Abstract
Globally, intracranial pressure (ICP) monitoring use in severe traumatic brain injury (sTBI) is inconsistent and susceptible to resource limitations and clinical philosophies. For situations without monitoring, there is no published comprehensive management algorithm specific to identifying and treating suspected intracranial hypertension (SICH) outside of the one ad hoc Imaging and Clinical Examination (ICE) protocol in the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST:TRIP) trial. As part of an ongoing National Institutes of Health (NIH)-supported project, a consensus conference involving 43 experienced Latin American Intensivists and Neurosurgeons who routinely care for sTBI patients without ICP monitoring, refined, revised, and augmented the original BEST:TRIP algorithm. Based on BEST:TRIP trial data and pre-meeting polling, 11 issues were targeted for development. We used Delphi-based methodology to codify individual statements and the final algorithm, using a group agreement threshold of 80%. The resulting CREVICE (Consensus REVised ICE) algorithm defines SICH and addresses both general management and specific treatment. SICH treatment modalities are organized into tiers to guide treatment escalation and tapering. Treatment schedules were developed to facilitate targeted management of disease severity. A decision-support model, based on the group's combined practices, is provided to guide this process. This algorithm provides the first comprehensive management algorithm for treating sTBI patients when ICP monitoring is not available. It is intended to provide a framework to guide clinical care and direct future research toward sTBI management. Because of the dearth of relevant literature, it is explicitly consensus based, and is provided solely as a resource (a “consensus-based curbside consult”) to assist in treating sTBI in general intensive care units in resource-limited environments.
Keywords: global health; ICP monitoring, intracranial hypertension; neurocritical care; sTBI
Introduction
Although this is not formally tabulated, it is reasonable to assume that the vast majority of severe traumatic brain injury (sTBI) patients globally are managed without intracranial pressure (ICP) monitoring at centers with limitations in both neurotrauma expertise and resources. Even in high-resource countries, such patients appear to be managed without monitoring in 23–89% of cases.1–5 Unfortunately, there is no recent literature to guide unmonitored sTBI management. Although resource availability is higher in high-income countries (HICs), routine sTBI management at non-specialist, non-academic centers will also be by teams with limited focus on neurotrauma (private practice neurosurgeons and general intensivists/traumatologists). The literature, however, is almost diametrically skewed, with most studies coming from neurotrauma centers with very specialized resources. The result is that physicians in low- and middle-income countries (LMICs) have almost no evidence-based guidance to assist in their management of sTBI. In addition, decisions about how to manage sTBI in these settings cannot be sidestepped or postponed in clinical practice pending future randomized trials.
For patients managed without monitoring of ICP, the only tested management protocol was incidentally provided by the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST:TRIP) trial,6 in which a treatment algorithm for unmonitored patients (imaging and clinical examination [ICE] protocol) was developed based on the combined experience of three of the initial site investigators and one published but untested care pathway.7 Because the BEST:TRIP trial found no outcome differences between the ICE group and a cohort managed based on monitored ICP, the ICE protocol appeared to be effective, although it was less efficient than the ICP monitoring protocol.6 Potential contributors to this inefficiency were the fixed minimum treatment duration for suspected intracranial hypertension (SICH) and the lack of decision support for escalating or tapering therapy. Refinement of the basic and linear ICE protocol might improve its efficiency and, perhaps, its influence on outcome.
The BEST:TRIP investigators are currently funded to prospectively investigate the effectiveness of the ICE protocol against care given in the absence of a shared protocol, and to then study the comparative effectiveness of a refined ICE protocol (Consensus REVised ICE [CREVICE] protocol) derived from a formal consensus process (R01 NS080648). In November 2015, having finished the ICE protocol versus usual care phase, we held the consensus conference in Buenos Aires, Argentina. The resulting CREVICE protocol is presented here.
It must be stressed that such a consensus approach is not a substitute for but a supplement to empirical evidence. It is responding to the absence of guiding literature in certain areas by providing what might be viewed as a group “curbside consult” generated based one shared experiences of a cohort of neurotrauma practitioners experienced in sTBI management under such conditions. As with any consultation, it carries no mandate to be followed. The target audience for this protocol is physicians treating sTBI in environments where ICP monitoring will not be part of the management approach, who might value some management advice beyond that of a single colleague or a Google search, when published evidence is lacking.
Methods
Details of the Delphi-method consensus development process are contained in the Supplementary Text. A brief description follows.
Forty-three intensivists and neurosurgeons with extensive experience managing sTBI without ICP monitoring comprised the consensus working group (CWG). It included investigators from the ongoing protocol research study (4 from protocol sites and 11 from no-protocol sites), plus a group from outside the study. Four CWG members attended by videoconference, but for technical reasons, did not vote. All contributors are currently involved in routinely managing sTBI patients in the absence of ICP monitoring, and have ≥5 years' experience doing so. Study investigators participated in the conference, but did not vote, as they lacked clinical experience managing unmonitored TBI patients.
Web-based polling cycles prior to the meeting determined discussion topics and some initial voting questions. The actual meeting occurred in November 2015. Table 1 lists the topics addressed. Anonymous electronic voting was used. Unless specifically modified by the group, the voting cycle was limited to three iterations, interspersed with discussion intervals. An element formed part of the final recommendations only if 80% approval was attained. We used small group sessions to address complex issues, with the whole CWG modifying and voting on small-group recommendations.
Table 1.
Issues Targeted for Consensus Development
| Issues targeted for consensus development: • Initiating and escalating therapy ○ Determine indications for initiating treatment of suspected intracranial hypertension (SICH) ○ Modify initial treatment of suspected intracranial hypertension ▪ How many agents ▪ Which agent(s) ○ Refine the definition of “neuroworsening” ○ Determine indications for consideration of escalating treatment ○ Develop a hierarchy of treatment modalities (based on perceived risk:benefit ratio in treating patients without definite intracranial hypertension) • Tapering ongoing SICH therapy ○ Specify methods of tapering therapy ○ Determine indications for consideration of initiating treatment tapering • Other areas ○ Set MAP targets or treatment ranges ○ Determine timing and indications for CT imaging ○ Classify CT imaging after surgical evacuation of a mass lesion ○ Review and modify basic treatment goals for TBI (not directly focused on ICP) |
MAP, mean arterial pressure; CT, computed tomography; TBI, traumatic brain injury; ICP, intracranial pressure.
One special small group session (Treatment-Tapering Exercise) addressed tendencies surrounding tapering therapy in commonly encountered categories of non-deteriorating patients. Four groups each rated their likelihood of initiating weaning of ongoing SICH therapy for specified combinations of clinical, imaging, and injury evolution parameters (Table 2). They rated these tendencies using a traffic light rating scheme, green favoring tapering, red opposing it, and yellow representing caution/uncertainty. Individual groups' recommendations were combined, and the whole CBG group refined and ratified the composite.
Table 2.
Parameters Included in the Exercise to Determine Clinical Practice Patterns in Tapering SICH Therapy
| Category | Parameter | Subtype | Definitions |
|---|---|---|---|
| Clinical | |||
| Pupils | |||
| Normal | |||
| Anisocoria, stable from admission | |||
| GCS motor score | 6 | Following commands | |
| 5 | Localizing | ||
| 4 | Withdrawing from noxious stimulation | ||
| 1-3 | No response, decerebrate or decorticate posturing | ||
| Imaging | |||
| CT Diagnosis | Modified Marshall Classification | ||
| DI I or II | |||
| DI III | |||
| EML / DI I or II | |||
| EML / DI III | |||
| Evolution | |||
| Time following injury | |||
| < 24 h | |||
| 24-48 h | |||
| 48-72 h | |||
| > 72 h |
SICH, suspected intracranial hypertension; GCS, Glasgow Coma Scale; CT, computed tomography; EML, evacuated mass lesions.
All recommendations were incorporated verbatim into the final protocol, which was submitted for approval to all voting members. Details of the Delphi-method consensus development process are contained in the supplementary section.
Results
The final protocol was approved by 97% of voting participants. Figures 1 and 2 present the CREVICE protocol algorithms. Detailed descriptions of the protocol are presented in English and Spanish in the supplementary section.
FIG. 1.
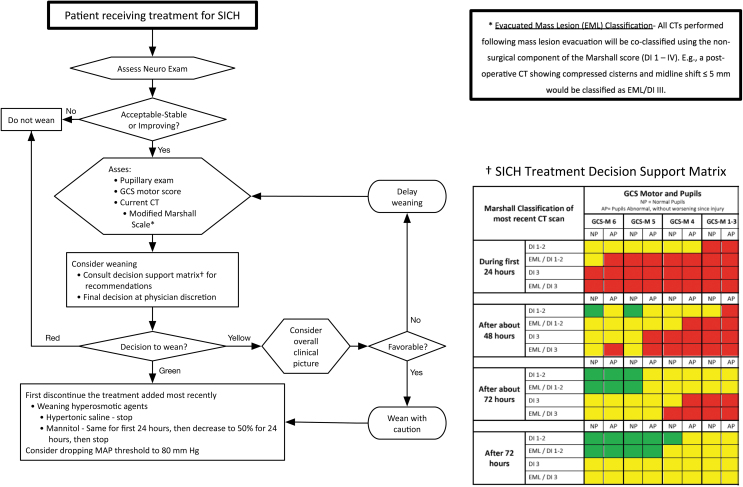
Graphic representation of the Consensus REVised Imaging and Clinical Examination (CREVICE) protocol for the treatment of suspected intracranial hypertension (SICH). The flow chart represents individual steps involved in the decision to treat for SICH, the choice of therapeutic agents, and the evaluation of the need for treatment escalation. The definition of “neuroworsening” is presented in a box at the upper right. The indications for initiating treatment for SICH are in the box at the lower left. The recommended therapeutic agents and their ranking into treatment tiers are contained in the box at the lower right. See text and supplementary section for further details.
FIG. 2.
- Green: it is recommended to begin to taper therapy.
- Red: it is recommended not to taper therapy.
- Yellow: this represents an intermediate status, wherein tapering therapy may be considered, but caution is recommended. The treating physician may choose to begin decreasing therapy, not to do so at that time, or to get more information to help in the decision. The decision to begin tapering treatment in patients in the “yellow” category should be accompanied by close observation as to the success of that decision.
The three areas of particular focus in creating this algorithm involved initiating, escalating, and tapering therapy.
Initiating therapy (Fig. 1)
Intracranial hypertension is suspected and treatment is recommended in the presence of one major or two minor criteria.
Major criteria are
-
1.
Compressed cisterns (computed tomographic [CT] classification of Marshall diffuse injury III)8
-
2.
Midline shift >5 mm (Marshall diffuse injury IV)8
-
3.
Unevacuated mass lesion.
Minor criteria are
-
1.
Glasgow Coma Scale (GCS) motor score ≤4
-
2.
Pupillary asymmetry
-
3.
Abnormal pupillary reactivity
-
4.
CT classification of Marshall diffuse injury II (i.e., basal cisterns are present with midline shift 0–5 mm and/or high- or mixed-density lesion ≤25 cm3
The CWG ranked individual therapeutic modalities according to perceived risk:benefit ratios, using a three-tiered hierarchy. Items were excluded if deemed contraindicated or there was no resolution after three voting cycles. Tiers were hierarchical; treatments with more favorable risk:benefit ratios were listed in lower tiers. This approach is bounded by three principles.
It is not necessary to use all modalities in a lower tier before moving to the next tier.
There is no rank ordering within a tier (except that hypertonic saline is recommended as the first hyperosmolar agent).
If considered advantageous, tiers can be skipped when advancing treatment (e.g., early decompressive craniectomy in selected cases).
Escalating therapy (Fig. 1)
Escalation of therapy (or adding another treatment) should be considered for the following
-
1.
Neuroworsening
a. Decrease in the motor GCS of 1 or more points
b. New loss of pupil reactivity
c. Interval development of pupil asymmetry of >2 mm or bilateral mydriasis
d. New focal motor deficit
e. Herniation syndrome (e.g., Cushing's triad)
-
2.
No improvement or worsening on follow-up CT imaging
-
3.
No acceptable response to initial therapy
The “modified definition of neuroworsening” now includes signs of the herniation syndrome (e.g. Cushing's triad) and a lower threshold for GCS motor score deterioration (≥ 1 point). Neuroworsening requires an immediate therapeutic response.
Tapering therapy (Fig. 2)
Figure 2 illustrates the decision pathway guiding consideration of weaning stable or improving patients from ongoing SICH treatment. The CWG developed decision support matrices (see Methods) to assist in these considerations. Decisions are based on the modified Marshall classification of the most recent CT scan and the clinical status (patient's GCS motor score and pupillary examination). Evaluations occur during the first 24 h, then at ∼48 and 72 h, and then at >72 h. The recommendations as to whether to begin tapering therapy are interpreted using a traffic light analogy.
Green: it is recommended to begin to taper therapy.
Red: it is recommended not to taper therapy.
Yellow: this represents an intermediate status, wherein tapering therapy may be considered but caution is recommended. The treating physician may choose to begin decreasing therapy, to not do so at that time, or to get more information to help in the decision. The decision to begin tapering treatment in patients in the “yellow” category should be accompanied by close observation as to the success of that decision.
Strong recommendations (green or red) indicate situations in which all four groups had the same recommendation. Matching the clinical and imaging situation and post-injury day to its matrix counterpart allows the clinician to determine the CWG's propensity to start weaning under those conditions.
When weaning therapy, the CWG recommended that the last treatment initiated be tapered/stopped first. Scheduled hypertonic saline does not need to be reduced gradually. It can be stopped when no longer indicated. A scheduled mannitol regimen should be maintained for the first 24 h following the decision to taper, then decreased to 50% for 24 h, then stopped.
Other areas
The CWG set mean arterial pressure (MAP) targets for the initial treatment and for after neurological and systemic abnormalities had stabilized. The initial target is 90 mm Hg. The target during tapering of MAP elevation treatment is 80 mm Hg (see supplementary section D.1. E.2., K.1-2).
The CWG modified the ICE protocol CT imaging schedule. If the initial CT is obtained within 4h of injury, a second CT is indicated within the next 12 h. In all cases, follow-up CT imaging is recommended at 24 and 72 h after injury, and as needed based on the clinical situation or to facilitate decision making. (Fig. 1; supplementary section C.1.)
The CWG modified the use of the Marshall CT classification9 scheme. Under the new protocol, the classification of any CT scan performed after the evacuation of a mass lesion includes the Marshall classifications for both (1) the surgical evacuation (i.e., evacuated mass lesions [EML]), and (2) an appended description of the post-operative CT image interpreted independently of the prior surgery. For example, a post-operative scan showing compressed/absent cisterns with midline shift ≤5 mm and no high- or mixed-density lesion >25 cc should be designated as EML/DI III. The decision matrices for tapering therapy use these modified Marshall Scores. (Fig. 2; supplementary section C.2.b).
The CWG maintained the transfusion threshold at a hemoglobin level of 7 g/dL but supported the option to choose a higher threshold in individual cases (supplementary section B.12.).
Discussion
The key finding of our consensus project among a large and diverse group of experienced neurotrauma specialists is that we were able to develop a comprehensive management algorithm for sTBI in settings lacking invasive ICP monitoring. Importantly, this management algorithm is in high agreement with current decision-making processes in sTBI populations at multiple LMIC sites, as evidenced by the high level of agreement (97%) with the final protocol among the consensus group.
Evidence to guide management of sTBI in environments where resource limitations preclude invasive ICP monitoring is lacking. However, decisions about how to manage sTBI in these settings cannot be sidestepped or postponed in clinical practice pending future randomized trials. In this context, our consensus-based protocol represents a valuable guidance for clinicians in low-resource environments.
Lacking any published protocol or substantive literature for non-ICP-monitored sTBI management, the three original site principal investigators developed the ICE protocol ad hoc for the BEST:TRIP trial.10 They were all Bolivian and familiar with each other's practices. Although the ICE protocol was effective,6 the many queries that arose during the trial, and secondary analysis of individual patient management courses, suggested numerous aspects that were either overly simplified, incomplete or absent, or controversial. One question was whether the treatment variability and controversies from the BEST:TRIP trial reflected strong beliefs or were simply practice variations. We hypothesized that simple practice variations would wash out if subjected to a consensus process, whereas strong beliefs would either prevent consensus, or at least establish the foundations underlying those beliefs. The CWG's high success in achieving consensus suggests strong convergence of beliefs and experiences underlying treatment, despite the absence of a solid literature foundation and the numerous institutions and widespread geographical locations represented within the group. The optimistic, albeit untested, assumption is that observed clinical success has reinforced many of these common practices.
Although the ICE protocol produced outcomes similar to the ICP-monitored protocol in the BEST:TRIP trial, it averaged 50% more ICP treatments and 1.4 more SICH-treatment-directed intensive care unit (ICU) days.6,10 Potential contributors to this inefficiency included the fixed SICH treatment duration (6 days) and the absence of initiation and tapering protocols for SICH therapy. We desired to augment the ability to adjust treatment intensity according to the perceived severity of SICH, both initially and over the management course. This required delineating discrete treatment elements, defining decision points, and providing support for the required decision making. The goal is targeted therapy and developing distinct algorithmic elements for future analysis and modification.
Table 1 presents our outline for addressing these issues. When possible, we modified the ICE protocol. Otherwise, we developed new concepts.
Initiating or escalating therapy (Fig. 1)
When ICP monitoring is available, there is weak evidence that the decision to monitor ICP can be based on the early clinical and imaging predictors of intracranial hypertension.11 When the question is whether to treat SICH (without monitoring), the relevance of data from monitored patients is nebulous. Without ICP monitoring, initiating SICH treatment is based on the degree of suspicion that a critical level of intracranial hypertension exists. The relative risks of over-treating a patient with acceptable ICP must be balanced against the risks of not treating a potentially harmful level of intracranial hypertension. Evidence from the ICE protocol arm of the BEST:TRIP trial is not helpful because it lacks objective indications for starting treatment. Because directly relevant literature is lacking, we decided to base the current recommendations for initiating SICH treatment entirely on the consensus experience of the CWG. We reasoned that their knowledge of the monitor-based literature, filtered through their clinical experience, should provide an acceptable initial set of recommendations. Their efficacy could subsequently be analyzed using outcome data. This accommodates the possibility that the effects of these treatments may not solely be related to their influence on ICP.
The strongest indications for initiating SICH treatment are CT based. There was much discussion about whether single clinical findings, particularly pupillary abnormalities, should be a stand-alone (major) criterion. A consensus majority agreed that, for example, pupillary asymmetry with normal reactivity and a negative CT in a briskly localizing patient would not automatically activate SICH treatment. Therefore, such findings were designated minor criteria, requiring a second supportive indication. However, physician discretion was also strongly supported as a criterion.
Lacking ICP data, the role of individual and trended CT imaging in clinical decision making is augmented. Many LMICs have strong financial constraints on obtaining CT imaging, including preclusion if a patient is unable to pay at the time. Setting minimal specific CT criteria attempts to leverage trauma centers to consider such imaging non-discretionary.
In the interest of matching therapeutic intensity to disease severity (targeted therapy), the CWG recommended initiating treatment with a single agent (hyperosmolar therapy) and developed specific indications for escalating treatment. Worsening or lack of improvement on follow-up imaging, or lack of clinical response to the initial intervention, should explicitly prompt consideration of increasing treatment. Addition of a second agent now represents escalation. By not setting a minimum duration for hyperosmolar treatment, the CWG rendered treatment length subject to the new escalation/tapering criteria, also supporting targeting.
Specific indicators for treatment modification are critical to this approach. The CWG defined these as (1) neuroworsening, (2) no improvement, or worsening on CT imaging, or (3) no response to initial therapy. CT worsening is straightforward, but lack of CT improvement or lack of clinical response to initial therapy were also included, to encompass cases in which persistence of undesirable initial status might be considered treatment failure. Neuroworsening implies high-probability, critical intracranial hypertension, so the CWG increased its sensitivity by lowering the ICE GCS motor (GCSm) trigger to ≥1 point and adding systemic signs of herniation (Cushing triad) to the definition. Neuroworsening mandates considering emergent temporizing maneuvers in addition to longer-term management changes.
To guide the process of therapy escalation and weaning (not addressed in the ICE protocol), individual interventions were organized into a three-tiered, hierarchical system based on perceived risk:benefit ratio in the absence of quantitative ICP feedback. Tier 1 contains treatments felt safest, Tier 2 contains treatments of intermediate risk:benefit ratio, and Tier 3 contains treatments with higher associated risks. Lower-tier treatments should be considered before those in a higher tier, although there are no constraints on skipping tiers when this is felt to be indicated (e.g., choosing decompressive craniectomy as the initial treatment in a patient with a Marshall DI IV CT9). It is not necessary to use all lower tier interventions prior to advancing, and there is no rank ordering within a tier, except for starting with a scheduled hyperosmolar agent.
Hypertonic treatment remained as the recommended first intervention. Hypertonic saline was preferred over mannitol (82.5% consensus) but the choice remains elective. Notably, as many LMIC centers cannot directly measure serum osmolarity, the ability to follow serum sodium levels is an added consideration favoring saline use.
The CWG recommended avoiding some interventions. These included proscriptions such as long-term epilepsy prophylaxis, steroids, and profound hyperventilation. They also include lumbar cerebrospinal fluid (CSF) drainage, which requires presence of a functioning external ventricular catheter (an ipso facto ICP monitor). Finally, the risk:benefit ratios of diuretics and continuous mannitol infusion were deemed unacceptable in this setting.
The absence of ICP data strongly influenced the CWG's interpretation of risk:benefit ratios. There was decreased willingness to accept treatment-associated risks when the ability to detect treatment toxicity is limited and targeted abnormality is only suspected. An example is hyperventilation to a PaCO2 < 30 mm Hg. For neuromuscular blockade, the loss of the examination in the absence of ICP monitoring counterbalanced its perceived utility in treating SICH. Its utility remained unresolved, despite multiple voting iterations.
Tapering therapy (Fig. 2)
Data are lacking on the appropriate timing, methods, and indications for tapering of ICP therapy for monitored or unmonitored patients. When ICP data are available, the definition of acceptable ICP and the duration of stability required to allow weaning remain controversial. Absent monitoring, such decisions must be based on clinical and imaging indicators. Lacking evidentiary guidance, the CWG considered that knowing the tendencies of experienced clinicians under similar conditions might assist decision making. The Treatment Tapering Exercise (Methods) attempted to establish a “virtual CWG consult tool” to describe consensus-based dispositions toward weaning therapy in particular groups of patients with stable-to-improving clinical and radiographical circumstances, at various times following injury (Table 2). The use of the traffic-light model attempts to represent the group's weaning tendencies in a simple, easily understood fashion.
This exercise represents a single, isolated encounter, and does not incorporate the physician's experience of the patient's evolution. It also posited that the clinical examination and CT classification are stable or improving, not worsening. Constructing this tool prompted the recommendation of earlier follow-up CT imaging than in the ICE protocol. This construct also provides a research framework for testing and refining parameters for weaning treatment. In practice, it is only a guide; it does not mandate management decisions in any fashion.
Other areas
The CWG desired an initial MAP threshold targeting an adequate cerebral perfusion pressure (CPP) in the face of moderate intracranial hypertension, but remaining at or below the CPP threshold of 70 mm Hg reported as hazardous.11–13 They selected a MAP threshold of 90 mm Hg. They reserved weaning vasopressors and hypervolemia to a lower threshold of 80 mm Hg for patients not at risk for systemic hypotension, with resolved or absent SICH.
When ICP data are not available, there is increased emphasis on the role of CT imaging in guiding assessment for SICH. The Marshall CT classification9 is strongly oriented toward intracranial hypertension in diffuse injuries. However, the classification of “surgical mass lesions” (> 25 cc) into EML or non-evacuated mass lesions (NEML) loses this orientation. The CWG modified the Marshall CT classification scheme for the EML classification so that it includes the same ICP-oriented interpretations used for diffuse injuries (Fig. 2; supplementary section C.2.b). This untested approach addresses the estimation of post-operative SICH using established tools. Its value as an actual predictor of intracranial hypertension will require empirical testing in the setting of ICP monitoring. There are no treatments directly linked to this classification, although it is used in treatment tapering matrices.
Some CWG members considered the American College of Surgeons Committee on Trauma hemoglobin level recommendations14 of 7 g/dL to be too low for brain injury in the absence of resources to monitor brain oxygenation. However, no alternate value met the 80% consensus threshold. The final recommendation supports 7 g/dL, but allows higher thresholds at the discretion of the physician.
Caveats
The target audience for this protocol is the large global population of physicians treating sTBI in environments where ICP monitoring is not employed. It is meant to be particularly useful to those who face a lower volume of sTBI, who desire guidance where relevant published evidence is weak or absent. It is not designed as an “alternative approach” when ICP monitoring is available.
This protocol is based on the consensus experience of 43 active, experienced intensivists and neurosurgeons who regularly manage sTBI without ICP monitoring. There is no evidentiary basis for either the overall premise of manipulating ICP in the absence of monitoring, or on the comparative or absolute efficacy of any individual agent used under such circumstances. This protocol might best be viewed as a “multi-physician sTBI management consultation,” with the caveats that the recommendations have not been rigorously tested. It is offered as a more explicit and rigorous alternative to curbside consultation with a local colleague or an Internet search on TBI management. The actual health benefits, side effects, and risks are unknown, and close clinical monitoring of the effects of individual steps, as well as the protocol in general, is strongly recommended. No implication is made as to the advisability of following this protocol in any individual case, and its modification or discontinuation is supported in if untoward effects are observed. Potential uses of this protocol include adoption (as a whole or in parts), modification to adapt to local environments, or as a template.
Although the plural of anecdote is not evidence, prolonged experience may bestow wisdom. The situational wisdom of an individual or close-knit group is subject to many confounding influences: personal, environmental, or stochastic. Combining multiple such individuals or groups in a Delphi-process-based consensus environment presumes that the commonalities of their “observational wisdoms” will compound, whereas the associated idiosyncrasies will not. Although there is no guarantee of this occurring, and no simple means of measuring its success, we believe that the absence of rigorous evidence and the strong clinical need for direction in this instance justifies the use of this algorithm-generating method as an initial approach toward a testable protocol. Implementation of the CREVICE protocol is ongoing, and outcome and efficiency will be compared with that of the ICE protocol and no protocol using a before/after design (R01-NS080648).
Funding Information
The conference and ongoing validation study are National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) funded through the Fogarty International Center (grant number NS080648).
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Aiolfi A., Khor D., Cho J., Benjamin E., Inaba K., and Demetriades D. (2018). Intracranial pressure monitoring in severe blunt head trauma: does the type of monitoring device matter? J. Neurosurg, 128, 828–833 [DOI] [PubMed] [Google Scholar]
- 2. Hesdorffer D.C., and Ghajar J. (2007). Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J. Trauma 63, 841–848 [DOI] [PubMed] [Google Scholar]
- 3. Myburgh J.A., Cooper D.J., Finfer S.R., Venkatesh B., Jones D., Higgins A., Bishop N., and Higlett T. (2008). Epidemiology and 12-month outcomes from traumatic brain injury in australia and new zealand. J. Trauma 64, 854–862 [DOI] [PubMed] [Google Scholar]
- 4. Sahjpaul R., and Girotti M. (2000). Intracranial pressure monitoring in severe traumatic brain injury—results of a Canadian survey. Can. J. Neurol. Sci. 27, 143–147 [PubMed] [Google Scholar]
- 5. Stocchetti N., Penny K.I., Dearden M., Braakman R., Cohadon F., Iannotti F., Lapierre F., Karimi A., Maas A. Jr., Murray G.D., Ohman J., Persson L., Servadei F., Teasdale G.M., Trojanowski T., and Unterberg A. (2001). Intensive care management of head-injured patients in Europe: a survey from the European brain injury consortium. Intensive Care Med. 27, 400–406 [DOI] [PubMed] [Google Scholar]
- 6. Chesnut R.M., Temkin N., Carney N., Dikmen S., Rondina C., Videtta W., Petroni G., Lujan S., Pridgeon J., Barber J., Machamer J., Chaddock K., Celix J.M., Cherner M., and Hendrix T. (2012). A Trial of Intracranial-Pressure Monitoring in Traumatic Brain Injury. N. Engl. J. Med. 367, 2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinosa-Aguilar A., Reyes-Morales H., Huerta-Posada C.E., de Leon I.L., Lopez-Lopez F., Mejia-Hernandez M., Mondragon-Martinez M.A., Calderon-Tellez L.M., Amezcua-Cuevas R.L., and Rebollar-Gonzalez J.A. (2008). Design and validation of a critical pathway for hospital management of patients with severe traumatic brain injury. J. Trauma 64, 1327–1341 [DOI] [PubMed] [Google Scholar]
- 8. Marshall L.F., Marshall S.B., and Klauber M.R. (1991). A new classification of head injury based on computerized tomography. J. Neurosurg. 75, S14-20 [Google Scholar]
- 9. Marshall L.F., Bowers-Marshall S., Klauber M.R., van Berkum-Clark M., Eisenberg H.M., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1991). A new classification of head injury based on computerized tomography. J. Neurosurg. 75, S14–S20 [Google Scholar]
- 10. Chesnut R.M., Temkin N., Dikmen S., Rondina C., Videtta W., Petroni G., Lujan S., Alanis V., Falcao A., la Fuenta G., Gonzalez L., Jibaja M., Lavarden A., Sandi F., Merida R., Romero R., Pridgeon J., Barber J., Machamer J., and Chaddock K. (2018). A method of managing severe traumatic brain injury in the absence of intracranial pressure monitoring: the imaging and clinical examination protocol. J. Neurotrauma 35, 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W., Bell M.J., Bratton S.L., Chesnut R., Harris O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., Vavilala M.S., Wilberger J., Wright D.W., and Ghajar J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 12. Bratton S., Bullock R., Carney N., Chesnut R., Coplin W., Ghajar J., Clifton G., Hammond F., Harris O., Härtl R., Maas A., Manley G., Marion D., Narayan R., Nemecek A., Newell D., Pitts L., Rosenthal G., Rosner M., Schouten J., Servadei F., Shutter L., Stocchetti N., Timmons S., Ullman J., Videtta W., Walters B., Wilberger J., and Wright D. (2007). Guidelines for the management of severe brain injury: 2007 revision. J. Neurotrauma 24, Suppl. 1, S1–106 [Google Scholar]
- 13. Robertson C.S., Valadka A.B., Hannay H.J., Contant C.F., Gopinath S.P., and Cormio M. (1999). Prevention of secondary ischemic insults after severe head injury. Crit. Care Med. 27, 2086–2095 [DOI] [PubMed] [Google Scholar]
- 14. American College of Surgeons Committee on Trauma (2015). Best Practices in the Management of Traumatic Brain Injury https://www.facs.org/quality-programs/trauma/tqp/center-programs/tqip/best-practice (Last accessed February19, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.