Abstract
Objective:
This study examined lexical and neuroanatomic correlates of reading errors in individuals with spatial neglect, defined as a failure to respond to stimuli in the side of space opposite a brain lesion, causing functional disability.
Method:
One hundred and ten participants with left spatial neglect after right-hemisphere stroke read aloud a list of 36 words. Reading errors were scored as “contralesional” (error in the left half of the word) or as “other”. The influence of lexical processing on neglect dyslexia was studied with a stepwise regression using word frequency, orthographic neighborhood (number of same length neighbors that differ by 1 letter), bigram and trigram counts (number of words with the same 2- and 3-letter combinations), length, concreteness, and imageability as predictors. MRI/CT images of 92 patients were studied in a voxelwise lesion-symptom analysis (VLSM).
Results:
Longer length and more trigram neighbors increased, while higher concreteness reduced, the rate of contralesional errors. VLSM revealed lesions in the inferior temporal sulcus, middle temporal and angular gyri, precuneus, temporal pole, and temporo-parietal white matter associated with the rate of contralesional errors.
Conclusions:
Orthographic competitors may decrease word salience, while semantic concreteness may help constrain the selection of available word options when it is based on degraded information from the left side of the word.
Public Significance Statement:
Reading impairments arising after stroke represent a devastating problem, restricting an individual’s life participation, independence and quality of life. In this study, we examined reading impairments in neglect dyslexia, a symptom characterized by reading errors in the half of the word opposite a brain lesion. To help improve the current understanding of this symptom, we identified specific word characteristics and stroke locations that are associated with increased rates of neglect dyslexia reading errors.
Keywords: Spatial Neglect, Right-Brain Stroke, Neglect Dyslexia, Reading, MRI, Lesion Symptom Mapping
1. Introduction
“My husband suffered right-hemisphere stroke several years ago, which caused spatial neglect. Since then, he has had trouble regaining his independence. Once, he accidentally walked into the ladies’ room of a restaurant, because he only noticed MEN on the sign that read WOMEN. “
-- Episode shared by a caregiver in an interview with one of the authors.
Spatial neglect, which can arise after stroke, causes or exacerbates functional disabilities (Adair & Barrett, 2008; Chen, Chen, Hreha, Goedert, & Barrett, 2015; Chen, Hreha, Kong, & Barrett, 2015). The affected individuals may allocate insufficient attention to the contralesional side of space, i.e., the hemi-space contralateral to the lesioned cerebral hemisphere; ipsilesional perceptual-attentional deficits and spatial deficits affecting representational and motor “aiming” functions, have also been observed (Barrett, 2014; Barrett & Burkholder, 2006; Corbetta & Shulman, 2011; Heilman, Watson, & Valenstein, 2012; Hillis, 2006; Parton, Malhotra, & Husain, 2004; Sacchetti, Goedert, Foundas, & Barrett, 2015). As illustrated by the example above, a disabling symptom that is frequently associated with spatial neglect is neglect dyslexia (ND) rendering individuals unable to complete simple daily activities that require reading (for a comprehensive review of neglect dyslexia, see Vallar, Burani, & Arduino, 2010). In the inpatient rehabilitation setting, where patients are typically admitted after acute stroke care, approximately 50-70% of individuals with right-brain stroke have spatial neglect (Chen, Chen, et al., 2015), and 37.5% of those with spatial neglect may have ND (Lee et al., 2009). ND primarily affects contralesional stimuli (Vallar et al., 2010) and, thus, presents as reading errors in the left part of horizontal letter strings in patients with right brain damage. Two of the most common error types in neglect dyslexia are substitution errors, in which a single segment is replaced and word length is preserved (e.g., NOVEL instead of LEVEL), and omission errors, in which letters in the beginning of a word may be omitted (e.g., reading MEN on a sign that says WOMEN). In both error types, up to 60% of errors comprise another word suggesting an interaction of linguistic and visuospatial processing (Ptak, Di Pietro, & Schnider, 2012). However, the exact contribution of lexical and sub-lexical factors to reading errors in ND is still not clear. Further, although it is known that presence of ND is associated with right occipito-temporo-parietal lesions (Lee et al., 2009; Ptak et al., 2012), the relationship of specific cortical and white matter lesions with the rate of contralesional reading errors in ND, while controlling for other types of errors, is still unexamined.
The interaction between linguistic and visuospatial processing was demonstrated in prior studies of ND, however these studies emphasized the whole-word level (Arduino, Burani, & Vallar, 2002, 2003; Brunn & Farah, 1991; Weinzierl, Kerkhoff, van Eimeren, Keller, & Stenneken, 2012). For example, individuals with ND read words more accurately, than nonwords (Brunn & Farah, 1991; Chatterjee, 1995; Frassinetti, Angeli, Meneghello, Avanzi, & Ladavas, 2002; Worthington, 1996); high-frequency words more accurately, than low-frequency words (Arduino et al., 2002; Caramazza & Hillis, 1990b); words with few orthographic neighbors (words of the same length, which differ from the target word by a single letter) more accurately, than words with many orthographic neighbors (Riddoch, Humphreys, Cleton, & Fery, 1990); and regular words better, than irregular words (Hillis & Caramazza, 1990; Vallar et al., 2010). Because reading is made possible through activation of multiple information processing components, including orthographic, phonological, and semantic (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001; Perry, Ziegler, & Zorzi, 2007; Pritchard, Coltheart, Palethorpe, & Castles, 2012; Seidenberg & McClelland, 1989), the interaction between visuospatial and linguistic processing likely involves multiple levels (e.g., orthographic vs. semantic; lexical vs. sub-lexical). Likewise, different levels of spatial processing may be involved (perceptual-attentional, representational, and occulo-motor aiming) (Barrett & Burkholder, 2006; Fortis, Goedert, & Barrett, 2011). Furthermore, the particular effects of the interaction between visuospatial and linguistic processing in patients with spatial neglect may depend on the degree of the spatial cognitive impairment (Arduino et al., 2002; Brunn & Farah, 1991).
Several models have been put forward to unify the cognitive mechanisms of reading and visuospatial processing observed in patterns of ND reading errors (Anderson, 1999; Brunn & Farah, 1991; Caramazza & Hillis, 1990a; Hillis & Caramazza, 1995; Mozer & Behrmann, 1990). For example, a cognitive model by Brunn and Farah (1991) proposes that the distribution of spatial attention during reading is not all-or-none in spatial neglect. According to Brunn and Farah (1991), “fully attended, high-quality stimulus information from the ipsilesional side of the letter string, and partially attended, low-quality stimulus information from the contralesional side of the letter string may be sufficient to allow the reading process to begin.” (p.71, Brunn & Farah, 1991). The early partial encoding of a letter string leads to partial but sufficient evidence for the presence of a word, which in turn, causes further reallocation of attention to the entire letter string, facilitating word reading. Thus, these authors suggest that the influence of visuospatial and linguistic processing may be reciprocal and that the severity of perceptual-attentional spatial processing deficits may account for ND errors (Caramazza & Hillis, 1990a; Haywood & Coltheart, 2000; Hillis & Caramazza, 1995; Hillis, Newhart, Heidler, Marsh, et al., 2005; Vallar et al., 2010). However, to account for the full extent of the available reading data in ND, a model may require specification of an interactive word recognition process (Harm & Seidenberg, 2004; McClelland & Rumelhart, 1981), with excitatory and inhibitory connections among lexical and sub-lexical processing components. For example, the computational model MORSEL (Mozer & Behrmann, 1990) proposes that after information about the word’s visual features is filtered by an attentional module, it is relayed to a network for detecting spatially invariant (e.g., with respect to size or position) representations. This network activates letter clusters, and is connected to a set of lexico-semantic units, which can boost activation of whole words. This model architecture can explain why real words are read more accurately than nonwords and why high-frequency words are read more accurately than low-frequency words. Not only can it explain some of the available ND reading data, it can also help make novel predictions about behavioral and neural phenomena observed in ND. For example, interactive activation of letter clusters in the MORSEL model suggests a potential mechanism for the influence of sub-lexical orthographic units (e.g., 2-letter bigrams and 3-letter trigrams) on word activation, which has yet to be demonstrated in ND patients. The model also predicts through connectivity of letter clusters to lexico-semantic units, that semantic variables can influence reading accuracy in ND. Although semantic variables, such as imageability (how easily a word brings an image to mind) and concreteness have only been investigated in single case studies (Nichelli, Venneri, Pentore, & Cubelli, 1993; Riddoch et al., 1990), a fully interactive account of visuospatial and semantic processing would predict that semantic variables should affect reading accuracy among all neglect patients. Furthermore, the hierarchical architecture of the MORSEL model is consistent with the involvement of distinct neuroanatomical components, where damage may produce impaired reading performance. However, the role of these neuroanatomic correlates still needs to be clarified with respect to their specific role in the interaction between visuospatial and linguistic processing.
Thus, the present study has two objectives: 1) To investigate linguistic factors modulating ND-related reading errors, in order to generate novel hypotheses about the role of sub-lexical processing, and 2) to identify the neuroanatomical correlates of ND reading errors. In the first objective, we built our thinking on previous case studies (Hillis & Caramazza, 1990; Riddoch et al., 1990), with the goal of evaluating whether the models created in these cases would inform our analysis of reading performance in a cohort of 110 individuals with spatial neglect after right-hemisphere stroke, who were tested on a single-word reading task. Consistent with prior studies, we hypothesized that word length, an orthographic variable, would be positively associated with the rate of left-sided reading errors. In contrast, we expected that word frequency, a variable that may affect both orthographic and semantic levels of processing, would be negatively associated with reading errors, and that words with higher frequency would be read more accurately. We also, however, wished to learn more about lexical-spatial interaction by examining orthographic competitors. Previous case studies showed that a word’s orthographic neighborhood density (or the number of same length words, which differ from the target word by a single letter) affected the rate of contralesional reading errors (Arguin & Bub, 2010; Riddoch et al., 1990). However, no study to date examined sub-lexical orthographic competitors (at the level of 2- and 3-letter clusters). We hypothesized that reading words with more orthographic competitors would increase the rate of left-sided reading errors by interfering with selection of the appropriate orthographic form. In line with the predictions of an interactive account of visuospatial and linguistic processing, we further hypothesized that semantic concreteness and imageability would decrease errors by facilitating the selection of appropriate orthographic and phonological representations.
In a second objective of this study, we wished to identify the neuroanatomical correlates of contralesional reading errors in ND. Previous studies established that ND is associated with right occipito-temporo-parietal lesions (Lee et al., 2009; Ptak et al., 2012), and it is clear that ND can be associated with lesions to parts of temporal and parietal cortex traditionally implicated in spatial neglect (Mort et al., 2003; Samuelsson, Jensen, Ekholm, Naver, & Blomstrand, 1997; Vallar & Perani, 1986). We sought to expand the current understanding of ND and, thus, our lesion-symptom analysis included a number of advancements. First, our analyses are based on one of the largest samples of participants with spatial neglect (N=110; N=92 with neuroimaging data) currently available in the literature. This sample size improved our power to uncover novel lesion-symptom associations. Second, instead of dichotomizing participants into groups based on a criterion (Lee et al., 2009; Ptak et al., 2012), we employed a within-participant design, analyzing continuous error rate data from the entire group of participants. This allowed us to establish a more precise link between specific brain lesions and the rate of ND reading errors. In contrast, comparing patients with and without ND may highlight regions implicated in impaired reading, as well as those relating to other types of impairments, which may contribute to, but not directly relate to reading error rate. Third, we used voxelwise lesion-symptom mapping (VLSM), enabling us to uncover anatomically precise links between brain correlates and patterns of reading deficits.
2. Methods
2.1. Participants
The participants in this study took part in ongoing prospective studies of spatial neglect and completed a reading assessment described here as part of behavioral assessment at study entry. One hundred and ten individuals undergoing inpatient rehabilitation met study participation criteria: 1) first stroke with no history of other psychiatric, developmental, or neurological conditions, 2) clinically-defined stroke in the right hemisphere, and 3) left-sided spatial neglect. Participants were diagnosed with spatial neglect if they scored 129 or below on the Behavioral Inattention Test – Conventional Subtest (BIT-c) (Halligan et al., 1990), or above 5 on the Catherine Bergego Scale (CBS) (Azouvi et al., 1996). The CBS may be more sensitive to detect spatial neglect than the BIT-c, because it captures additional spatial functional disability related to movements and performance (Azouvi et al., 2002; Goedert et al., 2012b; Pitteri et al., 2018). Participants were 28 to 90 years old (M = 65, SD = 14) and included 42 women (38.2%) and 68 men (61.8%). Eighty participants had visual field assessment and 10 of these (12.5%) had left hemianopia. All participants provided a written informed consent prior to study participation.
2.2. Materials and Procedures
All procedures were approved by the Kessler Foundation Institutional Review Board. Prior to reading assessment, participants were screened for neuropsychological deficits by a trained examiner to assess their attention, memory, language, and emotional functioning. Spatial neglect was assessed using BIT-c including three cancellation tasks (line, star, and letter cancellation), line bisection, and two drawing tasks (figure and shape copying and representational drawing) (B. Wilson, Cockburn, & Halligan, 1987). We also assessed spatial neglect-related functional performance deficits with the Kessler Foundation Neglect Assessment Process (KF-NAP®), a standardized method for administration of the Catherine Bergego Scale (Azouvi et al., 1996; CBS). The CBS via KF-NAP assesses spatial neglect based on observation of activities of daily living in 10 categories including gaze orientation, limb awareness, auditory attention, personal belongings, dressing, grooming, navigation, collisions, meals, and cleaning after meals (Chen, Chen, et al., 2015; Chen & Hreha, 2015).
We assessed reading aloud accuracy using two lists of 36 words. The words consisted of 4, 6, or 8 letters, comprising 1 to 4 syllables, and ranged in frequency between 1 and 343.87 instances per million (Medler & Binder, 2005; M. Wilson, 1987). Two lists were used in order to increase the overall number of sampled words to 72, while also trying to minimize testing time for each individual participant (see Table 1 for a complete list of words). The two lists were matched on a number of measures including length, number of syllables, frequency, familiarity, imageability, concreteness, position constrained bigram and trigram counts, and orthographic neighborhood density (see Table 2 for word characteristics). These characteristics were also used in a regression model with the goal to identify which of the orthographic and semantic word properties reliably predict the rate of contralesional reading errors. The orthographic measures were taken from MCWord database (Medler & Binder, 2005). Constrained bigram count refers to the number of words that share the same position-constrained 2-letter combination with the target word (e.g., brisk, brown, break). Similarly, constrained trigram count is the number of words that share the same 3-letter combinations in the same position with the target word (e.g., submit, subset, subtle). Orthographic neighborhood density is the number of orthographic neighbors a word has. An orthographic neighbor is a word of the same length that differs from the target word by a single letter. For example, breed, dread, and break are orthographic neighbors of the word “bread”. Semantic measures were obtained from MRC Psycholinguistic Database (M. Wilson, 1987), and included imageability (how easily a word brings an image to mind, e.g., high-imageability – red, low-imageability – repertoire), familiarity (how familiar a word is, low-familiarity – dove, high-familiarity – door) and concreteness (whether the word represents a concrete entity, e.g., concrete – chair, abstract – friendship).
Table 1.
Word Lists
| List 1 | List 2 |
|---|---|
| absent | audacity |
| advice | bind |
| afraid | bomb |
| anchor | breath |
| bend | bull |
| center | bush |
| cold | campuses |
| concerns | catalyst |
| dogmatic | catholic |
| door | clip |
| duck | corporal |
| episodes | danger |
| excess | division |
| fumble | dove |
| gone | edit |
| hungry | effort |
| inferior | external |
| lone | headache |
| matching | island |
| method | kite |
| minister | lend |
| opinions | length |
| poem | odor |
| purposes | pirate |
| riot | pope |
| ruin | quaint |
| ship | reason |
| sketch | region |
| sneeze | severe |
| soothing | siblings |
| straight | smartest |
| system | soup |
| theories | stutters |
| tractors | tongue |
| unit | tragic |
| womb | tributes |
Table 2.
Word characteristics.
| Measure | List 1 | List 2 | p value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | ||
| Length | 36 | 6 (1.66) | 4-8 | 36 | 6 (1.66) | 4-8 | 0.999 |
| Number of Syllables | 36 | 1.86 (0.68) | 1-3 | 36 | 1.86 (0.83) | 1-4 | 0.999 |
| Frequency | 36 | 54.71 (84.30) | 1-343.87 | 36 | 28.60 (42.33) | 1-227.56 | 0.101 |
| Orthographic Neighborhood Density | 36 | 3.31 (4.96) | 0-18 | 36 | 3.56 (5.22) | 0-16 | 0.836 |
| Constrained Bigram Count | 36 | 97.73 (114.22) | 10.33-460.86 | 36 | 80.33 (70.00) | 2.33-265 | 0.438 |
| Constrained Trigram Count | 36 | 25.01 (53.94) | 1-245.5 | 36 | 15.85 (20.43) | 0-100.17 | 0.344 |
| Familiarity | 23 | 529.91 (66.66) | 320-630 | 18 | 532.11 (50.32) | 415-601 | 0.908 |
| Imageability | 23 | 468.39 (106.27) | 300-632 | 18 | 498.28 (117.66) | 285-643 | 0.399 |
| Concreteness | 21 | 444.67 (116.34) | 291-615 | 17 | 478.76 (129.15) | 286-634 | 0.398 |
Participants were seated comfortably facing a 17-inch laptop computer. A microphone was clipped at the collar of the participant’s shirt. The viewing distance was about 53.5 cm. Depending on length, words occupied between 4.3 and 10.1 degrees of visual angle, thus, falling entirely within the foveal and parafoveal region. The center of the screen was at the participant’s eye level. Words were presented in white on a black background using the Microsoft PowerPoint. Each reading trial consisted of, in the following order, a color circle at the center of the screen, a word at the center of the screen, and a blank screen. The color circle, 0.6 inch in diameter, was used to bring the participant’s attention to the center of the screen. Once the participant named the color (blue, yellow, green, or red), the examiner pressed a mouse key to present the next slide containing the word. The word was presented in lowercase using a 44-point Arial font. A space was inserted between letters; for example, the word “lotion” was presented as “l o t i o n”. Letter spacing has previously been shown to facilitate reading (Chung, 2002). Once the participant read the word aloud, the examiner pressed the mouse key to end the trial by showing the blank black screen. There was no time limitation, and the response was not timed.
The participant had two practice trials following the instruction “First, name the color and a word will appear. Read the word. Ready?” which was presented on the screen and read by the examiner. After the practice trials, the same instruction appeared. The participant then proceeded to complete 36 trials. For each stimulus, an experimenter recorded the participant’s response verbatim and noted if the item was correct. If participants were unable to read a word aloud, they were asked to spell it aloud and the experimenter recorded the spelling (8.89% of all responses). Errors were later coded as contralesional (error in the left half of the word) or other (error elsewhere in the word) by three independent coders. Any disagreements in coding were resolved through discussion. To facilitate comparison to published studies, we identified patients with and without ND. For example, Lee et al. (2009) considered patients who made three or more errors on the left side of 25 words (>12% of stimuli) as having ND. Using this criterion, we classified participants as having ND if they made five or more contralesional errors.
2.3. Lesion Mapping
We had previously obtained, with participants’ authorization, their radiological records and brain scans from hospitals where they received acute care for stroke. MRI or CT Images were available for 92 of the 110 participants. Lesion mapping was done by hand using MRICron (Rorden, Karnath, & Bonilha, 2007). Lesions were visualized and mapped on a 2 mm3 anatomical template (available in MRICron) by trained staff and inspected by a neurologist in a procedure previously described (Chen, Goedert, Shah, Foundas, & Barrett, 2014). Areas of hyperintensity surrounding the lesion that appeared on Fluid Attenuated Inversion Recovery (FLAIR) MRI images, whenever these images were available were included in the lesion mask. Additionally, any bilateral, sub-clinical lacunar lesions that appeared on multiple anatomical images, and contained more than 15 2mm3 voxels, were mapped as part of the lesion mask. No participant had a clinically defined stroke in the left hemisphere, based on the inspection of medical records and on the examination of the brain scan by a neurologist (AMB).
2.4. Analysis
Behavioral analyses were carried out using SPSS version 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Voxelwise Lesion Symptom Mapping (VLSM) was carried out using VLSM2 software version 2.55 (Bates et al., 2003). Binary lesion masks for each participant, coregistered to the anatomical template, and behavioral data were entered into the VLSM2 software. Voxels lesioned in at least 10 patients were included in the analysis. The rate of contralesional errors, calculated as the proportion of words with contralesional errors relative to the total number of words, was used as the main predictor of interest. Log lesion volume was used as a co-variate to control for the overall stroke severity. We also used the rate of other errors as an additional co-variate. This allowed us to control for the common variance in error rate, which was due to processing difficulty unrelated to the interaction between reading and spatial processing. We used a voxelwise statistical threshold of p < 0.005. This p-value, when combined with a high threshold for including voxels lesioned in 10 or more participants, constitutes a strict statistical threshold.
3. Results and Discussion
3.1. Participant Characteristics
See Table 3 for detailed characteristics of the patient sample. Based on the criteria outlined in the Method section, all 110 participants had spatial neglect according to at least one of the tests. Specifically, 89 (81%) participants were identified according to the BIT-c, and 103 (97%) participants according to the CBS via KFNAP. In this sample, 27 (24.5%) participants made 5 or more contralesional reading errors, and thus identified as having ND. This indicates that the prevalence of ND may not be as high as reported previously (37.5%) by Lee et al. (2009). However, we did not use phrases or text to assess ND, which may be more sensitive to reading deficits in patients with spatial neglect (Galletta, Campanelli, Maul, & Barrett, 2014).
Table 3.
Characteristics of the patient sample.
| Measure | n | Mean | SD | Range |
|---|---|---|---|---|
| Age (in years) | 110 | 65.0 | 14.2 | 28-90 |
| Education (in year) | 110 | 13.7 | 3.0 | 4-21 |
| Lesion Size (in cm3) | 92 | 118.5 | 114.8 | 2.1-488.1 |
| Days Post Stroke | 110 | 21.59 | 20.40 | 5-209 |
| Boston Naming Test – Short (max score = 15) (Kaplan et al., 1983) | 72 | 12.2 | 3.0 | 3-15 |
| Geriatric Depression Scale (max score = 30) (Yesavage et al., 1982) | 110 | 6.9 | 5.2 | 0-26 |
| Depression as indicated by the score on the GDS (>13/30) | 15 (14%) | |||
| Mini Mental Status Exam (max score = 30) (Folstein et al., 1975) | 107 | 24.5 | 4.3 | 9-30 |
| Controlled Oral Word Association Test (Loonstra et al., 2001) | 103 | 22.1 | 11.6 | 1-53 |
| Behavioral Inattention Test (Halligan et al., 1990) | 110 | 86.9 | 42.1 | 8-145 |
| Spatial neglect as indicated by the score on the BIT (< 129/146) | 89 (81%) | |||
| Catherine Bergego Scale via the Kessler Foundation Neglect Assessment Process (Chen et al., 2015) | 106 | 14.39 | 7.79 | 0-30 |
| Spatial neglect as indicated by the score on the KF-NAP (>5/30) | 103 (97%) | |||
3.2. Spatial Neglect Severity and Reading Errors
To replicate the relationship between spatial neglect severity and ND reading errors established in prior studies (Beschin et al., 2014; Lee et al., 2009; Ptak et al., 2012), we tested if spatial neglect severity predicted the rate of reading errors, separately for contralesional and other errors. A multiple regression analysis was used to assess this relationship with the BIT-c as a measure of neglect severity, while controlling for days post stroke, age, MMSE score, and years of education. Controlling for these confounding variables, BIT-c score was a significant predictor of the rate of contralesional reading errors (F(3, 106) = 15.38, p < 0.001; BIT: b1 = −0.003, standardized b1 = −0.540, t = −6.58, p < 0.001). Higher score on the BIT-c (less severe spatial neglect) was associated with fewer reading errors. The BIT-c was not a significant predictor of the rate of other reading errors (p=.26), which was instead predicted by the MMSE score (F(3, 106) = 3.49, p < 0.01; b1 = −0.003, standardized b1 = −0.221 t = −1.99, p < 0.05). Similar results were obtained when log lesion volume (a proxy for overall stroke severity) was used as an additional covariate, and when only patients without hemianopia were considered. Thus, participants who had greater severity of spatial neglect demonstrated a higher rate of contralesional reading errors, independent of lesion volume or visual perceptual abnormalities. On the other hand, participants who had lower MMSE score showed a higher rate of other reading errors.
Reading might be co-supported by non-spatial right-brain systems, as is oral language (Crosson et al., 2009). For this purpose, we examined the rate of contralesional errors in spatial neglect patients and correlated it with the rate of other errors (r = .37, p < 0.001). This correlation remained significant when controlling for the BIT-c score (r = .29, p < 0.005) or presence of hemianopia (r = .32, p < 0.005). These correlations suggested that both types of reading errors had common variance due to factors other than spatial impairment, possibly due to the effect of other right brain systems on impaired reading mechanisms, or other factors not yet identified. To identify lesions associated with each type of error, we controlled for other reading error rate when examining the neural correlates of contralesional error rate, and vice versa.
3.3. Neural Correlates of Contralesional and Other Reading Errors
3.3.1. Contralesional Reading Errors
The lesion coverage map is shown in Figure 1. Controlling for lesion volume and the rate of other reading errors, contralesional reading errors were associated with lesions in several brain areas, including precuneus, thought to be involved in visuo-spatial imagery and episodic memory retrieval (Cavanna & Trimble, 2006), inferior temporal sulcus and temporal pole, thought to support semantic and object-centered processing (Graves, Desai, Humphries, Seidenberg, & Binder, 2010; Hillis, Newhart, Heidler, Barker, et al., 2005; Jackson Ralph, & Pobric, 2015; Lambon Ralph, Pobric, & Jefferies, 2009; Lauro, Tettamanti, Cappa, & Papagno, 2008; Noppeney & Price, 2002; Price, Moore, Humphreys, & Wise, 1997) (Table 4). While inferior temporal and parietal cortex have previously been associated with ND (Lee et al., 2009; Ptak et al., 2012), the association of anterior temporal lobe with ND represents a novel finding. Other areas implicated in semantic processing, such as the angular gyrus (Binder, Desai, Graves, & Conant, 2009; Binder et al., 1997; Price, 2012; Price & Mechelli, 2005) and middle temporal gyrus (Tune & Asaridou, 2016), were also identified in this analysis. Consistent with previous studies (Lee et al., 2009; Ptak et al., 2012), a large proportion of lesioned voxels was found in the right white matter, including inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculus, as well as periventricular white matter affecting posterior thalamic radiation (including optic radiation). While lesions to these white matter locations have been previously associated with ND, our study also found a novel association with the splenium of the corpus callosum. The splenium supports interhemispheric transfer of sensory information (Fabri, Pierpaoli, Barbaresi, & Polonara, 2014). A voxelwise T map for this analysis is shown in Figure 2A.
Figure 1.
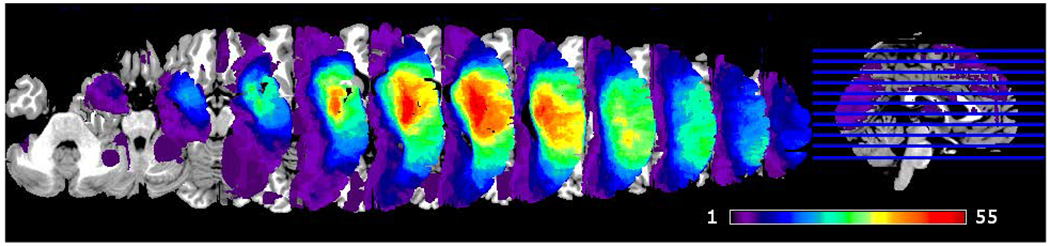
Lesion coverage map. Color bar represents lesion overlap among participants (1 – single participant, 55 - 55 participants with lesion in the area). Image in neurological convention.
Table 4.
VLSM results: The top 10 significant clusters of lesioned areas associated with higher rate of contralesional reading errors. Results thresholded at voxel threshold p < 0.005. Probabilistic anatomical labels are based on Harvard-Oxford Cortical Structural Atlas and JHU White-Matter Tractography Atlas available in FSLview.
| Cluster | Anatomical Region | N voxels | Peak x | Peak y | Peak z | Max T |
|---|---|---|---|---|---|---|
| 1 | 10% Precuneus Cortex, 8% Supracalcarine Cortex, 2% Cuneal Cortex; 13% Forceps major, 2% Inferior Fronto-Occipital Fasciculus R; Splenium of Corpus Callosum | 9073 | 25 | −55 | 19 | 5.44 |
| 2 | 44% Temporal Pole; 1% Inferior Longitudinal Fasciculus R | 1928 | 52 | 21 | −24 | 4.20 |
| 3 | 39% Lateral Occipital Cortex, superior division, 14% Angular Gyrus, 2% Middle Temporal Gyrus, temporooccipital part, 1% Lateral Occipital Cortex, inferior division; 2% Superior longitudinal fasciculus R | 182 | 43 | −61 | 22 | 3.17 |
| 4 | 22% Inferior Temporal Gyrus, posterior division, 20% Middle Temporal Gyrus, posterior division, 1% Middle Temporal Gyrus, temporooccipital part; 1% Superior longitudinal fasciculus (temporal part) R, 1% Superior longitudinal fasciculus R | 88 | 57 | −27 | −15 | 2.94 |
| 5 | 45% Middle Temporal Gyrus, posterior division, 14% Inferior Temporal Gyrus, posterior division, 3% Middle Temporal Gyrus, anterior division, 1% Inferior Temporal Gyrus, anterior division | 86 | 62 | −17 | −22 | 2.66 |
| 6 | 24% Frontal Orbital Cortex, 17% Temporal Pole, 4% Parahippocampal Gyrus, anterior division; 5% Right Amygdala | 49 | 25 | 8 | −22 | 3.47 |
| 7 | 29% Insular Cortex, 6% Frontal Orbital Cortex, 3% Temporal Pole | 42 | 38 | 14 | −16 | 3.18 |
| 8 | 19% Intracalcarine Cortex, 1% Cuneal Cortex; 21% Forceps major; 4% Inferior Fronto-Occipital Fasciculus R’ 2% Inferior Longitudinal Fasciculus R | 38 | 20 | −77 | 10 | 2.71 |
| 9 | 100% Right Parietal White Matter; Posterior Corona Radiata | 17 | 20 | −47 | 30 | 3.35 |
| 10 | 40% Cuneal Cortex, 19% Precuneous Cortex, 3% Supracalcarine Cortex | 16 | 18 | −72 | 26 | 3.48 |
Figure 2.
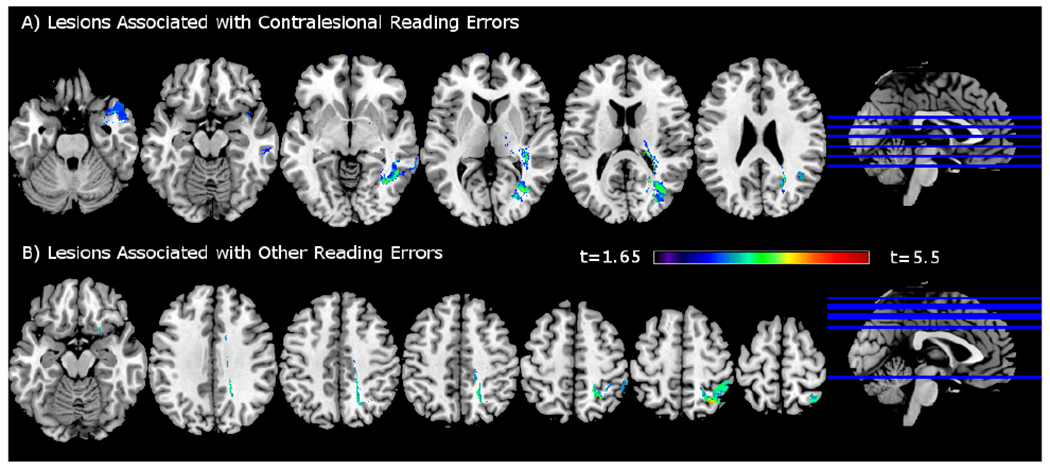
VLSM results. A). Lesions associated with higher contralesional reading errors, controlling for rate of other reading errors and log lesion volume. B). Lesions associated with higher other error rate, controlling for the rate of contralesional reading errors and log lesion volume. T map with voxelwise threshold of p < 0.005. Images in neurological convention.
3.3.1. Other Reading Errors
Controlling for lesion volume and the rate of contralesional reading errors, other reading errors were associated with lesions primarily in the right superior parietal lobule (SPL), part of the distributed spatial attention network (Corbetta & Shulman, 2002, 2011; Husain & Rorden, 2003; Mesulam, 1981), thought to support shifting of spatial attention (Molenberghs, Mesulam, Peeters, & Vandenberghe, 2007). While the SPL is not typically associated with reading, in previous studies of healthy people, it was activated bilaterally in tasks that focused on visual categorization of letter strings. It was also less active in people with developmental dyslexia, who did not have a brain injury, as compared to typical readers (Lobier, Peyrin, Le Bas, & Valdois, 2012; Reilhac, Peyrin, Demonet, & Valdois, 2013). This suggests that the right SPL may play a role in orthographic and phonological processing. In addition, parts of the cingulate gyrus, inferior frontal, orbital, pre- and postcentral, lateral occipital and inferior parietal cortex were involved. Neither pre-, nor postcentral gyrus have previously been implicated in ND reading errors. However, right postcentral gyrus was shown to be less active in people with developmental dyslexia compared to typical readers (Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008), while right precentral gyrus was shown to be involved in semantic processing of spoken words (Price, 2012). Anterior cingulate gyrus is thought to help suppress unintended responses during word retrieval. Frontal orbital cortex is involved in learning of object/pattern discriminations, while lateral occipital cortex supports visual object recognition (Price, 2012). Lesions also affected subcortical white matter, including superior longitudinal fasciculus, the cingulum bundle and inferior fronto-occipital fasciculus (see Table 5 for a complete list of areas). Similar lesions in the inferior parietal white matter in prior studies were associated with non-spatial reaction time deficits in spatial neglect patients (Samuelsson, Hjelmquist, Jensen, Ekholm, & Blomstrand, 1998). A voxelwise T map for this analysis is shown in Figure 2B.
Table 5.
VLSM results: The top 10 significant clusters of lesioned areas associated with higher rate of other reading errors. Results thresholded at voxel threshold p < 0.005. Probabilistic anatomical labels are based on Harvard-Oxford Cortical Structural Atlas and JHU White-Matter Tractography Atlas available in FSLview.
| Cluster | Anatomical Region | N voxels | Peak x | Peak y | Peak z | Max T |
|---|---|---|---|---|---|---|
| 1 | 48% Lateral Occipital Cortex, superior division | 4335 | 31 | −65 | 60 | 4.95 |
| 2 | 7% Cingulate Gyrus, anterior division, 1% Paracingulate Gyrus; 4% Cingulum (cingulate gyrus) R | 215 | 14 | 13 | 32 | 3.46 |
| 3 | 39% Frontal Orbital Cortex, 2% Parahippocampal Gyrus, anterior division, 1% Temporal Pole, 1% Insular Cortex | 37 | 25 | 9 | −17 | 3.06 |
| 4 | 55% Frontal Orbital Cortex, 18% Insular Cortex | 21 | 29 | 16 | −18 | 3.63 |
| 5 | 26% Lateral Occipital Cortex, superior division, 15% Superior Parietal Lobule, 9% Angular Gyrus; 2% Superior longitudinal fasciculus R | 11 | 31 | −58 | 42 | 2.90 |
| 6 | 2% Precuneous Cortex; 3% Inferior fronto-occipital fasciculus R, 1% Cingulum (hippocampus) R | 11 | 21 | −52 | 32 | 2.98 |
| 7 | 16% Precentral Gyrus, 1% Cingulate Gyrus, posterior division | 10 | 16 | −21 | 47 | 2.68 |
| 8 | 32% Lateral Occipital Cortex, superior division, 15% Superior Parietal Lobule, 10% Angular Gyrus; 2% Superior longitudinal fasciculus R | 9 | 34 | −58 | 44 | 2.80 |
| 9 | 35% Supramarginal Gyrus, posterior division, 30% Superior Parietal Lobule, 8% Angular Gyrus, 4% Postcentral Gyrus, 1% Supramarginal Gyrus, anterior division; 1% Superior longitudinal fasciculus R | 8 | 42 | −41 | 50 | 2.74 |
| 10 | 27% Frontal Pole, 12% Frontal Orbital Cortex, 1% Inferior Frontal Gyrus, pars triangularis; 3% Inferior fronto-occipital fasciculus R | 7 | 43 | 37 | −9 | 2.65 |
3.4. Word Characteristics and Reading Errors
We first explored the pattern of correlations among linguistic properties of each word and the corresponding rate of contralesional and other errors averaged across participants. The uncorrected upper triangular of the correlation matrix is presented in Figure 3 for data visualization. We also performed multiple comparisons correction for the correlation tests that involved the rate of contralesional and the rate of other errors using False Discovery Rate (FDR; Benjamini & Hochberg, 1995). The FDR significance level was calculated to be q=0.032. The rate of contralesional errors was positively associated with word length (r = 0.60, p < 0.001, q = 0.004), bigram count (r = 0.51, p < 0.001, q = 0.007), and trigram count (r = 0.46, p < 0.001, q = 0.01), suggesting that the rate of errors increased for longer words and for words with many orthographic competitors. The rate of contralesional errors was negatively correlated with word concreteness (r = −0.36, p < 0.05, q = 0.029) and imageability (r = −0.39, p < 0.05, q = 0.021), suggesting that more concrete words that easily bring an image to mind yield lower rate of contralesional errors. The rate of other errors was positively correlated with length (r = 0.30, p <0.05, q = 0.025), bigram count (r = 0.42, p <0.001, q = 0.014) and trigram count (r = 0.26, p < 0.05, q = 0.032).
Figure 3.
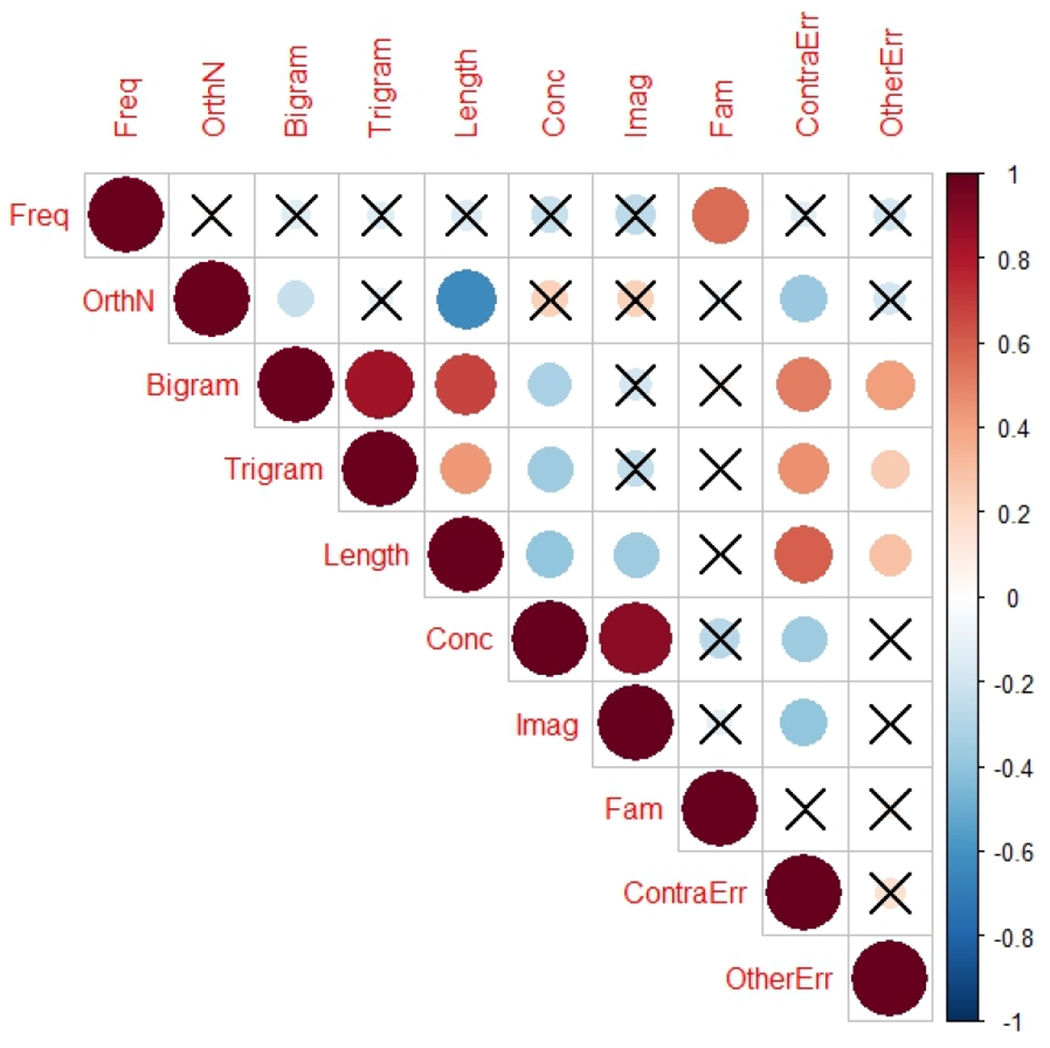
Bivariate correlations of word properties and reading errors. Correlations not significant at p < 0.05 are crossed out. OrthN – orthographic neighborhood density, Conc – concreteness, Imag – imageability, Freq – frequency, Bigram – constrained bigram count, Trigram – constrained trigram count, length – word length as number of letters, ContraErr – rate of contralesional reading errors, OtherErr – rate of other reading errors.
To account for any common variance in the influence of linguistic variables on performance we used a stepwise regression analysis to predict the rate of reading errors. We first focused on measuring the contribution of orthographic factors. The variables used in this analysis were word frequency, orthographic neighborhood density, constrained bigram and trigram counts, and word length. Word length and trigram count were significant predictors of contralesional errors, F(2, 71) = 23.40, p < 0.001, accounting for 64% of the variance. Longer length and greater number of trigram neighbors produced more contralesional errors (Figure 4). Bigram count was a significant predictor of other reading errors, F(1, 71) = 14.72, p < 0.001, accounting for 42% of the variance. We studied the semantic variables, concreteness and imageability, in a separate stepwise regression analysis. Among the semantic variables, greater rating of word concreteness was associated with a reduced rate of contralesional errors, F(1, 36) = 5.31, p < 0.05. The rate of other reading errors was not associated with semantic variables. The latter finding is consistent with our lesion-deficit analysis, where the rate of contralesional errors was associated with more extensive damage to temporo-parietal brain areas thought to subserve semantic processing.
Figure 4.
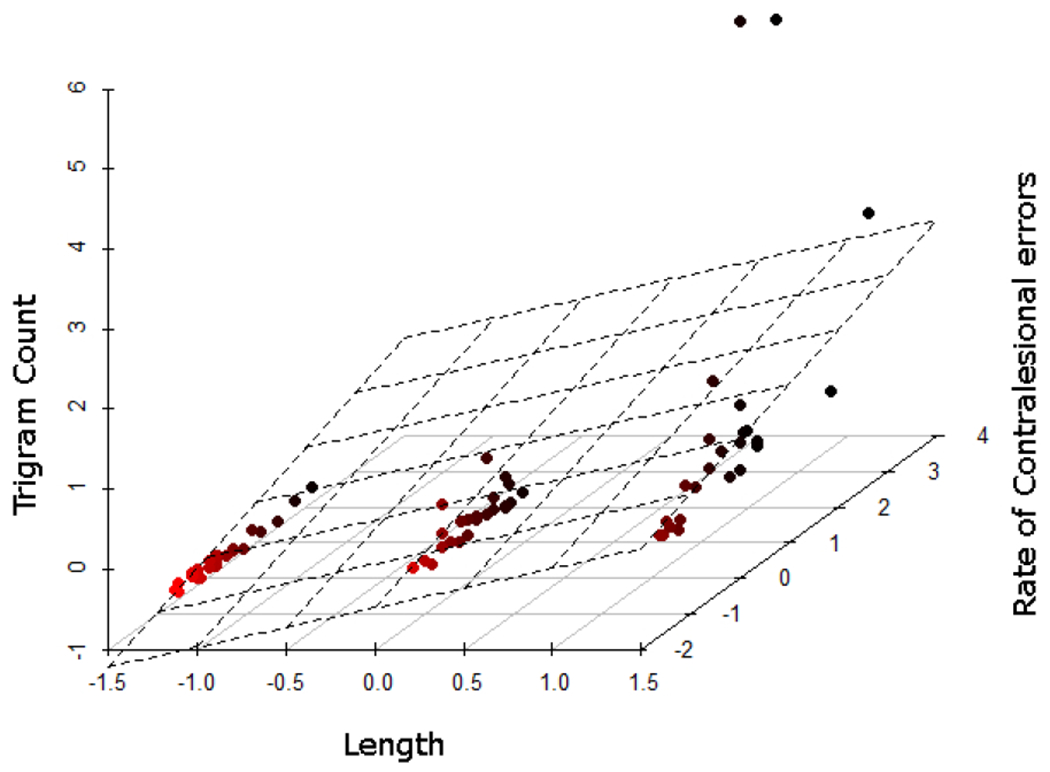
Item-wise multiple regression plot: Increased word length and trigram count amplify contralesional reading errors. Three-dimensional plane is superimposed on mean-centered regression coefficients to facilitate visualization.
To further understand the observed effects of trigram count and concreteness on contralesional reading errors, we tested if neglect severity modulated those effects. We created 2 new variables to define the size of both effects. First, a median split was performed on the trigram counts and the ratings of concreteness. The trigram count below 7 was considered low and above 7 was considered high. The concreteness rating below 449 was considered low and a rating above that number was considered high. We then calculated the error rate difference for words with high vs. low trigram count and high vs. low concreteness. This was done by subtracting the rate of contralesional errors for low from high trigram count words and for low from high concreteness words for each participant. Thus, the 2 new variables were “trigram effect” (i.e., trigram-based error rate difference) and “concreteness effect” (concreteness-based error rate difference). We then conducted two separate pairwise correlation analyses across participants for those two variables with the BIT-c and KF-NAP scores. Only the trigram effect was associated with spatial neglect severity. Specifically, trigram effect was positively correlated with the BIT (r=−0.49) and negatively correlated with the CBS via KF-NAP (r=0.33 p<.001). This result suggested that patients with more severe spatial neglect (as indicated by lower BIT-c and higher CBS scores) produced more contralesional reading errors in words with many trigram competitors (such as reason (really, prison) and system (hostel, mystic), as compared to words with fewer trigram competitors (such as hungry and anchor). This result is consistent with the idea that the interaction between visuospatial and linguistic processing in patients with spatial neglect depends on the degree of spatial impairment, which may be attentional as suggested by previous research (Arduino et al., 2002; Brunn & Farah, 1991).
4. General Discussion
In this study, we demonstrate that the rate of neglect dyslexia reading errors varies as a function of the word’s lexical and sub-lexical orthographic and lexico-semantic characteristics. Consistent with prior studies, we found that the rate of contralesional reading errors increased for longer words. We also demonstrate, for the first time, that words with more orthographic trigram competitors produce more ND reading errors and that the size of the trigram effect correlates with spatial neglect severity. Although semantic variables have been examined in past case studies of ND (Arguin & Bub, 2010; Riddoch et al., 1990), our study is the first to show that high concreteness reduces the rate of contralesional reading errors. Using voxel-wise lesion symptom analysis with continuous error rate data and the largest to-date sample of spatial neglect participants, we showed that ND reading errors are associated with lesions in the precuneus, temporal pole, inferior temporal sulcus, middle temporal gyrus, and angular gyrus. A large proportion of lesions involved the temporo-parietal white matter, including the splenium of the corpus callosum. Several of these brain regions overlap with previously identified lesion correlates of ND (Lee et al., 2009; Ptak et al., 2012). However, temporal pole and the splenium have not been previously implicated in studies of ND. We next discuss implications of these results in the context of previous work in word reading and spatial neglect. Please see Fig. 5 for a graphical representation of our reading model discussed below.
Figure 5.
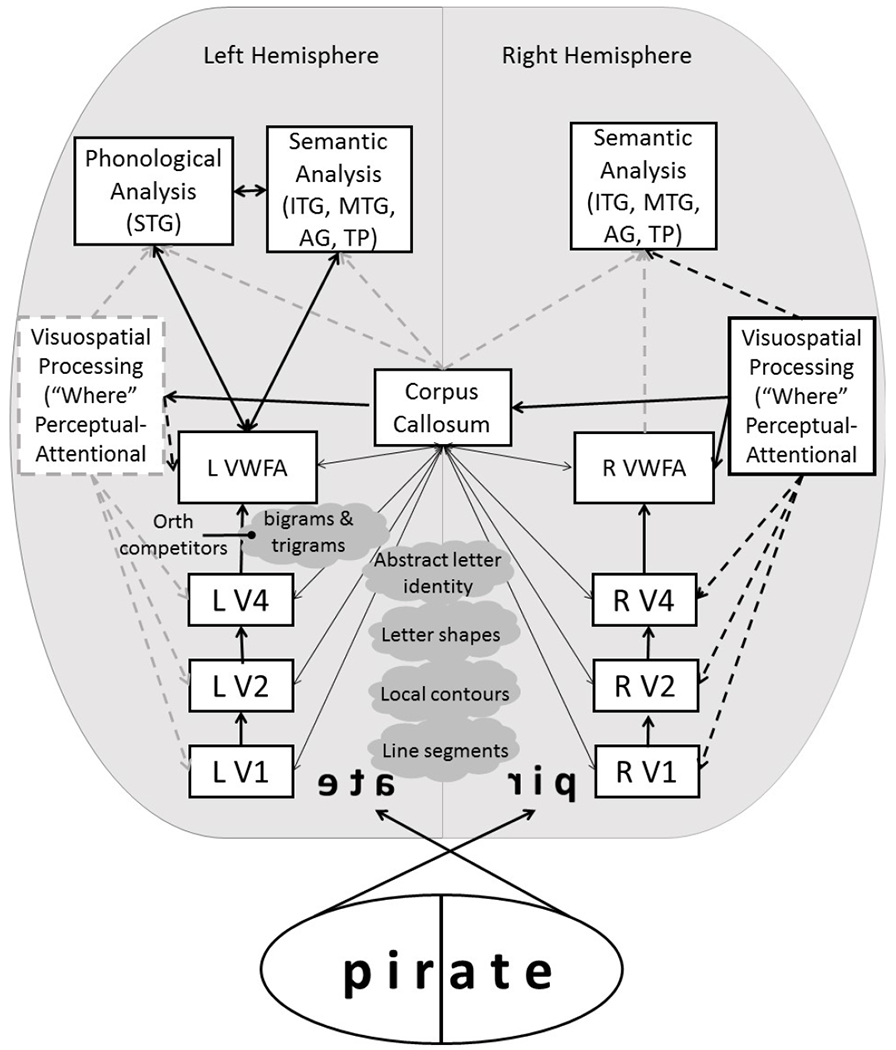
Tentative neurocognitive model of visuospatial and linguistic processing during single word reading. See Cohen et al., (2003) for a similar model. Contralateral hemisphere processes each half of the centrally presented word through a cascade of activation in the visual areas. Activation of bigrams and trigrams (2- and 3-letter clusters) is modulated by inhibition form orthographic competitors. Bigram, trigram, and word form information is processed in the ventral occipitotemporal cortex up to the level of the left Visual Word Form Area (VWFA). For word segments on the left, information is relayed to the left VWFA through interhemispheric connections via the corpus callosum. VWFA projects to brain structures involved in phonological and semantic processing. Thus, reading arises as an interactive activation of orthographic, phonological and semantic representations in the left hemisphere. The right hemisphere homologue of the VWFA supports abstract letter identification and higher order association areas in the right hemisphere may support semantic analysis. Visuospatial processing interacts with word recognition at each processing level. Arrow weight and outline represents the relative role of a given input in the reading process. (STG – superior temporal gyrus; ITG – inferior temporal gyrus; MTG – middle temporal gyrus; AG – angular gyrus, TP – temporal pole; V1-V4 – primary visual and visual association areas).
4.1. Orthographic Encoding in ND
The left hemisphere’s reading function may be relatively unaffected by word length, and capable of parallel letter recognition. This was shown under conditions of free foveal reading where the optimal gaze position falls left of the midline (Nazir, 2000). In contrast, the right hemisphere may process each additional letter in a serial fashion. For example, Lavidor et al. (2001) demonstrated that lexical decisions to briefly presented words (150 ms) were slower with each additional letter to the left, but not to the right, of a central fixation. Thus, longer words could increase spatial attentional processing demands on the right hemisphere during the earliest stages of visual word recognition (letter and word length analysis in retinotopic visual areas V1-V4) (Cohen et al., 2003; Mechelli, Friston, & Price, 2000), where the contralateral hemisphere processes each half of the centrally presented word. In this study, participants made more errors on the left side of longer words. It is possible that the normally slow serial processing of letters in the right hemisphere was exacerbated by spatial neglect. Thus, letters entering the contralesional, neglected, left hemi-field could have received deficient perceptual-attentional orthographic processing (Brunn & Farah, 1991).
Trigram competitors likely affect the next stage of orthographic processing where interhemispheric connectivity is important. In this stage, processing of letter clusters continues in the inferior occipitotemporal cortex culminating in the posterior fusiform gyrus, where a functionally-defined left visual word form area (VWFA) supports abstract word-form representations (e.g., apple and apple have the same font-invariant abstract representation) (Cohen et al., 2000; Cohen et al., 2002; Dehaene et al., 2004; Glezer, Jiang, & Riesenhuber, 2009). Sensitivity to progressively more complex orthographic units (single letters, bigrams, trigrams, quadrigrams, etc.) increases along the posterior to anterior axis of the left occipitotemporal cortex up to the whole word level in the VWFA (Taylor, Davis, & Rastle, 2019; Vinckier et al., 2007). For word segments appearing in the left visual field, information is relayed to the left hemisphere through interhemispheric connections in the splenium of the corpus callosum and the posterior horns of the lateral ventricles (Cohen et al., 2003). In people with spatial neglect, less orthographic information may be available to the right fusiform cortex. Furthermore, our lesion-symptom analysis revealed that the biggest cluster of lesions associated with ND extended subcortically to the splenium. Lesions to the splenium and periventricular white matter may have disrupted the transfer of orthographic information from the right fusiform cortex to the left VWFA and this undoubtedly increased the likelihood of misreading the left part of the word. This account assumes that refixation within a word (i.e., a second fixation where eye gaze is maintained on a particular location) does not occur, even when the exposure duration is long enough to allow it. This is possible, considering that words were presented such that they fell entirely within the foveal and parafoveal region, reducing the likelihood of saccades (Blais et al., 2009). Supporting the important role of the splenium in reading, Marsh and Hillis (2005) reported a reversible reading deficit associated with transient hypoperfusion of this area. Splenium lesions can also be associated with chronic spatial neglect (Lunven et al., 2015), although many of patients with damage to this region also have cingulate or ventral temporal damage based on the arterial perfusion of splenial regions (Kim, 2016).
Words with common trigrams may pose a particular challenge for patients with spatial neglect. Trigrams are longer orthographic units, which are more likely to be divided across the visual fields, making identification of any particular trigram more difficult. In addition, words and their competitors where a trigram occurs entirely in one hemi-field will be difficult to disambiguate for a patient with left spatial neglect, who may be less able to use information from the left side of the word (e.g. demand vs. strand; preset vs. preach). It is not surprising, therefore, that contralesional reading errors were more likely for words with higher number of trigram competitors.
4.2. Semantic Processing in ND
Visuospatial processing is necessary for word recognition, because each letter identity has to be encoded with its allocentric spatial location. Semantic processing can influence the distribution of spatial attention by modulating a word’s salience (Anderson, 1999) or its threshold for activation through interactive connections between letter clusters and lexico-semantic units (Mozer & Behrmann, 1990). For example, attention is captured by real words compared to pronounceable but meaningless pseudowords, which is reflected as higher reading accuracy for real words among neglect patients (Arduino et al., 2002; Brunn & Farah 1991; Worthington, 1996). Similarly, attention may be captured by real words contained within longer words, especially when the longer words are of low frequency, supporting interactive co-activation between visuospatial, orthographic, and semantic processing (Reinhart et al., 2016). In this study, we expected that semantically rich, concrete representations would improve reading in neglect dyslexia. In line with this hypothesis, words with high level of concreteness yielded fewer contralesional errors. One potential mechanism for this improvement is that words with high level of concreteness may be more readily available for access than abstract words. In healthy adults, greater semantic concreteness is associated with higher reading accuracy and shorter response time (Balota, Cortese, Sergent-Marshall, Spieler, & Yap, 2004; Cortese & Schock, 2013; Strain, Patterson, & Seidenberg, 1995; Taylor, Duff, Woollams, Monaghan, & Ricketts, 2015; Yap, Pexman, Wellsby, Hargreaves, & Huff, 2012). Similarly, in individuals with spatial neglect, highly concrete words may be easier to retrieve especially given an incomplete orthographic representation. In addition, some of the ability to read concrete words in spatial neglect may rely on the right hemisphere. Observations of split-brain and left hemispherectomy patients suggest that the right hemisphere is able to comprehend spoken and written language, and is capable of rudimentary production (Searleman, 1977). Consistent with the concreteness effect found in this study, some split-brain and left hemispherectomy patients are also able to read concrete and high imageability words better than abstract words (Coslett & Saffran, 1998).
4.3. Neuroanatomical correlates of ND
Our lesion-symptom analysis shows that more than one brain area is associated with ND. We found that the splenium, periventricular and temporo-parietal white matter, temporal pole, precuneus, angular gyrus, inferior temporal sulcus, and posterior middle temporal gyrus were among areas where lesions predicted a greater rate of contralesional errors. In part, these findings overlap with the existing literature on the neural substrates of ND. Specifically, previous studies also identified the inferior and middle temporal and inferior parietal regions, as well as white matter lesions, as important for ND, lending greater confidence to this relationship (Beschin, Cisari, Cubelli, & Della Sala, 2014; Lee et al., 2009; Ptak et al., 2012). Our analysis also yielded new associations, such as the greater rate of contralesional reading errors in patients with splenium and temporal pole lesions. While the splenium supports interhemispheric connectivity (Fabri et al., 2014), the right temporal pole is important for semantic processing (Lambon Ralph et al., 2009), and our findings of concreteness effects on the rate of contralesional errors are consistent with this association.
Our findings add more evidence to the literature suggesting the importance of middle and inferior temporal and parietal cortices in reading comprehension and in visuospatial processing. When recruited on the left side of the brain, these regions were implicated in semantic processing (Binder et al., 2009; Binder et al., 1997; Graves, Desai, et al., 2010; Price, 2012). Right angular gyrus and precuneus were also active in tasks tapping into semantics (Graves, Binder, Desai, Conant, & Seidenberg, 2010). In addition, right temporal and inferior parietal regions belong to a network of areas important for spatial attention and cognition and are often found to be lesioned in patients with spatial neglect (Chechlacz et al., 2012; Karnath, Rorden, & Ticini, 2009; Mort et al., 2003; Ptak & Schnider, 2011; Samuelsson et al., 1998; Samuelsson et al., 1997; Vallar & Perani, 1986). For example, right angular and middle temporal gyri lesions are associated with spatial asymmetry in performance on the representational drawing task (Kenzie et al., 2015). The important role of these areas in both visuospatial and linguistic processing is consistent with our novel finding of the influence of semantic variables on ND. While the role of semantics has been considered in prior case studies of ND, our study was the first to show that in a large sample of spatial neglect participants contralesional reading errors were modulated by the words’ semantic properties.
5. Study limitations and future directions
Our study investigated ND in a large group of right-hemisphere stroke survivors with left spatial neglect using a single-word reading task. We did not include participants with left-hemisphere strokes or with ipsilesional spatial neglect. However, it is important to note that 89% of published case studies show a definite lateral asymmetry in ND to the left side of words (Vallar et al., 2010). Nevertheless, future studies should include patients with left-hemisphere stroke and reading deficit in the right side of words as ND in this group remains understudied. In this study, we did not systematically document leukoaraiosis, defined as pathologically appearing white matter, because participants’ brain images were obtained from different acute care hospitals, which used different scanning sequences. Leukoaraiosis is strongly related to ischemic stroke and is associated with worse long-term outcomes and cognitive disturbances (Marek, Horyniecki, Fraczek, & Kluczewska, 2018). It would be important to include multiple white matter pathology assessments in future work to obtain information on the contribution of this pathology to ND.
A limitation of the present study was that all the letters were presented in a typical way (i.e., right side up) and in the same location (i.e., aligned with body center at the eye level), confounding the body-centered, stimulus-centered, and object-centered reference frames (Hillis & Caramazza, 1995; Medina et al., 2009). Moreover, we did not attempt to dissociate “Where” spatial perceptual-attentional, spatial representational, or spatial motor-intentional “Aiming” processes, or examine other dissociable aspects of the spatial neglect syndrome, to understand the mechanisms of visuospatial-linguistic interaction in more detail. Thus, our findings may reflect influences of several types of spatial deficits that can produce ND. As we review above, we found semantic influences on ND, and associated lesions in brain regions important to spatial representation, which suggest that both perceptual-attentional “Where” and representational “Where” spatial cognition contribute to ND. Future studies should analyze the primary stage of spatial cognitive processing at which our patients had deficits, to determine if this supports a spatial representational mechanism of ND (Barrett, 2014). We also did not assess spatial or spatial-syntactic properties of the words being read (Barrett & Craver-Lemley, 2008; Chatterjee, Maher, & Heilman, 1995). In future group studies it would be useful to include spatial-syntactic tasks for cross-comparison.
In this study, we characterized people as having spatial neglect using instruments with excellent predictive validity to identify functional disability. Spatial motor “Aiming” and motor exploratory deficits may be particularly important to real-life behaviors (Barrett & Muzaffar, 2014; Goedert et al., 2012a). These deficits may affect oculomotor movements as well as body, limb and head movements (Barrett, 2018). In this study, participants’ eye movements were not monitored by an eye tracker. While we expect that our patients had directional hypokinesia of leftward scanning and saccades (Primativo et al., 2015), these variables were not measured in the present study. Future studies measuring eye movements and relating these patterns of eye movements to spatial Aiming bias assessed by other tasks, as well as reading errors, can clarify this issue. Also, including specific tasks to evaluate non-spatial deficits associated with spatial neglect (Robertson et al., 2001) will help ensure that right hemisphere associations with ND are correctly attributed to spatial neglect, versus right brain-associated deficits. Lastly, examining the functional impact of reading problems on daily life will be very important to bring spatial functional deficits and reading functional deficits together in studies of activity and life participation.
6. Conclusions
In this study, we studied reading errors in a large sample of individuals with spatial neglect. We demonstrated for the first time that the occurrence of reading errors in the contralesional (left) side of words increased with the number of sub-lexical orthographic competitors and decreased with word concreteness. Shorter concrete words, with fewer orthographic neighbors seem to be optimal for reducing the rates of contralesional reading errors. We confirmed the role if inferior parietal and temporal lesions in ND and identified novel lesion correlates in the splenium and temporal pole. We argue that disruption of the splenium leads to a loss of information from the contralesional side of the word and, thus, produces reading errors. Lesions in areas like the temporal pole, which are thought to subserve semantic processing, may mediate the observed effect of word concreteness on reading accuracy in ND. Future comprehensive models of reading should consider the effects and interactions of spatial attention mechanisms and visuospatial processing on reading performance.
Acknowledgements
Data collection for this project was supported by NIH/NICHD/NCMRR grant K24-HD062647, R01NS055808-04, K02-NS47099, and NIDILRR grant 901F0037 to AMB (clinicaltrials.gov: NCT00990353, NCT00350012, NCT00989430). Contents of this manuscript do not necessarily represent the policy of NIDILRR or the Administration for Community Living, and one should not assume endorsement by the federal government. We thank Anne Foundas for providing a validation assessment of the lesion maps used in this study.
References
- Adair JC, & Barrett AM (2008). Spatial neglect: Clinical and neuroscience review: A wealth of information on the poverty of spatial attention. Annals of the New York Academy of Sciences, 1142, 21–43. doi: 10.1196/annals.1444.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B (1999). A computational model of neglect dyslexia. Cortex, 35(2), 201–218. doi: 10.1016/s0010-9452(08)70794-9 [DOI] [PubMed] [Google Scholar]
- Arduino LS, Burani C, & Vallar G (2002). Lexical effects in left neglect dyslexia: A study in Italian patients. Cognitive Neuropsychology, 19(5), 421–444. doi: 10.1080/026432902440000013 [DOI] [PubMed] [Google Scholar]
- Arduino LS, Burani C, & Vallar G (2003). Reading aloud and lexical decision in neglect dyslexia patients: A dissociation. Neuropsychologia, 41(8), 877–885. doi: 10.1016/s0028-3932(03)00015-0 [DOI] [PubMed] [Google Scholar]
- Arguin M, & Bub D (2010). Lexical constraints on reading accuracy in neglect dyslexia. Cognitive Neuropsychology, 14(5), 765–800. doi: 10.1080/026432997381448 [DOI] [Google Scholar]
- Azouvi P, Marchal F, Samuel C, Morin L, Renard C, LouisDreyfus A, … Bergego C (1996). Functional consequences and awareness of unilateral neglect: Study of an evaluation scale. Neuropsychological Rehabilitation, 6(2), 133–150. doi: 10.1080/713755501 [DOI] [Google Scholar]
- Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, … French Collaborative Study Group on Assessment of Unilateral, N. (2002). Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. Journal of Neurology Neurosurgery and Psychiatry, 73(2), 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Cortese MJ, Sergent-Marshall SD, Spieler DH, & Yap M (2004). Visual word recognition of single-syllable words. Journal of Experimental Psychology: General, 133(2), 283–316. doi: 10.1037/0096-3445.133.2.283 [DOI] [PubMed] [Google Scholar]
- Barrett AM (2014). Perceptual-attentional “where” and motor-intentional “aiming” spatial bias In Chatterjee A & Coslett HB (Eds.), The Roots of Cognitive Neuroscience: Behavioral Neurology and Neuropsychology. New York: Oxford University Press. [Google Scholar]
- Barrett AM, & Burkholder S (2006). Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. Journal of Rehabilitation Research and Development, 43(3), 337–345. doi: 10.1682/JRRD.2005.01.0015 [DOI] [PubMed] [Google Scholar]
- Barrett AM, & Craver-Lemley CE (2008). Is it what you see, or how you say it? Spatial bias in young and aged subjects. Journal of the International Neuropsychological Society, 14(4), 562–570. doi: 10.1017/s1355617708080764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AM, & Muzaffar T (2014). Spatial cognitive rehabilitation and motor recovery after stroke. Current Opinion in Neurology, 27(6), 653–658. doi: 10.1097/wco.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, & Dronkers NF (2003). Voxel-based lesion-symptom mapping. Nature Neuroscience, 6(5), 448–450. doi: 10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B, Statistical methodology, 57(1), 289–300. [Google Scholar]
- Beschin N, Cisari C, Cubelli R, & Della Sala S (2014). Prose reading in neglect. Brain and Cognition, 84(1), 69–75. doi: 10.1016/j.bandc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. doi: 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, & Prieto T (1997). Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience, 17(1), 353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn JL, & Farah MJ (1991). The relation between spatial attention and reading: Evidence from the neglect syndrome. Cognitive Neuropsychology, 8(1), 59–75. doi: 10.1080/02643299108253367 [DOI] [Google Scholar]
- Caramazza A, & Hillis AE (1990a). Levels of representation, co-ordinate frames, and unilateral neglect. Cognitive Neuropsychology, 7(5-6), 391–445. doi: 10.1080/02643299008253450 [DOI] [Google Scholar]
- Caramazza A, & Hillis AE (1990b). Spatial representation of words in the brain implied by studies of a unilateral neglect patient. Nature, 346(6281), 267–269. doi: 10.1038/346267a0 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Chatterjee A (1995). Cross-over, completion and confabulation in unilateral spatial neglect. Brain, 118, 455–465. doi: 10.1093/brain/118.2.455 [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Maher LM, & Heilman KM (1995). Spatial characteristics of thematic role representation. Neuropsychologia, 33(5), 643–648. doi: 10.1016/0028-3932(94)00134-b [DOI] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Roberts KL, Bickerton WL, Lau JKL, & Humphreys GW (2012). The prognosis of allocentric and egocentric neglect: Evidence from clinical scans. PLoS ONE, 7(11), e47821. doi: 10.1371/journal.pone.0047821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chen CC, Hreha K, Goedert KM, & Barrett AM (2015). Kessler Foundation Neglect Assessment Process uniquely measures spatial neglect during activities of daily living. Archives of Physical Medicine and Rehabilitation, 96(5), 869–876. doi: 10.1016/j.apmr.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Goedert KM, Shah P, Foundas AL, & Barrett AM (2014). Integrity of medial temporal structures may predict better improvement of spatial neglect with prism adaptation treatment. Brain Imaging and Behavior, 8(3), 346–358. doi: 10.1007/s11682-012-9200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, & Hreha K (2015). KF-NAP 2015 Manual Retrieved from https://www.kflearn.org/courses/kf-nap-2015-manuals
- Chen P, Hreha K, Kong Y, & Barrett AM (2015). Impact of spatial neglect in stroke rehabilitation: Evidence from the setting of an inpatient rehabilitation facility. Archives of Physical Medicine and Rehabilitation, 96(8), 1458–1466. doi: 10.1016/j.apmr.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL (2002). The effect of letter spacing on reading speed in central and peripheral vision. Investigative Ophthalmology and Visual Science, 43(4), 1270–1276. [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, & Michel F (2000). The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain, 123(Pt 2), 291–307. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, & Dehaene S (2002). Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain, 125(Pt 5), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehéricy S, Samson Y, Obadia M, … Dehaene S (2003). Visual word recognition in the left and right hemispheres: Anatomical and functional correlates of peripheral alexias. Cerebral Cortex, 13(12), 1313–1333. doi: 10.1093/cercor/bhg079 [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, & Ziegler J (2001). DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review, 108(1), 204–256. doi: 10.1037//0033-295x.108.1.204 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34, 569–599. doi: 10.1146/annurev-neuro-061010-113731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese MJ, & Schock J (2013). Imageability and age of acquisition effects in disyllabic word recognition. Quarterly Journal of Experimental Psychology, 66(5), 946–972. doi: 10.1080/17470218.2012.722660 [DOI] [PubMed] [Google Scholar]
- Coslett HB, & Saffran EM (1998). Reading and the right hemisphere: Evidence from acquired dyslexia In Beeman MJ & Chiarello C (Eds.), Right Hemisphere Language Comprehension. New York: Psychology Press. [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, … White KD (2009). Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain and Language, 111(2), 73–85. doi: 10.1016/j.bandl.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, & Cohen L (2004). Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychological Science, 15(5), 307–313. doi: 10.1111/j.0956-7976.2004.00674.x [DOI] [PubMed] [Google Scholar]
- Fabri M, Pierpaoli C, Barbaresi P, & Polonara G (2014). Functional topography of the corpus callosum investigated by DTI and fMRI. World Journal of Radiology, 6(12), 895–906. doi: 10.4329/wjr.v6.i12.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis P, Goedert KM, & Barrett AM (2011). Prism adaptation differently affects motor-intentional and perceptual-attentional biases in healthy individuals. Neuropsychologia, 49(9), 2718–2727. doi: 10.1016/j.neuropsychologia.2011.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassinetti F, Angeli V, Meneghello F, Avanzi S, & Ladavas E (2002). Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain, 125(Pt 3), 608–623. [DOI] [PubMed] [Google Scholar]
- Galletta EE, Campanelli L, Maul KK, & Barrett AM (2014). Assessment of neglect dyslexia with functional reading materials. Topics in Stroke Rehabilitation, 21(1), 75–86. doi: 10.1310/tsr2101-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, & Riesenhuber M (2009). Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron, 62(2), 199–204. doi: 10.1016/j.neuron.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Chen P, Botticello A, Masmela JR, Adler U, & Barrett AM (2012a). Psychometric evaluation of neglect assessment reveals motor-exploratory predictor of functional disability in acute-stage spatial neglect. Archives of Physical Medicine and Rehabilitation, 93(1), 137–142. doi: 10.1016/j.apmr.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Chen P, Botticello A, Masmela JR, Adler U, & Barrett AM (2012b). Psychometric evaluation of neglect assessment reveals motor-exploratory predictor of functional disability in acute-stage spatial neglect. Archives of Physical Medicine and Rehabilitation, 93, 137–142. doi: 10.1016/j.apmr.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Binder JR, Desai RH, Conant LL, & Seidenberg MS (2010). Neural correlates of implicit and explicit combinatorial semantic processing. Neuroimage, 53(2), 638–646. doi: 10.1016/j.neuroimage.2010.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, & Binder JR (2010). Neural systems for reading aloud: A multiparametric approach. Cerebral Cortex, 20(8), 1799–1815. doi: 10.1093/cercor/bhp245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm MW, & Seidenberg MS (2004). Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review, 111(3), 662–720. doi: 10.1037/0033-295X.111.3.662 [DOI] [PubMed] [Google Scholar]
- Haywood M, & Coltheart M (2000). Neglect dyslexia and the early stages of visual word recognition. Neurocase, 6(1), 33–44. doi: 10.1093/neucas/6.1.33 [DOI] [Google Scholar]
- Heilman KM, Watson RT, & Valenstein E (2012). Neglect and related disorders In Heilman KM & Valenstein E (Eds.), Clinical Neuropsychology (5th ed., pp. 296–348). New York: Oxford University. [Google Scholar]
- Hillis AE (2006). Neurobiology of unilateral spatial neglect. Neuroscientist, 12(2), 153–163. doi: 10.1177/1073858405284257 [DOI] [PubMed] [Google Scholar]
- Hillis AE, & Caramazza A (1990). The effects of attentional deficits on reading and spelling In Caramazza A (Ed.), Cognitive neuropsychology and neurolinguistics: Advances in models of cognitive function and impairment (pp. 211–275). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Hillis AE, & Caramazza A (1995). A framework for interpreting distinct patterns of hemispatial neglect. Neurocase, 1(3), 189–207. doi: 10.1080/13554799508402364 [DOI] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, & Degaonkar M (2005). Anatomy of spatial attention: Insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience, 25(12), 3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Marsh EB, Barker P, & Degaonkar M (2005). The neglected role of the right hemisphere in spatial representation of words for reading. Aphasiology, 19(3–5), 225–238. doi: 10.1080/02687030444000705 [DOI] [Google Scholar]
- Husain M, & Rorden C (2003). Non-spatially lateralized mechanisms in hemispatial neglect. Nature Reviews Neuroscience, 4(1), 26–36. doi: 10.1038/nrn1005 [DOI] [PubMed] [Google Scholar]
- Jackson RL, Ralph MAL, & Pobric G (2015). The timing of anterior temporal lobe involvement in semantic processing. Journal of Cognitive Neuroscience, 27(7), 1388–1396. doi: 10.1162/jocn_a_00788 [DOI] [PubMed] [Google Scholar]
- Karnath HO, Rorden C, & Ticini LF (2009). Damage to white matter fiber tracts in acute spatial neglect. Cerebral Cortex, 19(10), 2331–2337. doi: 10.1093/cercor/bhn250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzie JM, Girgulis KA, Semrau JA, Findlater SE, Desai JA, & Dukelow SP (2015). Lesion sites associated with allocentric and egocentric visuospatial neglect in acute stroke. Brain Connectivity, 5(7), 413–422. doi: 10.1089/brain.2014.0316 [DOI] [PubMed] [Google Scholar]
- Kim JS (2016). Posterior cerebral artery disease In Grotta J, Albers G, Broderick J, Lo E, Sacco R, & Wong L (Eds.), Stroke: Pathophysiology, Diagnosis, and Management (pp. 393–394): Elsevier. [Google Scholar]
- Lambon Ralph MA, Pobric G, & Jefferies E (2009). Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex, 19(4), 832–838. doi: 10.1093/cercor/bhn131 [DOI] [PubMed] [Google Scholar]
- Lauro LJR, Tettamanti M, Cappa SF, & Papagno C (2008). Idiom comprehension: A prefrontal task? Cerebral Cortex, 18(1), 162–170. doi: 10.1093/cercor/bhm042 [DOI] [PubMed] [Google Scholar]
- Lavidor M, Ellis AW, Shillcock R, & Bland T (2001). Evaluating a split processing model of visual word recognition: Effects of word length. Cognitive Brain Research, 12(2), 265–272. doi: 10.1016/s0926-6410(01)00056-8 [DOI] [PubMed] [Google Scholar]
- Lee BH, Suh MK, Kim EJ, Seo SW, Choi KM, Kim GM, … Na DL (2009). Neglect dyslexia: Frequency, association with other hemispatial neglects, and lesion localization. Neuropsychologia, 47(3), 704–710. doi: 10.1016/j.neuropsychologia.2008.11.027 [DOI] [PubMed] [Google Scholar]
- Lobier M, Peyrin C, Le Bas JF, & Valdois S (2012). Pre-orthographic character string processing and parietal cortex: A role for visual attention in reading? Neuropsychologia, 50(9), 2195–2204. doi: 10.1016/j.neuropsychologia.2012.05.023 [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, & Eden GF (2008). A metes-analysis of functional neuroimaging studies of dyslexia In Eden GF & Flower DL (Eds.), Learning, Skill Acquisition, Reading, and Dyslexia (Vol. 1145, pp. 237–259). [DOI] [PubMed] [Google Scholar]
- Marek M, Horyniecki M, Fraczek M, & Kluczewska E (2018). Leukoaraiosis: New concepts and modern imaging. Polish Journal of Radiology, 83, e76–e81. doi: 10.5114/pjr.2018.74344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EB, & Hillis AE (2005). Cognitive and neural mechanisms underlying reading and naming: Evidence from letter-by-letter reading and optic aphasia. Neurocase, 11(5), 325–337. doi: 10.1080/13554790591006320 [DOI] [PubMed] [Google Scholar]
- McClelland JL, & Rumelhart DE (1981). An interactive activation model of context effects in letter perception: I. An account of basic findings. Psychological Review, 88(5), 375–407. doi: 10.1037//0033-295x.88.5.375 [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, & Price CJ (2000). The effects of presentation rate during word and pseudoword reading: a comparison of PET and fMRI. Journal of Cognitive Neuroscience, 12 Suppl 2, 145–156. doi: 10.1162/089892900564000 [DOI] [PubMed] [Google Scholar]
- Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, Davis C, … Hillis AE (2009). Neural substrates of visuospatial processing in distinct reference frames: Evidence from unilateral spatial neglect. Journal of Cognitive Neuroscience, 21(11), 2073–2084. doi: 10.1162/jocn.2008.21160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler D, & Binder J (2005). MCWord: An on-line orthographic database of hte English language. Retrieved from http:/www.neuro.mcw.edu/mcword
- Mesulam MM (1981). A cortical network for directed attention and unilateral neglect. Annals of Neurology, 10(4), 309–325. doi: 10.1002/ana.410100402 [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, & Vandenberghe RR (2007). Remapping attentional priorities: Differential contribution of superior parietal lobule and intraparietal sulcus. Cerebral Cortex, 17(11), 2703–2712. doi: 10.1093/cercor/bhl179 [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, & Husain M (2003). The anatomy of visual neglect. Brain, 126, 1986–1997. doi: 10.1093/brain/awg200 [DOI] [PubMed] [Google Scholar]
- Mozer MC, & Behrmann M (1990). On the interaction of selective attention and lexical knowledge: A connectionist account of neglect dyslexia. Journal of Cognitive Neuroscience, 2(2), 96–123. doi: 10.1162/jocn.1990.2.2.96 [DOI] [PubMed] [Google Scholar]
- Nazir TA (2000). Traces of print along the visual pathway In Kennedy A, Radach R, Heller D, & Pynte J (Eds.), Reading as a Perceptual Process (pp. 3–22): Elsevier Science Ltd. [Google Scholar]
- Nichelli P, Venneri A, Pentore R, & Cubelli R (1993). Horizontal and vertical neglect dyslexia. Brain and Language, 44(3), 264–283. doi: 10.1006/brln.1993.1018 [DOI] [PubMed] [Google Scholar]
- Noppeney U, & Price CJ (2002). Retrieval of visual, auditory, and abstract semantics. Neuroimage, 15(4), 917–926. doi: 10.1006/nimg.2001.1016 [DOI] [PubMed] [Google Scholar]
- Parton A, Malhotra P, & Husain M (2004). Hemispatial neglect. J Neurol Neurosurg Psychiatry, 75(1), 13–21. [PMC free article] [PubMed] [Google Scholar]
- Perry C, Ziegler JC, & Zorzi M (2007). Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychological Review, 114(2), 273–315. doi: 10.1037/0033-295X.114.2.273 [DOI] [PubMed] [Google Scholar]
- Pitteri M, Chen P, Passarini L, Albanese S, Meneghello F, & Barrett AM (2018). Conventional and functional assessment of spatial neglect: Clinical practice suggestions. Neuropsychology, 32(7), 835–842. doi: 10.1037/neu0000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage, 62(2), 816–847. doi: 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, & Mechelli A (2005). Reading and reading disturbance. Current Opinion in Neurobiology, 15(2), 231–238. doi: 10.1016/j.conb.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, & Wise RJS (1997). Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience, 9(6), 727–733. doi: 10.1162/jocn.1997.9.6.727 [DOI] [PubMed] [Google Scholar]
- Primativo S, Arduino LS, Daini R, De Luca M, Toneatto C, & Martelli M (2015). Impaired oculo-motor behaviour affects both reading and scene perception in neglect patients. Neuropsychologia, 70, 90–106. doi: 10.1016/j.neuropsychologia.2015.02.020 [DOI] [PubMed] [Google Scholar]
- Pritchard SC, Coltheart M, Palethorpe S, & Castles A (2012). Nonword reading: Comparing dual-route cascaded and connectionist dual-process models with human data. Journal of Experimental Psychology-Human Perception and Performance, 38(5), 1268–1288. doi: 10.1037/a0026703 [DOI] [PubMed] [Google Scholar]
- Ptak R, Di Pietro M, & Schnider A (2012). The neural correlates of object-centered processing in reading: A lesion study of neglect dyslexia. Neuropsychologia, 50(6), 1142–1150. doi: 10.1016/j.neuropsychologia.2011.09.036 [DOI] [PubMed] [Google Scholar]
- Ptak R, & Schnider A (2011). The attention network of the human brain: Relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention. Neuropsychologia, 49(11), 3063–3070. doi: 10.1016/j.neuropsychologia.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Reilhac C, Peyrin C, Demonet JF, & Valdois S (2013). Role of the superior parietal lobules in letter-identity processing within strings: fMRI evidence from skilled and dyslexic readers. Neuropsychologia, 51(4), 601–612. doi: 10.1016/j.neuropsychologia.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Reinhart S, Schunck A, Schaadt AK, Adams M, Simon A, & Kerkhoff G (2016). Assessing neglect dyslexia with compound words. Neuropsychology, 30(7), 869–873. doi: 10.1037/neu0000307 [DOI] [PubMed] [Google Scholar]
- Riddoch JM, Humphreys G, Cleton P, & Fery P (1990). Interaction of attentional and lexical processes in neglect dyslexia. Cognitive Neuropsychology, 7(5-6), 479–517. doi: 10.1080/02643299008253452 [DOI] [Google Scholar]
- Rorden C, Karnath HO, & Bonilha L (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. doi: 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Sacchetti DL, Goedert KM, Foundas AL, & Barrett AM (2015). Ipsilesional neglect: Behavioral and anatomical correlates. Neuropsychology, 29(2), 183–190. doi: 10.1037/neu0000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson H, Hjelmquist EK, Jensen C, Ekholm S, & Blomstrand C (1998). Nonlateralized attentional deficits: An important component behind persisting visuospatial neglect? Journal of Clinical and Experimental Neuropsychology, 20(1), 73–88. doi: 10.1076/jcen.20.1.73.1481 [DOI] [PubMed] [Google Scholar]
- Samuelsson H, Jensen C, Ekholm S, Naver H, & Blomstrand C (1997). Anatomical and neurological correlates of acute and chronic visuospatial neglect following right hemisphere stroke. Cortex, 33(2), 271–285. doi: 10.1016/s0010-9452(08)70004-2 [DOI] [PubMed] [Google Scholar]
- Searleman A (1977). A review of right hemisphere linguistic capabilities. Psychological Bulletin, 84(3), 503–528. doi: 10.1037//0033-2909.84.3.503 [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, & McClelland JL (1989). A distributed, developmental model of word recognition and naming. Psychological Review, 96(4), 523–568. doi: 10.1037/0033-295x.96.4.523 [DOI] [PubMed] [Google Scholar]
- Strain E, Patterson K, & Seidenberg MS (1995). Semantic effects in single-word naming. Journal of Experimental Psychology-Learning Memory and Cognition, 21(5), 1140–1154. doi: 10.1037/0278-7393.21.5.1140 [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Davis MH, & Rastle K (2019). Mapping visual symbols onto spoken language along the ventral visual stream. Proceedings of the Nationall Academy of Sciences of the United States of America. doi: 10.1073/pnas.1818575116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JSH, Duff FJ, Woollams AM, Monaghan P, & Ricketts J (2015). How word meaning influences word reading. Current Directions in Psychological Science, 24(4), 322–328. doi: 10.1177/0963721415574980 [DOI] [Google Scholar]
- Tune S, & Asaridou SS (2016). Stimulating the semantic network: What can TMS tell us about the roles of the posterior middle temporal gyrus and angular gyrus? Journal of Neuroscience, 36(16), 4405–4407. doi: 10.1523/jneurosci.0194-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Burani C, & Arduino LS (2010). Neglect dyslexia: A review of the neuropsychological literature. Experimental Brain Research, 206(2), 219–235. doi: 10.1007/s00221-010-2386-0 [DOI] [PubMed] [Google Scholar]
- Vallar G, & Perani D (1986). The anatomy of unilateral neglect after right-hemisphere stroke lesions: A clinical CT-scan correlation study in man. Neuropsychologia, 24(5), 609–622. doi: 10.1016/0028-3932(86)90001-1 [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, & Cohen L (2007). Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron, 55(1), 143–156. doi: 10.1016/j.neuron.2007.05.031 [DOI] [PubMed] [Google Scholar]
- Weinzierl C, Kerkhoff G, van Eimeren L, Keller I, & Stenneken P (2012). Error types and error positions in neglect dyslexia: Comparative analyses in neglect patients and healthy controls. Neuropsychologia, 50(12), 2764–2772. doi: 10.1016/j.neuropsychologia.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, & Halligan PW (1987). Behavioural Inattention Test. London: Thames Valley Test Company. [Google Scholar]
- Wilson M (1987). MRC Psycholinguistic Database: Machine Usable Dictionary. Retrieved from http://websites.psychology.uwa.edu.au/school/MRCDatabase/uwa_mrc.htm
- Worthington AD (1996). Cueing strategies in neglect dyslexia. Neuropsychological Rehabilitation, 6(1), 1–17. doi: 10.1080/713755496117 [DOI] [PubMed] [Google Scholar]
- Yap MJ, Pexman PM, Wellsby M, Hargreaves IS, & Huff MJ (2012). An abundance of riches: Cross-task comparisons of semantic richness effects in visual word recognition. Frontiers in Human Neuroscience, 6, 72. doi: 10.3389/fnhum.2012.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]


