Abstract
Arf GAPs are a family of proteins with a common catalytic domain that induces hydrolysis of GTP bound to the small GTP-binding protein Arf. The proteins are otherwise structurally diverse. Several subtypes of Arf GAPs have been found to be targets of oncogenes and to control cell proliferation and cell migration. The latter effects are thought to be mediated by coordinating changes in actin remodeling and membrane traffic. In this chapter, we discuss Arf GAPs that have been linked to oncogenesis and the molecular mechanisms underlying the effects of these proteins in cancer cells. We also discuss the enzymology of the Arf GAPs related to possible targeted inhibition of specific subtypes of Arf GAPs.
I. INTRODUCTION
Carcinogenesis is a complex process involving changes in cell proliferation, apoptosis, migration, and adhesion. Signaling pathways controlling each of these cellular activities have been identified. The mechanisms by which the activities are coordinated are still being discovered. Arf GAP proteins, identified as regulators of Arf family GTP-binding proteins, are interfaces between signaling pathways. The Arf GAPs also function as scaffolds and have intrinsic activities, such as bending membranes, which may directly contribute to the aberrant behavior of cancer cells.
II. SIGNALS INFLUENCED BY Arf GAPs
Proliferative and migration signals that are influenced by Arf GAPs are initiated by receptor tyrosine kinases (RTKs). These are transmembrane proteins such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) (Blume-Jensen and Hunter, 2001; Hunter, 2000; Pawson and Scott, 1997; Schlessinger, 2000). In normal physiology, tyrosine kinase activity in these proteins is activated by ligand binding. At least seven polypeptide ligands, including epidermal growth factor (EGF) and transforming growth factor α (TGFα), bind to EGFR. The peptide platelet-derived growth factor (PDGF) binds to PDGFR. The receptors autophosphorylate, which creates binding sites for adaptor proteins and signaling proteins that contain SH2 and PTB domains. Proteins recruited to the membrane either by direct interaction with RTKs or indirectly include nonreceptor tyrosine kinases such as the oncogene Src, phospholipase Cγ, phosphatidylinositol 3-kinase, and exchange factors for Ras family GTP-binding proteins and Rho family GTP-binding proteins. Ras·GTP stimulates the MAP kinase pathway, leading to changes in transcriptional activity and, consequently, cell proliferation. Ras·GTP also stimulates PI3K, which generates the signaling lipid phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 activates the serine/threonine kinase Akt, which inhibits apoptosis and stimulates protein synthesis and cell proliferation. RhoA·GTP, Rac1·GTP, and Cdc42·GTP are generated, which act through different classes of effectors to alter the actin cytoskeleton and change transcription leading to proliferation.
III. CELLULAR ADHESIVE STRUCTURES AFFECTED BY Arf GAPs
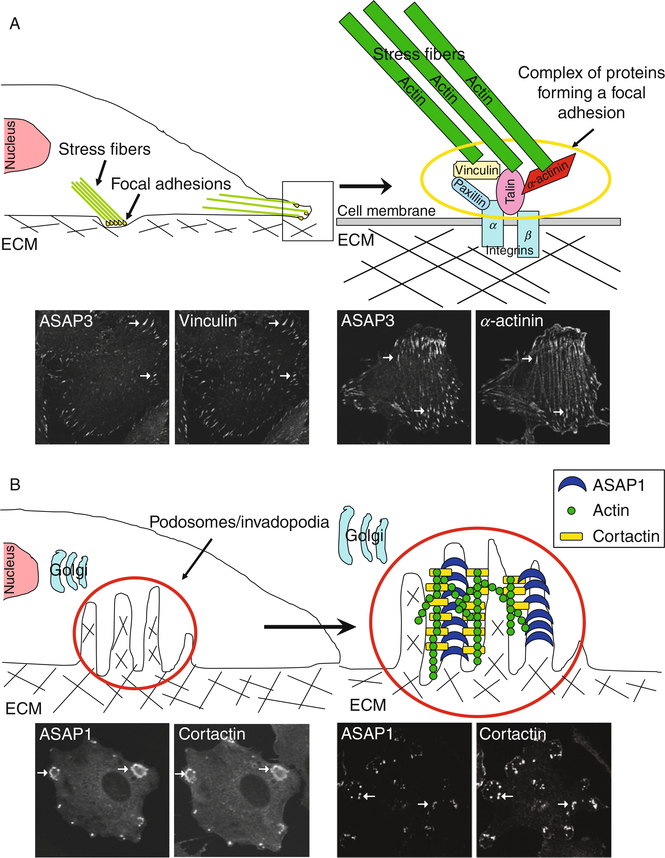
Cellular adhesive structures mediate cell movement and are involved in cellular signaling. At least three adhesive structures are affected by Arf GAPs (Fig. 1).
Fig. 1.
Schematic representation of cellular adhesive structures regulated by Arf GAPs. (A) Focal adhesions (FA). The structures are illustrated in panels using ASAP3 which colocalizes with FA markers such as vinculin (left panels) and α-actinin (right panels) in U118 glioblastoma cells. (B) Invadopodia and podosomes. These structures are induced by Src activation. To visualize invadopodia and podosomes, cells were transfected with plasmids directing the expression of active Src. Under this condition, invadopodia and podosomes were detected using ASAP1 and cortactin in NIH3T3 fibroblasts (left panels, arrows indicate podosomes) and MDA-MB-231 breast cancer cells (right panels, arrows indicate invadopodia).
A. Focal Adhesions
Focal adhesions (FAs) are the best characterized of the adhesive structures affected by Arf GAPs (Weaver, 2006; Yamaguchi et al., 2006). They are points of attachment of the actin cytoskeleton to the extracellular matrix (ECM). FAs contain transmembrane heterodimeric proteins called integrins. The extracellular portion of integrins bind to proteins in the ECM, such as fibronectin. The cytoplasmic part of integrin binds to bundles of actin filaments called stress fibers (Burridge and Chrzanowska-Wodnicka, 1996; Hynes, 1999, 2002). Other proteins associated with FAs include FAK, paxillin, vinculin, integrin-linked kinase, tensin, talin, and actinin.
FAs are dynamic structures that undergo a maturation process. Most of the components, excluding zyxin, are found in structures called focal complexes, at the edge of the cell. These mature, on a time scale of minutes, into FAs that appear as linear structures behind the edge of the cell at the end of actin stress fibers (Etienne-Manneville and Hall, 2002; Hall, 1998; Jaffe and Hall, 2005; Mackay and Hall, 1998; Ridley and Hall, 1992). FAs undergo further maturation to become more stable structures located more centrally in the cell. The process of maturation requires actin polymerization, stress fibers, and contraction of the stress fibers. Even in FAs that appear to be stable, the component proteins turn over on a time scale of under a minute.
B. Invadopodia and Podosomes
The other two adhesive structures affected by Arf GAPs are invadopodia and podosomes. Invadopodia, found in cancer cells, are sites of both attachment to and degradation of the ECM. Podosomes are highly related structures first identified in Rous-sarcoma virus-transformed fibroblasts (Buccione et al., 2004; Davidpfeuty and Singer, 1980; Marchisio et al., 1987; Tarone et al., 1985; Weed and Parsons, 2001) and later reported to be present in cells of the monocytic lineage, such as macrophages, osteoclasts, and dendritic cells, and in epithelial, endothelial, and smooth muscle cells (Gimona, 2003; Gimona and Buccione, 2006; Marchisio et al., 1984, 1987; Spinardi and Marchisio, 2006). Podosomes mediate migration and invasion, which is necessary, for instance, for the function of phagocytes. Invadopodia and podosomes contain integrins, cortactin, polymerized actin, and matrix metalloproteinases. Invadopodia appear as puncta or small rings on the ventral surface of cells (Ayala et al., 2006; Bowden et al., 1999; Buccione et al., 2004; Linder and Aepfelbacher, 2003; Spinardi and Marchisio, 2006; Weaver, 2006; Yamaguchi et al., 2006). In podosomes, the puncta may coalesce to form rosettes (Buccione et al., 2004; Linder and Aepfelbacher, 2003; Spinardi and Marchisio, 2006). Like FAs, podosomes and invadopodia are dynamic structures.
IV. THE SUBSTRATES FOR THE Arf GAPs: Arf FAMILY GTP-BINDING PROTEINS
ADP-ribosylation factor (Arf) family GTP-binding proteins are the substrates for Arf GAPs. Six genes encode Arf proteins in mammals, five in human (Donaldson, 2003; Donaldson et al., 2005; Gillingham and Munro, 2007; Kahn et al., 2006; Logsdon and Kahn, 2003; Moss and Vaughan, 1998; Souza-Schorey and Chavrier, 2006). Based on primary sequence, these have been divided into class 1 (Arf1, Arf2, and Arf3), class 2 (Arf4 and Arf5), and class 3 (Arf6) (Logsdon and Kahn, 2003; Moss and Vaughan, 1998). The name for these proteins comes from the pathologic activity for which they were identified: Arfs function as cofactors for cholera toxincatalyzed ADP-ribosylation of the heterotrimeric G protein Gs (Kahn and Gilman, 1984, 1986). This activity does not appear to be related to the function of Arf proteins in normal physiology. Most work on Arf proteins in mammals has focused on Arf1 and Arf6. They regulate membrane traffic and the actin cytoskeleton and are involved in cell migration (Donaldson, 2003; Gillingham and Munro, 2007; Randazzo et al., 2000a; Souza-Schorey and Chavrier, 2006).
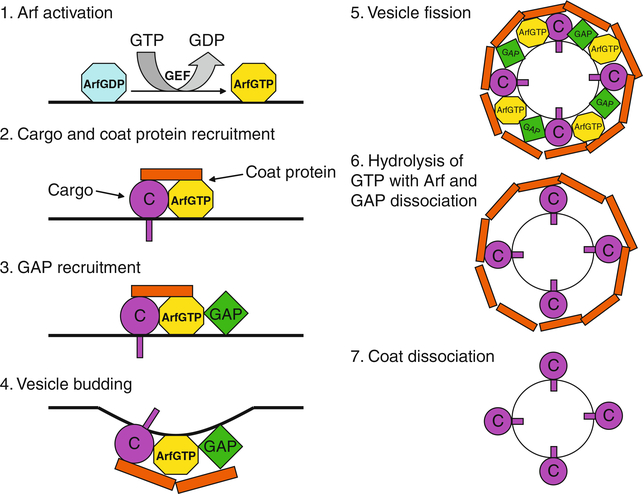
The molecular basis for function in membrane traffic is best described for Arf1 (Nie and Randazzo, 2006; Nie et al., 2003b; Randazzo et al., 2000a; Rothman, 2002; Spang, 2002; Springer et al., 1999) (Fig. 2). In the prevailing paradigm, Arf1·GTP binds to both membranes and coat proteins, recruiting coat proteins to the membrane surface. The coat proteins trap cargo, polymerize, and drive the formation of transport intermediates. Arf1·GTP is converted to Arf1·GDP by hydrolyzing GTP. Arf1·GDP does not bind either membranes or coat protein with high affinity. Consequently, the coat protein dissociates forming a transport intermediate that is competent to dock with and fuse to a target membrane. Arf1·GTP is able to interact with other proteins that may be effectors, such as PI 4-kinase (Godi et al., 1999; Jones et al., 2000; Krauss et al., 2003; Nie et al., 2003b; Randazzo et al., 2000a). Activating this enzyme at the Golgi apparatus is necessary for transport through and maintenance of the Golgi stack.
Fig. 2.
Model for Arf1 function in membrane traffic. Arf is activated by guanine nucleotide exchange factor (GEF) that catalyzes GTP exchange for GDP at the membrane. Next, the activated Arf·GTP recruits coat protein. The coat protein·Arf·GTP complex traps cargo (C). GTPase-activating protein (GAP) is then recruited to membrane sites through binding to the coat protein·Arf·GTP complex, triggering membrane deformation and subsequent vesicle formation. Fission releases the newly formed coated vesicle from the membrane. After vesicle budding, GAP hydrolyzes GTP on Arf, leading to the inactivation of Arf and subsequent dissociation of Arf and GAP from the cargo-containing vesicle. Coat proteins eventually dissociate from the vesicle surface, allowing the vesicle to dock and fuse with acceptor membranes.
The molecular basis for Arf6 control of membrane traffic is not strictly analogous to that for Arf1. The effect of Arf6 on membrane traffic and actin remodeling is mediated, at least in part, by enzymes that metabolize signaling lipids (Nie et al., 2003b). Arf6 binds to and activates phospholipase D (Brown et al., 1993; Cockcroft et al., 1994), which hydrolyzes phosphatidylcholine to generate phosphatidic acid (PA). Arf6 also binds to and activates phosphatidylinositol (PI) 4-kinase and phosphatidylinositol 4-phosphate (PI4P)-5 kinase (Anderson et al., 1999; Balla, 1998; Honda et al., 1999; Jones et al., 2000; Perez-Mansilla et al., 2006), resulting in the production of phosphatidylinositol 4,5-bisphosphate (PIP2). PIP2 has regulatory functions and is a precursor for the signaling molecules PIP3, diacylglycerol, and inositol 1,4,5 trisphosphate. The effects of Arf6 on PA and PIP2 have been found to be necessary for Arf6-dependent membrane traffic and actin remodeling (Brown et al., 2001; Cockcroft et al., 1994; Fensome et al., 1996; O’Luanaigh et al., 2002). Arfaptin is another Arf6 effector (Cherfils, 2001; Kanoh et al., 1997; Tarricone et al., 2001; Williger et al., 1999). Arfaptin is a BAR domain protein that binds to Rac·GDP. Rac·GDP is displaced by Arf6·GTP, which may be a mechanism by which Rac·GTP levels are locally regulated. Arf6 also binds to class 2 Rab11-binding proteins FIP3 and FIP4 (Fielding et al., 2005; Hickson et al., 2003; Horgan et al., 2004, 2007). The molecular basis of action of FIP3 and FIP4 is still being discovered. Arf6 also regulates an ACAP1/clathrin coat, which controls the endocytosis of transferrin and cell adhesion molecules called integrins (Dai et al., 2004; Li et al., 2005, 2007).
Arf6 has been implicated in the invasive behavior of cancer cells. Arf6 associates with invadopodia in MDA-MB-231 cells, which is a cell line derived from an invasive mammary carcinoma (Hashimoto et al., 2004; Sabe, 2003). Reducing Arf6 expression with siRNA had no effect on cell viability, but the cells were less migratory and invasive than controls. Dominant negative or constitutively active Arf6 or Arf1 also inhibited migration and invasion, whereas expressing analogous mutants of Arf5 had no effect. Similar results were obtained in a melanoma cell line (Tague et al., 2004). Cells expressing constitutively active Arf6 were more invasive than controls, and cells expressing dominant negative Arf6 were less invasive. The level of invasiveness correlated with activation of Erk. Arf6 is activated downstream of the EGFR by the exchange factor BRAG2, which was required for EGF-induced migration and invasion of a mammary carcinoma cell line (Hiroi et al., 2006; Morishige et al., 2008). These results are consistent with a model in which Arf6, activated by a growth factor, regulates Erk. Erk regulates the formation of invadopodia.
The function of Arfs requires cycling between the GTP- and GDP-bound forms of the protein. Arfs, however, have very low exchange rates and no detectable intrinsic GTPase activity. The cycle of GTP binding and hydrolysis is dependent on accessory proteins called guanine nucleotide exchange factors (GEFs), such as BRAG2, which was described above, and GTPase-activating proteins called GAPs.
V. THE Arf GAP FAMILY
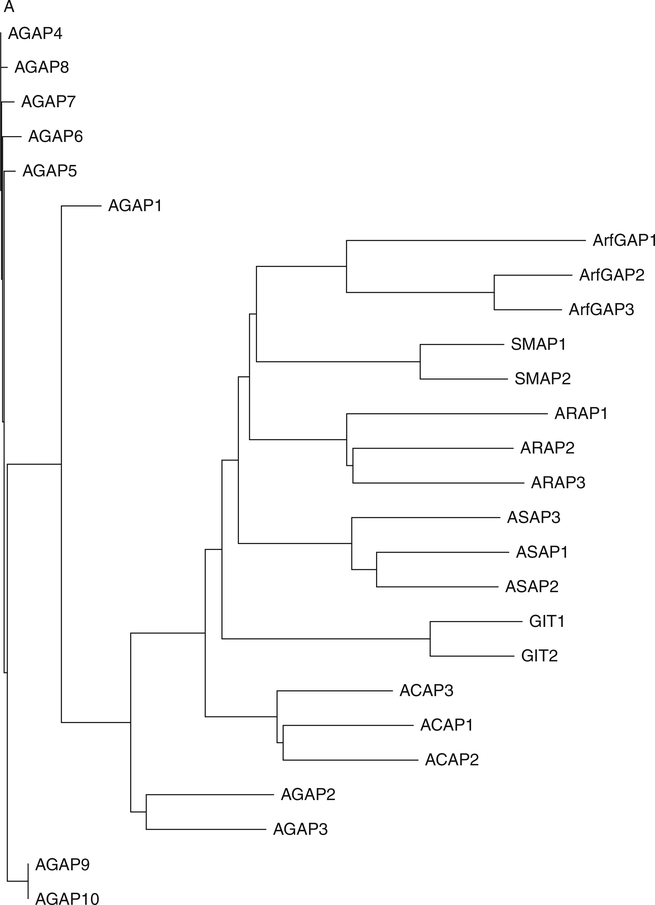
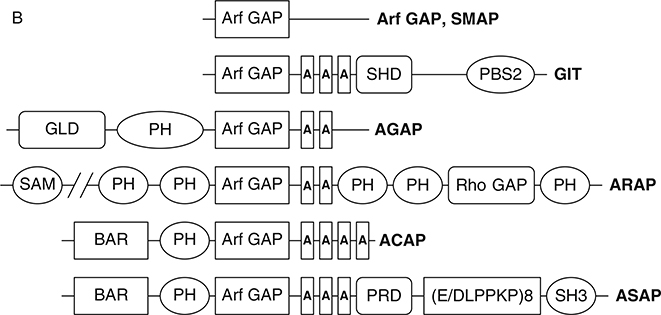
Thirty-one genes encoding proteins with the Arf GAP catalytic domain have been identified in humans (Kahn et al., 2008; Fig. 3). Arf GAPs were first identified on the basis of the enzymatic activity of inducing the hydrolysis of GTP that is bound to Arf family GTP-binding proteins (Brown et al., 1998; Cukierman et al., 1995; Makler et al., 1995; Randazzo and Kahn, 1994). Most Arf GAPs have multiple domains consistent with complex regulation and with multiple distinct functions (Inoue and Randazzo, 2007; Nie and Randazzo, 2006; Randazzo and Hirsch, 2004; Randazzo et al., 2007). Phylogenetic analysis of the Arf GAP domains or categorization based on domain structure yields a similar subclassification of the family (see Fig. 3A and B) (Kahn et al., 2008). The 10 major subtypes are called Arf GAP1-type, Arf GAP2/3, ADAPs, SMAPs, AGFGs, Gits, ASAPs, ACAPs, ARAPs, and AGAPs. The Arf GAP1, Arf GAP2/3, ADAP, SMAP, AGFG, and Git subtypes have the catalytic domain at the extreme N-terminus of the protein. The ASAPs, ACAPs, ARAPs, and AGAPs have an Arf GAP domain sandwiched between PH and Ank repeat domains. Based on this feature, these four subtypes are sometimes called AZAP for Arf GAP, Ank repeat, and PH domains. The “Z” stands for additional domains identifying a particular subtype. SMAPs and Gits have been implicated in oncogenesis and are the subject of excellent reviews (de Curtis, 2001; Meyer et al., 2005; Tanabe et al., 2006; Turner et al., 2001). We will focus on the AZAP group of Arf GAPs. The AZAPs have been, in some cases, directly implicated in oncogenesis (Ahn and Ye, 2005; Ehlers et al., 2005; Liu et al., 2007; Onodera et al., 2005). Furthermore, they are the targets of oncogenes, mediate or influence signaling pathways that are often disrupted or usurped in cancer cells, and regulate cellular adhesive structures that contribute to cell migration and invasion.
Fig. 3.
Arf GAP family. (A) Arf GAP family phylogram. The phylogram, edited using Tree-View, was obtained from the multiple sequence alignment of Arf GAP domains using ClustalW2. The branch lengths in the phylogram are proportional to the estimated divergence along each branch. Accession #s for human Arf GAPs: Arf GAP1 = NM_018209; Arf GAP2 = NM_032389; Arf GAP3 = NM_014570; Git1 = NM_014030; Git2 = NM_057169; ASAP1 = NM_018482; ASAP2 = NM_003887; ASAP3 = NM_017707; ACAP1 = NM_014716; ACAP2 = NM_012287; ACAP3 = NM_030649; AGAP1 = NM_014914; AGAP2 = NM_014770; AGAP3 = NM_031946; AGAP4 = NM_133446; AGAP5 = XM_001132588; AGAP6 = NM_001077665; AGAP7 = NM_001077685; AGAP8 = NM_001077686; AGAP9 = XM_001716810; AGAP10 = XM_001714786; ARAP1 = NM_015242; ARAP2 = NM_015230; ARAP3 = NM_022481; SMAP1 = AY055004; SMAP2 = NM_022733. (B) Schematic of human Arf GAPs. All Arf GAPs have a conserved Arf GAP domain. The other domains are as follows: Ank, ankyrin repeats; BAR, Bin/Amphiphysin/Rvs; PBS, paxillin-binding site; PH, pleckstrin homology; SAM, sterile alpha motif; SH3, Src-homology 3; SHD, Spa2 homology domain; PRD, proline rich; GLD, GTP-binding domain.
Four Arf GAP subtypes are within the AZAP group: ASAPs, ACAPs, ARAPs, and AGAPs. Each has a number of alternate names as detailed in recent reviews and in a commentary on the nomenclature of Arf GAPs (Gillingham and Munro, 2007; Inoue and Randazzo, 2007; Kahn et al., 2008; Table I). The nomenclature is based on the domain structure of the proteins that are the prototypes of each group. The ASAPs contain BAR, PH, Arf GAP, ankyrin repeat, and proline-rich SH3-binding motifs. ASAP1 contains a tandem repeat of E/DLPPKP. ASAP1 and ASAP2 also contain an SH3 domain, which is the basis for the “S” in the name, but ASAP3 does not. If the nomenclature is retained, “S” can stand for “SH3 binding site.” There are three ACAPs. They contain BAR, PH, Arf GAP, and ankyrin repeat domains. The “C” stands for a predicted coiled coil domain, which has now been identified as a BAR domain. There are three ARAPs, which contain SAM, 5 PH, Arf GAP, ankyrin repeat, Rho GAP, and Ras association domains. The “R” stands for Rho GAP. The AGAPs are the largest group with 11 members. They contain a GTP-binding protein-like domain and a split PH domain in addition to the Arf GAP and Ank repeat domains. The GTP-binding domain of AGAP1, for instance, has 28% identity with HaRas. The AGAPs and ASAPs have each been implicated in carcinogenesis. ARAPs and ACAPs have been implicated in cell behaviors important to cell migration and cancer-cell invasion and for cell signaling but have not been directly implicated in cancer.
Table I.
The Human AZAP-type Arf GAP Proteins and Their Alternative Names Found in the Literature
| AZAPs | Accepted names | Alternative names |
|---|---|---|
| ASAP | ASAP1 | DDEF1, AMAP1, PAG2, PAGβ |
| ASAP2 | DDEF2, AMAP2, PAG3, PAP | |
| ASAP3 | DDEFL1, UPLC1, ACAP4 | |
| AGAP | AGAP1 | CENTG2, GGAP1 |
| AGAP2 | CENTG1, GGAP2, PIKE-A | |
| AGAP3 | CENTG3, CRAG, MRIP1 | |
| AGAP4 | CTGLF1, MRIP2 | |
| ARAP | ARAP1 | CENTD2 |
| ARAP2 | CENTD1, PARX | |
| ARAP3 | CENTD3, DRAG1 | |
| ACAP | ACAP1 | CENTB1 |
| ACAP2 | CENTB2 | |
| ACAP3 | CENTB3 | |
VI. Arf GAP SUBTYPES IMPLICATED IN CARCINOGENESIS
A. AGAP Proteins in Glioblastoma
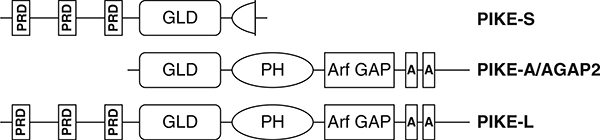
AGAPs were the first Arf GAPs implicated in cancer. Snyder and colleagues (Ye et al., 2000) identified a rat protein that was a splice variant of AGAP2, called PIKE-S, in a two-hybrid screen for proteins that bound to 4.1 N, a neuronal membrane cytoskeleton protein that mediates the antimitotic actions of NGF. PIKE-S is identical to the N-terminal GTP-binding domain and part of the PH domain of AGAP2 but has a limited amount of unrelated sequence in place of most of the PH domain and the Arf GAP and ankyrin repeat domains (Fig. 4). The sequence of PIKE-S includes an N-terminal extension containing three SH3 domain-binding motifs also found in the splice variant of AGAP2 referred to as PIKE-L (Ahn and Ye, 2005; Ahn et al., 2004a,b; Liu et al., 2007). PIKE-S is found in the brain and localizes exclusively to the nucleus, where it appears to function in mitogenic and antiapoptotic signaling.
Fig. 4.
Domain structures of three PIKE isoforms. PIKE-L, an alternatively spliced form of PIKE, is several hundred amino acids longer than the original form of PIKE, designated as PIKE-S. In addition to the GTP-binding domain (GLD) shared by PIKE-S and PIKE-L, PIKE-L contains an Arf GAP domain and two Ankyrin repeats. PIKE-S contains three proline-rich-domains (PRD) in the N-terminus, the GLD domain and a partial PH domain in the C-terminus. PIKE-A/AGAP2 contains the GTPase, PH, Arf GAP, and Ankyrin repeats domains present in PIKE-L but lacks the proline-rich domains (PRD) at the N terminus.
Because of the homology of PIKE-S to Ras, function analogous to Ras was investigated. PIKE-S was found to bind GTP or ATP with high affinity (10–40 nM) and a 1:1 stoichiometry (Ye et al., 2000). GTP binding to PIKE-S in cells was increased by addition of NGF. PIKE-S bound to PI 3-kinase, a known target of Ras, dependent on GTP. The mode of interaction with PI 3-kinase, however, was more complex than described for Ras. PIKE-S·GTP simultaneously bound both the p85 catalytic and the p110 regulatory subunits of PI 3-kinase. The proline-rich domains of PIKE-S mediated binding to p110 and the Ras-like domain mediated binding to the catalytic p85 subunit. The association with both subunits was required to increase PI 3-kinase activity, leading to increased PIP3 production and activation of the antiapoptotic protein kinase Akt/PKB.
The signaling pathway leading from the NGF receptor to PIKE-S in the nucleus was further defined with the identification of PLCγ1 as a PIKE-S-binding partner (Ye et al., 2002). PLCγ1 was found to bind to the third proline-rich domain (SH3-binding motif) of PIKE-S, via the SH3 domain of PLCγ1. The association resulted in accelerated nucleotide exchange on PIKE-S; thus, PLCγ1 functions as a GEF. Snyder and colleagues proposed a model in which NGF triggers PLCγ1 translocation to the nucleus (Ye et al., 2002). PLCγ1 activates PIKE-S, which activates PI3-kinase, leading to the production of PIP3 and activation of the antiapoptotic kinase Akt/PKB.
PIKE-S is not found in humans, but humans do have two splice variants containing Arf GAP domains. Proteins with the Ras-like domain of PIKE-S, PH, Arf GAP, and ankyrin repeat domains, called AGAP2/GGAP2, were reported by Nie et al. (2002) and Liu and colleagues (Xia et al., 2003). AGAP2 has also been called PIKE-A (Ahn et al., 2004a,b). A third splice variant, PIKE-L, has the N-terminal proline-rich motifs found in PIKE-S in addition to the GTP-binding protein like PH, Arf GAP, and ankyrin repeat domains (Ahn et al., 2004a; Liu et al., 2007; Ye and Snyder, 2004). AGAP2 is a member of the AGAP subfamily of Arf GAPs (Inoue and Randazzo, 2007; Kahn et al., 2008). The family is composed of 11 genes. AGAP1 and AGAP2 function as Arf1 GAPs that are activated by phosphoinositides (Nie et al., 2002). In contrast to PIKE-S, AGAP1 and AGAP2 localized to an endocytic compartment (Nie et al., 2003a, 2005). The PH domains of AGAP1 and AGAP2 bind to clathrin adaptor proteins, which are proteins important for membrane traffic. AGAP1 specifically binds clathrin adaptor AP-3 and regulates endocytic traffic from the TGN to lysosomes. AGAP2 specifically binds to AP-1 and affects transferrin recycling. Based on these results, AGAPs were proposed to function as a component of clathrin coats, which regulates membrane traffic.
The Ras-like domain of AGAP1/GGAP1 and AGAP2/GGAP2 has been reported to function like Ras (Xia et al., 2003). In experiments using IP-purified protein, AGAP1, similar to PIKE-S, bound GTP with high affinity and hydrolyzed GTP at about 0.01/min. The t1/2 for GDP dissociation was about 10 min. The GAP domain of the protein, through an intramolecular interaction, bound to the Ras-like domain and stimulated GTPase activity by about tenfold. As reported for PIKE-S, AGAP1/GGAP1 was found to stimulate the Ras pathway. In these experiments, activation of the c-fos serum response element was used as a reporter for the Ras pathway.
Further examination of signaling through AGAP2/PIKE-A and PIKE-L revealed differences from PIKE-S (Ahn and Ye, 2005; Ahn et al., 2004a,b; Chan and Ye, 2007; Liu et al., 2007; Ye, 2006). As described above, PIKE-S is thought to activate Akt consequent to binding to and activating PI 3-kinase. In contrast, PIKE-A/AGAP2 did not bind to PI 3-kinase. Instead, PIKE-A bound to and directly activated Akt, bypassing the need for PI 3-kinase activation. PIKE-L could also bind directly to Akt. Also different than the interaction of PIKE-S with PI 3-kinase, GTP binding to AGAP2/PIKE-A does not appear to regulate association with Akt (Ahn et al., 2004a). AGAP2/PIKE-A binding to Akt was found to depend on either GTP or GDP (Ahn et al., 2004b). Paradoxically, mutants of PIKE that would be predicted to reduce binding affinity for guanine nucleotide increased the binding of PIKE to Akt.
In summary, PIKE-S, PIKE-L, and PIKE-A/AGAP2 were all found to be important activators of Akt. For PIKE-S, the activation is dependent on GTP binding and is mediated by activation of PI 3-kinase. For PIKE-A, the activation is independent of the nucleotide bound and is mediated by direct binding to Akt.
AGAP2/PIKE-A has been linked to oncogenesis. AGAP2/PIKE-A, but not PIKE-L or PIKE-S, was found amplified in a number of human glioblastoma cell lines. Overexpression of AGAP2/PIKE-A increased Akt activity and promoted invasion of two glioblastoma cell lines (Ahn et al., 2004b). In other studies, 154 tumors and corresponding normal tissues were examined (Ahn et al., 2004a). PIKE-A/AGAP2 cDNAwas elevated in multiple cancers, including breast, lung, ovary, kidney, bladder, vulva, uterus, and cervical. In NIH 3T3 fibroblasts and glioblastoma cell lines, expression of AGAP2/PIKE-A enhanced Akt activity, accelerated cell growth, and induced anchorage independent growth. The data support the idea that AGAP2 is oncogenic due to binding and activating Akt.
The evidence that AGAP2/PIKE-A has a role in oncogenesis is compelling; however, details of the mechanism remain to be determined. The literature contains conflicting reports about properties of AGAP2 important to its function as a proto-oncogene. For instance, Ye and colleagues (Hu et al., 2005) reported robust and specific binding of PIP3 to PIKE-A/AGAP2 that was critical to the function of the protein. Nie et al. (2002) determined affinities of AGAPs for phosphoinositides. The proteins bound phosphoinositides without specificity for the position of the phosphate group on the inositol ring. Furthermore, the dissociation constant for PIP3 was greater than 10 μM, which is considered low-affinity binding. The nucleotide-binding properties of AGAP2 and the ability to function as Ras are also controversial. Snyder and colleagues (Ye et al., 2000) and Liu and colleagues (Xia et al., 2003) reported that the AGAP family members bind GTP with high affinity, approximating the dissociation constant to be in the 10 nM range, and to hydrolyze GTP at a rate of ~0.01/min, similar to Ras. However, Nie et al. (2002) could not detect binding with 1 μM or less nucleotide. On the basis of saturation kinetics carried out with highly purified AGAP, Declan and colleagues estimated the dissociation constant to be 500 μM and the catalytic rate of GTP hydrolysis to be 10/min (Soundararajan et al., 2007), each about a 1000-fold different than the parameter estimates provided by Liu and colleagues (Xia et al., 2003) and Snyder and colleagues (Ye et al., 2000). With a dissociation constant of 500 μM, it is hard to understand how an effect of PLC on exchange rates could have been detected on the time scales of the experiments reported (Ye et al., 2002). Snyder and colleagues and Liu and colleagues report that the Ras-like domain of PIKE and other AGAPs activated Ras effectors (Ahn and Ye, 2005; Xia et al., 2003; Ye and Snyder, 2004; Ye et al., 2000); Nie and colleagues were not able to detect an effect of AGAP on a number of Ras effectors (Nie et al., 2002). Finally, Liu and colleagues report that the GAP domain functions as a GAP for the Ras-like domain in an intramolecular reaction (Xia et al., 2003). In contrast, Nie et al. (2002, 2003a, 2005) report that the GAP domain functions with Arf1·GTP.
B. ASAPs and Cell Invasion
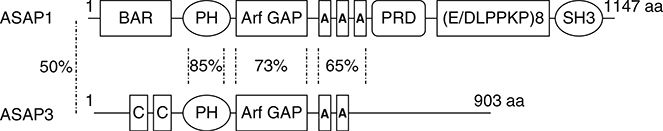
Two ASAP subtype proteins (Fig. 5) have been implicated in carcinogenesis. They are thought to contribute to the invasive and metastatic phenotype by regulating cellular adhesive structures and the associated actin cytoskeleton.
Fig. 5.
Domain structures of the ASAP-type proteins of Arf GAPs. Amino acid sequence similarity between full-length ASAP1 and ASAP3 and each identified domain is indicated.
1. ASAP1
ASAP1 was identified on the basis of Arf GAP activity and, separately, in screens for Src-binding proteins (Brown et al., 1998; King et al., 1999). ASAP1 was found to bind to and be phosphorylated by Src family proteins and focal adhesion kinase (FAK) and to associate with FAs (Brown et al., 1998; Liu et al., 2002, 2005; Randazzo et al., 2000b). Studies in uveal melanoma were the first to reveal a role of ASAP1 in cancer (Ehlers et al., 2005). The gene encoding ASAP1 is on chromosome 8q24.1, near the locus for the myc oncogene. The region of chromosome 8 containing ASAP1 is amplified in approximately half of uveal melanomas. These are class 2 tumors that have high invasive potential. After failing to detect changes in expression of myc, Harbour and colleagues (Ehlers et al., 2005) found that ASAP1 message and proteins levels correlated with invasive potential in the class 2 uveal melanomas. Expressing recombinant ASAP1 increased the migration rate of cells derived from class 1 (noninvasive) uveal melanomas. Additional studies on the role of ASAP1 in the invasive behavior of uveal melanoma have been slowed because of difficulty in culturing cells from class 2 tumors.
The role of ASAP1 in invasion was also examined in breast and prostate cancer. ASAP1 levels correlated with invasive potential in primary tumors (Lin et al., 2008; Onodera et al., 2005). Similar to uveal melanoma, copy number of the ASAP1 gene was increased in primary prostate cancer specimens (Lin et al., 2008), although this has not been reported for breast cancer. Different than the case for uveal melanoma, mammary carcinoma and prostate carcinoma cell lines could be established from the invasive tumors and were also found to contain high expression levels of ASAP1 (Onodera et al., 2005; Sabe et al., 2006). Consistent with a function in invasion, ASAP1 was found in and was required for the formation of invadopodia in mammary carcinoma cell lines (Bharti et al., 2007; Onodera et al., 2005) and in analogous structures called podosomes in Src-transformed fibroblasts (Bharti et al., 2007).
Examination of colorectal carcinoma revealed that the potential function of ASAP1 in uveal melanoma and prostate cancer may not extend to all tumor types. Like uveal melanoma, amplification of chromosome 8q24.1 is frequently observed in colorectal carcinoma. However, in this case, amplification of the myc gene correlated with more advanced stages of cancer, whereas changes in the ASAP1 gene did not (Buffart et al., 2005).
ASAP1 affects cell migration through association with cellular adhesive structures. Initial studies focused on the function of ASAP1 in FAs. Two proteins contribute to ASAP1 targeting to FAs. ASAP1 is a binding partner of FAK. The interaction is mediated by the SH3 domain of ASAP1 and is necessary for ASAP1 targeting to FAs in rat embryo fibroblasts (Liu et al., 2002). Crk/CrkL, an adaptor protein, mediated ASAP1 association with FAs in platelets (Oda et al., 2003). It binds to the proline-rich SH3-binding motifs present between the Ank repeat and E/DLPPKP repeat domains. ASAP1 overexpression was found to affect the paxillin content, but not vinculin content, of FAs (Liu et al., 2002, 2005). The effect was dependent on Arf GAP activity. Reduced ASAP1 expression also reduced the association of paxillin with FAs and increased levels of Arf1·GTP, which led to the conclusion that the rate of the GTP binding and hydrolysis cycle on Arf1 regulated the association of paxillin, and possibly other proteins, with FAs (Liu et al., 2005).
The effects of ASAP1 on cell movement are complex. Overexpressing ASAP1 has been reported to accelerate (Furman et al., 2002) and to reduce cell migration rates (Liu et al., 2005). Reduction of ASAP1 levels also reduced migration of fibroblasts (Furman et al., 2002; Liu et al., 2002, 2005). Reduction of ASAP1 has been reported to slow migration of breast and prostate cancer cells (Lin et al., 2008; Onodera et al., 2005) and, from another laboratory, to have no effect on the migration of cultured breast cancer cells (Ha et al., 2008). Thus, the role of ASAP1 in cell migration may be specific to particular cell types and experimental conditions.
The biochemistry related to the function of ASAP1 in invadopodia and podosomes has been examined in several reports. One unresolved issue is related to ASAP1 splice variants and cortactin. ASAP1 has two major splice variants. ASAP1a contains three proline-rich SH3-binding motifs between the ankyrin repeats and the tandem repeats of E/DLPPKP. The middle motif is atypical, composed of a string of six prolines. The other splice variant, ASAP1b, does not contain the middle, atypical proline-rich SH3-binding motif. It has been reported that the middle motif, and consequently ASAP1a but not ASAP1b, binds to cortactin (Onodera et al., 2005). The ASAP1a–cortactin complex drives formation of invadopodia. Perturbation of the interaction with a peptide composed of the atypical SH3-binding motif disrupted invadopodia formation (Hashimoto et al., 2006; Onodera et al., 2005). In contrast, another group reports that both ASAP1a and ASAP1b bound to cortactin and supported invadopodia and podosome formation. A peptide composed of the atypical proline-rich SH3-binding motif neither bound cortactin nor affected the formation of podosomes (Bharti et al., 2007). The role of an atypical proline motif (PXXXPR) between the E/DLPPKP repeat and SH3 domains, which binds to Cin85, is also unclear. It was reported to be critical for the formation of invadopodia (Nam et al., 2007). However, it did not contribute to the formation of podosomes (Bharti et al., 2007). Some of the difference between groups could be attributed to cell type differences or the differences between podosomes and invadopodia.
The contribution of three other structures within ASAP1 to podosome formation has been examined (Bharti et al., 2007). The tyrosine that is phosphorylated by Src was found to be critical to podosome formation. ASAP1 with the tyrosine mutated to phenylalanine functioned as a dominant negative, blocking podosome formation. ASAP1 with a glutamate in place of the tyrosine supported podosome formation, but was not sufficient to drive podosome formation. The SH3 domain of ASAP1, which binds to FAK and is necessary for binding to cortactin, was also found to be critical to podosome formation. Recombinant ASAP1 with the SH3 deleted or containing point mutations within the SH3 domain functioned as a dominant negative, blocking formation of podosomes in fibroblasts expressing activated Src. The BAR domain also has a role in podosome formation. Recombinant ASAP1 that did not have the BAR domain could not support podosome formation but did not function as a dominant negative protein.
The role of the Arf GAP domain for invadopodia formation has been examined (Bharti et al., 2007, and unpublished observations). Two types of mutants in the Arf GAP domain have been generated. In one type, Arf GAP activity is reduced or absent, but the mutant can still bind to Arf1·GTP. A second type of mutant is unable to bind Arf1·GTP. Mutants that can bind to Arf1·GTP support the formation of podosomes. In contrast, those mutant that cannot bind Arf1·GTP inhibit the formation of podosomes, functioning as dominant negative mutants. These results have been taken to support the idea that ASAP1 functions as an Arf effector.
Because ASAP1 is in invadopodia, it was anticipated to affect invasion. This hypothesis was tested in an invasive breast cancer cell line, MDA-MB-231. Reduction of ASAP1 expression, using siRNA, slowed invasion. Disrupting interaction with cortactin, using an SH3-binding peptide, slowed invasion as did disrupting the interaction of ASAP1 with CIN85 (Hashimoto et al., 2006; Nam et al., 2007; Onodera et al., 2005). However, there may be some variation in the effect of ASAP1 in particular cell lines. Another group, also working with MDA-MB-231, did not detect an effect of reducing ASAP1 expression on invasion (Ha et al., 2008).
In summary, ASAP1 contributes to the regulation of cellular adhesive structures involved in cell migration. The effect of ASAP1 in cancer invasion is cell type specific. The data are compelling that ASAP1 contributes to the invasive behavior in uveal melanoma. The role of ASAP1 in the behaviors of cancers of other origins is not well understood at this time.
2. ASAP3
ASAP3, previously called Upregulated in Liver Cancer 1 (UPLC1), DDEFL1, and ACAP4 (Fang et al., 2006; Okabe et al., 2004), was identified in analyses of expression profiles of clinical hepatocellular carcinomas (HCCs) using cDNA microarrays. ASAP3 is similar to ASAP1 and ASAP2 in structure but it does not contain an SH3 domain at the C-terminus (Fig. 5). Because it does not have an SH3 domain, the protein was called ACAP4 in one report. However, phylogenetic analysis indicates that the protein is an ASAP subtype Arf GAP and, therefore, has been renamed ASAP3 (Ha et al., 2008; Kahn et al., 2008) (Fig. 3A).
The identification of ASAP3 in expression profiles for HCCs led to the examination in cell lines derived from HCCs to determine a link to cancer. The relative expression of ASAP3 was reported to be high in HCC (Okabe et al., 2004) and correlated with cell proliferation and migration (Fang et al., 2006). These observations led to the hypotheses that high expression of ASAP3 causes increased cell proliferation and invasive behavior of cancer cells. The effect of overexpressing ASAP3 on cell proliferation has not been consistent (Fang et al., 2006; Ha et al., 2008). Whereas one group found that expressing ASAP3 in NIH 3T3 fibroblasts increased proliferation, another group did not find any effect. Similarly, decreasing ASAP3 expression was found to slow proliferation in one report and to have no effect on proliferation in another report. The differences could be attributable to assay conditions or cell lines. Also, the positive effects observed were modest so it is possible that the assay used by one group lacked the sensitivity to detect changes in cell proliferation induced by ASAP3.
ASAP3 may control the interaction between cell adhesion structures and the actin cytoskeleton to regulate cell migration and invasion. ASAP3, like ASAP1, associates with FAs and circular dorsal ruffles, two structures involved in adhesion and cell migration (Buccione et al., 2004). Unlike ASAP1, ASAP3 is not found in invadopodia. Also unlike ASAP1, overexpressing recombinant ASAP3 or reducing expression levels of ASAP3 did not affect the morphology of FAs, although a change in distribution of FAs was detected in some cell types. Reduction of ASAP3 expression decreased the number of actin stress fibers that bind to FAs and reduced the levels of phosphomyosin, which associates with and mediates the retraction of actin stress fibers (Ha et al., 2008).
The effect of ASAP3 on movement of cells derived from a mammary carcinoma, MDA-MB-231, has been examined. Migration, assessed by a wound-healing assay and by a transwell migration assay, was slowed by reducing ASAP3 expression. Similarly, reduction of ASAP3 slowed migration through matrigel, taken as a measure of invasion. In the same experiments, reduction of ASAP1 had no effect (Ha et al., 2008). The molecular bases for these effects are still being determined. Based on the available data, a model has been proposed in which ASAP3 controls the composition of FAs. Loss of ASAP3 results in the exclusion of actin-binding sites. Without the actin-binding sites, FAs cannot bind actin stress fibers, which prevents maturation of the FAs and prevents contraction that is necessary for cell movement.
In summary, ASAP3 affects migration and invasion of MDA-MB-231 cells, a mammary carcinoma cell line. ASAP3 does not have a large effect on cell proliferation and apoptosis has not been examined. The molecular basis for the effect on migration and invasion has not been explored.
VII. Arf GAP SUBTYPES THAT AFFECT SIGNALING OR ADHESION BUT HAVE NOT BEEN IMPLICATED IN ONCOGENESIS
A. The ARAPs
Three genes encode ARAP proteins in humans (Inoue and Randazzo, 2007; Kahn et al., 2008). The ARAPs contain SAM, 5 PH, Arf GAP, ankyrin repeat, Rho GAP, and Ras association domains. The “R” stands for Rho GAP. Both the Arf GAP and Rho GAP domains contribute to the cellular effects of the ARAPs. Initial reports examined the effects of ARAP1 and ARAP3 on cell spreading and membrane ruffling (Krugmann et al., 2002, 2004, 2006; Miura et al., 2002; Stacey et al., 2004). Recent work has provided some molecular details about the mechanisms by which the ARAPs may affect cell movement. ARAP1 affects the endocytic traffic of EGFRs (Yoon, Lee and Randazzo, submitted) and ARAP2 affects the formation of FAs (Yoon et al., 2006).
1. ARAP1
ARAP1 associates with the Golgi apparatus (Miura et al., 2002) and, in cells treated with growth factors, a Rab5 pre-endosomal compartment involved in the endocytic traffic of EGFR (Yoon, Lee, and Randazzo, submitted). Reduced ARAP1 expression leads to more rapid internalization of EGFR following EGF treatment and reduced signaling through Erk and Jnk. On the basis of these results, the authors concluded that ARAP1 is involved in attenuation of signals from the EGFR but the molecular basis remains to be determined. Current studies are examining the role of the Rab5-containing pre-endosome in signaling.
2. ARAP2
ARAP2 is differentfrom the other ARAPs in having a glutamine in place of the highly conserved catalytic arginine found in all known active Rho GAPs. The Rho GAP domain does not induce the hydrolysis of GTP bound to RhoA but it does bind specifically to RhoA·GTP. ARAP2 has PIP3-dependent Arf GAP activity that is specific for Arf6. ARAP2 is required for the formation of FAs. ARAP2 function to support FA formation depends on both the Arf GAP activity and the ability to bind to RhoA·GTP (Yoon et al., 2006). These results are consistent with a role of ARAP2 in cell migration and/or invasion, but this function remains to be tested.
B. ACAPs and the Regulation of Integrin
ACAP proteins contain BAR, PH, Arf GAP, and ankyrin repeat domains (Inoue and Randazzo, 2007; Jackson et al., 2000). In vitro and in vivo analyses indicate that ACAP1 and ACAP2 have a substrate preference for Arf6 over Arf1 and Arf5. ACAP1 is the most extensively studied of the ACAPs. Although not implicated in oncogenesis, ACAP1 does affect signaling pathways important to the behavior of cancer cells. It binds to phenylalanine-based sorting signals in transferrin receptor and promotes recycling of transferrin receptor (Dai et al., 2004). ACAP1 also binds to integrin β1 (Li et al., 2005). The interaction is dependent on the phosphorylation of ACAP1 by Akt, and is regulated by EGFR. The interaction between ACAP1 and integrins regulates integrin recycling and controls cell migration.
VIII. COMPARATIVE ENZYMOLOGY OF THE Arf GAPs
PH domains distinguish the AZAP group from other Arf GAPs. The PH domains have been found to be critical for catalytic activity in ASAPs. Deletion of the PH domain of ASAP1 reduces activity to 1/100,000th that of wild-type protein (Che et al., 2005; Kam et al., 2000; Luo et al., 2007). Similarly, deletion of the PH domain of ASAP2 and ASAP3 results in proteins with 1/1000th–1/10,000th the activity of the wild-type protein (Ha et al., 2008). The role of the PH domain in other AZAP-type Arf GAPs has not been characterized. However, the role of phosphoinositides has been examined. The activity of Arf GAP1, which does not have a PH domain, is not affected by PIP2 or PIP3 (Randazzo, 1997). In contrast, ASAP1 is activated 10,000-fold by PIP2 (Che et al., 2005; Kam et al., 2000). ASAP2 and ASAP3 are similar to ASAP1 (Andreev et al., 1999; Ha et al., 2008). The Arf GAP activity of ARAPs is specifically activated by PIP3 (Krugmann et al., 2002; Miura et al., 2002; Stacey et al., 2004; Yoon et al., 2006). AGAPs and ACAPs have been found to be activated by phosphoinositides but the effect is not specific for a particular phosphoinositide (Jackson et al., 2000; Nie et al., 2002).
The contribution of the PH domain for the GAP activity of AZAP proteins indicated that the AZAPs might differ catalytically from Arf GAPs that do not have PH domain. Consistent with this expectation, the amino terminus of Arf was found to be a critical determinant for interaction with ASAP1 and AGAP1 but not for Arf GAP1 (Yoon et al., 2004). Studies focusing on structural determinants with the substrate Arf for interaction with GAP also revealed catalytic differences between AZAP subtypes (Luo et al., 2005). A point mutation within switch 1 of Arf1, changing isoleucine 46 to aspartate, had a large effect on interaction with ASAP1 but not AGAP1 or Arf GAP1. These results were the basis for the conclusion that the GAPs, despite a similar catalytic fold, had distinct binding determinants for their substrates.
Examination of the enzymology of Arf GAP1 and ASAP1 revealed additional differences between the proteins (Luo and Randazzo, 2008; Luo et al., 2007). The catalytic constants of ASAP1 and Arf GAP1 are more than 200-fold different. Structural studies indicate that the PH domain of ASAP1 forms part of the substrate-binding site, contributing both to binding and to orienting the substrate in the catalytic pocket. Arf GAP1 does not contain a PH domain (Luo and Randazzo, 2008). Differences also reside in the catalytic Arf GAP domain (Luo and Randazzo, 2008; Luo et al., 2007). Mutation of tryptophan 479 within the Arf GAP domain of ASAP1 reduced activity to 1/4000th that of wild-type protein. The analogous mutation in Arf GAP1, tryptophan 32, reduced activity to one third that of wild-type protein, leading to the conclusion that this tryptophan is critical for ASAP1 but not Arf GAP1 catalytic activity.
IX. CONCLUSIONS
Thirty-one genes in humans encode Arf GAPs, proteins that induce the hydrolysis of GTP bound to the Arf family GTP-binding proteins. Of the 31 Arf GAPs, 4 (ASAP1, ASAP3, AGAP2, and SMAP1) have been found to be oncogenic. Five others (ARAP1, ARAP1, ACAP1, Git1, and Git2) have been found to affect signaling pathways that are often disrupted in cancer, but none of these has been directly implicated in oncogenesis. The effect of a particular Arf GAP may be specific for a tumor type. For instance, ASAP3, but not ASAP1, was found to affect the invasion of mammary tumor cells whereas ASAP1 contributes to the invasion of uveal melanoma and prostate cancer.
The molecular basis for Arf GAP action in cells is still being defined. Part of the action is through a common catalytic activity of inducing hydrolysis of GTP bound to Arf. Differences in function may arise from different sites of action or Arf isoform specificity. The Arf GAPs may also have other functions, either linked to or independent of Arf, as suggested by the structural diversity of these proteins. For instance, ARAPs, which have a Rho GAP domain, also exert effects by regulating Rho family GTP-binding proteins. If, as the early work indicates, AZAPs are critical mediators of cell migration related to metastasis and invasion of specific cell types and the proteins can be molecularly distinguished, the Arf GAPs may be valuable therapeutic targets for the treatment of cancer.
ACKNOWLEDGMENTS
This work was supported by the intramural program at the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
REFERENCES
- Ahn JY, and Ye K (2005). PIKE GTPase signaling and function. Int. J. Biol. Sci. 1, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JY, Hu YX, Kroll TG, Allard P, and Ye KQ (2004a). PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc. Natl. Acad. Sci. USA 101, 6993–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JY, Rong R, Kroll TG, Van Meir EG, Snyder SH, and Ye KQ (2004b). PIKE (Phosphatidylinositol 3-Kinase Enhancer)-A GTPase stimulates akt activity and mediates cellular invasion. J. Biol. Chem. 279, 16441–16451. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, and Loijens JC (1999). Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J. Biol. Chem. 274, 9907–9910. [DOI] [PubMed] [Google Scholar]
- Andreev J, Simon JP, Sabatini DD, Kam J, Plowman G, Randazzo PA, and Schlessinger J (1999). Identification of a new Pyk2 target protein with Arf-GAP activity. Mol. Cell. Biol. 19, 2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala I, Baldassarre M, Caldieri G, and Buccione R (2006). Invadopodia: A guided tour. Eur. J. Cell. Biol. 85, 159–164. [DOI] [PubMed] [Google Scholar]
- Balla T (1998). Phosphatidylinositol 4-kinases. Biochim. Biophys. ACTA 1436, 69–85. [DOI] [PubMed] [Google Scholar]
- Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, and Randazzo PA (2007). Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol. Cell. Biol. 27, 8271–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Jensen P, and Hunter T (2001). Oncogenic kinase signalling. Nature 411, 355–365. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, and Mueller SC (1999). An invasionrelated complex of cortactin, paxillin and PKC mu associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18, 4440–4449. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, and Sternweis PC (1993). ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase-D activity. Cell 75, 1137–1144. [DOI] [PubMed] [Google Scholar]
- Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, and Randazzo PA (1998). ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell. Biol. 18, 7038–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, and Donaldson JG (2001). Phosphatidylinositol 4, 5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccione R, Orth JD, and McNiven MA (2004). Foot and mouth: Podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5, 647–657. [DOI] [PubMed] [Google Scholar]
- Buffart TE, Coffa J, Hermsen MAJA, Carvalho B, van der Sijp JRM, Ylstra B, Pals G, Schouten JP, and Meijer GA (2005). DNA copy number changes at 8q11–24 in metastasized colorectal cancer. Cell. Oncol. 27, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, and Chrzanowska-Wodnicka M (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–518. [DOI] [PubMed] [Google Scholar]
- Chan CB, and Ye KQ (2007). PIKE GTPase are phosphoinositide-3-kinase enhancers, suppressing programmed cell death. J. Cell. Mol. Med. 11, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che MM, Boja ES, Yoon HY, Gruschus J, Jaffe H, Stauffer S, Schuck P, Fales HM, and Randazzo PA (2005). Regulation of ASAP1 by phospholipids is dependent on the interface between the PH and Arf GAP domains. Cell. Signal. 17, 1276–1288. [DOI] [PubMed] [Google Scholar]
- Cherfils J (2001). Structural mimicry of DH domains by Arfaptin suggests a model for the recognition of Rac-GDP by its guanine nucleotide exchange factors. FEBS Lett. 507, 280–284. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Thomas GMH, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong Q, and Hsuan JJ (1994).Phospholipase-D - A downstream effector of Arf in granulocytes. Science 263, 523–526. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Huber I, Rotman M, and Cassel D (1995). THE ARF1 GTPase-activating protein - zinc-finger motif and Golgi complex localization. Science 270, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Dai J, Li J, Bos E, Porcionatto M, Premont RT, Bourgoin S, Peters PJ, and Hsu VW (2004). ACAP1 promotes endocytic recycling - Short article by recognizing recycling sorting signals. Dev. Cell 7, 771–776. [DOI] [PubMed] [Google Scholar]
- Davidpfeuty T, and Singer SJ (1980). Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous-Sarcoma virus. Proc. Natl. Acad. Sci. USA 77, 6687–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I (2001). Cell migration: GAPs between membrane traffic and the cytoskeleton. EMBO Rep. 2, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG (2003). Multiple roles for Arf6: Sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 278, 41573–41576. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Honda A, and Weigert R (2005). Multiple activities for Arf1 at the Golgi complex. Biochim. Biophys. ACTA 1744, 364–373. [DOI] [PubMed] [Google Scholar]
- Ehlers JP, Worley L, Onken MD, and Harbour JW (2005). DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin. Cancer Res. 11, 3609–3613. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, and Hall A (2002). Rho GTPases in cell biology. Nature 420, 629–635. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Miao Y, Ding X, Deng H, Liu SQ, Wang FS, Zhou RH, Watson C, Fu CH, Hu QC, Lillard JW, Powell M, et al. (2006). Proteomic identification and functional characterization of a novel ARF6 GTPase-activating protein, ACAP4. Mol. Cell. Proteomic 5, 1437–1449. [DOI] [PubMed] [Google Scholar]
- Fensome A, Cunningham E, Prosser S, Tan SK, Swigart P, Thomas G, Hsuan J, and Cockcroft S (1996). ARF and PITP restore GTP gamma S-stimulated protein secretion from cytosol-depleted HL60 cells by promoting PIP2 synthesis. Curr. Biol. 6, 730–738. [DOI] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu XZ, Hickson GRX, Srivastava S, Baldwin SA, Prekeris R, and Gould GW (2005). Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 24, 3389–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman C, Short SM, Subramanian RR, Zetter BR, and Roberts TM (2002). DEF-1/ASAP1 is a GTPase-activating protein (GAP) for ARF1 that enhances cell motility through a GAP-dependent mechanism. J. Biol. Chem. 277, 7962–7969. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, and Munro S (2007). The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 23, 579–611. [DOI] [PubMed] [Google Scholar]
- Gimona M (2003). The vascular smooth muscle cell cytoskeleton. Nat. Cell Biol. 5, 598. [Google Scholar]
- Gimona M, and Buccione R (2006). Adhesions that mediate invasion. Int. J. Biochem. Cell. Biol. 38, 1875–1892. [DOI] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, andDe Matteis MA (1999). ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4, 5)P-2 on the Golgi complex. Nat. Cell Biol. 1, 280–287. [DOI] [PubMed] [Google Scholar]
- Ha VL, Bharti S, Inoue H, Vass WC, Campa F, Nie Z, de Gramont A, Ward Y, and Randazzo PA (2008). ASAP3 is a focal adhesion-associated Arf GAP that functions in cell migration and invasion. J. Biol. Chem. 283, 14915–14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Onodera Y, Hashimoto A, Tanaka M, Hamaguchi M, Yamada A, and Sabe H (2004). Requirement for Arf6 in breast cancer invasive activities. Proc. Natl. Acad. Sci. USA 101, 6647–6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Hirose M, Hashimoto A, Morishige M, Yamada A, Hosaka H, Akagi KI, Ogawa E, Oneyama C, Agatsuma T, Okada M, Kobayashi H, et al. (2006). Targeting AMAP1 and cortactin binding bearing an atypical src homology 3/proline interface for prevention of breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 103, 7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson GRX, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, and Gould GW (2003). Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell 14, 2908–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi T, Someya A, Thompson W, Moss J, and Vaughan M (2006). GEP(100)/BRAG2: Activator of ADP-ribosylation factor 6 for regulation of cell adhesion and actin cytoskeleton via E-cadherin and alpha-catenin. Proc. Natl. Acad. Sci. USA 103, 10672–10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, and Kanaho Y (1999). Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Horgan CP, Walsh M, Zurawski TH, and McCaffrey MW (2004). Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem. Biophys. Res. Commun. 319, 83–94. [DOI] [PubMed] [Google Scholar]
- Horgan CP, Oleksy A, Zhdanov AV, Lall PY, White IJ, Khan AR, Futter CE, McCaffrey JG, and McCaffrey MW (2007). Rab11-FIP3 is critical for the structural integrity of the endosomal recycling compartment. Traffic 8, 414–430. [DOI] [PubMed] [Google Scholar]
- Hu YX, Liu ZX, and Ye KQ (2005). Phosphoinositol lipids bind to phosphatidylinositol 3 (P13)-kinase enhancer GTPase and mediate its stimulatory effect on P13-kinase and Akt signalings. Proc. Natl. Acad. Sci. USA 102, 16853–16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T (2000). Signaling - 2000 and beyond. Cell 100, 113–127. [DOI] [PubMed] [Google Scholar]
- Hynes RO (1999). Cell adhesion: Old and new questions. Trends Biochem. Sci. 24, 33–37. [Google Scholar]
- Hynes RO (2002). Integrins: Bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Inoue H, and Randazzo PA (2007). Arf GAPs and their interacting proteins. Traffic 8, 1465–1475. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie ZZ, Miura K, Foroni L, Sun JL, Hsu VW, Donaldson JG, and Randazzo PA (2000). ACAPs are Arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol 151, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, and Hall A (2005). Rho GTPases: Biochemistry and biology. Ann. Rev. Cell Dev. Biol. 21, 247–269. [DOI] [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgen CP, Kondo H, Irvine RF, and Cockcroft S (2000). Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4, 5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem. 275, 13962–13966. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Buford E, Inoue H, Logsdon JM, Nie Z, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, and Cassel D (2008). Consensus nomenclature for the human arf GAP domain-containing proteins. J. Cell. Biol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, and Gilman AG (1984). ADP-ribosylation of Gs promotes the dissociation of its alpha-Subunit and beta-Subunit. J. Biol. Chem. 259, 6235–6240. [PubMed] [Google Scholar]
- Kahn RA, and Gilman AG (1986). The protein cofactor necessary for ADP-ribosylation of Gs by cholera-toxin is itself a GTP binding-protein. J. Biol. Chem. 261, 7906–7911. [PubMed] [Google Scholar]
- Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, and Schurmann A (2006). Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL and SAR proteins. J. Cell. Biol. 172, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JL, Miura K, Jackson TR, Gruschus J, Roller P, Stauffer S, Clark J, Aneja R, and Randazzo PA (2000). Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1 - Evidence for the pleckstrin homology domain functioning as an allosteric site. J. Biol. Chem. 275, 9653–9663. [DOI] [PubMed] [Google Scholar]
- Kanoh H, Williger BT, and Exton JH (1997). Arfaptin 1, a putative cytosolic target protein of ADP-ribosylation factor, is recruited to Golgi membranes. J. Biol. Chem. 272, 5421–5429. [DOI] [PubMed] [Google Scholar]
- King FJ, Hu ED, Harris DF, Sarraf P, Spiegelman BM, and Roberts TM (1999). DEF-1, a novel Src SH3 binding protein that promotes adipogenesis in fibroblastic cell lines. Mol. Cell. Biol. 19, 2330–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, and Haucke V (2003). ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type I gamma. J. Cell Biol. 162, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, et al. (2002). Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol. Cell 9, 95–108. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Williams R, Stephens L, and Hawkins PT (2004). ARAP3 is a P13K- and rap-regulated GAP for RhoA. Curr. Biol. 14, 1380–1384. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Andrews S, Stephens L, and Hawkins PT (2006). ARAP3 is essential for formation of lamellipodia after growth factor stimulation. J. Cell Sci. 119, 425–432. [DOI] [PubMed] [Google Scholar]
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, and Hsu VW (2005). Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta 1 to control cell migration. Dev. Cell 9, 663–673. [DOI] [PubMed] [Google Scholar]
- Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, Kandror KV, and Hsu VW (2007). An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 178, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Watahiki A, Bayani J, Zhang F, Liu L, Ling V, Sadar MD, English J, Fazli L, So A, Gout PW, Gleave M, et al. (2008). ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res. 68, 4352–4359. [DOI] [PubMed] [Google Scholar]
- Linder S, and Aepfelbacher M (2003). Podosomes: Adhesion hot-spots of invasive cells. Trends Cell Biol. 13, 376–385. [DOI] [PubMed] [Google Scholar]
- Liu YH,Loijens JC,Martin KH,Karginov AV,andParsons JT(2002).The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol. Biol. Cell 13, 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yerushalmi GM, Grigera PR, and Parsons JT (2005). Mislocalization or reduced expression of Arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J. Biol. Chem. 280, 8884–8892. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu Y, Hao C, Rempel SA, and Ye K (2007). PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene 26, 4918–4927. [DOI] [PubMed] [Google Scholar]
- Logsdon JM, and Kahn RA (2003). The Arf Family tree In “Arf family GTPases” (Kahn RA, Ed.), pp. 1–21. Kluwer Academic Publishers, Dordrecht. [Google Scholar]
- Luo R, and Randazzo PA (2008).Kinetic analysisof Arf GAP1indicates a regulatoryrole for coatomer. J. Biol. Chem. M802268200. [Google Scholar]
- Luo RB, Jacques K, Ahvazi B, Stauffer S, Premont RT, and Randazzo PA (2005). Mutational analysis of the Arf1 center dot GTP/Arf GAP interface reveals an Arf1 mutant that selectively affects the Arf GAP ASAP1. Curr. Biol. 15, 2164–2169. [DOI] [PubMed] [Google Scholar]
- Luo R, Ahvazi B, Amariei D, Shroder D, Burrola B, Losert W, and Randazzo PA (2007). Kinetic analysis of GTP hydrolysis catalysed by the Arf1-GTP-ASAP1 complex. Biochem. J. 402, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DJG, and Hall A (1998). Rho GTPases. J. Biol. Chem. 273, 20685–20688. [DOI] [PubMed] [Google Scholar]
- Makler V, Cukierman E, Rotman M, Admon A, and Cassel D (1995). ADP-ribosylation factor-directed GTPase-activating protein - Purification and partial characterization. J. Biol. Chem. 270, 5232–5237. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Capasso O, Nitsch L, Cancedda R, and Gionti E (1984). Cytoskeleton and adhesion patterns of cultured chick-embryo chondrocytes during cell spreading and Rous-Sarcoma virus transformation. Exp. Cell Res. 151, 332–343. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Cirillo D, Teti A, Zamboninzallone A, and Tarone G (1987). Rous-Sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dotlike organization of cytoskeletal proteins involved in microfilament membrane interactions. Exp. Cell Res. 169, 202–214. [DOI] [PubMed] [Google Scholar]
- Meyer C, Schneider B, Reichel M, Angermueller S, Strehl S, Schnittger S, Schoch C, Jansen MWJC, van Dongen JJ, Pieters R, Haas OA, Dingermann T, et al. (2005). Diagnostic tool for the identification of MLL rearrangements including unknown partner genes. Proc. Natl. Acad. Sci. USA 102, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Jacques KM, Stauffer S, Kubosaki A, Zhu KJ, Hirsch DS, Resau J, Zheng Y, and Randazzo PA (2002). ARAP1: A point of convergence for Arf and Rho signaling. Mol. Cell 9, 109–119. [DOI] [PubMed] [Google Scholar]
- Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, Wei S, Hashimoto A, Yamada A, Yano H, Mazaki Y, Kodama H, et al. (2008). GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat. Cell Biol. 10, 85–92. [DOI] [PubMed] [Google Scholar]
- Moss J, and Vaughan M (1998). Molecules in the ARF orbit. J. Biol. Chem. 273, 21431–21434. [DOI] [PubMed] [Google Scholar]
- Nam JM, Onodera Y, Mazaki Y, Miyoshi H, Hashimoto S, and Sabe H (2007). CIN85, a Cbl-interacting protein, is a component of AMAP1-mediated breast cancer invasion machinery. EMBO J. 26, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie ZZ, and Randazzo PA (2006). Arf GAPs and membrane traffic. J. Cell Sci. 119, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Nie ZZ, Stanley KT, Stauffer S, Jacques KM, Hirsch DS, Takei J, and Randazzo PA (2002). AGAP1, an endosome-associated, phosphoinositide-dependent ADP- ribosylation factor GTPase-activating protein that affects actin cytoskeleton. J. Biol. Chem. 277, 48965–48975. [DOI] [PubMed] [Google Scholar]
- Nie Z, Boehm M, Boja E, Vass W, Bonifacino J, Fales H, and Randazzo PA (2003a). SpecificRegulationof the adaptor protein complexAP-3 by the Arf GAPAGAP1. Dev. Cell 5, 513–521. [DOI] [PubMed] [Google Scholar]
- Nie ZZ, Hirsch DS, and Randazzo PA (2003b). Arf and its many interactors. Curr. Opin. Cell Biol. 15, 396–404. [DOI] [PubMed] [Google Scholar]
- Nie ZZ, Fei J, Premont RT, and Randazzo PA (2005). The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J. Cell Sci. 118, 3555–3566. [DOI] [PubMed] [Google Scholar]
- Oda A, Wada I, Miura K, Okawa K, Kadoya T, Kato T, Nishihara H, Maeda M, Tanaka S, Nagashima K, Nishitani C, Matsuno K, et al. (2003). CrkL directs ASAP1 to peripheral focal adhesions. J. Biol. Chem. 278, 6456–6460. [DOI] [PubMed] [Google Scholar]
- Okabe H, Furukawa Y, Kato T, Hasegawa S, Yamaoka Y, and Nakamura Y (2004). Isolation of development and differentiation enhancing factor-like 1 (DDEFL1) as a drug target for hepatocellular carcinomas. Int. J. Oncol. 24, 43–48. [PubMed] [Google Scholar]
- O’Luanaigh N, Pardo R, Fensome A, len-Baume V, Jones D, Holt MR, and Cockcroft S (2002). Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13, 3730–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, Wada H, Matsuura N, and Sabe H (2005). Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 24, 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, and Scott JD (1997). Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Perez-Mansilla B, Ha VL, Justin N, Wilkins AJ, Carpenter CL, and Thomas GMH (2006). The differential regulation of phosphatidylinositol 4-phosphate 5-kinases and phospholipase D1 by ADP-ribosylation factors 1 and 6. Biochim. Biophys. Acta 1761, 1429–1442. [DOI] [PubMed] [Google Scholar]
- Randazzo PA (1997). Resolution of two ADP-ribosylation factor 1 GTPase-activating proteins from rat liver. Biochem. J. 324, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, and Hirsch DS (2004). Arf GAPs: Multifunctional proteins that regulate membrane traffic and actin remodelling. Cell. Signal. 16, 401–413. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, and Kahn RA (1994). GTP hydrolysis by ADP-ribosylation factor (Arf) is dependent on both an Arf GAP and acid phospholipids. J. Biol. Chem. 269, 10758–10763. [PubMed] [Google Scholar]
- Randazzo PA, Nie Z, Miura K, and Hsu V (2000a). Molecular aspects of the cellular activities of ADP-ribosylation factors. Sci. STKE 2000(59), RE1. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, and Cooper JA (2000b). The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97, 4011–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Inoue H, and Bharti S (2007). Arf GAPs as regulators of the actin cytoskeleton. Biol. Cell 99, 583–600. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, and Hall A (1992). The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth-factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Rothman JE (2002). The machinery and principles of vesicle transport in the cell. Nat. Med. 8, 1059–1062. [DOI] [PubMed] [Google Scholar]
- Sabe H (2003). Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 134, 485–489. [DOI] [PubMed] [Google Scholar]
- Sabe H, Onodera Y, Mazaki Y, and Hashimoto S (2006). ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr. Opin. Cell Biol. 18, 558–564. [DOI] [PubMed] [Google Scholar]
- Schlessinger J (2000). Cell signaling by receptor tyrosine kinases. Cell 103, 211–225. [DOI] [PubMed] [Google Scholar]
- Soundararajan M, Yang X, Elkins JM, Sobott F, and Doyle DA (2007). The centaurin + −1 GTPase-like domain functions as an NTPase. Biochem. J. 401, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Schorey C, and Chavrier P (2006). ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347–358. [DOI] [PubMed] [Google Scholar]
- Spang A (2002). ARF1 regulatory factors and COPI vesicle formation. Curr. Opin. Cell Biol. 14, 423–427. [DOI] [PubMed] [Google Scholar]
- Spinardi L, and Marchisio PC (2006). Podosomes as smart regulators of cellular adhesion. Europ. J. Cell Biol. 85, 191–194. [DOI] [PubMed] [Google Scholar]
- Springer S, Spang A, and Schekman R (1999). A primer on vesicle budding. Cell 97, 145–148. [DOI] [PubMed] [Google Scholar]
- Stacey TTI, Nie ZZ, Stewart A, Najdovska M, Hall NE, He H, Randazzo PA, and Lock P (2004). ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J. Cell Sci. 117, 6071–6084. [DOI] [PubMed] [Google Scholar]
- Tague SE, Muralidharan V, and Souza-Schorey C (2004). ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc. Natl. Acad. Sci. USA 101, 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Kon S, Natsume W, Torii T, Watanabe T, and Satake M (2006). Involvement of a novel ADP-ribosylation factor GTPase-activating protein, SMAP, in membrane trafficking: Implications in cancer cell biology. Cancer Sci. 97, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, and Marchisio PC (1985). Rous-Sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 159, 141–157. [DOI] [PubMed] [Google Scholar]
- Tarricone C, Xiao B, Justin N, Walker PA, Rittinger K, Gamblin SJ, and Smerdon SJ (2001). The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways. Nature 411, 215–219. [DOI] [PubMed] [Google Scholar]
- Turner CE, West KA, and Brown MC (2001). Paxillin-ARF GAP signaling and the cytoskeleton. Curr. Opin. Cell Biol. 13, 593–599. [DOI] [PubMed] [Google Scholar]
- Weaver AM (2006). Invadopodia: Specialized cell structures for cancer invasion. Clin. Exp. Metastasis 23, 97–105. [DOI] [PubMed] [Google Scholar]
- Weed SA, and Parsons JT (2001). Cortactin: Coupling, membrane dynamics to cortical actin assembly. Oncogene 20, 6418–6434. [DOI] [PubMed] [Google Scholar]
- Williger BT, Provost JJ, Ho WT, Milstine J, and Exton JH (1999). Arfaptin 1 forms a complex with ADP-ribosylation factor and inhibits phospholipase D. FEBS Lett. 454, 85–89. [DOI] [PubMed] [Google Scholar]
- Xia CZ, Ma WB, Stafford LJ, Liu CY, Gong LM, Martin JF, and Liu MY (2003). GGAPs, a new family of bifunctional GTP-binding and GTPase-activating proteins. Mol. Cell. Biol. 23, 2476–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Pixley F, and Condeelis J (2006). Invadopodia and podosomes in tumor invasion. Euro. J. Cell Biol. 85, 213–218. [DOI] [PubMed] [Google Scholar]
- Ye KQ (2006). PIKE GTPase-mediated nuclear signalings promote cell survival. Biochim. Biophys. ACTA 1761, 570–576. [DOI] [PubMed] [Google Scholar]
- Ye KQ, and Snyder SH (2004). PIKE GTPase: A novel mediator of phosphoinositide signaling. J. Cell Sci. 117, 155–161. [DOI] [PubMed] [Google Scholar]
- Ye KQ, Hurt J, Wu FY, Fang M, Luo HBR, Hong JJ, Blackshaw S, Ferris CD, and Snyder SH (2000). PIKE: A nuclear GTPase that enhances PI3Kinase activity and is regulated by protein 4.1N. Cell 103, 919–930. [DOI] [PubMed] [Google Scholar]
- Ye KQ, Aghdasi B, Luo HBR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, and Snyder SH (2002). Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415, 541–544. [DOI] [PubMed] [Google Scholar]
- Yoon HY, Jacques K, Nealon B, Stauffer S, Premont RT, and Randazzo PA (2004). Differencesbetween AGAP1,ASAP1and Arf GAP1 in substraterecognition:Interactionwith the N-terminus of Arf1. Cell. Signal. 16, 1033–1044. [DOI] [PubMed] [Google Scholar]
- Yoon H-Y, Lee J-S, and Randazzo PA (submitted). ARAP1 regulates endocytosis of EGFR. [DOI] [PMC free article] [PubMed]
- Yoon HY, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, and Randazzo PA (2006). ARAP2 effects on the actin cytoskeleton are dependent on Arf6specific GTPase-activating protein activity and binding to RhoA-. J. Cell Sci. 119, 4650–4666. [DOI] [PubMed] [Google Scholar]