Abstract
The pancreatic islet is a highly vascularized micro-organ, and rapid revascularization postislet transplantation is important for islet survival and function. However, the various mechanisms involved in islet revascularization are not fully understood, and we currently lack good in vitro platforms to explore this. Our aim for this study was to generate perfusable microvascular networks in a microfluidic chip device, in which islets could be easily integrated, to establish an in vitro platform for investigations on islet–microvasculature interactions. We compared the ability of mesenchymal stem cells (MSCs) and fibroblasts to support microvascular network formation by human umbilical vein endothelial cells (HUVECs) and human induced pluripotent stem cell-derived endothelial colony-forming cell in two-dimensional and three-dimensional models of angiogenesis, and tested the effect of different culture media on microvessel formation. HUVECs that were supported by MSCs formed patent and perfusable networks in a fibrin gel, whereas networks supported by fibroblasts rapidly regressed. Network morphology could be controlled by adjusting relative cell numbers and densities. Incorporation of isolated rat islets demonstrated that islets recruit local microvasculature in vitro, but that the microvessels did not invade islets, at least during the course of these studies. This in vitro microvascularization platform can provide a useful tool to study how various parameters affect islet integration with microvascular networks and could also be utilized for studies of vascularization of other organ systems.
Impact statement
To improve pancreatic islet graft survival and function posttransplantation, rapid and adequate revascularization is critical. Efforts to improve islet revascularization are demanding due to an insufficient understanding of the mechanisms involved in the process. We have applied a microfluidics platform to generate microvascular networks, and by incorporating pancreatic islets, we were able to study microvasculature–islet interactions in real time. This platform can provide a useful tool to study islet integration with microvascular networks, and could be utilized for studies of vascularization of other organ systems. Moreover, this work may be adapted toward developing a prevascularized islet construct for transplantation.
Keywords: pancreatic islets, mesenchymal stem cells, microvasculature, islet revascularization, chip culture, microvascular network
Introduction
One of the most significant challenges facing the field of tissue engineering is the ability to vascularize tissues.1,2 All cells require a sufficient supply of nutrients and oxygen, as well as the ability to remove waste,3,4 and a functional microvasculature is needed to ensure proper function and survival of engineered biological tissues that are too large to be maintained by diffusion.5,6 Generating perfusable microvascular networks in vitro will be an important development toward successful engineering of organs and tissues,7,8 as well as for reliable modeling of microvasculature and organ systems.9 Currently, multiple in vitro systems mimicking characteristics of various organs have been established.10 However, many current systems do not fully integrate a three-dimensional (3D) microvasculature, and a vascularized model could consequently greatly improve their physiological relevance.11
Several approaches have emerged for engineering in vitro microvessels. One method involves endothelialization of micropatterned hydrogel channels or polydimethylsiloxane (PDMS) devices to make in vitro perfusable vessel analogs.9,11 These models have been used to study several aspects of endothelial cell (EC) biology, including EC migration, vascular barrier function, inflammatory response, thrombosis, tumor cell interactions, and response to biomolecular and mechanical stimuli.12–17 However, since these tubules are formed by the attachment of EC monolayers to channels in a predefined pattern, these models have only limited ability to replicate in vivo development microvascular networks.9 Moreover, these models cannot fully reconstitute the distinctive attributes and dynamics of endothelial responses in vivo.
Another approach to model 3D microvasculature relies on the ability of ECs to self-assemble into networks.18 Great advances have been made over recent years in developing 3D perfusable microvascular networks, where utilizing the morphogenic properties of EC eliminates the need for premade architecture to support the newly formed blood vessels.5,11 Suspending cells in hydrogels such as fibrin or collagen more closely mimics vessel formation in vivo.11 The inherent angiogenic ability of ECs, supported by cells secreting angiogenic growth factors, extracellular matrix (ECM) proteins, and proteases, allows for the dynamic development and remodeling of microvascular networks.19
EC tube formation is regulated by coordinated crosstalk between ECs and other cell types,20 and stromal cell-derived factors are important for EC sprouting and lumen formation.21 A number of cell types from the stromal compartment, such as fibroblasts, vascular smooth muscle cells, and mesenchymal stem cells (MSCs), have been shown to support self-assembly of microvascular networks in hydrogels.22–24 Even though the specific dimensions in self-assembled microvascular networks cannot be strictly controlled, microvascular network morphology can be modified by adjusting parameters such as cell-seeding density, hydrogel composition, and by the addition of growth factors.5
Microvascular network morphology and attributes are regulated by the surrounding environment. There are significant differences between the microvasculature structure and microvascular ECs of different organs,25,26 since every organ demonstrates specific vascular characteristics and functions.27 For instance, pancreatic islet vasculature shows considerable differences from that of the exocrine pancreas. Islet capillaries are highly fenestrated, are wider than those in the exocrine pancreas, and are present at a considerably higher vascular density than in the exocrine organ.28 Islets make up only 1% of the pancreas, but they receive up to 15% of the pancreatic blood supply.29 The higher degree of vascularization is important for the islet's ability to quickly secrete insulin in response to fluctuating blood glucose levels.29 Even though the outcomes of pancreatic islet transplantation have improved over the recent years,30–32 many challenges remain. Most islet transplants are done by intraportal vein injection,29 wherein infused islets are lodged in the liver vasculature. This site is suboptimal since it evokes early inflammatory reactions33 and exposes cells to a relatively hypoxic environment,34 resulting in substantial islet loss posttransplantation.35 Up to 60% of the transplanted islet mass is estimated to be destroyed following the infusion into the portal vein.36
Pancreatic islet isolation disrupts the connections between the islet vasculature and surrounding tissue, and intraislet EC content decreases rapidly in culture, partially as a consequence of hypoxia and lack of nutrients in the devascularized islets.37 After islet transplantation, the reestablishment of blood flow to transplanted islets requires at least several days.29 Therefore, ischemia and inadequate blood supply are likely contributors to the significant islet death occurring in the immediate posttransplant period.37 Hence, rapid and adequate revascularization is important for islet graft survival and function and could improve the outcomes of islet transplantation.29,38,39 Various methods to improve engraftment of transplanted islets have been suggested, including cotransplantation with other cell types,40–43 alternative sources for insulin producing cells,44 gene therapy,45 and the addition of ECM proteins and growth factors.46 Moreover, alternative transplant sites are being explored. The subcutaneous site, with its accessibility, large size, and ease of monitoring, may be an option.44 However, the subcutaneous option suffers from inadequate vascularization and poor oxygen tension, and islet transplantation into an unmodified subcutaneous site has never reversed diabetes in humans or animals.47,48 To improve the subcutaneous microenvironment, several approaches have been tried, including the use of encapsulation devices,49 polymers50 and meshes,51 cotransplantation with growth factors,51 MSCs52 or fibroblasts,53 as well as transient priming by temporary placement of a catheter.48 Beyond these approaches, the prevascularization of islets in vitro could possibly improve islet survival in vivo, by speeding inosculation with the host after implantation. Takahashi et al.39 showed that in vitro self-condensation of islets with ECs improves islet function in culture, as well as posttransplant reperfusion and engraftment.
The objective of our study was to establish a platform for the study of microvascular interaction with pancreatic islets. Despite recent advances, efforts to improve revascularization of transplanted islets have been hampered due to an insufficient understanding of the mechanisms involved in the process, including the responsible cells, ligands, and receptors.38 For this, good in vitro models are useful. The platform should support the development of patent and perfusable microvessels in a hydrogel system, in which pancreatic islets could be easily integrated, and should allow for real-time monitoring to closely follow islet–network interactions over time. Finally, the platform should also provide flexibility to investigate parameters that could potentially affect microvascular network development and islet integration and should be easy to reproduce.
We have integrated microvascular networks engineered into a commercially available microfluidic chip, along with pancreatic islets, to produce a platform that will improve our understanding of islet revascularization. This setup could be adapted for the study of microvascularization of other organ systems and tissues. Ultimately, this work may be adapted toward developing a prevascularized islet construct for transplantation.
Materials and Methods
Cells and culture
GFP- and RFP-tagged human umbilical vein endothelial cells (HUVECs) were purchased from Angio-Proteomie (Boston, MA). Cells were seeded onto rat tail collagen type I (Corning)-coated (50 μg/mL) tissue culture flasks and cultured in EBM-2 with complete EGM-2 MV Microvascular Endothelial Cell Growth Medium SingleQuot supplements (Lonza). Cells were passaged at 80% confluence, and passage 3–4 cells were used for all experiments.
Human adipose-derived MSCs were isolated from superficial or abdominal adipose tissue from healthy donors aged 10, 25, 35 (female), and 15 (male), undergoing elective reparative plastic surgery at New Haven Hospital. The isolation procedure has been described earlier.54 Cells from multiple donors were not grouped. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% Penicillin–Streptomycin (Pen-Strep; Gibco) and 10% fetal bovine serum (FBS; HyClone), and were passaged at 80% confluence. Passage 2–4 cells were used for all experiments.
Human lung fibroblasts were received as a generous gift from Will Chang at the Department of Medicine and Section of Nephrology, Yale University. Cells were maintained in M199 supplemented with 1% Pen-Strep and 10% FBS. Cells were passaged at 80% confluence. Passage 3–5 cells were used for all experiments.
tdTomato-tagged human induced pluripotent stem cell-derived endothelial colony-forming cells (iPSC-ECFCs) were a generous gift from Mervin Yoder, Department of Pediatrics, Indiana University School of Medicine. These cells have been thoroughly characterized earlier, and have been shown to maintain a stable endothelial phenotype and to not undergo replicative senescence for 18 passages in vitro.55 Cells were maintained in VascuLife VEGF Endothelial Medium supplemented with complete VascuLife VEGF LifeFactors Kit (LifeLine Technology, Walkersville, MD) on collagen-coated tissue culture flasks. Cells were passaged at 80% confluence. Cells from passage 11 were used for this study.
Rat islet isolation
This study was approved by the Yale University Institutional Animal Care and Use Committee. All animal care complied with the Guide for the Care and Use of Laboratory Animals. Human tissues and human cell populations that produced the human MSC were obtained using protocols approved by the Yale University Human Investigation Committee, and were discarded tissues, not categorized as human subjects research.
Sprague Dawley rats were euthanized by intraperitoneal injection of ketamine/xylazine at 75 mg/kg Ketamine and 5 mg/kg Xylazine. Pancreata were excised and digested in 1.5 mg/mL collagenase P (Sigma-Aldrich) for 20 min in a shaking 37°C water bath. Collagenase digestion was stopped with a solution composed of HBSS (ThermoFisher) containing 10% FBS and 1% Pen-Strep. The digest was further homogenized by passage through a 14 G syringe needle followed by filtration through a 500 μm nylon mesh. The digested pancreas was washed three times with HBSS solution, and islets were purified using Histopaque -1077 (Sigma-Aldrich) density gradient centrifugation. After additional washes to eliminate residual exocrine tissue and Histopaque solution, the islet pellet was resuspended in 10 mL RPMI 1640 medium (Gibco) supplemented with 10% FBS and 1% Pen-Strep, and cultured on uncoated dishes in a humidified 37°C CO2 incubator. Medium was changed on day 1, and then every 3 days. Islet function, in terms of insulin secretion, was assessed using Mercodia rat insulin enzyme-linked immunosorbent assays (ELISAs; Mercodia).
Two-dimensional angiogenesis assay
For stromal cell-EC coculture, adipose-derived human MSCs or human fibroblasts and HUVECs or iPSC-ECFCs were mixed at a ratio of 4:1 and plated at 1 × 105 cells/cm2 on tissue culture plastic. Fluorescently labeled ECs allowed the monitoring of cell behavior in real time over the course of the experiments.
To determine an optimal medium for two-dimensional (2D) angiogenesis assay, we tested three different cell culture media. These were the following: EBM-2 with added EGM-2 MV Microvascular Endothelial Cell Growth Medium SingleQuots supplements (Lonza); VascuLife VEGF Endothelial Medium supplemented with complete VascuLife VEGF LifeFactors Kit (LifeLine Technology); and MCDB131 (Gibco) supplemented with 10% FBS, 1% Pen-Strep and FGF2 vial from EGM2 bullet kit (Lonza). Medium was changed every day. Images were taken at days 3 and 6 with a Leica DM6000 fluorescent microscope (Leica Microsystems).
Microvascular network formation in fibrin
HUVECs or iPSC-ECFC, combined with MSC or fibroblasts, were resuspended in EGM basal medium with thrombin and aprotinin, and mixed with fibrinogen to yield a final fibrin concentration of 2 mg/mL. Final concentrations of thrombin and aprotinin were 1 U/mL and 25 μg/mL, respectively. The ratio of ECs (either HUVEC or iPSC-ECFC) to MSC/fibroblast cells was 5:1 for all experiments. For experiments comparing cell types, total cell number was 2.4 million/mL of fibrin gel. For investigations on the effects of cell number on network and lumen formation, the cell numbers were 3, 6, and 9 million/mL of fibrin gel. When looking at microvasculature recruitment to islets, 2.4 million cells/mL were used. Islets were included in the cell mixture before resuspension in basal medium for islet studies, and then mixed with fibrinogen to form the fibrin gel encapsulating the islets and vascular cells. The cell/fibrinogen mixture was quickly injected into the middle gel channel of the DAX 1 3D microfluidic Cell Culture chip (AIM Biotech). The chips were incubated at 37°C for 30 min before adding EGM-2MV medium to both medium channels. For perfusion assays, medium channels were coated with fibronectin (Sigma) at 10 μg/mL for 1 h after the gel had solidified, and HUVECs (2 million/mL) were added and left for a minimum of 4 h to allow cell attachment. Medium was then changed to remove unattached cells, and thereafter changed twice daily. Confocal images were taken with a Leica SP5 microscope.
Microvascular network analysis
To determine the effect of cell number on microvascular network formation, network density was analyzed using FIJI/ImageJ software. Images of cell networks (whole AIM Biotech chips, tiled images, n = 5) were converted to 8-bit images and an RFP fluorescence threshold was applied to render the images binary. Area coverage (vessel area) was determined as fluorescent area divided by total area of the gel. For further analysis, the FIJI plugin Angiogenesis Analyzer developed by Gilles Carpentier was used. Average vessel diameter was calculated as total length of vessels divided by size of vessel area in mm2. Quantification of vascular density was calculated as total vessel length/total gel area (mm2).
To study microvessel interaction with islets, microvascular networks on and immediately around the islets were analyzed. The region analyzed contained the islet and the area extending 100 μm around the islet. In a single experiment, the same islets were analyzed at days 1 and 4. Average network coverage of total chips (n = 6) were also analyzed at days 1 and 4, as a benchmark of capillary density in the absence of intervening islets. Area coverage vas calculated as described above.
Immunostaining of AIM Biotech chips
Fibrin gels were processed for staining after 5 days of culture. Gels were fixed with 4% PFA for 15 min and blocked for 2 h at room temperature (RT) in phosphate-buffered saline (PBS) containing 0.75% glycine (OmniPur) and 5% bovine serum albumin (Gemini Bio-Products). Primary antibody against ACTA2 (M0851; Dako) was diluted 1:100 in blocking buffer, and gels were incubated overnight at 4°C. After washing the gels for 3 × 5 min in PBS, fluorescence-conjugated secondary antibody (goat anti-mouse Alexa Fluor 555, A21424; Invitrogen) was diluted 1:400 in blocking buffer and incubated with the gels for 1 h at RT. Gels were then washed repeatedly in PBS and imaged by confocal microscopy (Leica SP5).
Microparticle perfusion
Thirty microliters medium was aspirated from both ports of one medium channel to create a pressure drop across the gel. Ten microliters 1 mg/mL green fluorescent microparticles were added to one port of the opposing medium channel, allowing the microparticles to flow into the medium channel and into the microvascular network. Images were taken with a Leica DM6000 microscope.
Generation of pancreatic islets reaggregates
Rat islets were washed with PBS and digested in Accutase cell detachment solution (STEMCELL Technologies) for 20 min in a 37°C shaking water bath. Enzyme activity was quenched by addition of RPMI 1640 medium supplemented with 10% FBS and 1% Pen-Strep, and the cell suspension was passed through a 40 μm cell strainer. The cells were collected by centrifugation, counted, and resuspended in EGM2-MV containing 2.4 mg/mL methyl cellulose (Sigma). Preparation of methyl cellulose medium has been described elsewhere.56 Spheroids were prepared by the hanging drop method by suspending 35 μL droplets containing 1000 islet cells on the inside lid of a cell culture dish. For islet-HUVEC spheroids (composite spheroids), the droplets contained 1000 islet cells +100/250/500 HUVECs. Spheroids were formed within 3 days of hanging drop culture.
Statistics
Data were analyzed by a two-way Student's t-test and are expressed as group mean ± standard error of the mean. A p-value of <0.05 was considered statistically significant. The R package ggplot257 was used for plotting.
Results
Effect of culture medium on 2D microvascular network formation
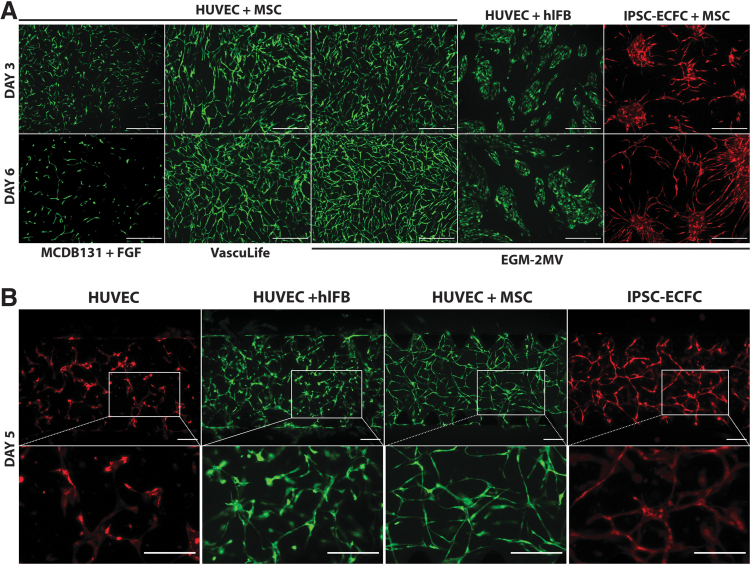
To evaluate the effect of culture medium type on microvascular network formation, HUVECs and MSCs were cocultured on tissue culture plastic in three different culture media. EGM-2MV and VascuLife VEGF Endothelial Medium both contain undisclosed concentrations of the growth factors FGF-β, VEGF-A, IGF-1, and EGF, in addition to hydrocortisone and ascorbic acid. We also tested MCDB131 that was supplemented with only FGF-β to provide a “minimal medium” comparator. By day 3, HUVECs in all three culture conditions had changed morphology from cobblestone to elongated cells and were starting to form interconnected networks (Fig. 1A, upper panels). These networks were notably denser in the growth factor supplemented media, indicating a faster proliferation rate and/or an impact on EC phenotype. By day 6, the networks cultured in either VascuLife or EGM-2MV had developed and stabilized, forming fully interconnected networks covering the entire culture area (Fig. 1A, lower panels). By contrast, networks cultured in minimal medium had regressed. These results indicate that growth factor-supplemented medium is required for microvascular networks formation by HUVECs in a 2D coculture model with MSCs. Since EGM-2MV gave smoother and denser networks in this assay, as assessed by microscopy, we chose this medium for subsequent experiments.
FIG. 1.
Effect of cell types and culture medium on microvascular network formation in 2D and 3D. (A) Representative images of vascular networks formed by HUVECs and iPSC-ECFC cocultured with MSCs or human lung fibroblasts in different culture media. Images were taken after 3 and 6 days of culture. Scale bars, 500 μm. (B) Representative images of vascular networks formed by HUVECs alone, HUVECs+human lung fibroblasts, HUVECs+adipose-derived MSCs, and iPSC-ECFC+adipose-derived MSCs in a fibrin gel. Images were taken after 5 days of culture. Scale bars, 200 μm. 2D, two-dimensional; 3D, three-dimensional; HUVECs, human umbilical vein endothelial cells; iPSC-ECFC, induced pluripotent stem cell-derived endothelial colony-forming cell; MSCs, mesenchymal stem cells.
Effect of cell types on microvascular network formation
As fibroblasts are widely used in coculture models with ECs, we investigated two different isolations of human lung fibroblasts in our 2D model to assess their potential to support microvascular network formation by HUVECs. Cultured in EGM-2MV, the HUVECs did not acquire a tube-forming phenotype at either days 3 or 6 (Fig. 1A) as assessed by elongated morphology, but instead remained clustered in cobblestone colonies. We obtained similar results from both fibroblast isolations, demonstrating that human lung fibroblasts cannot induce an angiogenic phenotype in HUVECs in these culture conditions.
Assessing the angiogenic potential of iPSC-ECFC, we cocultured iPSC-ECFC with MSCs in EGM-2MV. This resulted in mostly cobblestone-shaped ECs clustering together, with some elongated cells extending from the clusters (Fig. 1A, rightmost panels). By day 6, these elongated cells had increased in number, and formed larger areas of interconnected networks between the cell clusters. Our results demonstrate a clear EC phenotype of the iPSC-ECFCs, and indicate that cells with some in vitro angiogenic capacity can be derived from iPSCs. However, HUVECs were found to be superior in terms of network-forming ability in this assay.
Effect of cell type on microvascular network formation in 3D
To investigate the ability of cells to form stable microvascular networks in a fibrin gel, HUVECs were cultured alone, with MSCs, or with fibroblasts in a 2% fibrin gel in the AIM Biotech DAX 1 3D microfluidic Cell Culture chip in EGM2-MV medium (Fig. 1B). Chips with iPSC-ECFC and MSC were also cultured. HUVECs alone failed to form stable networks in the fibrin gel. The cells were found to elongate and form tentative branches with poor interconnection, and by day 5, most branches had regressed. HUVECs cultured with both fibroblasts and MSCs did form networks, both of which were still present to various extents at day 5. However, HUVECs cultured with MSCs displayed a more consistent elongated morphology and formed smoother branches than did HUVECs cultured with fibroblasts. In the fibroblast-supported networks, not all parts of the cell culture were interconnected, and the networks started to regress by day 4. iPSC-ECFC cultured with MSCs formed stable and mostly interconnected networks, although a proportion of the cells did not join in the network formation (Fig. 1B, rightmost panels). These results demonstrate that HUVECs require a supporting cell type to form a stable microvascular network in this assay, and that MSCs performed better than fibroblasts in this regard. iPSC-ECFCs did also form networks in coculture with MSCs, however, the HUVEC-MSC combination generated the most stable and interconnected networks.
To investigate whether the MSCs were directly interacting with the EC networks, we probed the fibrin gels for ACTA2 expression, which is expressed by MSCs only. Immunofluorescent staining revealed that ACTA2-positive cells were consistently present alongside of the HUVEC structures throughout the networks (Supplementary Fig. S1). The observation that MSCs are found adjacent to the ECs indicates a likely interaction between the cell types perhaps suggesting that MSCs have a role in stabilizing the newly formed microvascular networks.
Effect of cell density on microvascular network formation
To assess the effect of cell density on formation of microvascular networks, fibrin gels were seeded with 3, 6, and 9 million total cells/mL, at a HUVEC:MSC ratio of 5:1. By day 5, all visible HUVECs were participating in stable interconnected networks (Fig. 2A, B). Area coverage, as calculated as the area of the chip with RFP fluorescent cells, significantly increased with increasing cell number (3 million vs. 6 million cells/mL: p < 0.001; 6 million vs. 9 million cells/mL: p < 0.01, n = 5) (Fig. 2C). The average vessel diameter also significantly increased, from 22.8 ± 1.0 μm with 3 million cells/mL, to 28.2 ± 0.9 μm with 6 million cells and 38.7 ± 2.6 μm for 9 million cells/mL (3 million vs. 6 million cells/mL: p < 0.01; 6 million vs. 9 million cells/mL: p < 0.01, n = 5) (Fig. 2D). The network density (Fig. 2E), calculated as tubule length/mm2 showed a significant change from 3 to 6 million cells (p < 0.01, n = 5), whereas further increasing cell number to 9 million did not significantly change the length of the network. These results demonstrate that cell density can impact the morphology of microvascular networks grown in a fibrin gel, where higher cell densities yield networks with larger average vessel diameter.
FIG. 2.
Effect of cell number and cell type ratio on microvascular network. (A, B) Representative images of microvascular networks formed in a fibrin gel by increasing number of cells. Ratio of HUVEC to MSC is 5:1. Scale bars, 250 μm. (C) Quantification of vessel area of the fibrin gel shown in percentage of RFP-HUVEC signal of the total area. All values are means from five gels plus ± SEM. 3 million versus 6 million cells/mL: p < 0.001; 6 million versus 9 million cells/mL: p < 0.01. (D) Average vessel diameter calculated as total length of vasculature to area coverage. 3 million versus 6 million cells/mL: p < 0.01; 6 million versus 9 million cells/mL: p < 0.01. (E) Quantification of vascular density calculated as vessel length/mm2. Significant increase from 3 to 6 million cells, p < 0.01, **p < 0.01, ***p < 0.001. SEM, standard error of the mean.
Lumen formation in microvascular networks
Confocal imaging of 9 million/mL vascular networks revealed consistent lumen formation throughout the network (Fig. 3A; for projections, see Supplementary Video S1A, B). Orthogonal views revealed multiple clear circular shapes, verifying that the networks had intact lumens. Furthermore, addition of microparticles to the medium channel of the chip resulted in perfusion into the network (Fig. 3B).
FIG. 3.
Microvascular networks form perfusable lumens. (A) Orthogonal projections of z-stack images of RFP-HUVECs cultured with AT-MSCs in a fibrin gel for 5 days. Images were composed from 0.9 μm serial confocal images (72 slices) through the z-plane of the cells. Left panel shows stacked xy projection, and the side and bottom panels show yz and xz projections. Yellow crosshairs indicate intersection of yz and yx planes. Rightmost panel shows single slice from stack at z-depth as indicated by the x and y axis crosshairs within the projections. Scale bars, 100 μm. For lumen projections, see Supplementary Video S1A and B. (B) Fluorescent microparticles added to the medium channel enter the microvascular network, demonstrating that the engineered networks are perfusable. Scale bars, 25 μm. AT, adipose tissue.
Recruitment of microvasculature by rat islets in vitro
To generate an in vitro platform for the study of pancreatic islet interaction with microvasculature, isolated rat pancreatic islets were combined with our microvascular networks in the microfluidics device. Islet functionality was quantified before chip embedding using insulin ELISA tests demonstrating that islets are capable of secreting 33 ± 2 pg/islet/min of insulin over the course of 20 h, which is comparable to previously reported values in literature 57 ± 9 pg/islet/min.58 Immediately after seeding, cells were evenly distributed in the gels. After days in culture, cells gradually formed interconnected networks throughout the gel and around the islets (Fig. 4A, B; for projections of B, see Supplementary Video S2A, B). Analyzing area coverage of the microvascular network immediately surrounding the islets at days 1 and 4, the average coverage increased significantly (p-value <0.0001, n = 37 islets), from 12.3% ± 0.5% to 20.0% ± 0.9% (Fig. 4C). In comparison, the average microvascular area coverage for whole chips (the entire gels, including the area surrounding the islets) were 10.9% ± 0.35% and 12.7% ± 1.06% at days 1 and 4, and the change was not significant (p = 0.066, n = 6 gels). As the network densities increased significantly around the islets, but not for the entire gel, our results indicate that islets are attracting microvasculature to their immediate surroundings.
FIG. 4.
Microvascular network recruitment to islets. (A) Progression of microvascular network formation around a rat islet day by day. Cells were evenly dispersed in the gel immediately after seeding, and gradually formed a mostly interconnected network by day 5, assembling around the islets. Two million RFP-HUVECs were cocultured with AT-MSCs at a 5:1 ratio in a fibrin gel with integrated GFP rat islets. Scale bars, 200 μm. (B) Confocal image of microvascular network surrounding an islet after 5 days of culture. For projections, see Supplementary Video S2A and B. Scale bar, 100 μm. (C) Distribution of vascular network coverage immediately around islets at days 1 and 4. For analysis of network area coverage around islets, the regions that were analyzed contained the islet and the area extending 100 μm from the islet boundary. FIJI was used to automatically threshold and analyze the images. The vascular density around the islets increased significantly from day 1 (12.3% ± 0.5%) to day 4 (20.0% ± 0.9%) (p-value <0.00001, N = 37 islets). Red horizontal lines indicate vascular area coverage throughout the entire fibrin gel, this change was not significant. (10.9% ± 0.35% and 12.7% ± 1.06% at days 1 and 4, respectively. p-value <0.065699, n = 6 gels.) ***p < 0.001.
Islet reaggregates spheroids
To test whether islet spheroids were more prone to efficient revascularization, we dissolved rat islets in accutase to break them down into single cells and reaggregated them into islet spheroids by the hanging drop methods. The hanging drop methods yielded evenly sized islet spheroids of defined sizes (Fig. 5A) and stained positive for insulin (Fig. 5D). Spheroids secreted insulin at 35 ± 1 pg/islet/min, which is comparable to the insulin secretion rate we observed for native islets of similar size. When incorporated into the chips, the islet spheroids were recruiting networks to their surface, but just as for native islets, showed few signs of invasion (Fig. 5E). These results demonstrate that rat islets can be readily reaggregated into uniform insulin secreting spheroids, but the spheroids did not have improved EC invasion qualities compared to native islets.
FIG. 5.
Reaggregation of islet cells into spheroids with and without HUVECs. (A) Reaggregated islet cells form uniform spheroids. Scale bar, 200 μm. (B-C) Composite spheroids formed from 1000 islet cells +100/250 HUVECs are more irregular in shape and size. Scale bars, 200 μm. (D) Islet spheroids express insulin. Scale bar, 50 μm. (E) Islet spheroids embedded in vascularized chips recruit microvascular networks. Scale bar, 50 μm. (F) Increasing the ratio of HUVEC to islet cells does not increase the number of ECs in the spheroids. Most ECs are binding to the surface of a core spheroid formed almost exclusively of islet cells. Scale bar, 50 μm. (G) Composite spheroids embedded in a vascularized chip recruit microvascular networks, but we did not observe enhanced vascularization. Scale bar, 50 μm. ECs, endothelial cells.
Composite islet-HUVEC spheroids
Since ECs seemed to have difficulties invading into islets to form intraislet microvasculature, we theorized that we could improve revascularization by directly incorporating ECs during the reaggregation protocol. This would allow ECs to start inside the spheroids and migrate outward which circumvents the need for ECs to invade into the islets. To test whether incorporation of HUVECs into the islet spheroids could promote vascularization, we generated composite spheroids of various islet cell-HUVEC ratios. The resulting spheroids were not as uniform as spheroids composed of pancreatic islets only and were more irregular in shape and size (Fig. 5B, C). The different cells also seemed to have a preference to interact with its own cell type, and the resulting spheroids were mainly composed of a core of islet cells surrounded by smaller EC aggregates (Fig. 5F). Also, increased HUVEC to islet cell ratio did not seem to yield spheroids containing more ECs. Incorporation of the composite spheroids into the chips unfortunately did not show any evidence of improved vascular invasion (Fig. 5G).
Discussion
In the present study, we successfully developed a functional microvascular network in a microfluidic device, where HUVECs that were supported by adipose-derived MSCs formed interconnected microvessels. The generated microvasculature was patent and perfusable, thereby potentially allowing for delivery of nutrients and oxygen via the luminal space. By adjusting the cell-seeding density, we can control microvascular morphology, including the microvessel diameter and network density, demonstrating the potential to generate networks that are tailored to specific applications.
In the absence of MSCs, we observed only impaired network formation and stability, confirming the stimulatory effect of MSCs on angiogenesis.59 Immunofluorescent staining revealed that MSCs inhabit a perivascular position in the microvascular networks, supporting previous findings that MSCs can act in a pericyte-like manner under these types of coculture conditions,60–64 and provide stabilizing effects on microvascular networks in vitro and in vivo.65,66 Microvascular networks also formed in a coculture of MSCs and iPSC-ECFCs, although these networks did not appear to be fully interconnected. In addition, iPSC-ECFC were only partially induced to form networks in a 2D assay, possibly reflecting a lack of angiogenic potential or a need for further stimuli than were provided in this set of studies.
Fibroblasts, such as MSCs, promote angiogenesis through the production of ECM, growth factors, and proteases,67–70 and have been shown to support 3D network formation by HUVECs.5,71 We were therefore surprised to observe a rapid regression of HUVEC-fibroblasts networks, preventing the networks from reaching a fully interconnected and stable state. Moreover, human lung fibroblasts were unable to support endothelial network formation in 2D coculture assays in an angiogenic cell culture medium. Evensen et al.72 observed a similar difference between human dermal fibroblasts and other stromal cell types, while other investigators have found that human dermal fibroblasts do induce network formation of HUVECs in the same 2D model.73 Newman et al.21 compared human lung fibroblast populations having different angiogenic abilities and found that a number of ECM proteins are more abundant in fibroblasts that readily induce endothelial sprouting in a fibrin model of angiogenesis. Shortage of secreted ECM proteins from fibroblasts compared with MSCs, and thus insufficient structural support for the ECs to form networks, could possibly explain why networks failed to form in the 2D model. As the fibrin gel itself provides some structural support, this could explain the difference in angiogenic potential of fibroblasts between these two platforms. Fibroblast heterogeneity21,74 or donor genotype, as well as 2D versus 3D culture conditions affecting fibroblast morphology and gene expression,75–77 could also explain these contrasting results.
Combining islets with our engineered microvascular networks, we show that microvasculature is actively recruited to pancreatic islets in vitro. Islets are known to secrete angiogenic factors such as VEGF,78 which is also likely necessary for revascularization of islets in vitro. Rat VEGF has been shown to be a potent mitogen for HUVECs,79 although it is not known whether rat VEGF supports HUVECs as efficiently as human VEGF. Rapid revascularization of islets posttransplantation is considered important for islet graft survival and function and could improve the outcomes of islet transplantation.29,37,38 Moreover, secretion of VEGF and proteases by MSCs supports vascularization and could promote EC migration into the islets.59,80 MSCs have been shown to improve islet vascularization after islet transplantation,44,80–83 and MSCs from various sources have been reported to improve islet survival and function both in vivo and in vitro.84–90 Another possible explanation for the recruitment of networks to the islet surface could be matrix modulation by the islets. Without the addition of aprotinin to the fibrin gel, we observed local fibrin breakdown around the islets (data not shown), and the addition of aprotinin was necessary to delay matrix degradation. It is possible that the islets were still exerting some effect on the local surrounding matrix that potentially encouraged cellular migration into these areas. Local matrix degradation is a key early step in physiological angiogenesis,91 and MSCs are also known to contribute to EC migration by producing proteases, thereby facilitating EC sprouting and outgrowth.59 Altered matrix composition could thus also help explain the EC recruitment to the islet surface.
In the recent years, important steps have been made toward in vitro perfusion of organs and organ-like structures. Sobrino et al.64 successfully generated perfused tumor spheroids by incorporating tumor cells into microphysiological system containing a perfused microvessel network.92–94 Developing this platform further, Shirure et al.95 implanted tumor organoids adjacent to an already developed microvessel network, more accurately representing tumor angiogenesis. Others have also been successful in vascularizing nontumor cell spheroids, with angiogenic ingrowth and perfusion of HUVEC-fibroblast-MSC composite spheroids.96,97 Moreover, work from the Kamm lab98 has demonstrated both engineered 3D neuronal and perfusable vascular networks in a microfluidic device, using a stem cell-based approach to derive motor neurons and ECs. In their macro setup of the experiment, where HUVECs were used instead of iPS-EC, they found that most of the HUVECs formed networks around the spheroids, as did we, although they did observe some invasion. Moreover, work from the same laboratory has resulted in a perfusable model of the blood–brain barrier.99
Despite advances in vascularizing other organoid systems, the successful generation of a vascularized and perfused islet-on-a-chip model has not been seen to date. Providing islets with a readily available microvascular network supported by MSCs may speed up the revascularization process, as well as improve islet survival until revascularization is complete. By demonstrating that this process can be started in vitro by attraction of vascular cells to the islets, our model could represent a first step toward creating a vascularized islet construct for transplantation. Prevascularization could also facilitate integration of the construct upon implantation. Allowing fibrin constructs to develop microvascular networks in vitro before implantation, as opposed to a construct containing newly suspended cells, has been shown to significantly improve anastomosis with host vasculature.100
The fibrin base of the construct could also have a positive effect on islet outcome. In addition to promoting angiogenesis, fibrin has been shown to enhance islet function and survival.101,102 Fibrin is biocompatible and biodegradable,103,104 and is also the body's natural structural scaffold for wound healing.
Even though we observed consistent microvasculature recruitment to islets, we only rarely observed invasion of ECs into the islets themselves. In addition, our efforts to create islet spheroids incorporated with ECs unfortunately did not seem to improve vascularization. Lack of invasion and penetrating revascularization of islets in vitro could have several possible explanations. Our model may benefit from incorporation of flow which could provide more physiological conditions replicating natural blood flow in the body. Mechanical stimulation has been shown to impact vasculature remodeling and development,105 although no differences in network morphology were observed between perfusion and static conditions in a recent study on microvascular network formation in a microfluidic chip device.98 In addition, our in vitro model does not incorporate all of the physiological signals and signaling molecules found in vivo that influence islet revascularization.
For future investigations, alternative sources for ECs should also be considered. HUVECs are widely used for in vitro studies and have demonstrated an ability to form neovasculature and microvascular networks, but differ from microvascular EC in some important aspects, including differences in cell surface receptors, cytoskeletal, and secreted proteins.106–108 In addition, for potential clinical applications, an autologous cell source is desirable. One possible source for autologous microvascular ECs is iPSCs. These cells have an extensive capacity for self-renewal, and can be used to derive tissue-specific cells, including different EC subtypes.109 iPSC-derived vascular endothelium has been shown to be highly plastic,110 and can be directed toward specific EC subtypes in response to biomechanical cues.111 Although we and others112 found iPSC-ECFCs to be outperformed by HUVECs in terms of angiogenic ability, iPSC-derived ECs have previously shown more plasticity in modulating their phenotype in response to flow, compared with HUVECs.17 The use of IPSC-EC would warrant further studies, and may present a viable alternative in the future of islet transplantation. Moreover, generation of EC-islet spheroids might still be an interesting approach to promote in vitro vascularization, although more work is needed to improve the reaggregation process to ensure optimal incorporation of ECs. Outgrowth from composite EC-islet spheroids has been demonstrated by others, which attests to the feasibility of this approach.80
To conclude, we applied a commercially available microfluidic platform to generate perfusable microvascular networks, and by incorporating pancreatic islets, we were able to study microvasculature–islet interactions in real time. This platform can provide a useful tool to study how various parameters affect islet integration with microvascular networks, and could also be utilized for studies of vascularization of other organ systems. Moreover, this work may be adapted toward developing a prevascularized islet construct for transplantation.
Supplementary Material
Disclosure Statement
L.E.N. is a founder and shareholder in Humacyte, Inc., which is a regenerative medicine company. Humacyte, Inc. produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. L.E.N.'s spouse has equity in Humacyte, Inc. and L.E.N. serves on Humacyte's Board of Directors. L.E.N. is an inventor on patents that are licensed to Humacyte, Inc. and that produce royalties for L.E.N. L.E.N. has received an unrestricted research gift to support research in her laboratory at Yale. Humacyte, Inc. did not influence the conduct, description, or interpretation of the findings in this report. The other authors report no conflicts.
Funding Information
This work was supported by Yale University, and by NIH R01 HL127386 and by an unrestricted research gift from Humacyte, Inc. (both to L.E.N). M.H.R was supported by a Fulbright grant and Gidske and Peter Jacob Soerensen's Research Fund.
Supplementary Material
References
- 1. Nerem R.M. Tissue engineering: the hope, the hype, and the future. Tissue Eng 12, 1143, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Gomes M.E., Rodrigues M.T., Domingues R.M.A., and Reis R.L.. Tissue engineering and regenerative medicine: new trends and directions-a year in review. Tissue Eng Part B Rev 23, 211, 2017 [DOI] [PubMed] [Google Scholar]
- 3. Chang W.G., and Niklason L.E.. A short discourse on vascular tissue engineering. NPJ Regen Med 2, 7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sipe J.D. Tissue engineering and reparative medicine. Ann N Y Acad Sci 961, 1, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Whisler J.A., Chen M.B., and Kamm R.D.. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng Part C Methods 20, 543, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shafiee A., and Atala A.. Tissue engineering: toward a new era of medicine. Annu Rev Med 68, 29, 2017 [DOI] [PubMed] [Google Scholar]
- 7. Novosel E.C., Kleinhans C., and Kluger P.J.. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63, 300, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Chan J.M., Zervantonakis I.K., Rimchala T., Polacheck W.J., Whisler J., and Kamm R.D.. Engineering of in vitro 3D capillary beds by self-directed angiogenic sprouting. PLoS One 7, e50582, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim S., Lee H., Chung M., and Jeon N.L.. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13, 1489, 2013 [DOI] [PubMed] [Google Scholar]
- 10. Fatehullah A., Tan S.H., and Barker N.. Organoids as an in vitro model of human development and disease. Nat Cell Biol 18, 246, 2016 [DOI] [PubMed] [Google Scholar]
- 11. Lee H., Chung M., and Jeon N.L.. Microvasculature: an essential component for organ-on-chip systems. MRS Bull 39, 51, 2014 [Google Scholar]
- 12. Chrobak K.M., Potter D.R., and Tien J.. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 71, 185, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Chung S., Sudo R., Mack P.J., Wan C.R., Vickerman V., and Kamm R.D.. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9, 269, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Price G.M., Wong K.H., Truslow J.G., Leung A.D., Acharya C., and Tien J.. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31, 6182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song J.W., and Munn L.L.. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 108, 15342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Y., Chen J., Craven M., et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 109, 9342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sivarapatna A., Ghaedi M., Xiao Y., et al. Engineered microvasculature in PDMS networks using endothelial cells derived from human induced pluripotent stem cells. Cell Transplant 26, 1365, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haase K., and Kamm R.D.. Advances in on-chip vascularization. Regen Med 12, 285, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ucuzian A.A., Gassman A.A., East A.T., and Greisler H.P.. Molecular mediators of angiogenesis. J Burn Care Res 31, 158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes C.C. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol 15, 204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newman A.C., Chou W., Welch-Reardon K.M., et al. Analysis of stromal cell secretomes reveals a critical role for stromal cell-derived hepatocyte growth factor and fibronectin in angiogenesis. Arterioscler Thromb Vasc Biol 33, 513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterson A.W., Caldwell D.J., Rioja A.Y., Rao R.R., Putnam A.J., and Stegemann J.P.. Vasculogenesis and angiogenesis in modular collagen-fibrin microtissues. Biomater Sci 2, 1497, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kniazeva E., Kachgal S., and Putnam A.J.. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng Part A 17, 905, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegen A., Blois A., Tiron C.E., et al. Efficient in vivo vascularization of tissue-engineering scaffolds. J Tissue Eng Regen Med 5, e52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kassab G.S. Scaling laws of vascular trees: of form and function. Am J Physiol Heart Circ Physiol 290, H894, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Nolan D.J., Ginsberg M., Israely E., et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26, 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uwamori H., Ono Y., Yamashita T., Arai K., and Sudo R.. Comparison of organ-specific endothelial cells in terms of microvascular formation and endothelial barrier functions. Microvasc Res 122, 60, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henderson J.R., and Moss M.C.. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol 70, 347, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Pepper A.R., Gala-Lopez B., Ziff O., and Shapiro A.M.. J. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol 2013, 352315, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barton F.B., Rickels M.R., Alejandro R., et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anazawa T., Okajima H., Masui T., and Uemoto S.. Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg 3, 34, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCall M., and Shapiro A.M.. Update on islet transplantation. Cold Spring Harb Perspect Med 2, a007823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennet W., Groth C.G., Larsson R., Nilsson B., and Korsgren O.. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci 105, 125, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Carlsson P.O., Palm F., Andersson A., and Liss P.. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50, 489, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Gamble A., Pepper A.R., Bruni A., and Shapiro A.M.. J. The journey of islet cell transplantation and future development. Islets 10, 80, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shapiro A.M., Ryan E.A., and Lakey J.R.. Diabetes. Islet cell transplantation. Lancet 358 (Suppl), S21, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Nyqvist D., Köhler M., Wahlstedt H., and Berggren P.-O.. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 54, 2287, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Brissova M., and Powers A.C.. Revascularization of transplanted islets: can it be improved? Diabetes 57, 2269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takahashi Y., Sekine K., Kin T., Takebe T., and Taniguchi H.. Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep 23, 1620, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coppens V., Heremans Y., Leuckx G., et al. Human blood outgrowth endothelial cells improve islet survival and function when co-transplanted in a mouse model of diabetes. Diabetologia 56, 382, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Kang S., Park H.S., Jo A., et al. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes 61, 866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oh B.J., Oh S.H., Jin S.M., et al. Co-Transplantation of bone marrow-derived endothelial progenitor cells improves revascularization and organization in islet grafts. Am J Transplant 13, 1429, 2013 [DOI] [PubMed] [Google Scholar]
- 43. Figliuzzi M., Cornolti R., Perico N., et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc 41, 1797, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Moore S.J., Gala-Lopez B.L., Pepper A.R., Pawlick R.L., and Shapiro A.J.. Bioengineered stem cells as an alternative for islet cell transplantation. World J Transplant 5, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X., Meloche M., Verchere C.B., et al. Improving islet engraftment by gene therapy. Journal of transplantation 2011, 594851, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsuchiya H., Sakata N., Yoshimatsu G., et al. Extracellular matrix and growth factors improve the efficacy of intramuscular islet transplantation. PLoS One 10, e0140910, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simeonovic C.J., Dhall D.P., Wilson J.D., and Lafferty K.J.. A comparative study of transplant sites for endocrine tissue transplantation in the pig. Aust J Exp Biol Med Sci 64 (Pt 1), 37, 1986 [DOI] [PubMed] [Google Scholar]
- 48. Pepper A.R., Gala-Lopez B., Pawlick R., Merani S., Kin T., and Shapiro A.M.. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 33, 518, 2015 [DOI] [PubMed] [Google Scholar]
- 49. Wang W., Gu Y., Hori H., et al. Subcutaneous transplantation of macroencapsulated porcine pancreatic endocrine cells normalizes hyperglycemia in diabetic mice. Transplantation 76, 290, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Juang J.H., Bonner-Weir S., Ogawa Y., Vacanti J.P., and Weir G.C.. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 61, 1557, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Wang W., Gu Y., Tabata Y., et al. Reversal of diabetes in mice by xenotransplantation of a bioartificial pancreas in a prevascularized subcutaneous site. Transplantation 73, 122, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Veriter S., Gianello P., Igarashi Y., et al. Improvement of subcutaneous bioartificial pancreas vascularization and function by coencapsulation of pig islets and mesenchymal stem cells in primates. Cell Transplant 23, 1349, 2014 [DOI] [PubMed] [Google Scholar]
- 53. Perez-Basterrechea M., Briones R.M., Alvarez-Viejo M., et al. Plasma-fibroblast gel as scaffold for islet transplantation. Tissue Eng Part A 15, 569, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Wu W., Le A.V., Mendez J.J., Chang J., Niklason L.E., and Steinbacher D.M.. Osteogenic performance of donor-matched human adipose and bone marrow mesenchymal cells under dynamic culture. Tissue Eng Part A 21, 1621, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prasain N., Lee M.R., Vemula S., et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol 32, 1151, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Korff T. Methods in Endothelial Cell Biology (Augustin, H.G., ed.). Berlin Heidelberg: Springer, 2004, pp. 55–62 [Google Scholar]
- 57. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer Verlag, 2016 [Google Scholar]
- 58. Zawalich W.S., and Zawalich K.C.. Glucose-induced insulin secretion from islets of fasted rats: modulation by alternate fuel and neurohumoral agonists. J Endocrinol 166, 111, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Ghajar C.M., Blevins K.S., Hughes C.C., George S.C., and Putnam A.J.. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng 12, 2875, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Armulik A., Abramsson A., and Betsholtz C.. Endothelial/pericyte interactions. Circ Res 97, 512, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Ball S.G., Shuttleworth C.A., and Kielty C.M.. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med 11, 1012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dhar K., Dhar G., Majumder M., et al. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer 9, 209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Charbord P., Livne E., Gross G., et al. Human bone marrow mesenchymal stem cells: a systematic reappraisal via the genostem experience. Stem Cell Rev 7, 32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sobrino A., Phan D.T., Datta R., et al. 3D microtumors in vitro supported by perfused vascular networks. Sci Rep 6, 31589, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamamoto K., Tanimura K., Mabuchi Y., et al. The stabilization effect of mesenchymal stem cells on the formation of microvascular networks in a microfluidic device. J Biomech Sci Eng 8, 114, 2013 [Google Scholar]
- 66. Au P., Tam J., Fukumura D., and Jain R.K.. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111, 4551, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kellouche S., Mourah S., Bonnefoy A., et al. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res 313, 486, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Griffith C.K., Miller C., Sainson R.C., et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 11, 257, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Berthod F., Germain L., Tremblay N., and Auger F.A.. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol 207, 491, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Newman A.C., Nakatsu M.N., Chou W., Gershon P.D., and Hughes C.C.. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22, 3791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li Y., Pi Q.-M., Wang P.-C., et al. Functional human 3D microvascular networks on a chip to study the procoagulant effects of ambient fine particulate matter. RSC Adv 7, 56108, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Evensen L., Micklem D.R., Blois A., et al. Mural cell associated VEGF is required for organotypic vessel formation. PLoS One 4, e5798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kunz-Schughart L.A., Schroeder J.A., Wondrak M., et al. Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol 290, C1385, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Lynch M.D., and Watt F.M.. Fibroblast heterogeneity: implications for human disease. J Clin Invest 128, 26, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fluck J., Querfeld C., Cremer A., Niland S., Krieg T., and Sollberg S.. Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol 110, 153, 1998 [DOI] [PubMed] [Google Scholar]
- 76. Kessler D., Dethlefsen S., Haase I., et al. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem 276, 36575, 2001 [DOI] [PubMed] [Google Scholar]
- 77. Sung K.E., Su X., Berthier E., Pehlke C., Friedl A., and Beebe D.J.. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One 8, e76373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brissova M., Shostak A., Shiota M., et al. Pancreatic islet production of vascular endothelial growth factor—a is essential for islet vascularization, revascularization, and function. Diabetes 55, 2974, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Conn G., Soderman D.D., Schaeffer M.T., Wile M., Hatcher V.B., and Thomas K.A.. Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line. Proc Natl Acad Sci U S A 87, 1323, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johansson U., Rasmusson I., Niclou S.P., et al. Formation of composite endothelial cell-mesenchymal stem cell islets: a novel approach to promote islet revascularization. Diabetes 57, 2393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Figliuzzi M., Bonandrini B., Silvani S., and Remuzzi A.. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells 6, 163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ito T., Itakura S., Todorov I., et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89, 1438, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Berman D.M., Willman M.A., Han D., et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59, 2558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park K.S., Kim Y.S., Kim J.H., et al. Influence of human allogenic bone marrow and cord blood-derived mesenchymal stem cell secreting trophic factors on ATP (adenosine-5′-triphosphate)/ADP (adenosine-5'-diphosphate) ratio and insulin secretory function of isolated human islets from cadaveric donor. Transplant Proc 41, 3813, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Lu Y., Jin X., Chen Y., et al. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct 28, 637, 2010 [DOI] [PubMed] [Google Scholar]
- 86. de Souza B.M., Bouças A.P., Oliveira F.D., et al. Effect of co-culture of mesenchymal stem/stromal cells with pancreatic islets on viability and function outcomes: a systematic review and meta-analysis. Islets 9, 30, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boumaza I., Srinivasan S., Witt W.T., et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 32, 33, 2009 [DOI] [PubMed] [Google Scholar]
- 88. Scuteri A., Donzelli E., Rodriguez-Menendez V., et al. A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS One 9, e84309, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chao K.C., Chao K.F., Chen C.F., and Liu S.H.. A novel human stem cell coculture system that maintains the survival and function of culture islet-like cell clusters. Cell Transplant 17, 657, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Karaoz E., Genc Z.S., Demircan P.C., Aksoy A., and Duruksu G.. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis 1, e36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Neve A., Cantatore F.P., Maruotti N., Corrado A., and Ribatti D.. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int 2014, 756078, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hsu Y.H., Moya M.L., Abiri P., Hughes C.C., George S.C., and Lee A.P.. Full range physiological mass transport control in 3D tissue cultures. Lab Chip 13, 81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hsu Y.H., Moya M.L., Hughes C.C., George S.C., and Lee A.P.. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 13, 2990, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Moya M.L., Hsu Y.H., Lee A.P., Hughes C.C., and George S.C.. In vitro perfused human capillary networks. Tissue Eng Part C Methods 19, 730, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shirure V.S., Bi Y., Curtis M.B., et al. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip 18, 3687, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nashimoto Y., Hayashi T., Kunita I., et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr Biol (Camb) 9, 506, 2017 [DOI] [PubMed] [Google Scholar]
- 97. Sano E., Mori C., Nashimoto Y., Yokokawa R., Kotera H., and Torisawa Y.S.. Engineering of vascularized 3D cell constructs to model cellular interactions through a vascular network. Biomicrofluidics 12, 042204, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Osaki T., Sivathanu V., and Kamm R.D.. Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci Rep 8, 5168, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Campisi M., Shin Y., Osaki T., Hajal C., Chiono V., and Kamm R.D.. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen X., Aledia A.S., Ghajar C.M., et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A 15, 1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Beattie G.M., Montgomery A.M., Lopez A.D., et al. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes 51, 3435, 2002 [DOI] [PubMed] [Google Scholar]
- 102. Riopel M., Trinder M., and Wang R.. Fibrin, a scaffold material for islet transplantation and pancreatic endocrine tissue engineering. Tissue Eng Part B Rev 21, 34, 2015 [DOI] [PubMed] [Google Scholar]
- 103. Fang H., Peng S., Chen A., Li F., Ren K., and Hu N.. Biocompatibility studies on fibrin glue cultured with bone marrow mesenchymal stem cells in vitro. J Huazhong Univ Sci Technolog Med Sci 24, 272, 2004 [DOI] [PubMed] [Google Scholar]
- 104. Shaikh F.M., Callanan A., Kavanagh E.G., Burke P.E., Grace P.A., and McGloughlin T.M.. Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 188, 333, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Gimbrone M.A., Anderson K.R., and Topper J.N.. The critical role of mechanical forces in blood vessel development, physiology and pathology. J Vasc Surg 29, 1104, 1999 [DOI] [PubMed] [Google Scholar]
- 106. Adams R.H., Wilkinson G.A., Weiss C., et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13, 295, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bonnefoy A., Harsfalvi J., Pfliegler G., Fauvel-Lafeve F., and Legrand C.. The subendothelium of the HMEC-1 cell line supports thrombus formation in the absence of von Willebrand factor and collagen types I, III and VI. Thromb Haemost 85, 552, 2001 [PubMed] [Google Scholar]
- 108. Salcedo R., Resau J.H., Halverson D., et al. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J 14, 2055, 2000 [DOI] [PubMed] [Google Scholar]
- 109. Wilson H.K., Canfield S.G., Shusta E.V., and Palecek S.P.. Concise review: tissue-specific microvascular endothelial cells derived from human pluripotent stem cells. Stem Cells 32, 3037, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Adams W.J., Zhang Y., Cloutier J., et al. Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports 1, 105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sivarapatna A., Ghaedi M., Le A.V., Mendez J.J., Qyang Y., and Niklason L.E.. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials 53, 621, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bezenah J.R., Kong Y.P., and Putnam A.J.. Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Sci Rep 8, 2671, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.