BACKGROUND:
Several studies of critically ill patients reported that fluid resuscitation with hydroxyethyl starch (HES) solutions damages the kidneys, but their use for surgical patients is debated. Because different HES preparations have different safety profiles, we sought to determine whether 6% third-generation HES 130/0.4 was associated with renal morbidity when used for surgical patients.
METHODS:
We identified adults enrolled in a Japanese nationwide medical database who underwent surgery between 2014 and 2016, with HES 130/0.4 or without it (controls). These groups were balanced with propensity score matching in a 1:1 ratio without replacement by multivariable logistic regression with 36 covariates, including demographic characteristics, preoperative comorbidities, and anesthetic/surgical procedures. The primary outcome was the incidence of acute kidney injury (AKI) in patients receiving intraoperative HES and controls. Secondary outcomes were assessing whether HES was associated with worsening AKI stage, the incidence of renal-replacement therapy (RRT), hospital length-of-stay, and in-hospital 30-day mortality. Tertiary outcomes include the use of vasoactive agents and the fluid requirement on the day of surgery. Comparative analysis was made with χ2, Mann-Whitney U test, or the ordinal logistic regression analysis.
RESULTS:
Of 76,048 patients in the database, 58,425 were eligible: 9542 received HES and 48,883 controls. Propensity score matching identified 8823 matched pairs. The incidence of AKI was 6.2% (548/8823) in the HES group and 5.6% (492/8823) in controls (odds ratio [OR], 1.12; 95% confidence interval [CI], 0.99–1.27; P = .07). Compared to controls, HES was not associated with worsening AKI stage (OR, 0.89; 95% CI, 0.79–1.01; P = .08). The incidence of RRT was lower in the HES group than that in controls (0.2% vs 0.4%, respectively; OR, 0.51; 95% CI, 0.29–0.91; P = .02). Median [interquartile range] hospital stay was 1 day longer in the HES group (12 [8–21] vs 11 [7–20] days; P < .001), but in-hospital 30-day mortality did not differ between groups (0.5% vs 0.6%, respectively: OR, 0.83; 95% CI, 0.56–1.24; P = .36). The use rate of vasoactive agents and the median net fluid requirement on the day of surgery were higher in the HES group (80.5% vs 70.0%: P < .001, 88.1 vs 73.6 mL/kg; P < .001, respectively) compared to controls.
CONCLUSIONS:
The present study did not demonstrate that 6% HES 130/0.4 increased the incidence and the severity of postoperative AKI. It was associated with a lower incidence of RRT when used for surgical patients.
KEY POINTS.
Question: Is 6% third-generation hydroxyethyl starch (HES) 130/0.4 associated with postoperative renal morbidity?
Findings: 6% HES 130/0.4 was not associated with increased odds of acute kidney injury, but was associated with decreased odds of renal-replacement therapy.
Meaning: 6% HES 130/0.4 can be safely used as a volume expander for surgical patients.
See Article, p 1616
Fluid resuscitation with hydroxyethyl starch (HES) has been associated with renal damage in critically ill patients.1–3 The Crystalloid versus Hydroxyethyl Starch Trial (CHEST) study was a randomized controlled trial (RCT) of 7000 critically ill patients in an intensive care unit treated with a third-generation, waxy maize-derived solution of 6% HES 130/0.4 (the same formulation we tested) or with normal saline (controls). HES was associated with an increased need for renal-replacement therapy (RRT), despite the fact that 90-day mortality and the incidence of acute kidney injury (AKI) were as good as or better than those in patients treated with normal saline.3
Many of the most common colloid and crystalloid solutions were in widespread use before drugs began to require regulatory approval in the 1970s.4 As a result, safety data for these solutions were limited until the late 1990s,4 at which time evidence began showing that HES was often associated with important adverse events.1–8 In 2013, the Food and Drug Administration believed this evidence to be strong enough that it issued a Black Box warning for the use of HES for critically ill patients.9
Evaluating the safety of HES should address 2 concerns. First, the many formulations of HES have different basic ingredients, such as maize or potato, different concentrations and solvents, different mean molecular weights, different degrees of molar substitution, and different C2/C6 ratios (the carbon ratio, which affects the pattern of hydroxylethylation). Each of these characteristics has pharmacokinetic implications.10 However, despite these differences, studies on the safety of HES rarely identify the specific formulation used, and meta-analyses have not differentiated between the various formulations when evaluating the safety of HES, which may confound the results.7,8 Thus, the safety profile of third-generation HES 130/0.4 may be different from that of the older formulations of HES.10 Second, the pathophysiological conditions of patients in the operating room differ from those in an intensive care unit. We, anesthesiologists in Japan, have been using HES for simple hypovolemic hypotension caused by bleeding or anesthetic vasodilation during surgery with no apparent problems. In the intensive care unit, however, we use it more carefully and longer for more complex pathological conditions than we do in the operating room. Many studies have found no association between HES 130/0.4 and kidney damage in surgical patients.11–16
Given our experience and the results of the previous studies, we hypothesized that the third-generation HES 130/0.4 would not be associated with adverse renal effects in surgical patients. Then, we sought to determine whether HES 130/0.4 was associated with postoperative renal morbidity and mortality, especially with the incidence and the severity of AKI and RRT in a large retrospective cohort study.
METHODS
The study protocol was reviewed by the Toho University Ohashi Medical Center institutional review board (Ref: H16105), which waived formal approval and the requirement for written informed consent because the data were fully deidentified. The study protocol was registered with the UMIN Clinical Trial Registry of the Japanese University Hospital Medical Information Network on June 30, 2017 (http://www.umin.ac.jp/ctr/index-j.htm: registry number: UMIN000027896).
Study Design and Data Sources
We conducted a retrospective propensity-matched cohort study using data collected between January 2014 and December 2016 by the Japanese Medical Database for Healthcare Reimbursement (the Diagnosis Procedure Combination/Per-Diem Payment System).17 This nationwide database contains data on patient demographic information and clinical characteristics, including primary diagnosis, comorbidities, complications during hospitalization, medical interventions (including type of surgery, type and amount of fluid support, and prescribed transfusions), expenditures, and outcomes. However, because the full database does not include laboratory values, we used a subset of the database maintained by Medical Data Vision Corporation (Tokyo, Japan) that also contains laboratory values for 16,870,000 patients, from about 16% of the acute care hospitals in Japan.
Study Population
Eligible patients were hospitalized for surgery between January 2014 and December 2016, were at least 18 years old, had undergone general or regional anesthesia or both, and had both preoperative and postoperative serum creatinine concentrations recorded in the database. Patients were assigned to the HES group if they received any amount of HES 130/0.4 on the day of surgery and to the control group if they had not received HES 130/0.4 on the day of surgery. Patients were excluded if they received: either dextran or any HES formulations other than HES 130/0.4 during their hospital stay; HES 130/0.4, albumin, or any blood products during the 7 days before surgery; and HES 130/0.4 before or only after the day of surgery. We excluded patients with stage 5 chronic kidney disease (defined as an estimated glomerular filtration rate (eGFR)18 <15 mL·minute−1·1.73 m−2 or dialysis-dependent19), who died within 2 days after surgery, or who underwent multiple surgeries within 30 days before or after the index surgery. Patients who underwent surgery at university hospitals were not included because data collected from university hospitals during the study period did not include laboratory values, including serum creatinine concentrations, which are necessary to diagnose AKI. The US Food and Drug Association has not approved HES for patients with sepsis, but patients with preoperative septicemia were included because the use of HES for these patients is not contraindicated in Japan.
Statistical Methods
Data are summarized as medians and interquartile ranges or as numbers and percentages. We adjusted for the following variables as covariates in the following analyses: patient demographic characteristics, preoperative comorbidities, types of surgery, anesthetic methods and the duration of anesthesia, and transfusion requirements (Table 1). The preoperative comorbidities in the covariates were identified from a study reporting that these comorbidities were risk factors for AKI.20 Comorbidity data were extracted from the database with the International Classification of Disease, 10th Revision codes (Table 2).
Table 1.
Covariates Used in Propensity Score Matching in a Study of the Renal Morbidity of 6% HES 130/0.4 Administered on the Day of Surgery
| Covariate | Level of Measurement |
|---|---|
| Age, y | Continuous |
| Male sex | Binary |
| Body mass index, kg/m2 | Continuous |
| Hospital capacity | Ordinal |
| <200 beds | |
| 200–499 beds | |
| ≥500 beds | |
| Year of treatment | Ordinal |
| 2014 | |
| 2015 | |
| 2016 | |
| Preoperative serum creatinine, mg/dL | Continuous |
| Preoperative radiocontrast use | Binary |
| Emergency surgery | Binary |
| Preoperative comorbidities | |
| Myocardial infarction | Binary |
| Congestive heart failure | Binary |
| Peripheral arterial disease | Binary |
| Cerebrovascular disease | Binary |
| Chronic obstructive lung disease | Binary |
| Chronic liver disease | Binary |
| Portal hypertension | Binary |
| Ascites | Binary |
| Diabetes mellitus | Binary |
| Malignancy | Binary |
| Arrhythmia | Binary |
| Valvular heart disease | Binary |
| Hypertension | Binary |
| Chronic kidney disease | Binary |
| Anemia | Binary |
| Septicemia | Binary |
| Types of surgerya | |
| Cardiovascular with CPB | Binary |
| Cardiovascular without CPB | Binary |
| Open thoracic | Binary |
| Open gastrointestinal | Binary |
| Open hepatobiliary | Binary |
| Open orthopedic | Binary |
| Open gynecologic/urologic/obstetric | Binary |
| Craniotomy | Binary |
| Miscellaneous | Binary |
| Anesthetic management | |
| Anesthesia duration, min | Continuous |
| Anesthetic method | Categorical |
| General anesthesia | |
| Regional anesthesia | |
| General with regional anesthesia | |
| Transfusion volume on the day of surgery, mL | Ordinal |
| No transfusion | |
| 1–500 | |
| 501–1000 | |
| >1000 |
Abbreviations: CPB, cardiopulmonary bypass; HES, hydroxyethyl starch.
aTypes of surgery were counted as binary because some patients received multiple types of surgery on the day of surgery.
Table 2.
International Classification of Disease, 10th Revision, Preoperative Comorbidity Codes in a Study of the Renal Morbidity of 6% HES 130/0.4 Administered on the Day of Surgery
| Myocardial infarction | I21.x, I22.x, I25.2 |
| Congestive heart failure | I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5-I42.9, I43.x, I50.x, P29.0 |
| Peripheral arterial disease | I70.x, I71.x, I73.1, I73.8, I73.9, I77.1,I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 |
| Cerebrovascular disease | G45.x, G46.x, H34.0, I60.x-I69.x |
| Chronic obstructive lung disease | I27.8, I27.9, J40.x-J47.x, J60.x-J67.x, J68.4, J70.1, J70.3 |
| Chronic liver disease | B18.x, K70.0-K70.3, K70.9, K71.3-K71.5, K71.7, K73.x, K74.x, K76.0, K76.2-K76.4, K76.8, K76.9, Z94.4, I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K72.1, K72.9, K76.5, K76.6, K76.7 |
| Portal hypertension | K766 |
| Ascites | A183, C786, I898, N289, R18 |
| Diabetes mellitus | E10.0, E10.1, E10.6, E10.8, E10.9,E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9, E10.2-E10.5, E10.7, E11.2-E11.5, E11.7, E12.2-E12.5, E12.7, E13.2-E13.5, E13.7, E14.2-E14.5, E14.7 |
| Malignancy | C00.x-C26.x, C30.x-C34.x, C37.x-C41.x, C43.x, C45.x-C58.x, C60.x-C76.x, C81.x-C85.x, C88.x, C90.x-C97.x, C77.x-C80.x |
| Arrhythmia | I44.1-I44.3, I45.6, I45.9, I47.x-I49.x, R00.0, R00.1, R00.8, T82.1, Z45.0, Z95.0 |
| Valvular heart disease | A52.0, I05.x-I08.x, I09.1, I09.8, I34.x-I39.x, Q23.0-Q23.3, Z95.2-Z95.4 |
| Hypertension | I10.x, I11.x-I13.x, I15.x |
| Chronic kidney disease | I12.0, I13.1, N03.2-N03.7, N05.2-N05.7, N18.x, N19.x, N25.0, Z49.0-Z49.2, Z94.0, Z99.2 |
| Anemia | D50.0, D50.8, D50.9, D51.x-D53.x |
| Septicemia | A02.1, A20.7, A22.7, A24.1, A26.7, A28.8. A32.7, A39.4, A40.x, A41.x, A42.7, A54.8, B00.7, B34.9, B37.7 |
Abbreviation: HES, hydroxyethyl starch.
To deal with potential confounders, we used propensity score (PS) matching.21 We used multivariable logistic regression to model receiving HES 130/0.4 administration (versus not) on the day of surgery as a function of the 36 covariates in Table 1, and used the model to estimate the PS for each patient as the probability of receiving HES 130/0.4 on the day of surgery. The nearest-neighbor matching method was used, and 1:1 matching was performed with the “without replacement” sampling method. Specifically, each patient who received HES was paired with a patient in the control group with the descending method (which means that the pairing begins with a patient with the highest PS in the HES group to the lowest). That is, a patient in the control cohort was matched with a patient in the HES group having the nearest PS and was selected if the caliper was within 0.2 of the standard deviation of the PS logit. The caliper range is the maximum tolerated difference between patients matched on a covariate. A caliper within 0.2 of the standard deviation of the PS logit is widely accepted as appropriate.21 Patients who could not be matched were not included when assessing the relationship between HES and outcome.
To examine the balance of baseline variables between the cohorts, the standardized difference22 (the difference in means or proportions divided by the pooled standard deviation) was calculated before and after PS matching.23 When the standardized difference was <10%, we considered the groups to be balanced on the covariate.24
Assessing the Association Between HES and Outcomes
The primary outcome was the incidence of AKI within 7 days after surgery in the HES and control groups, where AKI was defined by the serum creatinine concentration set by the Kidney Disease: Improving Global Outcomes criteria.25 Only the creatinine criterion was applied because of the lack of urine output data in the database. Thus, stage 1 AKI was defined as an increase in creatinine concentration that was 1.5–1.9 times the baseline preoperative concentration or by an absolute increase of 0.3 mg/dL from baseline. Likewise, stage 2 was defined as a relative increase of 2.0–2.9 times baseline, and stage 3, as a relative increase of 3 times the baseline value, an absolute increase of 4.0 mg/dL from baseline, or initiation of RRT within 7 days after surgery.25 The age-limit criterion (<18-year old) in the Global Outcomes statement was not used because it was an exclusion criterion.
Secondary outcomes were assessing whether HES was associated with worsening AKI stage, the incidence of RRT initiated within 21 days after surgery, postoperative in-hospital 30-day mortality, and length of postoperative hospital stay with the Hodges-Lehman median difference. The postoperative days of patients who died in the charged hospital were not counted in the length of hospital stay. Death after hospital discharge could not be detected because the database contained no data after discharge. Anesthetic and postoperative management variables; the use of vasoactive agents (ephedrine, phenylephrine, dopamine, dobutamine, norepinephrine, or epinephrine); net fluid requirement; the infusion volume of crystalloid and albumin; and the number of patients who received albumin on the day of surgery were reported as the tertiary outcomes.
We assessed the interaction between HES infusion and the following 4 risk factors on the incidence of AKI: different amounts of HES (1–20, 21–40, >40 mL/kg), cardiac or noncardiac surgery, 3 levels of preoperative eGFR (≥60, 45–59, and 15–44 mL·minute−1·1.73 m−2), and presence or absence of preoperative septicemia (if listed as a preoperative comorbidity in the database). Patients in the 3 subsets of different amounts of HES were compared to controls who did not receive HES but who were otherwise matched in the PS analysis to the patients receiving HES.
Outcomes were compared between groups after PS matching was performed. Categorical variables were analyzed with χ2 tests. Continuous variables were analyzed with Mann-Whitney U tests because Kolmogorov-Smirnov tests found no normal distributions. To assess whether HES was associated with worsening AKI stage, we performed ordinal logistic regression analysis on the ordinal variable defined as stage 0 (no AKI), 1, 2, and 3 of AKI. Interactions between the risk factors and HES infusion on the incidence of AKI were assessed with the Breslow-Day test.
The sample size was the total number of eligible patients seen during the 3-year study period; no a priori sample size calculation was performed. However, based on an expected incidence of AKI of 5% in the control group, our sample size (8823 pairs) had 83% power to detect a 1% absolute difference in the incidence of AKI between the HES and control group at the 0.05 level.
Data were analyzed with the SAS software program (SAS ver9.4 TS1M6, SAS Institute, Cary, NC), which contains the new SAS official macroprogram for PS matching procedure. Alpha was set at .05, and all tests were 2-tailed.
RESULTS
Among the 16,870,000 patients in the Medical Data Vision database, 76,048 patients were treated surgically under general or regional anesthesia, or both, and had data on perioperative serum creatinine concentrations. Of these, 58,425 met the eligibility criteria: 9542 (16%) who received HES 130/0.4 and 48,883 (84%) who had not. Of these, 8823 patients receiving HES were successfully matched with an equal number of controls (Figure 1).
Figure 1.
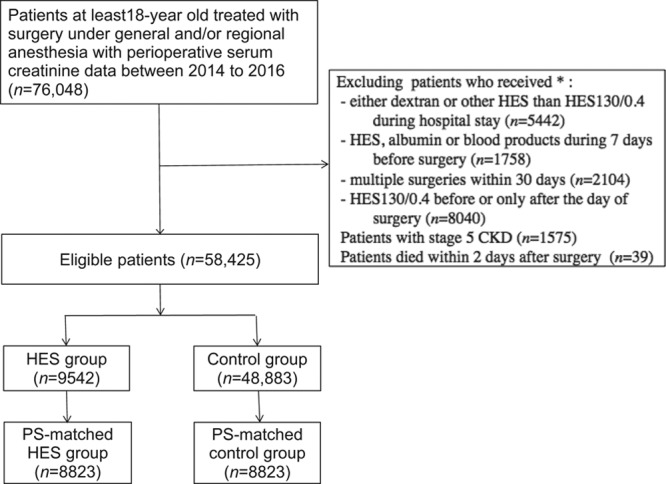
Sample selection process for a propensity-matched study of patients receiving fluid replacement on the day of surgery with or without 6% HES 130/0.4 to determine any associations between HES and the incidence of acute kidney injury. *Multiple exclusion criteria were applied because some patients met 2 or more exclusion criteria. CKD indicates chronic kidney disease; HES, hydroxyethyl starch; PS, propensity score.
The standardized differences of the covariates before and after matching show that the markedly heterogeneous cohorts before matching became relatively homogeneous after matching; that is, the number of covariates with standardized differences exceeding 10% was reduced from 18 to 0 after matching (Table 3). Patient background (demographic characteristics, preoperative creatinine concentrations, rate of radiocontrast use, and comorbidities), hospital size and year of treatment, and intraoperative variables (type of surgery, anesthesia duration and technique, and transfusion) were all balanced after matching.
Table 3.
Standardized Differences of Covariates Before and After Propensity Score Matching in a Study of the Renal Morbidity of 6% HES 130/0.4 Administered on the Day of Surgery
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Covariate | HES Group (n = 9542) | Control Group (n = 48,883) | Standardized Difference % | HES Group (n = 8823) | Control Group (n = 8823) | Standardized Difference % |
| Age, median (IQR), y | 68 (56–77) | 66 (51–76) | 10.7 | 68 (55–77) | 68 (55–76) | 1.6 |
| Male sex, n (%) | 4639 (48.6) | 20,997 (43.0) | 11.4 | 4179 (47.4) | 4104 (46.5) | 1.7 |
| BMI, median (IQR), kg/m2 | 22.9 (20.5–25.4) | 22.9 (20.5–25.6) | 2.2 | 22.9 (20.5–25.5) | 22.9 (20.5–25.5) | 0.3 |
| Hospital capacity, n (%) | 23.1 | 1.2 | ||||
| <200 beds | 129 (1.4) | 1200 (2.5) | 120 (1.4) | 451 (5.1) | ||
| 200–499 beds | 6936 (72.7) | 29,230 (59.8) | 6392 (72.4) | 5865 (66.5) | ||
| ≥500 beds | 2477 (26.0) | 18,453 (37.7) | 2311 (26.2) | 2507 (28.4) | ||
| Year of treatment, n (%) | 30.4 | 1.4 | ||||
| 2014 | 1310 (13.7) | 13,891 (28.4) | 1250 (14.2) | 1633 (18.5) | ||
| 2015 | 3828 (40.1) | 16,874 (34.5) | 3584 (40.6) | 2882 (32.7) | ||
| 2016 | 4404 (46.2) | 18,118 (37.1) | 3989 (45.2) | 4308 (48.8) | ||
| Preoperative sCr, median (IQR), mg/dL | 0.70 (0.57–0.85) | 0.69 (0.57–0.85) | 3.0 | 0.69 (0.56–0.85) | 0.69 (0.57–0.84) | 1.1 |
| Received preoperative radiocontrast, n (%) | 524 (5.5) | 3248 (6.6) | 4.8 | 480 (5.4) | 491 (5.6) | 0.5 |
| Preoperative comorbidities, n (%) | ||||||
| Myocardial infarction | 333 (3.5) | 940 (1.9) | 9.7 | 278 (3.2) | 252 (2.9) | 1.7 |
| Congestive heart failure | 1035 (10.8) | 4164 (8.5) | 7.9 | 907 (10.3) | 910 (10.3) | 0.1 |
| Peripheral artery disease | 880 (9.2) | 2565 (5.2) | 15.4 | 763 (8.6) | 744 (8.4) | 0.8 |
| Cerebrovascular disease | 999 (10.5) | 4648 (9.5) | 3.2 | 897 (10.2) | 903 (10.2) | 0.2 |
| COPD | 908 (9.5) | 4093 (8.4) | 4.0 | 815 (9.2) | 833 (9.4) | 0.7 |
| Chronic liver disease | 1110 (11.6) | 4945 (10.1) | 4.9 | 984 (11.2) | 961(10.9) | 0.8 |
| Portal hypertension | 7 (0.1) | 9 (0.0) | 2.6 | 4 (0.0) | 4 (0.0) | 0.0 |
| Ascites | 336 (3.5) | 1310 (2.7) | 4.9 | 294 (3.3) | 296 (3.4) | 0.1 |
| Diabetes mellitus | 2632 (27.6) | 11,508 (23.5) | 9.3 | 2370 (26.9) | 2364 (26.8) | 0.2 |
| Malignancy | 4536 (47.5) | 17,625 (36.1) | 23.4 | 4064 (46.1) | 4010 (45.4) | 1.2 |
| Arrhythmia | 910 (9.5) | 5079 (10.4) | 2.8 | 840 (9.5) | 867 (9.8) | 1.0 |
| Valvular heart disease | 945 (9.9) | 3266 (6.7) | 11.7 | 851 (9.6) | 853 (9.7) | 0.1 |
| Hypertension | 3632 (38.1) | 16,774 (34.3) | 7.8 | 3270 (37.1) | 3301 (37.4) | 0.7 |
| Chronic kidney disease | 208 (2.2) | 994 (2.0) | 1.0 | 181 (2.1) | 180 (2.0) | 0.1 |
| Anemia | 1622 (17.0) | 5539 (11.3) | 16.3 | 1442 (16.3) | 1509 (17.1) | 2.0 |
| Septicemia | 172 (1.8) | 629 (1.3) | 4.2 | 150 (1.7) | 168 (1.9) | 1.5 |
| Types of surgerya, n (%) | ||||||
| Cardiovascular with CPB | 252 (2.6) | 251 (0.5) | 17.1 | 203 (2.3) | 181 (2.1) | 1.7 |
| Cardiovascular without CPB | 521 (5.5) | 707 (1.4) | 22.1 | 435 (4.9) | 393 (4.5) | 2.3 |
| Open thoracic | 106 (1.1) | 340 (0.7) | 4.4 | 94 (1.1) | 88 (1.0) | 0.7 |
| Open gastrointestinal | 1507 (15.8) | 3727 (7.6) | 25.6 | 1355 (15.4) | 1388 (15.7) | 1.0 |
| Open hepatobiliary | 895 (9.4) | 1222 (2.5) | 29.4 | 690 (7.8) | 610 (6.9) | 3.5 |
| Open orthopedic | 1995 (20.9) | 13,359 (27.3) | 15.1 | 1939 (22.0) | 2050 (23.2) | 3.0 |
| Open gynecologic/urologic/obstetric | 1835 (19.2) | 4009 (8.2) | 32.5 | 1691 (19.2) | 1799 (20.4) | 3.1 |
| Craniotomy | 189 (2.0) | 839 (1.7) | 2.0 | 183(2.1) | 199 (2.3) | 1.2 |
| Miscellaneous | 2619 (27.4) | 24,899 (50.9) | 49.6 | 2533 (28.7) | 2405 (27.3) | 3.2 |
| Emergency surgery, n (%) | 240 (2.5) | 955 (2.0) | 3.8 | 224 (2.5) | 224 (2.5) | 0.0 |
| Anesthesia duration, median (IQR), min | 227 (152–325) | 142 (97–206) | 74.3 | 218 (148–305) | 206 (138–296) | 4.3 |
| Anesthesia technique, n (%) | 42.4 | 3.7 | ||||
| General anesthesia | 4947 (51.8) | 32,661 (66.8) | 4632 (52.5) | 4655 (52.8) | ||
| Regional anesthesia | 768 (8.0) | 6055 (12.4) | 762 (8.5) | 746 (8.5) | ||
| Both general and regional | 3827 (40.1) | 10,167 (20.8) | 3429 (38.9) | 3422 (38.8) | ||
| Transfusion on day of surgery, n (%) | 45.1 | 9.2 | ||||
| No transfusion | 8059 (84.5) | 47,470 (97.1) | 7800 (88.4) | 8048 (91.2) | ||
| 1–500, mL | 444 (4.7) | 751 (1.5) | 387 (4.4) | 271 (3.1) | ||
| 501–1000, mL | 321 (3.4) | 296 (0.6) | 239 (2.7) | 192 (2.2) | ||
| >1000, mL | 718 (7.5) | 366 (0.7) | 397 (4.5) | 312 (3.5) | ||
Bold values (with standardized differences>10%) show imbalanced characteristics.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; HES, hydroxyethyl starch; IQR, interquartile range; sCr, serum creatinine.
aStandardized difference was calculated for each type of surgery because some patients underwent multiple procedures on the day of surgery.
No data for exposure or outcome variables were missing. However, in the covariates, data on body mass index were not available for 662 (1.1%) patients before matching, and the duration of anesthesia was missing for 1217 (2.1%) before matching. All patients missing data for these covariates were excluded from the matching process. The HES infusion volume was missing for 124 (0.2%) patients before matching, and total infusion volume was missing for 495 (0.9%) before matching, but all patients had complete data after matching.
The incidence of AKI in the HES group did not differ significantly from that in controls: 6.2% (548/8823) vs 5.6% (492/8823), respectively; odds ratio (OR), 1.12; 95% confidence interval (CI), 0.99–1.27; P = .07. HES was not associated with worsening AKI stage (OR, 0.89; 95% CI, 0.79–1.01; P = .08). The incidence of RRT was lower in the HES group than in controls; 0.2% vs 0.4%, respectively (OR, 0.51; 95% CI, 0.29–0.91; P = .02). Median (interquartile range) hospital stay was 1 day longer in the HES group than in controls (12 [8–21] vs 11 [7–20] days, respectively; P < .001), but the Hodges-Lehman median difference and 95% CI for the hospital stay between groups were 0 and 0–1. The incidence of 30-day mortality did not differ between the groups (0.5% vs 0.6%, respectively; OR, 0.83; 95% CI, 0.56–1.24; P = .36) (Table 4).
Table 4.
Outcomes Before and After PS Matching in a Study of the Renal Morbidity of 6% HES 130/0.4 Administered on the Day of Surgery
| Outcome | Before PS Matching | After PS Matching | Odds Ratio (95% CI) | P | ||
|---|---|---|---|---|---|---|
| HES Group (n = 9542) | Control Group (n = 48,883) | HES Group (n = 8823) | Control Group (n = 8823) | |||
| AKI, n (%) | 671 (7.0) | 1434 (2.9) | 548 (6.2) | 492 (5.6) | 1.12 (0.99–1.27) | .07 |
| Worsening AKI stage, n (%) | 0.89 (0.79–1.01) | .08 | ||||
| Stage 0 | 8871 (93.0) | 47,449 (97.1) | 8275 (93.9) | 8331 (94.4) | ||
| Stage 1 | 549 (5.8) | 1200 (2.5) | 457(5.2) | 397 (4.4) | ||
| Stage 2 | 81 (0.8) | 138 (0.3) | 59 (0.7) | 51 (0.6) | ||
| Stage 3 | 41 (0.4) | 96 (0.2) | 32 (0.4) | 44 (0.5) | ||
| Patients on RRT, n (%) | 27 (0.3) | 65 (0.1) | 18 (0.2) | 35 (0.4) | 0.51(0.29–0.91)a | .02a |
| RRT duration, n (%) | ||||||
| 1–27 d | 25 (0.3) | 56 (0.1) | 16 (0.2) | 32 (0.4) | … | … |
| 28–89 d | 2 (0) | 9 (0) | 2 (0) | 3 (0) | … | … |
| ≥90 d | 0 | 0 | 0 | 0 | … | … |
| Length postoperative hospital stay (d) median (IQR)b | 12 (8–21) | 9 (5–16) | 12 (8–21) | 11 (7–20) | … | <.001a |
| In-hospital 30-d mortality n (%)c | 53 (0.6) | 159 (0.3) | 44 (0.5) | 53 (0.6) | 0.83 (0.56–1.24) | .36 |
| Use of any vasoactive agent, n (%) | 7748 (81.2) | 29,957 (61.3) | 7106 (80.5) | 6172 (70.0) | 1.78 (1.66–1.91)a | <.001a |
| Ephedrine | 5707 (59.8) | 22,714 (46.5) | 5279 (59.8) | 4879 (55.3) | ||
| Phenylephrine | 5302 (55.6) | 12,276 (25.1) | 4800 (54.4) | 3061 (34.7) | ||
| Dopamine | 1418 (14.9) | 1122 (2.3) | 1199 (13.6) | 423 (4.8) | ||
| Dobutamine | 396 (4.2) | 403 (0.8) | 317 (3.6) | 211 (2.4) | ||
| Norepinephrine | 574 (6.0) | 648 (1.3) | 448 (5.1) | 367 (4.2) | ||
| Epinephrine | 858 (9.0) | 8216 (16.8) | 820 (9.3) | 1084 (12.3) | ||
| Fluid summary, mL/kg/patient, median (IQR) | ||||||
| Net fluid on the day of surgery | 91.3 (67.4–130.3) | 58.8 (43.1–79.6) | 88.1 (66.2–123.5) | 73.6 (54.3–100.5) | … | <.001a |
| Crystalloid | 77.4 (55.0–113.1) | 58.7 (43.1–79.5) | 74.6 (53.8–107.0) | 73.3 (54.2–100.0) | … | |
| HES 130/0.4 | 11.1 (8.4–17.6) | … | 10.8 (8.3–16.9) | … | … | |
| Albumind | 9.1 (6.2–13.6) | 8.5 (5.4–12.2) | 8.9 (5.9–13.2) | 8.8 (5.8–13.0) | … | |
| Number of patients given albumin, n (%) | 1029 (10.8) | 901 (1.8) | 749 (8.5) | 530 (6.0) | 1.45 (1.29–1.63) | <.001a |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; HES, hydroxyethyl starch 130/0.4; IQR, interquartile range; PS, propensity score; RRT, renal-replacement therapy.
aStatistical significance at the .05 level.
bHodges-Lehman median difference (95% CI): 0 (0–1).
cThe postoperative days of patients who died in the charged hospital were not counted in length of postoperative hospital stay.
d5% albumin equivalent.
Vasoactive agents were more often used in patients receiving HES (80.5% vs 70.0%; OR, 1.78; 95% CI, 1.66–1.91; P < .001), and the median (interquartile range) net fluid requirement was higher in the HES group (88.1 [66.2–123.5] vs 73.6 [54.3–100.5] mL/kg, respectively; P < .001). The proportion of patients receiving albumin was higher in the HES group (8.5% vs 6.0%; P < .001, respectively). In the control group, 530 patients received albumin, and 94% of the remaining 8293 (=8823 − 530) controls were supposedly given only crystalloid solutions (Table 4) because the formulations of colloids used in Japan are albumin, HES 130/0.4, HES 70/0.5, and dextran, and receiving the latter 2 was an exclusion criterion (Figure 1).
Subgroup analyses found no interaction between HES infusion and the patients with high or low risk of AKI in any subgroups, with the exception of those with different eGFRs (the P value for interaction was. 004). There was no interaction between the incidence of AKI with HES and amounts of HES received (P = .41), cardiac versus noncardiac surgery (P = .53), or whether or not patients had preoperative septicemia (P = .72) (Figure 2).
Figure 2.
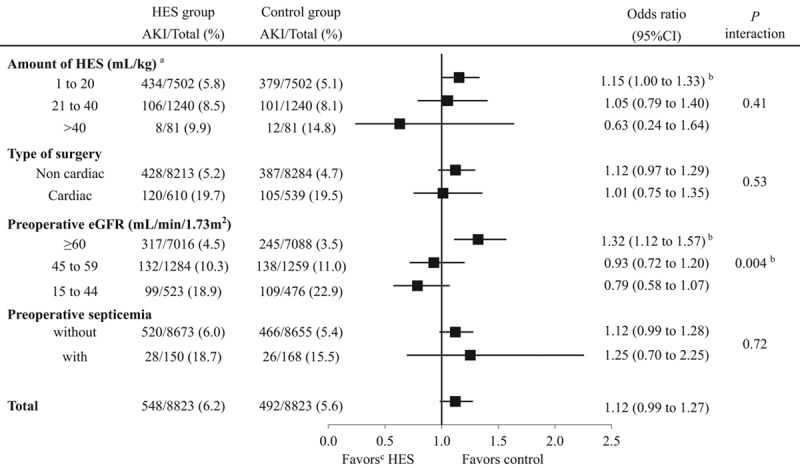
Subgroup analyses for a propensity-matched study of patients receiving fluid replacement on the day of surgery with or without 6% HES 130/0.4 to determine any associations between HES and the incidence of acute kidney injury. aPatients in the 3 HES infusion-volume subsets were compared to controls who did not receive HES but who were otherwise matched in the PS analysis to the patients receiving HES (which indicates the same denominators between the HES and the control group; 7502, 1240, and 81). bStatistically significant at the .05 level. cFavors mean a lower incidence of AKI. AKI indicates acute kidney injury; CI, confidence interval; eGFR; estimated glomerular filtration rate; HES, hydroxyethyl starch.
DISCUSSION
Our hypothesis was that 6% HES 130/0.4 would not be associated with adverse renal effects in surgical patients. In this retrospective cohort study with 8823 PS-matched pairs of surgical patients, we found that the incidence of AKI in patients receiving HES did not differ significantly from that of controls. Neither did AKI stage increase in patients after receiving HES nor in-hospital 30-day mortality. Although their median hospital stay was 1 day longer, the robust and unbiased estimator (Hodges-Lehman median difference) indicated that the median difference of the days between groups was 0 and 95% CI was 0–1. Therefore, this 1-day difference may be clinically negligible. In addition, patients receiving HES had a lower incidence of RRT.
Two large cohort studies of surgical patients (1 with more than a million patients and 1 with 40,000 patients) found that first-generation HES 670/0.7 was associated with an increased risk of AKI.5,6 Two systematic reviews, neither of which found that HES had negative effects, avoided concluding that HES was safe because the review included many small studies and older formulations of HES.7,8 In addition, 5 studies (2 systematic reviews, 2 small RCTs, and 1 follow-up study) evaluated the newest, third-generation HES 130/0.4 for surgical patients, and none found an increase in the risk of renal damage.11–14,16 One of these systematic reviews of 4529 pooled surgical patients reported no evidence that third-generation HES was associated with adverse renal effects or the need for RRT.11
Anesthesiologist might have preferentially chosen HES over a crystalloid preparation for highly invasive, hemorrhagic, and complex procedures. This can be explained in Table 3 by large imbalances before PS matching between the HES and the control group in the percentage of cardiovascular surgery with cardiopulmonary bypass (CPB) (2.6% vs 0.5%, respectively) or without CPB (5.5% vs 1.4%), the anesthesia duration (227 vs 142 minutes), and the transfusion rate (15.5% vs 2.9%). To deal with these confounding biases, we performed a PS-matching procedure and mostly succeeded. However, “surgical invasiveness” may not be completely balanced. That is, the higher rates of transfusion and vasoactive agent use, the higher fluid requirements, the higher rate of albumin use in the HES group may indicate that the HES group underwent more-invasive surgical procedures. Especially, the number of patients in the 3 categories of transfusion volume on the day of surgery (1–500 mL, 501–1000 mL, and >1000 mL) was higher in the HES group than in controls, even after the standardized difference was reduced from 45.1 to 9.2 by PS matching. This standardized difference of 9.2 was the highest among the covariates (Table 3). Dopamine, one of the most common catecholamine to treat severe hemodynamic instability, was used more frequently in the HES group (Table 4). Judging from these findings and our clinical experience, the direction of this confounding may favor the control group. That is, if surgical invasiveness had been equally balanced between 2 groups, the incidence of AKI (and also that of RRT) in controls would have become higher than what we found.
A recent PS-matched study in a single university hospital using HES 130/0.4 for surgical patients had findings similar to ours, although the number of matched patients was much smaller than ours (1084 vs 8823 pairs, respectively). This study demonstrated lower incidence of RRT in the HES group than in controls with no different incidence of AKI.15 In our study, as in the previous study, the incidence of AKI did not differ between groups, but the incidence of RRT was lower (0.2% vs 0.4%; P = .02) in the HES group. This discrepancy merits consideration.
Our findings indicate that HES 130/0.4 increases serum creatinine concentrations to some extent but that its effects might be limited to glomerular filtration in the renal cortex, where the glomeruli are located. However, other renoprotective mechanisms of HES 130/0.4 that might prevent more serious damage should be considered. In a porcine study, crystalloids decreased microvascular oxygenation in the renal cortex and the outer medulla by about 65% (P < .05) and in the inner medulla by about 30% (P < .05) after hemodilution from a hematocrit of 30% to 15%. In contrast, microvascular oxygenation remained unaltered with 6% HES 130/0.4.26 In a randomized trial of 30 patients, after cardiac surgery, the crystalloid-induced increase in GFR was associated with impaired renal oxygen demand and supply, an impairment not seen in patients receiving 6% HES 130/0.42.27 These findings lead us to speculate that HES would reduce GFR and increase serum creatinine concentrations, but may maintain medullary microcirculation and renal oxygenation while avoiding excessive interstitial edema by maintaining plasma oncotic pressure and consequently avoiding serious kidney damage.
Subgroup analyses found a significant interaction between HES infusion and the different levels of preoperative eGFR on the incidence of AKI, but not in the other factors (the different amounts of HES, cardiac surgery, and preoperative septicemia). However, Figure 2 illustrates that the more the risk of AKI increases, the more the OR decreases, except for patients with or without preoperative septicemia, because the patients are sorted from low risk to high risk of AKI in each subgroup. In other words, high-risk patients receiving the highest volume of HES, those undergoing cardiac surgery, and those with the lowest preoperative eGFRs had the lowest OR in each subgroup. This finding may support our speculation that HES 130/0.4 could avoid serious kidney damage in high-risk patients. However, subgroup analysis is an explorative measure to search the topics of the next investigation because it does not control for confounders and biases; therefore, these results are not robust and should be carefully interpreted.
Strengths and Limitations of the Study
Because this study was a retrospective cohort study, we could not regulate any potential confounders in advance. The present PS-matching study made patient demographic and surgical characteristics similar in 2 groups (Table 3). Although PS matching mimics some of the particular characteristics of an RCT, it does not allow the same control over bias and confounding. However, the number of patients involved in the present study (8823 pairs) may have a substantial statistical power.
The present study focused on the newest waxy-maize derived 6% HES 130/0.4, which has been used in surgeries and intensive care units throughout Japan with no apparent problems. Many studies have proposed confounding results because they treated different formulations of HES as a “HES.”5–8 Different products make different effects.10 Therefore, the present results should not be generalized for the older HES preparations and vice versa.
The incidence of RRT (0.2%–0.4%) and in-hospital 30-day mortality (0.5%–0.6%) in our study were lower than those of other studies: 2%–3% for RRT and 1%–2% for mortality in one study11 and 2%–3% for RRT and 2%–5% for mortality in another study.15 A possible explanation is that 70% of our patients had relatively minor surgeries (orthopedic: 22%, gynecologic/urologic/obstetric: 19%, miscellaneous: 29%; Table 3). Because minor surgery is associated with a lower risk of renal damage, given lower surgical invasiveness, assessing the direct effect of HES on postoperative renal function may be better. However, this approach has a clear risk of selection bias, so the results should be generalized carefully.
Intraoperative hypotension and blood loss are the main risks for AKI, but the database we used does not contain these data. We could speculate about the incidence of hypotension from the rate of vasopressor use and blood loss from the amount of transfusion. Low urine output is one of the Global Outcomes criteria for AKI. We could not include this criterion because data on urine output were not included in the database. Although other studies have used the same criteria we used,6,15 the absence of data on blood pressure, blood loss, and urine output could have affected our results.
The manufacturer and distributor of HES 130/0.4 in Japan funded this study. Although we believe that our study was unbiased by this fact, some studies have documented a strong relationship between funding source and the study results.28,29 Thus, this potential conflict of interest should be considered as a possible bias.
CONCLUSIONS
In this large, propensity-matched study to investigate the association between HES 130/0.4 and postoperative renal morbidity, we found that neither the incidence and the severity of postoperative AKI nor 30-day mortality differed between surgical patients treated with 6% HES 130/0.4 and their matched controls who did not receive HES. Median hospital stay in the HES group was 1 day longer than that of controls, but the incidence of RRT was lower.
ACKNOWLEDGMENTS
Hideki Miyao appreciates Mr Satoru Kamoshita from Otsuka Pharmaceutical Factory Inc, Tokyo, Japan, for helping us make the initial study design and select the statistical methods.
DISCLOSURES
Name: Hideki Miyao, MD, PhD.
Contribution: This author helped to develop the draft of initial study design and analysis plan, revised the draft, and was a major contributor in writing the manuscript.
Conflicts of Interest: H. Miyao has received honoraria and speaker’s fees for the fluid therapy in meetings and congresses from Otsuka Pharmaceutical Factory Inc, Japan. He was also a paid medical consultant for Fresenius Kabi, Japan until 2015.
Name: Yoshifumi Kotake, MD, PhD.
Contribution: This author helped to develop the initial study design and analysis plan, analyzed the data, interpreted the results, and wrote the first draft of the manuscript.
Conflicts of Interest: Y. Kotake has received an honorarium and speaker’s fees from Edwards Lifesciences and a speaker’s fee from Otsuka Pharmaceutical Factory, the distributor of HES 130/0.4, who also financially supported his participation in this study. He has also received speaker’s fees from the Japanese Blood Product Organization, GE Healthcare Japan, MSD, Ono Pharmaceuticals, and an unrestricted research grant and speaker’s fee from Nihon Koden Corporation, a medical device corporation.
This manuscript was handled by: Tong J. Gan, MD.
FOOTNOTES
GLOSSARY
- AKI =
- acute kidney injury
- BMI =
- body mass index
- CI =
- confidence interval
- CHEST =
- Crystalloid versus Hydroxyethyl Starch Trial
- COPD =
- chronic obstructive pulmonary disease
- CPB =
- cardiopulmonary bypass
- eGFR =
- estimated glomerular filtration rate
- HES =
- hydroxyethyl starch
- IQR =
- interquartile range
- OR =
- odds ratio
- PS =
- propensity score
- RCT =
- randomized controlled trial
- RRT =
- renal-replacement therapy
- sCr =
- serum creatinine
- UMIN =
- Japanese University Hospital Medical Information Network
Published ahead of print 16 December 2019.
Funding: This study was funded by Otsuka Pharmaceutical Factory Inc (Tokushima, Japan), who distributes 6% HES 130/0.4, and by Fresenius Kabi Japan K. K. (Tokyo, Japan) who manufactures it. These 2 companies supported the cost for data extraction and statistical analyses by Medical Data Vision Corp (Tokyo, Japan). Otsuka Pharmaceutical Factory, together with the authors, developed the initial study design and analysis plan, but neither Otsuka Pharmaceutical Factory nor Fresenius Kabi was involved in interpreting the results or preparing the manuscript, aside from paying an external, independent professional medical editor to review early drafts of the manuscript.
Conflicts of Interest: See Disclosures at the end of the article.
Trial registration: UMIN000027896 and the date of registration was June 30, 2017, at http://www.umin.ac.jp/ctr/index-j.htm.
Reprints will not be available from the authors.
REFERENCES
- 1.Brunkhorst FM, Engel C, Bloos F, et al. ; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. [DOI] [PubMed] [Google Scholar]
- 2.Perner A, Haase N, Guttormsen AB, et al. ; 6S Trial Group; Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 3.Myburgh JA, Finfer S, Bellomo R, et al. ; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 4.Hartog CS, Welte T, Schlattmann P, Reinhart K. Fluid replacement with hydroxyethyl starch in critical care–a reassessment. Dtsch Arztebl Int. 2013;110:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opperer M, Poeran J, Rasul R, Mazumdar M, Memtsoudis SG. Use of perioperative hydroxyethyl starch 6% and albumin 5% in elective joint arthroplasty and association with adverse outcomes: a retrospective population based analysis. BMJ. 2015;350:h1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashy BK, Podolyak A, Makarova N, Dalton JE, Sessler DI, Kurz A. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology. 2014;121:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillies MA, Habicher M, Jhanji S, et al. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis. Br J Anaesth. 2014;112:25–34. [DOI] [PubMed] [Google Scholar]
- 8.Raiman M, Mitchell CG, Biccard BM, Rodseth RN. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33:42–48. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health & Human Services: Vaccines, Blood & Biologics FDA Safety Communication. FDA safety communication: boxed warning on increased mortality and severe renal injury, and additional warning on risk of bleeding, for use of hydroxyethyl starch solutions in some settings. Available at: http://www.fffenterprises.com/assets/downloads/Article-FDASafetyCommunicationBoxedWarning6-13.pdf. Accessed August 25, 2019.
- 10.Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H. Hydroxyethyl starches: different products–different effects. Anesthesiology. 2009;111:187–202. [DOI] [PubMed] [Google Scholar]
- 11.Linden P, James M, Mythen M, Weiskopf RB. Safety of modern starches used during surgery. Anesth Analg. 2013;116:35–48. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Jacob M, Vicaut E, Guidet B, Van Aken H, Kurz A. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology. 2013;118:387–394. [DOI] [PubMed] [Google Scholar]
- 13.Kancir AS, Pleckaitiene L, Hansen TB, Ekeløf NP, Pedersen EB. Lack of nephrotoxicity by 6% hydroxyethyl starch 130/0.4 during hip arthroplasty: a randomized controlled trial. Anesthesiology. 2014;121:948–958. [DOI] [PubMed] [Google Scholar]
- 14.Kancir AS, Johansen JK, Ekeloef NP, Pedersen EB. The effect of 6% hydroxyethyl starch 130/0.4 on renal function, arterial blood pressure, and vasoactive hormones during radical prostatectomy: a randomized controlled trial. Anesth Analg. 2015;120:608–618. [DOI] [PubMed] [Google Scholar]
- 15.Pagel JI, Rehm M, Kammerer T, et al. Hydroxyethyl starch 130/0.4 and its impact on perioperative outcome: a propensity score matched controlled observation study. Anesth Analg. 2018;126:1949–1956. [DOI] [PubMed] [Google Scholar]
- 16.Joosten A, Delaporte A, Mortier J, et al. Long-term impact of crystalloid versus colloid solutions on renal function and disability-free survival after major abdominal surgery. Anesthesiology. 2019;130:227–236. [DOI] [PubMed] [Google Scholar]
- 17.Yasunaga H, Ide H, Imamura T, Ohe K. Influence of Japan’s new diagnosis procedure combination-based payment system on the surgical sector: does it really shorten the hospital stay? Surg Today. 2006;36:577–585. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, Imai E, Horio M, et al. ; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 19.Craig RG, Hunter JM. Recent developments in the perioperative management of adult patients with chronic kidney disease. Br J Anaesth. 2008;101:296–310. [DOI] [PubMed] [Google Scholar]
- 20.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yand D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. Paper 335-2012. Paper presented at: Proceedings of SAS Global Forum Conference; April 22–25, 2012; Cary, NC; SAS Institute Inc; Available at: http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed August 25, 2019. [Google Scholar]
- 24.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 25.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konrad FM, Mik EG, Bodmer SI, et al. Acute normovolemic hemodilution in the pig is associated with renal tissue edema, impaired renal microvascular oxygenation, and functional loss. Anesthesiology. 2013;119:256–269. [DOI] [PubMed] [Google Scholar]
- 27.Skytte Larsson J, Bragadottir G, Krumbholz V, Redfors B, Sellgren J, Ricksten SE. Effects of acute plasma volume expansion on renal perfusion, filtration, and oxygenation after cardiac surgery: a randomized study on crystalloid vs colloid. Br J Anaesth. 2015;115:736–742. [DOI] [PubMed] [Google Scholar]
- 28.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454–465. [DOI] [PubMed] [Google Scholar]
- 29.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


