Abstract
The present global health emergency involving the emergence and rapid spread of a novel coronavirus has prompted the world scientific community to consider how it can help to fight this growing viral pandemic. With few safe and effective drugs available to combat this threat to humanity and the normal functioning of our society, the oligonucleotide research community is uniquely positioned to apply its technology and expertise to help alleviate the crisis, thanks to its capacity for rational drug design, swift development cycles, and pursuing targets undruggable by conventional treatment strategies.
Keywords: coronavirus, virus–host interactions, chemistry optimization
Coronavirus disease 2019 (COVID-19) is a swiftly evolving global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is closely related but not identical to the coronaviruses (CoVs) that caused SARS and Middle East Respiratory Syndrome (MERS) in past decades. Owing to a relatively high case fatality rate, global coordination of health care systems and extreme government-instituted quarantine measures have been implemented against the pandemic. Presently, no effective drug treatments for the disease have been readily identified; however, a host of compounds—about 12 in total—are being tested, including drugs already approved for use against HIV and malaria, a number of experimental compounds shown to exhibit antiviral activity in animal testing, and even antibody-rich plasma taken from those patients who have successfully recovered from the virus (Fig. 1). Given the substantial need for and willingness to try new therapeutic approaches during this crisis, oligonucleotide drugs developed either as antiviral or symptom-alleviating approaches to COVID-19 could prove to be an attractive option due to their rational design and relative speed of development compared with traditional approaches.
FIG. 1.
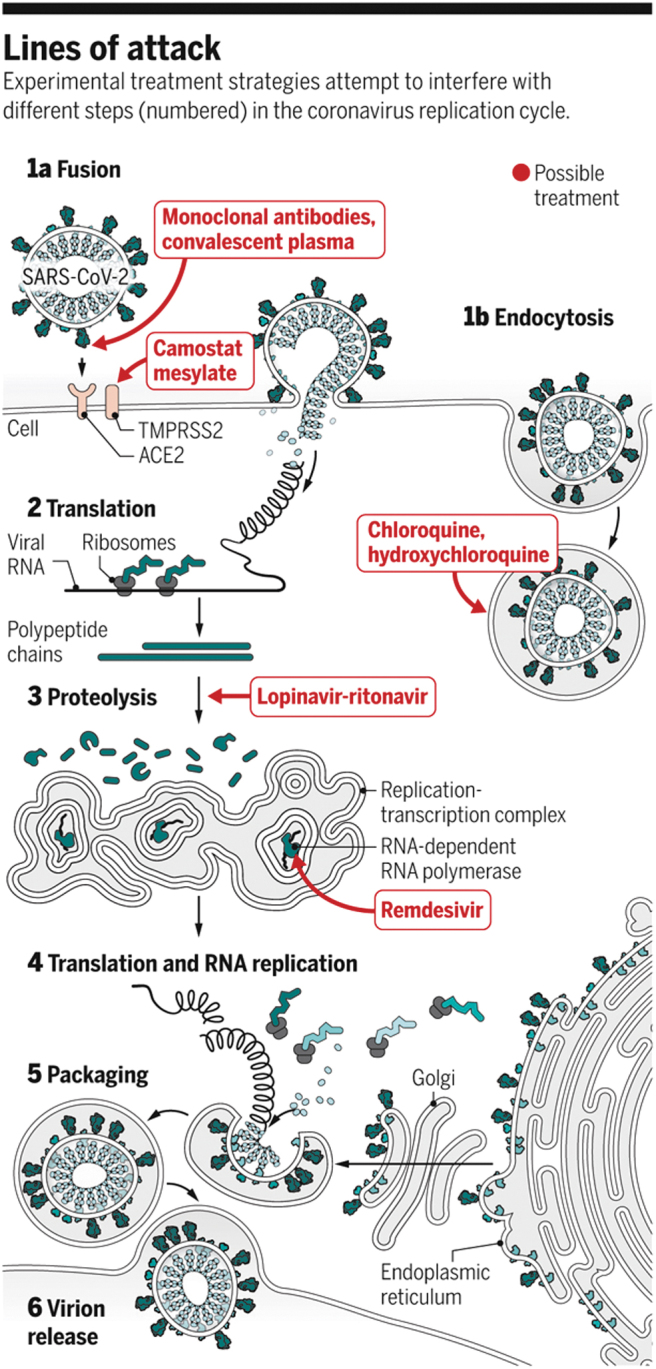
CoV infectious pathway and targets of drugs currently being tested. CoVs, coronaviruses. Reprinted with permission from AAAS [1].
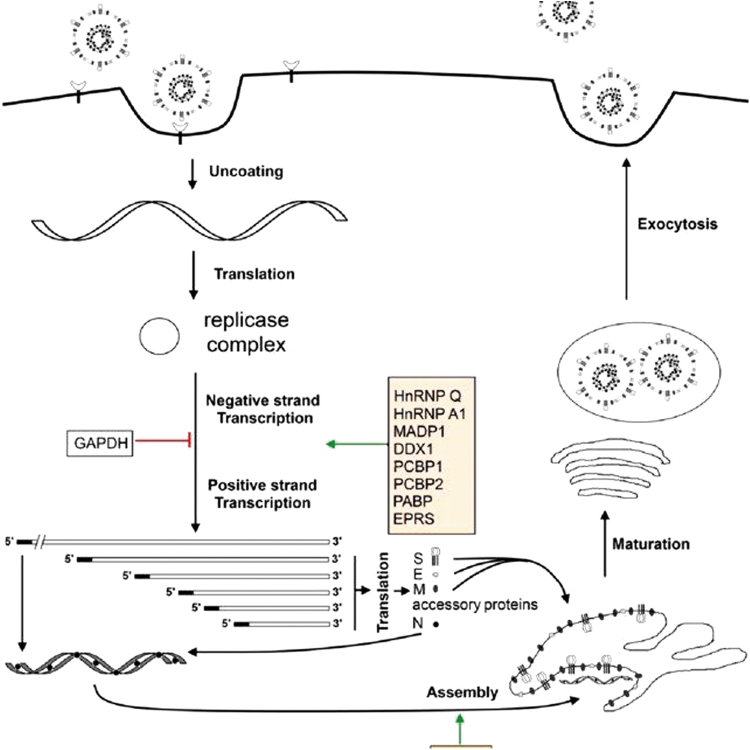
The complete viral genome of SARS-CoV-2 (29,903 nucleotides) revealed the virus is most closely related (89.1% nucleotide similarity) to a group of SARS-like CoVs previously found in bats in China. CoVs in general are characterized by unusually large RNA genomes such as that of SARS-Cov-2, and exhibit a unique replication strategy, as they possess a nonsegmented positive-sense RNA genome containing a 5′ cap structure along with a 3′ poly (A) tail, allowing it to be read by replicase polyproteins for translation. Two-thirds of this genome is occupied by genes encoding nonstructural proteins (NSPs), whereas the remainder accounts for structural and accessory proteins. RNA replication and transcription are facilitated by a leader sequence and untranslated region at the 5′ end of the genome, which contain multiple stem loop structures. Structurally, CoVs are pleomorphic or spherical in form and are characterized by club-shaped glycoprotein projections throughout their surface. The most important structural proteins of CoVs are spike (S) protein, membrane (M) protein, envelope (E) protein, and the nucleocapsid (N) protein. Two viral replicase polyproteins (PP1a and PP1ab) are produced that are further processed into 16 mature NSPs (Fig. 2).
FIG. 2.
Life cycle of a CoV. Reproduced under open access Creative Common CC BY license from Zhong [2].
SARS-CoV-2 binding to host cells is achieved through spike protein–host cell protein interaction with angiotensin converting enzyme-2 (ACE-2) in SARS-CoV-2. After receptor recognition, the virus genome with its nucleocapsid is released into the cytoplasm of the host cells. Production of PP1a and PP1ab facilitates control of host ribosomes for the translation of viral proteins, forming the replication transcription process. These polyproteins are further processed into 16 NSP byproducts that each has specific functions such as suppression of host gene expression by NSP1 and NSP2, formation of a multidomain complex by NSP3, protease activity by NSP5, transmembrane protein formation by NSP4 and NSP6, primase activity by NSP6 and NSP8, and RNA binding by NSP9, the dimeric form of which is important for viral infection. All of these NSPs have an important role in replication and transcription, making each a potential drug target. Structural proteins, meanwhile, such as M, E, and S are entered into the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) complex and there forms the structure of the viral envelope, whereas the N protein binds with the replicated RNA genome, forming the ribonucleoprotein complex. The capsid is formed by the M, E, and S proteins, with the formed viral particle emerging from the ERGIC and forming a vesicle that fuses with the plasma membrane and releases the newly birthed virion into the extracellular space. The immune response to this infection causes a surge of inflammatory cytokines and chemokines, causing damage to lung tissue, deterioration of lung function, and finally failure of the lungs as well as other major organs in the most severe cases.
Antiviral strategies to combat SARS-CoV-2 are currently nonexistent, and methods to combat the deleterious effect of cytokine storm in response to the infection within the lungs are still underdeveloped. Viable oligonucleotide-based therapeutic strategies can, therefore, opt to target either the virus itself by inhibiting its spike protein or interfering with its replicative proteins through protein–nucleic acid interaction—as in an aptamer—or by directly targeting its large genome through small interfering RNA (siRNA) or antisense oligonucleotide (ASO)-mediated gene silencing. Alternatively, gene silencing (or activation) approaches can be taken to reduce the inflammatory effects in the lungs and other organs that lead to mortality in severe cases of COVID-19.
An immediate consideration is the huge size of the viral genome in CoVs, offering many possible targets for oligo-based therapeutics since they function through simple Watson–Crick base pairing. siRNAs and ASO strategies against the genome itself stand out as obvious approaches, especially since a combinatorial approach can be taken with either technology wherein a cocktail of multiple oligos against key regions of the CoV genome is introduced, improving chances of success and lessening the chance of mutational escape. The Rossi laboratory, in collaboration with others, used such an approach with some success in HIV, wherein to combat this problem we developed a combinatorial oligonucleotide-based gene therapy strategy targeting both the virus and host dependency factors using a self-inactivating lentiviral vector. This method combined three angles of attack, each with a unique mechanism of action: an siRNA to target mRNA of key HIV regulatory proteins Tat and Rev, a nucleolar-localizing RNA decoy to sequester Tat protein, and a ribozyme targeting CCR5, a chemokine receptor on the surface of white blood cells utilized HIV as one of two coreceptors to infect T cells. Although an altered overall approach would be required in the context of the radically different biology of a CoV compared with a retrovirus, the core principle of engaging the virus at separate and diverse stages of its lifestyle is key consideration when dealing with the rapid mutation rate of any RNA-based virus, and one that is made viable with oligonucleotide-based methods. We speculate that highly conserved regions within the replicase polyprotein-encoding regions of the SARS-CoV-2 genome could make particularly attractive targets for gene silencing. On the host side, temporarily silencing ACE-2 in infection-susceptible tissues could offer a viable strategy as well, as mouse knockout studies suggest animals lacking the gene remain viable (although not without serious deleterious side effects, especially to the cardiovascular system). In terms of lethality, the most problematic proteins presented by SARS-CoV-2 are its structural proteins, which are generally responsible for patient immune responses, in some cases so strong that patients die from lung inflammation and CD8 depletion. Given this, a gene-silencing approach against these proteins in particular—which account for only a third of the viral genome—might ultimately be the best mode in which to preserve human life. However, it remains uncertain as to whether CoVs trigger innate immunity through Toll Like receptors (TLRs) or even trigger RNAi responses in the host. Before developing such a strategy, it will be important to understand the entire constellation of RNAs in host cells after infection. In our experience, an effective approach that was used to better understand host factors involved in the context of the HIV infectious cycle was to use semirandom siRNA libraries to knock down host cell transcripts followed by HIV challenges. These screens identified many host dependency factors required for HIV to complete its replicative cycle, and I would suggest that this type of screen is a good place to start studies of the SARS-CoV-2 replicative cycle. This semirandom screening approach can also be tested with DNA oligos or CRISPR-Cas9.
Another possibility is that of using aptamers against ACE-2 as inhibitory agents; this prospect is bolstered by the relatively fast development time available to aptamers through protein-based SELEX, which can reveal sequence candidates with high target specificity and sensitivity in a relatively short amount of time. A further consideration is that aptamers conjugated to siRNAs or microRNAs (miRNAs) can be used to selectively target these antiviral agents to target cells and tissues, offering an avenue of delivery for silencing strategies previously outlined. Combining aptamers with antiviral oligos could be a powerful approach to therapeutic treatment and should be considered for CoV therapies. The challenge will be delivery of the aptamers—those that are used therapeutically need to be injected by IV and are cleared rapidly. However, other forms of delivery offering superior pharmacokinetic profiles are being pursued, such as by our group's development of an aptamer suitable for subcutaneous administration.
An alternative—though perhaps less viable given concerns about scalability and safety—is to utilize a gene therapy approach in which RNA oligos are encoded in a viral vector such as adeno-associated virus (AAV). For this approach, shRNAs can be fused to small PolIII promoters such as tRNA genes. The PolIII transcripts will be exported to the cytoplasm where the shRNAs will function in the RNAi pathway to knock down expression of both viral RNAs and host dependency factor RNAs.
Small RNAs can also be targeted to the nucleus for silencing or activating host gene transcription. Although gene activation using shRNAs has received a dearth of attention compared with silencing, this method shows great promise to hit many previously undruggable diseases. For example, activation of master regulatory genes such as CCAAT enhancer binding protein α modulates myeloid cell expression of inflammatory cytokines. Small double-stranded RNAs targeting the CEBP α promoter have been clinically tested in liver cancer patients by MiNA Therapeutics, with results to date showing a strong safety profile accompanied by suppression in myeloid cell cytokine expression and mobilization of CD8+ T cells. Given that the host immune response—specifically in the lungs—has been implicated as the major cause of lethality in severe cases of COVID-19, targeting master regulators of the immune system such as CEBP α with activating shRNAs could be a life-saving strategy for intubated patients. In addition, the small activating RNA approach should also be used to turn on antiviral gene responses such as interferons or RNAseL.
miRNAs constitute another strong area of consideration, since they naturally regulate host dependency factors, allowing the virus to be elucidated and exploited by augmenting their expression. miRNAs with potential to down regulate viral proteins and host gene responses that support infection can be identified via RNA seq analysis followed by informatics; once elucidated, a therapeutic strategy can be devised wherein these miRNAs can be ectopically delivered to CoV-infected cells.
A final thought on potential modes of treatment is that of activating innate immunity. Oligos with appropriate sequence and backbone modifications can be used to target TLRs to trigger protective interferon production. Interferons are so important as antiviral agents that viruses such as herpes simplex virus (HSV) and cytomegalovirus (CMV) have evolved proteins that block interferon activities. Conversely, oligos can perhaps be used to dampen innate immune responses to prevent inflammation. RNAs with specific sequence motifs can activate TLR7, whereas DNA oligos with CpG motifs activate TLR9. In addition, 5′ triphosphate-containing RNAs activate the retinoic acid-inducible gene-I (RIGI) TLR. When activated, each of these TLRs signals type 1 interferon gene expression, which can be an effective antiviral strategy.
Of course, the greatest challenge for oligo-based treatment strategies in this setting is delivery. The majority of oligo therapeutic applications utilize systemic delivery through intravenous or subcutaneous injection. In this circumstance, what is ideally needed is a form of inhalation delivery to get the oligos directly into the airways and lungs. A number of groups have pursued such a method of delivery in the past decade for respiratory diseases, and its effective implementation in patients with COVID-19 could enable many of the aforementioned strategies to be truly efficacious.
In summary, oligo-based therapies introduce a galaxy of possible applications to SARS-CoV-2 and other CoV infections (Table 1). Oligo therapeutics are easy to design, cost-effective to manufacture, and already possess many optimizing chemical modifications identified for use in other diseases, thereby simplifying design, production, and medicinal chemistry optimization during a crisis when rapid drug development is of utmost importance. As veterans of the field, the Rossi group feels it would be incredibly rewarding to witness oligo utilization to treat COVID-19 and other CoV infections.
Table 1.
Coronavirus Disease-19 and Treatment Strategies
| World society for virology COVID-19 statistics |
| https://www.ws-virology.org/sars-cov2_covid_19/ |
| Race to find COVID-19 treatments accelerates |
| https://science.sciencemag.org/content/367/6485/1412 |
| The proximal origin of SARS-CoV-2 |
| https://www.nature.com/articles/s41591-020-0820-9 |
| A new coronavirus associated with human respiratory disease in China |
| https://www.nature.com/articles/s41586-020-2008-3 |
| Drug targets for corona virus: A systematic review |
| https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074424/ |
| Coronaviruses: An overview of their replication and pathogenesis |
| https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4369385/ |
| Angiotensin-converting enzyme 2 is a key modulator of the renin angiotensin system in health and disease |
| https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3321295/ |
Author Disclosure Statement
D.R. is the interim president of iSTAT Therapeutics, Inc., which is developing products related to research described in this article. D.R. and iSTAT Therapeutics may financially benefit if the company is successful in marketing its products that are related to this research.
Funding Information
No funding was received for this article.
References
- 1. Kupferschmidt K and Cohen. J. 2020. Science 367:1412–1413. DOI: 10.1126/science.367.6485.1412 https://science.sciencemag.org/content/sci/367/6485/1412/F1.large.jpg [DOI] [PubMed]
- 2. Zhong Y, Yong WT and Liu. DX (2012). Recent progress in studies of arterivirus- and coronavirus-host interactions. Viruses 4:980–1010. DOI: 10.3390/v4060980 [DOI] [PMC free article] [PubMed] [Google Scholar]