In response to a Request for Applications to support meetings to develop a novel roadmap for the Department of Veterans Affairs with focus on gastrointestinal (GI) illnesses that primarily affect returning veterans, a team of GI experts was invited to address these medical conditions affecting Veterans. A meeting was held on May 17, 2019, in San Diego, California, focused at developing a roadmap for targeting gut-microbe interactions in service-related GI diseases of veterans. This meeting was funded by Office of Research and Development (Dr Sharma and Dr Iqbal) and led by Dr Pradeep Dudeja and Dr Jasmohan Bajaj. Initial discussion based on the talks from Drs. Bajaj and Dudeja focused on the current state of gut microbiome data in veterans’ diseases and the overall significance of gut microbiome. It was agreed that, to date, there are no systematic, controlled, longitudinal, and sufficiently powered studies available on the role of gut microbiome in GI illnesses of veterans. Also, few studies examining the microbiome in Gulf War illnesses (GWI) veterans and traumatic brain injury (TBI) are underway. The meeting focused on the role of gut microbiome in (1) diarrheal diseases, pain, and the gut–brain axis, (2) liver diseases, and (3) inflammatory bowel diseases (IBD). The overall discussion was focused on the potential role of gut microbiome in some of the key veterans service-related diseases GWI, post-traumatic stress disorder (PTSD) and TBI and its connections to the 3 focus areas.
Focus Group 1: Gut–Microbe Interactions in Diarrheal Diseases, Pain, and Gut–Brain Axis
Diarrheal illnesses are one of the most important diseases encountered during deployment. Our veterans have reported as high as 76% incidence of diarrheal illnesses during deployment to Iraq and Afghanistan, resulting in major job performance impairment.1 Most cases were associated with food- or water-borne infections or related to deployment-related stress.2 Many of these acute diarrheal illnesses become chronic ailments afflicting our veterans, such as functional bowel disorders including diarrhea-predominant irritable bowel syndrome (IBS) or have a nonspecific etiology related to GWI. In these diarrheal illnesses, chronic deployment-related stress and an altered microbiome appear to be common features.
Results from animal models, clinical outcomes of treatment, and individual susceptibility to diarrheal diseases have established a key role of gut microbiota in pathogenesis of these diseases. For example, Citrobacter rodentium (the murine counterpart of the human enteropathogenic Escherichia coli) infection only occurs in certain susceptible mouse strains and this phenotype can be transferred via microbial transfer.3 Similarly, diarrhea due to recurrent Clostridioides (formerly Clostridium) difficile infection in humans can be treated effectively by fecal microbial transplantation (FMT) from healthy donors. Furthermore, repeated bouts of infectious gastroenteritis combined with exposure to chronic stress increases the risk for developing IBS, which can be up to 6-fold higher.4 It is, therefore, critical to model the service-related illnesses in experimental animals and to systematically investigate the role of the gut microbiome in veterans’ diseases (eg, GWI, PTSD, and TBI) and the associated GI symptoms. Before planning detailed translational studies, it is also critical to first establish the microbial signatures of these diseases in veterans using systematic, longitudinal and well-powered randomized trials.
The key diarrheal pathogens in veterans include E coli, Clostridioides difficile, Campylobacter jejuni, nontyphoid Salmonella, Shigella, Norovirus, and West Nile virus.2 However, the pathophysiology of diarrhea in veterans’ diseases with respect to the role of gut–microbe interactions has not been investigated. Therefore, there are 3 critical needs to address these issues: (1) perform studies in humanized mice utilizing gut microbiota from veterans with GWI, PTSD, and TBI, (2) elucidate the pathophysiology of IBS-associated diarrhea in veterans (IBS-associated diarrhea is multifactorial and incorporates alterations in GI barrier function, motility, sensory function, brain-gut axis dysfunction, genetics and microbial dysbiosis), and (3) design effective therapies for these veterans’ diseases to improve the current therapeutic regimens. It is anticipated that modelling veterans’ diseases using state-of-the-art in vivo and in vitro approaches will provide better targets for therapeutic interventions. These include using humanized animal models to investigate host–pathogen interactions and the ion transport basis of diarrhea, as well as human gut-derived enteroid and enteroid-derived monolayers. Strategies to restore the microbiome using biotherapeutics such as FMT, synbiotics, and improved probiotics and postbiotics may be important and novel ways to combat these diseases.
This focus area also discussed the importance of brain-gut microbiota axis in the pathogenesis of IBS,5,6 the role of corticotropin-releasing factor and its receptor, the role of probiotics in reducing visceral pain and diarrhea associated with IBS, newer targets such as nerve growth factor and its receptor tropomyosin receptor kinase A in IBS/IBD pain, and the use of animal models to study microbiome-gut-brain interactions in visceral hypersensitivity in veterans’ diseases. Graft versus host disease diarrhea, which is affected by gut microbiota, is another important targetable area because war-related chemical exposure (eg, agent orange) in veterans increases the risk of developing hematological malignancies that require bone marrow or hematopoietic stem cell transplantation.7
The overarching goal of the focus group 1 will be to investigate the pathophysiological basis of diarrhea associated with GI tract infections, IBD, IBS, and graft versus host disease in the setting of dysbiosis parallel to veteran’s diseases and design novel strategies to restore a healthy microbiome to combat these illnesses. Key parameters that need to be examined include (1) gut–microbe interactions, (2) epithelial barrier function, (3) gut immune responses, and (4) gut–brain dysfunction/visceral pain. The studies pertaining to the barrier function and immune responses need to be carried out in collaboration with focus group 3.
Focus Group 2: Liver Diseases
Liver disease is epidemic in veterans due to the high prevalence of chronic hepatitis C, obesity and alcohol use disorder.8 Wartime injuries are a major contributor to the development of these predominantly lifestyle-associated etiologies of chronic liver disease. These can be varied but most often are related to depression and PTSD. PTSD and depression can further worsen the course of chronic liver disease by impacting medication adherence, clinic visits, potential candidacy for liver transplantation, and can worsen life expectancy.9 In animal models, PTSD is linked with differences in gut microbial composition, which can, in turn, modulate aggressive behavior and exert a negative influence on the mice that suffer because of this aggression.10,11 Moreover, these changes can be transmitted by fecal transplant in rodent models, setting the stage for gut microbial modulation as an important avenue in PTSD.10,11 Human studies are currently lacking in veterans with PTSD; however, patients with noncombat PTSD show altered gut microbial composition compared with those exposed to similar trauma but who did not develop PTSD.12,13
The interaction between PTSD, depression and the onset of liver disease in veterans is a relevant question since several lifestyle-associated disorders can propagate liver disease. Prominent among these are alcohol use disorder and nonalcoholic fatty liver disease, which are associated with the metabolic syndrome and obesity.9 These illnesses are comorbid with PTSD and depression, which can affect the microbial composition, function, and subsequently brain function. By the time veterans present with liver disease to the hepatology clinics, their disease process is a sum of liver disease, PTSD, depression, and comorbid substance abuse.14 Current therapies for PTSD involve group sessions, antidepressant and antianxiety therapy, and supportive psychotherapy.15 Although these approaches can potentially help many veterans, their adherence is suboptimal and those with liver disease have altered pharmacodynamics of prescribed drugs. Therefore, beneficially altering the gut microbiota could present another therapeutic approach, the greatest need for which being those with PTSD and chronic liver disease. Therefore, a greater understanding of the gut–liver–brain axis and its modulation is an important step in the roadmap of studying gut microbiota in Veteran’s war-related diseases. Gut microbiota influence both precirrhotic and cirrhotic stages of liver diseases.16 Gut microbial modulation by dietary and xenobiotic (including alcohol) intake can produce metabolically active bile acids, ammonia, xenobiotics and short-chain fatty acids (SCFA).16 These, in turn, affect the gut-brain axis in the setting of liver disease. In patients with cirrhosis, alteration of the gut–brain axis often manifests in the form of hepatic encephalopathy.16 Cognitive impairment is greater in veterans with cirrhosis and PTSD compared to veterans with cirrhosis without PTSD.14
There is, therefore, a major need to beneficially alter the gut microbiota composition and function in human and animal models of PTSD and chronic liver disease. Basic, translational, and clinical investigators need to use state-of-the-art novel approaches that include animal models, organoids and clinical trials. The roadmap will call on investigators to focus on gut microbiota as (a) major modulator(s) of the gut–brain axis in liver disease and PTSD, (b) propagators of liver injury in conjunction with worsening brain injury, (c) a targetable focus for therapy using FMT experiments in germ-free and specific pathogen-free mice with demonstrable impact on behavior, neuroinflammation, and gene expression in the brain, and (d) target for beneficial impact using individual microbial function modification, phage therapy and development of new probiotics. This roadmap will help to prioritize strategies for addressing the major burden of PTSD and cirrhosis, which has the potential to beneficially modulate liver disease in veterans.
Focus Group 3: IBD
IBD cause substantial morbidity and a 2- to 3-fold increase in IBD prevalence has been reported in US veterans.17 Although the cause of this increased prevalence is not known, this results in a high burden for the VA system. IBD involves a complex interaction between genes and environment, that includes an altered microbiome.18 Despite advances in understanding specific risk genes, increasing IBD incidence in areas such as China and India emphasizes the interaction between genetic and environmental factors. Among environmental factors, psychological stress has been implicated in inducing and/or promoting IBD. Early studies strongly associated IBD with psychological stress, and the role of stress has been demonstrated in experimental colitis in animals.19 Now, psychoneuro-endocrine-immune modulation through the brain–gut axis is well-recognized in IBD susceptibility and chronicity.20 Patients with inactive IBD had more relapses if their depression scores were increased. Service-related diseases, TBI, GWI, and PTSD may hold clues to increased frequency of IBD.
The association between psychological stress and gut dysbiosis may be another key in IBD development.21 Deployment-related psychological disorders in veterans are associated with gut dysbiosis, and dysregulation of the gut–brain axis, and immune function.13 However, it remains unclear what instigates changes in the gut microbiome and/or gut–brain communication to increase IBD susceptibility.
Dysbiosis, an altered balance of beneficial and aggressive resident microbiota, is a consistent finding in IBD patients. The expansion of aggressive resident strains, such as Enterobacteriaceae, is felt to drive TH1 and TH17 immune responses that mediate the tissue injury of IBD, as demonstrated in selective colonization and human fecal transplant to germ- free mice. Human FMT to genetically susceptible mice represents an extremely powerful strategy to determine functional properties of dysbiotic microbial populations in veterans, which can be further dissected by selective colonization of cultured strains in gnotobiotic mice.
Dietary imbalances and dysregulated gut barrier integrity can help to determine gut dysbiosis and drive mucosal inflammation. Factors associated with military deployment and war-related syndromes, including chronic stress, infections, food intolerance/allergies, and toxins, and nonsteroidal anti-inflammatory drugs are associated with a leaky gut.22 Disrupting the epithelial barrier/gut microbiome equilibrium may promote disease. Genetic determinants of gut barrier function and immunoregulation can modulate mucosal epithelial/immune homeostasis, and susceptibility to IBD and colitis-associated colon cancer.23 It remains to be determined how stress, gut barrier dysfunction, and immune activation may induce susceptibility to gut inflammation in veterans with war-related syndromes.
Nutrition is critical in regulating the gut barrier. SCFA, metabolic byproducts of gut microbiota fermentation of dietary substrates, are an essential fuel source for colonic epithelial cells and are effective in mouse models of colitis and IBD patients.24 SCFA and other protective bacterial metabolites, such as indoles and sphingolipids, are decreased in IBD patients. Other metabolic regulators, especially amino acids and polyamines, have recently been investigated in human IBD.25 How military deployment affects SCFAs, amino acids, polyamines, and the microbiome, and whether this is involved in immune/epithelial dysregulation and IBD needs to be investigated.
There is a significant need to investigate IBD pathogenesis in veterans, especially those with coexistent PTSD and TBI. This includes determining relationships between stress, gut microbiome, and IBD by physiologic and pathologic simulation with FMT from patients into germ-free murine IBD models. These efforts should be combined with determining transcriptional signatures at the single-cell level in patients and mouse models to determine the microbial interactions and cell types and behaviors that determine disease phenotypes. We hypothesize that by combining high-level expertise in clinical IBD, biosample procurement, metabolomics, single-cell RNA sequencing and analysis, multiplex tissue immunofluorescence, and FMT for modeling of dysbiosis/stress/barrier dysregulation/IBD, we can define causes for susceptibility to IBD in veterans and impact this major clinical problem.
Conclusions
Gut microbe–host interactions can modulate several diseases experienced by veterans, including functional GI disorders, IBD, infectious and IBD associated diarrhea and liver diseases. Additionally, an altered gut microbiome in response to deployment related diseases such as PTSD, GWI, and TBI can further exacerbate GI and liver diseases. Most of these disorders are painful, debilitating, and chronic. Their symptoms can continue to affect the quality of life long after a soldier’s tour is over, and the stress or infection subsides. Therefore, there is a critical need to understand the mechanisms underlying these disorders and identify novel therapeutic targets. This field meeting explored and identified several multidisciplinary, multi-institutional, collaborative options to develop a roadmap (summarized in Figure 1) specifically focused on gut–microbe interactions in service-related GI diseases to identify novel and translatable therapeutic targets.
Figure 1.
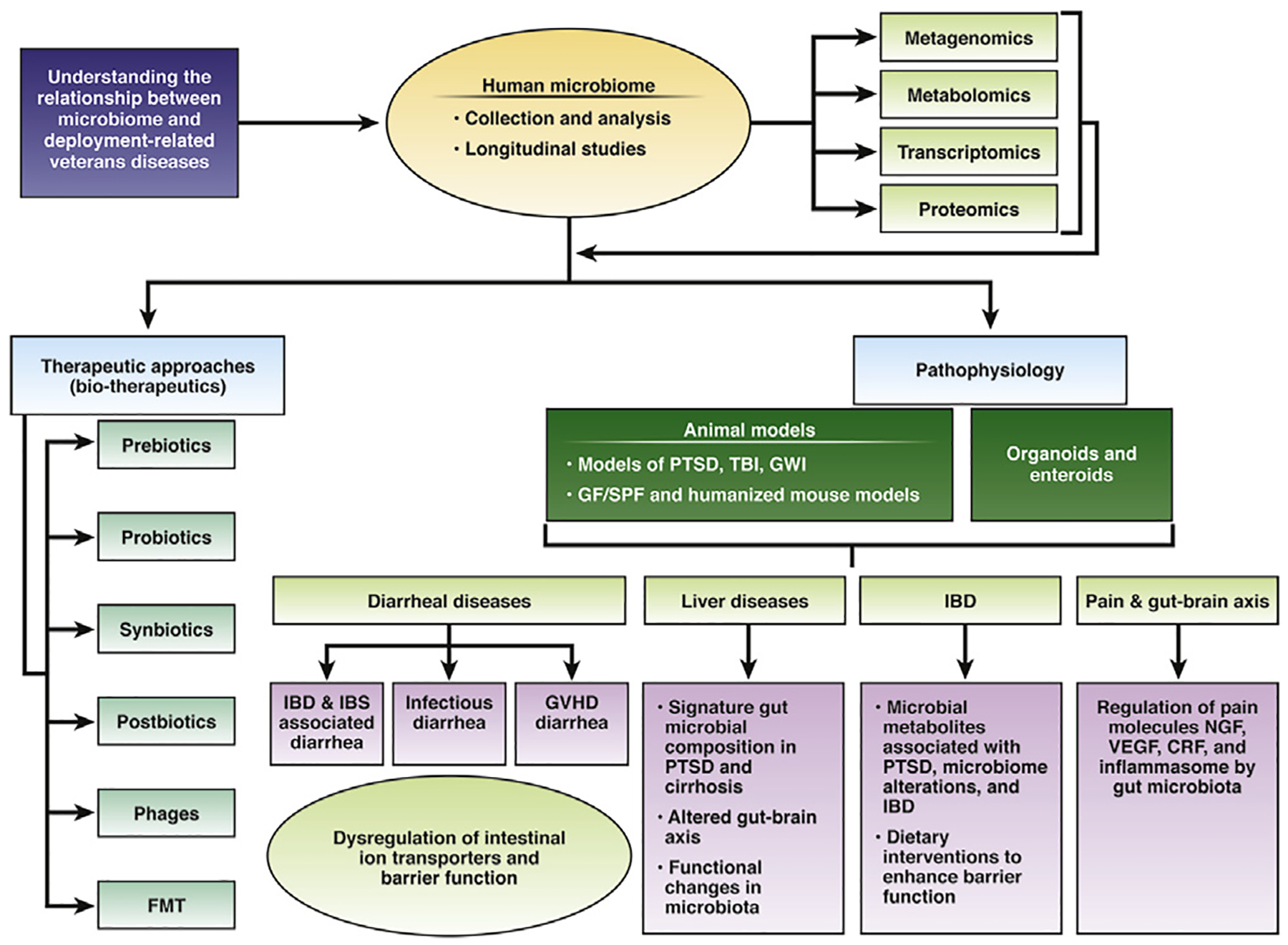
Roadmap of targeting gut microbes in service-related gastrointestinal and liver diseases in veterans.
Acknowledgments
Other contributors and attendees in the Appendix.
Funding
Sponsored by Veterans Affairs Central Office through a Field-Based Planning.
Abbreviations used in this paper:
- FMT
fecal microbial transplantation
- GI
gastrointestinal
- GWO
Gulf War veterans’ medically unexplained illnesses
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- PTSD
post-traumatic stress disorder
- SCFA
short-chain fatty acids
- TBI
traumatic brain injury
Appendix: Other Attendees and Contributors
Dr Zafar Iqbal, Veterans Affairs Central Office, Washington, DC
Dr Amar B. Singh, University of Nebraska Medical Center and VA Medical Center,
Dr Keith T. Wilson, Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, Nashville, TN
Dr Yvette Taché, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Dr Joseph Pisegna, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Dr Beverley Greenwood-Van Meerveld, University of Oklahoma Health Sciences Center and VA Medical Center, Oklahoma City, OK
Dr R. Balfour Sartor, University of North Carolina, Chapel Hill, NC
Dr Gail Hecht, Loyola University Medical Center and Hines VA Medical Center, Maywood, IL
Dr Huiping Zhou, Virginia Commonwealth University and Hunter Holmes McGuire VA Medical Center, Richmond, VA
Dr Phillip Hylemon, Virginia Commonwealth University and Hunter Holmes McGuire VA Medical Center, Richmond, VA
Dr Hee-Jeong Im, University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center, Chicago, IL
Dr Jonathan Jacobs, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Dr Jonathan Skupsky, University of California and VA Medical Center, Irvine, CA
Dr Jun Sun, University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center, Chicago, IL
Dr Jihane Benhammou, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Dr M Nedim Ince, University of Iowa and Iowa City VAHCS
Dr Lori A Coburn, Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, Nashville, TN
Dr Lisa Brenner, VA Rocky Mountain Mental Illness Research Education and Clinical Center, Denver, CO
Dr Nasia Safdar, University of Wisconsin and Madison VA, Madison, WI
Dr Patrick M. Gillevet, George Mason University, Manassas, VA
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2019.07.060.
References
- 1.Putnam SD, Sanders JW, Frenck RW, et al. Self-reported description of diarrhea among military populations in operations Iraqi Freedom and Enduring Freedom. J Travel Med 2006;13:92–99. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AE, Sivitz LB, Black RE. Infectious diseases In: Mitchell AE, Black RE, eds. Gulf War and health. volume 5 Washington, DC: The National Academies Press, 2007. [Google Scholar]
- 3.Ghosh S, Dai C, Brown K, et al. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol 2011;301:G39–G49. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MS, Welsh M, Porter CK, et al. The epidemiology of irritable bowel syndrome in the US military: findings from the Millennium Cohort Study. Am J Gastroenterol 2016;111:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pusceddu MM, Gareau MG. Visceral pain: gut microbiota, a new hope? J Biomed Sci 2018;25:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moloney RD, Johnson AC, O’Mahony SM, et al. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther 2016;22:102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredricks DN. The gut microbiota and graft-versus-host disease. J Clin Invest 2019;129:1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471–1482 e5; quiz e17–8. [DOI] [PubMed] [Google Scholar]
- 9.Forehand JA, Peltzman T, Westgate CL, et al. Causes of excess mortality in veterans treated for posttraumatic stress disorder. Am J Prev Med 2019;57:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson-Leary J, Zhao C, Bittinger K, et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry 2019. March 4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Gautam A, Kumar R, Chakraborty N, et al. Altered fecal microbiota composition in all male aggressor-exposed rodent model simulating features of post-traumatic stress disorder. J Neurosci Res 2018;96:1311–1323. [DOI] [PubMed] [Google Scholar]
- 12.Hemmings SMJ, Malan-Muller S, van den Heuvel LL, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 2017;79:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclercq S, Forsythe P, Bienenstock J. Posttraumatic stress disorder: does the gut microbiome hold the key? Can J Psychiatry 2016;61:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burroughs TK, Wade JB, Ellwood MS, et al. Effect of Post-traumatic stress disorder on cognitive function and covert hepatic encephalopathy diagnosis in cirrhotic veterans. Dig Dis Sci 2018;63:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Veterans Affairs. PTSD: National Center for PTSD. Available: www.ptsd.va.gov/professional/treat/essentials/dsm5_ptsd.asp#one.
- 16.Acharya C, Bajaj JS. The microbiome in cirrhosis and its complications. Clin Gastroenterol Hepatol 2019; 17:307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou JK, Kramer JR, Richardson P, et al. The incidence and prevalence of inflammatory bowel disease among U. S. veterans: a national cohort study. Inflamm Bowel Dis 2013;19:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology 2001;120:622–635. [DOI] [PubMed] [Google Scholar]
- 19.Qiu BS, Vallance BA, Blennerhassett PA, et al. The role of CD4þ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 1999;5:1178–1182. [DOI] [PubMed] [Google Scholar]
- 20.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013; 144:36–49. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Li L, Xia Y, et al. The gut microbiota heterogeneity and assembly changes associated with the IBD. Sci Rep 2019;9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konig J, Wells J, Cani PD, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 2016;7:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad R, Kumar B, Chen Z, et al. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/beta-catenin signaling. Oncogene 2017; 36:6592–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504:446–450. [DOI] [PubMed] [Google Scholar]
- 25.Scoville EA, Allaman MM, Brown CT, et al. Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics 2018;14. [DOI] [PMC free article] [PubMed] [Google Scholar]


