Abstract
Purpose:
This study aimed to evaluate the clinical features and outcomes of osteosarcoma to identify prognostic factors and determine new strategies to improve overall survival.
Patients and Methods:
We retrospectively analyzed 12 cases of osteosarcoma treated at our hospital from 2012 to 2017. Tumor site, tissue type, stage, treatments, adverse effects, postoperative limb function, surgical margin, and final outcomes were evaluated.
Results:
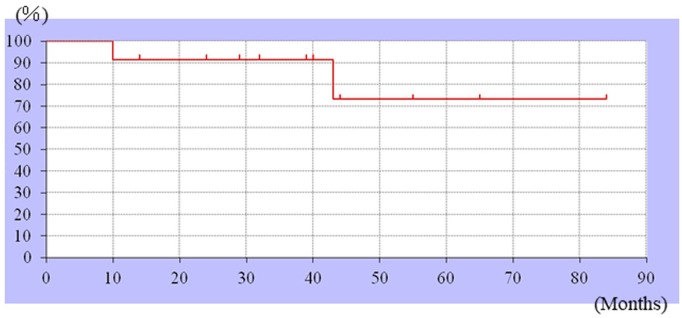
All patients received chemotherapy, and 10 underwent wide resection. The Musculoskeletal Tumor Society scores were more than good in all cases, and the 3-year survival rate was 73.3%. Two patients are alive with disease, eight have remained disease-free, and two died of the disease. Three of the four recurrent cases involved the pelvis.
Conclusion:
The treatment of primary osteosarcoma with wide resection in our department, therefore, yielded favorable outcomes. However, improved treatment strategies are needed for pelvic and advanced cases.
Keywords: Clinical outcome, osteosarcoma, comprehensive treatment, heavy particle radiation, pelvic
Introduction
Osteosarcoma is the most common malignant tumor of the bone and is derived from primitive mesenchymal cells. Usually, the tumors originate from bone and rarely from soft tissue.1 Osteosarcoma commonly affects children, adolescents, and young adults and accounts for 15% of all solid cancers in this age group.1–3 Although the survival rate of patients with osteosarcoma has been extremely poor in the past few decades, it has dramatically improved with the establishment of chemotherapy.4 In general, cisplatin, doxorubicin, high-dose methotrexate, and ifosfamide are considered the active agents against osteosarcoma.1 Advances in biomedical engineering have led to a major shift toward limb-preserving surgery instead of amputation surgery. Options for reconstruction after limb-preserving tumor resections include endoprosthetic implants or reconstruction by liquid nitrogen. In this way, surgical treatments have also improved the quality of life of the survivors, mainly because of preservation surgeries performed for the extremities to preserve the affected limbs.5 These findings show that the treatment for osteosarcoma is evolving. Although new treatment approaches have been developed, thus far, no curative treatment protocol for patients with unresectable osteosarcomas has been developed. Therefore, treatment strategies have room for improvement.1,6 Most recently, heavy particle radiation (HPR) therapy has been used to treat osteosarcoma.7 However, there have been a few reports on the outcomes of HPR treatment for resectable or unresectable osteosarcoma.7 This study aimed to investigate the results of treatments in osteosarcoma patients treated comprehensively in our department.
Patients and methods
The present retrospective study evaluated 12 patients (eight men and four women) with osteosarcoma who were treated in our department from March 2012 to March 2017 (Table 1). The median age of the patients was 22.5 years (range 8–74 years), and the average follow-up period was 39.5 months (range 10–84 months). The tumor site, tissue type, stage, treatments, postoperative limb function, effects and side effects of chemotherapy, surgical margin, survival, and final outcomes were researched. Patients with poor prognosis were evaluated in depth. The Enneking staging system was used for staging the disease,8 and adverse effects were assessed using the National Cancer Institute Common Toxicity Criteria (version 1).9 The surgical margins in the resected specimens were categorized as R0, R1, or R2 based on previous classifications.10 The Musculoskeletal Tumor Society (MSTS) score was used for limb function evaluation.11 The survival rate and event-free survival were calculated. The biopsies assessed in this study were all from patients with osteosarcoma treated at our hospital. All patients provided written informed consent to be included in this study.
Table 1.
Clinical features of patients with primary osteosarcoma treated comprehensively in our department.
| Patient number | Age (years)/sex | Site | Histological type | Stage | Treatment | Surgical margin | CT | CT response | Local recurrence | Metastasis | Metastasectomy | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17/M | Tibia | Conventional | 3 | CT, WR | R1 | NECO-95J | SD | + | + | − | 43 | DOD |
| 2 | 32/M | Pelvic | Conventional | 2B | CT, HPR | – | NECO-95J | PR | − | + | + | 55 | AWD |
| 3 | 8/M | Humerus | Fibroblastic | 3 | CT, WR, VBG | R0 | NECO-95J | PR | − | − | − | 29 | CDF |
| 4 | 52/M | Pelvic | Conventional | 2B | CT, WR, HPR | R1 | NECO-95J | PD | + | − | − | 32 | AWD |
| 5 | 28/F | Sacrum | Chondroblastic | 2B | CT, HPR | – | NECO-95J | PD | − | − | − | 10 | DOD |
| 6 | 14/M | Femur | Conventional | 2A | CT, WR | R0 | NECO-95J | CR | − | − | − | 24 | CDF |
| 7 | 13/F | Femur | Conventional | 2B | CT, WR | R0 | NECO-95J | PD | − | − | − | 65 | CDF |
| 8 | 15/F | Femur | Osteoblastic | 2A | CT, WR | R0 | NECO-95J | SD | − | − | − | 84 | CDF |
| 9 | 36/F | Tibia | Chondroblastic | 2A | CT, WR | R0 | NECO-95J | SD | − | − | − | 40 | CDF |
| 10 | 51/M | Humerus | Conventional | 1A | CT, WR | R0 | IA | SD | − | − | − | 44 | CDF |
| 11 | 74/F | Radius | Fibroblastic | 2B | CT, WR, LNB | R0 | IA | SD | − | − | − | 14 | CDF |
| 12 | 16/M | Tibia | Conventional | 2B | CT, WR, LNB | R0 | NECO-95J | PD | − | − | − | 39 | CDF |
NECO-95J: neoadjuvant chemotherapy for osteosarcoma – 95J; SD: stable disease; DOD: dead of the disease; HPR: heavy particle radiation; AWD: alive with disease; CDF: continuously disease-free; PD: progressive disease; IA: ifosfamide and doxorubicin; CR: complete response; PR: partial response; VBG: vascularized bone graft; LNB: liquid nitrogen treated bone; WR: wide resection; CT: chemotherapy.
Statistical analysis
The survival rate was determined using the Kaplan–Meier method. Statistical analysis was performed with StatMate (ATMS, Tokyo, Japan) software for Windows, version 5.01 as previously described.12
Results
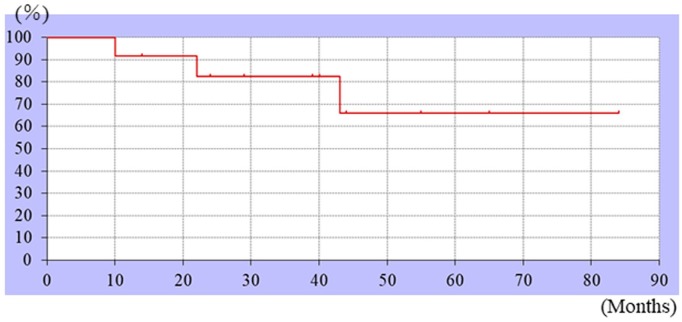
The tumor sites included the femur (n = 4), pelvis (n = 2), tibia (n = 3), humerus (n = 2), and radius (n = 1). Based on the histopathology, seven cases were classified as conventional, two as fibroblastic, two as chondroblastic, and one as an osteoblastic tumor. Among the 12 patients, one, three, six, and two patients had stage 1A, 2A, 2B, and 3 tumors, respectively. The treatments administered included chemotherapy, wide resection, and artificial joint replacement (Table 1). All enrolled patients received chemotherapy (10 patients: NECO-95J protocol13 and two patients: IA (ifosfamide and doxorubicin)). As surgical treatment, wide resection was performed in 10 cases. HPR was performed in the other two cases. For reconstruction, tumor-type arthroplasty was performed in six cases. Vascularized fibular bone graft was also performed in one case. Moreover, liquid nitrogen–treated bone was used in two cases. The MSTS scores (survey of nine cases with extremity reconstruction) were excellent for seven patients and good for two patients. The 3-year survival rate was 73.3% (Figure 1). The event-free survival rate was 66% (Figure 2). The outcomes of chemotherapy (with magnetic resonance imaging (MRI))14 included the following: complete response in one patient, partial response in two patients, stable disease (SD) in five patients, and progressive disease (PD) in four patients. The adverse events included mild to moderate neutropenia in all 12 patients and moderate kidney disorder (tubule injury) in two patients. No irreversible side effects were observed. The surgical margin was evaluated in 10 patients who underwent wide resection. While a positive microscopic margin (R1) was observed in two patients, a negative margin (R0) was seen in the other eight patients. One patient who underwent surgery with liquid nitrogen treatment developed a fracture 22 months after the first operation. Revision surgery was performed, which resulted in a non-union joint. Ten patients were found to be continuously disease-free (CDF). Of the two patients treated with chemotherapy and heavy ion beam irradiation, one was alive with disease (AWD; living with lung metastasis), while the other was dead of the disease (DOD). Of the two patients treated with heavy ion beam irradiation without surgery, one had two tumors. One tumor exhibited PD as evaluated by MRI after heavy ion beam irradiation, and the other exhibited SD. One patient still had a remaining tumor mass in the retroperitoneum and the other had lung metastasis after HPR. Therefore, both patients after HPR were not eligible for surgical treatment. In the other patient, a lung metastasis was detected 19 months after treatment, for which lower lobular resection was performed. Lung metastasis recurred, and a partial metastasectomy was performed, following which no more metastasis has been observed. The two patients who were AWD (including one patient with recurrence and another with lung metastasis) and two who were DOD were evaluated in greater detail. Three of these four patients (75%) had tumors in the pelvic region, and the tumor in the fourth patient originated in the tibia. The tumors originating in the tibia all had a positive microscopic margin (R1). Tumors in two of these four patients were unresectable because invasion around the main tumor was observed, whereas they were resectable in the other two patients.
Figure 1.
Kaplan–Meier curves showing the survival rate. The 3-year survival rate was 73.3%.
Figure 2.
Kaplan–Meier curves showing the event-free survival rate. The 3-year event-free survival was 66%.
Discussion
In our hospital, osteosarcoma patients are treated comprehensively for limb preservation. In this study, we evaluated the clinical outcomes of these treatments and found them to be favorable. Consistent with the results of previous studies, histologically, the common types of osteosarcomas seen were conventional, fibroblastic, and chondroblastic,13 and the most common stage was the Enneking stage 2B.5,15
Recent studies have shown that the 5-year survival rate for primary osteosarcomas is approximately 65%–70%,16,17 although a survival rate of approximately 90% has also been reported.18 Recent advances in chemotherapy and surgical treatments for osteosarcoma have improved the survival rates compared to those in the 1970s.19 One more most recent study showed that the 3-year survival rate was 79%20 and the event-free survival rate at 3 years was 69.5%.21 Similarly, in our hospital, the event-free survival rate at 3 years was 66% and the 3-year survival rate was 73.3%, with eight of the 10 cases that were originally resectable showing good outcomes (CDF). These findings suggest that osteosarcoma has been appropriately treated by a multidisciplinary approach and is expected to be curable.
According to a recent meta-analysis, the methotrexate, doxorubicin, and cisplatin regimen remains the preferred option for osteosarcoma chemotherapy.22 The most common grade 3/4 adverse event reported is neutropenia.22 Ten of the 12 patients in our study were administered chemotherapy according to the NECO-95J protocol, which resulted in a good histological response, was comparable to induction chemotherapy, and had a favorable overall survival23 (78.7%). Elderly patients were treated using the IA protocol. We observed no irreversible adverse events (Table 2).
Table 2.
Chemotherapy toxic effects, worst grade per patient.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Hematological | |||||
| White blood cells | 1 | 4 | 4 | 3 | 0 |
| Neutrophils | 0 | 5 | 4 | 3 | 0 |
| Platelets | 11 | 1 | 0 | 0 | 0 |
| Biochemical | |||||
| Creatinine | 0 | 2 | 1 | 0 | 0 |
| AST | 10 | 1 | 1 | 0 | 0 |
| ALT | 9 | 3 | 0 | 0 | 0 |
| Clinical | |||||
| Nausea | 10 | 2 | 0 | 0 | 0 |
| Vomiting | 3 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | 0 |
| Mucositis | 0 | 0 | 0 | 0 | 0 |
| Alopecia | 12 | 0 | 0 | 0 | 0 |
| Fever | 2 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 0 | 0 | 0 |
| Neurological | 0 | 0 | 0 | 0 | 0 |
| Cardiac | 0 | 0 | 0 | 0 | 0 |
AST: aspartate aminotransferase; ALT: alanine aminotransferase.
Tumor-type arthroplasty and liquid nitrogen treatment are the common methods used for reconstruction after wide resection.24 In the past few decades, limb salvage surgery has evolved,5,25 and good limb function has been reported after remodeling using both tumor-type joint replacement and liquid nitrogen treatment of the bone (95% MSTS score: ⩾good).5,26,27 With tumor-type arthroplasty, revision surgery is usually needed for a long-term follow-up, especially if the patient is a child.28 In this study, none of the patients who underwent arthroplasty required revision surgery. The MSTS score after tumor arthroplasty was generally good. However, with liquid nitrogen treatment, bone fractures, infections, soft tissue recurrences, and nerve palsy have been reported as surgical complications.29 Fractures and bone non-union are common following liquid nitrogen–treated bone reconstruction.30 Liquid nitrogen–treated bone combined with vascularized fibular bone graft has been shown to form a better union,31 but, in this study, the patient who underwent this treatment had a fracture and finally non-union.
The 5-year survival rate for osteosarcomas occurring in unresectable sites is estimated to be almost 0%.32 In contrast, the 5-year survival rate for patients with unresectable osteosarcomas treated with heavy particle radiotherapy is 33%.33 However, the effects of this treatment could not be determined in our institution.
In general, osteosarcomas metastasize mainly to the lungs.34 Previous studies have reported that approximately 10%–50% of osteosarcoma patients have lung metastasis at diagnosis,14,35,36 while another study reported that the incidence of lung metastasis in osteosarcoma patients is approximately 15%.37 Lung metastasis has a significant impact on the prognosis of osteosarcoma patients.34 Surgery and chemotherapy can significantly improve the survival of patients with metastases.34 In 2008, Chen et al.38 estimated that the 5-year survival rate of osteosarcoma patients with lung metastasis treated by pulmonary metastasectomy was 31%. In this study, we had one patient with lung metastasis who was treated surgically and is AWD. These findings suggest that aggressive surgical treatments result in favorable outcomes.
Pelvic osteosarcoma has a higher rate of local recurrence than recurrence in the limbs.39 In addition, inadequate margins can lead to poor prognosis.39 Other studies have also shown that the recurrence rate of osteosarcomas with an inadequate margin (⩾R1) is high and leads to poor prognosis.40 Moreover, given the same margin, pelvic osteosarcomas are more likely to recur locally than in the extremities.41 In this study, pelvic osteosarcoma patients with inadequate margins (⩾R1) also had a poor prognosis.
The major limitation of this study is the small sample size. Due to the short follow-up period, the 5-year survival rate and event-free survival rate could not be calculated. In addition, the number of unresectable cases was small, and therefore, a comparison with resectable cases was not made. However, we could address clinical outcomes of patients with osteosarcoma treated comprehensively with HPR treatment. As the number of unresectable cases is expected to increase over time, further assessments can be done in the future.
Conclusion
The treatment outcomes of osteosarcoma in our institution were good. Aggressive and comprehensive treatment of osteosarcomas provides a favorable prognosis.
Acknowledgments
The authors thank Editage (www.editage.jp) for English language editing.
Footnotes
Author contributions: K.H., S.N., and N.O. conceptualized and designed the study; K.H., N.O., and M.A. carried out the analysis and interpretation of the data. All authors drafted the manuscript and approved the final version.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Ethics Committee of Kindai University Hospital (approved number 31-253, Osaka, Japan). Ethical approval was not sought for this study because this is a retrospective study and results from conventional treatment according to the guidelines.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all patients before the study.
ORCID iD: Kazuhiko Hashimoto  https://orcid.org/0000-0002-8332-0063
https://orcid.org/0000-0002-8332-0063
References
- 1. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol 2010; 21: 320–325. [DOI] [PubMed] [Google Scholar]
- 2. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. In: Jaffe N, Bruland O, Bielack S. (eds) Pediatric and Adolescent Osteosarcoma. Boston, MA: Springer, 2009, pp. 3–13. [Google Scholar]
- 3. Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol 2015; 33(27): 3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Yang J, Zhao N, et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett 2018; 16(5): 6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamal AF, Rubiansyah P. Clinical outcome of various limb salvage surgeries in osteosarcoma around knee: megaprosthesis, extracorporeal irradiation and resection arthrodesis. Ann Med Surg 2019; 42: 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Qin G, Liang X, et al. Targeting the CK1α/CBX4 axis for metastasis in osteosarcoma. Nat Commun 2020; 11: 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blattmann C, Oertel S, Schulz-Ertner D, et al. Non-randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non-resectable osteosarcoma. BMC Cancer 2010; 10: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enneking WF, Spanier SS, Goodman MA. The classic: a system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 2003; 415: 4–18. [DOI] [PubMed] [Google Scholar]
- 9. Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012; 13(10): 1045–1054. [DOI] [PubMed] [Google Scholar]
- 10. Gundle KR, Kafchinski L, Gupta S, et al. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol 2018; 36(7): 704–709. [DOI] [PubMed] [Google Scholar]
- 11. Steffner RJ, Jang ES. Staging of bone and soft-tissue sarcomas. J Am Acad Orthop Surg 2018; 26: e269–e278. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto K, Nishimura S, Hara Y, et al. Clinical outcomes of patients with primary malignant bone and soft tissue tumor aged 65 years or older. Exp Ther Med 2019; 17: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein MJ, Siegal GP. Osteosarcoma. Am J Clin Pathol 2006; 125: 555–581. [DOI] [PubMed] [Google Scholar]
- 14. Ferguson WS, Harris MB, Goorin AM, et al. Presurgical window of carboplatin and surgery and multidrug chemotherapy for the treatment of newly diagnosed metastatic or unresectable osteosarcoma: Pediatric Oncology Group Trial. J Pediatr Hematol Oncol 2001; 23(6): 340–348. [DOI] [PubMed] [Google Scholar]
- 15. Huang T-L, Chen T-H, Chen WY-K, et al. Allograft arthrodesis of the knee in high-grade osteosarcoma. J Chinese Med Assoc 2005; 68: 425–430. [DOI] [PubMed] [Google Scholar]
- 16. Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 2006; 32(6): 423–436. [DOI] [PubMed] [Google Scholar]
- 17. Tsuchie H, Emori M, Nagasawa H, et al. Prognosis of primary osteosarcoma in elderly patients: a comparison between young and elderly patients. Med Princ Pract 2019; 28(5): 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuchiya H, Tomita K, Mori Y, et al. Marginal excision for osteosarcoma with caffeine assisted chemotherapy. Clin Orthop Relat Res 1999(358): 27–35. [PubMed] [Google Scholar]
- 19. Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am 2016; 47: 283–292. [DOI] [PubMed] [Google Scholar]
- 20. Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 2019; 109: 36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morsy AM, Ahmed BM, Rezk KM, et al. Age and tumor location predict survival in nonmetastatic osteosarcoma in upper Egypt. J Pediatr Hematol Oncol 2020; 42(2): e66–e78. [DOI] [PubMed] [Google Scholar]
- 22. Yu D, Zhang S, Feng A, et al. Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy. Medicine 2019; 98(19): e15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwamoto Y, Tanaka K, Isu K, et al. Multiinstitutional phase II study of neoadjuvant chemotherapy for osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop Sci 2009; 14(4): 397–404. [DOI] [PubMed] [Google Scholar]
- 24. Albergo JI, Gaston CL, Aponte-Tinao LA, et al. Proximal tibia reconstruction after bone tumor resection: are survivorship and outcomes of endoprosthetic replacement and osteoarticular allograft similar? Clin Orthop Relat Res 2017; 475: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Traven SA, Brinton DL, Walton ZJ, et al. A propensity-score matched analysis of limb salvage vs amputation for osteosarcoma. J Surg Oncol 2019; 120(7): 1252–1258. [DOI] [PubMed] [Google Scholar]
- 26. Yoshida Y, Osaka S, Tokuhashi Y. Analysis of limb function after various reconstruction methods according to tumor location following resection of pediatric malignant bone tumors. World J Surg Oncol 2010; 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gede EWI, Ida Ayu AA, Setiawan I, Gn Y, et al. Outcome of bone recycling using liquid nitrogen as bone reconstruction procedure in malignant and recurrent benign aggressive bone tumour of distal tibia: a report of four cases. J Orthop Surg 2017; 25(2): 713940. [DOI] [PubMed] [Google Scholar]
- 28. Futani H. Long-term follow-up after limb salvage in skeletally immature children with a primary malignant tumor of the distal end of the femur. J Bone Joint Surg Am 2006; 88(3): 595–603. [DOI] [PubMed] [Google Scholar]
- 29. Subhadrabandhu S, Takeuchi A, Yamamoto N, et al. Frozen autograft-prosthesis composite reconstruction in malignant bone tumors. Orthopedics 2015; 38(10): e911–e918. [DOI] [PubMed] [Google Scholar]
- 30. Moran M, Stalley PD. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high grade primary bone sarcomas. Arch Orthop Trauma Surg 2009; 129(10): 1339–1345. [DOI] [PubMed] [Google Scholar]
- 31. Nishida Y, Tsukushi S, Wasa J, et al. Vascularized fibular flaps enhance histological repair in pasteurized autogenous bone graft. Ann Plast Surg 2011; 67(4): 416–420. [DOI] [PubMed] [Google Scholar]
- 32. Jaffe N. Osteosarcoma: review of the past, impact on the future. Cancer Treat Res 2009; 152: 239–262. [DOI] [PubMed] [Google Scholar]
- 33. Matsunobu A, Imai R, Kamada T, et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 2012; 118(18): 4555–4563. [DOI] [PubMed] [Google Scholar]
- 34. Huang X, Zhao J, Bai J, et al. Risk and clinicopathological features of osteosarcoma metastasis to the lung: a population-based study. J Bone Oncol 2019; 16: 100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bentzen SM. Prognostic factor studies in oncology: osteosarcoma as a clinical example. Int J Radiat Oncol Biol Phys 2001; 49(2): 513–518. [DOI] [PubMed] [Google Scholar]
- 36. Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol 1993; 11(3): 449–453. [DOI] [PubMed] [Google Scholar]
- 37. Kaste SC, Pratt CB, Cain AM, et al. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis. Cancer 1999; 86(8): 1602–1608. [DOI] [PubMed] [Google Scholar]
- 38. Chen F, Miyahara R, Bando T, et al. Prognostic factors of pulmonary metastasectomy for osteosarcomas of the extremities. Eur J Cardiothorac Surg 2008; 34(6): 1235–1239. [DOI] [PubMed] [Google Scholar]
- 39. Chen Y, Shen Q, Gokavarapu S, et al. Osteosarcoma of head and neck: a retrospective study on prognostic factors from a single institute database. Oral Oncol 2016; 58: 1–7. [DOI] [PubMed] [Google Scholar]
- 40. Bertrand TE, Cruz A, Binitie O, et al. Do surgical margins affect local recurrence and survival in extremity, nonmetastatic, high-grade osteosarcoma? Clin Orthop Relat Res 2016; 474: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He F, Zhang W, Shen Y, et al. Effects of resection margins on local recurrence of osteosarcoma in extremity and pelvis: systematic review and meta-analysis. Int J Surg 2016; 36(Pt. A): 283–292. [DOI] [PubMed] [Google Scholar]