre and control mice shown in C and D, data are pooled from 4 – 7 experiments (N = 5 (spleen and lung); N = 4 (LN), N = 7 (liver)). Each circle or square represents one mouse of the indicated genotypes. *, P<0.05; **, P<0.01; ***, P<0.001 determined by pairwise Student t-test.
DAG-mediated signaling is required for MAIT cell terminal maturation/maintenance
MAIT cells sequentially mature from CD24+CD44− stage 1 to CD24−CD44− stage 2 and finally to CD24−CD44+ stage 3 in the thymus [14]. In WT thymus, both stages 1 and 2 account for less than 5% of MAIT cells, indicating efficient maturation of MAIT cells to effector lineages. In GFP-DgkzΔNLS-Cd4Cre thymus stages 1 and 2, MAIT cell percentages increased, but in stage 3 they decreased (Figure 3A, 3B). Because total thymic MAIT cell numbers decreased severely in GFP-DgkzΔNLS-Cd4Cre mice, stages 1 and 2 MAIT cell numbers in these mice were similar to control mice. However, stage 3 MAIT cell numbers in GFP-DgkzΔNLS-Cd4Cre mice drastically decreased (Figure 3C). In the spleen, stage 1 MAIT cells were undetectable, and stage 2 MAIT cells accounted for about 10% of total MAIT cells in WT mice (Figure 3A, 3B). In GFP-DgkzΔNLS-Cd4Cre spleen, stage 2 percentages increased, accompanying a 54% increase of stage 2 MAIT cell numbers; however, in stage 3, both percentages and numbers decreased drastically (Figures 3A–3C). Thus, enhanced DGKζ function results in a selective developmental blockage of MAIT cell maturation from stage 2 to stage 3 in both thymus and spleen, suggesting that DAG-mediated signaling promotes late stage MAIT cell maturation. Additionally, an increase in stage 2 MAIT cells in the spleen in GFP-DgkzΔNLS-Cd4Cre mice suggests that MAIT cells also complete stage 2 to stage 3 maturation in the peripheral organs and that such maturation also requires DAG-mediated signaling, which DGK activity regulates.
Figure 3. DAG-mediated signaling is important for MAIT cell maturation/maintenance.
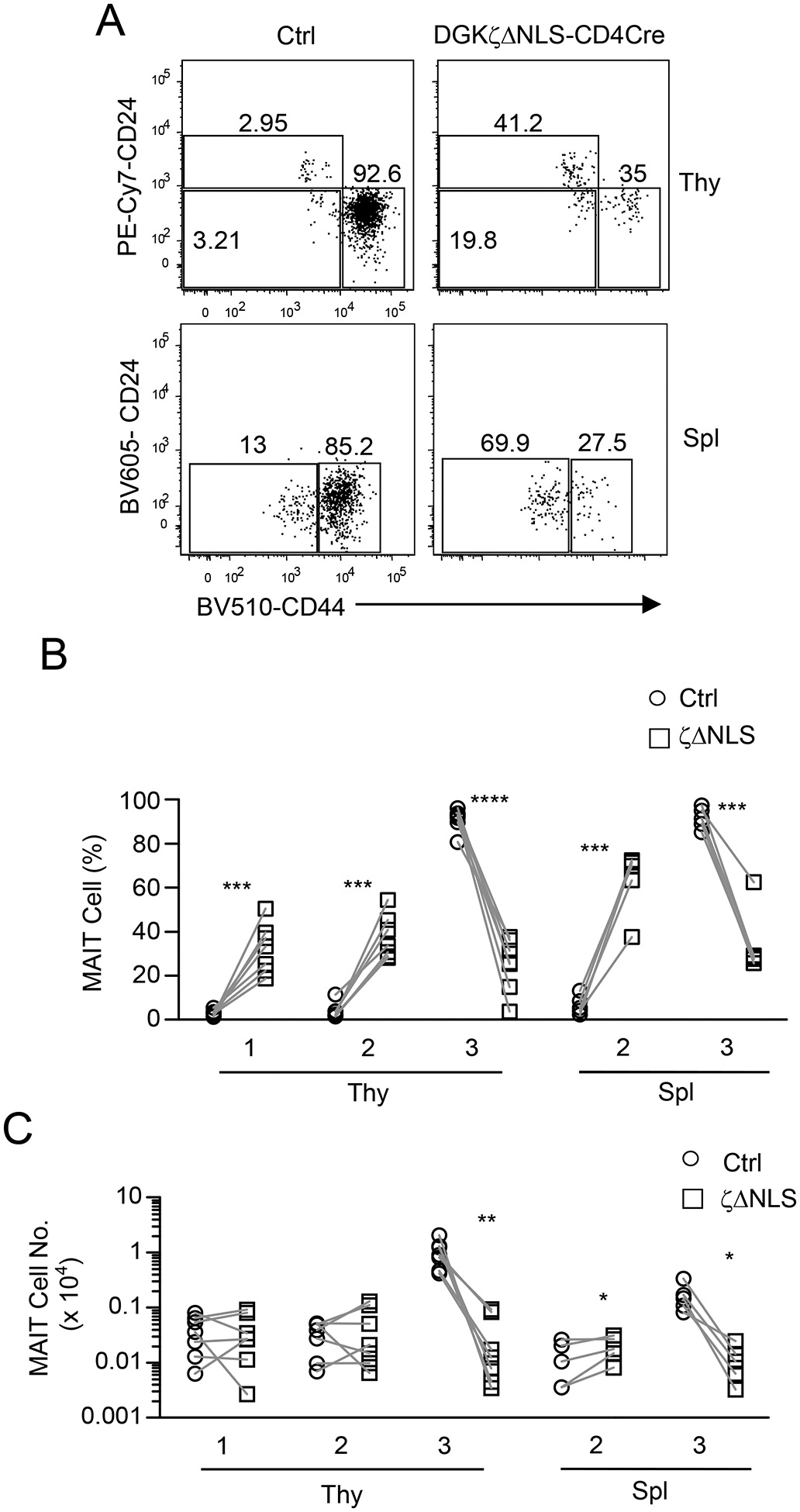
Thymocytes and splenocytes from GFP-DgkzΔNLS-Cd4Cre and control mice were enriched for and then were stained for MAIT cells as shown in Figure 1 with the addition of anti-CD24 and -CD44 antibodies. A. Representative dot plots show CD24 and CD44 expression in live gated Lin− TCRβ+MR1-Tet+ MAIT cells. Data shown are representative of at least five experiments. B, C. Scatter plots show stage 1 (CD24+CD44−), stage 2 (CD24−CD44−), and stage 3 (CD24−CD44+) MAIT cell percentages (B) and numbers (C). Data shown are pooled from seven experiments for thymus and five experiments for spleen. N = 7 for thymus and N = 5 for spleen for both test and control mice. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001 determined by pairwise Student t-test.
DAG-mediated signaling intrinsically promotes late stage MAIT cell maturation
MAIT cells were positively selected by MR1 expressed on CD4+CD8+ DP thymocytes [49]. Expression of DGKζΔNLS in GFP-DgkzΔNLS-Cd4Cre mice could affect DP thymocytes to extrinsically influence affect MAIT cell development. To rule out this possibility, we irradiated CD45.1+ WT mice and reconstituted them with a mixture of bone marrow (BM) cells from CD45.1+CD45.2+ GFP-DGKζΔNLS-CD4Cre mice and CD45.2+ WT control mice at a 1:1 ratio. Eight weeks after reconstitution, the GFP-DGKζΔNLS/WT ratios of donor-derived CD4+CD8+ DP thymocytes and B220+ splenic B cells were close to 1:1 (Figure 4A), suggesting equal reconstitution of hematopoietic stem cells from the donor mice. However, GFP-DGKζΔNLS-derived MAIT cells were greatly underrepresented in the recipients’ thymus and peripheral organs (Figure 4B, 4C). In the thymus, the GFP-DGKζΔNLS/WT ratios of stages 1 and 2 MAIT cells were close to 1:1; however, the ratio was greatly reduced in stage 3 MAIT cells (Figure 4D, 4E). Thus, enhanced DGKζ activity intrinsically inhibits MAIT cell generation by triggering a blockade between stages 2 and 3. The more severe decreases in GFP-DGKζΔNLS/WT ratios in the peripheral organs further support that DAG-mediated signaling plays a role for late stage MAIT cell maturation in the periphery.
Figure 4. Enhanced DGKζ function intrinsically inhibits MAIT cell maturation.
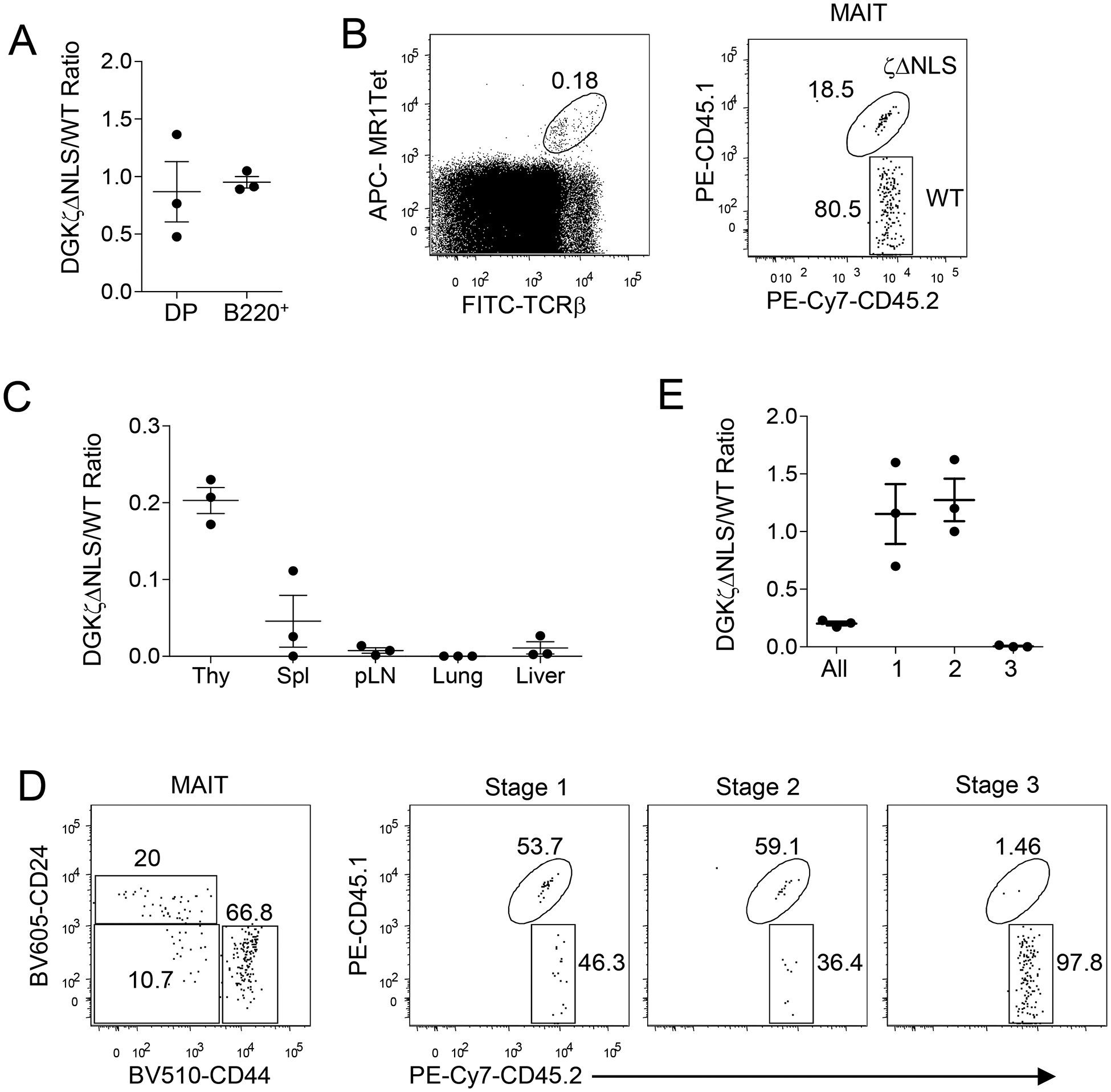
Lethally irradiated CD45.1+ WT recipient mice were reconstituted with a mixture of CD45.2+ WT and CD45.1+CD45.2+ GFP-DgkzΔNLS-Cd4Cre BM cells and were analyzed 6–8 weeks later. A. DGKζΔNLS to WT ratios of DP thymocytes and B220+ splenic B cells. B. Representative dot plots show MAIT cell staining of thymocytes (left panel) and CD45.1 and CD45.2 staining in thymic MAIT cells (right panel). C. DGKζΔNLS to WT ratios of MAIT cells in the indicated organs. D. Representative dot plots show CD24 and CD44 staining of thymic MAIT cells (left panel) and CD45.1 and CD45.2 staining of stage 1–3 thymic MAIT cells. E. DGKζΔNLS to WT ratios of stage 1–3 thymic MAIT cells. Each circle represents one recipient mouse. Data shown are presentative of (B, D) or are pooled from (A, C, E) three experiments with one recipient mouse examined in each experiment. Horizontal bars represent mean ± SEM (N = 3).
Effects of DGKζ or DGKα deficiency on MAIT cell development
DGKα and DGKζ are the major isoforms of the DGK family expressed in T cells. To further investigate the physiological functions of DGKs in MAIT cell development, we examined DGKα- and ζ-deficient mice. Deficiency of either DGKα or ζ does not obviously affect total cellularity in the thymus, spleen, or LNs or total numbers of liver mononuclear cells (MNCs) and total lung TCRαβ+ T cell numbers (Supplemental Figure 1). As Figure 5 shows, MAIT cell percentages and numbers in Dgka−/− mice were similar to their Dgka+/− littermate controls (Figures 5A, 5B). However, they decreased by 80% and 64%, respectively, in Dgkz−/− mice compared with their Dgkz+/− littermate controls (Figures 5C, 5D). Such decreases of MAIT cells in Dgkz−/− but not in Dgka−/− thymus were not caused by the enrichment process, as we obtained similar results by directly staining and analyzing MAIT cells from these mice without pre-enrichment (Figures 5E–5H). Thus, deficiency of DGKζ but not DGKα can impede MAIT cell development.
Figure 5. Effects of DGKα or ζ deficiency on thymic MAIT cell numbers.
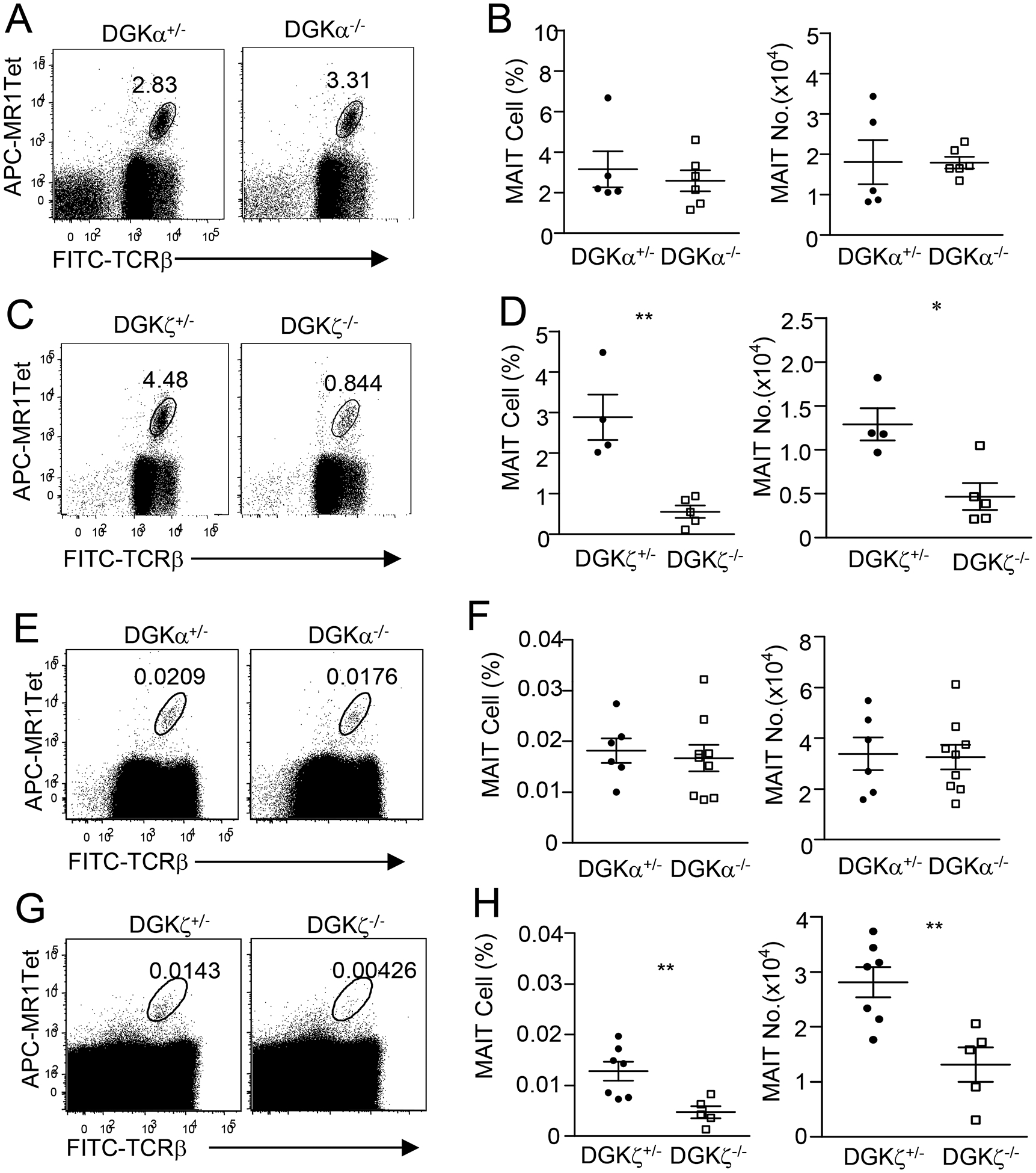
Thymocytes from 8–10-week-old mice enriched for MAIT cells with MR1-Tet (A–D) or total thymocytes (E–H) were stained with fluorescently labeled anti-TCRβ, MR1-Tet, and other antibodies similar to Figure 1. A, C, E, H. Dot plots show TCRβ and MR1-Tet straining in live gated Lin− cells from Dgka−/− and Dgka+/− control mice (A,E) or Dgkz−/− and Dgkz+/− control mice (C, G). B, D, F, H. Scatter plots represent mean ± SEM of MAIT cell percentages and numbers of Dgka−/− (B,F) and Dgkz−/− (D,H) mice and control mice. For A and B, data shown are representative of or pooled from five experiments (N = 5 for DGKα+/−, N = 6 for DGKα−/−). For C and D, data shown are representative of or pooled from four experiments (N = 4 for DGKζ+/−, N = 5 for DGKζ−/−). For E and F, data shown are representative of or pooled from six experiments (N = 6 for DGKα+/−, N = 9 for DGKα−/−). For G and H, data shown are representative of or pooled from five experiments (N = 7 for DGKζ+/−, N = 5 for DGKζ−/−). Dgka+/− and Dgkz+/− mice had similar numbers of MAIT cells to WT mice and were used as control. *, p<0.05; **, p<0.01 determined by unpaired Student t-test.
Severe decreases of MAIT cells in DGKα and ζ double-deficient mice
To further determine if DGKα and ζ may perform overlapping functions during MAIT cell development, we generated and analyzed Dgka−/−zf/f-Cd4Cre (DKO) mice. Although total thymic cellularity was not obviously altered in DKO mice, there were noticeable decreases of CD4+CD8−TCRβ+ and CD4−CD8+TCRβ+ single positive (SP) mature T cells in these mice compared with controls (manuscript in preparation), which is consistent with previous observations in Dgka and Dgkz germline double-deficient mice [39]. Interestingly, DKO mice displayed severe decreases of MAIT cells in both percentages and numbers in the thymus examined without enrichment (Figures 6A, 6B) or after enrichment (Figures 6C, 6D).
Figure 6. Severe decreases of thymic MAIT cells in DGKα and ζ double-deficient mice.
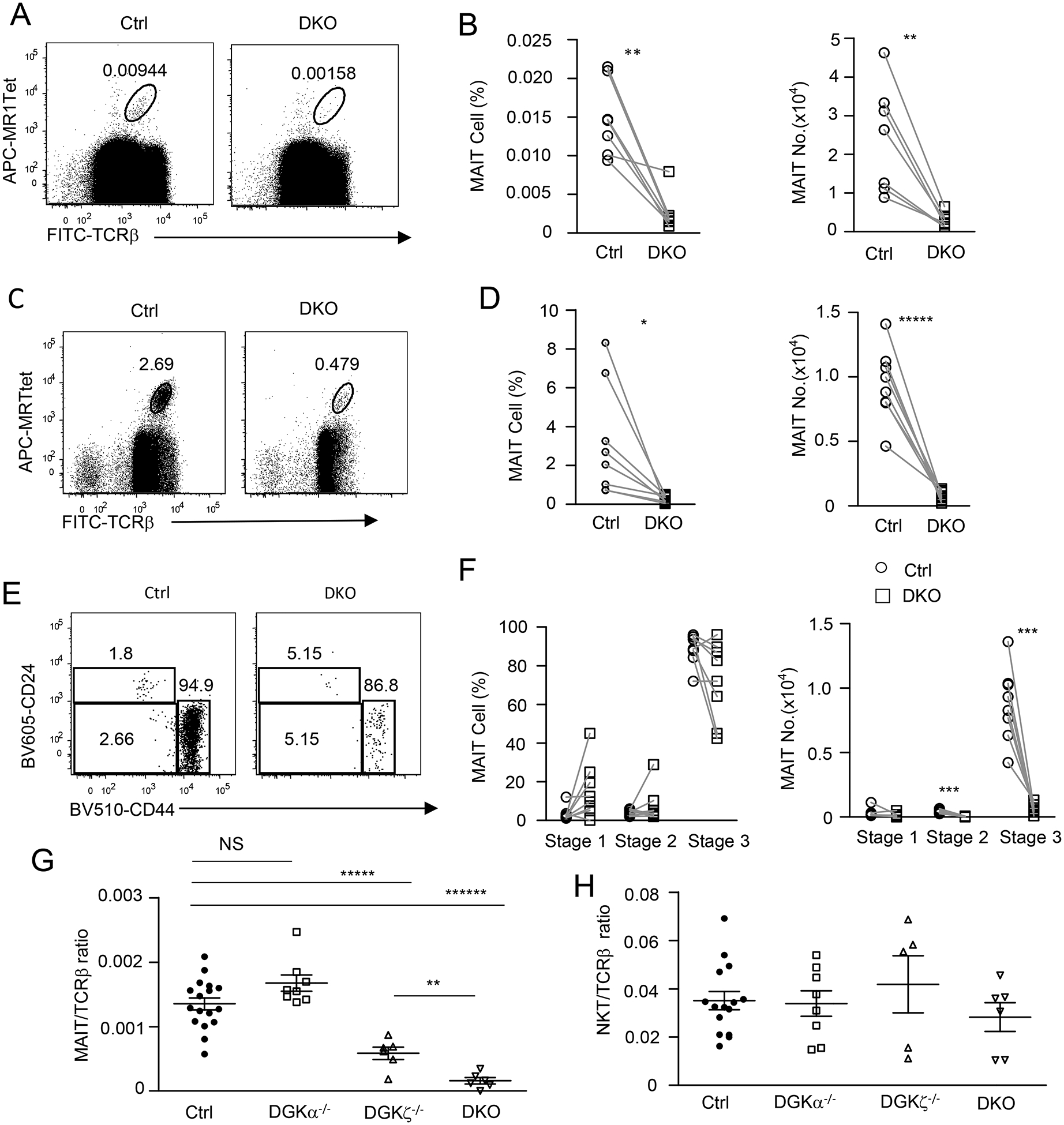
Thymocytes from 8–10-week-old Dgka−/−Dgkzf/f-CD4Cre (DKO) mice and DGKα+/− or WT control mice were similarly stained and analyzed for MAIT cells without (A, B) or after MR1-Tet enrichment (C, D) as in Figure 1 and Figure 2. A, C. Dot plots show TCRβ and MR1-Tet straining in live gated Lin− cells without enrichment (A) or after enrichment (C). B, D. Scatter plots show MAIT cell percentages and numbers without enrichment (B) and after enrichment (D). E. Representative dot plots show CD24 and CD44 expression in live gated Lin− TCRβ+MR1-Tet+ MAIT cells. F. Scatter plots show stage 1–3 MAIT cell percentages and numbers. G. MAIT to cαβT cell ratios in the thymus (horizontal bars represent mean ± SEM). H. iNKT to cαβT cell ratios in the thymus (horizontal bars represent mean ± SEM). For A and B, data shown are representative of or pooled from seven experiments (N = 7 for both WT and DKO mice). For C - F, data shown are representative of (C, E) or pooled from (D, F) eight experiments (N = 8 for both WT and DKO mice). For G, data shown are pooled from at least six experiments (N = 17 for control, N = 8 for DGKα−/−, N = 8 for DGKζ−/−, and N = 6 for DKO). For H, data shown are pooled from at least five experiments (N = 15 for control, N = 8 for DGKα−/−, N = 5 for DGKζ−/−, and N = 6 for DKO). Each circle, square, or triangle represents one mouse of the indicated genotypes. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001 determined by pairwise Student t-test (B, D, F) and unpaired Student t-test (G, H).
Within DKO MAIT cells, the relative ratios of stage 1 and 3 MAIT cells appeared to have increased and decreased, respectively. However, such differences were not statistically significant (Figures 6E, 6F; p>0.05). The absolute number of DKO stage 1 MAIT cells did not change, while stage 2 MAIT cells decreased slightly and stage 3 MAIT cells decreased drastically in comparison with controls. Together, these observations reveal that DGKα also plays an important role in promoting MAIT cell development in the thymus by functioning redundantly or synergistically with DGKζ and that the absence of both DGKα and ζ activity may result in MAIT cell developmental defects at stages 2 and 3.
Because DGK activity also affects cαβT cell and iNKT cell development [39, 47], we calculated thymic MAIT and iNKT to cαβT and ratios in individual mice to compare their sensitivity to decreased DGK activity. MAIT to cαβT ratios did not change in Dgka−/− mice but decreased 40% in Dgkz−/− mice and 80% in DKO mice (Figure 6G). In contrast, iNKT to cαβT ratios were not obviously different between these mice (Figure 6H). Thus, MAIT cell development is most sensitive to dysregulation of DGK-controlled signaling.
Severe decreases of MAIT cells in the peripheral organs of DGKα and ζ double- but not single-deficient mice
In Dgka−/− mice, MAIT cell percentages and numbers in the spleen, LNs, lung, and liver were not obviously different from those of Dgka+/− control mice (Supplemental Figure S2). In Dgkz−/− mice, whereas MAIT cell percentages and numbers in those organs tended to decline slightly, such decreases were not statistically significant (p>0.05, Supplemental Figure 3). However, in DGKαζ DKO mice, both MAIT cell percentages and numbers in those organs decreased severely (Figure 7). Thus, impaired MAIT cell development in DKO mice resulted in a scarcity of MAIT cells in the peripheral organs.
Figure 7. Severe decreases of MAIT cells in the peripheral organs in DGKαζ DKO mice.
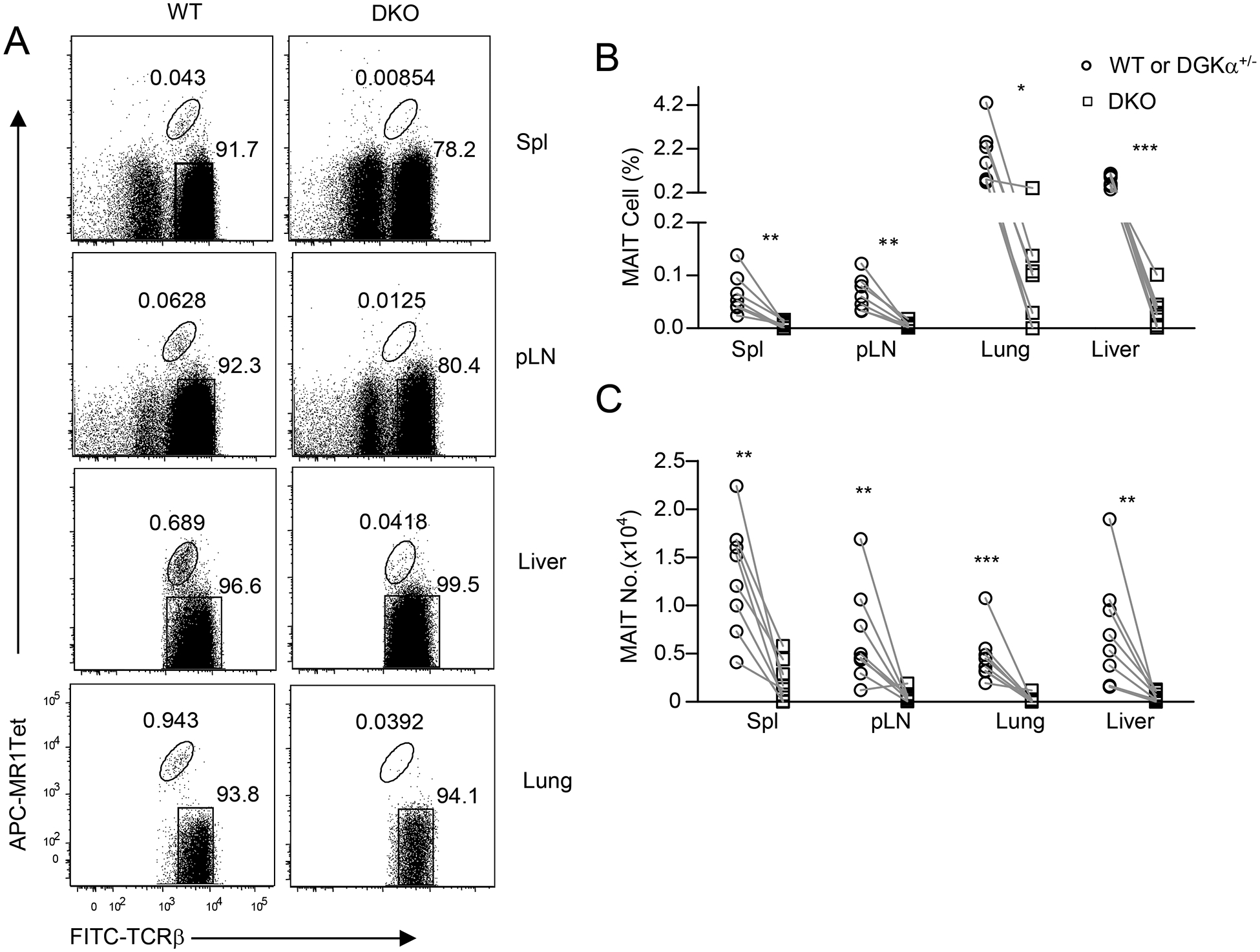
Single cell suspensions of the spleen, pLNs, lung, and MNCs of the liver from 8–10-week-old Dgka−/−Dgkzf/f-CD4Cre (DKO) and DGKα+/− or WT control mice were directly stained with MR1-Tet, anti-TCRβ, CD44, CD24, CD45, and lineage antibodies. A. Dot plots show TCRβ and MR1-Tet straining in live gated Lin− cells. For lung and liver, only TCRβ+ cells were gated and shown. B. MAIT cell percentages. C. MAIT cell numbers. Data shown are representative or pooled from eight experiments (N = 8 for both control and DKO mice). Each circle and square represents one control and DKO mouse respectively. *, P<0.05; **, P<0.01; ***, P<0.001 determined by pairwise Student t-test.
DGKα and ζ double deficiency intrinsically inhibits MAIT cell development
To determine if impaired MAIT cell development in DKO mice is cell autonomous, we generated irradiation chimeric mice reconstituted with a mixture of WT and DKO BM cells. Eight weeks after reconstitution, the ratios of CD45.2+ DKO to CD45.1+ WT CD4+CD8+ DP thymocytes and splenic B220+ B cells in the recipient mice were close to 2:1 (Figures 8A, 8B). However, MAIT cells were almost entirely generated from WT but not from DKO BM hematopoietic stem cells (Figures 8C, 8D). These data indicated that DGKα and ζ intrinsically promote MAIT maturation.
Figure 8. DGKα and ζ intrinsically promote MAIT cell development.
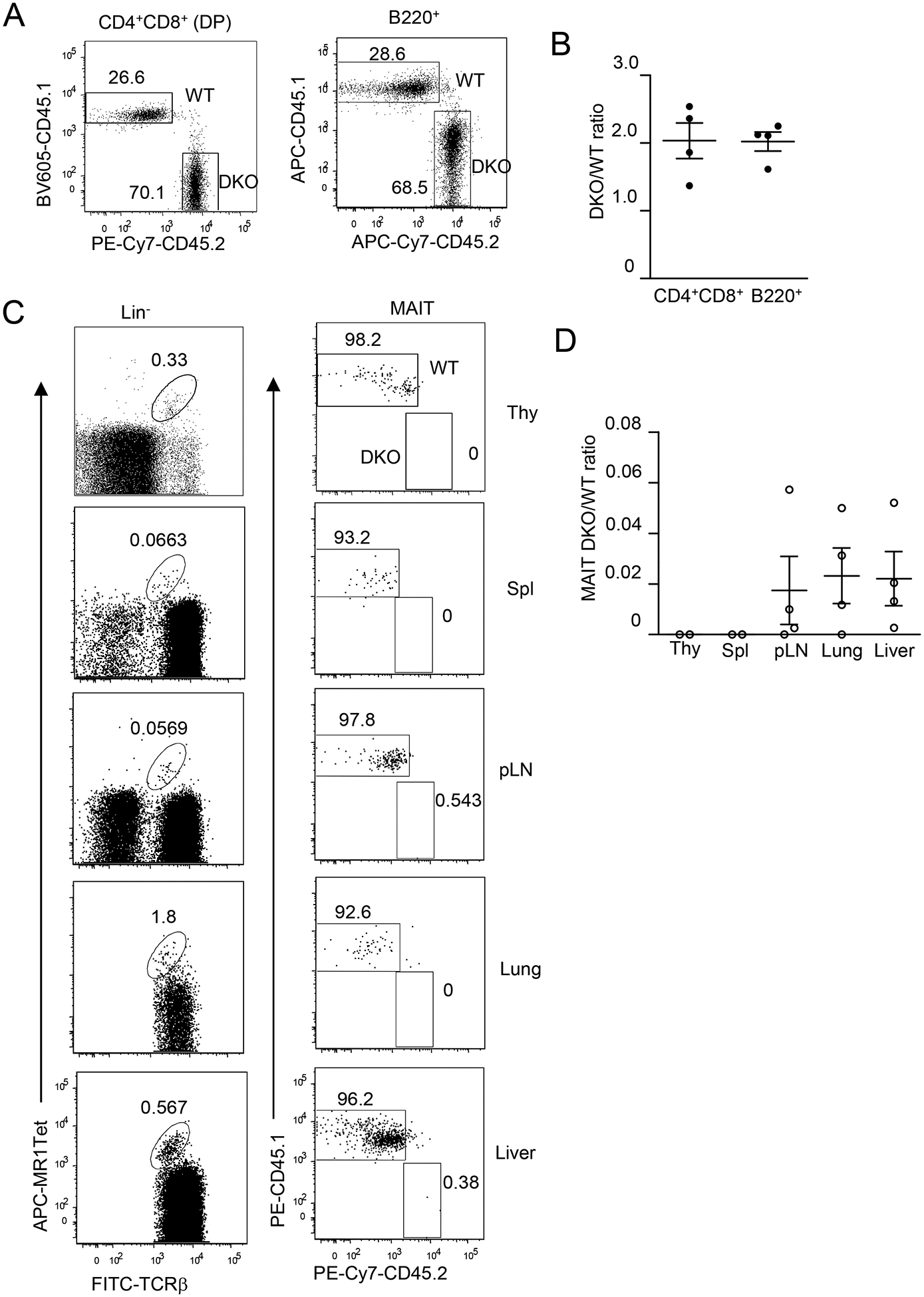
Irradiated CD45.1+CD45.2+ WT recipient mice were reconstituted with CD45.1+ WT and CD45.2+ DKO BM cells and were analyzed 8 weeks later. A. Representative dot plots showing CD45.1 and CD45.2 staining in live gated CD4+CD8+ DP thymocytes and B220+ splenocytes. B. DKO to WT ratios of DP thymocytes and B220+ splenic B cells. C. Representative dot plots showing MAIT cell staining in live gated Lin− cells (Thy, Spl, and pLNs) or in Lin−TCRβ+ cells (lung and liver) and CD45.1 and CD45.2 staining in MAIT cells. D. DKO to WT ratios of MAIT cells. Data shown represent or are pooled from four experiments with one recipient mouse examined in each experiment. Each circle represents one recipient mouse. Horizontal bars represent mean ± SEM (N = 4).
DISCUSSION
MAIT cells can recognize and quickly respond to bacterial derived metabolites and may play important roles in the homeostasis of the immune system, host defense, and pathogenesis of many diseases. Although it has been suggested that the TCR signal is crucial for MAIT cell development [4, 6, 15, 16], signal pathways downstream of the TCR and their regulations in MAIT cells have not been identified. Using genetically manipulated mice with either enhanced DGKζ activity or absence of DGKα and/or ζ activities in T cells, we demonstrated here that DAG-mediated signaling is not only critical but also must be tightly regulated by DGKs for proper MAIT cell maturation.
We have demonstrated that overexpression of WT DGKζ in developing thymocytes causes modest decreases in MAIT cells dependent on its kinase activity, suggesting that either decreased DAG or increased PA levels prevent MAIT cell maturation. DGKζ contains an NLS that can mediate its nuclear location; however, the functional importance of DGKζ nuclear translocation is unclear. By comparing GFP-DgkzΔNLS-Cd4Cre and GFP-Dgkzwt-CD4Cre mice, we have found that DGKζΔNLS contains a superior capability to inhibit DAG-mediated signaling in developing thymocytes, indicating that shuttling DGKζ between the cytoplasm and nucleus serves as a mechanism to control DGKζ function and that sequestration of DGKζ in the nucleus may prevent it from accessing its substrate DAG after TCR engagement (manuscript in submitted). Our data showing much severer decreases in thymic MAIT cells in GFP-DgkzΔNLS-Cd4Cre mice than in GFP-Dgkzwt-CD4Cre mice further support that DGKζΔNLS is a gain-of-function mutation and that DAG-mediated signaling is critical for MAIT cell development.
DAG binds to and regulates the activities and cytoplasmic localizations of many effector molecules with RasGRP1 and PKCθ being demonstrated important for cαβT, iNKT, and Treg development and/or function in mice and/or humans [17, 18]. Most recently, patients with loss of function mutations in RASGRP1 were found to have drastically decreased peripheral blood MAIT cells as well as iNKT cells [50]. Together, the observations in patients and in our mice suggest a crucial role of the DAG-RasGRP1 pathway for the generation/maintenance of MAIT cells in both mice and humans. It is interesting to note that DAG signal leads to mTOR activation via both the RasGRP1-Ras-Erk1/2 and PKCθ-CARMA1 pathways [20, 27]. Reduced mTOR activation could also contribute to MAIT cell defect in GFP-DgkzΔNLS-Cd4Cre mice as mTOR is crucial for late stage MAIT cell development (manuscript submitted). Of note, DGKs convert DAG to PA, which itself can serve as an important second messenger to control the activity of multiple signal molecules. Although we favor the argument that impairment of MAIT cell generation with enhanced DGKζ function results from reduced DAG signaling, that elevated PA in developing MAIT cells inhibits their maturation may also be possible.
It has been proposed that MAIT cells egress from the thymus after complete maturation. Although virtually all MAIT cells in the liver and lung are CD44+ stage 3 cells, we can consistently detect very low percentages of CD24−CD44− stage 2 MAIT cells in the spleen in WT mice. Because MAIT cells are very rare, and because and both stage 1 and 2 MAIT cells account for less than 5% of MAIT cells in mice, distinguishing these cells from noise has been difficult, as most thymocytes are CD24+ and most splenic T cells are CD44− that can fall into stage 1 and 2 MAIT cells in the thymus and spleen, respectively. The relative enrichment of CD24−CD44− TCRβ+MR1-Tet+ cells in the spleen of GFP-DgkzΔNLS-Cd4Cre mice suggests that these cells are true stage 2 MAIT cells and that a small portion of stage 2 MAIT cells may complete maturation to stage 3 effector lineages in the periphery. DGKζΔNLS may inhibit late stage MAIT cell maturation both in the thymus and periphery.
Our data demonstrate that enhanced DGKζ activity inhibits MAIT cell maturation from stage 2 to 3 without grossly affecting maturation to stage 1 and 2. It is well known that the DAG-mediated RasGRP1-Ras-Erk1/2 pathway is important for positive selection of cαβT cells and iNKT cells at the DP stage in the thymus [26, 30]. The relatively normal generation of stage 1 and stage 2 MAIT cells in GFP-DgkzΔNLS-Cd4Cre thymus was surprising. One possibility is that early and late MAIT cell maturation have differential DAG signal requirements, with the late stage relying on more intensive DAG-mediated signaling than do early stages. The other possibility is that GFP-DGKζΔNLS protein is expressed at lower levels in DP thymocytes, from which MAIT cells are generated, than in SP thymocytes and MAIT cells, diminishing its negative impact on DAG levels and its downstream pathways. Within MAIT cells, stage 1 expresses the lowest GFP-DGKζ, which may also limit the ability of GFP-DGKζ to affect early MAIT cell development. Additionally, early and late stage MAIT cells may have different abilities to adapt to reduced DAG signaling. Developing MAIT cells from DP thymocytes may overcome reduced DAG signaling by expressing iVα19TCRs with elevated affinity to the MR1 ligands. In contrast, the TCR repertoire in late stage MAIT cells is fixed, which, coupled with increased GFP-DGKζΔNLS protein expression, may lead to obvious maturation defects.
We have showed that deficiency of DGKζ but not DGKα causes moderate decreases of MAIT cells in the thymus, indicating that DGKζ plays a more important role in MAIT cell development than does DGKα. However, DGKα also participates in MAIT cell development as deficiency of both DGKα and ζ leads to a more severe reduction of MAIT cells than of DGKζ single-knockout mice. Functional redundancy of these two DGK isoforms has also been observed in cαβT cell development [39]. Interestingly, in DGKα and ζ DKO thymus, both stage 2 and stage 3 MAIT cell numbers are decreased, suggesting that dysregulation of DAG signaling exerts broad effects on MAIT cell development/homeostasis. Thus, our data reveal that DAG signal needs to be tightly fine-tuned to ensure proper MAIT cell development as either reduced or excessive DAG signaling is detrimental to MAIT cell maturation. While enhanced DGKζ function could downregulate DAG and downstream pathways such as RasGRP1 and mTOR to cause MAIT cell maturation defect, deficiency of DGKα and ζ leads to dysregulation of multiple signal pathways such as RasGRP1-Ras-Erk1/2, PKCθ-IKKα/β/γ-NFκB, and mTOR pathways [27, 39, 42]. Uncontrolled activation of the IKKβ, Ras, and mTORC1 impairs early and late stage iNKT cell maturation [47, 51], which could also impede MAIT cell development. Additional studies are necessary to illustrate the mechanisms of defective MAIT cell maturation caused by DGKαζ deficiency.
DGKα express at the highest level of mRNA among the DGK family in T cells (Immgen.org). The lack of obvious defect in MAIT cell development in DGKα deficient but not in DGKζ deficient mice is intriguing. It is interesting to note that the weak effect of DGKα deficiency is not limited to MAIT cells; it also applies to cαβT cells, regulatory T cells, and B cells [43, 44, 52, 53]. These two enzymes contain different structural features that may regulate their activities, limit their functions to specific signal pathways and biological processes, and control their subcellular localizations. DGKα contains EF-hand motifs and its activity requires calcium signal, which may limit its ability to compensate the loss of DGKζ activity on MAIT and other cells in conditions that calcium signal is not triggered. In contrast, DGKζ enzymatic activity is constitutively active, which may allow it to participate in broader developmental processes and to compensate for the loss of DGKα activity.
MATERIALS AND METHODS
Mice
Dgka−/− and Dgkz−/− mice were reported previously [33, 37]. Dgkzf/f mice were generated by the insertion of LoxP sites flanking exons 10–14 in the locus [42]. Cd4Cre mice were acquired from Charles River Laboratory. Rosa26-DGKζKI mice were generated by knocking the coding sequences for EGFP-DDKζWT, EGFP-DGKζΔNLS, or EGFP-DGKζKD DGKζ into the Rosa26 locus together with a chicken β-actin promoter and a floxed transcription stop cassette 5’ of the EGFP-DGKζ fusion gene (manuscript submitted). All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of Duke University. Single cell suspensions of the thymus, spleen, LNs, and liver MNCs were prepared as previous described [30]. Single cell suspensions of the lung were made after collagenase digestion as reported previously [54].
Antibodies and tetramers
Fluorochrome-conjugated anti-CD45.2 (clone 104), CD45.1 (A20), TCR-β (clone H57–597), TCRγδ (clone GL3), NK1.1 (clone PK136), CD44 (clone IM7), CD24 (clone M1/69), Gr1 (clone RB6–8C5), CD11b (clone M170), CD11c (clone N418), F4/80 (clone BM8), B220 (clone RA3–6B2), TER119/Erythroid Cells (clone TER-119), and CD3e (clone 145–2C11) were purchased from Biolegend. PE- or APC-conjugated 5-OP-RU loaded MR1 tetramer was kindly provided by the NIH tetramer facility. Cell death was identified using the Live/Dead Fixable Violet Dead Cell Stain (Invitrogen) or 7-AAD.
Staining MAIT cells and flow cytometry
Enrichment of MAIT cells from thymocytes and splenocytes was performed as previously reported [48]. For MAIT cell analysis, single cell suspensions with or without MR1-Tet enrichment were stained with anti-TCRβ, CD24, CD44, and other antibodies, and for unenriched cells, PE- or APC-conjugated 5-OP-RU loaded MR1-Tet at room temperature for 30 minutes. Lineage markers, including TCRγδ, CD11b, CD11c, F4/80, B220, Gr1, Ter119, and, in some experiments, PBS-57 loaded CD1d-tetramer were included to exclude other cell lineages. MAIT cells were gated on live Lin− TCRβ+MR1-Tet+ cells. Stained cells were collected using a BD FACScanto II or LSRFortessa™ and analyzed using the FlowJo software. Flow cytometry analyses were adhered to the ‘Guidelines for the use of flow cytometry and cell sorting in immunological studies’. Gating strategies are shown in supplemental Figure S4.
Generation of chimeric mice
CD45.1+ or CD45.1+CD45.2+ WT mice in C57BL/6 background were irradiated with a single dose of 800 rad X-Ray and intravenously injected with 10–15 million of a mixture of BM cells from CD45.2+ WT mice and Cd45.1+CD45.2+ GFP-DGKζΔNLS-CD4Cre mice at 1:1 ratio or from CD45.1+ WT and CD45.2+ DKO mice at a 2:1 ratio, respectively. Recipient mice were euthanized and analyzed 8 weeks later.
Statistical analysis
Data were presented as mean ± SEM and analyzed for statistical differences using the Prism 5/GraphPad software. Comparisons were made using pairwise or unpaired two-tailed Student t-test. P-values less than 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the NIH Tetramer Facility for providing MR1- and CD1d-Tetramers, the Transgenic and Knockout Mice Facility at the Duke Cancer Institute for generating Rosa26-DGKζ knocking mice, and the Flow Cytometry Facility at the Duke Cancer Institute for service. Research in this manuscript is supported by NIAID, NIH (R01AI079088 and R01AI101206 for XPZ). YP, JX, SZ, LL, ZH, and HT designed and performed experiments and analyzed the data. WD and CW generated critical reagents. YP and SZ prepared the figures. YC, LM, and JG participated in data analysis. XPZ conceived the project, designed experiments, participated in data analysis, and wrote the paper.
Abbreviation list:
- MAIT
mucosal associated invariant T cells
- DAG
Diacylglycerol
- DGK
Diacylglycerol kinase
- DP
double positive
- SP
signal positive
- LN
lymph node
- BM
bone marrow
- cαβT cell
conventional αβT cells
- DKO
DGKα and ζ double deficient
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C and Lantz O, Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 2011. 32: 212–218. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J and Moody DB, The burgeoning family of unconventional T cells. Nat Immunol 2015. 16: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 3.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, et al. , MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012. 491: 717–723. [DOI] [PubMed] [Google Scholar]
- 4.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S and Lantz O, Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003. 422: 164–169. [DOI] [PubMed] [Google Scholar]
- 5.Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SB, Meehan B, Chen Z, et al. , Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med 2015. 212: 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawachi I, Maldonado J, Strader C and Gilfillan S, MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol 2006. 176: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 7.Napier RJ, Adams EJ, Gold MC and Lewinsohn DM, The Role of Mucosal Associated Invariant T Cells in Antimicrobial Immunity. Front Immunol 2015. 6: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franciszkiewicz K, Salou M, Legoux F, Zhou Q, Cui Y, Bessoles S and Lantz O, MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol Rev 2016. 272: 120–138. [DOI] [PubMed] [Google Scholar]
- 9.Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, Piedavent M and Friese MA, CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol 2014. 44: 3119–3128. [DOI] [PubMed] [Google Scholar]
- 10.Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, Somsouk M, et al. , Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013. 121: 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, Park KJ, et al. , Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol 2014. 193: 3891–3901. [DOI] [PubMed] [Google Scholar]
- 12.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, et al. , Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011. 117: 1250–1259. [DOI] [PubMed] [Google Scholar]
- 13.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, et al. , Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013. 210: 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koay HF, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, Russ BE, et al. , A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol 2016. 17: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 15.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, et al. , Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009. 7: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croxford JL, Miyake S, Huang YY, Shimamura M and Yamamura T, Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol 2006. 7: 987–994. [DOI] [PubMed] [Google Scholar]
- 17.Krishna S and Zhong X, Role of diacylglycerol kinases in T cell development and function. Crit Rev Immunol 2013. 33: 97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merida I, Andrada E, Gharbi SI and Avila-Flores A, Redundant and specialized roles for diacylglycerol kinases alpha and zeta in the control of T cell functions. Sci Signal 2015. 8: re6. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, et al. , PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 2000. 404: 402–407. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton KS, Phong B, Corey C, Cheng J, Gorentla B, Zhong X, Shiva S and Kane LP, T cell receptor-dependent activation of mTOR signaling in T cells is mediated by Carma1 and MALT1, but not Bcl10. Sci Signal 2014. 7: ra55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsland BJ, Soos TJ, Spath G, Littman DR and Kopf M, Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med 2004. 200: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, et al. , SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity 2004. 21: 693–706. [DOI] [PubMed] [Google Scholar]
- 23.Kwon MJ, Ma J, Ding Y, Wang R and Sun Z, Protein kinase C-theta promotes Th17 differentiation via upregulation of Stat3. Journal of immunology 2012. 188: 5887–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W and Sun Z, Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol 2008. 46: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roose JP, Mollenauer M, Gupta VA, Stone J and Weiss A, A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol 2005. 25: 4426–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL and Stone JC, RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol 2000. 1: 317–321. [DOI] [PubMed] [Google Scholar]
- 27.Gorentla BK, Wan CK and Zhong XP, Negative regulation of mTOR activation by diacylglycerol kinases. Blood 2011. 117: 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golec DP, Henao Caviedes LM and Baldwin TA, RasGRP1 and RasGRP3 Are Required for Efficient Generation of Early Thymic Progenitors. J Immunol 2016. 197: 1743–1753. [DOI] [PubMed] [Google Scholar]
- 29.Kortum RL, Sommers CL, Pinski JM, Alexander CP, Merrill RK, Li W, Love PE and Samelson LE, Deconstructing Ras signaling in the thymus. Mol Cell Biol 2012. 32: 2748–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen S, Chen Y, Gorentla BK, Lu J, Stone JC and Zhong XP, Critical roles of RasGRP1 for invariant NKT cell development. J Immunol 2011. 187: 4467–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Ci X, Gorentla B, Sullivan SA, Stone JC, Zhang W, Pereira P, et al. , Differential requirement of RasGRP1 for gammadelta T cell development and activation. J Immunol 2012. 189: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK and Koretzky GA, Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem 2002. 277: 31089–31098. [DOI] [PubMed] [Google Scholar]
- 33.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA and Zhong XP, Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol 2006. 7: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 34.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, et al. , T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol 2006. 7: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Moreno M, Garcia-Lievana J, Soutar D, Torres-Ayuso P, Andrada E, Zhong XP, Koretzky GA, et al. , FoxO-dependent regulation of diacylglycerol kinase alpha gene expression. Mol Cell Biol 2012. 32: 4168–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA and Merida I, T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol 2003. 170: 2877–2883. [DOI] [PubMed] [Google Scholar]
- 37.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H and Koretzky GA, Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol 2003. 4: 882–890. [DOI] [PubMed] [Google Scholar]
- 38.Baldanzi G, Pighini A, Bettio V, Rainero E, Traini S, Chianale F, Porporato PE, et al. , SAP-mediated inhibition of diacylglycerol kinase alpha regulates TCR-induced diacylglycerol signaling. J Immunol 2011. 187: 5941–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW and Zhong XP, Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A 2008. 105: 11909–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almena M, Andrada E, Liebana R and Merida I, Diacylglycerol metabolism attenuates T-cell receptor signaling and alters thymocyte differentiation. Cell Death Dis 2013. 4: e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruffo E, Malacarne V, Larsen SE, Das R, Patrussi L, Wulfing C, Biskup C, et al. , Inhibition of diacylglycerol kinase alpha restores restimulation-induced cell death and reduces immunopathology in XLP-1. Sci Transl Med 2016. 8: 321ra327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Zhang P, Krishna S, Wang J, Lin X, Huang H, Xie D, et al. , Unexpected positive control of NFkappaB and miR-155 by DGKalpha and zeta ensures effector and memory CD8+ T cell differentiation. Oncotarget 2016. 7: 33744–33764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin J, O’Brien TF, Grayson JM and Zhong XP, Differential regulation of primary and memory CD8 T cell immune responses by diacylglycerol kinases. J Immunol 2012. 188: 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi RP, Schmidt AM, Das J, Pytel D, Riese MJ, Lester M, Diehl JA, et al. , The zeta isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling. Sci Signal 2013. 6: ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung IY, Kim YY, Yu HS, Lee M, Kim S and Lee J, CRISPR/Cas9-Mediated Knockout of DGK Improves Antitumor Activities of Human T Cells. Cancer Res 2018. 78: 4692–4703. [DOI] [PubMed] [Google Scholar]
- 46.Riese MJ, Wang LC, Moon EK, Joshi RP, Ranganathan A, June CH, Koretzky GA and Albelda SM, Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res 2013. 73: 3566–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen S, Wu J, Srivatsan S, Gorentla BK, Shin J, Xu L and Zhong XP, Tight regulation of diacylglycerol-mediated signaling is critical for proper invariant NKT cell development. J Immunol 2011. 187: 2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie J, Pan Y, Tao H, Wang P, Chen Y, Gao J and Zhong X, Deficiency of Mucosal-Associated Invariant T Cells in TCRJα18 Germline Knockout Mice. ImmunoHorizons 2019. 3: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, Soudais C and Lantz O, Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol 2013. 191: 6002–6009. [DOI] [PubMed] [Google Scholar]
- 50.Winter S, Martin E, Boutboul D, Lenoir C, Boudjemaa S, Petit A, Picard C, et al. , Loss of RASGRP1 in humans impairs T-cell expansion leading to Epstein-Barr virus susceptibility. EMBO Mol Med 2018. 10: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J, Yang J, Yang K, Wang H, Gorentla B, Shin J, Qiu Y, et al. , iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J Clin Invest 2014. 124: 1685–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avila-Flores A, Arranz-Nicolas J, Andrada E, Soutar D and Merida I, Predominant contribution of DGKzeta over DGKalpha in the control of PKC/PDK-1-regulated functions in T cells. Immunol Cell Biol 2017. 95: 549–563. [DOI] [PubMed] [Google Scholar]
- 53.Wheeler ML, Dong MB, Brink R, Zhong XP and DeFranco AL, Diacylglycerol kinase zeta limits B cell antigen receptor-dependent activation of ERK signaling to inhibit early antibody responses. Sci Signal 2013. 6: ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng W, Yang J, Lin X, Shin J, Gao J and Zhong XP, Essential Role of mTORC1 in Self-Renewal of Murine Alveolar Macrophages. J Immunol 2017. 198: 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


