Abstract:
Current guidelines recommend the consideration of positive inotropes in patients with acute decompensated heart failure (ADHF) who have low cardiac index and evidence of systemic hypoperfusion or congestion. However, there is no evidence detailing the first line agent for the management of ADHF. The purpose of this study was to compare the safety and efficacy of dobutamine to milrinone for the treatment of ADHF. This was a single-center, retrospective study at a tertiary academic medical center, approved by Partner's Health Care Institutional Review Board. Patients included in this study were those admitted with ADHF who received dobutamine or milrinone from June 2015 to July 2017. A total of 95 dobutamine and 40 milrinone patients were included in the analysis. Median hospital length of stay was 12 days in the dobutamine group versus 10 days in the milrinone group (P = 0.34). Rehospitalization within 30 days occurred in 29.5% of patients in the dobutamine group versus 17.5% of patients in the milrinone group (P = 0.15). Median intensive care unit length of stay was 4.5 days in the dobutamine group versus 10 days in the milrinone group (P < 0.01). All other minor end points including all-cause mortality, progression to renal failure within 72 hours, rehospitalization in 90 days, and urine output within 72 hours of therapy were not found to be statistically significant. In addition, a post hoc analysis compared major and minor outcomes between milrinone and dobutamine using linear and logistic regression with adjustment for baseline characteristics. There were not any statistically significant findings in the post hoc analysis. Overall, there were no statistically significant differences in outcomes between the 2 groups other than longer intensive care unit length of stay in the milrinone group.
Key Words: acute decompensated heart failure, positive inotropes, dobutamine, milrinone
INTRODUCTION
Acute decompensated heart failure (ADHF) is the leading cause of hospitalizations in patients older than 65 years. More than 1 million hospitalizations are attributed to heart failure each year. There is an approximate 50% incidence of recurrent hospitalizations at 6 months and 20% within 30 days in this patient population. In addition, there is approximately a 30% risk of mortality within 1 year of being diagnosed with ADHF.1–3 Current guidelines recommend the short-term use of continuous positive inotropic agents in patients presenting with low blood pressure and significantly depressed cardiac output to maintain systemic perfusion and preserve end organ performance.1
Dobutamine and milrinone are 2 positive inotropic agents that have similar clinical effects yet different pharmacologic and pharmacokinetic profiles including mechanism of action and half-lives. Dobutamine is a beta1-adrenergic receptor agonist while milrinone is a phosphodiesterase 3 inhibitor, thus possessing more vasodilatory effects.3–6 Both dobutamine and milrinone have a rapid onset of action within 10 minutes (min) but differ in that dobutamine has a half-life of approximately 2 minutes while milrinone has a half-life of about 2.4 hours.6 Overall, there is limited and inconclusive evidence comparing the efficacy of milrinone versus dobutamine.7–9 The purpose of this study was to compare the safety and efficacy of dobutamine as compared to milrinone for the treatment of ADHF.
METHODS
Study Design and Data Collection
This was a single-center, retrospective study performed at a tertiary academic medical center. This study was approved by the Partner's Healthcare Institutional Review Board. All data were obtained from electronic medical records.
Patients were included if they were hospitalized for ADHF and received dobutamine or milrinone from June 2015 to July 2017. Patients were excluded if they had cardiac surgery during the same hospitalization, had a ventricular assist device, or if they received both milrinone and dobutamine during the same hospitalization. Patients were also excluded if they were concurrently receiving other agents with positive inotropic effects that included norepinephrine, epinephrine, and moderate- to high-dose dopamine, defined as doses >5 µg/kg/min.
Collected baseline characteristics included age, sex, race, weight, body mass index, left ventricular ejection fraction (>40% or ≤40%), and creatinine clearance calculated by the Cockcroft–Gault formula. Other data collected included medical history such as stroke or atrial fibrillation and home medications. Baseline data including troponin and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were collected when available. Of note, if troponin levels were less than 0.01 ng/mL, these values were counted as zero for statistical analysis. Loop diuretic doses administered within 72 hours (h) of positive inotrope initiation were collected in intravenous furosemide equivalents.
Study End Points
Major end points of this study included hospital length of stay and rehospitalization within 30 days. These 2 outcomes were chosen as major end points because they are considered key outcome measures for ADHF patients by the Center of Medicaid and Medicare Services.10 Minor end points included intensive care unit (ICU) length of stay, all-cause mortality within 30 days, urine output within 72 hours of therapy, rehospitalization within 90 days, and progression to renal failure within 72 hours. Renal failure was defined using the risk, injury, failure, loss of kidney function, and end-stage kidney disease (RIFLE) criteria.11 Patients were not considered to have progression to renal failure if they met RIFLE criteria before initiation of the study drug.
Safety end points included occurrence of new-onset tachycardia and new-onset arrhythmias with the absence of tachycardia or arrhythmias before initiation of dobutamine or milrinone. Other safety end points included symptomatic hypotension, symptomatic chest pain, and hypersensitivity or infusion reactions. All end points were assessed if they occurred within 72 hours of study drug initiation and if the patient did not have the condition before study drug initiation.
New-onset tachycardia was defined by a heart rate ≥110 beats/min with absence of tachycardia at baseline. New-onset arrhythmias were defined as a diagnosis by electrocardiogram or chart documentation of arrhythmias. Symptomatic hypotension was defined as documented systolic blood pressure ≤90 mm Hg with chart documentation of dizziness. Chest pain was defined by chart documentation of chest pain.
Statistical Analysis
Continuous data were compared using Student's t-test and Mann–Whitney U statistical testing as appropriate. Categorical data were compared using χ2 test and Fisher's exact test as appropriate. Statistical significance was set at a level of P < 0.05. The primary analysis was an unadjusted comparison of major, minor, and safety outcomes between milrinone and dobutamine. In addition, a post hoc analysis compared major and minor outcomes between milrinone and dobutamine using linear and logistic regression with adjustment for baseline characteristics that differed significantly in univariate analysis. Specifically, the regression models adjusted for age, sex, creatinine clearance, dyslipidemia, stroke, coronary artery disease, troponin, and NT-proBNP. Statistical analyses were performed using SPSS and STATA software.
RESULTS
Overall, 1385 patient charts were evaluated for inclusion, and 135 patients were included in the final analysis, 95 in the dobutamine group, and 40 in the milrinone group. Of the 1250 patients who were excluded, 859 received both dobutamine and milrinone within the same hospitalization, 185 received cardiac surgery within the same hospitalization, 110 received concurrent vasoactive agents, 51 had a ventricular assist device, and 45 were on home inotropic therapy (Fig. 1).
FIGURE 1.
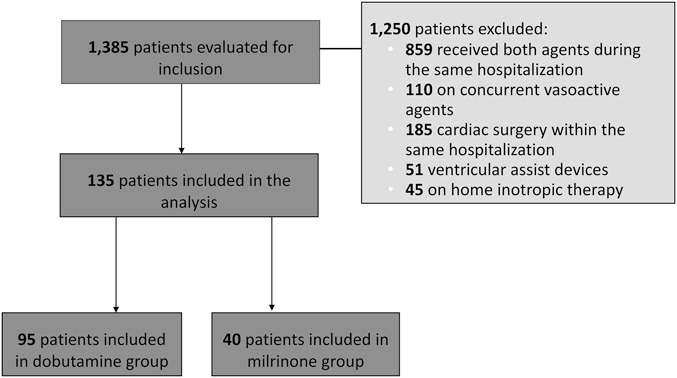
Patient Enrollment.
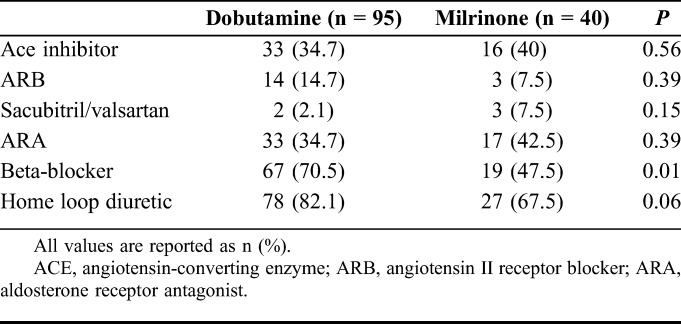
There was no statistically significant difference between sex, body mass index, or left ventricular ejection fraction between the 2 groups (Table 1). Patients in the dobutamine group had a higher median age [69 years; Interquartile range (IQR): 56.2–78.7] as compared to the milrinone group (58 years; IQR 49.3–64.5) (P < 0.01). Dobutamine patients also had a lower median creatinine clearance (35.5 mL/min; IQR: 20.6–48.0) as compared to the milrinone group (63.2 mL/min; IQR: 37.6–86.4) (P < 0.01). Patients in the dobutamine group had a higher incidence of coronary artery disease (55.8% vs. 35.0%, P = 0.03) and stroke (46.3% vs. 20%, P < 0.01) as compared to the milrinone group (Table 1). Home medications were similar between the 2 groups; however, more dobutamine patients were on beta-blockers at home compared with milrinone patients (70.5% vs. 47.5%, P = 0.01) (Table 2). This may be due to the higher incidence of atrial fibrillation in the dobutamine group (46.3% vs. 20%, P < 0.01).
TABLE 1.
Baseline Characteristics and Medical History
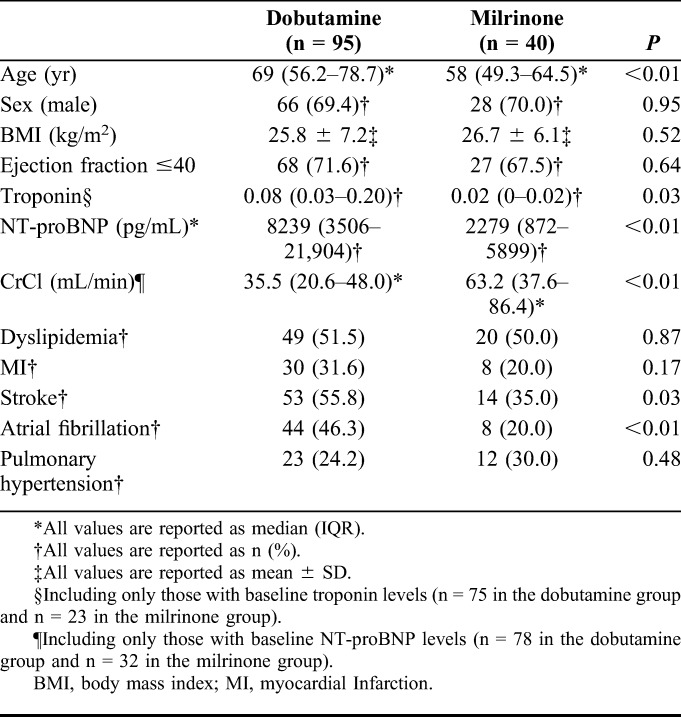
TABLE 2.
Home Medications
In patients who had troponin and NT-proBNP laboratory values available, those in the dobutamine group had significantly higher baseline troponin and NT-proBNP values when compared with the milrinone group. Median baseline troponin was 0.08 ng/mL (IQR: 0.03–0.20) in the dobutamine group versus 0.02 ng/mL in the milrinone group (IQR: 0–0.02) (P = 0.03). Median baseline NT-proBNP was 8239 pg/mL (IQR: 3506–21,904) in the dobutamine group versus 2279 pg/mL (IQR: 872–5899) in the milrinone group (<0.01). Doses of loop diuretics received within 72 hours of positive inotrope initiation were similar between groups.
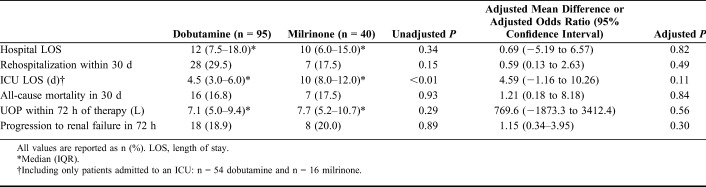
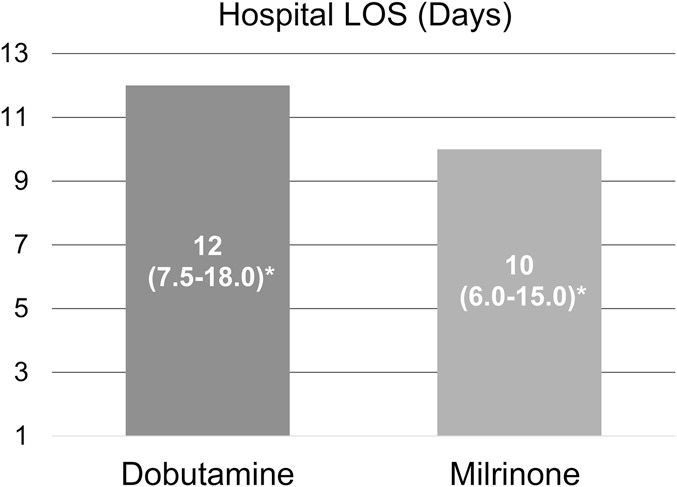
Both major end points were not significantly different between the milrinone and dobutamine groups (Fig. 2). Median hospital length of stay was 12 days (IQR: 7.5–18.0) in the dobutamine group versus 10 days in the milrinone group (IQR: 6.0–15.0) (unadjusted P = 0.34). Rehospitalization within 30 days occurred in 29.5% of patients in the dobutamine group versus 17.5% of patients in the milrinone group (P = 0.15). In patients who were admitted to the ICU, median ICU length of stay was 4.5 days (IQR: 3.0–6.0) in the dobutamine group versus 10 days in the milrinone group (IQR: 8.0–12.0) (P < 0.01). There were similar outcomes in all other minor end points between the 2 groups.
FIGURE 2.
Major End Points.
When the post hoc linear and logistic regressions were performed adjusting for differences in baseline characteristics, there were no significant differences found in any major or minor outcomes (Table 3). All safety outcomes were similar between the 2 groups (Table 4).
TABLE 3.
Major, Minor, and Safety End Points
TABLE 4.
Safety End Points
DISCUSSION
In our retrospective study assessing the efficacy and safety of dobutamine versus milrinone in ADHF patients who have significantly depressed cardiac output with systemic hypoperfusion, we found no differences in major outcomes. There are, however, many differences in baseline patient characteristics. Patient demographics often drive prescribing practices and may help to explain certain trends in agent selection and differences in outcomes. The median age of patients in the dobutamine group was 11 years higher than the age of patients in the milrinone group. Age has been directly correlated with increased risk of mortality in ADHF patients; however, this was not observed in our study.12 Older age or lower blood pressure may have driven the clinician to use dobutamine because of its faster onset of action and shorter duration, making it easier to titrate and respond to hemodynamic changes. As milrinone is renally eliminated, both the package insert and institution-specific guidelines suggest using caution when prescribing milrinone in patients with impaired renal function. In our study, the dobutamine group had a lower baseline creatinine clearance as compared to the milrinone group which coincides with current recommendations.3 As milrinone is renally eliminated, significant renal dysfunction may have led clinicians to choose dobutamine over milrinone. Dobutamine patients had a higher incidence of history of stroke and coronary artery disease, as well as higher troponin and NT-proBNP levels at baseline when compared with the milrinone group. Both coronary artery disease and stroke have been associated with worse outcomes in ADHF patients, such as increased risk of hospitalization and mortality.13,14 Previous literature also suggests that higher BNP levels predict worsening prognosis in ADHF. Therefore, these factors may lead dobutamine patients to be more susceptible to worse outcomes at baseline.15
Our patient selection criteria were comparable with other retrospective studies assessing dobutamine and milrinone in patients with ADHF.7,9 Similarly to these previous studies, we excluded patients who received both agents during the same hospitalization. Although using a combination of both agents is a common strategy, there is a lack of evidence regarding dual inotropic therapy as compared to the use of a single agent. Our study excluded patients on both agents so that we could investigate the safety and efficacy profiles of one individual agent over the other. One major difference in our patient population compared with other studies was that we also excluded patients on concurrent vasoactive agents. By excluding these patients, we eliminated those who presented with a mixed shock picture as opposed to ADHF with a cold and wet profile.16 These vasoactive agents, such as epinephrine, also have positive inotropic effects, and concurrent administration may interfere with evaluating the efficacy of dobutamine or milrinone. Previous studies comparing dobutamine and milrinone in patients with ADHF did not specifically exclude these patients, which may explain some of the differences in our results.7–9
Although there were no statistically significant differences found in either major outcome, there was a trend toward a longer overall hospital length of stay and higher incidence of rehospitalization within 30 days in the dobutamine group. The aforementioned baseline patient characteristics may also be contributing factors in these trends. Our results were similar to another single-center retrospective review which compared the clinical efficacy and economic costs of dobutamine-based (n = 269) versus milrinone-based (n = 60) therapy in patients with ADHF. This study found no difference in mortality or hospital length of stay between these 2 groups, although there was significantly reduced direct drug costs in the dobutamine arm. Of note, this study consisted only of patients with an ejection fraction of 35% or less, whereas our study did not exclude patients based on ejection fraction. This study stated that they excluded patients with systemic illness, cardiogenic shock, and sepsis; however, they did not specifically comment on use of vasopressors.9
Milrinone patients were found to have a statistically longer ICU length of stay as compared to the dobutamine group in the unadjusted primary analysis. Of note, when controlling for differences in baseline characteristics, there was no difference found in ICU length of stay. Given the increased age, higher incidence of comorbidities, and elevated baseline NT-proBNP of the dobutamine group, this is an unexpected finding that warrants further investigation. Our post hoc regression analysis results were similar to another retrospective analysis of 15,230 patients from the Acute Decompensated Heart Failure National Registry (ADHERE). This study showed no difference in ICU length of stay when comparing patients who received dobutamine versus those who received milrinone.8
No statistically significant differences were found in any other minor outcomes, including all-cause mortality in 30 days. Our study differed with another retrospective cohort study comparing out of hospital mortality in ADHF patients who received dobutamine (n = 306) or milrinone (n = 194). In this study, dobutamine was associated with increased heart failure-associated out of hospital mortality rates compared with milrinone, and a higher incidence of all-cause mortality in the first 2 weeks after hospital discharge. Of note, 53.9% and 59.8% of the dobutamine and milrinone patients were on vasopressors (norepinephrine or epinephrine), respectively.7 As mentioned above, these medications also have positive inotropic effects and therefore may interfere with analyzing the effects of dobutamine and milrinone. Our mortality results also differed from the analysis of the ADHERE registry. Patients who received dobutamine were 24% more likely to experience in-hospital mortality as compared to milrinone.8 Explanations for any differences between dobutamine or milrinone in this study or any previous studies are unknown.
There are also several small studies that compare the use of these 2 agents in cardiac surgery populations and patients awaiting heart transplant while our study specifically excluded these patient populations, allowing for a more homogeneous population of ADHF patients.17,18 Given the large burden that ADHF poses on the health care system and the lack of current evidence, we have chosen to explore patients hospitalized for ADHF specifically.
There were several limitations to this study. This was a retrospective chart review with a small sample size of 135 patients. In addition, several outcomes of this study could have been underestimated due to loss of follow-up, including hospital readmission rates or all-cause mortality. There was not a specific protocol or algorithm to determine which inotropic agent to use; therefore, the choice of agent was based on physician preference. Clinicians most likely chose an agent based on the pharmacologic characteristics of the medication in addition to the hemodynamic profile of their patients. In addition, location of training and overall physician experience with either agent may affect their decision, as well as several patient-specific considerations such as renal function or hemodynamics. There may have been other unknown confounding factors that could have affected the patient outcomes that were not initially captured, such as the stage of heart failure. Baseline blood pressure was not collected in this study which may have provided further insight into prescriber preferences of dobutamine or milrinone. Finally, there were several notable differences in baseline characteristics of the 2 groups as outlined above. Perhaps, using different patient characteristics in guiding the selection of dobutamine versus milrinone will overall provide similar outcomes.
CONCLUSIONS
This study did not demonstrate any significant difference between dobutamine and milrinone other than a longer ICU length of stay in the milrinone group. When adjusting for differences in baseline characteristics, there were no significant difference between the 2 groups. This is mostly consistent with previous studies comparing these agents. Further evaluation of the optimal strategy in choosing positive inotropic agents in larger, prospective trials may be necessary.
ACKNOWLEDGMENTS
Leo Buckley, PharmD.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC. The Acute Decompensated Heart Failure National Registry (ADHERE™): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(suppl 7):S21–S30. [PubMed] [Google Scholar]
- 3.Scott MC, Winters ME. Congestive heart failure. Emerg Med Clin North Am. 2015;33:553–562. [DOI] [PubMed] [Google Scholar]
- 4.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. [DOI] [PubMed] [Google Scholar]
- 5.Dobutamine Hydrochloride in 5% Dextrose Injection Package Insert. Deerfield, IL: Baxter Healthcare Corp.; 2014. [Google Scholar]
- 6.Primacor (Milrinone Injection) Package Insert. Bridgewater, NJ: Sanofi-Aventis US LLC; 2006. [Google Scholar]
- 7.King JB, Shah RU, Sainski-Nguyen A, et al. Effect of inpatient dobutamine versus milrinone on out-of-hospital mortality in patients with acute decompensated heart failure. Pharmacotherapy. 2017;37:662–672. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. [DOI] [PubMed] [Google Scholar]
- 9.Yamani MH, Haji SA, Starling RC, et al. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: hemodynamic efficacy, clinical outcome, and economic impact. Am Heart J. 2001;142:998–1002. [DOI] [PubMed] [Google Scholar]
- 10.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira A, Parenica J, Park JJ, et al. ; GREAT (Global Research on Acute Conditions Team) Network. Clinical presentation and outcome by age categories in acute heart failure: results from an international observational cohort. Eur J Heart Fail. 2015;17:1114–1123. [DOI] [PubMed] [Google Scholar]
- 13.Purek L, Laule-Kilian K, Christ A, et al. Coronary artery disease and outcome in acute congestive heart failure. Heart. 2006;92:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pongmoragot J, Lee DS, Park TH, et al. ; Investigators of the Registry of the Canadian Stroke Network; University of Toronto Stroke Program for the Stroke Outcomes Research Canada (SORCan— www.sorcan.ca) Working Group. Stroke and heart failure: clinical features, access to care, and outcomes. J Stroke Cerebrovasc Dis. 2016;25:1048–1056. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai S, Adachi H, Hasegawa A, et al. Brain natriuretic peptide facilitates severity classification of stable chronic heart failure with left ventricular dysfunction. Heart. 2003;89:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah MR, Hasselblad V, Stinnett SS, et al. Hemodynamic profiles of advanced heart failure: association with clinical characteristics and long-term outcomes. J Card Fail. 2001;7:105–113. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen DV, Torp-Pedersen C, Skals RK, et al. Intraoperative milrinone versus dobutamine in cardiac surgery patients: a retrospective cohort study on mortality. Crit Care. 2018;22:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aranda JM, Jr, Schofield RS, Pauly DF, et al. Comparison of dobutamine versus milrinone therapy in hospitalized patients awaiting cardiac transplantation: a prospective, randomized trial. Am Heart J. 2003;145:324–329. [DOI] [PubMed] [Google Scholar]