Abstract
Aim:
Feminising hormone therapy with estradiol is used to align an individual’s physical characteristics with their gender identity. Given considerable variations in doses of estradiol therapy administered as gender-affirming hormone therapy (GAHT), we aimed to assess if body mass index (BMI) correlated with estradiol dose/concentration and assess the correlation between estradiol dose and estradiol concentrations.
Methods:
In a retrospective cross-sectional study, we analysed transgender individuals attending a primary or secondary care clinic in Melbourne, Australia who were prescribed oral estradiol valerate for at least 6 months and had estradiol dose and concentration available. Estradiol concentration was measured by immunoassay. Outcomes were the correlation between estradiol dose and BMI, and estradiol dose and estradiol concentration.
Results:
Data were available for 259 individuals {median age 25.8 [interquartile range (IQR) 21.9, 33.5] years}. Median duration of estradiol therapy was 24 (15, 33) months. Median estradiol concentration was 328 (238, 434) pmol/l [89 (65, 118) pg/ml] on 6 (4, 8) mg estradiol valerate. Median BMI was 24.7 (21.8, 28.6) kg/m2. There was a weak positive correlation between estradiol dose and estradiol concentration (r = 0.156, p = 0.012). There was no correlation between BMI and estradiol concentration achieved (r = −0.063, p = 0.413) or BMI and estradiol dose (r = 0.048, p = 0.536). Estradiol concentrations were within the target range recommended in consensus guidelines in 172 (66%) individuals.
Conclusion:
Estradiol dose was only weakly correlated with estradiol concentration, suggesting significant interindividual variability. Prescription of estradiol dose should not be based upon an individual’s BMI, which did not correlate with estradiol concentration achieved. In all, 66% of individuals achieved estradiol concentrations recommended in Australian consensus guidelines with a relatively high oral estradiol dose.
Keywords: estradiol, gender dysphoria, gender identity, transgender
Introduction
Transgender, including gender diverse and non-binary, individuals who seek feminisation of physical characteristics (hereafter termed transfeminine individuals) are typically treated with estradiol with or without anti-androgen to increase serum estradiol concentration and decrease serum testosterone concentration into the respective female reference ranges. This results in development of feminine physical characteristics, including softening of skin, a decrease in facial and body hair growth, breast development, and changes in body composition manifested by body fat redistribution and decreased muscle mass.1,2
Estradiol is most commonly administered via the oral or transdermal route,3 and oral estradiol valerate is the most common first-line feminising treatment in clinicians experienced in transgender healthcare in Australia.4 Several consensus guidelines give recommendations for estradiol concentrations to allow titration of estradiol therapy.1,5 The Australian ‘Position statement on the hormonal management of adult transgender and gender diverse individuals’ recommends targeting estradiol concentrations of 250–600 pmol/l (68–163 pg/ml) (GRADE 2D recommendation) based on local cross-sectional data.5 Notably, given the lack of data, this is an approximate guide and the position statement states that the value of biochemical testing in addition to clinical assessment is unclear. It is also unclear if body mass index (BMI) should be a consideration during estradiol prescribing.
As such, the aim of this retrospective study in adult transfeminine individuals on established estradiol therapy was to assess, firstly, the correlation between BMI and serum estradiol concentration, and secondly the relationship between estradiol dose and serum estradiol concentration. In order to assess implementation of current guidelines in clinical practice, we also aimed to assess the proportion of individuals achieving estradiol concentrations within the range now recommended by consensus guidelines.
Methods
A retrospective cross-sectional analysis was undertaken of consultations for gender-affirming hormone therapy (GAHT) at Equinox Gender Diverse Clinic, a primary care clinic specialising in transgender health, and an Endocrine Specialist Centre, a secondary care clinic, in Melbourne, Australia. Data were obtained from consultations between 1 January 2011 and 21 October 2019. The audit was approved by the Austin Health Human Research Ethics Committee (LNR/17/Austin/102) and Thorne Harbour Health (THH/CREP 19/015), who waived the need for informed consent.
Transfeminine individuals treated with oral estradiol for at least 6 months who had estradiol dose and estradiol concentration documented in their medical records were included in this retrospective cross-sectional analysis. For individuals with multiple estradiol concentrations available, the most recent prior to sex reassignment surgery was included. The primary outcome of interest was to establish the correlation between BMI and estradiol concentration, and estradiol dose and estradiol concentration. We also aimed to establish the proportion of individuals achieving estradiol concentrations in consensus guidelines.
As data were obtained retrospectively, sex steroid concentrations were performed using immunoassay available at several laboratories used as standard care in clinical practice. Multiple National Association of Testing Authorities (NATA, the national accreditation body for Australia) accredited laboratories were used. Time of blood sampling with respect to estradiol dosing was not systematically recorded in clinical notes and was therefore unavailable for analysis in this study.
Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences, Chicago, IL, USA). As data were not normally distributed, values are reported as median [interquartile range (IQR)]. Differences between groups were tested using the Mann–Whitney U test or Kruskal–Wallis test followed by Dunn’s post hoc comparisons. Spearman’s rank correlation coefficient was used to assess the correlation between variables. For all analyses, the level of significance was set at p < 0.05.
Results
A total of 390 transfeminine individuals were included in our analysis. After excluding individuals treated with concurrent transdermal estradiol (n = 34), ethinyl estradiol (n = 12), estradiol implant (n = 4), anti-androgen monotherapy (n = 3) or gonadotropin-releasing hormone (GnRH) subcutaneous implant (n = 3), 334 individuals treated with oral estradiol valerate were left for analysis. Of these, 259 were treated with oral estradiol valerate for at least 6 months and had estradiol concentration available prior to sex reassignment surgery.
In all, 73 individuals were treated with oral estradiol valerate without anti-androgen, 95 with spironolactone, 87 with cyproterone acetate, 2 with both cyproterone acetate and spironolactone, and 2 with bicalutamide. Concurrent progesterone was prescribed in 80 individuals, and concurrent finasteride in 12 individuals.
Median age of individuals was 25.8 (21.9, 33.5) years and duration of estradiol therapy was 24 (15, 33) months. Median BMI was 24.7 (21.8, 28.6) kg/m2. Median estradiol concentration achieved was 328 (238, 434) pmol/l [89 (65, 118) pg/ml] on 6 (4, 8) mg estradiol valerate.
There was no difference in the estradiol concentrations achieved between those treated with estradiol without anti-androgen, estradiol with spironolactone, or estradiol with cyproterone acetate (p = 0.264) (Table 1). There was no difference in the estradiol concentrations achieved between groups at each estradiol valerate dose (data not shown). There was no difference in the estradiol concentrations achieved between those treated with [331 (259, 453) pmol/l] or without [325 (238, 423) pmol/l] concurrent progesterone therapy (p = 0.354).
Table 1.
Characteristics by treatment group.
| Estradiol without anti-androgen (n = 73) | Estradiol with spironolactone (n = 95) | Estradiol with cyproterone acetate (n = 87) | p value | |
|---|---|---|---|---|
| Age (years) | 27.9 (22.8, 38.6) | 25.4 (20.9, 30.2) | 25.4 (21.8, 30.2) | 0.058 |
| Duration of GAHT (months) | 18.1 (12.9, 29.7) | 24.4 (15.0, 32.4) | 24.3 (15.5, 38.3) | 0.130 |
| Estradiol valerate dose (mg) | 6 (4, 6) | 6 (6, 8) | 6 (4, 8) | 0.015 |
| Estradiol concentration (pmol/l) | 332 (225, 416) | 355 (271, 456) | 304 (230, 417) | 0.264 |
| BMI (kg/m2) | 25.3 (22.7, 28.7) | 24.3 (21.6, 28.2) | 24.7 (20.7, 28.9) | 0.353 |
There was a weak positive correlation between estradiol dose and estradiol concentration (r = 0.156, p = 0.012) (Figure 1). There was no correlation between BMI and estradiol concentration achieved (r = –0.063, p = 0.413) (Figure 2) or BMI and estradiol dose (r = 0.048, p = 0.536).
Figure 1.
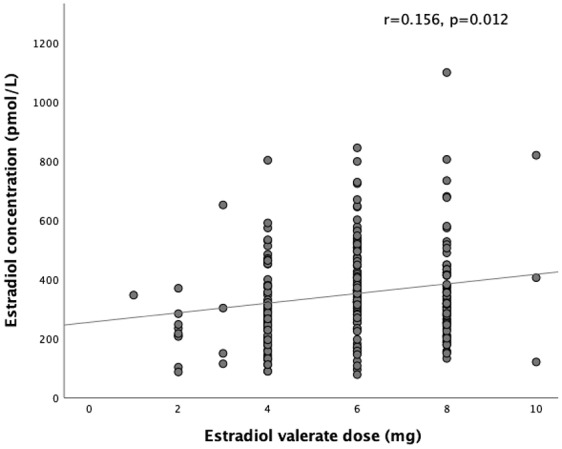
Correlation between estradiol valerate dose and estradiol concentration.
BMI, body mass index.
Figure 2.
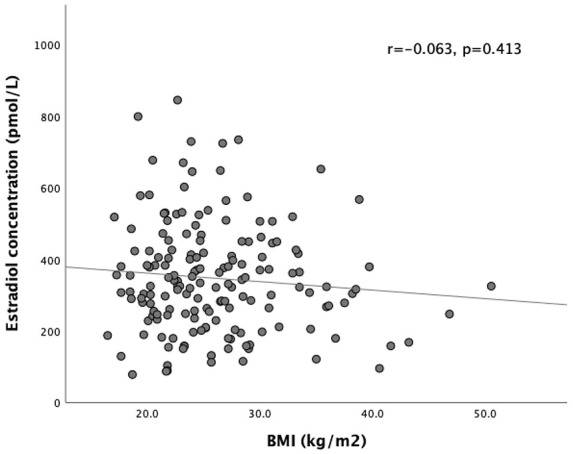
Lack of correlation between BMI and estradiol concentration.
BMI, body mass index.
Table 2 demonstrates the estradiol concentrations achieved and the proportion of individuals achieving recommended estradiol concentrations in consensus guidelines for each estradiol valerate dose. A total of 172 (66%) individuals had estradiol concentrations within the target range of 250–600 pmol/l5; 70 (27%) individuals had estradiol concentrations below target, and 17 (7%) above target. However, if using the Endocrine Society Clinical Practice Guidelines target of 367–734 pmol/l (100–200 pg/ml),1 95 (35%) individuals reached target concentrations with 158 (61%) below target and 6 (2%) above target.
Table 2.
Estradiol concentration by oral estradiol valerate dose.
| Estradiol valerate dose (mg) | Number of individuals | Estradiol concentration (pmol/l) | Number of individuals reaching target in Australian guidelines | Number of individuals reaching target in Endocrine Society guidelines |
|---|---|---|---|---|
| 1 | 2 | 246 (196, 297) | 1 (50%) | 0 (0%) |
| 2 | 9 | 217 (207, 248) | 2 (22%) | 1 (11%) |
| 3 | 4 | 226 (141, 390) | 1 (25%) | 1 (25%) |
| 4 | 63 | 293 (207, 381) | 39 (62%) | 20 (32%) |
| 6 | 111 | 362 (299, 470) | 83 (75%) | 51 (46%) |
| 8 | 67 | 319 (249, 423) | 45 (67%) | 25 (37%) |
| 10 | 3 | 406 (263, 613) | 1 (33%) | 2 (66%) |
Median (IQR) are presented.
IQR, interquartile range.
Discussion
In this retrospective cross-sectional analysis of transfeminine individuals treated with oral estradiol valerate, there was no correlation between BMI and estradiol concentration. There was a weak positive correlation between estradiol valerate dose and estradiol concentration with significant interindividual variability. In all, 66% of individuals achieved estradiol concentrations within Australian consensus guideline recommendations of 250–600 pmol/l, with small numbers achieving supraphysiological concentrations.
Association between BMI and estradiol concentration
Observational studies have demonstrated discrepant results with respect to the impact of BMI on estradiol concentration in women treated with menopausal hormone therapy. BMI was found to be positively associated with estradiol concentration in a nested case-control study evaluating the influence of sex steroids on breast cancer risk,6 whereas increasing BMI was associated with a lower odds of achieving an estradiol concentration greater than 165 pmol/l in a cross-sectional analysis of 309 post-menopausal women.7 Other studies have found no association.8,9
In a previous retrospective analysis of 184 transfeminine individuals, BMI was positively associated with estradiol concentration, but the variance attributable to BMI was small.10 BMI did not influence estradiol dose.10 In another retrospective cohort of 84 individuals, there was no statistically significant correlation between BMI and the estradiol concentration achieved though there was a trend toward a positive correlation.11 However, of the individuals who achieved estradiol concentration within the target range, there was a significant negative correlation between estradiol dose and BMI.11 The median BMI reported in both studies was significantly higher than our cohort, which could contribute to the discrepancy between previous studies and the current findings.
Association between estradiol dose and estradiol concentration
The association between estradiol dose and estradiol concentration in transfeminine individuals was evaluated in two previous retrospective analyses. Both studies reported a positive correlation between estradiol dose and the estradiol concentration achieved.10,11 Spironolactone was reported to reduce the effectiveness of estradiol dosing achieving desired estradiol concentrations.10 However, our study did not find a difference in estradiol concentration achieved between anti-androgen groups, and further prospective studies are required. The weak association between estradiol dose and serum estradiol concentration highlights significant interindividual variability, where different individuals achieve similar serum estradiol concentrations on wide estradiol doses.
Estradiol concentrations and consensus guidelines
No studies have examined the optimal estradiol concentrations in transfeminine individuals. Based on cross-sectional data,12 Australian consensus guidelines recommend targeting trough estradiol concentrations of 250–600 pmol/l,5 whereas the Endocrine Society Clinical Practice Guidelines recommend a target range of 367–734 pmol/l based on the physiological range of pre-menopausal women.1 The estradiol assay used and timing of blood testing in relation to the estradiol dose are other important considerations when interpreting the estradiol concentration.
Estradiol concentrations are best measured via liquid chromatography–mass spectrometry (LC-MS) given that immunoassays lack selectivity and precision, particularly at low estradiol concentrations.13 The clinician must also consider the estradiol concentration in relation to the timing of the estradiol dose, given that peak estradiol concentration is generally 4–5 hours post-dose with an elimination half-life of 14–22 h.14 In one study in which transfeminine individuals were treated with 2 mg oral estradiol valerate, there was a peak median estradiol concentration of 189 (99) pmol/l with 24-h post-dose concentration of 56 (154) pmol/l.15 No large study has evaluated the pharmacokinetic parameters at doses used in feminising hormone therapy regimens.
Various clinical guidelines also give recommendations for estradiol dosing. The Endocrine Society Clinical Practice Guidelines recommend estradiol dosing of 2–6 mg daily,1 whereas the European Network for Investigation of Gender Incongruence (ENIGI) use an oral estradiol valerate dose of 2 mg twice daily.16 Despite a median estradiol valerate dose higher than that used in ENIGI, only one-third of individuals in our cohort achieved estradiol concentrations recommended in international guidelines.
Retrospective analyses have documented the proportion of individuals achieving estradiol concentrations in consensus guidelines; 77/136 (55.7%) achieved an estradiol concentration in the Endocrine Society Clinical Practice Guidelines target range on 4–8 mg/day oral estradiol valerate.10 Only 21/136 (15.4%) achieved target estradiol concentrations on 4 mg/day; 13 individuals (9.6%) did not achieve target estradiol concentration at 8 mg oral estradiol valerate. Similarly, 35 (41%) individuals were able to achieve estradiol concentrations within the Endocrine Society Clinical Practice Guidelines in a separate analysis.11 The estradiol valerate doses that achieved target estradiol concentrations were not reported, but the entire cohort had a dose range 1–10 mg/day.
Clinical implications
The value of biochemical monitoring is currently unclear, and no prospective study has been designed to compare the effectiveness of different routes of estradiol administration or varying estradiol concentrations. Prospective studies from the ENIGI cohort have reported that estradiol concentration did not predict breast development or more feminine changes in body composition in transfeminine individuals.2,17 However, higher estradiol concentrations have been associated with a higher lumbar spine bone mineral density.18 Ultimately, the best assessment of hormonal efficacy is the clinical response.19
Ensuring adequate feminisation also needs to be weighed against the potential risks of escalating estradiol doses. There is evidence in post-menopausal women that higher oral estradiol doses are associated with an increased prevalence of venous thromboembolic (VTE) events.20 However, recent evidence in transfeminine individuals has demonstrated a low incidence of VTE.21
No prospective studies have assessed the effectiveness of different estradiol concentration targets on clinical features of feminisation or potential adverse events. Until further data are available, current guidelines provide clinicians with target ranges that allow suppression of testosterone while avoiding supraphysiological estradiol concentrations. Further research is required.
Limitations
This study has several limitations. The most critical of which is the lack of patient-reported outcomes or objective measures of feminisation such as breast development. Although most clinicians monitor trough estradiol concentrations, the concentrations reported represent a single time point as part of routine clinical care so are not strictly collected in a standardized manner, and we do not have data on compliance with therapy. We cannot be certain that an individual would have achieved stable dosing by 6 months but the clinical practice of clinicians at these clinics is to up-titrate over a period of approximately 3–6 months so the 6 month cut-off was an arbitrary timepoint to ensure that the gradual titration often seen at initiation of feminising hormone therapy was not captured. Data on medications that could influence estradiol metabolism such as cytochrome P450 3A4 (CYP3A4) modulators were not collected.22 Given that bloods were taken as standard care, estradiol was measured via immunoassay and not liquid chromatography tandem mass spectrometry (LCMS/MS). However, all were performed using NATA-accredited laboratories.
Conclusion
No correlation between BMI and serum estradiol concentration is apparent, and a weak positive correlation between estradiol dose and serum estradiol concentration exists; 66% of transfeminine individuals treated with oral estradiol valerate had a serum estradiol concentration within the target range recommended by the Australian consensus guidelines. Reassuringly, few individuals had supraphysiological estradiol concentrations. Given the limited evidence base, it remains reasonable for clinicians to consider other factors, such as clinical feminisation, patient satisfaction, and potential risks of escalating estradiol dose, in making dosing decisions. Further prospective longitudinal studies comparing various estradiol concentration targets or doses and clinical features of feminisation are required.
Footnotes
Author contribution(s): Brendan J Nolan: Conceptualization; Formal analysis; Methodology; Writing-original draft; Writing-review & editing.
Adam Brownhill: Conceptualization; Investigation; Resources; Writing-review & editing.
Ingrid Bretherton: Investigation; Resources; writing-review & editing.
Peggy Wong: Investigation; Resources; Writing-review & editing.
Susan Fox: Investigation; Resources; Writing-review & editing.
Peter Locke: Investigation; Resources; Writing-review & editing.
Nicholas D Russell: Investigation; Resources; Writing-review & editing.
Mathis Grossmann: Methodology; Supervision; Writing-review & editing.
Jeffrey D Zajac: Methodology; Supervision; Writing-review & editing.
Ada S. Cheung: Conceptualization; Methodology; Supervision; Writing-review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Brendan Nolan is supported by the Royal Australasian College of Physicians Fellows Research Entry Scholarship. Ada Cheung is supported by an Australian Government National Health and Medical Research Council Early Career Fellowship (#1143333) and receives research support from the Viertel Charitable Foundation Clinical Investigator Award, Endocrine Society of Australia Postdoctoral Award and the Royal Australasian College of Physicians.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ada S. Cheung  https://orcid.org/0000-0001-5257-5525
https://orcid.org/0000-0001-5257-5525
Contributor Information
Brendan J. Nolan, Department of Medicine, Austin Health, The University of Melbourne, Studley Road, Heidelberg, Victoria 3084, Australia; Department of Endocrinology, Austin Health, Heidelberg, Victoria, Australia.
Adam Brownhill, Equinox Gender Diverse Clinic, Thorne Harbour Health, Fitzroy, Victoria, Australia.
Ingrid Bretherton, Department of Endocrinology, Austin Health, Heidelberg, Victoria, Australia; Department of Medicine, Austin Health, University of Melbourne, Heidelberg, Victoria, Australia.
Peggy Wong, Equinox Gender Diverse Clinic, Thorne Harbour Health, Fitzroy, Victoria, Australia.
Susan Fox, Equinox Gender Diverse Clinic, Thorne Harbour Health, Fitzroy, Victoria, Australia.
Peter Locke, Equinox Gender Diverse Clinic, Thorne Harbour Health, Fitzroy, Victoria, Australia.
Nicholas Russell, Department of Endocrinology, Austin Health, Heidelberg, Victoria, Australia; Department of Medicine, Austin Health, University of Melbourne, Heidelberg, Victoria, Australia.
Mathis Grossmann, Department of Endocrinology, Austin Health, Heidelberg, Victoria, Australia; Department of Medicine, Austin Health, University of Melbourne, Heidelberg, Victoria, Australia.
Jeffrey D. Zajac, Department of Endocrinology, Austin Health, Heidelberg, Victoria, Australia Department of Medicine, Austin Health, University of Melbourne, Heidelberg, Victoria, Australia.
Ada S. Cheung, Department of Endocrinology, Austin Health, Heidelberg, Victoria, Australia Department of Medicine, Austin Health, University of Melbourne, Heidelberg, Victoria, Australia.
References
- 1. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017; 102: 3869–3903. [DOI] [PubMed] [Google Scholar]
- 2. Klaver M, de Blok CJM, Wiepjes CM, et al. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol 2018; 178: 163–171. [DOI] [PubMed] [Google Scholar]
- 3. Cheung AS, Ooi O, Leemaqz S, et al. Sociodemographic and clinical characteristics of transgender adults in Australia. Transgend Health 2018; 3: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bretherton I, Thrower E, Grossmann M, et al. Cross-sex hormone therapy in Australia: the prescription patterns of clinicians experienced in adult transgender healthcare. Intern Med J 2019; 49: 182–188. [DOI] [PubMed] [Google Scholar]
- 5. Cheung AS, Wynne K, Erasmus J, et al. Position statement on the hormonal management of adult transgender and gender diverse individuals. Med J Aust 2019; 211: 127–133. [DOI] [PubMed] [Google Scholar]
- 6. Tworoger SS, Missmer SA, Barbieri RL, et al. Plasma sex hormone concentrations and subsequent risk of breast cancer among women using postmenopausal hormones. J Natl Cancer Inst 2005; 97: 595–602. [DOI] [PubMed] [Google Scholar]
- 7. Gavaler JS. Oral hormone replacement therapy: factors that influence the estradiol concentrations achieved in a multiracial study population. J Clin Pharmacol 2002; 42: 137–144. [DOI] [PubMed] [Google Scholar]
- 8. Lambrinoudaki I, Armeni E, Rizos D, et al. Sex hormones in postmenopausal women receiving low-dose hormone therapy: the effect of BMI. Obesity (Silver Spring) 2011; 19: 988–993. [DOI] [PubMed] [Google Scholar]
- 9. Waaseth M, Bakken K, Dumeaux V, et al. Hormone replacement therapy use and plasma levels of sex hormones in the Norwegian Women and Cancer postgenome cohort - a cross-sectional analysis. BMC Womens Health 2008; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leinung MC, Feustel PJ, Joseph J. Hormonal treatment of transgender women with oral estradiol. Transgend Health 2018; 3: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez JD, Kendjorsky K, Narla A, et al. Assessment of gender-affirming hormone therapy requirements. LGBT Health 2019; 6: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angus L, Leemaqz SY, Ooi O, et al. Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. Endocr Connect 2019; 8: 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Denver N, Khan S, Homer NZM, et al. Current strategies for quantification of estrogens in clinical research. J Steroid Biochem Mol Biol 2019; 192: 105373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarvinen A, Kainulainen P, Nissila M, et al. Pharmacokinetics of estradiol valerate and medroxyprogesterone acetate in different age groups of postmenopausal women. Maturitas 2004; 47: 209–217. [DOI] [PubMed] [Google Scholar]
- 15. Hiransuthikul A, Janamnuaysook R, Himmad K, et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc 2019; 22: e25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dekker MJ, Wierckx K, Van Caenegem E, et al. A European network for the investigation of gender incongruence: endocrine part. J Sex Med 2016; 13: 994–999. [DOI] [PubMed] [Google Scholar]
- 17. de Blok CJM, Klaver M, Wiepjes CM, et al. Breast development in transwomen after 1 year of cross-sex hormone therapy: results of a prospective multicenter study. J Clin Endocrinol Metab 2018; 103: 532–538. [DOI] [PubMed] [Google Scholar]
- 18. Wiepjes CM, de Jongh RT, de Blok CJ, et al. Bone safety during the first ten years of gender-affirming hormonal treatment in transwomen and transmen. J Bone Miner Res 2019; 34: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend 2012; 13: 165–232. [Google Scholar]
- 20. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ 2019; 364: k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnold JD, Sarkodie EP, Coleman ME, et al. Incidence of venous thromboembolism in transgender women receiving oral estradiol. J Sex Med 2016; 13: 1773–1777. [DOI] [PubMed] [Google Scholar]
- 22. Blode H, Zeun S, Parke S, et al. Evaluation of the effects of rifampicin, ketoconazole and erythromycin on the steady-state pharmacokinetics of the components of a novel oral contraceptive containing estradiol valerate and dienogest in healthy postmenopausal women. Contraception 2012; 86: 337–344. [DOI] [PubMed] [Google Scholar]


