Abstract
Scope
Plant polyphenols are widespread in the American diet, yet estimated intake is uncertain. We examine the application of the Polyphenol Explorer® (PED) database to quantify polyphenol and ellagitannin (ET) intake of men with prostate cancer and tested the implementation of diets restricted in polyphenols or ETs.
Methods and results
Twenty-four men enrolled in a 4-week trial were randomized to usual, low-polyphenol or low-ET diet. Estimated polyphenol and ET intakes were calculated from 3-day diet records utilizing the PED. Urine and plasma metabolites were quantified by UPLC-MS. Adherence to the restricted diets was 95% for the low polyphenol and 98% for low-ET diet. In the usual diet, estimated dietary polyphenol intake was 1568 ± 939 mg/day, with coffee/tea beverages (1112 ± 1028 mg/day) being the largest contributors and estimated dietary ET intake was 12 ± 13 mg/day. The low-polyphenol and low-ET groups resulted in a reduction of total polyphenols by 45% and 85%, respectively, and omission of dietary ETs. UPLC analysis of urinary host and microbial metabolites reflect ET intake.
Conclusion
PED is a useful database for assessing exposure to polyphenols. Diets restricted in total polyphenol or ET intake are feasible and UPLC assessment of ET metabolites is reflective of dietary intake.
Keywords: Black raspberries, Ellagitannins, Polyphenol explorer database, Polyphenols, Urolithins
1. Introduction
Systematic reviews of the accumulated scientific literature on diet, nutrition, and cancer support public health recommendations for cancer prevention [1,2]. Consistently, a plant-based diet enriched in fruits, vegetables, and whole grains is the foundation of a cancer prevention dietary pattern [1, 2]. Multiple classes of bioactive components present in plant foods are hypothesized to impact humans via diverse mechanisms to reduce cancer risk. Polyphenols represent a large group of bioactives and includes over 500 chemically distinct classes of compounds [3] and a number of dietary polyphenols have been shown in laboratory studies to have anti-cancer bioactivity [4–7], including studies of prostate cancer (PCa) [8–12]. The chemical complexity of polyphenols presents a challenge for epidemiologists to estimate intake of polyphenols as a group or individual polyphenols in human studies. In recent years, there has been progressive improvement in standardized laboratory methods for analysis of food polyphenols [13]. Much like nutrients and other non-nutrient bioactives, we need to remain cognizant of the fact that content in a specific food item will vary among varieties of plant species, with growing conditions, time of harvest, and food processing [14–16]. Progress in the assessment of polyphenol intake in humans is one critical missing link in our understanding of how fruits and vegetables may impact health and particularly cancer risk.
The Polyphenol Explorer® database (PED) was established in 2009 as a resource to assist investigators in the assessment of polyphenol content of foods and estimation of human exposures [11]. With continual updates, the PED is the most comprehensive polyphenol database, containing over 450 foods and 500 polyphenols [11]. The PED includes quantitative data from the published literature as assessed by two different methods, the Folin–Ciocalteu (FC) assay or chromatography. The database is currently the most inclusive resource to translate dietary intake data from clinical studies or epidemiologic investigations to estimates of exposure to total polyphenols or specific classes of compounds [3, 13]. Although the PED is not conclusive of all foods typically consumed in the American diet, it currently serves as our most comprehensive tool for assessment
Our research team is preparing for a phase II clinical trial testing the ability of polyphenol rich foods to modulate human prostate carcinogenesis. In order to proceed with experimental designs that will allow us to precisely evaluate the metabolites of polyphenols by targeted metabolomic strategies and to assess biomarkers of bioactivity we must first define the baseline exposure in men of the age range targeted for clinical trials. We also aim to address the feasibility of developing and implementing controlled diets that are low in total polyphenols or a specific class of polyphenols called ellagitannins (ET)s. Thus, based upon the PED we have defined the predicted major contributors to polyphenol intake in free-living American men and specifically to ET intake. In addition, we developed a low-polyphenol and low-ET dietary pattern and assessed feasibility and compliance in a clinical trial of 4-weeks duration. In parallel, we examine profiles of metabolites by HPLC-MS. Several studies examining the impact of ET rich food products have instituted low-polyphenol diets to minimize background dietary polyphenols and increase analytic precision within biological samples [6, 7, 17], which is a formidable challenge for adherence in studies of any significant duration. Thus, our efforts to define usual intake of polyphenol classes using the current PED followed by a comparison of the usual diet to the low-polyphenol and low-ET dietary patterns for compliance and biomarker assessment will provide data that will inform the design of future clinical trials aimed at evaluating the biological activity of food products rich in polyphenols and ETs for PCa prevention.
We have chosen to focus upon ETs based upon basic and human studies suggesting that these compounds or metabolites may impact carcinogenesis [9, 11, 18–30]. In the large 51 000 man Health Professional’s study, it was reported that 0.5 cup/week of strawberries consumed was associated with a lowered risk of PCa (RR 0.80; 95%CI = 0.57–1.10; P-trend = 0.005) [31]. Black raspberries (BRB) are consumed too infrequently to be quantified from epidemiologic studies yet have a similar phytochemical pattern to strawberries, but contain a much greater concentration of ETs [8]. Our efforts benefit from improvements in the analytic tools to assess ETs and metabolites, yet quantification of specific compounds as biomarkers reflecting a dose response of individual exposure to dietary ETs has not been fully validated [32]. Dimethylellagic acid (DMEA) and urolithin derivatives are proposed ET metabolites that may serve as biomarkers, yet the pathways by which these compounds are produced is not fully understood [32–36].
The goals of this study are as follows: (i) to apply the PED to 3-day diet records to estimate intake of polyphenols and ETs in free-living men with PCa and the major foods contributing to intake, (ii) to develop two dietary interventions aimed at reducing either total polyphenol intake or ET-rich foods, (iii) to test adherence to a low-polyphenol or low-ET diet during a 4-week clinical trial, (iv) to compare estimated polyphenol intake based upon the two assays available in the PED database (FC assay and Chromatography assays), and (v) to measure changes in ET metabolites by dietary intervention and determine relationships to dietary exposure.
2. Materials and methods
2.1. Study population
Study participants (N = 58) were recruited from the multidisciplinary Prostate Cancer Clinic of The James Cancer Hospital at The Ohio State University. Men had biopsy-proven clinically localized adenocarcinoma of the prostate and had selected a radical prostatectomy for curative treatment of their PCa. Men were ineligible if they were currently receiving treatment for PCa or if they had a history of any digestive, metabolic, or malabsorptive disorders requiring a specialized diet. Written informed consent was obtained from the patient to participate in this study. Patient dietary records were collected and analyzed under approval from The James Cancer Hospital/The Ohio State University Cancer Institutional Review Board. The study was registered at clinicaltrials.gov (NCT01823562).
2.2. Intervention, diet education, and assessment
The first 24 men enrolled into the study were randomized to one of three diets: regular diet (control group), low-polyphenol diet or low-ET diet (Fig. 1) and are the focus of the analyses presented. The remaining men we assigned to an intervention trial and these results will be reported in subsequent publications. The control group of eight men was asked to continue their typical intake and to record their intake of polyphenol and ET-rich foods and beverages on a daily food checklist, which was based upon a literature review of dietary sources of polyphenols and/or ET foods in addition to data available in the PED (Supporting Information data A) [37, 38]. The daily food checklist was designed to ensure we were capturing usual intake of polyphenol and ET-rich foods from the 3-day diet records. The low-polyphenol diet was designed to omit or limit the major foods identified as being a rich dietary sources of polyphenols based upon the PED [37–39]. Values used to differentiate between low-, moderate- and high-dietary sources of polyphenols were established by determining natural tertiles of the top 100 foods in the PED [38]. Men were educated to abstain from foods containing greater than 50 mg/serving of total polyphenols with the exception of 8 ounces of coffee (215 mg/100g) or tea (101 mg/100g) (Supporting Information data B) [39]. Foods containing 10–50 mg/serving of polyphenols were limited to a daily maximum of two servings and foods containing less than 10 mg/serving were unrestricted. Men were asked to document daily servings of high (>50 mg/serving) and moderate (10–50 mg/serving) polyphenol-rich foods per day on the daily food checklist (Supporting Information data B) [39]. The low-ET diet was designed to omit any known dietary sources of ETs. Dietary ETs were identified through an in-depth literature search [8, 37, 40–42] and through the use of the PED [3,38,39]. Men documented all ET-rich foods consumed in the daily food checklist (Supporting Information data C). In addition to checklists noted above for documented their intake of polyphenol-rich and/or ET-rich foods (Supporting Information data A–C) all men were instructed to complete a 3-day diet record on two non-consecutive week days and one weekend day.
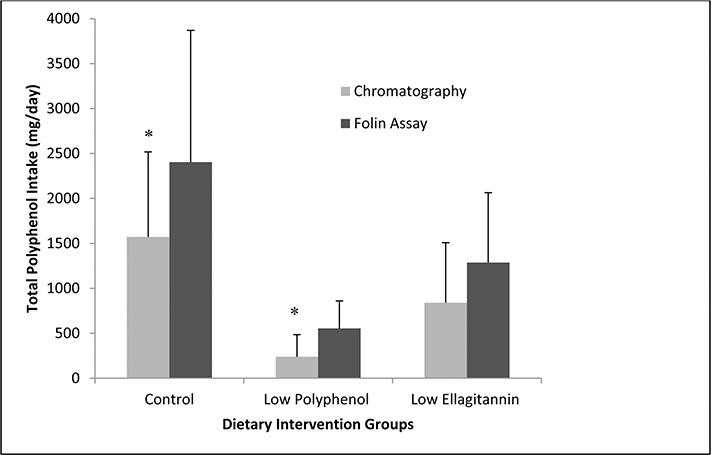
Figure 1.
Comparison in estimated polyphenol intake between and within intervention groups. Data on both methodologies included (Chromatography versus Folin Ciocalteu Assay). Data are means ± SD from subjects completing a 3-day diet record (n = 8 per group) to estimate total polyphenol intake. Records analyzed in the Polyphenol Explorer Database® *P values < 0.05 for differences between groups analyzed by chromatography and Folin Ciocalteu, aanalyzed by Students t-test (Control: p = 0.014; Low Polyphenol: p = 0.003; Low ellagitannin: p = 0.055). Differences between the polyphenol intake in the three diet groups within each of the estimation methods were evaluated with ANOVA (Chromatography: p = 0.004; Folin Ciocalteu: p = 0.003)
2.3. Dietary compliance
A daily food checklist (Supporting Information data A–C) was developed to document the consumption of polyphenol and ET-rich foods. This approach accounts for exposure to polyphenol-rich dietary components, such as jams and fruit-based sauces, in the quantification of compounds. Compliance to the intervention diets was determined by calculating the percentage of days on study where the dietary restriction was adhered to exactly as instructed as determined from self-reported records. Data is reported as percent compliant days on study.
2.4. Diet analysis
Three-day diet records collected during the study period were entered into the Nutrition Data System for Research (2012 version) for macro- and micronutrient diet analysis. To estimate total polyphenol content, all foods reported on three-day diet records were entered into the PED Version 3.5 to retrieve mean polyphenol content values expressed as mg/100g food fresh weight for all foods available in the database. Total polyphenol content was considered the sum of all individual polyphenols from chromatography without previous hydrolysis (Reverse Phase and Normal Phase High Performance Liquid Chromatography), except foods contained in a complex food matrix identified by Perez-Jimenez et al. where chromatography after hydrolysis was used and proanthocyanidin oligomers and polymers were measured by normal-phase HPLC [39]. Total polyphenol intake is reported as average +/− SD milligrams per day. All foods containing trace amounts of polyphenols such as animal foods were excluded from the PED and therefore, were excluded from analysis [39].
To estimate total ET content, all foods reported on three-day diet records were entered into the PED Version 3.5 to retrieve mean ET content values expressed as mg/100g food fresh weight for all foods available in the database. Dietary ET intake was estimated using the PED data from chromatographic analysis after hydrolysis and reported as average milligrams per day.
Because the PED lists whole foods only and not mixed dishes (like spaghetti) or few multi-ingredient foods (like pastries), it was necessary to estimate relative content of whole food ingredients in many foods documented by study participants. In order to do this consistently, we employed the USDA recipe database to calculate quantities of polyphenol containing ingredients in mixed foods.
Total dietary phenolic intake was estimated using data from the FC assay collected in the PED. Foods consumed, for which the PED did not have FC measures of polyphenol content, were excluded from the current analysis (bread, pumpkin, grape juice, cinnamon, walnut, peas, blackberries, rice, bran, chocolate milk, applesauce, soy sauce, pasta, strawberry jam, vinegar, oat bran, tofu and lime).
2.5. Urine collection
Each subject provided a spot urine sample at baseline and a 24-h urine sample after dietary intervention. The entire urine volume was collected by the subjects in Simport Urisafe® 4-L urine container. The 24-h urine samples were preserved with boric acid. Prior to being frozen, spot and 24-h urine samples were separated into 5 mL aliquots and 250 μL of 5% formic acid was added to each tube. Urine samples were immediately stored at −80°C until analysis.
2.6. Urine sample extraction for UPLC-MS/MS analysis
Urine samples were thawed and 0.5 mL was diluted with 0.5 mL of 2M sodium acetate buffer at a pH of 5.5. Glucuronidase/sulfatase (S9626, Sigma Chem. Co., St Louis, MO) was added (10 μL of 54 mg/mL) and incubated for 2 h at 37°C. Samples were extracted twice with 3 volumes of diethyl ether. The supernatants were pooled and dried under nitrogen. The residue was dissolved with 200 μL methanol in an ultrasonic bath. The solution was then filtered through a 0.2 μm nylon syringe filter for injection into the HPLC-MS/MS.
2.7. Plasma sample extraction for UPLC-MS/MS analysis
Plasma samples were thawed and homogenized using probe sonication with 1 mL of acetonitrile added to 0.5 mL plasma. Samples were centrifuged and the supernatant collected. 1 mL 2:1 acetonitrile:water was added to re-suspend the pellet with probe sonication. The supernatants were pooled and dried under nitrogen. The residue was re-suspended in 0.5 mL water, and then homogenized with 0.5 mL of 2M sodium acetate buffer at a pH of 5.5. The mixture was then combined with 10 μL of 54 mg/mL glucuronidase/sulfatase (S9626, Sigma Chem. Co., St. Louis, MO) and incubated for 2 h at 37°C. Samples were extracted twice with 3 volumes of diethyl ether. The supernatants were pooled and dried under nitrogen. The dry residue was dissolved with 200 μL methanol in the ultrasound bath. The solution was then filtered through a 0.2 μm nylon syringe filter for injection into the HPLC-MS/MS.
2.8. UPLC-ESI-MS/MS analysis
All samples were analyzed using the Nutrient & Phytochemical Analytic Shared Resource at The Ohio State University. Samples (5 μL) of digested extract were injected onto an UPLC system (Acquity UPLC, Waters Corp., Milford, MA) and separated on a 50×2.1 mm ID BEH C18 1.7um column with a linear gradient of 1% (v/v) formic acid in water versus 1% (v/v) formic acid in acetonitrile. The composition was held at 10%B for 0.5 min and then increased linearly to 55.5%B by 4 min, through 88.9%B by 5 min, and re-equilibrated through 6.5 min. Column temperature was 40°C and flow rate of 0.75 mL/min.
UPLC eluate was introduced to a triple quadrupole mass spectrometer (Quattro Ultima, Waters Corp., Beverley, MA) via an electrospray probe operated in positive ion mode without splitting flow. Standards of urolithin D, C, A, B, methyl A, and dimethyl A were used for external calibration. MS/MS transitions included 261>171, 199 for urolithin D, 245>155, 183 for urolithin C, 229>128, 157 for urolithin A, 259>183 for methyl urolithin C, 213>115, 141 for urolithin B, 243>171, 184 for methyl urolithin A, and 257>198 for dimethyl urolithin A. Dimethyl ellagic acid was monitored in electrospray negative polarity during the same analysis and 329>299, 314 transitions were used for detection. MS source parameters included 500°C desolvation temperature, 800 L/h desolvation gas (N2), and 3×10−3 mBar collision gas pressure (Ar).
Baseline urine analysis was determined from a spot urine collection and metabolite profiles were normalized to urinary creatinine levels. Final urinary analysis was based on a 24-h urine collection and metabolite profiles were normalized to 24-h urine volumes.
2.9. Identification and quantification of ET metabolites
Urolithin A, B, C, D in addition to hydroxyl urolithin C, dimethyl ellagic acid, methyl urolithin A and dimethyl urolithin A were targeted for identification and quantification in both urine and plasma samples. Methyl urolithin C and dimethyl ellagic acid were tentatively identified according to their accurate mass (run separately on a QTOF instrument, 6550, Agilent Technologies, Santa Clara, CA), fragmentation, and UV absorption consistent with structure. Responses of these two compounds are reported in response units and due to the linearity of response can be used for comparative purposes between samples. Tentative detection of hydroxy urolithin C was made based on it being isobaric with urolithin D and similar chromatography and fragmentation. The MS/MS transitions for urolithin D afforded signals for hydroxyl urolithin C with a peak eluting just after the urolithin D standard. Experimental agents were synthesized according to reported literature protocols [43–45]. In general, these compounds were prepared through condensation reactions of appropriately substituted 2-bromobenzoic acids with either resorcinol for the preparation of urolithins A, B, and C or 2,3-dimethoxyphenol in the case of urolithin D. Demethylation of aromatic methoxy substituents was subsequently accomplished with hydrobromic acid in acetic acid to complete the syntheses of urolithins A, C, and D. Dimethyl urolithin A was prepared through methylation of the corresponding phenol of methy urolithin A [44]. The chemical identity of all compounds was confirmed based on obtained HRMS and NMR data. The NMR spectral properties of all compounds were found to be identical to reported values for urolithin A, methyl urolithin A, dimethyl urolithin A [44], urolithin B [46], urolithin C [47], and urolithin D [48].
2.10. Statistical analysis
Data are presented as means (± SD) for normally distributed continuous variables, medians and interquartile ranges (IQR) for variables not normally distributed, and percentages for categorical variables. The mean intake of all polyphenol subclasses and total polyphenols in this cohort were determined and stratified by diet intervention. Differences between groups were tested by analyses of covariance (ANOVA), and differences below the probability level (p < 0.05) were considered significant. When the normality assumption of ANOVA was violated the non-parametric Kruskal-Wallis test was used to test for significant differences between the groups. Statistical analyses were performed with STATA software (Stata/IC 12.0; College Station, TX).
3. Results
3.1. Study population
The average age of the 24 men enrolled was 64 years (range 49 to 79) with mean body mass index of 30.5 kg/m2 (Table 1). There were no significant differences in age and anthropometric measurements between the three groups at baseline (data not shown). Analysis of the 3-day diet records indicated no significant differences between groups for energy, fat, carbohydrate, protein or fiber intake (p = 0.659, p = 0.598, p = 0.948, p = 0.362 and p = 0.068, respectively) (Table 1). There was significantly greater dietary intake in fruit and grain intake in the control group compared to the low-polyphenol and low-ellagitannins groups (p = 0.005 and p = 0.003, respectively) (data not shown).
Table 1.
Measured anthropometric assessments and smoking status at baseline and estimated dietary intakes in all subjects during the study perioda)
| Control (n = 8) | Low polyphenol (n = 8) | Low ellagitannin (n = 8) | All subjects (N = 24) | p-value | |
|---|---|---|---|---|---|
| Anthropometrics | |||||
| Age (years) | 66 ± 7 | 65 ± 8 | 62 ± 7 | 64 ± 7 | 0.444 |
| Weight (kg)b) | 83.9 ± 23.9 | 100.4 ± 18.4 | 98.5 ± 15.0 | 94 ± 20 | 0.202 |
| BMI (kg/m2)b) | 27.6 ± 5.7 | 31.4 ± 4.9 | 32.4 ± 5.5 | 30.5 ± 5.6 | 0.188 |
| Dietary componentsc) | |||||
| Energy (kcal) | 2064 ± 262 | 1886 ± 393 | 2052 ± 579 | 2001 ± 420 | 0.659 |
| Fat (g) | 72 ± 12 | 74 ± 19 | 84 ± 36 | 77 ± 24 | 0.598 |
| Carbohydrate (g) | 89 ± 12 | 86 ± 33 | 89 ± 12 | 88 ± 21 | 0.948 |
| Protein (g) | 267 ± 77 | 219 ± 56 | 234 ± 67 | 240 ± 67 | 0.362 |
| Fiber (g) | 24 ± 11 | 15 ± 7 | 16 ± 5 | 18 ± 9 | 0.068 |
| Smoking statusd) | 0.396 | ||||
| Never smoker, n (%) | 4 (50) | 4 (50) | 7 (88) | 15 (66) | |
| Former smoker, n (%) | 2 (25) | 2 (25) | 1 (12) | 5 (21) | |
| Current smoker, n (%) | 2 (25) | – | – | 2 (8) | |
| Unknown, n (%) | – | 2 (25) | – | 2 (8) |
All values are means ± SDs. Differences between dietary intervention groups were analyzed by ANOVA. No significant differences were found between groups.
Weights reported were taken at baseline.
Estimated dietary components were calculated from 3-day diet records. Eight men were excluded from analysis due to average caloric intake being <1000 or >4000 kcals/day.
kg = kilograms, m = meter, kcal = kilocalories, g = grams.
Data presented as frequency of detection and percent of the total cohort. Differences between dietary intervention groups were analyzed with Fisher’s exact test. No significant difference was found.
3.2. Dietary compliance
Dietary compliance was assessed for the low-polyphenol and low-ET diet groups. Men on the low-polyphenol diet complied with the dietary prescription for 95% of total days on study and men on the low-ET diet complied with the prescribed diet 98% of total days on the study (Table 2). Consumption of more than eight fluid ounces of polyphenol-rich beverages (i.e., coffee, tea, beer) was the major contributor to non-compliance in the low-polyphenol diet. Non-compliance on the low-ET diet was reflective of wine intake, typically consumed during religious practices which occurred in <1% of the days on study.
Table 2.
Duration of study prior to prostatectomy and reported compliance to dietary intervention in all subjects during the study perioda)
| Dietary group | Study duration (days) | Compliant with study dietb) (days) | Complianceb) (%) |
|---|---|---|---|
| Control | 22.0 (15.5–25.5) | 22.0 (15.5–25.2) | 100 |
| Low polyphenol | 24.5 (17.5–26.5) | 21.5 (17.5–26.0) | 95 |
| Low ellagitannin | 26.0 (25.0–28.5) | 25.5 (24.5–27.5) | 98 |
| Average across groups | 25.0 (18.5–27.0) | 25.0 (18.5–26.0) | 97 |
| p-value | 0.244 | 0.275 | 0.102 |
Values represent the median and IQR. Differences between dietary intervention groups (n = 8 per group) were analyzed with Kruskal-Wallis tests. No significant differences were found between groups.
Compliance = days compliant/study duration. Compliance with the study diet was based upon deviation from the expected restrictions and recorded as yes/no. Yes meant that the subject met all diet requirements for the day. No meant that the subject was non-compliant ≥ 1 time per day. All compliance data was self-reported.
3.3. Estimated total dietary polyphenols: Chromatography
Estimated total dietary polyphenols intake based on PED chromatography data was determined for all three groups. Estimated dietary polyphenol intake varied significantly between groups. The control diet group consumed 1568 ± 939 mg/day compared to the low-polyphenol and low-ET groups (238 ± 248 mg/day and 840 ± 667 mg/day, respectively; p = 0.004; Fig. 1). Diet education to reduce polyphenol-rich foods led to > 85% reduction in total polyphenols compared to the controls. Diet education for men on the ET-restricted diet led to a 45% reduction in estimated polyphenol intake compared to the control group. A significant reduction in total polyphenol intake in the two intervention groups were largely reflective of reduced coffee and/or tea consumption (p = 0.021; Table 3). The low-polyphenol diet group included a daily coffee and tea restriction (≤ 1c. per day), whereas this restriction was not necessary for a low-ET diet. The low-ET diet group had an eightfold higher consumption of tea/coffee compared to the low-polyphenol diet (Table 3). Furthermore, coffee and/or tea were the greatest contributors of polyphenol intake in all groups (control 70.9%, low polyphenol 40.1%, low ET 86.8%; Table 3).
Table 3.
Dietary contributions to estimated polyphenol intake during the study perioda)
| Sources of polyphenol intake |
|||||||
|---|---|---|---|---|---|---|---|
| Control |
Low polyphenol |
Low ellagitannin |
P-value | ||||
| mg/day | % | mg/day | % | mg/day | % | ||
| Food group | |||||||
| Coffee/tea | 1112 | 70.9 | 95 | 40.1 | 730 | 86.8 | 0.021 |
| Chocolate | 111 | 7.1 | 63 | 26.6 | 13 | 1.5 | 0.678 |
| Fruits (fresh/processed) | 121 | 7.7 | 5 | 2.1 | 45 | 5.4 | 0.112 |
| Juice | 76 | 4.8 | 27 | 11.4 | 22 | 2.6 | 0.501 |
| Alcoholic beverages | 50 | 3.2 | 2 | <1 | 3 | <1 | 0.812 |
| Grains | 38 | 2.4 | 10 | 4.2 | 8 | 1.0 | 0.157 |
| Vegetables | 39 | 2.5 | 31 | 13.1 | 18 | 2.1 | 0.229 |
| Oil/seasoning | 14 | <1 | 1 | <1 | <1 | <1 | 0.574 |
| Nuts/seeds/legumes | 7 | <1 | 3 | 1.3 | 2 | <1 | 0.238 |
| Total | 1568 | – | 237 | – | 841 | – | 0.003 |
Estimations calculated from 3-day diet records using the Polyphenol Explorer Database® and the chromatographic analysis available within the database. Calculations based on eight men per group.
When stratifying total polyphenol intake across all three diet groups by polyphenol subclasses, phenolic acids and flavonoids are the greatest contributors to total estimated polyphenol intake. In the control group, phenolic acids contribute 72% and flavonoids 24% of the estimated total polyphenols in the diet. Phenolic acids contributed to 52% of total polyphenols in the low-polyphenol group and 76% in the low-ET group, whereas flavonoids contributed to 44% and 23%, respectively. Despite differences in total polyphenol intake between groups, phenolic acids contributed to the greatest intake amongst the polyphenol subclasses. Interestingly amongst all groups, coffee is the main food source within the phenolic acids (control 93%; low polyphenol 76%; low ET 59%) (Table 4).
Table 4.
Foods contributing to the top three food sources or ≥5% of the estimated polyphenol subclasses during the study period
| Polyphenol subclassa) | Polyphenol intakeb) (mg/d per person) | Main food sourcesc) (% contribution to intake with in the polyphenol subclass) |
|---|---|---|
| Control | ||
| Phenolic acids | 1140.7 ± 989.3 | Coffee (93), Juice (2), Potato (2) |
| Flavonoids | 371.5 ± 191.4 | Chocolate (31), Tea (12), Apple (11), Berries (11), Juice (10), Wine (9), Oranges (5) |
| Stilbenes | 1.8 ± 3.1 | Wine (94), Strawberry (3), Juice (2) |
| Lignans | 0.2 ± 0.2 | Olive oil (67), Pineapple (18), Refined flour (10) |
| Other polyphenols | 53.9 ± 63.5 | Cereal (29), Coffee (24), Whole grain flour (20), Turmeric (15), Peas (7) |
| Total Polyphenols | 1568.1 ± 939.4 | |
| Low polyphenol | ||
| Phenolic acids | 122.8 ± 205.1 | Coffee (76), Potato (14), Tomato (3) |
| Flavonoids | 105.0 ± 157.0 | Chocolate (56), Juice (25), Whole grain flour (5) |
| Stilbenes | 0.1 ± 0.3 | Wine (98), Chocolate (2), Vinegar (0.1) |
| Lignans | 1.7 ± 4.8 | Flaxseed (97), Pineapple (2), Refined flour (1) |
| Other polyphenols | 7.6 ± 10.7 | Whole grain flour (58), Coffee (15), Juice (14), Vinegar (9) |
| Total Polyphenols | 238.0 ± 248.3 | |
| Low ellagitannin | ||
| Phenolic acids | 635.1 ± 692.7 | Coffee (59), Whole grain flour (25), Juice (8) |
| Flavonoids | 192.2 ± 169.7 | Tea (54), Apple (23), Juice (11), Chocolate milk (6) |
| Stilbenes | 0.8 ± 1.3 | Peanut butter (87), Wine (13) |
| Lignans | 0.03 ± 0.03 | Refined flour (65), Pineapple (35) |
| Other polyphenols | 12.3 ± 7.1 | Coffee (94), Tea (4), Potato (2) |
| Total Polyphenols | 840.4 ± 666.8 |
Polyphenol subclasses included in the Polyphenol Explorer® database
Values are means ± SD.
Foods contributing to the top 5% of each polyphenol subclass calculated from 3-day diet records. When there was less than three foods contributing to the top 5% of each polyphenol subclass, up to the top three food sources were included. Percent contribution = mg polyphenols per food/total mg polyphenols per day. Calculations based on eight men per group.
3.4. Estimated total dietary phenolics: FC assay
Estimated total dietary phenolics using data derived from the FC assay were determined for all three diet groups [39]. Estimated dietary phenolic intake varied significantly between groups. The control group consumed an estimated 2405 ± 1465 mg/day compared to the low-polyphenol and low-ET groups (554 ± 305 and 1288 ± 777 mg/day, respectively; p = 0.003; Fig. 1). Diet education aimed to restrict total polyphenol-rich foods led to a 77% reduction in phenolics compared to the control group, whereas the diet education to reduce total ET-rich foods led to nearly a 47% reduction in estimated polyphenol intake compared to the control group (Fig. 1).
3.5. Estimated total dietary ellagitannin: Chromatography after hydrolysis
Dietary ET intake was calculated based upon chromatography after hydrolysis [3] and estimated to be 12 ± 13 mg/day in the control group with a range of intake from 0 mg/day to a maximum of 35 mg/day (Table 5). Subjects in both dietary restriction groups reported no dietary intake of ETs on 3-day diet records; therefore, no sources of dietary ETs were reported in the low-polyphenol or low-ET diets. Within the control group, ET intake was estimated from five main foods sources: strawberries, blackberries, walnuts, pomegranate juice and preserves. Fresh berries contributed to nearly half of the estimated ET intake from the diet (42%) while nuts, fruit juices and preserves account for the other half of dietary ET intake (Table 5).
Table 5.
Dietary contributions to total estimated ellagitannin intake during the study perioda)
| Total estimated ellagitannins |
||
|---|---|---|
| Food group | mg/day | % |
| Blackberries/strawberries | 5.3 | 41.6 |
| Walnuts | 3.4 | 26.7 |
| Pomegranate juice | 3.5 | 27.6 |
| Strawberry jam | 0.5 | 4.1 |
Estimations calculated from 3-day diet records using the Polyphenol Explorer Database® using Chromatography after hydrolysis assay for estimation. Calculations based on eight men in the control group as the low-polyphenol and low-ET diets had zero values and were therefore, not reported.
3.6. Urinary and plasma ellagitannin metabolites
Men in the control group had the greatest quantity of ET metabolites in the urine (Table 6). A statistically significant difference in urolithin A was detected between groups with the highest range of detection noted in the control group and the highest median intake detected in the low-polyphenol group (p = 0.012; Table 6). Urolithin C was not detectable in any 24-h urine specimens of the men receiving a dietary intervention, but di-methyl ellagic acid was detected in four men in the control group (Table 6). Although urolithin B and D were detected in the 24-h urine specimens of all three groups, there was no statistically significant differences detected between groups (p = 0.255 and 0.719, respectively; Table 6).
Table 6.
Detected metabolites in spot urine specimens in all men at baseline prior to receiving a dietary intervention and in 24-h urine collection after receiving a dietary interventiona)
| Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | Control diet |
Low polyphenol |
Low ellagitannin |
p-valueb) |
||||
| Baseline | End of study | Baseline | End of study | Baseline | End of study | Baseline | End of study | |
| Urolithin A (nmol/mL) | 0.005 (0–11.1) | 0 (0–36.8) | 0.03 (0–0.17) | 3.8 (3.0–6.9) | 0.3 (0–11.7) | 0 (0–0) | 0.884 | 0.012 |
| Urolithin B (nmol/mL) | 0 (0–0.73) | 1.6 (0–47.3) | 0.02 (0.004–0.02) | 4.4 (2.7–5.4) | 0 (0–0.006) | 0.3 (0–2.8) | 0.160 | 0.255 |
| Urolithin C (nmol/mL) | 0 (0–0.004) | ND | 0 (0–0.04) | ND | 0 (0–0) | ND | 0.463 | – |
| Urolithin D (nmol/mL) | 0.03 (0.02–0.05) | 2.8 (1.8–5.1) | 0.02 (0.01–0.03) | 2.9 (2.1–4.1) | 0.03 (0.008–0.05) | 3.8 (2.4–6.1) | 0.564 | 0.719 |
| Di-Methyl Ellagic acid (arbitrary units) | 0 (0–150) | 11.6 (0–118) | 13.9 (0–26.9) | ND | 7.9 (0–116) | ND | 0.941 | – |
Values are medians and IQRs. Calculations based on eight men per group.
P-values are calculated using the non-parametric Kruskal–Wallis test.
ND = not detected.
Urolithin A, B, C, D, di-methyl ellagic acid were all included in the plasma analysis. Urolithin A and urolithin D were the only metabolites detected. Subnanomolar concentrations of urolithin A were detected in the baseline plasma specimens of five subjects while no urolithins were detected at the end of the study in any group. Due to the low-detection rates, statistical analysis was not possible.
4. Discussion
The data presented provides information that will assist investigators regarding the design and implementation of interventions with polyphenol-rich foods or polyphenol-based dietary patterns in human studies. We have employed valuable tool, which is being continuously updated, the PED to define the typical exposure to polyphenols in men with PCa who had undergone a prostatectomy and to a specific class of polyphenols known as ETs. We have further defined and evaluated a low-polyphenol or low-ET dietary pattern with similar excellent compliance over a period of several weeks, indicating that either of these interventions could be utilized as a control diet or background diet for studies of specific polyphenol rich foods focusing on bioactivity and mechanisms of action. Finally, we have documented the impact of these interventions on biomarkers of exposure using a targeted metabolomic HPLC-approach focusing upon ET metabolites.
Food-based intervention trials are typically more complex than pharmaceutical-based interventions for many reasons. Most critically, unlike drugs, a study population is often exposed to various amounts of a specific food or component under study. In the case of polyphenols which are found in many foods, a clinical intervention trial may require a control group is established with a restricted polyphenol intake. Several have attempted a total polyphenol depleted diet, an approach that may be possible and potentially useful for short term studies, but such an extreme diet is not easily achieved for longer studies of biomarkers relevant to cancer risk and prevention. For example, one study implemented a diet which is devoid of all fruits, vegetables, whole grains, seasonings and most beverages (including tea and coffee) other than milk, water and grain alcohol [17], which is challenging for adherence in studies of any significant duration. Thus, a controlled polyphenol diet, allowing modest intake, designed to greatly reduce exposure but provide for greater compliance can be considered. Other studies target a low-ET diet [28, 34], but there is no data comparing compliance with a low-polyphenol vs. a low-ET diet and their impact on systemic biomarkers of metabolites. One of our goals is to define a low-polyphenol dietary pattern and a low-ET dietary pattern that are feasible for longer term studies. The first step in this process is knowledge regarding the typical polyphenol consumption on the population of interest. The usual polyphenol intake of the American population is not well defined, and we quantitated intake in our control group. The PED, first released in 2009 and continually updated, is the most comprehensive polyphenol database available and constructed to allow researchers to extrapolate polyphenol intake in humans from dietary assessment data [13]. The strength and weaknesses of various dietary assessment tools are also a challenge for the precise estimation of polyphenol intake [49]. We chose to examine 3-day diet records in conjunction with a specific polyphenol screening questionnaire (Supporting Information data D). Describing the polyphenol signature of each food remains a challenge given that polyphenol content varies considerably based on the season of harvest, the plant variety, growing conditions and food processing after harvesting the crop [13]. Capturing this variability is problematic and, thus, limiting our ability to precisely define the exposure of individuals in clinical trials. This problem is not unique to polyphenols, and is a concern for many of the nutrients provided in the USDA or other food composition databases. At this point in time, the PED provides the “state-of-the-art” database for estimating intake in study populations where accurate food consumption data is available [37, 39].
Our estimate of total polyphenol intake of men consuming their usual diet was 1568 ± 939 mg/day which is slightly higher in magnitude, but similar in variation to that of the Finnish (863 ± 415 mg/day) [37] and French populations (1193 ± 510 mg/day) [39]. It is apparent that individuals vary substantially based upon the large standard deviations observed in multiple studies. Slightly higher estimated daily intakes of 1786 mg/day and 1626 mg/day were noted for men and women in Denmark, respectively, and lower intakes in Greece of 744 mg/day and 584 mg/day for men and women, respectively [50]. Variability is likely a result of differences across populations, but also in dietary assessment tools (FFQ vs. food diaries) and the methods used for quantification of polyphenols (PED vs. national food databases). The Finnish cohort utilized 48-hour dietary recalls and estimated polyphenol content from the national food database (Fineli) whereas the French cohort used the PED to estimate the polyphenol content from a 24-h dietary recall making it challenging for a direct comparison between our study and those reported in the literature. Most recently, in over 7000 Spanish men and women enrolled in a Mediterranean feeding trial quintiles were constructed of estimated polyphenol intake: <642, 642–749, 750–852, 853–995, and >995 mg/day (first to fifth quintile, respectively) [51]. The authors reported a 37% reduction in all-cause mortality when comparing the highest quintile of estimated polyphenol intake to the lowest (HR = 0.63; 95% CI 0.41 to 0.97; p-value = 0.12) [51]. Interestingly, although the methodology for dietary assessment was different from our analysis (FFQ vs. 3-day diet records), the estimated average polyphenol content in our control group is comparable to the fifth-quintile estimates in the Spanish study. Additional studies using improving diet assessment tools coupled with the continually updated PED are necessary to better define exposure to bioactive polyphenols overall, and specific polyphenols that may have unique effects on health and disease.
The contributions of food sources to polyphenol estimates within populations has been shown to vary by sex, age, region, body mass index (BMI), education level, smoking status, physical activity and dietary intake [50]. Males have been found to consume a greater quantity of polyphenols compared to women [37, 39, 50] and likely in part due to their greater caloric intake. Due to our modest sample size, we were not powered to detect an impact in smoking status, BMI or age. It is noted that non-alcoholic beverages (coffee, tea and juice) were the largest contributors of polyphenol intake which is similar in other populations [11,39,50]. Due to the high prevalence of coffee and/or tea intake within many populations, these foods must be carefully considered in the dietary design of future clinical trials aimed at manipulating total dietary polyphenol intake or polyphenols of specific classes.
One objective of the present trial is to define a control diet that would be low in polyphenols, yet feasible with high compliance over a period of several weeks, such that any intervention with a polyphenol rich food could be examined on a background of low total polyphenol exposure. After education to restrict dietary polyphenol intake, we were able to achieve intake at our goal of being similar to those of the lowest quintile of estimated human intake from various population studies [50,51]. Our data suggesting that we achieved a “low” -polyphenol diet with an estimated intake of 238 mg/day compared to the <642 mg suggested by other studies [51]. We found compliance to be excellent over the short duration of our study.
One of the classes of polyphenols that may impact prostate carcinogenesis based upon experimental data is the ETs [52]. Thus, we also designed a low-ET diet targeting the lowest quintile of typical population exposure, and based the intervention to exclude known sources of ET intake. Estimating individual polyphenol subclasses like ETs is also possible with the PED. No previous studies have estimated ET content in the American diet. Many studies exclude estimates of dietary ETs or report these estimates as total phenolic acids or hydroxybenzoic acids making it difficult for comparison to our estimates. The 2007 report by Ovaskainen et al. includes an estimation of dietary ETs in Finnish adults as 12 ± 37 mg/day, which is similar to our estimates in the present cohort. Interestingly, the largest dietary contribution to ET intake in the Finnish cohort was strawberries [37]. This was also true in our subset of men, which identifies a dietary target for adjusting dietary ET content in future clinical trials. In all groups, the estimated ET dietary intake was much lower than ET doses used in intervention studies (30–60 g/day) [6, 7, 17, 34, 53]. The controlled ET diet resulted in estimated polyphenol intake in the third quintile suggesting “moderate” polyphenol intake.
Given our interests in ETs and PCa, we also chose to examine ET metabolites detected in the urine relative to our dietary interventions. Many studying ETs, measure the presence of urolithins after exposure to a food product or extract rich in ETs. We employed a targeted metabolomics approach to detect and quantify the presence of ET and ET metabolites in the urine and plasma of the men receiving the dietary intervention or the control diet. Urinary ET metabolites were detected at a higher concentration in specimens of men in the control group at the end of the of controlled diet interventions. This suggests that urinary urolithins and DMEA may be used as a biomarker of exposure in free living men, but this relationship requires validation in a larger, more diverse population. Urinary urolithin A, the predominant ET metabolite detected in other reports has been quantified at concentrations as high as 50 000 nmol/day after receiving dietary sources of ETs or ellagic acid extracts [23, 54]. These concentrations are significantly higher than detected in our cohort of men consuming a usual American diet. The biological activity of ET metabolites varies based on in vitro and in vivo models [9, 11, 23]. Suggestive data has been reported on various metabotypes that have emerged from human, urinary and plasma urolithin data. These three metabotypes include individuals that produce only urolithin A (most predominant metabotype reported), those that produce isourolithin A and/or urolithin B in addition to urolithin A (second most prevalent metabotype reported) and those that do not produce any detectable urolithins [34]. Various factors may contribute to these metabotypes such as the present state of illness for subjects, and body habitus, but these associations are yet to be fully understood. Data has been reported on the association of BMI with various gut bacteria species and metabotypes that suggest overweight and obese individuals favor a metabotype in which isourolithin A and/or urolithin B in addition to urolithin A [46]. In our study, the average BMI suggests that our subjects fall into an overweight and obese range (Table 1) and therefore, could impact urolithin production. Full characterization of the gut microbiota and its association with urolithin production and various metabotypes is warranted to clarify this relationship and is a goal of future intervention studies. Thus, there is much to learn regarding the interface between dietary ET, impact on the gut microflora structure and function, and the resulting metabolites produced that in turn impact the host [55]. Men educated to restrict either total polyphenols or ETs had detected urolithins <5 nmol/day confirming the excellent dietary compliance data.
This data supports the utility of the PED for estimation of total polyphenols and subclasses such as ET. It is important to remain aware of analytical techniques reported in the database and employed by investigators in order to consider comparisons among studies. The PED uses five analytical categories: chromatography, chromatography after hydrolysis, normal phase HPLC, FC assay or the pH differential method [13]. Chromatography assays and the FC assay provide slightly different values when estimating dietary compounds, but both can provide estimates of the dietary characteristics [56]. The FC assay is an inexpensive, quick colorimetric assay for the quantification of phenolic content of a specimen or product yet it lacks specificity for defining individual dietary polyphenols [57]. The FC assay is historically reported for quantification of total phenolic content although it best represents the reducing capacity of a food item or food product [16, 57]. Chromatography data is likely to completely replace the FC data over time and has improved specificity for individual dietary polyphenols over the FC assay, but with the numerous polyphenol classes and the complex structures of these compounds, creation of a complete database including all foods and all known dietary polyphenols is currently unavailable [16]. We have presented the estimated intake using both the FC assay and chromatography and report similar changes in dietary patterns and quantities of estimated total phenolics (identified by the FC assay) and in total polyphenols (identified by the chromatography assay) between the three groups regardless of the analytical technique used. A limitation to this comparison includes that not all foods reported in the 3-day diet records were included in the PED and assessed by both methods. Therefore, some caution should be used when comparing these estimates.
The PED as applied to a 3-day diet records is a useful tool for the approximation of polyphenol intake in American men and shows a significant association with urinary metabolite profiles and dietary ET intake. We have developed feasible low-polyphenol and low-ET dietary patterns that can be implemented in clinical trials to reduce background exposure when the objective is to examine the metabolism or impact of specific polyphenol rich foods, extracts, or pure phytochemicals. These dietary patterns are more tolerable than diets devoid of all polyphenols or ETs, thus reduce subject burden and enhance accrual and compliance. Although the role of plant derived polyphenols in human health and disease is clearly complex, the new tools to assess exposure and characterize metabolism are a key step in the understanding their impact and mechanisms of action. The findings from this study should be replicated by others to ensure reliability in patient populations other than men with PCa and in larger groups of both males and females.
Supplementary Material
Acknowledgments
The authors would like to thank S. Rusnak, MS, RD and C. Spees, PhD, RD for assistance in the analysis of the dietary records of study participants. This study was supported by funding and resources available through The Ohio State University Comprehensive Cancer Center Molecular Carcinogenesis and Chemoprevention Program and the Nutrient and Phytochemical Analytic Shared Resource, the Center for Clinical and Translational Science via a Pilot Award in conjunction with the Food Innovation Center (Vodovotz), TL-1 pre-doctoral fellowship (Roberts), OSU-James Prostate Cancer Prevention Fund and the Bionutrition and Cancer Prevention Fund.
K.M.R., S.K.C., E.M.G., and J.M.T. designed the clinical trial; K.M.R, E.M.G., and R.A. enrolled and monitored participants, collected clinical data and specimens; K.R. and S.J.S. conducted the phytochemical analysis; K.M.R. and S.K.C. prepared the manuscript; A.H. provided all statistical support; J.T., E.G., J.G., Y.V., and R.A.,edited and revised the manuscript.
Abbreviations
- BRB
black raspberries
- ET
ellagitannin
- PED
Polyphenol Explorer® Database
- HPFS
health professionals follow-up study
- PCa
prostate cancer
- FC
Folin–ciocalteu
- FFQ
food frequency questionnaire
Footnotes
The authors declare no conflict of interest
Additional supporting information may be found in the online version of this article at the publisher’s web-site
5 References
- [1].Research, W.C.R.F.A.I. f.C., Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective, AICR: Washington, DC: 2007. [Google Scholar]
- [2].DeSalvo KB, Olson R, Casavale KO, Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [DOI] [PubMed] [Google Scholar]
- [3].Perez-Jimenez J et al. , Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [DOI] [PubMed] [Google Scholar]
- [4].Kresty LA et al. , Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr. Cancer 2006, 54, 148–156. [DOI] [PubMed] [Google Scholar]
- [5].Mallery SR et al. , Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. (Phila). 2011, 4, 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang LS et al. , A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. (Phila). 2014, 7, 666–674. [DOI] [PubMed] [Google Scholar]
- [7].Chen T et al. , Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev Res (Phila). 5, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seeram NP et al. , Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [DOI] [PubMed] [Google Scholar]
- [9].Seeram NP et al. , Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007, 55, 7732–7737. [DOI] [PubMed] [Google Scholar]
- [10].Lee ST et al. , Proteomic exploration of the impacts of pomegranate fruit juice on the global gene expression of prostate cancer cell. Proteomics. 12, 3251–3262. [DOI] [PubMed] [Google Scholar]
- [11].Kasimsetty SG et al. , Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J. Agric. Food Chem. 2009, 57, 10636–10644. [DOI] [PubMed] [Google Scholar]
- [12].Zamora-Ros R et al. , Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am. J. Clin. Nutr. 2014, 100, 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Neveu V et al. , Phenol-explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010, 2010, bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rothwell JA et al. , Effects of food processing on polyphenol contents: a systematic analysis using phenol-explorer data. Mol. Nutr. Food Res. 2015, 59, 160–170. [DOI] [PubMed] [Google Scholar]
- [15].Hager TJ, Howard LR, Prior RL, Processing and storage effects on the ellagitannin composition of processed blackberry products. J. Agric. Food Chem. 58, 11749–11754. [DOI] [PubMed] [Google Scholar]
- [16].Amarowicz R et al. , Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53 Suppl 2, S151–S183. [DOI] [PubMed] [Google Scholar]
- [17].Stoner GD et al. , Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [DOI] [PubMed] [Google Scholar]
- [18].Adams LS et al. , Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [DOI] [PubMed] [Google Scholar]
- [19].Adhami VM et al. , Oral infusion of pomegranate fruit extract inhibits prostate carcinogenesis in the TRAMP model. Carcinogenesis 33, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bi X et al. , Black raspberries inhibit intestinal tumorigenesis in apc1638+/− and Muc2−/− mouse models of colorectal cancer. Cancer Prev. Res. (Phila). 3, 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Casto BC et al. , Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002, 22, 4005–4015. [PubMed] [Google Scholar]
- [22].Chen T et al. , Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006, 66, 2853–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gonzalez-Sarrias A et al. , Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 54, 311–322. [DOI] [PubMed] [Google Scholar]
- [24].Harris GK et al. , Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2’-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [DOI] [PubMed] [Google Scholar]
- [25].Huang C et al. , Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res. 2006, 66, 581–587. [DOI] [PubMed] [Google Scholar]
- [26].Kresty LA et al. , Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119. [PubMed] [Google Scholar]
- [27].Mallery SR et al. , Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008, 68, 4945–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nunez-Sanchez MA et al. , Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [DOI] [PubMed] [Google Scholar]
- [29].Pantuck AJ et al. , Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006, 12, 4018–4026. [DOI] [PubMed] [Google Scholar]
- [30].Rodrigo KA et al. , Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr. Cancer 2006, 54, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Giovannucci E et al. , Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [DOI] [PubMed] [Google Scholar]
- [32].Garcia-Villalba R, Espin JC, Tomas-Barberan FA, Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J. Chromatogr. A 2016, 1428, 162–175. [DOI] [PubMed] [Google Scholar]
- [33].Cerda B, Tomas-Barberan FA, Espin JC, Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [DOI] [PubMed] [Google Scholar]
- [34].Tomas-Barberan FA et al. , Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [DOI] [PubMed] [Google Scholar]
- [35].Seeram NP et al. , Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [DOI] [PubMed] [Google Scholar]
- [36].Espin JC et al. , Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat. Med. 2013, 2013, 270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ovaskainen ML et al. , Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [DOI] [PubMed] [Google Scholar]
- [38].Perez-Jimenez J et al. , Identification of the 100 richest dietary sources of polyphenols: an application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64 Suppl 3, S112–S120. [DOI] [PubMed] [Google Scholar]
- [39].Perez-Jimenez J et al. , Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [DOI] [PubMed] [Google Scholar]
- [40].Clifford MN, Scalbert A, Ellagitannins - nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar]
- [41].Amakura Y et al. , High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J. Chromatogr. A 2000, 896, 87–93. [DOI] [PubMed] [Google Scholar]
- [42].Koponen JM et al. , Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [DOI] [PubMed] [Google Scholar]
- [43].Bialonska D et al. , Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [DOI] [PubMed] [Google Scholar]
- [44].Cozza G et al. , Urolithin as a converging scaffold linking ellagic acid and coumarin analogues: design of potent protein kinase CK2 inhibitors. Chem. Med. Chem. 2011, 6, 2273–2286. [DOI] [PubMed] [Google Scholar]
- [45].S G et al. , IV: Chemistry of two bioactive benzopyrone metabolites. J. Chem. Res. Synop. 1989, 350–351. [Google Scholar]
- [46].Pisani L et al. , Design, synthesis, and biological evaluation of coumarin derivatives tethered to an edrophonium-like fragment as highly potent and selective dual binding site acetylcholinesterase inhibitors. Chem. Med. Chem. 2010, 5, 1616–1630. [DOI] [PubMed] [Google Scholar]
- [47].Ito H, Iguchi A, Hatano T, Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J. Agric. Food Chem. 2008, 56, 393–400. [DOI] [PubMed] [Google Scholar]
- [48].BI A et al. , Sequential directed ortho metalation-boronic acid cross-coupling reactions. A general regiospecific route to oxygenated dibenzo[b,d]pyran-6-ones related to ellagic acid. J. Org. Chem. 1991, 56, 3763–3768. [Google Scholar]
- [49].Shim JS, Oh K, Kim HC, Dietary assessment methods in epidemiologic studies. Epidemiol. Health. 2014, 36, e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zamora-Ros R et al. , Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tresserra-Rimbau A et al. , Polyphenol intake and mortality risk: a re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gu J, Department of Food Science and Technology. The Ohio State University: Columbus, Ohio: 2015. [Google Scholar]
- [53].Stoner GD, Wang LS, Casto BC, Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tulipani S et al. , Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J. Agric. Food Chem. 2012, 60, 8930–8940. [DOI] [PubMed] [Google Scholar]
- [55].Cerda B et al. , Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [DOI] [PubMed] [Google Scholar]
- [56].Robbins RJ et al. , Analysis of flavanols in foods: what methods are required to enable meaningful health recommendations? J. Cardiovasc. Pharmacol. 2006, 47(Suppl 2), S110–S118; discussion S119–S121. [DOI] [PubMed] [Google Scholar]
- [57].Tan JB, Lim YY, Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.