Abstract
In light of the current novel coronavirus (COVID-19) pandemic, as well as other viral outbreaks in the 21st century, there is a dire need for new diagnostic and therapeutic strategies to combat infectious diseases worldwide. As a convergence science, tissue engineering has traditionally focused on the application of engineering principles to biological systems, collaboration across disciplines, and rapid translation of technologies from the benchtop to the bedside. Given these strengths, tissue engineers are particularly well suited to apply their skill set to the current crisis and viral outbreaks in general. This work introduces the basics of virology and epidemiology for tissue engineers, and highlights important developments in the field of tissue engineering relevant to the current pandemic, including in vitro model systems, vaccine technology, and small-molecule drug delivery. COVID-19 serves as a call to arms for scientists across all disciplines, and tissue engineers are well trained to be leaders and contributors in this time of need.
Impact statement
Given the steep mortality caused by the recent novel coronavirus (COVID-19) pandemic, there is clear need for advances in diagnostics and therapeutics for viral outbreaks. Tissue engineering has the potential for critical impact on clinical outcomes in viral outbreaks. Tissue engineers, if mobilized, could play key roles as leaders in the outbreak, given their ability to apply engineering principles to biological processes, experience in collaborative environments, and penchant for technological translation from benchtop to bedside. In this work, three areas pioneered by tissue engineers that could be applied to the current COVID-19 crisis and future viral outbreaks are highlighted.
Keywords: tissue engineering, biomaterials, pandemic, coronavirus, viral
Introduction
As of April 2020, the world is facing a pandemic of unfathomable proportions. As per the World Health Organization (WHO) on April 9, more than 1,500,000 patients worldwide have been diagnosed with the novel coronavirus disease 2019 (COVID-2019) caused by a laboratory-confirmed infection of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), with more than 84,000 global deaths at this time.1 Our hospital systems are rapidly filling with patients suffering from viral illness, and the capacity of resources such as emergency departments, inpatient wards, and intensive care units (ICUs) has become overwhelmed in some regions. There is a dire need for new diagnostic and therapeutic modalities. Early diagnosis is critical in establishing quarantine and limiting the spread of outbreaks; diagnostics later in the course of an epidemic remain important, particularly in determining established immunity. For those infected, there is a current paucity in validated antiviral therapies, and there is no vaccine at this time, although multiple efforts are under way. Even after the predicted resolution of COVID-19, the increasing frequency of viral outbreaks (including the 2003 Severe Acute Respiratory Syndrome Coronavirus,2 2014 Ebola virus,3 2015 Middle Eastern Respiratory Syndrome Coronavirus,4 and 2015 Zika virus outbreaks5) suggests that new mechanisms to combat viral infections are a high priority.
As a convergence science, tissue engineering is uniquely suited to offer solutions to complex clinical questions. Currently, tissue engineering–based technologies are being developed to revolutionize areas in medicine such as high-throughput drug discovery,6 personalized cancer therapy,7 immune modulation,8 and organ transplantation.9 Tissue engineers specialize in the application of engineering principles to biological systems, which facilitates the generation of fundamental knowledge as well as new technologies that could be key in a pandemic. In addition, tissue engineers are well versed in collaborative models,10 working closely alongside clinicians, biologists, chemists, physicists, mathematicians, veterinarians, and other specialists, which will be critical in a multidisciplinary approach to combating the virus known as SARS-CoV-2. Lastly, tissue engineering as a field has emphasized clinical translation, including creating workflows to optimize bringing the benchtop to the bedside,11 resulting in a $9 billion market, with 21 companies selling tissue engineering–based products in the United States alone as of 2017.12 In the current setting of limited clinical data and rising patient morbidity and mortality, tissue engineers would be welcomed and valuable allies in the COVID-19 pandemic.
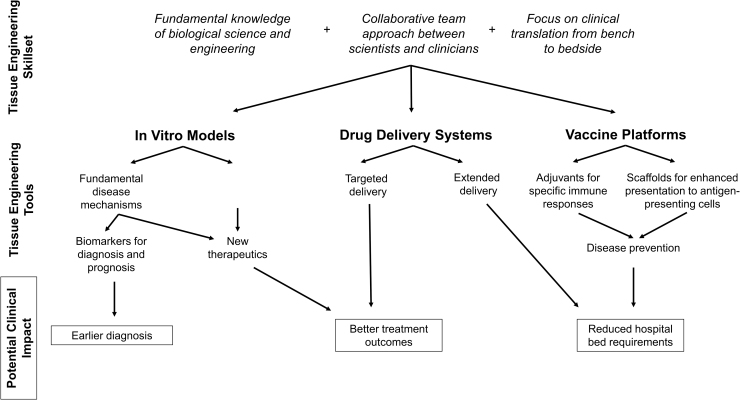
In this work, the potential impact of tissue engineering in improving clinical outcomes during the COVID-19 pandemic and future viral epidemics is explored (Fig. 1). Relevant background information regarding SARS-CoV-2 is briefly reviewed, and pertinent tissue engineering work is highlighted, including development of viral in vitro models, drug delivery systems, and vaccine platforms. Given the current state of the pandemic, it may be challenging to mobilize new efforts within the tissue engineering community in time to change practice in health care. New in vitro models, diagnostics, and therapeutics will need to be validated prior to safe implementation at the clinical level. In addition, many major academic centers are currently at limited capacity due to precautions to limit the spread of the pandemic. However, COVID-19 can act as a representative outbreak for which tissue engineers can learn from and begin preparations to lead the way to prevent and treat the next viral epidemic better.
FIG. 1.
Examples of how tissue engineering skills and tools may be leveraged to have an impact on clinical practice in the setting of a viral outbreak.
Our current understanding of SARS-CoV-2 is evolving and incomplete. The following information is based on current evidence and will likely change as the virus is more closely studied. Given the urgency of the pandemic, some of the references in this work have yet to receive peer review and should be interpreted with caution.
SARS-CoV-2 Background
Coronaviruses, or Orthocoronavirinae, are enveloped single-stranded RNA viruses. The virus gets its name from the projections, or “spikes,” emerging from its envelope that appear crown-like on electron micrography. The major components of SARS-CoV-2 are the envelope protein (E), membrane glycoprotein (M), spike protein (S), nucleocapsid protein (N), and its relatively large RNA genome of ∼30 kb.13,14 Enveloped viruses have a protective lipid bilayer with surface proteins and are generally more vulnerable to harsh environments than non-enveloped viruses. The spike protein is supposed to interact with human angiotensin-converting enzyme 2 (ACE2) membrane protein to induce fusion, endocytosis, and subsequent invasion into the host cell.15 Coronaviruses escape endosomes to the cytoplasm via acid-dependent cleavage of the S protein.14 RNA viruses, with few exceptions, replicate in the cytoplasm. The virus takes advantage of host ribosomes to replicate by translation and then assembly in the endoplasmic reticulum directed by M and E protein interactions. Viruses are then released by exocytosis to repeat the cycle of infection. Given this pathophysiology, potential targets being explored as therapeutics agents include blocking ACE2 interactions, altering endosome pH to prevent escape, inhibiting viral and/or host enzymes critical to replication, and downregulating host inflammation, given that an overexuberant response may lead to acute respiratory distress syndrome (ARDS).16
SARS-CoV-2 has the highest viral burden in the nares rather than the throat and is thought to be spread during coughing and aerosolization of droplets.17 Compared to SARS-CoV-1, spread of the virus appears to be more rapid due to higher asymptomatic carrier rates and a longer incubation time prior to symptom onset.18 Initial symptoms commonly include fever, cough, and fatigue and, less commonly, gastrointestinal manifestations.19 Current biomarkers suggestive of infection include elevated lactate dehydrogenase, ferritin, D-dimer, erythrocyte sedimentation rate, C-reactive protein, and absolute lymphopenia.20 In the United States, the most widely available diagnostic test is polymerase chain reaction based, although antibody-based assays are in development and are available in other countries.21 The virus primarily affects the lungs, causing ARDS in up to 5–10% of infected patients,22,23 although it has also been causing myocardial injury suspected to be due to high concentrations of ACE2 in cardiac tissue.15 Mortality is estimated to be 3–4%.18 Given the extreme global morbidity and mortality caused by the pandemic, there is a dire need for a better understanding of molecular mechanisms of host–virus interaction, more rapid methods to screen potential therapeutics, and platforms to facilitate safe clinical translation. These are all areas in which tissue engineers are primed to make significant contributions for COVID-19, the next coronavirus epidemic, or other future viral outbreaks.
In Vitro Models
Development of physiologically representative in vitro models of viral disease can assist in two critical roles during a pandemic: (1) better characterization and understanding of the host–pathogen interface and mechanisms of infection; and (2) as a platform for high-throughput screening of potential therapeutics. Currently, it is challenging and clinically necessary to predict the course of patients infected with SARS-CoV-2. Profiling biomarkers may allow for better risk stratification and resource allotment.24 More accurate in vitro modeling to understand host response as well as viral mechanisms for transmission and replication is critical for the identification of biomarkers to pursue as hypothesis-driven diagnostic and therapeutic targets. In addition, a physiologically relevant in vitro model has the potential to fill the critical need for improved drug candidate screening.
The current gold standard for screening antiviral therapeutics is based on static monolayer culture of Vero cells, an interferon-deficient aneuploid line of kidney epithelial cells originally isolated from an African green monkey.25,26 In a recent study, Vero cells were exposed to a library of 3000 drugs approved by the Food and Drug Administration and the Investigational New Drug program and then infected with SARS-CoV-2 to screen for potential therapeutics.27 Because Vero cells lack interferon, they are highly susceptible to viruses and allow for replication, and so they have been an attractive vehicle to screen compounds. However, interferon itself is an important regulator of host binding proteins involved in SARS-CoV-2.28 In general, the relevance of a drug's ability to inhibit viral infection of a malignant nonhuman primate (NHP) kidney cell lacking interferon production is uncertain. For example, while the antidepressant sertraline was found to have potent in vitro activity against Ebola in the Vero cell model,26 later testing in an in vivo NHP model failed to show protection against Ebola.29 Static cultures of xenograft monolayers fail to replicate many of the conditions facing viruses in vivo, including realistic extracellular matrix (ECM), three-dimensional cell–cell interfaces, and shear forces. Coronaviruses and other respiratory viruses, for example, can bind to the ECM components such as sialic acid to assist in infection of the host.30
In the last decade, tissue engineering has made significant advances regarding in vitro human cell culture models. Developments of induced pluripotent stem cells, CRISPR-Cas, microfluidics, 3D printing, and biomaterials have led to technologies such as tissue-on-a-chip and advanced bioreactor models containing co-cultures of cells from ectodermal, mesodermal, and endodermal lineages. These models, utilizing human cells, have been able to mimic complex pathophysiology such as generation of pulmonary edema upon exposure to inflammatory signals such as interleukin-2.31 For example, in a model to study influenza A virus, 3D tissue-engineered constructs more accurately recapitulated the host morphology of cultured human epithelial airway cells compared to 2D culture, and infection with major influenza strains resulted in upregulation of proinflammatory cytokines.32 One coronavirus-specific example of a tissue-engineered platform in which respiratory viruses have been studied is the rotation wall vessel bioreactor. These models simulate low physiologic shear stresses and frequently incorporate multiple pulmonary cell types, including co-culture of human mesenchymal bronchial tracheal cells and human bronchial epithelial cells, challenging them against respiratory syncytial virus and SARS-CoV-1.33 Unfortunately, SARS-CoV-1 did not replicate in this study.34 In another model, human pulmonary epithelial progenitor cells were grown on a collagen matrix in a serum-free media with a mesenchymal stroma and exposed to virus. It was demonstrated that stem cells were targeted by SARS-CoV-1, which may suggest why normal lung regeneration following viral infection is challenging.35
There are a number of exciting tissue-engineered human in vitro lung models currently available that could be leveraged for studying viral infection36,37 and established in vivo models for respiratory viruses, including NHPs, already in use.38–40 Elements that may improve the relevance of in vitro models include: (1) human rather than animal cell lines; (2) co-culture of multiple pulmonary cell lines; (3) 3D scaffolds that mimic native pulmonary architecture; and (4) culture methods that permit generation of ECM prior to viral inoculation. Additional head-to-head studies will need to be performed to determine if these components are necessary to capture the pathophysiology of viral infection. These platforms will be important in conducting hypothesis-driven research to understand the host–pathogen interface. Scaling these models to allow for high-throughput drug screening will offer important advantages over the current Vero-based methods to identify therapeutic candidates for in vivo translation rapidly during major infectious outbreaks.
Drug Delivery Systems
During the COVID-19 pandemic, one of the most significant strains on the health-care system has been the need for inpatient beds, both on general wards and in ICUs.41 As discussed previously regarding in vitro drug screens, there are currently limited therapeutics that have clear clinical evidence of improving outcomes such as days of hospitalization required, need for ICU stay, and need for intubation/ventilation. As new small molecule–based therapies come through the pipeline, tissue engineers can continue to design drug delivery systems to (1) target medications to specific organ systems to increase bioavailability, and (2) extend the release of medications so that frequent administration is not necessary.
Classes of molecules that have been suggested as possible therapies include repurposed small-molecule drugs, monoclonal antibodies, and oligonucleotide strategies.42 For example, based on a small clinical study,43 the combination of hydroxychloroquine (a small molecule traditionally used to treat malaria and lupus) and azithromycin (a macrolide antibiotic with anti-inflammatory properties) has been suggested as a means to reduce SARS-CoV-2 viral load, although these results are controversial.44 Poly(lactide) and poly(lactide-co-glycolide) (PLGA) microparticles can deliver azithromycin for up to 60 days with zero-order release kinetics.45 There are also formulations using fumaryl diketopiperazine microparticles to deliver azithromycin via intratracheal insufflation directly to the lungs, resulting in higher local concentrations in a murine pneumonia model compared to oral and intravenous routes of delivery.46 Given that the half-life of hydroxychloroquine is >40 days, extended drug delivery options may not be warranted.47 However, there have been successful delivery systems constructed from PLGA nanoparticles for specific cell targeting.48 While the efficacy of hydroxychloroquine and azithromycin in the prevention and treatment of SARS-CoV-2 remains an area of active study, the above examples serve to show that tissue engineers and biomaterials scientists have been working on drug delivery for decades with a variety of vehicles available for small molecules in general.
In addition to small molecules, monoclonal antibodies are an exciting class of medication, given their success in the treatment of Ebola virus.49 Antibodies are a natural part of humoral immunity and can be engineered to block specific ligands or receptors vital for viral function. These therapies generally need to be delivered intravenously to be successful. In the Ebola virus studies, for example, patients required one to three infusion sessions, depending on the antibody. A human monoclonal antibody was developed against SARS-CoV-1 and was demonstrated to be effective in a ferret model.38 Researchers have screened monoclonal antibodies designed against SARS-CoV-1 and have discovered cross-reactivity of at least one of the antibodies against SARS-CoV-2.50 Systems designs for the controlled extended release of antibodies may be advantageous over multiple infusion sessions for clinical practice. Nanoporous scaffolds coated with allylamine-based polymer were capable of releasing rituximab, a monoclonal antibody against B cells, for up to 30 days.51 Similarly, an alginate-based drug delivery system was able to deliver a human immunoglobulin G1 (IgG1) monoclonal antibody in a rat model for at least 28 days with a single dose of the system.52 In addition to the possibility of reducing administration to single dosing by extended release, there has also been development of ingestible injection systems to deliver biomacromolecules through autoinjection during gastric transit. These allowed for insulin delivery in a porcine model and may facilitate oral delivery of medications previously only efficacious in intravenous form.53
Lastly, short interfering RNA (siRNA) has also been explored as both prophylaxis and therapy for coronavirus infection.54 For sequences specific to SARS-CoV-1, siRNA was effective in a NHP model,39 resulting in diminished viral load and alveolar damage. Four intranasal doses were required over 5 days in treatment arms. The researchers reported not using additional vehicles to deliver their siRNA such as polyethylenimine due to the possibility of carrier-induced lung inflammation. However, more complex vehicles have since been developed by the field specifically for pulmonary usage, including mesoporous silica nanoparticles55 and cationic liposomes.56 As siRNA sequences, specific antibodies, and small molecules are identified that specifically mitigate SARS-CoV-2, tissue engineers and biomaterials scientists can continue their work in designing vehicles to target areas of high viral load specifically and extendedly. Even if this work may not come to fruition during the current pandemic, these vehicles may serve vital roles during future viral outbreaks.
Vaccine Platforms
The ability to vaccinate against specific pathogens has played a major role in preventative medicine for the last century. Vaccines exist both for respiratory viruses such as Influenzavirus and for bacteria such as Streptococcus pneumoniae and Haemophilus influenzae. While the success of respiratory viral vaccines varies from season to season, data suggest influenza vaccination generally results in lower probability of complications, including ICU stay, mechanical ventilation, and severe outcomes, especially in patients with comorbidities such as chronic obstructive pulmonary disease.57–59 Work is already underway to develop vaccines effective against SARS-CoV-2 to prevent disease and mitigate transmission.60
Successful vaccination against pathogens relies on presenting antigens and stimulating specific elements of the immune system to build recognition and memory in both humoral and cell-mediated branches. With advances in immunology, biomaterials and tissue engineering are being leveraged to elicit specific host immune responses to augment vaccination strategies.8,61 In many of these systems, the biomaterial acts as the drug delivery vehicle for the vaccine as well as the adjuvant. Furthermore, the field is characterizing how the size, shape, and other physicochemical properties of biomaterials affect the behavior of immune cells.62–64 The goals of many of these systems are to target antigen-presenting cells such as macrophages and dendritic cells and to drive specific responses such as Th1 or Th2 (different helper T classes). For example, conjugation of different receptors to protein-based particles can individually tune Th1 or Th2 response in a murine model.65 Biodegradable polymers, one of the primary workhorses as scaffold material in tissue engineering, have also been explored. PLGA nanoparticles drive Th1 immune response compared to no carriers and other biomaterials in a vaccine against Chagas disease in a murine model66 and have also been explored for targeted delivery to specific immune populations.67 PLGA microparticles in combination with a chitosan/peptide conjugate coating have also been used as a delivery system to target specific mucosal cells and to deliver a swine dysentery vaccine with elevated IgA and IgG production in mice.68 In another murine model, chitosan nanoparticles enhanced T-cell response for a Mycobacterium tuberculosis DNA vaccine.69 When chitosan was mannosylated to promote endocytosis, an intranasal vaccine increased IgG levels in a murine model.70 For influenza, chitosan has also been modified to create a thermoresponsive intranasal murine vaccine against H5N1l.71 Silver nanoparticles have also been used to deliver inactivated influenza vaccine locally with some specificity to lung immune cells, resulting in greater IgG titers and reduced mortality in a murine model.40 While there are currently fewer studies regarding coronavirus vaccines, there has been some success in mice in which nanoparticles were prepared from a SARS-CoV-1 peptide sequence, and subsequent sera was successful in preventing infection of Vero cells.72
In addition to various particle-based platforms, tissue engineering strategies have been harnessed to create scaffold systems for vaccination enhancement. With specific physicochemical properties, such as pore size, and profile of released recruitment signals such as granulocyte-colony stimulating factor, scaffolds of PLGA73,74 and mesoporous silica rods64,75 have been used to recruit and concentrate antigen-presenting cells to vaccine components. This strategy has primarily been applied to tumor vaccines and has demonstrated efficacy in animal models against melanoma and intracranial gliomas.73,76 It is currently undergoing a Phase I clinical trial of 23 patients with melanoma, which is estimated to complete in June 2020 (NCT01753089; ClinicalTrials.gov). This platform of scaffold-based vaccination has also shown efficacy against bacterial pathogens in porcine and murine models.77 Other examples of scaffold-based vaccine systems include those generated from respiratory syncytial virus that were effective in mice as well NHPs.78,79 These particle-based and scaffold-based vaccine systems may be promising in translation against SARS-CoV-2. However, the majority have only been studied in mouse models at this point in time, and significant translational efforts will need to be undertaken for clinical trials. Modular platforms in which different antigens can be plugged in77 may be very useful for rapid vaccine development in future pandemics.
Conclusion
The world faces a global health-care crisis of unheralded magnitude. The rate of infection and mortality from COVID-19 make it unlike any virus seen in this century. Physicians and scientists are banding together to combat the threat of SAR-CoV-2. Tissue engineers have a rare set of tools and can make substantial contributions to our understanding of viral disease and contribute toward the critical development of diagnostic and therapeutic platforms. Together, we can overcome this current pandemic and work to prevent and mitigate future viral outbreaks.
Acknowledgments
I would like to thank my co-residents at the Massachusetts General Hospital for their selfless service on the front lines in the treatment of patients in this pandemic.
Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this work.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. World Health Organization, 2020 [Google Scholar]
- 2. Bell D.M.; World Health Organization Working Group on Internationaland Community Transmission of SARS.. Public health interventions and SARS spread, 2003. Emerg Infect Dis 10, 1900, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomes M.F., y Piontti A.P., Rossi L., et al. Assessing the international spreading risk associated with the 2014 West African Ebola outbreak. PLoS Curr 6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho S.Y., Kang J.M., Ha Y.E., et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet 388, 994, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang C., Ortiz K., Ansari A., and Gershwin M.E.. The Zika outbreak of the 21st century. J Autoimmun 68, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazzocchi A., Soker S., and Skardal A.. 3D bioprinting for high-throughput screening: drug screening, disease modeling, and precision medicine applications. Appl Phys Rev 6, 011302, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fong E.L., Wan X., Yang J., et al. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77, 164, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vishwakarma A., Bhise N.S., Evangelista M.B., et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol 34, 470, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Khademhosseini A., and Langer R.. A decade of progress in tissue engineering. Nat Protoc 11, 1775, 2016 [DOI] [PubMed] [Google Scholar]
- 10. Fong E.L., Watson B.M., Kasper F.K., and Mikos A.G.. Building bridges: leveraging interdisciplinary collaborations in the development of biomaterials to meet clinical needs. Adv Mater 24, 4995, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tatara A.M., Ratcliffe A., Wong M.E., Kasper F.K., and Mikos A.G. Biomaterials in regenerative medicine: considerations in early process development. In: Translational Regenerative Medicine. Elsevier, 2015, p. 141 [Google Scholar]
- 12. Kim Y.S., Smoak M.M., Melchiorri A.J., and Mikos A.G.. An overview of the tissue engineering market in the United States from 2011 to 2018. Tissue Eng Part A 25, 1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chatterjee S. Understanding the nature of variations in structural sequences coding for coronavirus spike, envelope, membrane and nucleocapsid proteins of SARS-CoV-2 (March 28, 2020) [Google Scholar]
- 14. Fehr A.R., and Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Coronaviruses. Springer, 2015, p. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., and Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020 [Epub ahead of print]; DOI: 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo Y.-R., Cao Q.-D., Hong Z.-S., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res 7, 11, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New Engl J Med 382, 1177, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen S.F., and Ho Y.-C. SARS-CoV-2: a storm is raging. J Clin Invest 2020 [Epub ahead of print]; DOI: 10.1172/JCI137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J.J., Dong X., Cao Y.Y., et al. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy 2020. [Epub ahead of print]; DOI: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 20. Lippi G., and Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020 [Epub ahead of print]; DOI: 10.1515/cclm-2020-0198 [DOI] [PubMed] [Google Scholar]
- 21. To K.K.-W., Tsang O.T.-Y., Leung W.-S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020 [Epub ahead of print]; DOI: 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020 [Epub ahead of print]; DOI: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Remuzzi A., and Remuzzi G. COVID-19 and Italy: what next? Lancet 2020 [Epub ahead of print]; DOI: 10.1016/S0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie J., Hungerford D., Chen H., et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv 2020 [Epub ahead of print]; DOI: 10.1101/2020.03.28.20045997 [DOI] [Google Scholar]
- 25. Adcock R.S., Chu Y.K., Golden J.E., and Chung D.H.. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res 138, 47, 2017 [DOI] [PubMed] [Google Scholar]
- 26. Johansen L.M., DeWald L.E., Shoemaker C.J., et al. A screen of approved drugs and molecular probes identifies therapeutics with anti–Ebola virus activity. Scince Transl Med 7, 290ra89, 2015 [DOI] [PubMed] [Google Scholar]
- 27. Jeon S., Ko M., Lee J., et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. bioRxiv 2020 [Epub ahead of print]; DOI: 10.1101/2020.03.20.999730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ziegler C., Allon S.J., Nyquist S.K., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is enriched in specific cell subsets across tissues. Cell 2020 [Epub ahead of print]; DOI: 10.2139/ssrn.3555145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Honko A.N., Johnson J.C., Marchand J.S., et al. High dose sertraline monotherapy fails to protect rhesus macaques from lethal challenge with Ebola virus Makona. Sci Rep 7, 5886, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwegmann-Weßels C., and Herrler G.. Sialic acids as receptor determinants for coronaviruses. Glycoconj J 23, 51, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huh D., Leslie D.C., Matthews B.D., et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4, 159ra147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhowmick R., Derakhshan T., Liang Y., Ritchey J., Liu L., and Gappa-Fahlenkamp H.. A three-dimensional human tissue-engineered lung model to study influenza A infection. Tissue Eng Part A 24, 1468, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gardner J.K., and Herbst-Kralovetz M.M.. Three-dimensional rotating wall vessel-derived cell culture models for studying virus–host interactions. Viruses 8, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suderman M., McCarthy M., Mossell E., et al. Three-dimensional human bronchial-tracheal epithelial tissue-like assemblies (TLAs) as hosts for severe acute respiratory syndrome (SARS)-CoV infection. NASA/TP-2006-213723. NASA, 2006 [Google Scholar]
- 35. Ling T.Y., Kuo M.D., Li C.L., et al. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci U S A 103, 9530, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller A.J., and Spence J.R.. In vitro models to study human lung development, disease and homeostasis. Physiology (Bethesda) 32, 246, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nichols J.E., Niles J.A., Vega S.P., and Cortiella J.. Novel in vitro respiratory models to study lung development, physiology, pathology and toxicology. Stem Cell Res Ther 4 (Suppl 1), S7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ter Meulen J., Bakker A.B., van den Brink E.N., et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363, 2139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li B.-j., Tang Q., Cheng D., et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med 11, 944, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez-Guzman D., Le Guen P., Villeret B., et al. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials 217, 119308, 2019 [DOI] [PubMed] [Google Scholar]
- 41. Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020 [Epub ahead of print]; DOI: 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 42. Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res 9, 72, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gautret P., Lagier J.-C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020 [Epub ahead of print]; DOI: 10.1016/ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Kim A.H., Sparks J.A., Liew J.W., et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med 2020 [Epub ahead of print]; DOI: 10.7326/M20-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X., Chang S., Du G., et al. Encapsulation of azithromycin into polymeric microspheres by reduced pressure-solvent evaporation method. Int J Pharm 433, 79, 2012 [DOI] [PubMed] [Google Scholar]
- 46. Wang Q., Mi G., Hickey D., et al. Azithromycin-loaded respirable microparticles for targeted pulmonary delivery for the treatment of pneumonia. Biomaterials 160, 107, 2018 [DOI] [PubMed] [Google Scholar]
- 47. Tett S., Cutler D., Day R., and Brown K.. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol 27, 771, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mansilla E., Marin G.H., Nunez L., et al. The lysosomotropic agent, hydroxychloroquine, delivered in a biodegradable nanoparticle system, overcomes drug resistance of B-chronic lymphocytic leukemia cells in vitro. Cancer Biother Radiopharm 25, 97, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Mulangu S., Dodd L.E., Davey R.T. Jr., et al. A randomized, controlled trial of Ebola virus disease therapeutics. New Engl J Med 381, 2293, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian X., Li C., Huang A., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect 9, 382, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simovic S., Diener K., Bachhuka A., et al. Controlled release and bioactivity of the monoclonal antibody rituximab from a porous matrix: a potential in situ therapeutic device. Mater Lett 130, 210, 2014 [Google Scholar]
- 52. Schweizer D., Vostiar I., Heier A., et al. Pharmacokinetics, biocompatibility and bioavailability of a controlled release monoclonal antibody formulation. J Control Release 172, 975, 2013 [DOI] [PubMed] [Google Scholar]
- 53. Abramson A., Caffarel-Salvador E., Khang M., et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng B.-J., Guan Y., Tang Q., et al. Prophylactic and therapeutic effects of small interfering RNA targeting SARS coronavirus. Antivir Ther 9, 365, 2004 [PubMed] [Google Scholar]
- 55. Chen Y., Gu H., Zhang D.S.-Z., Li F., Liu T., and Xia W.. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials 35, 10058, 2014 [DOI] [PubMed] [Google Scholar]
- 56. Fehring V., Schaeper U., Ahrens K., et al. Delivery of therapeutic siRNA to the lung endothelium via novel lipoplex formulation DACC. Mol Ther 22, 811, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Demicheli V., Jefferson T., Ferroni E., Rivetti A., and Di Pietrantonj C.. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2, CD001269, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaselitz T.B., Martin E.T., Power L.E., and Cinti S.. Impact of vaccination on morbidity and mortality in adults hospitalized with influenza A, 2014–2015. Infect Dis Clin Pract 27, 328, 2019 [Google Scholar]
- 59. Poole P.J., Chacko E., Wood-Baker R.W., and Cates C.J. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev CD002733, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Chen W.H., Strych U., Hotez P.J., and Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep 2020 [Epub ahead of print]; DOI: 10.1007/s40475-020-00201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jones K.S. Biomaterials as vaccine adjuvants. Biotechnol Prog 24, 807, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Chen X., Yan Y., Mullner M., et al. Shape-dependent activation of cytokine secretion by polymer capsules in human monocyte–derived macrophages. Biomacromolecules 17, 1205, 2016 [DOI] [PubMed] [Google Scholar]
- 63. Veiseh O., Doloff J.C., Ma M., et al. Size-and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 14, 643, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li W.A., Lu B.Y., Gu L., Choi Y., Kim J., and Mooney D.J.. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials 83, 249, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gause K.T., Yan Y., O'Brien-Simpson N.M., et al. Codelivery of NOD2 and TLR9 ligands via nanoengineered protein antigen particles for improving and tuning immune responses. Adv Funct Mater 26, 7526, 2016 [Google Scholar]
- 66. Barry M.A., Wang Q., Jones K.M., et al. A therapeutic nanoparticle vaccine against Trypanosoma cruzi in a BALB/c mouse model of Chagas disease. Hum Vaccin Immunother 12, 976, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Primard C., Rochereau N., Luciani E., et al. Traffic of poly(lactic acid) nanoparticulate vaccine vehicle from intestinal mucus to sub-epithelial immune competent cells. Biomaterials 31, 6060, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Jiang T., Singh B., Li H.-S., et al. Targeted oral delivery of BmpB vaccine using porous PLGA microparticles coated with M cell homing peptide-coupled chitosan. Biomaterials 35, 2365, 2014 [DOI] [PubMed] [Google Scholar]
- 69. Feng G., Jiang Q., Xia M., et al. Enhanced immune response and protective effects of nano-chitosan-based DNA vaccine encoding T cell epitopes of Esat-6 and FL against Mycobacterium tuberculosis infection. PLoS One 8, e61135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70. Yao W., Peng Y., Du M., Luo J., and Zong L.. Preventative vaccine-loaded mannosylated chitosan nanoparticles intended for nasal mucosal delivery enhance immune responses and potent tumor immunity. Mol Pharm 10, 2904, 2013 [DOI] [PubMed] [Google Scholar]
- 71. Wu Y., Wei W., Zhou M., et al. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials 33, 2351, 2012 [DOI] [PubMed] [Google Scholar]
- 72. Pimentel T.A., Yan Z., Jeffers S.A., Holmes K.V., Hodges R.S., and Burkhard P.. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Design 73, 53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ali O.A., Doherty E., Mooney D.J., and Emerich D.. Relationship of vaccine efficacy to the kinetics of DC and T-cell responses induced by PLG-based cancer vaccines. Biomatter 1, 66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ali O.A., Emerich D., Dranoff G., and Mooney D.J.. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med 1, 8ra19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim J., Li W.A., Choi Y., et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol 33, 64, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ali O.A., Doherty E., Bell W.J., et al. Biomaterial-based vaccine induces regression of established intracranial glioma in rats. Pharm Res 28, 1074, 2011 [DOI] [PubMed] [Google Scholar]
- 77. Super M., Doherty E.J., Cartwright M.J., et al. Modular biomaterials vaccine technology protects against multiple pathogens and septic shock. bioRxiv 2020 [Epub ahead of print]; DOI: 10.1101/2020.02.25/964601 [DOI] [Google Scholar]
- 78. Correia B.E., Bates J.T., Loomis R.J., et al. Proof of principle for epitope-focused vaccine design. Nature 507, 201, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luo X., Liu T., Wang Y., et al. An epitope-specific respiratory syncytial virus vaccine based on an antibody scaffold. Angew Chem Int Ed Engl 54, 14531, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]