Abstract
Decompressive craniectomy (DC) in traumatic brain injury (TBI) has been suggested to influence cerebrovascular reactivity. We aimed to determine if the statistical properties of vascular reactivity metrics and slow-wave relationships were impacted after DC, as such information would allow us to comment on whether vascular reactivity monitoring remains reliable after craniectomy. Using the CENTER-TBI High Resolution Intensive Care Unit (ICU) Sub-Study cohort, we selected those secondary DC patients with high-frequency physiological data for both at least 24 h pre-DC, and more than 48 h post-DC. Data for all physiology measures were separated into the 24 h pre-DC, the first 48 h post-DC, and beyond 48 h post-DC. We produced slow-wave data sheets for intracranial pressure (ICP) and mean arterial pressure (MAP) per patient. We also derived a Pressure Reactivity Index (PRx) as a continuous cerebrovascular reactivity metric updated every minute. The time-series behavior of the PRx was modeled for each time period per patient. Finally, the relationship between ICP and MAP during these three time periods was assessed using time-series vector autoregressive integrative moving average (VARIMA) models, impulse response function (IRF) plots, and Granger causality testing. Ten patients were included in this study. Mean PRx and proportion of time above PRx thresholds were not affected by craniectomy. Similarly, PRx time-series structure was not affected by DC, when assessed in each individual patient. This was confirmed with Granger causality testing, and VARIMA IRF plotting for the MAP/ICP slow-wave relationship. PRx metrics and statistical time-series behavior appear not to be substantially influenced by DC. Similarly, there is little change in the relationship between slow waves of ICP and MAP before and after DC. This may suggest that cerebrovascular reactivity monitoring in the setting of DC may still provide valuable information regarding autoregulation.
Keywords: cerebrovascular reactivity, DC, decompressive craniectomy, PRx, TBI
Introduction
Cerebrovascular reactivity monitoring in neurocritical care is emerging as an important physiological parameter for prognosis in adult moderate/severe traumatic brain injury (TBI).1,2 To date, numerous studies have demonstrated the strong association between intracranial pressure (ICP) derived metrics of cerebrovascular reactivity, and global outcome at 6 months.3–9 Further, this association with outcome has been shown to provide additional prognostic information above models containing baseline demographic and standard physiological data captured in TBI.8 Finally, recent publications from the Collaborative European Neuro Trauma Effectiveness Research in TBI (CENTER-TBI) High Resolution Intensive Care Unit (ICU) Sub-Study cohort have provided some preliminary multi-center validation of this relationship.9,10
The Pressure Reactivity Index (PRx), derived from the correlation between vasogenic slow-waves in ICP and mean arterial pressure (MAP) is the most widely cited continuous measure of cerebrovascular reactivity in moderate/severe TBI.3,11 Experimental literature also provides some support for it as a measure of the lower limit of autoregulation,12–14 and critical thresholds exist in adult TBI associated with 6-month global outcome.4,8
Despite these strong links with outcome, a previous retrospective analysis conducted in the setting of decompressive craniectomy (DC) suggests that PRx behavior is altered after craniectomy.15 This study of 27 patients found that PRx was more positive after bone flap removal on the basis of grand mean PRx data both before and for the first 72 h after DC. The suggestion from these results was that craniectomy may induce a state of impaired autoregulation, and thus there has been some concern about the interpretation of continuously measured cerebrovascular reactivity post-craniectomy. Whereas, an alternative explanation is that after craniectomy the compliance of the cerebral system dramatically increases, the relationship between intracerebral volume and ICP diminishes, and PRx no longer carries valid information about cerebrovascular pressure reactivity.16 This previous work focused on averaged data from different time periods pre- and post-DC, not the statistical properties and behaviors of the signals pre- and post-DC. Such analysis of signal statistical properties may provide information as to whether continuous cerebrovascular reactivity metrics still carry reliable information regarding autoregulation post-craniectomy.
The goal of this study was to explore in more detail the impact of craniectomy on PRx and the relationship between vasogenic slow-waves of ICP and MAP, in a cohort of secondary DC patients from the CENTER-TBI High Resolution Intensive Care Unit (HR ICU) Sub-Study,17 using time-series analytical techniques. Assessing the statistical signal properties of the ICP, blood pressure, and their inter-relationship offers a more principled and physiological-model agnostic way to explore changes in underlying cerebrovascular reactivity. We aimed to determine if the statistical properties of vascular reactivity metrics and slow-wave relationships were impacted secondary to DC, as such information would allow us to comment on whether vascular reactivity monitoring remains reliable after craniectomy.
Methods
Patient population
Patients from the multi-center CENTER-TBI HR ICU cohort were included in this study. Only patients who underwent a secondary DC and had the following high-frequency physiology recording parameters were included: 1) at least 24 h of recording prior to DC, and 2) more than 48 h of recording post-DC. These patients were prospectively recruited between January 2015 and December 2017, from across 21 centers in the European Union (EU). All patients were admitted to an ICU for their TBI during the course of the study, and had high-frequency digital signals recorded from their ICU monitors during the course of their ICU stay. All patients suffered predominantly from moderate to severe TBI (moderate = Glasgow Coma Scale [GCS] score 9–12, and severe = GCS ≤8). A minority of patients suffered from non-severe TBI, with subsequent early deterioration leading to ICU admission for care and monitoring. All patients in this cohort had invasive ICP monitoring conducted in accordance with Brain Trauma Foundation (BTF) guidelines.18
Ethics
Data used in these analyses were collected as part of the CENTER-TBI study, which had individual national or local regulatory approval; the U.K. ethics approval is provided as an exemplar (IRAS No: 150943; REC 14/SC/1370). The CENTER-TBI study has been conducted in accordance with all relevant laws of the EU if directly applicable or of direct effect and all relevant laws of the country where the recruiting sites were located, including but not limited to, the relevant privacy and data protection laws and regulations (the “Privacy Law”), the relevant laws and regulations on the use of human materials, and all relevant guidance relating to clinical studies from time to time in force including, but not limited to, the ICH Harmonized Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) (ICH GCP) and the World Medical Association Declaration of Helsinki entitled “Ethical Principles for Medical Research Involving Human Subjects.” Informed consent by the patients and/or the legal representative/next of kin was obtained, accordingly to the local legislations, for all patients recruited in the core data set of CENTER-TBI and documented in the electronic case report form (e-CRF).
Data collection
As part of recruitment to the multi-center high-resolution ICU cohort of CENTER-TBI,17 demographics and clinical data were prospectively collected, and patients had high-frequency digital signals from ICU monitoring recorded throughout their ICU stay, with the goal of initiating recording within 24 h of injury. All digital ICU signals were further processed (see sections on Signal Acquisition and Signal Processing). For the purpose of this study, the following admission demographic variables were collected: age, sex, and admission Glasgow Coma Scale score (GCS total and motor), admission pupillary response, admission Marshall computed tomography (CT) grade, presence of subarachnoid hemorrhage on admission CT, presence of epidural hematoma on admission CT, history of pre-hospital hypoxia, history of pre-hospital hypotension, and admission glucose and hemoglobin values. CENTER-TBI data version 1.0 was accessed for the purpose of this study, using the Opal datamart software.19
Signal acquisition
Arterial blood pressure (ABP) was obtained through either radial or femoral arterial lines connected to pressure transducers. ICP was acquired via an intra-parenchymal strain gauge probe (Codman ICP MicroSensor; Codman & Shurtleff Inc., Raynham, MA), parenchymal fiber optic pressure sensor (Camino ICP Monitor, Integra Life Sciences, Plainsboro, NJ; https://www.integralife.com/), or external ventricular drain. All signals were recorded using digital data transfer or were digitized via an A/D converter (DT9801; Data Translation, Marlboro, MA) where appropriate, sampled at a frequency of 100 Hz or higher, using the ICM+ software (Cambridge Enterprise Ltd., Cambridge, United Kingdom; http://icmplus.neurosurg.cam.ac.uk) or Moberg CNS Monitor (Moberg Research Inc., Ambler, PA), or a combination of both. Signal artifacts were removed using automated methods prior to further processing or analysis.
Signal processing
Post-acquisition processing of the above signals was conducted using ICM+. Cerebral perfusion pressure (CPP) was calculated as MAP – ICP. Ten-second moving averages (updated every 10 sec to avoid data overlap) were calculated for all recorded signals: ICP and ABP (which produced MAP). Data sheets with the 10-sec mean values were created per patient for the purpose of the ICP and MAP slow-wave analysis.
PRx was derived using the Pearson correlation between 30 consecutive, 10-sec mean values for ICP and MAP, updated every minute. Data for PRx were provided in minute-by-minute comma-separated variable sheets.
Finally, both the 10-sec by 10-sec data (ICP and MAP), and the minute-by-minute data (for PRx) were divided for each patient into the following: 1) 24 h pre-DC, 2) first 48 h post-DC, and 3) beyond 48 h post-DC to the end of recording.
Data processing
Post-ICM+ processing was undertaken using R (R Core Team [2016]. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/). General summary data for the duration of each time period was produced per patient and included: proportion (%) time with ICP above both 20 mm Hg and 22 mm Hg, proportion (%) time with PRx above 0, proportion (%) time with PRx above +0.25, and proportion (%) time with PRx above +0.35. These thresholds were utilized based on previous publications suggesting their association with global outcome in adult TBI.4,8,18 Grand mean values of the physiological variables were also generated for each patient during the above-described three time periods around DC. Differences in these values between the three time periods were assessed using box plots and Mann-U testing, with alpha set at 0.05.
Statistical analysis
All statistical analysis was conducted using the R and XLSTAT (Addinsoft, New York, NY; https://www.xlstat.com/en/) add-on package to Microsoft Excel (Microsoft Office 15, version 16.0.7369.1323). Normality of continuous variables was assessed via Shapiro-Wilks test, confirming non-parametric distribution. Differences in the general summary values for the physiological measures (as described in the Data Processing section) between the three time periods were assessed using box plots and Mann-U testing, with alpha set at 0.05.
For time-series modeling, transformed data were utilized. ICP and MAP were transformed using a logarithmic transform, whereas PRx was transformed using a Fisher transform.20
PRx analysis
Using minute-by-minute Fisher transformed PRx data, the following analysis was conducted for each of the three time periods around DC, for each patient. For each patient, the optimal autoregressive integrative moving average (ARIMA) time-series structure was determined for PRx using the following methodology, similar to that reported in other articles from our group.21,22 First, autocorrelation function (ACF) and partial autocorrelation function (PACF) plots were produced, and both Augmented Dickey-Fuller (ADF) and Kwiatkowski-Phillips-Schmidt-Shin (KPSS) testing were conducted, for PRx measures, confirming non-stationarity. First-order differencing was then undertaken to remove all trend components, confirming stationarity by repeating the above mentioned plots and testing. Next, ARIMA models were built for PRx, keeping the differencing order of 1 (i,e., d = 1), and varying both the autoregressive and moving average orders (i.e., p and q, respectively) from 0 to 4, through all respective permutations. The Akaike Information Criterion (AIC) and log-likelihood (LL) were then tabulated for each of these models, for every patient, during each time period around DC. Using the AIC and LL, the optimal ARIMA structures for PRx were compared in each patient across the three time periods, with the lowest AIC and highest LL values indicating superior models. More details surrounding ARIMA modeling of time-series data can be found in the reference literature.21–24 The general Box-Jenkin's autoregressive moving average (ARMA) structure for PRx can be expressed as follows:
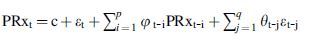
where c = constant, t = time “t”, i = integer, j = integer, p = autoregressive order, PRx = pressure reactivity index, q = moving average order, φ = autoregressive coefficient at time “t-i”, θ = moving average coefficient at time “t-j”, and ɛ = error term.
ICP and MAP slow-wave analysis
Transformed ICP and MAP slow waves were analyzed in the 10-sec by 10-sec data sheets, per patient. The time-series characteristics of ICP and MAP were first independently evaluated in each patient to determine then general ARIMA structure across the population for each. Then the co-variance of ICP and MAP slow waves were evaluated using multi-variate vector ARIMA (VARIMA) models. Such models explore the behavior of two time-series recorded simultaneously over time, and are derived via extending the standard Box-Jenkin's ARIMA models to multi-variate systems. Further description on this technique can be found in the References section.21–24 The vector autoregressive moving average model (VARMA) of first-order difference ICP and MAP can be represented by the following formula:
where C = constant vector, t = time “t”, i = integer, j = integer, p = VARMA autoregressive order, Yt = ICP or MAP at time t, q = VARMA moving average order, A = autoregressive coefficient matrix at “t-i”, B = moving average coefficient matrix at time “t-j”, E = error term vector.
We utilized first-order differenced and transformed ICP and MAP signals, to eliminate trend and seasonality, and employed basic VARMA models with an autoregressive order of 4 and a moving average order of 4, based on the findings from individual-patient ARIMA models of the transformed ICP and MAP, for each patient, confirming that such VARMA orders would encompass the variation seen in optimal ARIMA structure for ICP and MAP across the population. The coefficients derived from these VARMA models were then employed to derive impulse response function (IRF) plots between ICP and MAP. The IRF plots provide a descriptive graphical representation of the impact of ICP on MAP, and MAP on ICP, by using the generated VARIMA model and modeling a one standard deviation orthogonal impulse of one variable on the other, and vice versa. The plots depict how much from baseline the standard error of one variable fluctuates in response to the orthogonal impulse of the other variable, and how many lags in time it takes to recover back to baseline. The above was conducted for each individual patient, during each of the three time periods around DC (described previously).
Finally, the influence of slow waves of ICP and MAP on one another over time was assessed via Granger causality using stationary first-order differenced ICP and MAP data, with testing of both the impact of ICP on MAP, and the impact of MAP on ICP.25 This was tested in every patient, in all three time periods around DC. Both F-test statistic value and p-values were recorded, with alpha set at 0.05. We did not correct for multiple comparisons.
Results
Patient characteristics
Ten patients from the high-resolution ICU cohort met the defined inclusion criteria, with at least 24 h of ICM+ physiological data pre-DC, and more than 48 h post-DC. There were eight male patients, with a mean age of 34.0 ± 18.1 years and a median admission GCS motor score of 4 (interquartile range [IQR] 1–5). Three patients suffered pre-hospital hypotensive episodes, one had a pre-hospital hypoxic episode, and three patients had abnormal pupillary status (one with unilateral reactive pupil, two with bilateral unreactive pupils). Three patients had an epidural hematoma, seven had a traumatic subarachnoid hemorrhage, five had an acute subdural hematoma, and seven had contusions. The median Marshall CT score was 4 (IQR 3–6). The mean duration of physiological recording was 301.0 ± 126.0 h, with a 90.6 ± 46.5 h pre-DC and 210.4 ± 101.8 h post-DC of data. Beyond 48 h post-DC, there was a mean of 162.4 ± 101.8 h of physiological recording to analyze.
ICP and cerebrovascular reactivity pre- and post-DC
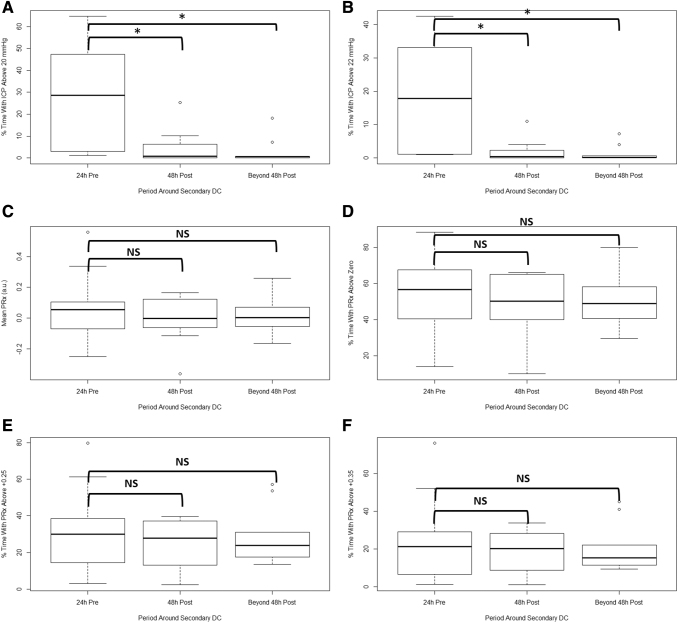
Physiological data from the minute-by-minute output files were summarized for each time period around the secondary DC: 1) 24 h pre-DC, 2) first 48 h post-DC, and 3) beyond 48 h post-DC. Table 1 provides a summary of the mean physiological values for each time period, and the p-values for the Mann-U test comparing: 1) 24 h pre-DC versus first 48 h post-DC, and 2) 24 h pre-DC versus beyond 48 h post-DC. Of note, ICP and the proportion of time above ICP threshold were significantly reduced post-DC. Cerebrovascular reactivity metrics, as measured through mean PRx and percent (%) time above PRx thresholds were not affected by the craniectomy across the three time periods. Figure 1 displays the box plots for selected ICP and PRx metrics across the three time periods around secondary DC.
Table 1.
Physiology between Three Time Periods around Secondary DC
| Physiology | 24 h pre-DC – mean (SD) | First 48 h post-DC – mean (SD) | Beyond 48 h post-DC – mean (SD) | Mann-U p-values for pre-DC vs. first 48 h post | Mann-U p-values for pre-DC vs. beyond 48 h post |
|---|---|---|---|---|---|
| Mean ICP (mm Hg) | 15.9 (5.2) | 12.4 (4.1) | 11.1 (4.0) | 0.002 | 0.043 |
| MAP (mm Hg) | 91.1 (10.3) | 82.1 (7.4) | 83.8 (7.3) | 0.002 | 0.166 |
| % time with ICP >20 mm Hg | 18.2 (24.0) | 4.8 (8.0) | 2.9 (5.8) | 0.011 | 0.0007 |
| % time with ICP | 12.0 (16.9) | 1.9 (3.5) | 1.3 (2.4) | 0.004 | 0.002 |
| >22 mm Hg | |||||
| Mean PRx (a.u.) | 0.070 (0.234) | -0.007 (0.156) | 0.06 (0.141) | 0.853 | 0.631 |
| % time with PRx >0 | 53.4 (22.6) | 47.2 (17.6) | 51.5 (16.2) | 0.481 | 0.739 |
| % time with PRx >+0.25 | 31.9 (23.9) | 25.8 (12.8) | 28.4 (15.4) | 0.853 | 0.971 |
| % time with PRx >+0.35 | 24.8 (23.7) | 19.8 (11.0) | 20.5 (12.8) | 0.900 | 0.971 |
Bold p-values are those reaching statistical significance.
a.u., arbitrary units; DC, decompressive craniectomy; ICP, intracranial pressure; Mann-U, Mann-Whitney-U test; MAP, mean arterial pressure; mm Hg, millimeters of mercury; PRx, Pressure Reactivity Index (correlation between ICP and MAP); SD, standard deviation; % = percent.
FIG. 1.
Box plots of ICP and PRx metrics across the three time periods. (A) Percent (%) time with ICP above 20 mm Hg. (B) Percent (%) time with ICP above 22 mm Hg. (C) Mean PRx. (D) Percent (%) time with PRx above 0. (E) Percent (%) time with PRx above +0.25. (F) Percent (%) time with PRx above +0.35. *, significant difference; DC, decompressive craniectomy; h, hours; ICP, intracranial pressure; MAP, mean arterial pressure; NS, non-significant; PRx, Pressure Reactivity Index (correlation between ICP and MAP).
Time-Series analysis of PRx pre- and post-craniectomy
The optimal ARIMA structure for PRx during the three defined time periods was assessed for each individual patient transformed data. Supplementary Appendix S1 provides the ARIMA model tables for each patient, across each time period around DC, reporting the AIC and LL for each model tested. The specific optimal time-series ARIMA model varied between patients, given the natural physiological heterogeneity seen between individuals. However, in general, across all patients, the time-series structure of PRx did not change going from pre-DC, to the first 48 h post-DC, and finally to beyond 48 h after DC. These findings support the notion that cerebrovascular reactivity may not be affected by DC.
ICP and MAP slow-wave time-series analysis
To explore the relationship between vasogenic slow-wave fluctuations in response to secondary DC, we employed both VARIMA multi-variate time-series modeling and Granger causality analysis across the three defined time periods in 10-sec log-transformed mean data.
ICP and MAP VARIMA models
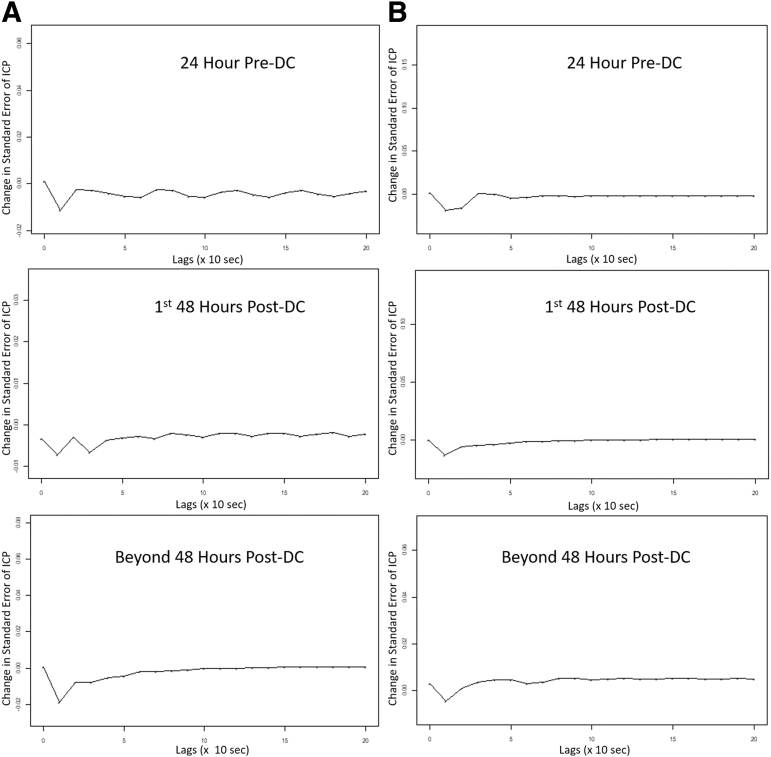
Using the VARIMA model with an autoregressive order of 4, an integrative/differencing factor of 1, and moving average order of 4, models were generated for each individual patient. With these models, the coefficients were then utilized to generate IRF plots. These IRF plots allowed us to visually determine the relationship between ICP and MAP, assessing the impact of one standard deviation impulse in MAP on ICP, using transformed data. Figure 2 displays two patient examples of IRF plots for MAP acting on ICP, across each of the three time periods around DC. In every patient, the IRF plots confirmed that no substantial change in the time-series relationship occurred as a result of DC, with one standard deviation impulse in MAP leading to a similar lagged time response in ICP standard error, regardless of the time period around craniectomy. These results support the findings of the above PRx analysis, which demonstrated no substantial impact on cerebrovascular reactivity secondary to DC.
FIG. 2.
ICP and MAP slow-wave VARIMA generated IRF plots—patient examples. The above plots display two patient examples (A and B) of typical IRFs for a one standard deviation impulse in MAP on the standard error in ICP, based on the VARIMA model of structure (4,1,4), derived in each individual patient. These plots suggest that craniectomy does not substantially impact the vasogenic slow-wave relationship between MAP and ICP. ICP and MAP are log transformed, and the Lag axis is reported in the number of 10-sec observations. DC, decompressive craniectomy; ICP, intracranial pressure; IRF, impulse response function; MAP, mean arterial pressure; min, minutes; VARIMA, vector autoregressive integrative moving average.
ICP and MAP Granger causality testing
Finally, to provide supporting evidence that the causal relationship between ICP and MAP did not change as a result of craniectomy, we performed Granger causality testing on each individual patient, across each time period around craniectomy, using de-trended transformed ICP and MAP data. Table 2 reports the Granger testing for each patient. For all but one patient, the causal relationship favored MAP on ICP, with Granger testing displaying higher F-test magnitudes for MAP on ICP, as opposed to ICP on MAP. This directional relationship did not change as a result of craniectomy, further suggesting limited impact of DC on the ICP and MAP vasogenic slow-wave association. Further, the mean F-test value did not significantly change for the MAP on ICP causal relationship, when comparing the 24 h pre-DC with the first 48 h post-DC (p = 0.280), and when comparing the 24 h pre-DC with the beyond 48 h post-DC (p = 0.248).
Table 2.
Granger Causality Testing for ICP and MAP across Three Time Periods around Secondary DC
| Patient | 24 h pre-DC |
First 48 h post-DC |
Beyond 48 Hours post-DC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP on ICP |
ICP on MAP |
MAP on ICP |
ICP on MAP |
MAP on ICP |
ICP on MAP |
|||||||
| Granger F statistic | P-value | Granger F statistic | P-value | Granger F statistic | P-value | Granger F statistic | P-value | Granger F statistic | P-value | Granger F statistic | P-value | |
| 1 | 98.19605 | p < 0.0001 | 33.92996 | p < 0.0001 | 249.9292 | p < 0.0001 | 107.721 | p < 0.0001 | 173.7906 | p < 0.0001 | 39.96617 | p < 0.0001 |
| 2 | 35.73278 | p < 0.0001 | 1.397988 | p < 0.0001 | 44.62436 | p < 0.0001 | 7.741133 | p < 0.0001 | 11.59793 | p < 0.0001 | 4.402093 | p < 0.0001 |
| 3 | 185.8105 | p < 0.0001 | 26.87107 | p < 0.0001 | 29.99126 | p < 0.0001 | 13.3402 | p < 0.0001 | 160.585 | p < 0.0001 | 13.62764 | p < 0.0001 |
| 4 | 100.1413 | p < 0.0001 | 22.48569 | p < 0.0001 | 91.10587 | p < 0.0001 | 39.22871 | p < 0.0001 | 838.0326 | p < 0.0001 | 8.319206 | p < 0.0001 |
| 5 | 181.545 | p < 0.0001 | 15.00727 | p < 0.0001 | 77.46647 | p < 0.0001 | 5.482175 | 0.0002 | 83.88176 | p < 0.0001 | 10.69612 | p < 0.0001 |
| 6 | 48.99229 | p < 0.0001 | 4.082754 | 0.0026 | 5.946278 | p < 0.0001 | 1.38636 | 0.2358 | 188.6362 | p < 0.0001 | 1.828569 | p < 0.0001 |
| 7 | 408.6103 | p < 0.0001 | 50.93941 | p < 0.0001 | 236.6314 | p < 0.0001 | 13.56572 | p < 0.0001 | 78.96369 | p < 0.0001 | 5.453439 | 0.0002 |
| 8 | 38.48715 | p < 0.0001 | 62.67107 | p < 0.0001 | 37.52064 | p < 0.0001 | 9.188934 | p < 0.0001 | 82.32697 | p < 0.0001 | 14.05842 | p < 0.0001 |
| 9 | 3.18963 | 0.0125 | 8.1914 | p < 0.0001 | 8.610713 | p < 0.0001 | 4.637555 | 0.000968 | 979.9123 | p < 0.0001 | 19.15756 | p < 0.0001 |
| 10 | 107.6338 | p < 0.0001 | 18.41431 | p < 0.0001 | 24.96126 | p < 0.0001 | 16.0318 | p < 0.0001 | 816.7331 | p < 0.0001 | 55.97435 | p < 0.0001 |
Table displays causal relationship between ICP and MAP to favor the direction of MAP influencing ICP. This relationship remains present regardless of the craniectomy, implying that the intimate association between ICP and MAP is unchanged with DC, and thus viable information regarding cerebrovascular reactivity derived from this relationship may still be carried in these signals. There is not a statistically significant difference in the mean F-statistic value when comparing the 24 h pre-DC with the first 48 h post-DC, and comparing the 24 h pre-DC with the data from beyond 48 h post-DC.
DC, decompressive craniectomy; MAP, mean arterial pressure; ICP, intracranial pressure.
Discussion
Through the evaluation of PRx metrics and the relationship between ICP and MAP vasogenic slow-waves during the three time periods around craniectomy, we have provided preliminary results suggesting that the statistical properties of cerebrovascular reactivity metrics and slow-wave relationships between ICP and MAP may not be affected. These results are somewhat contrary to the previous retrospective single-center exploration using grand average data for craniectomy patients,15 although they carry important implications for future studies on cerebrovascular reactivity in TBI as they suggest such vascular reactivity metrics may remain reliable measures post-craniectomy. Further, given the difference from the previous article on the subject, it may also suggest patient-by-patient heterogeneity in the response of the cerebral vasculature to DC, another aspect requiring future study. It must be emphasized that these results should be considered preliminary and require much further validation. Some important aspects deserve highlighting.
First, DC leads to a reduction in ICP and time above BTF-defined ICP thresholds. This is not a surprise given the main purpose for such surgical intervention is for ICP control, and this has been documented in numerous previous studies. However, cerebrovascular reactivity as measured through mean PRx and % time spent above PRx of 0, +0.25, and +0.35 was not statistically different as a result of DC. The current analysis implies that there may not be a substantial alteration in the relationship between vasogenic slow-waves in ICP and MAP. Such results are contrary to the previous study assessing the physiological impact of DC.15 Within our cohort, autoregulatory capacity (as measured through PRx) was quite impaired pre-DC, which may have mitigated the likelihood of any substantial change during the post-DC period, thus leading to the somewhat contrary results to previous literature for mean PRx metrics post-DC.
Further, such discrepancies likely stem from the methodology employed in previous work, where grand average summary values of raw minute-averaged physiology were assessed around craniectomy time. With the more complex methodologies employed within this current pilot study, the temporal course was explored thoroughly, leading to the interesting and important preliminary findings. The findings in this study were corroborated using multiple different statistical approaches including Mann-U testing, ARIMA, VARIMA, and Granger causality assessments. With that said, both studies were based on small cohorts of patients, and much larger studies of craniectomy patients are required to improve our understanding of the impact of craniectomy on cerebrovascular reactivity and other physiological metrics. Future work would also benefit from experimental models of DC, evaluating multi-modal monitoring-based cerebrovascular reactivity metrics against the lower limit and upper limits of autoregulation.
Second, the statistical time-series structure of PRx does not appear to substantially change as a result of DC. This finding may support the notions that PRx-based cerebrovascular reactivity may behave independent of craniectomy, may display a patient-specific response, and may still carry reliable information regarding cerebrovascular reactivity post-craniectomy, which is an important finding for future analysis of vascular reactivity and optimal CPP in TBI populations. However, these results do remain preliminary and require much further validation, and should thus not be considered definitive at this time.
Third, the statistical relationship between vasogenic slow-waves of ICP and MAP also does not appear to be affected by craniectomy, implying this relationship retains some reliable information regarding vascular reactivity. This was confirmed during both VARIMA IRF plot visualization and Granger causality testing for each individual patient. These results are in keeping with the findings from the PRx analysis in this project, suggesting that vascular reactivity metrics may still carry reliable information post-craniectomy. Although, again, these results are preliminary and should not be considered definitive at this point. There was a trend for a reduction in signal variance, as seen in Table 1, going from pre- to post-DC. However, given the small patient numbers, it is difficult to say at this time if such a trend is real or just a function of this particular small group of patients. Future work in the area would benefit from larger cohort sizes, where signal complexity pre- and post-DC can be more accurately commented on using potentially approximate or multi-scale entropy techniques to make more decisive comments on signal variability.
Finally, synthesizing all of the findings, this study suggests that cerebrovascular reactivity metrics and monitoring may still be of value and carry reliable information regarding vascular reactivity after craniectomy. The lack of significant change in statistical properties of PRx metrics and time-series behaviors of both PRx and ICP/MAP slow waves comparing pre- to post-DC states supports this notion. This concept is of importance for future investigation and research. In particular, if cerebrovascular reactivity measures are not drastically affected by craniectomy, this could suggest that reliable vascular reactivity metrics can be derived from ICP and MAP, and that individualized physiological targets, such as CPP optimum, may be considered in this population. Much further work is required to validate the findings of this study, as it is based on complex methodologies applied to a small cohort of patients. Further, the application of cerebrovascular reactivity monitoring after DC also requires more exploration, determining if there is a difference in response to therapies post-DC, or if the ability to determine CPP optimum is affected.
Future confirmatory studies will involve both prospective and retrospective archived data sets for secondary DC. We plan to explore the existing data sets from Canadian, Nordic, and other European collaborative initiatives in high-frequency digital physiology after TBI, while also taking a look at the data of patients from the RESCUE-ICP study,26 which has archived high-frequency physiology data available. Such initiatives will enable us to build up patient numbers for repeat analysis, and explore some of the above mentioned signal complexity relationships. Further, prospective data collection initiatives in high-resolution ICU data, such as those planned in Canada and through other European collaboratives, will allow for more complex multi-modal monitoring data sets, potentially allowing for comment on relationships between brain oxygen, cerebral blood flow (CBF), and metabolism pre- and post-DC. Finally, any future studies evaluating secondary DC would benefit from archiving of high-frequency digital physiology pre- and post-DC.
Limitations
Despite the interesting results of this study, there are important limitations that deserve highlighting. First, this study is only a pilot exploration into the impact of DC on statistical properties of cerebrovascular reactivity metrics and slow-wave relationships. The described analysis required high-frequency digital physiology to be recorded both at pre- and post-craniectomy, resulting in a type of data relatively unique and somewhat difficult to obtain. As such, given the small cohort, the results should be considered exploratory and not definitive, requiring much further validation, and are not necessarily generalizable to all craniectomy patients at this time.
Second, the statistical methodologies for modeling the time-series relationships of PRx and slow waves in ICP and MAP are complex, and burdensome. As such, future application in larger populations of craniectomy patients would require substantial computational resources, an important aspect to consider when planning such projects.
Third, this small cohort of patients did not have additional multi-modal monitoring information available pre- and post-DC. Particularly, invasive brain tissue oxygen (PbtO2), thermal diffusion CBF, and cerebral microdialysis would have been extremely valuable and interesting information to add to this data set. If present, some intelligent comments on the relationship between PRx, CBF, PbtO2, and cerebral metabolism could have been made. Future analysis and study of the impact of secondary DC on cerebral physiology should aim to include such complex multi-modal monitoring techniques.
Fourth, the results from this TBI cohort do not necessarily translate to other cohorts in which secondary DC is performed, particularly malignant ischemic stroke. Various studies have evaluated the utility of DC in malignant stroke, in relation to global patient outcome.27 To date, there have not been studies evaluating continuously measured cerebrovascular reactivity pre- and post-DC. It is quite possible that different statistical time-series relationships may be seen in this pathology. Such investigation into cerebrovascular reactivity pre- and post-DC for stroke is important, as one can imagine cerebrovascular response may dictate secondary complications both pre- and post-operatively, such as edema, ischemia, and hemorrhagic progression.
Fifth, PRx is considered a global cerebral metric of cerebrovascular reactivity, despite being derived from a focal/regional ICP measure. It is possible that there are significant hemispheric differences both pre- and post-DC. To date, there are no studies available evaluating continuously measured cerebrovascular reactivity pre- and post-DC. Study of non-DC TBI patients indicates the potential for hemispheric asymmetry, when evaluated using transcranial Doppler techniques.28 Future investigations into hemispheric differences pre- and post-DC would require either the use of bilateral invasive ICP monitoring, or the application of transcranial Doppler or near infrared spectroscopy techniques.
Finally, it is unknown if metrics derived from PRx, such as CPP optimum, are drastically affected after DC. This aspect needs further evaluation, as the ability to determine a CPP optimum value may be influenced.
Conclusion
PRx metrics and statistical time-series behavior appears to not be substantially influenced by DC. Similarly, there is little change in the relationship between slow waves of ICP and MAP, comparing physiology before and after DC. This implies that cerebrovascular reactivity monitoring in the setting of DC may still provide valuable information regarding autoregulation. Future work is required to explore the impact of DC on cerebrovascular reactivity.
Supplementary Material
Acknowledgments
Data used in preparation of this manuscript were obtained in the context of the CENTER-TBI study. The views expressed are those of the authors and not necessarily those of the U.K. National Health Service (NHS), National Institute for Health Research (NIHR), or the Department of Health and Social Care.
Contributor Information
Collaborators: the CENTER-TBI High Resolution ICU (HR ICU) Sub-Study Participants and Investigators, Audny Anke, Ronny Beer, Bo-Michael Bellander, Erta Biqiri, Andras Buki, Marco Carbonara, Arturo Chieregato, Giuseppe Citerio, Endre Czeiter, Bart Depreitere, Shirin Frisvold, Raimund Helbok, Stefan Jankowski, Danile Kondziella, Lars-Owe Koskinen, Geert Meyfroidt, Kirsten Moeller, David Nelson, Anna Piippo-Karjalainen, Andreea Radoi, Arminas Ragauskas, Rahul Raj, Jonathan Rhodes, Saulius Rocka, Rolf Rossaint, Juan Sahuquillo, Oliver Sakowitz, Ana Stevanovic, Nina Sundström, Riikka Takala, Tomas Tamosuitis, Olli Tenovuo, Peter Vajkoczy, Alessia Vargiolu, Rimantas Vilcinis, Stefan Wolf, and Alexander Younsi
The CENTER-TBI HR ICU Sub-Study Participants and Investigators
Audny Anke, University Hospital Northern Norway; Ronny Beer, Medical University of Innsbruck; Bo-Michael Bellander, Karolinska University Hospital; Erta Biqiri, Niguarda Hospital; Andras Buki, University of Pécs; Manuel Cabeleira, University of Cambridge; Marco Carbonara, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico; Arturo Chieregato, Niguarda Hospital; Giuseppe Citerio, ASST di Monza, Università Milano Bicocca; Endre Czeiter, University of Pecs; Marek Czosnyka, University of Cambridge; Bart Depreitere, University Hospitals Leuven; Ari Ercole, University of Cambridge; Shirin Frisvold, University Hospital Northern Norway; Raimund Helbok, University of Innsbruck; Stefan Jankowski, Sheffield Teaching Hospitals NHS Foundation Trust; Danile Kondziella, Region Hovedstaden Rigshospitalet; Lars-Owe Koskinen, Umeå University; David K. Menon, University of Cambridge; Geert Meyfroidt, University Hospitals Leuven; Kirsten Moeller, Region Hovedstaden Rigshospitalet; David Nelson, Karolinska University Hospital; Anna Piippo-Karjalainen, Helsinki University Central Hospital; Andreea Radoi, Vall d'Hebron University Hospital; Arminas Ragauskas, Kaunas University of Technology, Vilnius University; Rahul Raj, Helsinki University Central Hospital; Jonathan Rhodes, NHS Lothian, University of Edinburg; Saulius Rocka, Kaunas University of Technology, Vilnius University; Rolf Rossaint, University Hospital of Aachen; Juan Sahuquillo, Vall d'Hebron University Hospital; Oliver Sakowitz, Klinikum Ludwigsburg, University Hospital Heidelberg; Peter Smielewski, University of Cambridge; Ana Stevanovic, University Hospital of Aachen; Nino Stocchetti, Milan University, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico; Nina Sundström, Umea University; Riikka Takala, Turku University Central Hospital, University of Turku; Tomas Tamosuitis, Kaunas University of Health Sciences; Olli Tenovuo, Turku University Central Hospital, University of Turku; Peter Vajkoczy, Charité – Universitätsmedizin Berlin; Alessia Vargiolu, ASST di Monza; Rimantas Vilcinis, Kaunas University of Health Sciences; Stefan Wolf, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin Institute of Health; Alexander Younsi, University Hospital Heidelberg; and Frederick A. Zeiler, University of Cambridge, University of Manitoba.
Funding Information
CENTER-TBI is a large collaborative project with the support of the European Union 7th Framework Program (European Commission grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), OneMind (United States), and Integra LifeSciences Corporation (United States). The study also received additional support from the National Institute for Health Research (NIHR) Clinical Research Network.
FAZ is supported by the University of Manitoba VPRI Research Investment Fund (RIF), Winnipeg Health Sciences Center (HSC) Foundation, the University of Manitoba Rudy Falk Clinician-Scientist Professorship, the National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS) (R03 NS114335-01), the Canadian Institutes of Health Research (CIHR) (432061), and the University of Manitoba - Centre on Aging. DKM was supported by funding from the National Institute for Health Research (NIHR) through a Senior Investigator Award and the Cambridge Biomedical Research Center at the Cambridge University Hospitals National Health Service (NHS) Foundation Trust. TvE is supported by funding from the Hersenstichting Nederland (the Dutch Brian Foundation, grant number ps2014.06).
Author Disclosure Statement
PS and MC each receive a part of licensing fees for the software ICM+ (Cambridge Enterprise Ltd., United Kingdom) used for data collection and analysis in this study.
Supplementary Material
References
- 1. Czosnyka M., Miller C., and Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. (2014). Monitoring of cerebral autoregulation. Neurocrit. Care 21 Suppl. 2, S95–S102 [Google Scholar]
- 2. Le Roux P., Menon D.K., Citerio G., Vespa P., Bader M.K., Brophy G.M., Diringer M.N., Stocchetti N., Videtta W., Armonda R., Badjatia N., Böesel J., Chesnut R., Chou S., Claassen J., Czosnyka M., De Georgia M., Figaji A., Fugate J., Helbok R., Horowitz D., Hutchinson P., Kumar M., McNett M., Miller C., Naidech A., Oddo M., Olson D., O'Phelan K., Provencio J.J., Puppo C., Riker R., Robertson C., Schmidt M., Taccone F., and Neurocritical Care Society, and European Society of Intensive Care Medicine. (2014). Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care : a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 40, 1189–1209 [DOI] [PubMed] [Google Scholar]
- 3. Czosnyka M., Smielewski P., Kirkpatrick P., Laing R.J., Menon D., and Pickard J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–17; discussion 17–19. [DOI] [PubMed] [Google Scholar]
- 4. Sorrentino E., Diedler J., Kasprowicz M., Budohoski K.P., Haubrich C., Smielewski P., Outtrim J.G., Manktelow A., Hutchinson P.J., Pickard J.D., Menon D.K., and Czosnyka M. (2012). Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 16, 258–266 [DOI] [PubMed] [Google Scholar]
- 5. Steiner L.A., Czosnyka M., Piechnik S.K., Smielewski P., Chatfield D., Menon D.K., and Pickard J.D. (2002). Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 30, 733–738 [DOI] [PubMed] [Google Scholar]
- 6. Aries M.J.H., Czosnyka M., Budohoski K.P., Steiner L.A., Lavinio A., Kolias A.G., Hutchinson P.J., Brady K.M., Menon D.K., Pickard J.D., and Smielewski P. (2012). Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med. 40, 2456–2463 [DOI] [PubMed] [Google Scholar]
- 7. Donnelly J., Czosnyka M., Adams H., Cardim D., Kolias A.G., Zeiler F.A., Lavinio A., Aries M., Robba C., Smielewski P., Hutchinson P.J.A., Menon D.K., Pickard J.D., and Budohoski K.P. (2019). Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: a retrospective, single-center analysis. Neurosurgery 85, E75–E82 [DOI] [PubMed] [Google Scholar]
- 8. Zeiler F.A., Donnelly J., Smielewski P., Menon D.K., Hutchinson P.J., and Czosnyka M. (2018). Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J. Neurotrauma 35, 1107–1115 [DOI] [PubMed] [Google Scholar]
- 9. Zeiler F.A., Ercole A., Cabeleira M., Zoerle T., Stocchetti N,N., Menon D.K., Smielewski P., Czosnyka M., and CENTER-TBI High Resolution Sub-Study Participants and Investigators. (2019). Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a CENTER-TBI study. Acta Neurochir. (Wien) 161, 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeiler F.A., Ercole A., Cabeleira M., Carbonara M., Stocchetti N., Menon D.K., Smielewski P., and Czosnyka M. (2018). Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult TBI: a CENTER-TBI study. J. Neurotrauma 36, 1505–1517 [DOI] [PubMed] [Google Scholar]
- 11. Zeiler F.A., Donnelly J., Calviello L., Smielewski P., Menon D.K., and Czosnyka M. (2017). Pressure autoregulation measurement techniques in adult traumatic brain injury, Part II: a scoping review of continuous methods. J. Neurotrauma 34, 3224–3237 [DOI] [PubMed] [Google Scholar]
- 12. Brady K.M., Lee J.K., Kibler K.K., Easley R.B., Koehler R.C., and Shaffner D.H. (2008). Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39, 2531–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeiler F.A., Lee J.K., Smielewski P., Czosnyka M., and Brady K. (2018). Validation of intracranial pressure-derived cerebrovascular reactivity indices against the lower limit of autoregulation, Part II: experimental model of arterial hypotension. J. Neurotrauma 35, 2812–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeiler F.A., Donnelly J., Calviello L., Lee J.K., Smielewski P., Brady K., Kim D.-J., and Czosnyka M. (2018). Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation: Part I: experimental intracranial hypertension. J. Neurotrauma 35, 2803–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Timofeev I., Czosnyka M., Nortje J., Smielewski P., Kirkpatrick P., Gupta A., and Hutchinson P. (2008). Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J. Neurosurg. 108, 66–73 [DOI] [PubMed] [Google Scholar]
- 16. Aries M.J.H., Czosnyka M., Budohoski K.P., Kolias A.G., Radolovich D.K., Lavinio A., Pickard J.D., and Smielewski P. (2012). Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit. Care 17, 67–76 [DOI] [PubMed] [Google Scholar]
- 17. Maas A.I.R., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., Hill S., Legrand V., Sorgner A., and CENTER-TBI Participants and Investigators. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 18. Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W.J., Bell M.J., Bratton S.L., Chesnut R., Harris O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., Vavilala M.S., Wilberger J., Wright D.W., and Ghajar J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 19. Doiron D., Marcon Y., Fortier I., Burton P., and Ferretti V. (2017). Software application profile: Opal and Mica: open-source software solutions for epidemiological data management, harmonization and dissemination. Int. J. Epidemiol. 46, 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelly S., Bishop S.M., and Ercole A. (2018). Statistical signal properties of the Pressure-Reactivity Index (PRx). Acta Neurochir. Suppl. 126, 317–320 [DOI] [PubMed] [Google Scholar]
- 21. Zeiler F.A., Smielewski P., Stevens A., Czosnyka M., Menon D.K., and Ercole A. (2018). Non-invasive Pressure Reactivity Index using doppler systolic flow parameters: a pilot analysis. J. Neurotrauma 36, 713–720 [DOI] [PubMed] [Google Scholar]
- 22. Zeiler F.A., Smielewski P., Donnelly J., Czosnyka M., Menon D.K., and Ercole A. (2018). Estimating pressure reactivity using noninvasive doppler-based systolic flow index. J. Neurotrauma 35, 1559–1568 [DOI] [PubMed] [Google Scholar]
- 23. Chatfield C. (2016). The Analysis of Time Series: An Introduction, 6th ed. Chapman and Hall/CRC: Boca Raton, FL [Google Scholar]
- 24. Douc R., Moulines E., and Stoffer D. (2014). Nonlinear Time Series: Theory, Methods and Applications with R Examples, 1st ed. Chapman and Hall/CRC: Boca Raton, FL [Google Scholar]
- 25. Gao L., Smielewski P., Czosnyka M., and Ercole A. (2017). Early asymmetric cardio-cerebral causality and outcome after severe traumatic brain injury. J. Neurotrauma 34, 2743–2752 [DOI] [PubMed] [Google Scholar]
- 26. Hutchinson P.J., Kolias A.G., Timofeev I.S., Corteen E.A., Czosnyka M., Timothy J., Anderson I., Bulters D.O., Belli A., Eynon C.A., Wadley J., Mendelow A.D., Mitchell P.M., Wilson M.H., Critchley G., Sahuquillo J., Unterberg A., Servadei F., Teasdale G.M., Pickard J.D., Menon D.K., Murray G.D., Kirkpatrick P.J., and RESCUEicp Trial Collaborators. (2016). Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 375, 1119–1130 [DOI] [PubMed] [Google Scholar]
- 27. Beez T., Munoz-Bendix C., Steiger H.-J., and Beseoglu K. (2019). Decompressive craniectomy for acute ischemic stroke. Crit. Care 23, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt E.A., Czosnyka M., Steiner L.A., Balestreri M., Smielewski P., Piechnik S.K., Matta B.F., and Pickard J.D. (2003). Asymmetry of pressure autoregulation after traumatic brain injury. J. Neurosurg. 99, 991–998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.