Abstract
Background: The combination of herbal medicine with conventional treatment increases the survival rate of cancer patients, but the effect is not great. Hyperthermia may have a synergistic effect with herbal medicine alongside conventional medicine. Objective: To monitor the efficacy of hyperthermia together with Gun-Chil-Jung (GCJ) capsule for event-free survival (EFS) and overall survival (OS) for the treatment of various cancers. Methods: We collected data retrospectively on 54 cancer patients of all stages. They were divided into 4 groups according to each hyperthermia or GCJ treatment period. Hyperthermia with 0.46 MHz radiofrequency wave was applied a power of 50 to 100 W for 70 minutes. GCJ capsules were administered orally 3 times a day. Results: The median follow-up was 13.4 months, and 25 (55.6%) patients showed disease-related events. Hyperthermia with GCJ treatment was administered in combination group (n = 36, 66.7%) and traditional Korean medicine–only group (n = 17, 31.5%). The median EFS was 190 days, and the median OS was 390 days. The group of hyperthermia 7 times or fewer and GCJ more than 28 days showed longer EFS and OS. The analysis of superiority between hyperthermia and GCJ showed no significant difference (EFS, P = .55; OS, P = .364). Conclusions: The combination of hyperthermia 1 to 2 times a week with GCJ treatment may improve survival of cancer patients treated or being treated with conventional cancer therapies.
Keywords: hyperthermia, Gun-Chil-Jung, event-free survival, overall survival, combination therapy
Introduction
In Korea, the survival rate for patients with cancer has been increasing for decades.1 Despite positive outcomes in conventional medicine, cancer is still the number one cause of death in Korea.2 Therefore, cancer patients who received or planned to receive conventional cancer treatment tended to seek and choose as optional treatments various alternative therapies recommended by family members and acquaintances, in books, and on the internet.3 To alleviate cancer-related symptoms and to increase quality of life, about 78% of cancer patients in Korea and 91% in the United States have used complementary and alternative medicine (CAM) at least once after chemotherapy or radiation therapy.4 Cancer survivors often seek integrative cancer treatment on their own or on the recommendation of conventional health providers to alleviate cancer-related and conventional treatment–related symptoms and to enhance immune function.5 These facts indicate that the trend of cancer treatment is gradually moving toward an integrative approach.5 When herbal medicine treatment is combined with chemotherapy or radiation therapy, it suppresses tumor progression, increases the sensitivity of conventional cancer treatment, prevents damage to normal cells following conventional therapy, ensures immune function, and reduces the side effects from conventional treatment.6 In a prospective, randomized, controlled, multicentered study, add-on treatment of traditional Chinese herbal medicine to chemotherapy for patients with advanced lung cancer showed better outcomes for progression-free survival (PFS) than chemotherapy alone.7 Conventional treatment combined with herbal medicine, such as Herba Hedyotidis diffusa and Xiang-sha-Liu-Jun-Zi-Tang, showed an increased median overall survival (OS) of 4.5 months for patients with pancreatic cancer.8 Also, transarterial chemoembolization (TACE) combined with herbal medicine granules showed a better median OS of 3.3 months for patients with an inoperable hepatocellular carcinoma.9 However, in the CATLA study, the prolonged median PFS was only 3.5 months, and in another 2 studies, the median OS increased by only 3 to 5 months.7-9 Furthermore, when cancer patients have distant metastasis, their PFS and OS would be significantly decreased.10
As part of integrative cancer treatment, an increasing number of institutions or hospitals are looking at hyperthermia as a treatment modality for patients with cancer.11 Radiofrequency hyperthermia is a potent add-on approach to various cancer therapies and has also been used and studied as a chemo- and radiosensitizer since the 1970s.12 Hyperthermia has been shown to kill tumor cells, but not normal cells, because of its selective heat-trap mechanism for malignant cells.13-15 In vitro studies have shown that hyperthermia selectively inhibits the proliferation of malignant tumor cells and has little cytotoxic effect on normal cells in the same organ.16,17 Combined hyperthermia and radiation therapy for patients with advanced rectal and cervical cancers improved the local tumor control rate and the OS.18,19 Such combined therapy showed significant increases in the PFS and the OS for patients with glioblastoma muliforme,20 as well as improved PFS, OS, or complete response rate in patients with a wide range of malignancies, including head and neck cancer,21-23 lung cancer,24 breast cancer,25 pelvic malignancies,18,19,26,27 and skin cancer.28 Moreover, the use of hyperthermia with chemotherapy to treat patients with a high-risk soft tissue sarcoma, superficial bladder cancer, or esophageal cancer showed better PFS, disease-free survival, and OS than chemotherapy therapy alone.29-33 Over 20 randomized clinical trials have shown the benefits of using hyperthermia combined with radiation therapy or chemotherapy to treat patients with a wide range of cancers, including breast cancer, head and neck cancer, esophageal cancer, melanoma, and so on.34
While many hyperthermia studies have focused on relatively high temperatures, such as 43 to 45 °C, mild-temperature radiofrequency therapy has also shown positive effects for cancer treatment when combined with radiation therapy. Hyperthermia treatment at a mild temperature of 41.5 °C given on consecutive days increased radiosensitization by causing physiological changes in the tumor’s microenvironment.35 Generally, the selective heat-trap mechanism of the above 42 °C hyperthermia treatment on cancer cells results in an antiangiogenesis effect by causing deterioration of the tumor’s oxygenation status and consequently by activating apoptosis.15,36 Oxygenation is an important factor in the survival of malignant tumor cells and has an important impact on the progression of cancer due to oxygen transport to and distribution in tumor tissue.37 Relatively low frequencies are also known to be more advantageous in malignant tumor cell selectivity, and that higher selectivity increases the amount of energy absorbed by the target lesion.38 As we know, angiogenesis is a critical element for tumor growth, invasion, and distant metastasis.39 In contrast to high-temperature hyperthermia, which inhibits angiogenesis, mild-temperature hyperthermia might increase tumor perfusion and cause reoxygenation when combined with radiation therapy or chemotherapy.40 This aspect of mild-temperature radiofrequency therapy might contribute to radio- or chemosensitization.
Gun-Chil Jung (GCJ) is a Korean herbal medicine capsule extracted from the plant Rhus verniciflua Stokes (RVS) and has been traditionally prescribed for detoxifying and resolving blood stasis and masses.41 RVS extracts have been known to exhibit anticancer efficacy through the activation of cancer cell apoptosis, antiangiogenesis, and the growth-inhibitory mechanism.42 RVS extracts also have an antiproliferative and apoptotic effect on tumor cells, including the malignant cells of breast and colorectal cancers and those in hepatocellular carcinomas, osteosarcomas, and lymphomas.43-46 RVS extracts are also known to have anti-inflammatory, antioxidant, and immunomodulatory effects, in addition to their antitumor effect.47 GCJ mainly consists of several compounds, such as fisetin, sulfuretin, and fustin, which have apoptotic effects on various cancer cells.
The aim of this retrospective study on combined treatment with mild-temperature hyperthermia and the herbal medicine GCJ capsule was to investigate its effect on survival for patients treated or being treated with chemotherapy or radiation therapy. Furthermore, we wanted to figure out which treatment would affect the survival of patients because of the economic burden of combination treatment of hyperthermia with GCJ.
Materials and Methods
Eligibility Criteria
A retrospective study was conducted to identify all patients who had visited and been treated at 2 Integrative Cancer Treatment Clinical Centers, East-West Cancer Center (EWCC), located in Dunsan and Cheonan, from June 1, 2015, to August 31, 2017. The treatment periods for hyperthermia and GCJ at the EWCC were the same during that period. Follow-up on cancer-related events and death after treatment ended on August 31, 2018.
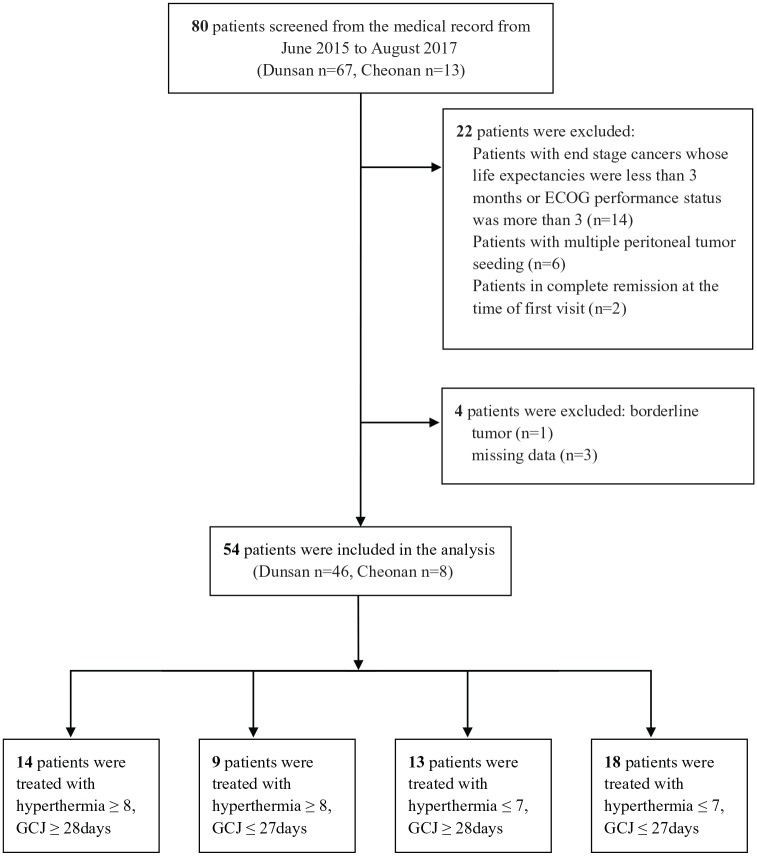
A retrospective review of the Registry of Patients was conducted based on electronic medical records. Inclusion criteria were as follows: patients who had a primary or metastatic malignant tumor and had visited Dunsan or Cheonan Daejeon University Korean medical hospitals, had visited the EWCC at least once as an inpatient/outpatient, patients who had been treated with chemotherapy or radiation therapy after having been diagnosed with various cancer types, and patients who had been treated with both hyperthermia and GCJ therapies at the EWCC. The following patients were excluded: patients with end-stage cancers whose life expectancies were less than 3 months or Eastern Cooperative Oncology Group (ECOG) performance status was more than 3, patients diagnosed with hematologic malignancies like lymphomas, patients with multiple peritoneal tumor seeding, and patients in complete remission at the time of first visit to the EWCC. From June 2015 to August 2018, patients treated with hyperthermia and GCJ were screened in 2 Korean medical hospitals; 54 of these patients met the inclusion criteria and were enrolled in the study (Dunsan n = 46, Cheonan n = 8; Figure 1). Data were evaluated from first visit to the EWCC until death or last follow-up.
Figure 1.
Patient recruitment flow chart in this retrospective study.
Abbreviations: ECOG, Eastern Cooperative Oncology Group Performance Status; GCJ, Gun-Chil-Jung.
All available medical records on the history of conventional therapy (chemotherapy, radiation therapy, and surgery), metastasis, progression, times of hyperthermia, period of GCJ treatment, and clinical laboratory test results were reviewed without contacting any of the patients. This retrospective study was approved by the Institutional Review Board of Dunsan Korean Medicine Hospital of Daejeon University (DKMHDU; Approval Number: DJDSKH-18-E-11-2).
Data Collection and Patient Classification
The basic and the clinical characteristics of patients included in this study included sex, age, date of first visit, patient’s history of cancer (primary site, numbers of metastatic extranodal lesions, ECOG, period from diagnosis of cancer to start of treatment, cancer stage, surgery, conventional therapy), treatment received at the EWCC (hyperthermia, GCJ therapy), and clinical laboratory test results for safety measures. Radiofrequency hyperthermia treatment was applied using a REMISSION 1 °C device (AdipoLABs Company, Seoul, Korea), which produced a 0.46-MHz radiofrequency wave. Hyperthermia was applied to the abdomen or the chest, depending on the site of the tumor, and each treatment time was 70 minutes by a doctor. Two 12-cm-diameter electrode terminals were applied to front and the back sides of tumor’s location. The hyperthermia was performed by a single practitioner, and the electrical power was increased gradually from 50 to 100 W because of patients’ different tolerances to the heat or navel pain often induced by hyperthermia. The Korean medicine doctors at the EWCC generally recommend hyperthermia to various types of cancer patients who are undergoing chemotherapy or radiation therapy because of its supposedly positive add-on effect to conventional therapies. However, patients with difficult economic circumstances tended to be reluctant to receive hyperthermia as an add-on treatment option.
Gun-Chil Jung capsule is an oral herbal medicine, RVS, which contains several bioactive ingredients, such as fisetin, fustin, butein, sulfuretin, and urushiol. The allergy-inducing compound urushiol was removed from RVS to avoid severe contact dermatitis in patients with sensitivities to urushiol. GCJ is a water extract of RVS with urushiol removed and is composed of fustin, fisetin, and sulfuretin (600 mg total per capsule). At the EWCC, the Korean medicine doctors routinely prescribe GCJ 3 times per day orally to various cancer patients because of its broad spectrum of antitumor and antimetastatic activities. In this study, all patients received the same daily dose of GCJ.
Three researchers independently collected, coded, and integrated all medical records data, after which they cross-checked one another’s data. To evaluate the effect of combination treatment and to determine which treatment was more effective, we classified the patients according to treatment period. At first, we hypothesized that EFS and/or OS would be improved if the patients received hyperthermia or GCJ treatment for more than 4 weeks. Therefore, we established the treatment protocol as 8 treatments with hyperthermia and 28 days of GCJ treatment over a 4-week period; a factor in this decision was the fact that the patients usually received hyperthermia twice per week. The included patients were divided into 4 groups: hyperthermia more than 8 times and GCJ more than 28 days (group A), hyperthermia more than 8 times and GCJ 27 days or fewer (group B), hyperthermia 7 times or fewer and GCJ more than 28 days (group C), and hyperthermia 7 times or fewer and GCJ 27 days or fewer (group D).
Outcome Measures and Statistical Analysis
For demographic and clinical characteristics, cross tabulations of demographic variables and test results for patients were analyzed to determine relative mortality rates and relative event incidence rates. The 95% confidence intervals of the median survivals were calculated and presented based on the EFS and the OS for each group. Kaplan-Meier’s method was used to estimate the survival distribution of each group and the log-rank test was used to compare the survival distributions between groups. CTCAE (Common Terminology Criteria for Adverse Events) version 5.0 was used to classify the type and the intensity of any adverse event. Statistical significance was defined as a P value of <.05.
Results
Demographic and Clinical Characteristics
A total of 54 patients met the criteria and were included in this retrospective study. The median follow-up was 13.4 months. Table 1 shows for each group and the characteristics. In this study, 55.6% of the patients were male and 44.4% were female, and 59.3% were younger than 60 years. Of the included patients, 57.4% had fewer than 2 extranodal metastasis sites compared with 33.3% with more than 2 sites. The majority of the patients (98.1%) had an ECOG grade 2 performance status, and for 61.1%, the time from first diagnosis to hyperthermia and GCJ treatment was less than 600 days, compared with 38.9%, for whom that time was more than 600 days. Primary tumor sites were the lungs (10, 18.5%), colon (6, 11%), liver (6, 11%), rectum (6, 11%), breast (5, 9%), pancreas (5, 9%), bile duct (4, 7.4%), cervix (2, 3.7%), esophagus (2, 3.7%), ovary (2, 3.7%), stomach (2, 3.7%), bladder (1, 1.8%), bone (1, 1.8%), maxilla (1, 1.8%), and kidneys (1, 1.8%).
Table 1.
Demographic Characteristics of the Sample Patients Plus EFS and OS Risks for Demographic Variables.
| Characteristics | No. of patients | % of Patients | EFS, HR (95% CI) | P | OS, HR (95% CI) | P |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 30 | 55.6 | 1 (0.69-0.97) | .160 | 1 (0.49-0.85) | .756 |
| Female | 24 | 44.4 | 0.81 (0.46-0.87) | 0.94 (0.42-0.83) | ||
| Age (year) | ||||||
| ≤60 | 32 | 59.3 | 1 (0.48-0.83) | .033 | 1 (0.35-0.71) | .030 |
| >60 | 22 | 40.7 | 1.38 (0.78-1.04) | 1.55 (0.64-0.99) | ||
| Stage | ||||||
| I to III | 7 | 13 | 1 (0.08-1.07) | .423 | 1 (0.07-0.92) | .434 |
| IV | 40 | 74.1 | 1.40 (0.67-0.93) | 1.58 (0.52-0.83) | ||
| Unknown | 7 | 13 | 1.25 (0.26-1.17) | 1.65 (0.26-1.17) | ||
| Number of extranodal sites | ||||||
| <2 | 31 | 57.4 | 1 (0.54-0.88) | .620 | 1 (0.43-0.79) | .716 |
| ≥2 | 18 | 33.3 | 1.17 (0.64-1.02) | 1.10 (0.43-0.91) | ||
| Unknown | 5 | 9.3 | 1.13 (0.24-1.36) | 1.31 (0.24-1.36) | ||
| ECOG | ||||||
| 2 | 53 | 98.1 | 1 (0.63-0.87) | .578 | 1 (0.51-0.77) | .467 |
| 1 | 1 | 1.9 | 1.33 | 1.56 | ||
| Diagnosis to 0 period (days) | ||||||
| <600 | 33 | 61.1 | 1 (0.60-0.91) | .972 | 1 (0.46-0.81) | .824 |
| ≥600 | 21 | 38.9 | 1 (0.56-0.96) | 1.04 (0.45-0.89) | ||
Abbreviations: EFS, event-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group performance status.
Table 2 shows the hazard ratio (HR) for an EFS and OS according to conventional cancer treatments. Of the included patients, 57.4% had been treated with only chemotherapy, compared with 3.7% who had been undergone only radiation therapy and 38.9% who had undergone both chemoradiation therapies. The category of conventional cancer treatment in this study included TACE therapy for patients with inoperable hepatocellular carcinoma. About two thirds of patients were included in the combination treatment group (n = 36, 66.7%), and about one third were included in the only traditional Korean medicine (TKM) group (n = 17, 31.5%). The combination treatment group is defined as concurrent use of conventional treatment with TKM treatment during a treatment period at EWCC. The prevention group is a patient who was diagnosed as complete remission after the first visit at EWCC who terminated conventional treatment but continued EWCC treatment. The majority (74.1%) of patients who visited the EWCC had stage IV cancer, while 13% presented with cancer in stages I to III. Table 3 shows the HR for an EFS and OS according to divided treatment group.
Table 2.
Treatment History of the Sample Patients Plus EFS and OS Risks for Demographic Variables.
| Treatments | No. of patients | % of patients | EFS, HR (95% CI) | P | OS, HR (95% CI) | P |
|---|---|---|---|---|---|---|
| Surgery | ||||||
| Yes | 36 | 66.7 | 1 (0.60-0.90) | .826 | 1 (0.44-0.78) | .430 |
| No | 18 | 33.3 | 1.04 (0.56-0.99) | 1.18 (0.49-0.95) | ||
| Conventional treatment history | ||||||
| Only chemotherapy | 31 | 57.4 | 1 (0.62-0.93) | .650 | 1 (0.50-0.85) | .433 |
| Only radiation treatment | 2 | 3.7 | 1.30 (1.00-1.00) | 1.47 (1.00-1.00) | ||
| Both treatments | 21 | 38.9 | 0.92 (0.50-0.92) | 0.84 (0.34-0.80) | ||
| EWCC treatment group | ||||||
| Combination group | 36 | 66.7 | 1 (0.60-0.90) | .176 | 1 (0.47-0.80) | .363 |
| TKM-only group | 17 | 31.5 | 1.09 (0.62-1.03) | 1.11 (0.46-0.95) | ||
| Prevention group | 1 | 1.9 | 0 | 0 | ||
Abbreviations: EFS, event-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; EWCC, East-West Cancer Center; TKM, traditional Korean medicine.
Table 3.
The HR for EFS and OS of the Groups.
| No. of patients | % of patients | EFS, HR (95% CI) | P | OS, HR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Hyperthermia ≥8 | ||||||
| GCJ ≥28 | 14 | 60.9 | 1 (0.65-1.07) | .301 | 1 (0.36-0.93) | .372 |
| GCJ ≤27 | 9 | 39.1 | 0.78 (0.28-1.05) | 0.69 (0.04-0.85) | ||
| Hyperthermia ≤7 | ||||||
| GCJ ≥28 | 13 | 41.9 | 1 (0.22-0.85) | .028 | 1 (0.15-0.78) | .008 |
| GCJ ≤27 | 18 | 58.1 | 1.65 (0.73-1.05) | 1.93 (0.73-1.05) | ||
| GCJ ≥28 | ||||||
| Hyperthermia ≥8 | 14 | 51.9 | 1 (0.65-1.07) | .075 | 1 (0.36-0.93) | .363 |
| Hyperthermia ≤7 | 13 | 48.1 | 0.63 (0.22-0.85) | 0.72 (0.15-0.78) | ||
| GCJ ≤27 | ||||||
| Hyperthermia ≥8 | 9 | 33.3 | 1 (0.28-1.05) | .174 | 1 (0.04-0.85) | .012 |
| Hyperthermia ≤7 | 18 | 66.7 | 1.33 (0.73-1.05) | 2.02 (0.73-1.05) | ||
Abbreviations: HR, hazard ratio; EFS, event-free survival; OS, overall survival; CI, confidence interval; GCJ, Gun-Chil-Jung.
Survival Analysis
At the end of the study follow-up (August 31, 2018), 25 patients (55.6%) showed disease-related events including tumor progression, recurrence, metastasis, and death. Of the 54 total patients, 26 (57.8%) had expired before the last follow-up. The data on patients who survived until the last follow-up were censored. The median EFS time of the 54 patients was 190 days, and the median OS time was 390 days. The results of the Kaplan-Meier analyses are presented in figures. Figures 2 and 3 show the EFS and OS time according to the treatment period of GCJ for patients treated with hyperthermia 7 times or fewer during the treatment period. The primary endpoint of this study is EFS, and surrogate endpoints are OS and natural killer (NK) cell test results for assessing immune function improvement. All patients included in this retrospective study had residual tumors at the start of EWCC treatment. Therefore, we did not consider disease-free survival, which means the duration of tumor remission status, to be an appropriate endpoint. Thus, we set EFS as the primary endpoint, which is an indicator that includes all events related to cancer, such as recurrence, metastasis, tumor progression, and death. Recently, EFS, rather than traditional OS, was used in the approval of targeted chemotherapeutic drugs such as panitumumab.48
Figure 2.
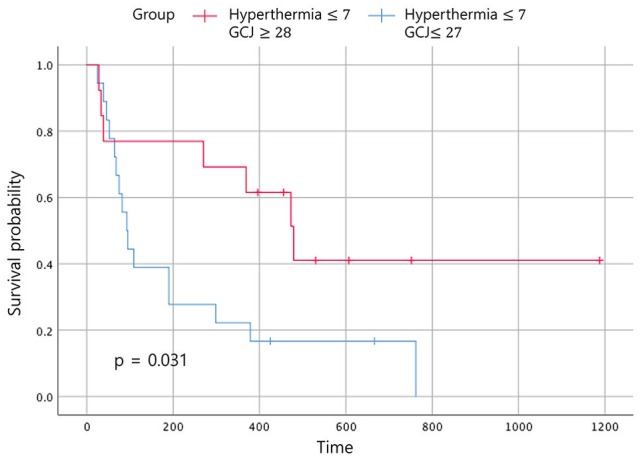
Event-free survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
Figure 3.
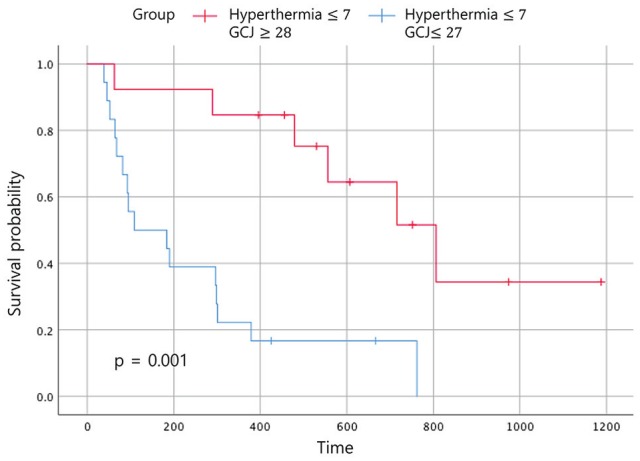
Overall survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
Statistically significant improvements in the EFS and the OS were noted in patients who had received GCJ treatment for more than 28 days versus 27 days or fewer (Figures 2 and 3; P = .03 and P = .001). Figure 4 shows that the OS for patients treated with hyperthermia more than 8 times and with GCJ for more than 28 days (group A) was higher than that for patients treated with hyperthermia 7 times or fewer and with GCJ for 27 days or fewer (group D). Furthermore, no statistically significant difference was observed in the EFS between patients treated with hyperthermia more than 8 times and with GCJ for more than 28 days (group A) and those treated with hyperthermia 7 times or fewer and with GCJ for 27 days or fewer (group D; Figure 5; P = .669).
Figure 4.
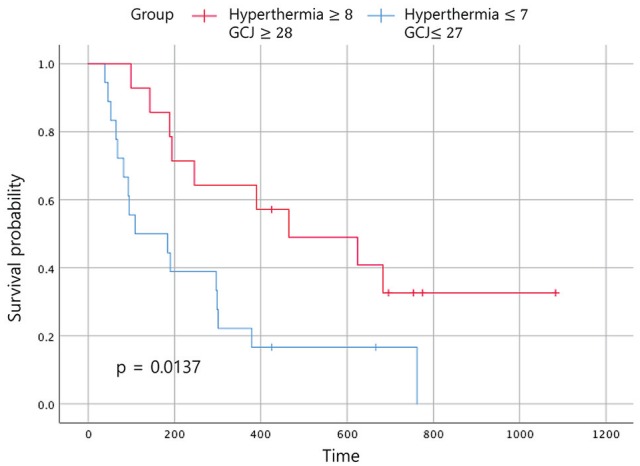
Overall survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
Figure 5.
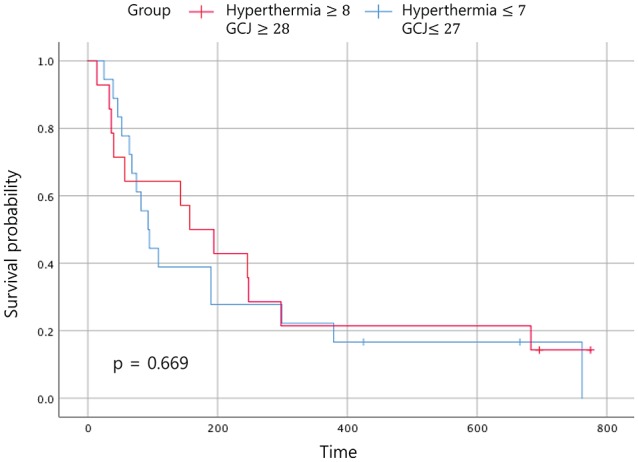
Event-free survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
When the 4 groups were analyzed according to the duration of treatment, patients treated with hyperthermia more than 8 times and with GCJ for more than 28 days (group A) did not show the best outcome for the OS (Figure 6; P = .002). The same analysis for the EFS time showed no statistically significant difference (Figure 7; P = .162). The most improved OS was seen for patients treated with hyperthermia 7 times or fewer and with GCJ for more than 28 days (group C).
Figure 6.
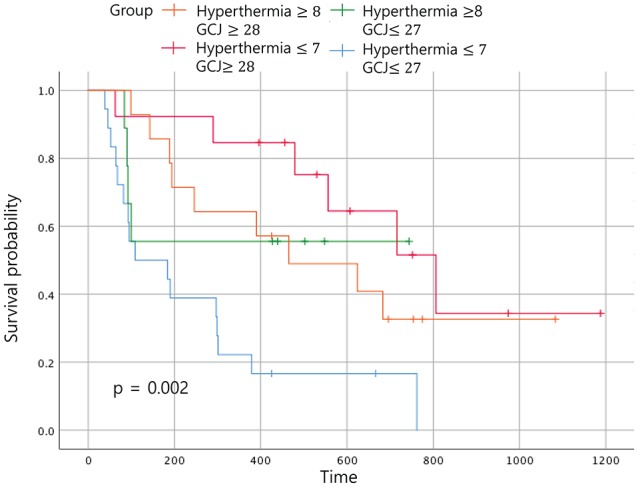
Overall survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
Figure 7.
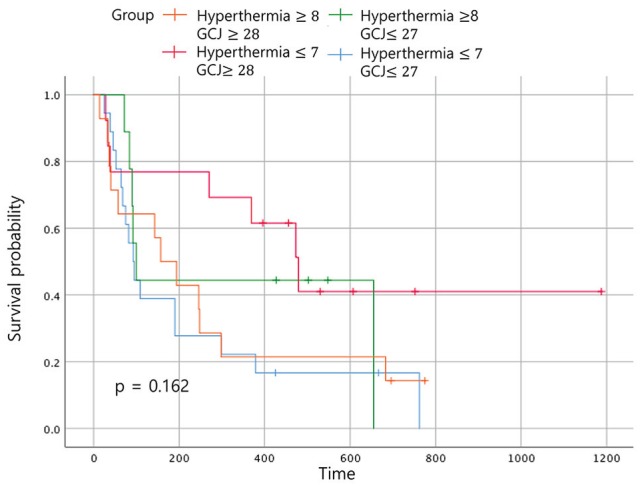
Event-free survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
In the Figure 6, when comparing the OS between the 4 groups according to the duration of treatment, the OS time of patients who had been treated with hyperthermia 7 times or fewer and with GCJ for more than 28 days (group C) was superior to the other 3 groups. The OS for patients treated with hyperthermia more than 8 times and with GCJ for more than 28 days (group A), which was expected to be the highest, was found to be the second highest followed by group C. As expected, the OS for the patients who had been treated with hyperthermia 7 times or fewer and with GCJ for 27 days or fewer (group D) was the lowest. Although one of our aims was to identify which treatment of hyperthermia and GCJ predominantly influences the EFS time or the OS time for patients with cancer, the results showed no statistically significant differences (Figures 8 and 9; P = .55 and P = .364).
Figure 8.
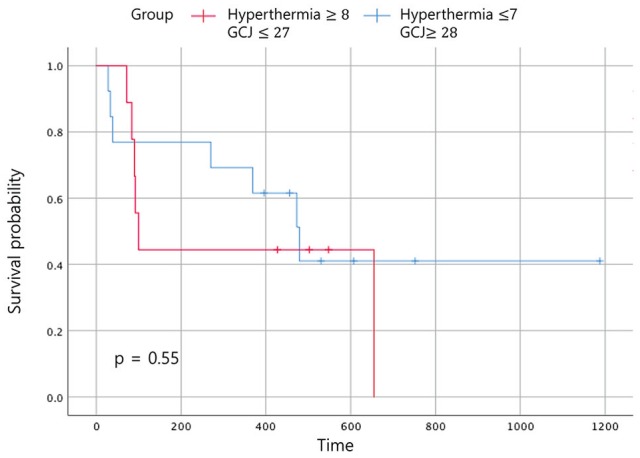
Event-free survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
Figure 9.
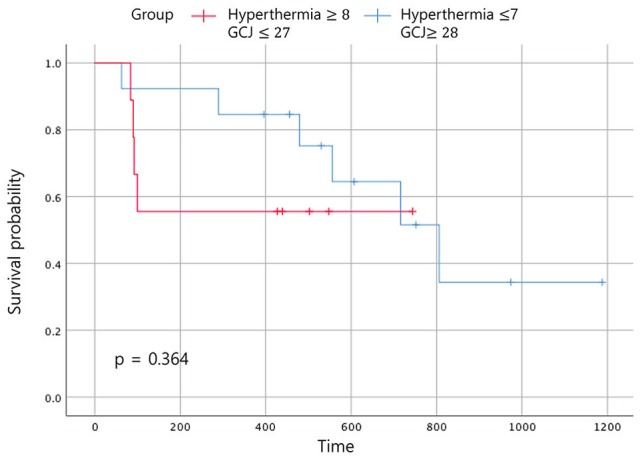
Overall survival according to the treatment period. Abbreviation: GCJ, Gun-Chil-Jung.
Adverse Effects and Safety
Assessable NK cell activity test results were collected only in 7 patients (13%), but even those showed no statistically significant changes. Clinical laboratory test results, such as aspartate transaminase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen (BUN), were insufficient to assess the significance of changes between before and after treatment. Adverse events occurred during treatment in 4 patients were the following: burns, pruritus, abdominal pain, and right chest pain. Except for pruritus, the other 3 side effects were considered to be adverse events of hyperthermia. All adverse events were assessed grade 1 according to CTCAE version 5.0, without sequelae.
Discussion
The results of this retrospective study showed that the patients who visited the EWCC mainly presented with stage IV cancer rather than cancer in relatively early stages. They also showed that combination treatment of hyperthermia with GCJ might have better survival benefits. When hyperthermia was administered 7 times or fewer and GCJ 28 days or more (group C), the EFS time and the OS time were better than they were when hyperthermia was administered 7 times or fewer and GCJ 27 days or fewer (group D). By contrast, when hyperthermia treatment was administered more than 8 times, no statistically significant difference in survival was noted between the patients who were administered GCJ for 27 days or fewer (group B) and those who were administered GCJ for more than 28 days (group A). Also, when GCJ was administered 27 days or fewer, no statistically different results were found for the EFS time and the OS time between the patients who had undergone hyperthermia 7 times or fewer (group D) and those who had undergone hyperthermia more than 8 times (group B). The tendency of positive outcomes for the patients who had taken GCJ for more than 28 days may not be free from bias because that medicine may have had more long-term use in patients with good prognosis and physical status.
Moreover, analyses that were conducted to determine which treatment of the 4 resulted in superior EFS and OS times showed no statistically significant difference between the patients treated with hyperthermia more than 8 times and with GCJ for 27 days or fewer (group B) and those treated with hyperthermia 7 times or fewer and with GCJ for more than 28 days (group C).
Hyperthermia has been recognized as a potential add-on treatment option for conventional cancer treatment because of its convincing clinical results, including those of several phase III trials for cancer patients.29,36,49,50 Adding hyperthermia to conventional cancer treatment has been considered to have a radio-, chemo-, or radio/chemosensitization effect. The mechanisms of synergistic cytotoxicity include enhanced perfusion, intracellular chemotherapeutic drug accumulation, S-phase cell cycle arrest, and reversal of chemotherapeutic agent or radiation therapy resistance.34 Mild-temperature hyperthermia also increased radiosensitization and, thus, increased cytotoxicity, which was not seen with mild-temperature hyperthermia monotherapy.15 Hyperthermia has the advantage of lowering the effective temperature when combined with radiation therapy.15 Hyperthermia also showed chemosensitization effects on alkylating agents, such as cyclophosphamide and ifosfamide, and on platinum-based agents, such as cisplatin, bleomycin, and nitrosourea.51 Hyperthermia combined with various chemotherapeutic drugs showed a chemosensitization effect at different temperatures of hyperthermia52 and was more effective when administered at different time intervals, depending on the type of chemotherapeutic drug.15
Combination treatment of herbal medicines and radiation therapy showed that some herbal medicines inhibited antiapoptotic proteins against caspase or bcl-2 activity during radiation therapy, thereby increasing radiosensitization.53 Thus, although not concurrently implemented, synergistic effects are expected only when hyperthermia and GCJ are merged into chemotherapy or radiation therapy with various time differences.
One of the active ingredients of GCJ capsule, fisetin, showed apoptotic activity for colon, prostate, and pancreatic cancer cells54-56 and showed a synergistic effect with chemotherapeutic drugs in triple-negative breast cancer, lung cancer, and melanoma cells.57-59 Sulfuretin, also an active ingredient of GCJ, showed an apoptotic effect on breast cancer cells through inhibiting the NF-κB signaling pathway and on leukemia cell through the Fas-mediated caspase-8–dependent pathway.60,61
The combined use of herbal medicine with hyperthermia may have an increased growth inhibitory effect on tumors. Marsdenia tenacissima herb extracts, which have shown inhibitory effects on lung, gastric, esophageal, and liver cancer cells,62-64 showed synergistic growth inhibitory and proapoptotic effects when combined with hyperthermia.13 Combining traditional Chinese medicine (TCM) and hyperthermia improves immune functions, such as CD3+, CD4+, and NK cells, compared with TCM alone or hyperthermia monotherapy, thereby contributing to a synergistic effect when used with conventional cancer therapies.65 Celastrol, the major bioactive ingredient of the herbal medicine Tripterygium wilfordii, activates heat-shock transcription factor 1 in various cancer cells, and heat-shock protein (HSP) exerts a chaperone effect. Therefore, when celastrol is combined with hyperthermia, a positive effect on the activated tumor’s immune function is expected.66
In general, many studies have reported the results of combined cancer therapy for high-temperature hyperthermia with chemotherapy or radiation therapy. Moreover, predictive mechanisms that can produce sufficiently positive antitumor effects even at mild temperatures have also been studied, and those results are consistent with the results of our study. High-temperature hyperthermia causes hypoxia mainly through direct injury to the blood vessels of the tumor, causing apoptosis, whereas mild-temperature hyperthermia increases blood perfusion and causes reoxygenation of the tumor.67 This mechanism, which is expressed differently from high-temperature hyperthermia, may result in increased radiosensitization when combined with radiation therapy by maintaining tumor vascular perfusion, while radiation therapy inhibits DNA repair of the malignant tumor.35 The combination of mild-temperature hyperthermia with chemotherapeutic agents has a synergistic effect of delaying tumor growth when combined with docetaxel, irinotecan, gemcitabine, and oxaliplatin.68 This result may also be due to mechanisms that ensure vascular perfusion of the tumor to allow the chemotherapeutic drugs to be better delivered with less drug resistance.
The temperature of the Remission 1 °C hyperthermia device used in this study was found to increase to 41.3 °C in direct measurement of the core temperature of 3 Yorkshire swine at a relatively low frequency of 0.43 MHz (data not shown). Therefore, the results of our study can be interpreted in terms of this mechanism which mild-temperature hyperthermia has. In addition, HSP, which is expressed when hyperthermia is applied to the body, is known to act as a chaperokine against cell damage caused by various stressors.69-71 However, growing evidence shows that HSP has an antigen-presenting activity, which plays an immunomodulating role in tumor cells, causing a positive effect when used to treat cancer.69-72 Thus, these various cellular immune responses induced by HSP can activate tumor immunity, allowing hyperthermia to contribute to cancer treatment. Hyperthermia also has the effect of activating dendritic cells and NK cell function through upregulating expression of HSP.73
A study on mild-temperature hyperthermia showed better outcome for tumor growth delay when intermittent hyperthermia was given in combination with radiation therapy rather than when daily hyperthermia was given, suggesting the possibility of an intermittent hyperthermia schedule being more effective in reoxygenation.35 Thus, we can speculate that this mechanism, which has not yet been completely identified, may be related to the better outcomes for survival when patients received hyperthermia treatment 7 times or fewer, as shown in our study.
However, in the analysis of the groups receiving hyperthermia treatment more than 8 times, the numbers of patients assigned to the groups were too small for any statistically significant result to be obtained satisfactorily. Therefore, concluding prematurely that hyperthermia treatment for 7 times or fewer has a positive influence survival outcomes is not possible, although this definitely needs more study. Thus, further research should be carried out with a large number of patients. In this retrospective study, because of the conditions that chemo- or radiation therapy with hyperthermia could not be administered concurrently and that the intervals between chemo- or radiation therapy and hyperthermia were very diverse, calculating the benefits of the combined treatment was difficult; thus, the hyperthermia treatment in this study may have played the role of only monotherapy especially in TKM-only group. However, for this reason, this analysis including the herbal medicine antiangiogenic prescription GCJ capsule has meaning.
Conclusions
In conclusion, combination of hyperthermia 1 to 2 times a week with GCJ treatment may improve EFS and OS of cancer patients treated or being treated with conventional cancer therapies. Further studies with a large-scale, prospective design in a multiclinical setting are required to clarify the benefits of this combination treatment for patients with cancer.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from Daejeon University’s Industry-Academic Cooperation Foundation of AdipoLABs Co (2016; Grant Number: 20180172).
ORCID iDs: Hyeong Joon Jun  https://orcid.org/0000-0003-4012-4107
https://orcid.org/0000-0003-4012-4107
Namhun Lee  https://orcid.org/0000-0002-5039-2423
https://orcid.org/0000-0002-5039-2423
Hwa-Seung Yoo  https://orcid.org/0000-0003-3738-3239
https://orcid.org/0000-0003-3738-3239
References
- 1. Jung K, Won Y, Kong H, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Statistics Korea. Preliminary Results of Birth and Death Statistics in 2018. Daejeon, South Korea: Statistics Korea; 2019. [Google Scholar]
- 3. Evans M, Shaw A, Thompson EA, et al. Decisions to use complementary and alternative medicine (CAM) by male cancer patients: information-seeking roles and types of evidence used. BMC Complement Altern Med. 2007;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim MJ, Lee SD, Kim DR, et al. Use of complementary and alternative medicine among Korean cancer patients. Korean J Intern Med. 2004;19:250-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52). doi: 10.1093/jncimonographs/lgx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends. 2010;4:297-307. [PubMed] [Google Scholar]
- 7. Jiao L, Xu J, Sun J, et al. Chinese herbal medicine combined with EGFR-TKI in EGFR mutation-positive advanced pulmonary adenocarcinoma (CATLA): a multicenter, randomized, double-blind, placebo-controlled trial. Front Pharmacol. 2019;10:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuo YT, Liao HH, Chiang JH, et al. Complementary Chinese herbal medicine therapy improves survival of patients with pancreatic cancer in Taiwan: a nationwide population-based cohort study. Integr Cancer Ther. 2018;17:411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu Y, Lang Q, Chen Z, et al. The efficacy for unresectable hepatocellular carcinoma may be improved by transcatheter arterial chemoembolization in combination with a traditional Chinese herbal medicine formula: a retrospective study. Cancer. 2009;115:5132-5138. [DOI] [PubMed] [Google Scholar]
- 10. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peeken JC, Vaupel P, Combs SE. Integrating hyperthermia into modern radiation oncology: what evidence is necessary? Front Oncol. 2017;7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad B, Kim S, Cho W, et al. Quantitative estimation of the equivalent radiation dose escalation using radiofrequency hyperthermia in mouse xenograft models of human lung cancer. Sci Rep. 2019;9:3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vancsik T, Kovago C, Kiss E, et al. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J Cancer. 2018;9:41-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiorentini G, Sarti D, Milandri C, et al. Modulated electrohyperthermia in integrative cancer treatment for relapsed malignant glioblastoma and astrocytoma: retrospective multicenter controlled study. Integr Cancer Ther. 2019;18:1534735418812691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33-56. [DOI] [PubMed] [Google Scholar]
- 16. Curley S, Palalon F, Sanders K, Koshkina N. The effects of non-invasive radiofrequency treatment and hyperthermia on malignant and nonmalignant cells. Int J Environ Res Public Health. 2014;11:9142-9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Overgaard J. Effect of hyperthermia on malignant cells in vivo: a review and a hypothesis. Cancer. 1977;39:2637-2646. [DOI] [PubMed] [Google Scholar]
- 18. Lutgens L, van der Zee J, Pijls-Johannesma M, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev. 2010;(3):CD006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev. 2009;(3):CD006269. [DOI] [PubMed] [Google Scholar]
- 20. Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287-295. [DOI] [PubMed] [Google Scholar]
- 21. Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia. 1990;6:479-486. [DOI] [PubMed] [Google Scholar]
- 22. Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163-169. [DOI] [PubMed] [Google Scholar]
- 23. Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Cancer Res Ther. 2010;6:492-496. [DOI] [PubMed] [Google Scholar]
- 24. Mitsumori M, Zhi-Fan Z, Oliynychenko P, et al. Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Clin Oncol. 2007;12:192-198. [DOI] [PubMed] [Google Scholar]
- 25. Group ICH, Vernon CC, Hand JW, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. Int J Radiat Oncol Biol Phys. 1996;35:731-744. [DOI] [PubMed] [Google Scholar]
- 26. van der Zee J, González DG, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet. 2000;355:1119-1125. [DOI] [PubMed] [Google Scholar]
- 27. Thomas GM. Concurrent chemotherapy and radiation for locally advanced cervical cancer: the new standard of care. Semin Radiat Oncol. 2000;10:44-50. [DOI] [PubMed] [Google Scholar]
- 28. Feyerabend T, Steeves R, Wiedemann GJ, et al. Local hyperthermia, radiation, and chemotherapy in locally advanced malignancies. Oncology. 1996;53:214-220. [DOI] [PubMed] [Google Scholar]
- 29. Issels R, Schlemmer M. Current trials and new aspects in soft tissue sarcoma of adults. Cancer Chemother Pharmacol. 2002;49:4-8. [DOI] [PubMed] [Google Scholar]
- 30. Trabulsi NH, Patakfalvi L, Nassif MO, et al. Hyperthermic isolated limb perfusion for extremity soft tissue sarcomas: systematic review of clinical efficacy and quality assessment of reported trials. J Surg Oncol. 2012;106:921-928. [DOI] [PubMed] [Google Scholar]
- 31. Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21:4270-4276. [DOI] [PubMed] [Google Scholar]
- 32. Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2011;107:912-918. [DOI] [PubMed] [Google Scholar]
- 33. Kitamura K, Ishida M, Kimura Y, Saeki H, Maehara Y, Sugimachi K. Early report of correlation between the thermal dosage and the treatment effect of hyperthermia in combination with chemoradiotherapy for esophageal cancer patients. Hepatogastroenterology. 2002;49:1560-1562. [PubMed] [Google Scholar]
- 34. Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol. 2014;41:714-729. [DOI] [PubMed] [Google Scholar]
- 35. Griffin RJ, Dings RP, Jamshidi-Parsian A, Song CW. Mild temperature hyperthermia and radiation therapy: role of tumour vascular thermotolerance and relevant physiological factors. Int J Hyperthermia. 2010;26:256-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys. 1989;16:535-549. [DOI] [PubMed] [Google Scholar]
- 37. Toma-Dasu I, Dasu A. Modelling tumour oxygenation, reoxygenation and implications on treatment outcome. Comput Math Methods Med. 2013;2013:141087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fiorentini G, Szasz A. Hyperthermia today: electric energy, a new opportunity in cancer treatment. J Cancer Res Ther. 2006;2:41-46. [DOI] [PubMed] [Google Scholar]
- 39. Nishimura Y, Murata R, Hiraoka M. Combined effects of an angiogenesis inhibitor (TNP-470) and hyperthermia. Br J Cancer. 1996;73:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia. 2005;21:761-767. [DOI] [PubMed] [Google Scholar]
- 41. Yoo HT, Roh JR. Compendium of Prescriptions From the Countryside (Hyangyakjipseongbang). Vol 1433 Seoul, Republic of Korea: Hangrimchulpansa; 1977. [Google Scholar]
- 42. Choi W, Jung H, Kim K, et al. Rhus verniciflua Stokes against advanced cancer: a perspective from the Korean Integrative Cancer Center. J Biomed Biotechnol. 2012;2012:874276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Son YO, Lee KY, Lee JC, et al. Selective antiproliferative and apoptotic effects of flavonoids purified from Rhus verniciflua Stokes on normal versus transformed hepatic cell lines. Toxicol Lett. 2005;155:115-125. [DOI] [PubMed] [Google Scholar]
- 44. Jang HS, Kook SH, Son YO, et al. Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta. 2005;1726:309-316. [DOI] [PubMed] [Google Scholar]
- 45. Lee JC, Lee KY, Kim J, et al. Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem Toxicol. 2004;42:1383-1388. [DOI] [PubMed] [Google Scholar]
- 46. Lee SO, Kim SJ, Kim JS, Ji H, Lee EO, Lee HJ. Comparison of the main components and bioactivity of Rhus verniciflua Stokes extracts by different detoxification processing methods. BMC Complement Altern Med. 2018;18:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim JH, Shin YC, Ko SG. Integrating traditional medicine into modern inflammatory diseases care: multitargeting by Rhus verniciflua Stokes. Mediators Inflamm. 2014;2014:154561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: Panitumumab (Vectibix). Oncologist. 2007;12:577-583. [DOI] [PubMed] [Google Scholar]
- 49. Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol. 1991;14:133-141. [DOI] [PubMed] [Google Scholar]
- 50. Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marmor JB. Interactions of hyperthermia and chemotherapy in animals. Cancer Res. 1979;39(6 pt 2):2269-2276. [PubMed] [Google Scholar]
- 52. Dahl O. Interaction of hyperthermia and chemotherapy. In: RD Issels, W Wilmanns, eds. Application of Hyperthermia in the Treatment of Cancer. Berlin, Germany: Springer; 1988:157-169. [DOI] [PubMed] [Google Scholar]
- 53. Jia L, Ma S, Hou X, et al. The synergistic effects of traditional Chinese herbs and radiotherapy for cancer treatment. Oncol Lett. 2013;5:1439-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2008;30:300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pal HC, Baxter RD, Hunt KM, et al. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015;6:28296-28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Touil YS, Seguin J, Scherman D, Chabot GG. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol. 2011;68:445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith ML, Murphy K, Doucette CD, Greenshields AL, Hoskin DW. The dietary flavonoid fisetin causes cell cycle arrest, caspase-dependent apoptosis, and enhanced cytotoxicity of chemotherapeutic drugs in triple-negative breast cancer cells. J Cell Biochem. 2016;117:1913-1925. [DOI] [PubMed] [Google Scholar]
- 60. Kim JM, Noh EM, Kwon KB, et al. Suppression of TPA-induced tumor cell invasion by sulfuretin via inhibition of NF-κB-dependent MMP-9 expression. Oncol Rep. 2013;29:1231-1237. [DOI] [PubMed] [Google Scholar]
- 61. Lee KW, Chung KS, Seo JH, et al. Sulfuretin from heartwood of Rhus verniciflua triggers apoptosis through activation of Fas, Caspase-8, and the mitochondrial death pathway in HL-60 human leukemia cells. J Cell Biochem. 2012;113:2835-2844. [DOI] [PubMed] [Google Scholar]
- 62. Fan W, Sun L, Zhou JQ, et al. Marsdenia tenacissima extract induces G0/G1 cell cycle arrest in human esophageal carcinoma cells by inhibiting mitogen-activated protein kinase (MAPK) signaling pathway. Chin J Nat Med. 2015;13:428-437. [DOI] [PubMed] [Google Scholar]
- 63. Han SY, Zhao W, Sun H, et al. Marsdenia tenacissima extract enhances gefitinib efficacy in non-small cell lung cancer xenografts. Phytomedicine. 2015;22:560-567. [DOI] [PubMed] [Google Scholar]
- 64. Li W, Yang Y, Ouyang Z, et al. Xiao-Ai-Ping, a TCM injection, enhances the antigrowth effects of cisplatin on Lewis lung cancer cells through promoting the infiltration and function of CD8. Evid Based Complement Alternat Med. 2013;2013:879512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pang CLK. The immune regulating effect of hyperthermia in combination with TCM on cancer patients. Oncothermia J. 2016;18:170-179. [Google Scholar]
- 66. Westerheide SD, Bosman JD, Mbadugha BN, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053-56060. [DOI] [PubMed] [Google Scholar]
- 67. Vujaskovic Z, Poulson JM, Gaskin AA, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 2000;46:179-185. [DOI] [PubMed] [Google Scholar]
- 68. Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463-468. [DOI] [PubMed] [Google Scholar]
- 69. Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperthermia. 2009;25:610-616. [DOI] [PubMed] [Google Scholar]
- 70. Repasky E, Issels R. Physiological consequences of hyperthermia: heat, heat shock proteins and the immune response. Int J Hyperthermia. 2002;18:486-489. [DOI] [PubMed] [Google Scholar]
- 71. Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia. 2005;21:713-716. [DOI] [PubMed] [Google Scholar]
- 72. Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother. 2001;50:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10:550-558. [PMC free article] [PubMed] [Google Scholar]