Abstract
The neuropeptide arginine-vasopressin (AVP) has long been implicated in the regulation of social behavior and communication in diverse taxa, often through its actions on the V1a receptor (V1aR) and in a sex-different and steroid-dependent way. One source of sex-different brain AVP is the steroid-sensitive and sexually-dimorphic AVP neurons in the bed nucleus of the stria terminalis (BNST), a cell population that regulates social behavior in a sex-dependent manner. Potential targets of these BNST-AVP cells include the lateral habenula (LHb) and dorsal raphe (DR), areas known to be important for social behavior, yet few studies have investigated AVP action within these regions. Consequently, to test if V1aR action in the LHb or DR controls social behavior in a sexually dimorphic manner, we administered a highly-specific V1aR antagonist (or saline vehicle) in the LHb or DR of C57BL/6 male and female mice and tested its effects on social investigation, social communication (urine marking, ultrasonic vocalizations), and territorial aggression. V1aR antagonism of the LHb or DR decreased male urine marking toward unfamiliar males, but not toward unfamiliar females. Additionally, V1aR blockade of the LHb decreased ultrasonic vocalizations generated in the presence of females. Social investigation, locomotion and aggressive behavior were not altered by V1aR antagonism in either area. Blocking V1aR in the LHb or DR of females had no effect, indicating V1aR action in the DR and LHb drives sex differences in social communication.
Introduction
Animals often display profound sex differences in social behavior and communication and especially in reproductively-oriented behaviors such as courtship displays, territorial marking, parenting, and copulation (Darwin 1871; Bradbury and Vehrencamp 1998; Scott et al. 2015; Song et al. 2018). In humans, dysfunction in social communication is a common feature of several psychopathologies, such as autism, that show substantial sex differences in prevalence and impact (Halladay et al., 2015). It is therefore plausible that sex differences in neural circuitry may contribute to sexually differentiated function and dysfunction in social behavior and communication. One of the largest and most evolutionarily conserved sex-different systems in the vertebrate brain is the male-biased and steroid-dependent expression of the neuropeptide vasopressin/vasotocin (AVP) (de Vries, 2008; de Vries and Panzica, 2006; Goodson and Bass, 2001). AVP, working through the vasopressin V1a receptor (V1aR), has been repeatedly implicated in modulation of social behaviors across vertebrate species, including humans, and often in a sex-different way (Choleris et al. 2009; Donaldson and Young 2008; Insel 2010; Duque-Wilckens et al. 2016; Guastella et al., 2010; Rilling et al., 2014). For example, AVP acts in various brain regions to alter vertebrate social communication (Goodson and Bass, 2001; Kelly and Goodson, 2013), maternal care (Bosch and Neumann, 2008), pair bonding (Carter et al., 1995; Jarcho et al., 2011; Young and Wang, 2004), and social recognition (Dantzer et al. 1988; Everts and Koolhaas 1999; Bielsky and Young 2004; Bielsky et al. 2004; Veenema et al. 2012; Johnson and Young 2017).
A number of brain regions involved in social and emotional behavior contain sex-different and steroid-sensitive AVP innervation and V1aR expression, such as the lateral septum (LS), ventral pallidum (VP), lateral habenula (LHb) and several hypothalamic and midbrain areas, including the dorsal raphe nuclei (DR) (Rood et al. 2013; Dumais and Veenema 2016). However, most examinations of the social role of AVP have focused on LS and, to a lesser degree, the VP and hypothalamus (Lim and Young 2004; Bielsky et al. 2005; Ophir et al. 2008; Gobrogge et al. 2009; Bredewold et al. 2014; Otero-Garcia et al. 2014; DiBenedictis et al. 2020). Consequently, we know little about the behavioral role of AVP in other areas previously implicated in social and emotional behavior, such as LHb and DR nuclei (Nagayasu 2017; Balázsfi et al. 2018; Yang et al. 2018; Congiu et al. 2019; Russo et al. 2019) even though they also contain dense, steroid-sensitive and sexually dimorphic AVP innervation (Rood et al. 2013). Moreover, AVP, acting through V1aR, indirectly excites DR serotonin neurons (Rood and Beck 2014) and recent work suggests that increased AVP is associated with decreased LHb output and decreased fear response in stressful situations (Zhang et al. 2016).
Most sex-different brain AVP innervation, including that of LHb and DR, likely originates from sex-different AVP-expressing neurons within the bed nucleus of the stria terminalis (BNST) (de Vries and Panzica, 2006), a key structure in the social behavior neural network. Therefore, we hypothesized that BNST-AVP inputs to DR and LHb regulate sexually differentiated effects of AVP on social communication. Indeed, selective lesions of BNST-AVP neurons reduces social investigation and alters social communication in males, but not females (Rigney et al. 2019). Consequently, we predict that blocking V1aR action in DR or LHb will recapitulate the sex-specific (male, but not female) deficits in social investigation and communication caused by BNST-AVP lesions. To test this, we assessed the effects of injecting a highly-selective V1aR antagonist (Manning et al. 2012) in the LHb or DR on male and female mouse communicative (ultrasonic vocalizations, urine marking, social investigation) and aggressive behaviors that are sexually differentiated (Kimura and Hagiwara 1985; Yang et al. 2013; Palanza and Parmigiani 2017; Zala et al. 2017).
Methods
Animals and husbandry
All mice were maintained at 22°C on a 12/12 h reverse light/dark cycle with food and water available ad libitum, housed in individually ventilated cages (Animal Care Systems), and provided with corncob bedding, a nestlet square, and a housing tube. All animal procedures were performed in accordance with the Georgia State University animal care and use committee regulations and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Subjects
Fifty-six male and female C57BL/6J mice between 4–8 weeks of age were obtained from Jackson Laboratories (stock # 000664) and were singly-housed for a minimum of one week prior to testing.
Stimulus animals
Adult CD1(ICR) mice obtained from Charles River Laboratories were used as stimuli for behavioral testing and to provide male and female subjects with social experience as strain differences between subjects and stimulus mice increase social investigation (Gheusi et al., 1994). Mice were used at 9 –16 weeks of age and were novel and unrelated to the subject to which they were exposed.
Female stimulus mice were group-housed, ovariectomized, implanted with an estradiol capsule (GDX+E), and given two sexual experiences before testing. Two groups of stimulus males were used for behavioral testing differed in their social experience, to either render them more competitive (“dominant”) or less competitive (“subordinate”). Male subordinate mice that were used in the home cage aggression tests and for providing aggressive experience to subjects were group-housed, gonadectomized (GDX), and subjected to two aggressive encounters as an intruder in a resident male’s home cage. Mice in the second group (dominant) were singly-housed, GDX, implanted with testosterone (GDX+T), and given two sexual experiences before testing; these males provided sexual experience to female subjects and served as stimulus animals in the three-chamber social tests.
Surgery
All surgeries were conducted using 1.5–3% isoflurane gas anesthesia in 100% oxygen with 3 mg/kg of carprofen given prior to surgery to reduce pain.
Gonadectomy and hormone treatment
Testes were cauterized and removed at the ductus deferens via a midline abdominal incision. SILASTIC capsules (1.5-cm active length; 1.02-mm inner diameter, 2.16-mm outer diameter; Dow Corning Corporation) were filled with crystalline T (Sigma) and inserted subcutaneously between the scapulae after gonadectomy; this procedure leads to physiologic levels of T (Barkley and Goldman, 1977; Matochik et al., 1994). To reduce aggression and promote submissive behavior in stimulus males (Beeman 1947), these mice were GDX but did not receive a T implant (GDX).
The ovaries of stimulus female mice were removed by cauterization at the uterine horn and attendant blood vessels. SILASTIC capsules (0.7-cm active length; 1.02-mm inner diameter, 2.16-mm outer diameter; Dow Corning Corporation) containing estradiol benzoate (E; diluted 1:1 with cholesterol) were implanted subcutaneously in the scapular region immediately following ovariectomy (GDX+E; Bakker et al. 2002; Ström et al. 2012). To induce sexual receptivity, stimulus females were injected subcutaneously with 0.1 mL of progesterone (500 μg dissolved in sesame oil, Sigma) 4 hours preceding sexual experience and behavioral testing (Veyrac et al. 2011).
Stereotaxic surgery and cannula implantation
Mice were positioned in a stereotaxic frame (David Kopf Instruments) with ear and incisor bars holding the skull level relative to bregma and lambda. After a midline scalp incision, a hand operated drill was used to make holes in the skull, exposing the dura. Subjects were then implanted (LHb animals bilaterally, DR animals unilaterally) with 26-gauge guide cannulas (Plastics One, Roanoke, VA) with a 1mm projection below the pedestal, fixed at a 15° angle 0.99mm anterior to bregma and 1.19mm lateral to the midline suture (LHb) or 4.3mm anterior to bregma and 0.78mm lateral to the midline suture (DR). DR injections were placed at a 15° angle to reduce the potential for V1aR antagonist crossing into the cerebral ventricle. Since crossing the ventricles was unavoidable in LHb injections, we analyzed a separate ‘miss’ group with cannula tracks ending in the lateral ventricle. Guide cannula were secured to the skull with dental cement and a skull screw, and dummy cannula of length equal to the guide cannula were inserted and screwed into place. All subjects were allowed to recover for at least 10 days prior to behavioral testing.
Drug injections
The highly-specific V1aR antagonist (d(CH2)5[Tyr(Me)2,Dab5] AVP; (a generous gift from M. Manning, University of Toledo)) was diluted in sterile saline to a final injected dose of 450ng/300nL and stored at −20°C until use. This antagonist, modified from the original Manning compound with the addition of diaminobutyric acid (Dab), is exceptionally selective for V1aR, eliciting no detectable anti-OT activity in vitro or in vivo (Chan et al. 1996; Manning et al. 2012); the dose selected here is effective in blocking AVP action in rodents (Lozić et al. 2016).
Forty five to sixty minutes prior to behavioral testing, subjects were briefly anesthetized (1.5–3% isoflurane gas) and a 33 gauge needle was inserted through the guide cannula, extending a total distance of 2.4 mm and 2.3 mm ventral from dura for LHb and DR animals, respectively. Subjects were then injected with 300 nL sterile saline (vehicle) or V1aR antagonist at 100nL/min (10 μL Hamilton syringe; Harvard Apparatus PHD 22/2000 syringe pump) via the guide cannula. The injection needle was left in place for one minute to allow the drug to diffuse away from the tip of the injection needle.
Social experience
As opposite-sex sexual experience and attaining competitive status (“social dominance”) promotes male and female communicative behaviors (Lumley et al. 1999; Roullet et al. 2011), male and female (adjusted for stage of estrous cycle; determined by vaginal lavage) mice received social experience (two sexual and two aggressive encounters on separate days) prior to testing.
Sexual experience
Subjects were given two opportunities to copulate with either a novel stimulus female (for male subjects) or a novel stimulus male (for female subjects). In the first interaction, a sexually experienced stimulus mouse was placed in the subject’s home cage overnight. In the second interaction, a novel stimulus mouse was placed in the subject’s home cage and removed 5 minutes after one ejaculation or 90 minutes in the absence of ejaculation. Subjects that did not engage in any sexual behavior (mounting, intromission, or ejaculation) during the second trial were removed from further testing.
Same-sex (aggressive) experience
Male and female subjects were exposed to two daily interactions with a stimulus animal of the same sex. Male subjects received two interactions with subordinate stimulus males that had 40 μL of freshly-collected GDX+T male urine (pooled from five males) applied to their backs to provide aggression-promoting cues to otherwise non-aggressive stimulus males (Beeman 1947; Connor and Winston 1972; Van Loo et al. 2001).
Stimulus males were placed in the subject’s home cage and removed after the subject’s first offensive attack (lunge with bite) within a 10 minute period. All subject males attacked the intruder male stimulus by the second encounter, and all subordinate stimulus males displayed submissive behavior, defined by defensive postures (e.g., on-back), fleeing, and non-social exploration (Koolhaas et al. 2013). Female subjects received two interactions with a group housed GDX stimulus female in their home cage over a 10 minute period. Female encounters rarely resulted in aggressive responses from either animal; however, if any aggressive behavior, though limited, occurred, the female was removed after the first offensive attack, much like following male-male interactions.
Experimental design
All testing occurred within the first 6 hours of the dark cycle under red light illumination. Subjects were habituated to the testing room and apparatus by handling and placing mice for 5 minutes in the three-chamber apparatus (see below) once a day for 3 days. On experimental days, subjects were adapted to the experimental room for 15 minutes before testing. All tests were scored by an experimenter blind to the drug treatment of the subject.
Subjects received a total of five injections of V1aR antagonist/vehicle across four tests for social communication in the three-chamber apparatus (two antagonist and two vehicle injections) and during one final test (antagonist or vehicle injection) within the subject’s home cage to measure any aggression during same-sex interactions. Each test was separated by at least three days. The order of treatment (saline, V1aR antagonist) and stimulus condition (male, female) was counterbalanced across subjects, except that subjects exposed to a stimulus type on the first test were then given that same stimulus type on the second test (Fig 1). Female subjects were tested irrespective of estrous cycle day; prior research indicates minimal effects of estrous cycle on female mouse communicative behavior (Maggio and Whitney 1985; Coquelin 1992; Moncho-Bogani et al. 2004). Following the final test, subjects were euthanized and injected through guide cannula with dilute India ink.
Figure 1.

Experimental timeline.
Social communication and behavior
Ultrasonic vocalizations (USV), urine marking, and social investigation by subjects was recorded in an acrylic three-chamber apparatus (Crawley 2007; Arakawa et al. 2008; Moy et al. 2009; Harvard Apparatus; dimensions: 20.3 × 42 × 22 cm). Instead of a solid floor, the apparatus was placed on absorbent paper (Nalgene Versi-dry paper, Thermo Fisher Scientific) so as to retain and accurately measure urine marking. Animals were tracked using motion detection software (ANY-maze, San Diego Instruments, RRID:SCR_014289). During testing with stimulus animals, subjects had access to a stimulus animal in a cage (8 cm (D), 18 cm (H); 3-mm diameter steel bars, 7.4 mm spacing) and an empty “clean” cage placed at opposite corners of the outermost chambers of the apparatus. The location of stimulus and clean cages was counterbalanced across animals. After placing the subject in the center of the middle chamber, we measured investigation of clean and stimulus cages, distance traveled throughout the apparatus, time spent in the chambers containing stimulus and clean cages as well as USVs and urine marking across a 5 minute trial. After testing, the apparatus and cages were thoroughly cleaned with 70% ethanol and allowed to dry before further testing.
Social investigation and USVs
Social investigation was defined as time spent sniffing within 2 cm of the stimulus or clean cage; climbing on the cage was not scored as investigation. USVs of subject and stimulus interactions were detected using a condenser microphone connected to an amplifier (UltraSoundGate CM16/CMPA, 10 –200 kHz, frequency range) placed 4 cm inside the apparatus and directly above the center compartment. USVs were sampled at 200 kHz (16-bit) with target frequency set to 70 kHz (UltraSoundGate 116Hb, Avisoft Bioacoustics). Recordings were then analyzed using a MATLAB (MathWorks, RRID:SCR_001622) plug-in that automates USV analysis (Van Segbroeck et al. 2017). Using this program, sonograms were generated by calculating the power spectrum on Hamming windowed data and then transformed into compact acoustic feature representations (Gammatone Filterbank). Each 200-ms window containing the maximum USV syllable duration was then clustered, via machine learning algorithms, into USV syllable types (repertoire units) based on time-frequency USV shape. Repertoire units that appeared as background noise were discarded. Both the number of all USV produced in each condition as well as the number of USV syllable types (using criteria previously described: short, composite, downward, upward, 1 frequency jump, modulated, multiple frequency jumps, u-shape, flat, chevron (Hanson and Hurley 2012)) were counted.
Urine marking
Following testing, the substrate sheet was allowed to dry for 1 hour and then sprayed with ninhydrin fixative (LC-NIN-16; Tritech Forensics Inc.) to visualize urine marks (Meyer 1957; Lehmann et al. 2013). After 24 hours, sheets were imaged (Sony DSC-S700 camera), binarized and analyzed using a computer-aided imaging software (ImageJ, RRID:SCR_003070). Urine marking was measured as both as the total area (cm2) covered and the total count of individual marks throughout the entire arena. Urine spots that were larger than 6 cm2 and directed toward corners were counted as eliminative “pools” and were excluded from analysis (Bishop and Chevins 1987).
Same-sex interactions and aggressive behavior
To measure territorial aggression, a subordinate male stimulus animal with applied urine (see above) was placed in a male subject’s home cage and then removed after the subject’s first offensive attack (lunge with bite) within a 10 minute period; the latency to first bite was recorded. Each female subject was presented with a GDX, group housed, female stimulus animal within the subject’s home cage for a 10 minute period. Though rare, if an offensive attack occurred, the stimulus animal was removed, much like following male-male interactions.
Tissue collection and histological analysis
Animals were euthanized by intraperitoneal injection of Beuthanasia-D (150 mg/kg) and subsequently injected through guide cannula with 300nL of dilute India ink. Brains were extracted and flash frozen on dry ice and stored at -80 °C until further processing. Coronal sections were cut at a thickness of 30 μm with a cryostat (Leica CM3050 S, Leica Biosystems) and analyzed for injection placement with reference to anatomical landmarks (ventricles, fiber tracts) and plotted on anatomical plates (Paxinos and Franklin 2012).
Statistical analysis
All data were analyzed and graphed in R (3.4.4; R Core Team, 2017). Social investigation, movement, USV, and urine marking data met the assumptions of parametric statistical tests. Therefore, we analyzed data on social investigation, movement (distance traveled, time in chambers containing stimulus and clean cages), number of USVs, and urine marking with mixed-model ANOVAs [between-subject factor: sex; within-subject factors: treatment (V1aR antagonist, vehicle), sex of stimulus (male, female)] followed by paired t tests assessing treatment effects. The number of specific USV syllables and aggressive behavior (latency to bite) were not normally distributed and could not be transformed, therefore, we analyzed treatment effects using pairwise Mann-Whitney U tests. All post hoc pairwise comparisons report Bonferroni-corrected p values and Cohen’s d for effect size when statistically significant. Results were considered statistically significant if p < 0.05.
Results
Histology
Subjects were included in the main LHb/DR analysis if the tip of the injection cannula was located within LHb bilaterally (10 males, 9 females; Fig. 2a) or the DR (10 males, 8 females; Fig. 2b). Eleven LHb targeted subjects (6 males, 5 females) were analyzed in a separate ‘miss’ group due to placement of injection needle tip outside the LHb, including subjects with only unilateral LHb cannulation (Fig. 2a, Table 5). Similarly, eight DR targeted subjects (males = 4, females = 4) were analyzed in a separate ‘miss’ group due to injection needle placement within the periaqueductal gray (PAG) (Fig. 2b, Table 5).
Figure 2.

Histological identification of injection sites. Coronal sections through the rostral-caudal extent of the mouse brain (relative to bregma) referenced to Paxinos and Franklin (2012). a. Lateral habenula (LHb) targeted subjects. Blue dots represent male subjects with bilateral injections into LHb (‘hits’, n=10), half-filled blue dots represent a subject with a unilateral LHb injection (‘unilateral hit’, n=1), and open blue dots represent bilateral injections outside of LHb (‘bilateral misses’, n=5). Orange dots represent female subjects with bilateral injections into LHb (‘hits’, n=9), half-filled orange dots represent a subject with a unilateral LHb injection (‘unilateral hit’, n=3), and open orange dots represent bilateral injections outside of LHb (‘bilateral misses’, n=5). b. Dorsal raphe (DR) targeted subjects. Blue dots represent male subjects with injections into DR (‘hits’, n=10) and open blue dots represent injections outside of DR (‘misses’, n=4). Orange dots represent female subjects with injections into DR (‘hits’, n=8) and open orange dots represent injections outside of DR (‘misses’, n=4).
Table 5.
Table of median (interquartile range) of urine mark (UM) counts, UM area (in pixels), USV counts, and social investigation (SI, seconds) produced by LHb ‘miss’ and DR ‘miss’ subjects. Male LHb ‘miss’ subjects (n=6) reduced the number of UM (counts, p=0.007) to female stimuli following infusion of V1aR antagonist compared to vehicle injections. Male DR ‘miss’ subjects (n=4) reduced the number of UM (counts, p=0.03) to male stimuli following infusion of V1aR antagonist compared to vehicle injections. Bold numbers represent significant differences between treatment groups.
| treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| stimulus | ||||||||
| LHb ‘miss’ subjects | ||||||||
| UM (count) | 70 (30–118) | 49 (31–134) |
(p=0.007) 14 (4–85) |
53 (0–112) | 0 (0–0) | 0 (0–16) | 0 (0–112) | 0(0–0) |
| UM (pixels) | 7640 (356019277) | 19871 (53628798) | 12042 (85135031) | 8934 (060452) | 0 (0–0) | 0 (0–3888) | 0 (0–323) | 0(0–0) |
| USV (count) | 463 (187–848) | 24 (9–132) | 400 (7–1256) | 23 (16–58) | 16 (7–70) | 24 (17–25) | 257 (1–398) | 16 (14–21) |
| SI (s) | 37 (25–64) | 23 (13–39) | 56 (26–91) | 16 (11–40) | 20 (17–26) | 8 (5–113) | 18 (12–33) | 13 (5–118) |
| DR ‘miss’ subjects | ||||||||
| UM (count) | 83 (34–118) | 101 (31–136) | 30 (0–179) |
(p=0.03) 28 (0–57) |
0 (0–0) | 0(0–0) | 0(0–0) | 0(0–7) |
| UM (pixels) | 24044 (1238862580) | 18865 (556832421) | 9509 (023348) | 4481 (08962) | 0 (0–0) | 0(0–0) | 0(0–0) | 0 (0–1975) |
| USV (count) | 25 (11–155) | 1 (0–2) | 68 (4–461) | 4 (1–14) | 5 (2–12) | 30 (1–49) | 7 (3–17) | 8 (4–17) |
| SI (s) | 45 (17–89) | 24 (6–36) | 39 (23–66) | 27 (3–30) | 26 (20–34) | 18 (11–25) | 24 (23–26) | 8 (7–19) |
V1aR antagonism within lateral habenula
LHb V1aR antagonism decreased male urine marking to males
Male subjects produced more urine marks than female subjects (F(1,17) = 21.25, p = 0.0003) with an interaction between sex of subject and treatment (F(1,17) = 5.37, p = 0.03) and a trend toward an interaction between sex of subject and sex of stimulus (F(1,17) = 4.0, p = 0.06). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased urine marks to male stimuli compared to when given vehicle (saline) injections (t(9) = 3.84, p = 0.004, d = 1.22; Fig. 3a); this effect was not observed in response to female stimuli (t(9) = 0.70, p = 0.50; Fig. 3b). Injections of V1aR antagonist in female subjects did not alter the number of urine marks to male stimuli (t(8) = 1.84, p = 0.10; Fig. 3c) or female stimuli (no marking; Fig. 3d) compared to vehicle injections.
Figure 3.
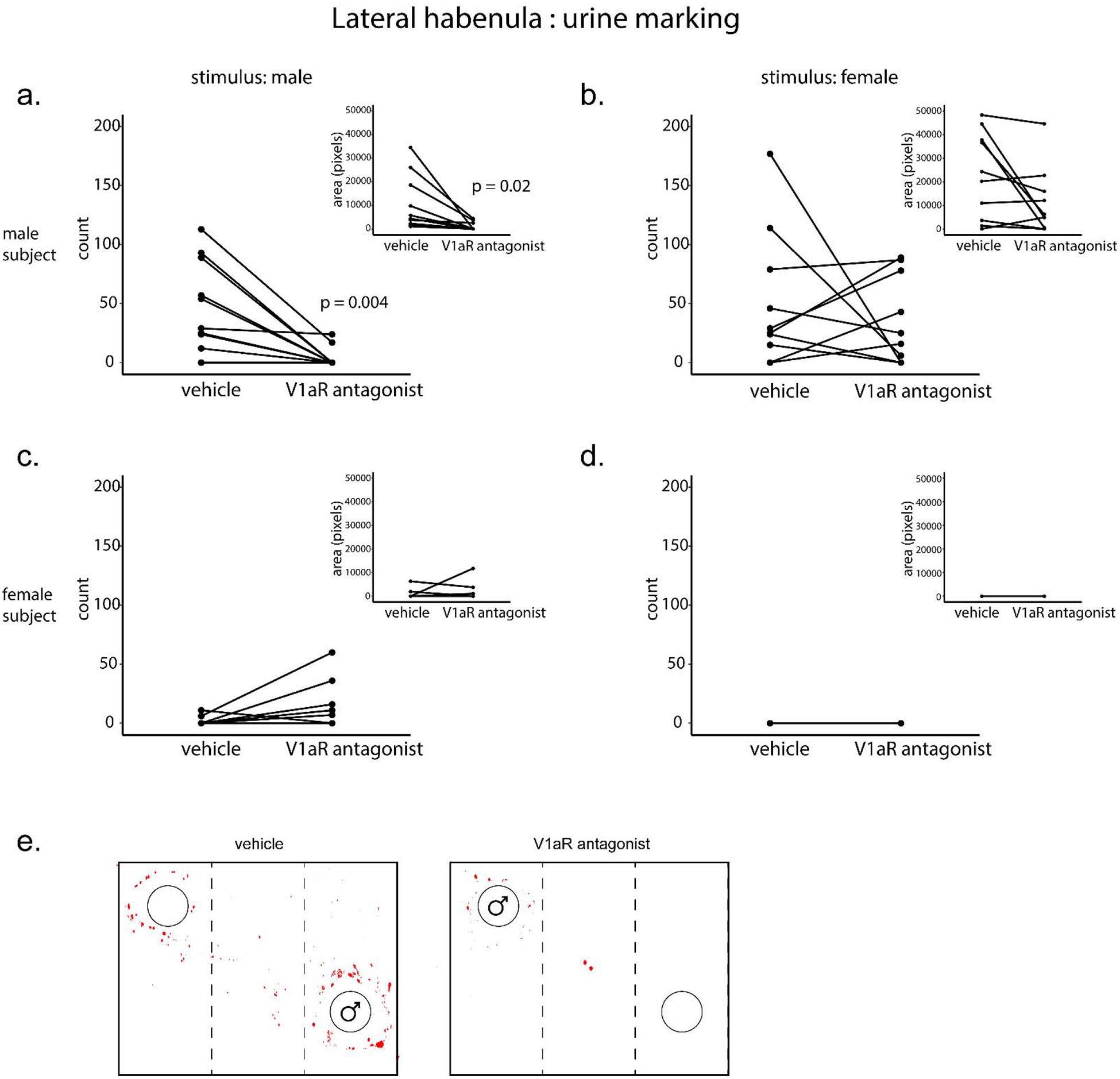
Effects of V1aR blockade in the lateral habenula (LHb) on urine marking in the presence of a confined male (a, c) or female (b, d) within a three-chamber apparatus. Urine marking was evaluated following infusion of V1aR antagonist (450ng/300nL) or vehicle (saline), counterbalanced. a-b. Male subjects (n=10) reduced the number of urine marks (counts, p=0.004) as well as area covered by urine marking (pixels, inset; p = 0.02) to (a) male, but not (b) female, stimuli following infusion of V1aR antagonist compared to vehicle injections. c-d. Female subjects (n=9) did not alter the number of urine marks or area covered by urine marking (inset) to male (c) or female (d) stimuli following infusion of V1aR antagonist compared to vehicle injections. e. Example images of reduced urine marking by a male subject (toward a male stimulus) following V1aR antagonist or vehicle injections. Each point and horizonal line represents individual within-subject data. Overlapping data are represented as one point/line.
Male subject’s urine marking covered more area than female subjects (F(1,17) = 14.63, p = 0.001) with interactions between sex of subject and sex of stimulus (F(1,17) = 7.41, p = 0.01) and sex of subject and treatment (F(1,17) = 6.37, p = 0.02). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased urine marking area to male stimuli compared to when given vehicle injections (t(9) = 2.79, p = 0.02, d = 0.9; Fig. 3a inset); this effect was not observed in response to female stimuli (t(9) = 2.13, p = 0.06; Fig. 3b inset). Injections of V1aR antagonist in the LHb of female subjects did not alter the urine marking area in the presence of male stimuli (t(8) = 0.83, p = 0.43; Fig. 3c inset) or female stimuli (no marking; Fig. 3d inset).
LHb V1aR antagonism decreased male ultrasonic vocalizations to females
There was no overall difference in USV production by sex of subject (F(1,17) = 0.13, p = 0.73; but there was an interaction between sex of subject and treatment (F(1,17) = 6.57, p = 0.02) and sex of stimulus and treatment (F(1,17) = 5.42, p = 0.03). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased USVs to female stimuli compared to when given vehicle injection (t(9) = 3.18, p = 0.01, d = 0.99; Fig. 4b); this effect was not observed in response to male stimuli (t(9) = 0.67), p = 0.52, Fig. 4a). As expected, males that received vehicle injections produced more USVs to female stimuli than to male stimuli (t(9) = 2.59, p = 0.029; however, when males received the V1aR antagonist, this difference disappeared (t(9) = 1.26, p = 0.24). V1aR injections into LHb of females did not change USV production in the presence of male stimuli (t(8) = 0.68, p = 0.52, Fig. 4c) or female stimuli (t(8) = 1.1, p = 0.30, Fig. 4d) compared to vehicle treatment. Additionally, V1aR antagonism did not change the distribution of USV syllable types in either males or females (Table 1).
Figure 4.

Effects of V1aR blockade in the lateral habenula (LHb) on USVs in the presence of a confined male (a, c) or female (b, d) within a three-chamber apparatus. USVs were evaluated following infusion of V1aR antagonist (450ng/300nL) or vehicle (saline), counterbalanced. a-b. Male subjects (n=10) did not alter USVs to (a) male stimuli, but did decrease USVs to (b) female stimuli (p=0.01), following infusion of V1aR antagonist into LHb compared to vehicle injections. c-d. Female subjects (n=9) did not alter USVs to male (c) or female (d) stimuli following infusion of V1aR antagonist compared to vehicle injections. Each point and horizonal line represents individual within-subject data. Overlapping data are represented as one point/line.
Table 1.
Table of median (interquartile range) counts of different USV syllable types in male and female subjects. V1aR antagonism in the LHb did not change the composition of USV syllable types produced compared to vehicle treatment.
| treatment | |||||||
|---|---|---|---|---|---|---|---|
| stimulus | |||||||
| short | 22 (0–56) | 24 (0–49) | 32 (0–72) | 13 (0–38) | 13 (0–38) | 11 (0–37) | 10 (0–32) |
| down | 5 (0–23) | 6 (0–27) | 2 (0–13) | 0 (0–13) | 0 (0–10) | 1 (0–13) | 0 (0–9) |
| upward | 5 (0–40) | 23 (0–50) | 3 (0–35) | 0 (0–29) | 0 (0–38) | 8 (0–46) | 0 (0–40) |
| flat | 20 (0–45) | 7 (0–27) | 9 (0–50) | 25 (7–100) | 28 (0–100) | 8 (0–100) | 27 (0–100) |
| modulated | 0(0–8) | 4 (0–18) | 3 (0–14) | 0 (0–25) | 2(0–8) | 4 (0–13) | 4 (0–15) |
| u shape | 9 (0–19) | 5 (0–13) | 3 (0–23) | 4 (0–25) | 0(0–8) | 5 (0–42) | 0 (0–8) |
| chevron | 0(0–8) | 3 (0–15) | 0 (0–5) | 0 (0–25) | 0 (0–29) | 4 (0–26) | 4 (0–29) |
| one frequency jump | 12 (0–28) | 9 (0–18) | 3 (0–18) | 0 (0–20) | 3 (0–20) | 3 (0–20) | 0 (0–16) |
| composite | 2 (0–14) | 2 (0–6) | 2 (0–9) | 0 (0–14) | 0(0–2) | 0(0–7) | 0 (0–4) |
LHb V1aR antagonism did not alter social investigation in males or females
Subjects spent more time investigating female stimuli than male stimuli (F(1,17) = 25.56, p = 0.001), regardless of treatment (F(1,17) = 0.01, p = 0.92) with no interaction between sex of subject and treatment (F(1,17) = 0.05, p = 0.82), or sex of stimulus and treatment (F(1,17) < 0.01, p = 0.99; Fig. 5a–d).
Figure 5.

Effects of V1aR blockade in the lateral habenula (LHb) on social investigation in the presence of a confined male (a, c) or female (b, d) within a three-chamber apparatus. Social investigation was evaluated following infusion of V1aR antagonist (450ng/300nL) or vehicle (saline), counterbalanced. a-b. Male subjects (n=10) did not alter social investigation to male (a) or female (b) stimuli following infusion of V1aR antagonist compared to vehicle injections. c-d. Female subjects (n=9) did not alter social investigation to male (c) or female (d) stimuli following infusion of V1aR antagonist compared to vehicle injections. Each point and horizonal line represents individual within-subject data. Overlapping data are represented as one point/line.
V1aR antagonism in LHb did not alter the amount or spatial distribution of activity
Overall, subjects in both treatment conditions traveled similar distances throughout the three-chamber apparatus (F(1,17) = 3.56 p = 0.08) with no evident differences between treatment conditions in time spent in the stimulus chambers (F(1,17) = 0.96, p = 0.34) but with an interaction between treatment and sex in time spent in the clean chamber (F(1,17) = 6.43, p = 0.02). Post hoc comparisons revealed that males receiving V1aR antagonist spent more time in the clean chamber zone in presence of female stimuli compared to when given vehicle injections (t(9) = 2.85, p = 0.01, d = 0.9; Table 2); this effect was not observed in response to male stimuli (t(9) = 0.65, p = 0.53; Table 2) or in female subjects (to male stimuli: (t(8) = 1.65, p = 0.14; to female stimuli: (t(8) = 1.65, p = 0.14; Table 2)).
Table 2.
Table of median (interquartile range) distance traveled (meters) and time spent (seconds) in stimulus or clean cage chamber. Subjects with V1aR antagonist infused into the LHb did not differ in distance traveled or time spent in stimulus chambers compared to when injected with vehicle (saline). However, males receiving V1aR antagonist spent more time in clean chambers during tests with female stimuli compared to when injected with vehicle. Bold numbers represent significant differences between treatment groups.
| treatment | |||||||
|---|---|---|---|---|---|---|---|
| stimulus | |||||||
| distance traveled (m) | 0.5 (0.4–0.66) | 0.57 (0.340.73) | 0.5 (0.390.83) | 0.52 (0.340.83) | 0.52 (0.340.83) | 0.68 (0.540.86) | 0.68 (0.54–0.86) |
| time in stimulus chamber (s) | 101 (15–138) | 174 (62–216) | 96 (13–143) | 151 (54–188) | 151 (54–188) | 140 (112–160) | 140 (112–160) |
| time in clean chamber (s) | 115 (49–193) |
p=0.02 82 (21–189) |
146 (99–222) | 72 (27–117) | 73 (27–116) | 92 (70–111) | 93 (70–111) |
V1aR antagonism within dorsal raphe
DR V1aR antagonism decreased male urine marking to males
Male subjects produced more urine marks than female subjects (F(1,16) = 15.02, p = 0.001) with a trend toward an interaction between sex of stimulus and treatment (F(1,16) = 4.4, p = 0.052). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased urine marks to male stimuli compared to when given vehicle injections (t(9) = 3.0, p = 0.015, d = 0.95; Fig. 6a); this effect was not observed in response to female stimuli (t(9) = 0.5, p = 0.63; Fig. 6b). Injections of V1aR antagonist in the DR of female subjects did not alter the number of urine marks in the presence of male stimuli (t(7) = 1.02, p = 0.92; Fig. 6c) or female stimuli (t(7) = 1.54, p = 0.17; Fig. 6d) compared to vehicle injections.
Figure 6.

Effects of V1aR blockade in the dorsal raphe (DR) on urine marking in the presence of a confined male (a, c) or female (b, d) within a three-chamber apparatus. Urine marking was evaluated following infusion of V1aR antagonist (450ng/300nL) or vehicle (saline), counterbalanced. a-b. Male subjects (n=10) reduced the number of urine marks (counts, p=0.015) as well as area covered by urine marking (pixels, inset; p = 0.03) to (a) male, but not (b) female, stimuli following infusion of V1aR antagonist compared to vehicle injections. c-d. Female subjects (n=8) did not alter the number of urine marks (counts) or area covered by urine marking (pixels, inset) to male (c) or female (d) stimuli following infusion of V1aR antagonist compared to vehicle injections. e. Example images of reduced urine marking by a male subject (toward a male stimulus) following V1aR antagonist or vehicle injections. Each point and horizonal line represents individual within-subject data. Overlapping data are represented as one point/line.
Male subject’s urine marking covered more area than female subjects (F(1,16) = 13.61, p = 0.002) with an interaction between sex of stimulus and treatment (F(1,16) = 9.99, p = 0.006) and sex of subject and sex of stimulus and treatment (F(1,16) = 6.69, p = 0.02). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased urine marking area to male stimuli compared to when given vehicle injections (t(9) = 2.57, p = 0.03, d = 0.81; Fig. 6a inset); this effect was not observed in response to female stimuli (t(9) = 1.15, p = 0.28; Fig. 6b inset). Injections of V1aR antagonist in the DR of female subjects did not alter the urine marking area in the presence of male stimuli (t(7) = 0.98, p = 0.36; Fig. 5c inset) or female stimuli (t(7) = 1.11, p = 0.3; Fig. 6d inset).
DR V1aR antagonism did not alter USVs in males or females
V1aR antagonism within DR did not change the number of USVs emitted in either female or male stimulus conditions (treatment: F(1,16) = 0.1, p=0.73; sex: F(1,16) = 4.07, p = 0.06; Fig. 7a–d). Additionally, injections of V1aR antagonist did not change the distribution of USV syllable types produced in these conditions (Table 3).
Figure 7.

Effects of V1aR blockade in the dorsal raphe (DR) on USVs in the presence of a confined male (a, c) or female (b, d) within a three-chamber apparatus. USVs were evaluated following infusion of V1aR antagonist (450ng/300nL) or vehicle (saline), counterbalanced. a-b. Male subjects (n=10) did not alter USVs to male (a) or female (b) stimuli following infusion of V1aR antagonist compared to vehicle injections. c-d. Female subjects (n=8) did not alter USVs to male (c) or female (d) stimuli following infusion of V1aR antagonist compared to vehicle injections. Each point and horizonal line represents individual within-subject data. Overlapping data are represented as one point/line.
Table 3.
Table of median (interquartile range) number of different USV syllable types in male and female subjects. V1aR antagonism in the DR did not change the distribution of USV syllable types produced compared to vehicle treatment.
| treatment | |||||||
|---|---|---|---|---|---|---|---|
| stimulus | |||||||
| short | 33 (0–73) | 17 (0–67) | 35 (0–78) | 13 (0–29) | 13 (0–22) | 15 (0–100) | 25 (3–89) |
| down | 6 (0–14) | 9 (0–21) | 0 (0–9) | 0 (0–25) | 0 (0–11) | 0 (0–11) | 0(0–6) |
| upward | 9 (0–25) | 25 (0–63) | 4 (0–36) | 10 (0–42) | 0 (0–40) | 0(0–32) | 6 (0–38) |
| flat | 25 (3–51) | 14(7–33) | 18 (0–75) | 47 (0–63) | 56 (0–91) | 39 (0–100) | 25 (0–68) |
| modulated | 0 (0–11) | 0 (0–12) | 0 (0–0) | 0 (0–17) | 0 (0–11) | 0(0–8) | 0 (0–17) |
| u shape | 0 (0–17) | 0 (0–8) | 0 (0–5) | 0 (0–0) | 0 (0–0) | 0(0–0) | 0(0–0) |
| chevron | 0(0–4) | 0 (0–4) | 0 (0–0) | 0 (0–0) | 0 (0–18) | 0(0–0) | 0 (0–18) |
| one frequency jump | 0 (0–29) | 0 (0–29) | 0 (0–14) | 24 (0–67) | 2 (0–20) | 11 (0–31) | 8 (0–21) |
| composite | 0(0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0(0–0) | 0(0–0) |
DR V1aR antagonism did not alter social investigation in males or females
Subjects spent more time investigating female stimuli than male stimuli (F(1,16) = 15.82, p = 0.001), regardless of treatment (F(1,16) = 3.01, p = 0.10); there was no interaction between sex of subject and treatment (F(1,16) = 0.07, p = 0.79), or sex of stimulus and treatment (F(1,16) = 0.04, p = 0.85; Fig. 8a–d).
Figure 8.

Effects of V1aR blockade in the dorsal raphe (DR) on social investigation in the presence of a confined male (a, c) or female (b, d) within a three-chamber apparatus. Social investigation was evaluated following infusion of V1aR antagonist (450ng/300nL) or vehicle (saline), counterbalanced. a-b. Male subjects (n=10) did not alter social investigation to male (a) or female (b) stimuli following infusion of V1aR antagonist compared to vehicle injections. c-d. Female subjects (n=8) did not alter social investigation to male (c) or female (d) stimuli following infusion of V1aR antagonist compared to vehicle injections. Each point and horizonal line represents individual within-subject data. Overlapping data are represented as one point/line.
V1aR antagonism in DR did not alter the amount or spatial distribution of activity
Overall, subjects in both treatment groups traveled similar distances throughout the three-chamber apparatus (F(1,16) = 0.01 p = 0.12); there were no differences between treatment groups in the amount of time subjects spent in the stimulus chamber zones (F(1,16) = 0.36, p = 0.56) or clean chamber zones (F(1,16) = 0.74, p = 0.40; Table 4).
Table 4.
Table of median (interquartile range) distance traveled (meters) and time spent (seconds) in stimulus or clean cage chamber. Subjects with V1aR antagonist infused into the DR did not differ in distance traveled, time spent in stimulus or clean chambers compared to when injected with vehicle (saline).
| treatment | |||||||
|---|---|---|---|---|---|---|---|
| stimulus | |||||||
| distance traveled (m) | 0.34 (0.30.46) | 0.36 (0.30.56) | 0.3 (0.280.53) | 0.47 (0.340.67) | 0.43 (0.380.66) | 0.55(0.43–0.6) | 0.49 (0.260.69) |
| time in stimulus chamber (s) | 145 (88–208) | 189 (135–229) | 97 (29–181) | 167 (105–171) | 158 (98–184) | 162 (142186) | 130 (105–202) |
| time in clean chamber (s) | 97 (29–181) | 78 (47–120) | 134 (59–164) | 94 (50–104) | 70 (55–147) | 78 (57–116) | 94 (74–119) |
V1aR antagonism in LHb or DR did not alter territorial aggression
The attack latency did not differ between treatment groups for LHb or DR animals (LHb: U = 14, p = 0.84; DR: U = 11, p = 0.84; Fig. 9). Female subjects did not attack female intruders.
Figure 9.

V1aR antagonism in LHb (n=5) (A) or DR (n=5) (B) did not alter onset of male-male aggression compared to vehicle (saline) injected subjects (LHb: n=5; DR: n=5). Bar graph and individual data points represent median and range of male subject’s latency to attack a subordinate intruder male.
Effects of V1aR antagonism on areas outside of LHb or DR
LHb or DR targeted subjects with placement of injection needle tip outside of LHb (unilateral hits included) or DR were analysed in a separate ‘miss’ group for urine marking, ultrasonic vocalization (USV), and social investigation behavior. In male subjects with LHb ‘misses’, a treatment and stimulus interaction was found in number of urine marks produced (F(1,9) = 5.76, p = 0.04) but not in urine marking area covered (F(1,9) = .001, p = 0.97). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased urine marks to female stimuli compared to when given vehicle injections (t(5) = 4.45, p = 0.007, d = 1.82; Table 5; this effect was not observed in response to male stimuli (t(5) = 1.57, p = 0.18; Table 5). Injections of V1aR antagonist in female subjects with LHb ‘misses’ did not alter urine mark number in the presence of male stimuli (t(4) = 1, p = 0.37) or female stimuli (t(4) = 1.07, p = 0.35; Table 5). No treament effect was found in USV production (F(1,9) = 0.58, p = 0.47) or social investigation (F(1,9) = 0.03, p = 0.87; Table 5). Therefore, data from LHb ‘misses’ did not match the pattern of behavior following direct LHb injections (reduced urine marking to males and reduced USVs to females).
Injections in subjects that missed the DR were all located above the DR and within the ventrolateral PAG and resulted in a treatment and sex interaction in number of urine marks produced (F(1,6) = 9.11, p = 0.02) as well as urine marking area (F(1,6) = 6.97, p = 0.04). Post hoc comparisons revealed that males receiving V1aR antagonist significantly decreased urine marks to male stimuli compared to when given vehicle injections (t(3) = 3.89, p = 0.03, d = 1.94; Table 5); this effect was not observed in response to female stimuli (t(3) = 0.65, p = 0.50) or urine marking area (to male stimuli: t(3) = 2.53, p = 0.09; to female stimuli: t(3) = 1.38, p = 0.26; Table 5). These results are partially similar to effects of direct DR injections: both reduced male urine marks to other males. V1aR injections in female subjects that missed the DR did not alter urine mark number in the presence of male stimuli (t(3) = 1, p = 0.39) or female stimuli (no marking; Table 5). No treatment effect was observed in USVs (F(1,6) = 0.82, p = 0.40) or SI responses (F(1,6) = 3.56, p = 0.11; Table 5).
Discussion
The importance of central AVP acting on V1aR in the modulation of rodent social behavior has been well-established (Lukas and Neumann 2013) and is often sexually-differentiated (Veenema et al. 2013; Dumais and Veenema 2016; Duque-Wilckens et al. 2016; Rigney et al. 2019). Here, we pharmacologically blocked V1aR in LHb or DR, areas known to have sex-different (male-biased) AVP expression (Rood et al. 2013) and broad involvement in social behavior (Luo et al. 2017; Soutschek 2018), and assessed changes in social behavior and communication. Our results indicate that sex differences in AVP innervation of LHb and DR may contribute to sexually dimorphic expression of social communication, as V1aR blockade in the LHb of males, but not females (who have less LHb and DR AVP innervation), reduced urine marking to unfamiliar males and production of ultrasonic vocalizations (USV) to unfamiliar, sexually receptive females, while V1aR antagonism in the DR of males, but not females, reduced urine marking to unfamiliar males. These changes occurred even though social investigation, locomotion, and territorial aggression were unaffected by V1aR blockade in either area, indicating specific effects on communicative behavior.
V1aR action in lateral habenula
The LHb has long been known to receive sex-different, hormone-sensitive AVP innervation (de Vries and Panzica 2006; de Vries 2008), likely from the BNST cells that show sexually dimorphic AVP expression (de Vries and Panzica 2006). Our previous findings demonstrate that BNST-AVP cells are important for male urine marking and social investigation of unfamiliar male (Rigney et al. 2019) as well as for detection of social novelty (Whylings et al. 2020).
Consequently, our present results suggest that BNST-AVP action on social communication is potentially mediated by the LHb, although the LHb does receive AVP axonal projections from other sources as well (Rood and De Vries, 2011, Zhang et al. 2018). We found that V1aR blockade in the LHb reduced communicative behaviors typical of dominant, territorial male mice (urine marking to unfamiliar males and USVs to unfamiliar females) without concomitant changes in social investigation, locomotion or territorial aggression toward subordinate males. This suggests that AVP may normally act in the LHb to promote specifically male-typical communication, rather than changing all aspects of social behavior. Our experiments do not allow us to fully exclude the possibility that changes in USVs following V1aR blockade in LHb in male subjects are due to changes in USV production by female stimuli. However, given the substantial male bias in USVs in opposite-sex interactions, it is likely that male subjects produced most of the USVs (Warren et al., 2018). Importantly, we did not observe V1aR antagonist effects on social investigation suggesting that AVP/V1aR action does not appear to alter the broader aversive function of LHb (Sparta et al. 2013; Golden et al. 2016; Benekareddy et al. 2018, but see Lecourtier et al. 2004). Moreover, the behavioral effects of V1aR antagonism are likely due to action on LHb directly as injections outside of LHb did not recapitulate the effects of LHb injections in that they did not reduce urine marking to unfamiliar males or production of USVs to unfamiliar, sexually receptive females. It should be noted that our misses were all very close to LHb and so it is possible that observed urine marking reductions in these offsite injections may still have allowed spread of antagonist to LHb.
Recent work on AVP action in LHb suggests that AVP may decrease defensive behavior in response to water deprivation (Zhang et al. 2016), potentially through hypothalamic AVP inputs (Zhang et al. 2018). This reduction in fear-responses, although correlative, combined with the present results, suggests an important role of AVP in LHb in promoting male-typical active responses to social competition and other biological threats. The exact source and nature of AVP action within LHb (BNST, medial amygdala, or hypothalamic) requires further examination.
V1aR action in dorsal raphe
The DR is interconnected with the BNST (Weissbourd et al. 2014; Garcia-Garcia et al. 2018) and contains more steroid-sensitive AVP expression in males than in females, suggesting that BNST AVP and/or medial amygdala AVP cells are the main source of AVP here (Rood et al. 2013). Similarly, sex differences in V1aR expression in DR have been noted in other species (Ross et al., 2019) and AVP has been found to indirectly excite DR serotonin neurons via V1aR action (Rood and Beck 2014), possibly through intrinsic connections of glutamatergic DR cells (Soiza-Reilly and Commons 2011). Our study is the first to examine the role of DR V1aR on social behavior and here we demonstrate that V1aR blockade within DR in males reduces urine marking toward unfamiliar males, but not unfamiliar females, without altering social investigation and other behaviors. The lack of social avoidance following V1aR antagonism is in contrast with previous studies showing that AVP injections within DR facilitate fear-motivated passive avoidance in rats (Kovács et al. 1979; Kovács et al. 1986). This discrepancy may reflect differences in task parameters (social vs. non-social; memory processes), species (rat vs. mouse) as well as the known differences in behavioral effect between exogenous stimulation of the AVP system verses blockade of endogenous action of V1aR action (Engelmann 2008).
V1aR blockade in the ventrolateral periaqueductal gray (PAG) overlying DR also reduced urine marks toward unfamiliar males. As this area is the only nearby region to also contain AVP fibers and V1aR expression, is it possible that V1aR activation in PAG can generate appropriate levels of urine marking in male mice. Although AVP innervation of this relatively caudal region of the PAG is likely of hypothalamic origin and not sex-different or steroid sensitive (Rood et al. 2013), it may play a role in social communication as AVP injected into the PAG stimulates scent marking in hamsters, a species lacking extrahypothalamic AVP (Hennessey et al. 1992). Indeed, the PAG is broadly involved in other aspects of mouse social communication, such as USVs (Tschida et al. 2019). It is also possible that V1aR antagonism could be acting in DR and PAG together to alter behavior since these structures are close in proximity. Additionally, our cannulation approach in LHb or DR does not avoid the possibility that the V1aR antagonist spread into the lateral ventricle (near LHb) or cerebral aqueduct (near DR). Yet, we feel that this is unlikely to fully account for our effects since LHb ‘misses’ did not replicate effects seen in LHb ‘hit’ subjects and a scenario of ventricular leak would require further downstream sex-differences in V1aR responsiveness. Nevertheless, additional experiments targeting different AVP cell populations (hypothalamic vs. limbic) will be required to disambiguate AVP/V1aR action in PAG from DR.
As sex differences in LHb and DR AVP fiber density likely originate from BNST cell bodies (de Vries 2008; Rood et al. 2013; Otero-Garcia et al. 2014), it is surprising that we did not observe alterations in male-male investigation following LHb/DR V1aR antagonism because deletion of BNST-AVP cells reduces male investigation of other males (Rigney et al. 2019). Similarly, BNST-AVP lesions did not alter USVs whereas V1aR blockade within LHb did. These apparent discrepancies may be due to several factors. First, AVP action at other BNST target structures, such as ventral pallidum, may drive social investigation, as V1aR activation in this area regulates male investigatory behavior and partner preference (Lim and Young 2004; DiBenedictis et al. 2020). Second, the source of AVP that regulates USVs in LHb may originate from medial amygdala AVP or hypothalamic AVP cells (Browne et al. 2018). Lastly, it is possible, given the cross-talk between OT and V1aR, that OT projections may be responsible for behavioral action (Song and Albers, 2017).
Our results indicate that the V1aR system plays a sexually dimorphic role in control of social communicative behaviors via LHb and, to a lesser extent, DR. This is largely in keeping with other findings that the AVP/V1aR system is sexually-differentiated not just in anatomy, but also across various domains of behavioral function and across species (Albers, 2015; Dumais and Veenema, 2016; Hammock, 2015), including humans (Meyer-Lindenberg et al. 2011; Rilling et al. 2014). Given that the LHb and DR are strongly interconnected (Pasquier et al. 1976; Aghajanian and Wang 1977; Kalén et al. 1986; Ferraro et al. 1996; Yang et al. 2008) and appear to work as a system to regulate emotional responding (Zhao et al. 2015; Dolzani et al. 2016), concurrent AVP action on V1aR on LHb and DR may coordinate strongly-competitive aspects of social communication, such as territorial scent marking. In contrast, less-competitive aspects of communication, such as sexually-motivated vocalizations, may require direct AVP/V1aR action more specifically at LHb (and perhaps other AVP targets). The present work highlights the need to explore less-investigated targets of sex-different AVP inputs as they may play significant roles in sex-different social and emotional behaviors.
Highlights.
Blocking vasopressin 1a receptor in the lateral habenula or dorsal raphe reduced male-typical communicative behavior toward males (urine marking)
Antagonism of vasopressin 1a receptor in lateral habenula decreased male-typical ultrasonic vocalizations to female stimuli in male but not female mice
Vasopressin 1a receptor antagonism did not alter other aspects of social behavior, including social investigation and territorial aggression) or locomotion
Acknowledgments
We thank Dr. Maurice Manning (University of Toledo) for his generous gift of the V1aR antagonist and Dr. Geert J. de Vries for helpful comments on the manuscript. This work was supported by National Institutes of Health [R21 MH111104] and the Center for Behavioral Neuroscience at Georgia State University.
Funding sources: This work was supported by NIH grant R21 MH111104.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors report no conflict of interest
Contributor Information
Nicole Rigney, Neuroscience Institute 145 Piedmont Ave SE, Atlanta, GA 30303, USA; Center for Behavioral Neuroscience 145 Piedmont Ave SE, Atlanta, GA 30303, USA; Georgia State University 145 Piedmont Ave SE, Atlanta, GA 30303, USA.
Rachael Beaumont, Neuroscience Institute 145 Piedmont Ave SE, Atlanta, GA 30303, USA; Center for Behavioral Neuroscience 145 Piedmont Ave SE, Atlanta, GA 30303, USA; Georgia State University 145 Piedmont Ave SE, Atlanta, GA 30303, USA.
Aras Petrulis, Neuroscience Institute 145 Piedmont Ave SE, Atlanta, GA 30303, USA; Center for Behavioral Neuroscience 145 Piedmont Ave SE, Atlanta, GA 30303, USA; Georgia State University 145 Piedmont Ave SE, Atlanta, GA 30303, USA.
References
- Aghajanian GK, Wang RY, 1977. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 122, 229–242. [DOI] [PubMed] [Google Scholar]
- Albers HE, 2015. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol 36, 49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ, 2008. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav. Brain Res 190, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S-I, Harada N, Balthazart J, 2002. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J. Neurosci 22, 9104–9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázsfi D, Zelena D, Demeter K, Miskolczi C, Varga ZK, Nagyváradi Á, Nyíri G, Cserép C, Baranyi M, Sperlágh B, Haller J, 2018. Differential Roles of the Two Raphe Nuclei in Amiable Social Behavior and Aggression - An Optogenetic Study. Front. Behav. Neurosci 12, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley MS, Goldman BD, 1977. The effects of castration and Silastic implants of testosterone on intermale aggression in the mouse. Horm. Behav 9, 32–48. [DOI] [PubMed] [Google Scholar]
- Beeman EA, 1947. The relation of the interval between castration and first encounter to the aggressive behavior of mice. Anat. Rec 99, 570. [PubMed] [Google Scholar]
- Benekareddy M, Stachniak TJ, Bruns A, Knoflach F, von Kienlin M, Künnecke B, Ghosh A, 2018. Identification of a Corticohabenular Circuit Regulating Socially Directed Behavior. Biol. Psychiatry 83, 607–617. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Ren X, Terwilliger EF, Young LJ, 2005. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47, 503–513. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Szegda KL, Westphal H, Young LJ, 2004. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29, 483–493. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ, 2004. Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565–1574. [DOI] [PubMed] [Google Scholar]
- Bishop MJ, Chevins PF, 1987. Urine odours and marking patterns in territorial laboratory mice (Mus musculus). Behav. Processes 15, 233–248. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID, 2008. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. U. S. A 105, 17139–17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL, 1998. Principles of Animal Communication, Second Edition Bradbury Jack W. and Vehrencamp Sandra L.. [Google Scholar]
- Bredewold R, Smith CJW, Dumais KM, Veenema AH, 2014. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Hammack R, Lucki I, 2018. Dysregulation of the Lateral Habenula in Major Depressive Disorder. Front. Synaptic Neurosci 10, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL, 1995. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev 19, 303–314. [DOI] [PubMed] [Google Scholar]
- Chan WY, Wo NC, Cheng LL, Manning M, 1996. Isosteric substitution of Asn5 in antagonists of oxytocin and vasopressin leads to highly selective and potent oxytocin and V1a receptor antagonists: new approaches for the design of potential tocolytics for preterm labor. J. Pharmacol. Exp. Ther 277, 999–1003. [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M, 2009. Neuroendocrinology of social information processing in rats and mice. Front. Neuroendocrinol 30, 442–459. [DOI] [PubMed] [Google Scholar]
- Congiu M, Trusel M, Pistis M, Mameli M, Lecca S, 2019. Opposite responses to aversive stimuli in lateral habenula neurons. Eur. J. Neurosci [DOI] [PubMed] [Google Scholar]
- Connor JL, Winston H, 1972. Genetic analysis of conditioned emotional responses in the mouse (Mus musculus L.). J. Comp. Physiol. Psychol 81, 37–44. [DOI] [PubMed] [Google Scholar]
- Coquelin A, 1992. Urine-marking by female mice throughout their reproductive cycle. Horm. Behav 26, 255–271. [DOI] [PubMed] [Google Scholar]
- Crawley JN, 2007. Mouse Behavioral Assays Relevant to the Symptoms of Autism. Brain Pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthé RM, Le Moal M, 1988. Septal vasopressin modulates social memory in male rats. Brain Res. 457, 143–147. [DOI] [PubMed] [Google Scholar]
- Darwin C, 1981. The descent of man, and selection in relation to sex. Princeton, NJ: Princeton University Press. [Google Scholar]
- De Vries GJ, 2008. Sex differences in vasopressin and oxytocin innervation of the brain. Prog. Brain Res 170, 17–27. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, 1983. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 273, 307–317. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC, 2006. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Cheung HK, Nussbaum ER, Veenema AH, 2020. Involvement of ventral pallidal vasopressin in the sex-specific regulation of sociosexual motivation in rats. Psychoneuroendocrinology 111, 104462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Amat J, Agster KL, Saddoris MP, Watkins LR, Maier SF, 2016. Activation of a Habenulo-Raphe Circuit Is Critical for the Behavioral and Neurochemical Consequences of Uncontrollable Stress in the Male Rat. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ, 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH, 2016. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol 40, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, Trainor BC, 2016. Inhibition of vasopressin V1a receptors in the medioventral bed nucleus of the stria terminalis has sex- and context-specific anxiogenic effects. Neuropharmacology 110, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M, 2007. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res 178, 123–127. [DOI] [PubMed] [Google Scholar]
- Engelmann M, 2008. Vasopressin in the septum: not important versus causally involved in learning and memory--two faces of the same coin? Prog. Brain Res 170, 389–395. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM, 1999. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav. Brain Res 99, 7–16. [DOI] [PubMed] [Google Scholar]
- Ferraro G, Montalbano ME, Sardo P, La Grutta V, 1996. Lateral habenular influence on dorsal raphe neurons. Brain Res. Bull 41, 47–52. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Canetta S, Stujenske JM, Burghardt NS, Ansorge MS, Dranovsky A, Leonardo ED, 2018. Serotonin inputs to the dorsal BNST modulate anxiety in a 5-HT1A receptor-dependent manner. Mol. Psychiatry 23, 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Bluth R-M, Goodall G, Dantzer R, 1994. Social and individual recognition in rodents” Methodological aspects and neurobiological bases. Behav. Processes 33, 59–88. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z, 2009. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. U. S. A 106, 19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, Bregman D, Khibnik L, Tai J, Rebusi N, Krawitz B, Chaudhury D, Walsh JJ, Han M-H, Shapiro ML, Russo SJ, 2016. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH, 2001. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Brain Res. Rev 35, 246–265. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB, 2010. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol. Psychiatry 67, 1220–1222. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, Singer AT, Taylor JL, Szatmari P, 2015. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EAD, 2015. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40, 24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM, 2012. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One 7, e40782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorn J, Ang VT, Jenkins JS, 1985. Effects of lesions in the hypothalamic paraventricular, supraoptic and suprachiasmatic nuclei on vasopressin and oxytocin in rat brain and spinal cord. Brain Res. 346, 51–57. [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Whitman DC, Albers HE, 1992. Microinjection of arginine-vasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus). Brain Res. 569, 136–140. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL, 2011. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 10, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2017. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev 76, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalén P, Pritzel M, Nieoullon A, Wiklund L, 1986. Further evidence for excitatory amino acid transmission in the lateral habenular projection to the rostral raphe nuclei: lesion-induced decrease of high affinity glutamate uptake. Neurosci. Lett 68, 35–40. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL, 2013. Behavioral relevance of species-specific vasotocin anatomy in gregarious finches. Front. Neurosci 7, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Hagiwara Y, 1985. Regulation of urine marking in male and female mice: effects of sex steroids. Horm. Behav 19, 64–70. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ, 2013. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp (77):e4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács GL, Bohus B, Versteeg DH, 1979. Facilitation of memory consolidation by vasopressin: mediation by terminals of the dorsal noradrenergic bundle? Brain Res. 172, 73–85. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Veldhuis HD, Versteeg DH, De Wied D, 1986. Facilitation of avoidance behavior by vasopressin fragments microinjected into limbic-midbrain structures. Brain Res. 371, 17–24. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH, 2004. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur. J. Neurosci 19, 2551–2560. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Geddes CE, Lee JL, Herkenham M, 2013. Urine Scent Marking (USM): A ovel Test for Depressive-Like Behavior and a Predictor of Stress Resiliency in Mice. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ, 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. [DOI] [PubMed] [Google Scholar]
- Lozić M, Tasić T, Martin A, Greenwood M, Šarenac O, Hindmarch C, Paton JF, Murphy D, Japundžić-Žigon N, 2016. Over-expression of V1A receptors in PVN modulates autonomic cardiovascular control. Pharmacological Research. [DOI] [PubMed] [Google Scholar]
- Lukas M, Neumann ID, 2013. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav. Brain Res 251, 85–94. [DOI] [PubMed] [Google Scholar]
- Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL, 1999. Social Stress Effects on Territorial Marking and Ultrasonic Vocalizations in Mice. Physiology & Behavior. [DOI] [PubMed] [Google Scholar]
- Luo J, Feng Q, Wei L, Luo M, 2017. Optogenetic activation of dorsal raphe neurons rescues the autistic-like social deficits in Shank3 knockout mice. Cell Res. 27, 950–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio JC, Whitney G, 1985. Ultrasonic vocalizing by adult female mice (Mus musculus). J. Comp. Psychol 99, 420–436. [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G, 2012. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol 24, 609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Sipos ML, Nyby JG, Barfield RJ, 1994. Intracranial androgenic activation of male-typical behaviors in house mice: motivation versus performance. Behav. Brain Res 60, 141–149. [DOI] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, Wildes CP, Ungless MA, Tye KM, 2016. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 164, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H, 1957. The ninhydrin reaction and its analytical applications. Biochem. J 67, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M, 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci 12, 524–538. [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Lanuza E, Lorente MJ, Martinez-Garcia F, 2004. Attraction to male pheromones and sexual behaviour show different regulatory mechanisms in female mice. Physiol. Behav 81, 427–434. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM, 2009. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 8, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayasu K, 2017. Elucidation of the Role of Dorsal Raphe Serotonergic Neurons in Mood Regulation Using Pharmacological and Viral Vector-based Approaches. Yakugaku Zasshi 137, 341–346. [DOI] [PubMed] [Google Scholar]
- Newman SW, 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci 877, 242–257. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM, 2008. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl. Acad. Sci. U. S. A 105, 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Garcia M, Martin-Sanchez A, Fortes-Marco L, Martínez-Ricós J, Agustin-Pavón C, Lanuza E, Martínez-García F, 2014. Extending the socio-sexual brain: arginine-vasopressin immunoreactive circuits in the telencephalon of mice. Brain Struct. Funct 219, 1055–1081. [DOI] [PubMed] [Google Scholar]
- Palanza P, Parmigiani S, 2017. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci. Biobehav. Rev 76, 134–143. [DOI] [PubMed] [Google Scholar]
- Pasquier DA, Anderson C, Forbes WB, Morgane PJ, 1976. Horseradish peroxidase tracing of the lateral habenular-midbrain raphe nuclei connections in the rat. Brain Res. Bull 1, 443–451. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, 2012. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Academic Press. [Google Scholar]
- Rigney N, Whylings J, Mieda M, de Vries G, Petrulis A, 2019. Sexually Dimorphic Vasopressin Cells Modulate Social Investigation and Communication in Sex-Specific Ways. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G, 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Beck SG, 2014. Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience 260, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJW, Woodbury ME, de Vries GJ, 2013. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J. Comp. Neurol 521, 2321–2358. [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ, 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J. Comp. Neurol 519, 2434–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AP, McCann KE, Larkin TE, Song Z, Grieb ZA, Huhman KL, Albers HE, 2019. Sex-dependent effects of social isolation on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5HT) 1a receptor binding and aggression. Horm. Behav 116, 104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet FI, Wöhr M, Crawley JN, 2011. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav. Brain Res 216, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AM, Lawther AJ, Prior BM, Isbel L, Somers WG, Lesku JA, Richdale AL, Dissanayake C, Kent S, Lowry CA, Hale MW, 2019. Social approach, anxiety, and altered tryptophan hydroxylase 2 activity in juvenile BALB/c and C57BL/6J mice. Behav. Brain Res 359, 918–926. [DOI] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T, 2015. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519–522. [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG, 2011. Glutamatergic drive of the dorsal raphe nucleus. J. Chem. Neuroanat 41, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Albers HE, 2017. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Kalyani M, Becker JB, 2018. Sex differences in motivated behaviors in animal models. Curr Opin Behav Sci 23, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek A, 2018. Neural Circuits Regulating Social Behavior: Highlighting the Causal Contribution of the Lateral Habenula. Biol. Psychiatry [DOI] [PubMed] [Google Scholar]
- Sparta DR, Jennings JH, Ung RL, Stuber GD, 2013. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav. Brain Res 255, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström JO, Theodorsson A, Ingberg E, Isaksson I-M, Theodorsson E, 2012. Ovariectomy and 17β-estradiol replacement in rats and mice: a visual demonstration. J. Vis. Exp e4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschida K, Michael V, Takatoh J, Han B-X, Zhao S, Sakurai K, Mooney R, Wang F, 2019. A Specialized Neural Circuit Gates Social Vocalizations in the Mouse. Neuron 103, 459–472.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V, 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol. Behav 72, 675–683. [DOI] [PubMed] [Google Scholar]
- Van Segbroeck M, Knoll AT, Levitt P, Narayanan S, 2017. MUPET-Mouse Ultrasonic Profile ExTraction: A Signal Processing Tool for Rapid and Unsupervised Analysis of Ultrasonic Vocalizations. Neuron 94, 465–485.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ, 2013. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology 38, 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ, 2012. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav 61, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Wang G, Baum MJ, Bakker J, 2011. The main and accessory olfactory systems of female mice are activated differentially by dominant versus subordinate male urinary odors. Brain Res. 1402, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MR, Spurrier MS, Roth ED, Neunuebel JP, 2018. Sex differences in vocal communication of freely interacting adult mice depend upon behavioral context. PLoS One 13, e0204527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L, 2014. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whylings J, Rigney N, Peters NV, de Vries GJ, Petrulis A, 2020. Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain Behav. Immun 83, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM, 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L-M, Hu B, Xia Y-H, Zhang B-L, Zhao H, 2008. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav. Brain Res 188, 84–90. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H, 2018. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, 2004. The neurobiology of pair bonding. Nat. Neurosci 7, 1048–1054. [DOI] [PubMed] [Google Scholar]
- Zala SM, Reitschmidt D, Noll A, Balazs P, Penn DJ, 2017. Sex-dependent modulation of ultrasonic vocalizations in house mice (Mus musculus musculus). PLoS One 12, e0188647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hernández VS, Swinny JD, Verma AK, Giesecke T, Emery AC, Mutig K, Garcia-Segura LM, Eiden LE, 2018. A GABAergic cell type in the lateral habenula links hypothalamic homeostatic and midbrain motivation circuits with sex steroid signaling. Transl. Psychiatry 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hernández VS, Vázquez-Juárez E, Chay FK, Barrio RA, 2016. Thirst Is Associated with Suppression of Habenula Output and Active Stress Coping: Is there a Role for a Non-canonical Vasopressin-Glutamate Pathway? Front. Neural Circuits 10, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhang B-L, Yang S-J, Rusak B, 2015. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav. Brain Res 277, 89–98. [DOI] [PubMed] [Google Scholar]


