Abstract
Current treatment of vitiligo is still a great challenge, since most cases of vitiligo have variable re-pigmentation outcomes due to their unpredictable responses to existing therapeutic regimens. There is an urgent need to identify this re-pigmentation process and to develop novel therapies. This review illustrates the most current research and latest understanding of vitiligo skin re-pigmentation and related regulatory mechanisms. Literature was collected from PubMed until January 2020, using the search terms including “vitiligo,” “re-pigmentation,” “phototherapy,” “narrow-band ultraviolet B, ” “excimer,” “fractional carbon dioxide laser,” and “melanocyte stem cells.” Literature was mainly derived from English articles. Article type was not limited. Emerging evidence suggests that patients with vitiligo present various re-pigmentation patterns following ultraviolet B phototherapy, which relies on different cell reservoirs from the perilesional margins and/or from uninvolved hair follicles to replenish functional melanocytes that are lost in vitiliginous skin. The following events are likely to be involved in this re-pigmentation process, including: 1) changes in the paracrine secretion and distribution of transforming growth factor-β1 in the bulge area and in the epidermis; 2) the enhanced transfer of dermal pro-melanogenic growth factors to the epidermis; and 3) the induction of a C-X-C motif chemokine ligand (CXCL) 12-enriched micro-environment that efficiently recruits CXCR4- or CXCR7-positive melanocytes. Ongoing studies on the cellular and molecular events underlying vitiligo re-pigmentation will help design new therapeutic strategies to improve treatment outcomes.
Keywords: Vitiligo, Re-pigmentation, Melanocyte, Ultraviolet B, Skin trauma
Introduction
Among pigmentary skin disorders, vitiligo is the most common skin disease of acquired depigmentation, affecting 0.5% to 1% of the population worldwide. Vitiligo is clinically characterized by well-defined depigmented macules and white patches of the skin, due to the chronic and progressive loss of functional melanocytes from the epidermis and/or hair follicles, which can be psychologically devastating and stigmatizing, especially in dark-skinned individuals.[1,2] Although autoimmune-mediated cell death is currently accepted as the leading cause for the disappearance of melanocytes in most cases of vitiligo, the molecular mechanism by which melanocyte-specific immune responses are triggered has never been clearly understood.[3] In the clinical setting, the treatment of vitiligo is challenging and often variable due to unpredictable responses of patients to various therapeutic regimens. Thus, the type and course of treatment needs to be tailored for each vitiligo patient.[4] Current treatment strategies for vitiligo are aimed at arresting disease progression in the active stage and stimulating re-pigmentation in the inactive stage of the disease. Re-pigmentation has been thought to involve a regenerative process, in which existing viable melanocyte precursors and/or melanocyte stem cells are dissociated from one area in the perilesional normal epidermis and/or unaffected hair follicles and are subsequently recruited to another area to repopulate the achromic skin.[5,6] It is interesting to observe that vitiligo re-pigmentation in some areas can commonly parallel active depigmentation of other areas in the same patient.[5] According to a recent report, this clinical phenomenon can be explained by the fact that the presence of melanocyte-specific resident memory T cells in the lesional skin tissues of vitiligo patients is closely related to disease flareups.[7] Therefore, a maintenance therapy consisting of the continuous application of anti-inflammatory agents (such as topical corticosteroids, topical calcineurin inhibitors, and/or topical Janus kinase inhibitors) can be suggested as a means to prevent new episodes of flare-up from the accumulation of these resident memory T cells.[8,9] With the exception of endeavors to attenuate and halt the local inflammatory response of vitiligo, ultraviolet B (UVB)-based phototherapy has been clinically proven to be more efficient to induce re-pigmentation.[5,10] Various re-pigmentation patterns, such as perifollicular, marginal, diffuse, and combined patterns, have been observed in vitiligo patients who have undergone UVB-based phototherapy, pointing out that the re-pigmentation patterns differ greatly depending on available source of melanocyte precursors or stem cells in the epidermis or in hair follicles. It seems plausible that the existence of viable residual melanocytes in vitiliginous lesions might explain the formation of diffuse re-pigmentation pattern.[11] More recent studies have indicated that the induction of therapeutic skin trauma in the lesional skin by dermabrasion, micro-needling, ablative fractional CO2 lasers and punch grafting also provide an effective treatment option in achieving re-pigmentation in difficult-to-treat areas of vitiligo patients. Here, we review recent advances in understanding the cellular and molecular mechanisms underlying re-pigmentation induced by UVB irradiation or by therapeutic skin trauma, with a focus on the initiation and acceleration of this process. Knowledge about the restoration of skin pigmentation may help develop novel therapeutic strategies to improve treatment outcomes for vitiligo.
Formation and Regulation of the Perifollicular Re-pigmentation Pattern
Re-pigmentation in human vitiligo occurs in different clinical patterns, of which the most frequent re-pigmentation pattern is the perifollicular type, which indicates that hair follicles are the main melanocyte reservoir for this pattern.[4,5] Clinical evidence also shows that vitiligo patients with depigmented skin (leukoderma) and leukotrichia (white hair) have poor responses to UVB phototherapy, whereas those with leukoderma and pigmented hair (black hair) seem to typically develop a perifollicular re-pigmentation pattern after UVB phototherapy.[12] It is widely assumed that mature melanocytes may be vulnerable to injury by oxidative stress due to the melanin deposits in their cytoplasm, since melanosomal proteins are subject to oxidative modifications and appear to be more immunogenic.[13,14] Several studies have demonstrated that the bulge region in hair follicles is a relatively safe niche that houses melanocyte stem cells[15] together with keratinocyte stem cells.[16] The bulge region is a part of the outer root sheath (ORS) that provides the insertion point for the arrector pili muscle and points to the bottom of the permanent portion of the hair follicle. Meanwhile, the bulge region is also one of the immune privileged sites of the body and consequently is protected from the potentially detrimental influences of immunologic inflammation. Keratinocytes in the bulge region secrete high levels of transforming growth factor beta-1 (TGF-β1) that maintain melanocyte stem cells in a quiescent (inactive) status via the down-regulated function of microphthalmia-associated transcription factor (MITF).[17] The niche-derived TGF-β1 cytokine also plays an immunosuppressive role in maintaining the immune privileged micro-environment surrounding the bulge region.[18]
Over the past few decades, many efforts have been devoted to investigating whether dormant melanocyte stem cells residing in the bulge region of hair follicles are awakened to participate in the perifollicular re-pigmentation. Goldstein et al[4] carried out a well-designed study using immunofluorescence to examine the melanocyte distribution in skin exposed to narrow band UVB (NB-UVB) using a panel of antibodies that recognize Dct (a lineage marker of melanocytes), c-Kit (a marker of proliferating melanocytes), and Tyrosinase (a marker of differentiated melanocytes). Their results revealed that there are three populations of melanocytes, Dct+/c-Kit−/TYR− (melanocyte stem cells), Dct+/c-Kit+/TYR− (melanoblasts, also known as transit-amplifying cells/TA cells) and Dct+/c-Kit+/TYR+ (mature melanocytes), found in serial sections of skin treated with NB-UVB.[4] The cellular events involved in formation of the perifollicular re-pigmentation pattern have been assumed to be: 1) dormant melanocyte stem cells (Dct+/c-Kit−/TYR−) residing in the bulge region of hair follicles are directly activated by NB-UVB[19]; 2) once the melanocyte stem cells have mobilized to exit from the bulge region into the supra-bulge region, they grow and differentiate in part as a consequence of the eliminated repression by the niche-derived TGF-β1 and become melanoblasts (TA cells, Dct+/c-Kit+/TYR−); and 3) these melanoblasts migrate upward through the ORS to the lesional epidermis and eventually differentiate into functional melanocytes (Dct+/c-Kit+/TYR+) [Figure 1]. Given the fact that the role of TGF-β1 in the bulge region is critical for the activation of melanocyte stem cells, one might conclude that the reduced inhibition by TGF-β1 in the bulge region of hair follicles improves the mobilization capacity of UVB to accelerate the process of perifollicular re-pigmentation. Moreover, α-melanocyte-stimulating hormone (α-MSH) derived from keratinocytes is also main hormonal signal that stimulates proliferation and differentiation of melanocytes/melanoblasts in the re-pigmented lesional skin after UVB exposures.[20,21] A recent pilot study demonstrated that afamelanotide, a synthetic form of α-MSH with 13 amino acids, in combination with NB-UVB phototherapy resulted in clinically apparent, statistically significant superior and faster perifollicular re-pigmentation as compared with NB-UVB monotherapy.[22]
Figure 1.
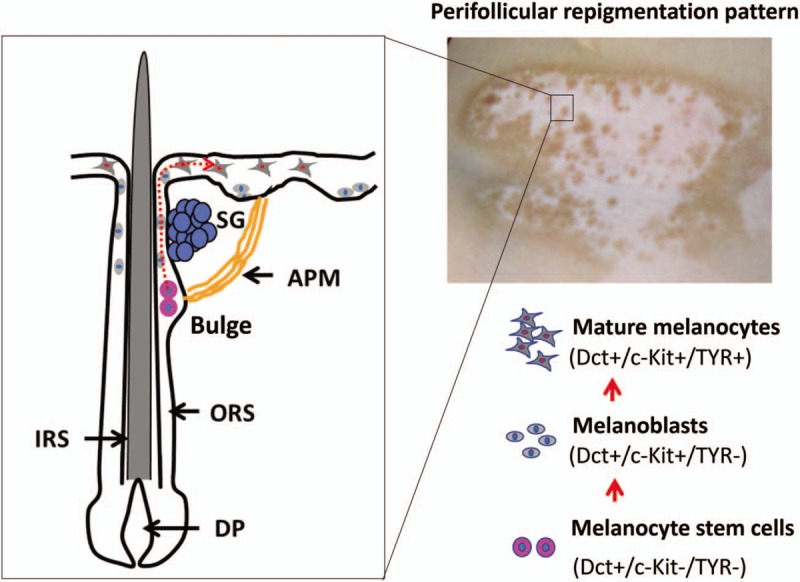
Schematic depiction of cellular events underlying the perifollicular re-pigmentation pattern. (Upper-right) A male patient with vitiligo on the right side of his neck was treated twice a week with 308 nm excimer light (EL) for 2 months, after which multiple pigmented dots were seen around the hair follicles. (Left) Schematic of the bulge region in hair follicles showing a relatively safe niche that houses melanocyte stem cells (Dct+/c-Kit−/TYR−). Those melanocyte stem cells are mobilized to exit the bulge region upon exposure to narrow-band UVB (NB-UVB) or/308 nm EL and to start their differentiation or proliferation program to become melanoblasts (Dct+/c-Kit+/TYR−, also called transit amplifying [TA] cells). These melanoblasts continuously migrate upward through the ORS to the lesional epidermis and eventually differentiate into functional melanocytes (Dct+/c-Kit+/TYR+). The red dotted line indicates the putative migration route of melanocyte stem cells. DP: Dermal papilla; IRS: Inner root sheath; ORS: Outer root sheath; APM: Arrector pili muscle; SG: Sebaceous gland.
Formation and Regulation of the Marginal Re-pigmentation Pattern
Although the perifollicular re-pigmentation pattern of vitiligo has been the subject to intensive investigation, little attention has been paid to formation of the marginal re-pigmentation pattern, in which unaffected melanocytes bordering the depigmented skin may be activated, allowing them to migrate into the leukoderma areas.[23] It is, however, an issue worth determining whether differentiated melanocyte division occurs in vivo, by which participates in formation of the marginal re-pigmentation pattern. Mature melanocytes are generally viewed as terminally differentiated cells that have lost the ability to divide under normal physiological conditions.[24] In addition, we cannot completely exclude the possibility that these terminally differentiated melanocytes potentially dedifferentiate and regain the capacity to divide after UVB irradiation.[25] The present findings add to the evidence suggesting that reduced levels of MITF activity by gene mutations promotes differentiated melanocyte division in zebrafish.[26] In spite of this, there should be a population of immature melanocytes that exist in normal human epidermal tissue that serves as the main cell source for marginal re-pigmentation of vitiliginous skin.
To determine whether immature melanocytes in the interfollicular epidermis (IFE) directly arise from hair follicles, Chou et al[19] carried out an elegant experiment using human scalp explant cultures to verify whether follicular melanocytes migrate to the epidermis in human skin. The epidermal melanocytes were completely removed by ablating the skin above the level of the sebaceous gland in the human scalp explants. The remaining denuded skin was cultured and analyzed to determine whether follicular melanocytes could migrate to the re-epithelialized skin. After culturing the explants for 8 days, melanocytes that were originally localized in the ORS of hair follicles were found in the epidermis, concomitant with re-epithelialization of the denuded skin. The results of that study showed that human follicular melanocytes had the ability to repopulate in the skin epidermis.[19] To date, several lines of evidence support the concept that the TGF-β1-enriched bulge region of hair follicles is the only niche harboring melanocyte stem cells.[15,17,19] While the majority of immature melanocyte precursors (also called melanoblasts) exit the bulge region and enter the IFE, it is possible that a small fraction of those cells reaches a bulge-like TGF-β1-enriched area in the epidermis and are kept in a quiescent state.[5,25] Accumulating evidence supporting the presence of a bulge-like TGF-β1-enriched area in human epidermis is as follows: 1) keratin 15 (K15)-positive keratinocytes exiting the basal layer enter the suprabasal layer and differentiate to keratin 10 (K10)-positive cells, and simultaneously increase their paracrine production of TGF-β1[27,28]; 2) Immunohistochemical staining also confirmed that there are at least two sub-populations of melanocytes with distinct maturation phenotypes existing in the human epidermis, that is, mature Sox 9-positive melanocytes and immature Sox10-positive melanocytes[29]; and 3) immature c-Kit-positive melanocytes are prominently distributed in the base of rete ridges.[30] Accordingly, we might further speculate that there is a TGF-β1-enriched area, which is similar to the bulge region of hair follicles, that is potentially localized in the vicinity of the base of epidermal rete ridges. As we know, differentiated suprabasal keratinocytes are stacked in rete ridges, which constitute the thickest part of human epidermis, that secrete high levels of TGF-β1 concentrated in the area surrounding the base of rete ridges [Figure 2]. Suppression of TGF-β1 production by keratinocytes induced by UVB irradiation causes a decreased inhibition of TGF-β1 on the growth and maturation of melanocytes,[26,31] thereby improving the marginal re-pigmentation in vitiligo.
Figure 2.
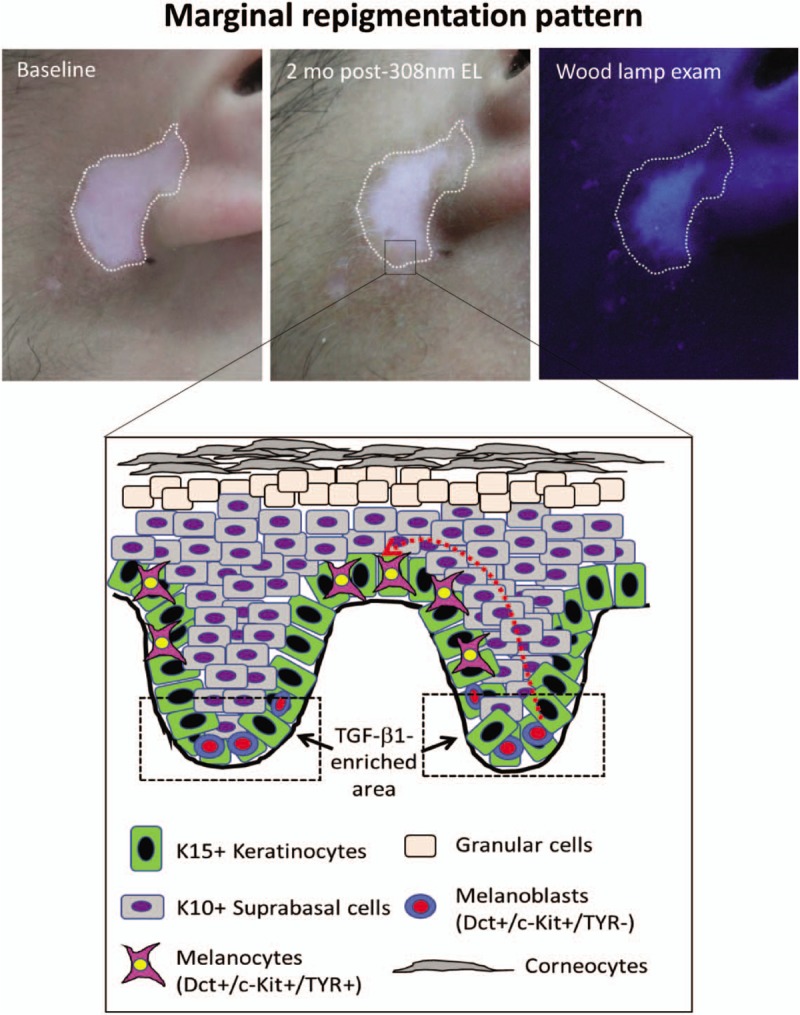
Schematic depiction of cellular events underlying the marginal re-pigmentation pattern. (Upper panel) A 20-year-old male with vitiligo on the front of his left earlobe was treated twice a week with the 308-nm excimer light (EL) for 2 months. Two months later, a hyperpigmented rim is seen at the border of the white macules and the area of leukoderma tended to be smaller compared to the baseline before phototherapy. (Lower panel) K15+ keratinocytes exit the basal layer to enter the suprabasal layer and then differentiate into K10+ keratinocytes. These TGF-β1-secreting keratinocytes stack in rete ridges to form the thickest parts of human epidermis, which creates a TGF-β1-concentrated micro-environment that is similar to the bulge region of hair follicles for maintaining the undifferentiated state of melanocytes in the epidermis. Exposure to NB-UVB or/308-nm EL inhibits the secretion of TGF-β1 by keratinocytes and impairs the TGF-β1-mediated growth suppression of melanocytes, thereby improving marginal re-pigmentation in vitiligo. The red dotted line indicates the putative migration route of melanocytes. NB-UVB: Narrow band ultraviolet B; TGF-β1: Transforming growth factor beta-1.
Micro-environment Favorable for Vitiligo Re-pigmentation
Apart from the mobilization and activation of melanocytes and/or stem cells from the epidermis and hair follicles, as discussed above, re-pigmentation of the skin also depends on a highly instructive and functional micro-environment to recruit and to reposition melanocytes in vitiliginous skin. Increasing evidence suggests that the mutual interplays of cytokines and chemokine networks in the treated skin, such as the reduced inhibition of TGF-β1 on melanocytes in the epidermis and in hair follicles, the increased transfer of pro-melanogenic growth factors from the dermis, and the formation of a CXCL12 chemokine-enriched micro-environment, play crucial roles in the process of vitiligo re-pigmentation.[32] We now discuss each of those points in more detail.
Reduced inhibition of TGF-β1 on melanocytes elicited by UVB
Several studies have shown that micro-RNA (miR)-203, an epidermal-specific miRNA, is highly expressed only in differentiated suprabasal keratinocytes via a transcriptional activation mechanism driven by asymmetric cell division.[33,34] The p63 and c-Jun genes have been identified as target genes that are directly regulated by miR-203.[35,36] miR-203 post-transcriptionally represses the expression of p63 and c-Jun genes in the basal layer of human epidermis, which is essential in initiating epithelial stratification and maintaining the proliferative potential of keratinocytes in the basal layer. In addition, recent studies have also identified a distal negative regulatory region upstream of the TGF-β1 transcript, which binds c-Jun to up-regulate TGF-β1 gene transcription.[36] Several previous reports have claimed that UVB irradiation of human keratinocytes can lead to a rapid increase in expression of the proto-oncogene c-Jun and a decrease in the autocrine production of TGF-β1.[37,38] Overall, these observations lead us to explore new therapeutic approaches that directly down-regulate the regional expression of TGF-β1 in the suprabasal layer to effectively awaken dormant melanocytes in skin exposed to UVB [Figure 3].
Figure 3.
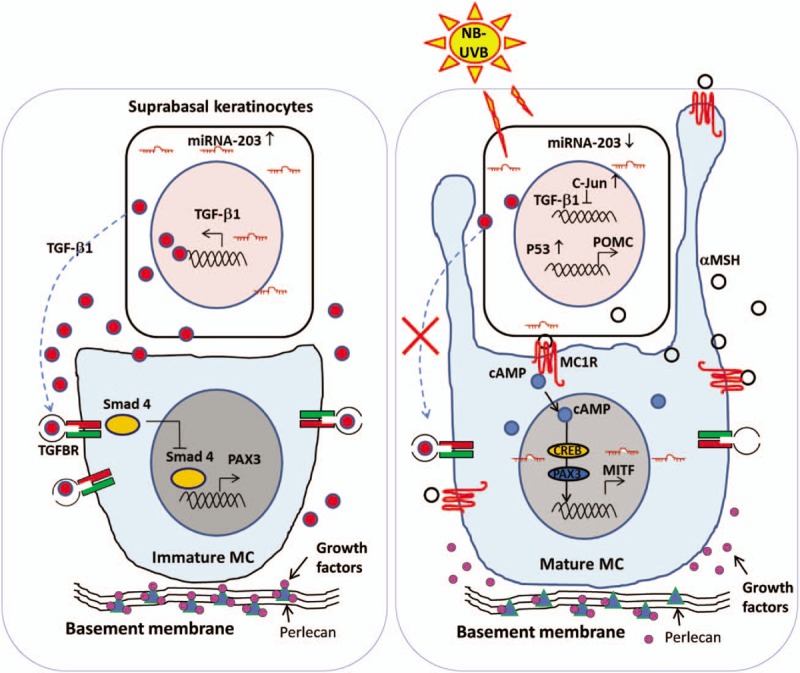
The micro-environment favorable for re-pigmentation. (Left panel) In the absence of NB-UVB irradiation, differentiated keratinocytes residing in the suprabasal layers of the skin express high levels of miR-203 via a transcriptional activation mechanism. miR-203 post-transcriptionally represses c-Jun and then increases TGF-β1 expression, forming a TGF-β1-enriched extracellular micro-environment. (Right panel) In response to NB-UVB, keratinocytes activate the classical pathway of the p53/αMSH/MC1R/MITF cascade and suppress TGF-β1-ligand secretion via the miR-203/c-Jun pathway, which stimulates the proliferation and differentiation of melanocytes. NB-UVB also activates heparanase to induce the release of heparan sulfate-binding growth factors, which synergistically facilitates the proliferation and melanogenesis of melanocytes. All references are cited in the text. α-MSH: α-Melanocyte-stimulating hormone; cAMP: Cyclic adenosine monophosphate; MC: Melanocytes; MITF: Microphthalmia-associated transcription factor; NB-UVB: Narrow band ultraviolet B; POMC: Proopiomelanocortin; TGF-β1: Transforming growth factor beta-1.
Increased transfer of pro-melanogenic growth factors from the dermis induced by UVB
The dermal-epidermal junction (DEJ), also termed the epidermal basement membrane zone, is an acellular zone that separates the epidermis from the underlying connective tissue dermis.[39] The basement membrane has long been considered a barrier against the unhindered passage of chemicals and pathogens into the body and of water and electrolytes out of the body. The basement membrane has been recently considered not only a mere static scaffold but rather a dynamic interface structure, in which several laminins, type IV collagen, nidogen and perlecan constitute a dynamic extracellular matrix molecule network.[40] It has been demonstrated that perlecan, a heparan sulfate proteoglycan with a large multivalent protein core, is a key structural constituent of basement membranes that also works as a storage reservoir of heparan sulfate-binding growth factors, such as fibroblast growth factors and hepatocyte growth factor in the basement membrane and in the dermal extracellular matrices.[41] Cleavage of heparan sulfate side chains from perlecan at the basement membrane by heparanase induces the release of heparan sulfate-binding growth factors, and indirectly facilitates melanocyte proliferation and melanogenesis.[42] Iriyama et al[43] reported that the hyperpigmentation of solar lentigos may be favored due to the heparanase-mediated reduction of heparan sulfate at the DEJ, which may increase the transfer of pro-melanogenic heparin-binding growth factors from the dermis to melanocytes. Low levels of heparinase were detected in sun-protected skin, whereas the expression level of heparanase was significantly up-regulated in skin exposed to UVB irradiation. Thus, the heparanase-induced degradation of heparan sulfate compromises the integrity and function of the DEJ, causing an increased diffusion of heparin-binding growth factors. Likewise, paracrine growth factors produced by keratinocytes in the IFE also easily diffuse downwards to the bulge region to stimulate the migration, proliferation and differentiation of melanocyte stem cells from hair follicles.
CXCL12 chemokine-enriched micro-environment induced by therapeutic skin trauma
Increasing evidence suggests that dermabrasion,[44] micro-needling,[45] ablative fractional CO2 lasers,[46] and punch grafting[41] are effective for inducing re-pigmentation of the skin in vitiligo patients who are resistant to conventional modalities such as UVB-based phototherapy, but the molecular mechanisms behind this favorable effect remain unknown. With the exception of simply enhanced trans-dermal drug delivery due to skin injuries caused by these therapeutic interventions,[47] these intentional skin injuries trigger a wound-healing process, which likely mobilizes and activates melanocyte stem cells and melanocyte precursors in the epidermis and in hair follicles. More recent studies have demonstrated that CXCL12 (SDF-1) is significantly up-regulated upon excisional or burn wounding of the skin.[48,49] The wounding-induced production of CXCL12 appears to arise from dermal fibroblasts and micro-vascular endothelium cells rather than from epidermal keratinocytes in the wound margins.[48] These events possibly form a concentration gradient of CXCL12 in the route of melanocyte movement and recruit CXCR4- or CXCR7-positive melanocytes or melanocyte precursors into the wound sites. Kovacs et al found that small size punch grafts (1.5 mm) were much better than larger punch grafts (3 mm) for effectively achieving re-pigmentation in vitiligo patients who underwent the punch grafting treatment.[41] It can be hypothesized that activation of the micro-environment can be better promoted by a larger number of smaller punch grafts compared to a smaller number of larger punch grafts. It remains to be investigated whether the level of CXCL12 in the smaller punch grafting sites is higher than that in the larger punch graft sites. Together, the injury and healing process caused by therapeutic skin trauma may create an environment favorable for the stimulation and activation of melanocytes. In fact, the pro-melanogenic growth factors produced by epidermal keratinocytes and the underlying dermal fibroblasts are all induced in the course of the skin healing process.
Conclusions
Vitiligo is a common skin disease with a large impact on the quality of life of patients and even on their entire family. Current treatments for vitiligo, including phototherapy, topical corticosteroids, topical calcineurin inhibitors, and surgical procedures, appear to have limited efficacies.[50,51] Complete re-pigmentation may take months (even years) to achieve and may not be achieved at all, especially in glabrous acral skin. Thus far, much has been revealed about the sources and reservoirs of cells in the perilesional margins and hair follicles for the replenishment of melanocytes that have been lost in vitiliginous skin.[52,53] Recent initial observations have provided a tantalizing insight into the micro-environment supportive of vitiligo re-pigmentation. It is clear that in epidermal homeostasis, melanocyte growth is rigorously controlled by keratinocytes via paracrine and/or autocrine signaling mechanisms. Only a low rate of melanocyte proliferation is permitted except for sunlight exposure or skin wounding, which could be partially ascribed to the increased transfer of pro-melanogenic growth factors from the dermis.[54] A greater understanding of these cellular and molecular mechanisms underlying skin re-pigmentation may help develop a novel therapeutic strategy to accelerate cutaneous re-pigmentation and improve satisfactory clinical outcomes in the treatment of vitiligo.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972919).
Conflicts of interest
None.
Footnotes
How to cite this article: Lei TC, Hearing VJ. Deciphering skin re-pigmentation patterns in vitiligo: an update on the cellular and molecular events involved. Chin Med J 2020;133:1231–1238. doi: 10.1097/CM9.0000000000000794
References
- 1.Taïeb A, Seneschal J, Mazereeuw-Hautier J. Special considerations in children with vitiligo. Dermatol Clin 2017; 35:229–233. doi: 10.1016/j.det.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet 2015; 386:74–84. doi: 10.1016/S0140-6736 (14)60763-7. [DOI] [PubMed] [Google Scholar]
- 3.Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin 2017; 35:257–265. doi: 10.1016/j.det.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, et al. Narrow band ultraviolet B treatment for human vitiligo is associated with proliferation, migration, and differentiation of melanocyte precursors. J Invest Dermatol 2015; 135:2068–2076. doi: 10.1038/jid.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birlea SA, Costin GE, Roop DR, Norris DA. Trends in regenerative medicine: repigmentation in vitiligo through melanocyte stem cell mobilization. Med Res Rev 2017; 37:907–935. doi: 10.1002/med.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Reilly-Pol T, Johnson SL. Melanocyte regeneration reveals mechanisms of adult stem cell regulation. Semin Cell Dev Biol 2009; 20:117–124. doi: 10.1016/j.semcdb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol 2019; 28:656–661. doi: 10.1111/exd.13879. [DOI] [PubMed] [Google Scholar]
- 8.Cavalié M, Ezzedine K, Fontas E, Montaudié H, Castela E, Bahadoran P, et al. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Invest Dermatol 2015; 135:970–974. doi: 10.1038/jid.2014.527. [DOI] [PubMed] [Google Scholar]
- 9.Passeron T. Medical and maintenance treatments for vitiligo. Dermatol Clin 2017; 35:163–170. doi: 10.1016/j.det.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Esmat S, Mostafa W, Hegazy RA, Shalaby S, Sheth V, Youssef R, et al. Phototherapy: the vitiligo management pillar. Clin Dermatol 2016; 34:594–602. doi: 10.1016/j.clindermatol.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Yang YS, Cho HR, Ryou JH, Lee MH. Clinical study of repigmentation patterns with either narrow-band ultraviolet B (NBUVB) or 308 nm excimer laser treatment in Korean vitiligo patients. Int J Dermatol 2010; 49:317–323. doi: 10.1111/j.1365-4632.2009.04332.x. [DOI] [PubMed] [Google Scholar]
- 12.Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol 2009; 54:313–318. doi: 10.4103/0019-5154.57604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XM, Zhou Q, Xu SZ, Wakamatsu K, Lei TC. Maintenance of immune hyporesponsiveness to melanosomal proteins by DHICA-mediated antioxidation: possible implications for autoimmune vitiligo. Free Radic Biol Med 2011; 50:1177–1185. doi: 10.1016/j.freeradbiomed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S, Liu XM, Dai X, Zhou Q, Lei TC, Beermann F, et al. Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: implication for skin photoprotection against UVA radiation. Free Radic Biol Med 2010; 48:1144–1151. doi: 10.1016/j.freeradbiomed.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 2005; 307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 16.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990; 61:1329–1337. doi: 10.1016/0092-8674 (90)90696-c. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 2010; 6:130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol 2008; 159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 19.Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med 2013; 19:924–929. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 2007; 128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Lim HW, Grimes PE, Agbai O, Hamzavi I, Henderson M, Haddican M, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol 2015; 151:42–50. doi: 10.1001/jamadermatol.2014.1875. [DOI] [PubMed] [Google Scholar]
- 22.Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol 2013; 149:68–73. doi: 10.1001/2013.jamadermatol.386. [DOI] [PubMed] [Google Scholar]
- 23.Lee DY, Kim CR, Park JH, Lee JH. The incidence of leukotrichia in segmental vitiligo: implication of poor response to medical treatment. Int J Dermatol 2011; 50:925–927. doi: 10.1111/j.1365-4632.2011.04914.x. [DOI] [PubMed] [Google Scholar]
- 24.Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol 2013; 30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kormos B, Belso N, Bebes A, Szabad G, Bacsa S, Széll M, et al. In vitro dedifferentiation of melanocytes from adult epidermis. PLoS One 2011; 6:e17197.doi: 10.1371/journal.pone.0017197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor KL, Lister JA, Zeng Z, Ishizaki H, Anderson C, Kelsh RN, et al. Differentiated melanocyte cell division occurs in vivo and is promoted by mutations in Mitf. Development 2011; 138:3579–3589. doi: 10.1242/dev.064014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y, Dong L, et al. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell 2008; 32:554–563. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Friedrichs J, Fink D, Mauch C, Kindler D, Hartmann W, Schüle R, et al. TGF-(1-dependent induction and nuclear translocation of FHL2 promotes keratin expression in pilomatricoma. Virchows Arch 2015; 466:199–208. doi: 10.1007/s00428-014-1692-5. [DOI] [PubMed] [Google Scholar]
- 29.Klar AS, Biedermann T, Michalak K, Michalczyk T, Meuli-Simmen C, Scherberich A, et al. Human adipose mesenchymal cells inhibit melanocyte differentiation and the pigmentation of human skin via increased expression of TGF-β1. J Invest Dermatol 2017; 137:2560–2569. doi: 10.1016/j.jid.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Grichnik JM, Ali WN, Burch JA, Byers JD, Garcia CA, Clark RE, et al. KIT expression reveals a population of precursor melanocytes in human skin. J Invest Dermatol 1996; 106:967–971. doi: 10.1111/1523-1747.ep12338471. [DOI] [PubMed] [Google Scholar]
- 31.Moustakas A. TGF-beta targets PAX3 to control melanocyte differentiation. Dev Cell 2008; 15:797–799. doi: 10.1016/j.devcel.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Boehnke K, Falkowska-Hansen B, Stark HJ, Boukamp P. Stem cells of the human epidermis and their niche: composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis 2012; 33:1247–1258. doi: 10.1093/carcin/bgs136. [DOI] [PubMed] [Google Scholar]
- 33.Jackson SJ, Zhang Z, Feng D, Flagg M, O’Loughlin E, Wang D, et al. Rapid and widespread suppression of self-renewal by microRNA-203 during epidermal differentiation. Development 2013; 140:1882–1891. doi: 10.1242/dev.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi R, Fuchs E. MicroRNA-mediated control in the skin. Cell Death Differ 2010; 17:229–235. doi: 10.1038/cdd.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing 'stemness’. Nature 2008; 452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonkoly E, Lovén J, Xu N, Meisgen F, Wei T, Brodin P, et al. MicroRNA-203 functions as a tumor suppressor in basal cell carcinoma. Oncogenesis 2012; 1:e3.doi: 10.1038/oncsis.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritz G, Kaina B. Activation of c-Jun N-terminal kinase 1 by UV irradiation is inhibited by wortmannin without affecting c-iun expression. Mol Cell Biol 1999; 19:1768–1774. doi: 10.1128/mcb.19.3.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garmyn M, Degreef H. Suppression of UVB-induced c-fos and c-jun expression in human keratinocytes by N-acetylcysteine. J Photochem Photobiol B 1997; 37:125–130. doi: 10.1016/s1011-1344 (96)07340-x. [DOI] [PubMed] [Google Scholar]
- 39.Fuoco C, Petrilli LL, Cannata S, Gargioli C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J Orthop Surg Res 2016; 11:86.doi: 10.1186/s13018-016-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley LC, Lohmer LL, Hagedorn EJ, Sherwood DR. Traversing the basement membrane in vivo: a diversity of strategies. J Cell Biol 2014; 204:291–302. doi: 10.1083/jcb.201311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacs D, Abdel-Raouf H, Al-Khayyat M, Abdel-Azeem E, Hanna MR, Cota C, et al. Vitiligo: characterization of melanocytes in repigmented skin after punch grafting. J Eur Acad Dermatol Venereol 2015; 29:581–590. doi: 10.1111/jdv.12647. [DOI] [PubMed] [Google Scholar]
- 42.Iriyama S, Ono T, Aoki H, Amano S. Hyperpigmentation in human solar lentigo is promoted by heparanase-induced loss of heparan sulfate chains at the dermal-epidermal junction. J Dermatol Sci 2011; 64:223–228. doi: 10.1016/j.jdermsci.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Iriyama S, Matsunaga Y, Takahashi K, Matsuzaki K, Kumagai N, Amano S. Activation of heparanase by ultraviolet B irradiation leads to functional loss of basement membrane at the dermal-epidermal junction in human skin. Arch Dermatol Res 2011; 303:253–261. doi: 10.1007/s00403-010-1117-5. [DOI] [PubMed] [Google Scholar]
- 44.Awad SS. Dermabrasion may repigment vitiligo through stimulation of melanocyte precursors and elimination of hyperkeratosis. J Cosmet Dermatol 2012; 11:318–322. doi: 10.1111/jocd.12010. [DOI] [PubMed] [Google Scholar]
- 45.Iriarte C, Awosika O, Rengifo-Pardo M, Ehrlich A. Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol 2017; 10:289–298. doi: 10.2147/CCID.S142450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doghaim NN, Gheida SF, El-Tatawy RA, Mohammed Ali DA. Combination of fractional carbon dioxide laser with narrow band ultraviolet B to induce repigmentation in stable vitiligo: a comparative study. J Cosmet Dermatol 2019; 18:142–149. doi: 10.1111/jocd.12553. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Wu Y, Zhang J, Gu H, Luan Q, Qian L, et al. Ablative fractional Co (2) laser aided delivery of long-acting glucocorticoid in the treatment of acral vitiligo: a multicenter, prospective, self-bilateral controlled study. J Dermatolog Treat 2019; 30:320–327. doi: 10.1080/09546634.2018.1509048. [DOI] [PubMed] [Google Scholar]
- 48.Avniel S, Arik Z, Maly A, Sagie A, Basst HB, Yahana MD, et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J Invest Dermatol 2006; 126:468–476. doi: 10.1038/sj.jid.5700069. [DOI] [PubMed] [Google Scholar]
- 49.Toksoy A, Müller V, Gillitzer R, Goebeler M. Biphasic expression of stromal cell-derived factor-1 during human wound healing. Br J Dermatol 2007; 157:1148–1154. doi: 10.1111/j.1365-2133.2007.08240.x. [DOI] [PubMed] [Google Scholar]
- 50.Webb KC, Tung R, Winterfield LS, Gottlieb AB, Eby JM, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol 2015; 173:641–650. doi: 10.1111/bjd.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulekar SV, Isedeh P. Surgical interventions for vitiligo: an evidence-based review. Br J Dermatol 2013; 169: Suppl 3: 57–66. doi: 10.1111/bjd.12532. [DOI] [PubMed] [Google Scholar]
- 52.Eleftheriadou V. Future horizons in vitiligo research: focusing on the recommendations of the Cochrane systematic review ’Interventions for vitiligo’ 2010. Br J Dermatol 2013; 169: Suppl 3: 67–70. doi: 10.1111/bjd.12530. [DOI] [PubMed] [Google Scholar]
- 53.van Geel N, Mollet I, Brochez L, Dutré M, De Schepper S, Verhaeghe E, et al. New insights in segmental vitiligo: case report and review of theories. Br J Dermatol 2012; 166:240–246. doi: 10.1111/j.1365-2133.2011.10650.x. [DOI] [PubMed] [Google Scholar]
- 54.Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol Symp Proc 2005; 10:153–163. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]


