Abstract
Daily rhythms in behavior, physiology, and metabolism are an integral part of homeostasis. These rhythms emerge from interactions among endogenous circadian clocks, ambient light-dark-, sleep-activity-, and eating-fasting cycles. Nearly the entire primate genome shows daily rhythms in expression in a tissue- and locus- specific manner. These molecular rhythms modulate several key aspects of cellular and tissue function with profound implications in public health, disease prevention and disease management. In modern societies light at night disrupts circadian rhythms which leads to further disruption of sleep and eating-fasting cycles. While acute circadian disruption may cause transient discomfort or exacerbate chronic diseases, chronic circadian disruption can enhance risks for numerous diseases. The molecular understanding of circadian rhythms is opening new therapeutic frontiers placing the circadian clock in a central role. Here we review recent advancements on how to enhance our circadian clock through behavioral interventions, timing of drug administration and pharmacological targeting of circadian clock components that are already providing new preventive and therapeutic strategies for several diseases including metabolic syndrome and cancer.
Keywords: Circadian rhythm disruption, chronotherapy, Time-restricted eating, shift-work, Rev-erb, circadian clock
The overlooked common sense — circadian rhythms are not solely driven by circadian clocks.
Ever since French Botanist Jean-Jacques d’Ortous de Mairan put a Mimosa pudica plant in his basement wine cellar and observed an endogenous rhythm in leaf movement [1], the interest in understanding circadian rhythms have been focused on the intrinsic rhythms generated by an endogenous circadian clock (see Glossary). However, during the focused pursuit of the endogenous circadian time keeping mechanism, a large proportion of the field has often overlooked that daily rhythms under normal living conditions emerge from an interaction between endogenous circadian clocks and various rhythmic behaviors and/or environmental factors. Sometimes, even in the complete absence of an endogenous circadian clock, some (if not all) 24 hours rhythms in physiology, metabolism and in activity-rest behavior can be driven by imposed rhythms in feeding-fasting or light-dark cycles [2]. In recent years, research on factors that influence endogenous circadian clocks, known as zeitgebers (time-givers), and clock outputs have converged on a few broad areas. Circadian clocks reciprocally regulate daily rhythms in hunger-satiety, activity-rest, and body temperature. One of the strongest zeitgebers is light. Ambient light information is transmitted through blue-light sensitive and melanopsin expressing retinal ganglion cells to entrain the master circadian oscillator present in the hypothalamus, the suprachiasmatic nucleus (SCN) (see Glossary), to the light-dark cycle [3]. The design of these emergent rhythms, in which the light-dark cycle, feeding-fasting, and activity-rest patterns (Figure 1) can modulate the phase and amplitude of circadian clocks, offers an adaptive advantage to animals. It allows them to adapt their circadian rhythms to changes in day length as in different seasons or availability of food. However, in modern societies, extended periods of electrical illumination after sunset, and associated reduction in sleep and increased availability of energy dense and appetizing diet have made both acute and chronic circadian rhythm disruption (CRD) widespread.
Figure 1. Circadian rhythms emerge from multiple factors including the circadian clock.
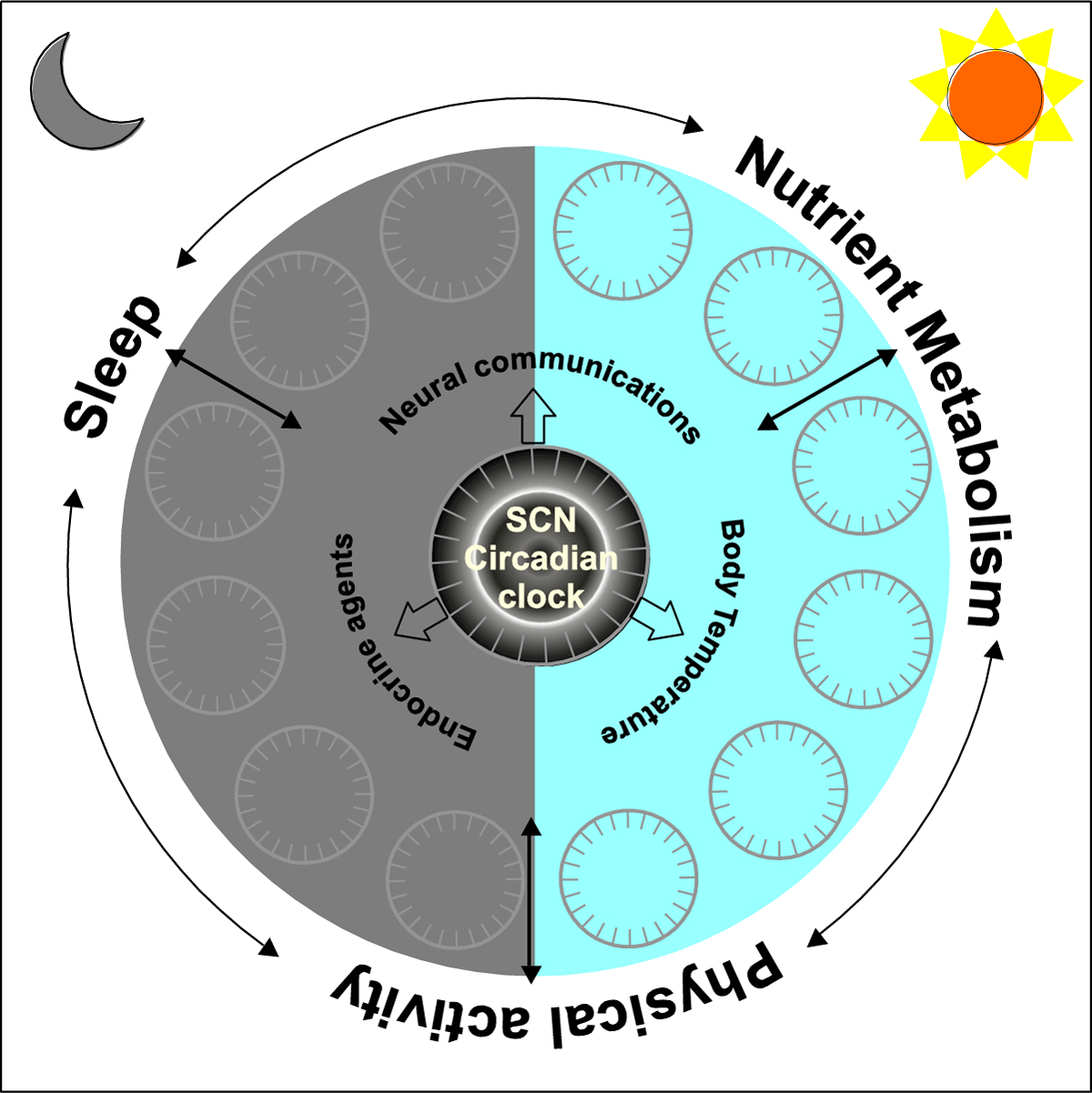
Schematic organization of various factors that interact to produce daily rhythms in behavior, physiology and metabolism. Network of cell autonomous circadian oscillators in the suprachiasmatic nucleus (SCN) directly or indirectly communicates with peripheral circadian clocks through neural communications, endocrine agents and body temperature rhythms. Both SCN and peripheral clocks interact to produce daily rhythms in sleep, physical activity, and nutrition metabolism, each of which can also feedback to central or peripheral clocks. Overall, both SCN and the peripheral circadian oscillators are influenced by ambient light-dark cycle.
There is a circadian time-code to the genome.
Circadian clocks are formed through transcription-translation feedback loops (TTFL) [4]. These TTFLs are comprised of more than a dozen different transcription factors, co-activators, and co-repressors that orchestrate a time-delayed transcriptional activation and repression sequence to generate and self-sustain a ~24 hours rhythm in transcription of the core clock components [2] (Text Box 1). In addition to the endogenous circadian oscillation of clock components, direct regulation by clock components and indirect interactions with transcription factors (clock controlled or other) can drive daily rhythms in transcription [2]. Whole genome transcriptional analyses have been powerful tools to identify transcripts that show circadian rhythm in their steady state level. Such an approach has led to the identification of thousands of rhythmic transcripts in different organs/tissues. Circadian transcriptome analyses of multiple organs/tissues from the same animals have revealed that nearly all protein coding genes in the genome display diurnal rhythms in a tissue specific manner [5, 6]. Although, rhythmic transcripts may not translate to rhythmic protein levels or the active form of the protein, numerous proteins, or their post-translational modified forms, exhibit robust rhythms in abundance [7, 8]. Therefore, it is safe to conclude that the expression or activity of almost every gene in the genome shows circadian modulation.
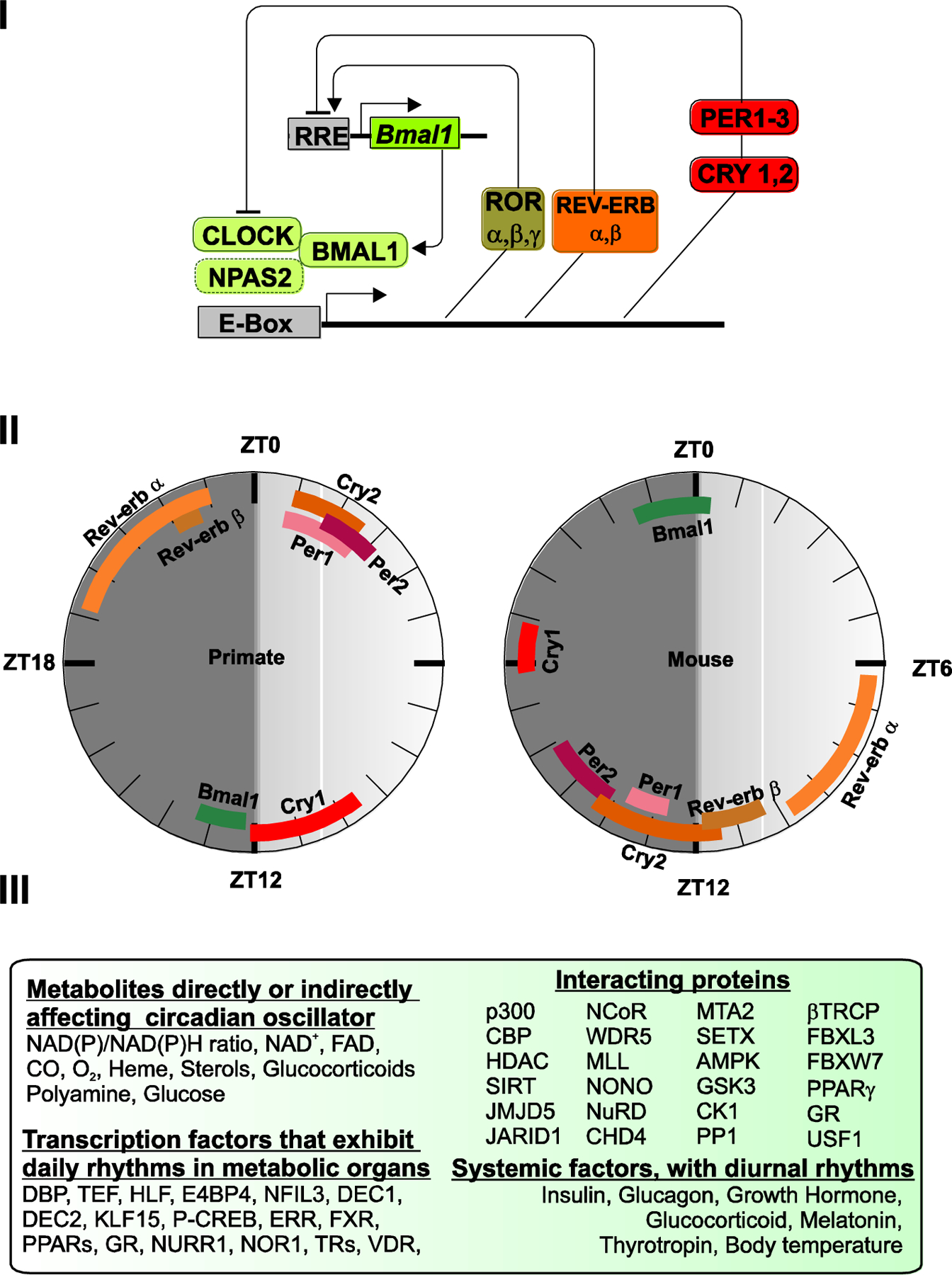
Text box 1. Circadian transcription-translation feedback loop.
In mammals, the circadian system is based on a cell-autonomous and self-sustaining molecular oscillator (Figure I). It is composed of two interlocking transcription-translation feedback loops. In the core loop that is conserved from Drosophila to humans, transcription factors, Circadian Locomotor Output Cycles Kaput (CLOCK) and BMAL1 (and their respective homologs Neuronal PAS domain containing protein 2 (NPAS2) and BMAL2 bind to the enhancer box (E-box) and function as transcriptional activators of the Cryptochrome (Cry1 and Cry2), and Period (Per1, Per2, Per3) genes. In turn, CRYs and PERs form complexes that repress CLOCK-BMAL1 activity, ultimately suppressing their own expression [2]. Eventually, CRYs and PERs are degraded, relieving CLOCK-BMAL1 repression and beginning the cycle anew. In a second loop, CLOCK and BMAL1 activate the transcription of Rev-erbα and Rev-erbβ and retinoic acid orphan receptor (Ror-α, -β, -γ) nuclear hormone receptors [79]. REV-ERBs and RORs are, respectively, transcriptional repressors and activators that bind to Ror elements present in the Bmal1 gene promoter, and whose interplay enforces rhythmic expression of Bmal1. Additionally, REV-ERBs and RORs also fine tune rhythmic expression of additional clock components [80]. Essentially, the transcriptional interplay among these 14 transcriptional regulators (Clock, Bmals, Npas2, Crys, Pers, Rors and Revs) gives rise to the self-sustained, near-24-hour, alternating activation-repression cycles that are the proverbial ticks of the clock.
The time of peak expression of many of these components are conserved across multiple tissues in diurnal non-human primates and in nocturnal mice (based on [6]) (Figure II). This reference peak time of expression of clock components will help understand how diseases or acute circadian perturbation affect the relative phases of clock components in one or more organs.
Several proteins interact with clock components to fine tune their post-translational modifications, stability, movement, degradation or modulate their activity. Similarly, several metabolites also interact with the clock components to modulate their function (Figure III). These interacting proteins and ligands expand the repertoire of targets that can be modulated by pharmacological agents. Additionally, several downstream transcription factors or endocrine factors also show diurnal rhythms yet have modest effect on the core clock. These offer pharmacological targets to modulate specific outputs of the circadian system.
Text Box Figure 1.
Pathways that maintain cellular homeostasis are under circadian regulation.
The number of transcripts with daily rhythms varies greatly from one tissue to another. In some tissues only a few hundred transcripts may show diurnal rhythms, while in other tissues from the same set of animals, a few thousand transcripts may cycle [5, 6]. Irrespective of these variations in the number of transcripts, functional annotation of rhythmic transcripts has revealed pathways that ensure the homeostasis of basic cellular processes are likely to be circadianly modulated in multiple tissues. These homeostatic functions include cell cycle regulation, proteostasis, nutrient sensing, mobilization and utilization, redox regulation, mitochondria function, detoxification, secretory function, stress response, cellular defense (immunity), and cellular communication [6] (Figure 2). Given the central roles of these pathways in cellular vitality, one may wonder if genome-wide circadian modulation in a tissue specific manner is important. For instance, why are circadian mutations in mice not lethal? It is important to point out that circadian modulation accounts for a modest change in expression of most of the genes. The genetic loss-of-function (LOF) of any one circadian clock component in experimental animals rarely abolishes expression of any essential gene. Nevertheless, the temporal regulation is important for normal health as circadian rhythm disruption can compromise fitness and elevates risk for several diseases. Accordingly, a close phenotypic analysis of circadian mutant mice reveals many chronic pathologies leading to early aging [9]. Conversely, complementing the age-dependent reduced expression of circadian clock component can extend healthy lifespan in Drosophila [10].
Figure 2. Hallmarks of circadian regulation.
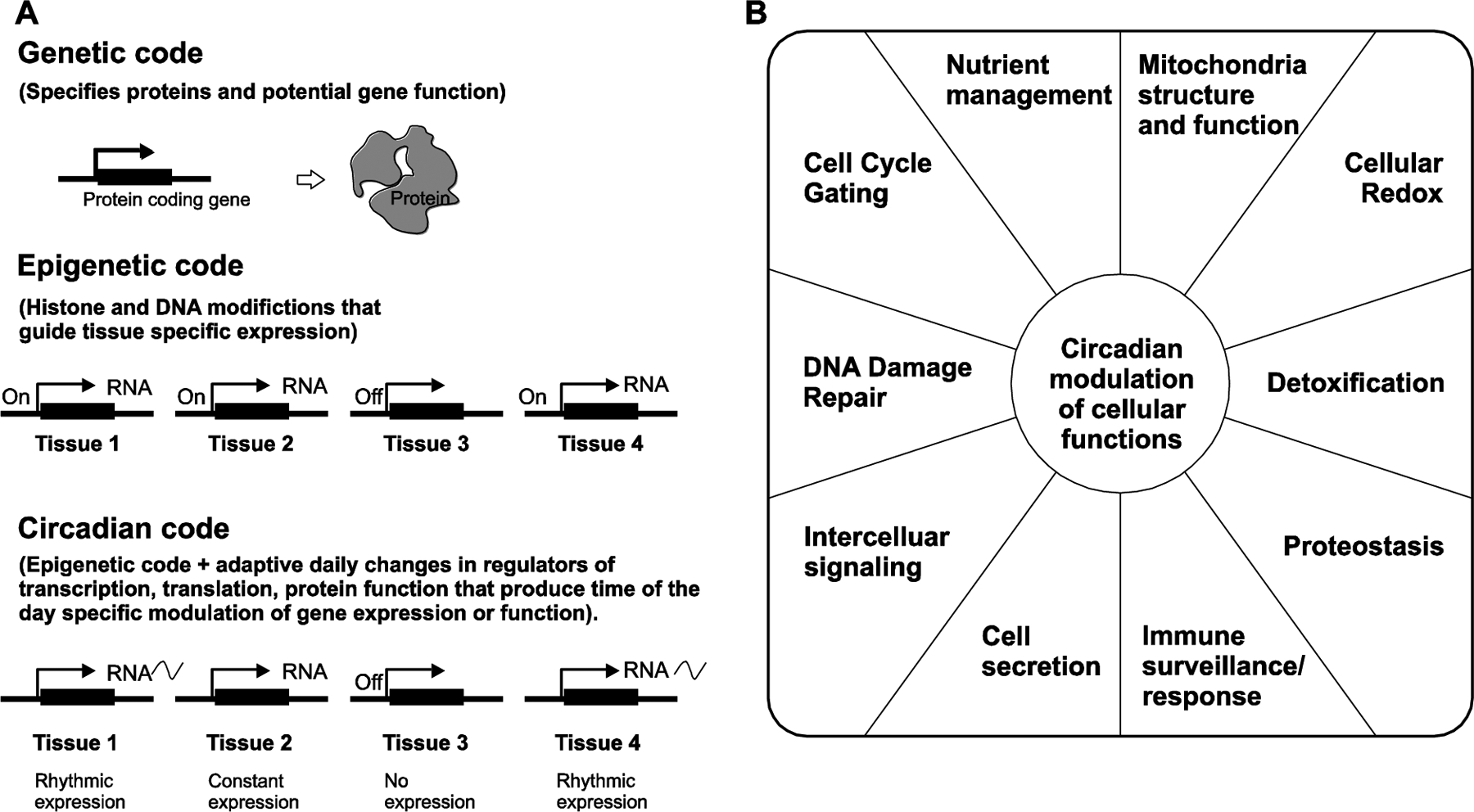
A. The genetic code denotes the set of protein coding genes in the genome. The epigenetic code instructs the expression of a subset of genes in the genome to be expressed in a given tissue that determines the tissue identity and function. Similarly, the circadian code instructs a subset of transcripts in every given tissue to be temporally regulated. The ensemble of rhythmic transcripts in different tissues determine the diurnal rhythms in physiology, metabolism and behavior. B. Functional annotation of rhythmic transcripts has revealed some of the basic cellular processes are circadianly modulated in multiple tissues. Some of these processes are depicted below.
Various flavors of circadian rhythm disruption.
In general, the term circadian rhythm disruption (CRD) is synonymously used with shift work or chronic jet lag lifestyle characterized by aberrant exposure to light and darkness, resultant sleep disruption and/or insufficient sleep, and changes in hunger, satiety, and eating time. Many of these challenges lead to an erratic eating pattern. This strongly implies that irrespective of having a genetically intact circadian clock, chronic disruption of circadian rhythm by human behavior can profoundly disrupt temporal homeostasis. Shiftwork is an extreme case of CRD. However, individual aspects of shift work (e.g. erratic eating or sleep patterns) are sufficient to disrupt circadian regulation of physiology, metabolism, and/or behavior. This indicates that a larger fraction of the population is prone to some aspect of CRD. Some of these disruptions may not appreciably affect the molecular circadian clock itself, but still significantly alter processes to indirectly affect circadian behavior.
CRD through suboptimal ambient light-dark environment.
Humans evolved near the equator, where the daylength does not change appreciably between summer and winter seasons. However, when humans ventured and settled in higher latitudes 30,00–40,000 years ago, they experienced the disruptive effects of prolonged light in summer and darkness in winter. The circadian clocks continue to function under conditions of constant darkness in long winter nights and, constant light in long summer days. However, insufficient light in winter increases the risk for depression, which may be due to insufficient light stimulated activation of melanopsin[11]. Constant light or long summer days can also suppress sleep through light suppression of melatonin. Modern societies pose two types of circadian rhythm disruption — extended light at nights and early waking. Extended evening electrical lighting can suppress melatonin and delay sleep onset, which coupled with social and professional demand to wake up early in the morning, reduces sleep duration or compromises sleep quality [12]. During the daytime, we spend >85% of time indoors. This lack of natural sunlight results in insufficient photostimulation of the melanopsin photoresponse pathway that can also disrupt circadian processes.
CRD through diet and eating pattern dysregulation.
Rodents with ad libitum access to calorie dense diet exhibit an erratic eating pattern. Rather than consolidating their food intake to the night as mice on normal chow do, they spread their caloric intake over 24 hours [13]. As feeding is a resetting and entraining cue for the circadian clock [14, 15], random eating patterns continuously disrupt circadian modulation of metabolic processes.
CRD through sedentary life.
Rodents with access to a running wheel voluntarily exercise during their wakeful period and show better consolidation of activity and rest [16]. This observation had raised the hypothesis that skeletal muscle function might affect circadian rhythms and/or sleep. A recent study has revealed that restoration of circadian clocks only in the skeletal muscle can restore normal sleep in mice [17]. While the mechanism by which skeletal muscle circadian clocks or voluntary wheel running activity improves circadian consolidation of sleep and activity remains to be investigated, it raises the hypothesis that sedentary lifestyle may contribute to CRD.
In addition to these CRDs, controlled clinical studies, animal studies and observational studies have lent support to a growing connection between CRD and increased risk for chronic diseases (Text Box 2).
Text Box 2. Connection between diseases and disruption of the circadian rhythm.
The link between circadian rhythm disruption and diseases is based on different types of correlational or causation studies.
Heritable circadian disorders in humans. These are examples of diseases that disturb the normal sleep-wake cycles affect the timing, duration or quality of sleep. Some examples are Smith-Magenis syndrome (SMS), Prader-Willie syndrome, familial advanced sleep-phase syndrome (FASPS), delayed sleep phase syndrome (DSPS), and short sleep [81, 82].
Disease susceptibility of circadian mutant animals. Mice carrying hypomorphic, or LOF mutations of circadian clock genes (Clock, Bmal1, Cry1, Cry2, Per1, Per2, Rev-erbs, Rors) often exhibit metabolic [83–88] inflammatory [89–93] and psychiatric diseases [94], cancer [95–99], and early aging [9, 100]. Similarly, mice that lack the blue light sensor melanopsin show signs of depression [55].
Disease prevalence among shift workers. People who work night shifts, or rotate between day and night shifts, have higher incidences of a number of diseases compared to people who typically work the day shift. These diseases range from obesity and diabetes, to diseases of the colon, infections, and certain types of cancer (breast, liver, colon, lung, skin, prostate cancer). Although correlational in nature, some of these connections have also been verified in controlled clinical conditions [101].
Controlled clinical studies. When the circadian rhythms of healthy human volunteers are intentionally disrupted, they show early signs of diseases. For example, sleep deprived healthy volunteers show disrupted hunger-satiety homeostasis and may exhibit glucose intolerance [102].
Chronic circadian disruption studies in animals. Wild type animals with normal circadian clock function when placed in conditions that disrupt their circadian clocks (e.g., constant light or simulated chronic jetlag or rotating shiftwork) succumb to metabolic diseases, show early sign of heart diseases, elevated inflammation, and even cancer. Some of these animals also become prone to pathogenic infection, and females often develop reproductive issues [25, 86, 103].
Therapeutic effects of restored rhythms. Restoring daily rhythm of light-dark, feeding-fasting, or sleep-wake can alleviate disease symptoms. For example, maintaining a daily rhythm of feeding and fasting — an eating pattern called time-restricted feeding (TRF) — can prevent or reverse obesity, metabolic diseases, heart disease [104], and even certain diseases of the brain such as the Huntington’s disease[60].
Circadian rhythms in disease symptoms. Autoimmune diseases, including eczema, asthma, and diseases of the gut such as acid reflux, becomes more severe at night. Similarly, inflammatory diseases, such as rheumatoid arthritis, are most painful early in the morning. These time-of-the-day fluctuations in disease severity indicates that the underlying pathology has a circadian component.
CRD increases risks for multiple diseases across our lifespan.
Acute and short-term CRD, as occurs during jetlag or one night of sleep deprivation, causes transient discomforts. These include increased irritability, excessive daytime sleepiness, and compromised mental acuity and they affect almost every age category (Figure 3, Key Figure). Although these symptoms do not warrant classification as a disease, they do affect mood, productivity and performance. Among some sensitized individuals with chronic psychiatric diseases, autoimmune diseases, gastrointestinal diseases, such acute CRD is reported to increase flare ups [18]. If acute CRD repeats a few times in a month or year, an individual may resort to pharmacological agents to cope with these transient discomforts to improve sleep at night, reduce fatigue during the day, reduce GI discomfort, and improve mood.
Figure 3. Circadian rhythm disruption and diseases across lifespan.
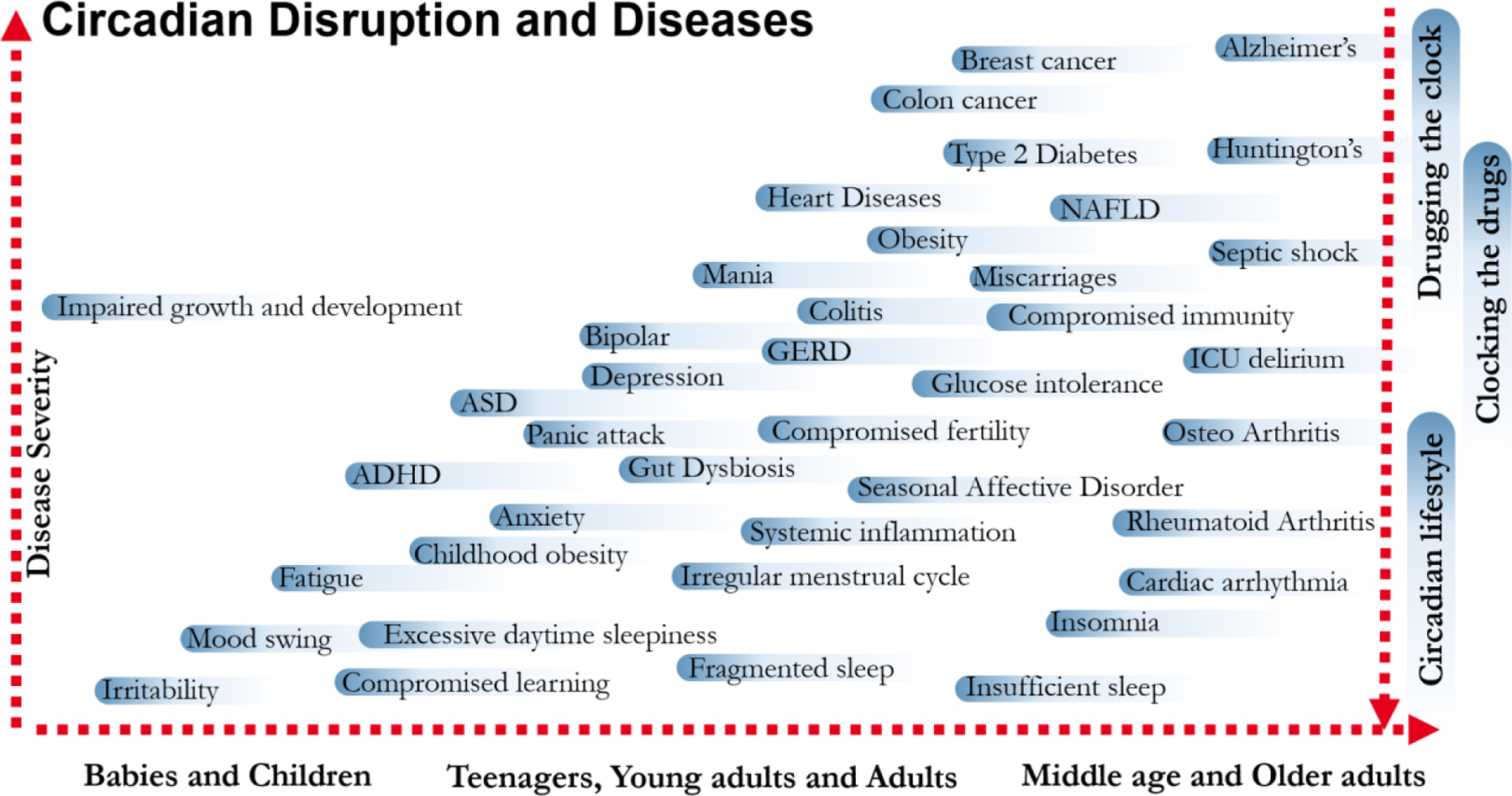
CRD can manifest in different diseases and disorders during the lifespan of an individual. In early childhood, disruption of the rhythm can cause irritability, mood swings and fatigue that can lead to impaired growth and development in extreme cases. As we age, chronic circadian rhythm disruption continuing over several weeks or months can cause mild disorders like fragmented sleep and compromised learning to an increased risk for a number of chronic diseases, including Type2 diabetes, Alzheimer’s etc. While milder forms of the diseases can be alleviated by behavioral adjustments to improve circadian rhythms, optimal timing of existing drugs or even pharmacological targeting of circadian clock components hold promise for treating some of the severe chronic diseases.
Chronic circadian rhythm disruption as in chronic jetlag or shiftwork lifestyle profoundly affects gastro-intestinal and metabolic organs. Parallel CRD studies in mice with chronic jetlag, misaligned feeding time, sleep deprivation, and animals carrying LOF alleles of circadian clock components, has revealed that such chronic genetic or behavioral CRD predisposes to a range of metabolic diseases including prediabetes, type 2 diabetes, obesity, hypertension, fatty liver disease, atherosclerosis, heart diseases, chronic kidney disease and cancer [19–24].
Chronic CRD also affects the reproductive system leading to a range of abnormalities including compromised fecundity, irregular menstrual cycle, and increased risk for miscarriages in both mice and humans [25–27]. Among newborn and juveniles, chronic CRD such as continuous light or erratic sleep and meal timing can have lasting impact on general development, epigenetically imprinted activity-sleep disruption [28], and increased risks for childhood obesity [29]. Chronic CRD due to life in higher latitudes predisposes humans to seasonal affective disorders [30]. The modern human population is mostly indoors and people in the tropical/equatorial latitudes lead a lifestyle similar to those in higher latitudes. Hence, chronic CRDs increases risk for depression, manic disorders, bipolar diseases, and exacerbates symptoms of Post-Traumatic Stress Disorder (PTSD) in the general population, irrespective of the geographical location [31].
Circadian rhythms have a role in many processes involved in the immune system homeostasis such as when the immune system functions as a barrier to pathogens, to the mobilization of immune cells to site of antigen presentation, immune response, and resolution of inflammation [32]. In epithelial cells including skin and intestinal epithelium, a circadian rhythm in repair and cell division is present to ensure sustained barrier function to prevent the entry of pathogens, antigens from pathogenic agents, and potential allergens [33]. In brain, the circadian clock components play an important role in maintaining the blood-brain barrier (BBB) function [34, 35]. There is circadian rhythm associated with the exit of differentiated immune cells from the bone marrow and trafficking of immune cells, both of which are important parts of immune surveillance [36, 37]. As immune response is an energetically expensive function, upon injury, an appropriate magnitude of immune response, and resolution of injury are carefully controlled by the circadian clock [38]. Specifically, direct inhibition of inflammatory regulators by the circadian clock components and circadian expression pattern of cytokines, chemokines, their receptors, phagocytosis, growth factor production, etc., ensures that acute inflammation that is necessary for neutralizing pathogenic challenge does not continue to become chronic inflammation [38]. Similarly, in brain, the phagocytic astrocytes that ensure local repair, are also put under check by circadian clock components [39, 40]. These regulations of the key pathways of the immune system by the circadian clock components may explain the disproportionately less or excessive immune responses that occur with CRD which leads to increased risk for several chronic inflammatory diseases, reduced response to vaccination, and increased susceptibility to bacterial and viral infections[41].
Circadian rhythms modulate cell cycle regulations at several nodes under three broad categories. First, by regulating nutrient homeostasis, redox regulation, autophagy, and xenobiotic metabolism, a robust circadian clock maintains a cellular environment that is not conducive for uncontrolled cell division [42]. Second, the circadian clock and cell cycle share several enzymes that mediate post-translational regulation. Such shared components and physical or functional interaction between circadian clock components and cell cycle regulators impose circadian gating of cell cycle. Finally, the circadian clock also modulates several signal transduction pathways that are pro-oncogenic. Due to such intimate interactions between circadian clocks and the cell cycle, several hallmarks of cancer are also hallmarks of chronic circadian rhythm disruption [43]. These hallmarks include chronic inflammation, autophagy upregulation, disrupted DNA damage repair and, increased glycolysis.
CRD also increases risks for many age-related diseases of the central nervous system (CNS) including Alzheimer’s disease, Huntington’s disease and Parkinson’s disease [40, 44]. Although the specific mechanisms are yet to be understood, both cell autonomous and systemic factors are likely involved. Bmal1 (a circadian clock component) knockout mice have been shown to exhibit symptoms of neurodegeneration [34, 39]. It is hypothesized that increased astrocytic inflammation and compromised BBB function in these knockout mice may contribute to onset or exacerbation of neurodegenerative disease [34, 39]. Cell autonomous functions of the circadian clock in protein folding, exocytosis, endocytosis, and reactive oxygen species (ROS) regulation may constitute the mechanism by which CRD elevates predisposition to neurodegenerative diseases.
Training the clock, clocking the drugs and drugging the clock
Behavioral and pharmacological approaches have begun to leverage the knowledge of circadian rhythms in health, to prevent or treat several chronic diseases. These interventions fall into three broad categories; (a) Interventions to maintain a robust circadian rhythm in feeding-fasting, sleep-wake or light-dark cycle (training the clock), (b) Optimizing the timing of the drugs to improve efficacy and reduce adverse side effects (clocking the drugs), and (c) Using small molecule agents that directly targets a circadian clock component or targets a part intimately associated with the circadian clock (drugging the clock) (Figure 3).
Training the clock:
Sleep researchers and physicians have been treating insomnia, insufficient sleep and long sleep latency for years with cognitive behavioral therapy, sleep hygiene, sleep medications including melatonin [45]. While exogenous melatonin is effective in improving sleep latency, it has modest or no effect in sustaining sleep. Moreover, it has a short half-life. Consequently, the newer drugs and slow-release formulation of melatonin are more effective in improving sleep [46]. Although such therapies are targeted towards treating sleep problems, improving sleep strengthens at least one major outputs of circadian rhythm and may indirectly improve other aspects of the daily rhythms leading to a better quality of life. Approaches to improve sleep by behavioral or pharmacological interventions have been extensively reviewed elsewhere [47].
Light is considered the primary enabler of circadian disruption. Light at night suppresses the sleep promoting hormone melatonin and the drive for sleep. Therefore, light at night delays sleep onset or compromises sleep quality, which in turn can increase food intake and perturb circadian clocks in peripheral organs. The discovery of a dominant role of blue light sensing melanopsin photopigment in circadian photoentrainment [48, 49] and melatonin suppression [50, 51] has led to informal suggestions on reducing evening light exposure and increasing daytime light exposure. However, electrical lighting guidelines for man-made environment are largely written for safety or for performing specific tasks (for proper illumination of work places, habitations, security purposes). The rise of new energy efficient LEDs (Light Emitting Diode) that produce higher intensity light and are relatively richer sources of blue light compared to the CFL (Compact Fluorescent Lamp) or tungsten lights they replace, pose new challenges. Many households, hospitals and, commercial buildings are rapidly switching to LEDs which are also lit late into the night. This can significantly suppress melatonin and disrupt the circadian clock. Rigorous studies are necessary to consider light as a therapeutic agent in man-made environment. These will hopefully help in setting and defining guidelines on the intensity and spectral composition of light used and the duration for which it is used in order to improve the health of the occupants [12, 52, 53]. Nowadays, numerous eye-glass manufacturing companies are marketing blue-blocking glasses with various health claims [54]. Excessive blocking of the blue light throughout the day may mimic the lack of melanopsin, which is known to make animals prone to depressive symptoms or SAD (Seasonal Affective Disorder) [55]. As seen in experimental settings [56] blocking of the blue light can also delay re-entrainment of circadian clock among shift workers or jet-travelers, further complicating health issues. Therefore, before these unregulated blue light-blocking eye-glasses enter the market, several studies will need to be performed on different patient groups and for various indications to understand how much and for how long the blue light-blocking is beneficial. Furthermore, there are pharmacological approaches to block melanopsin activation [57]. A new class of sulfonamides, called opsinamides, can act as competitive inhibitors of melanopsin by binding to the same pocket as its natural cis-retinal-based chromophore. Inhibition of melanopsin photosensitivity reduces photophobia in rodents. This proof-of-concept may offer “pharmacological darkness” to some patients who suffer from excessive light sensitivity leading to migraine pain or to patients in intensive care unit (ICU) who experience constant light. However, such drugs are currently in the preclinical stage.
The peripheral circadian rhythms are more profoundly affected by the timing of food intake than the timing of light [14, 15, 58]. Therefore, consolidating eating period to a defined interval under time-restricted feeding or eating (TRF or TRE) has emerged as another behavioral intervention to sustain robust circadian rhythms in peripheral organs and improve health [59]. TRE directly affects many nutrient sensing and utilization pathways in the liver including CREB (CAMP Responsive Element Binding Protein), mTOR (Mechanistic Target Of Rapamycin Kinase), FOXO (Forkhead Box O), PPARγ(Peroxisome Proliferator Activated Receptor Gamma) and SREBP (Sterol Regulatory Element Binding Transcription Factor) and supports their daily rhythms in alignment with the feeding-fasting cycle even in the complete absence of a functioning circadian oscillator [58]. Recently TRE has also been shown to improve brain health in Huntington’s disease [60]and reduce inflammation from an acute infection. Both studies were performed in mice [61]. Although numerous benefits of TRF have been documented, most of the rigorous studies are in pre-clinical animal models and a few human studies are preliminary pilot or feasibility studies [62, 63]. Currently there is less clarity about the duration and timing of eating with relation to sleep-wake or light-dark cycle, that might be beneficial in humans. Since a significant proportion of the adult population uses various performance enhancing compounds, supplements, over-the-counter (OTC) and prescription drugs, the interaction of TRE with these small molecules also needs further investigation.
A new development in connecting sleep-wake cycle with metabolic diseases is the discovery that melatonin may influence pancreas function. Melatonin secreted from the pineal glands at night time acts through melatonin receptors expressed in different tissues. The melatonin receptor MNTR1B (Melatonin Receptor 1B) is expressed in pancreatic islets. Upon binding to melatonin, it suppresses insulin release [46, 64–67]. The relevance of MNTR1B in glucose regulation is further strengthened by the observation that people carrying a variant of MNTR1B are more susceptible to weight gain upon late night eating [68]. This insight to the effect of melatonin on glucose regulation offers at least three approaches to patient care; (a) avoid high carb diet before bedtime when melatonin levels begin to rise, (b) optimize timing of melatonin or melatonin receptor agonists to improve sleep without compromising metabolic health, and (c) combine genetic tests with human behavior or pharmacology to optimize health.
Clocking the drugs.
There are many health conditions where the timing of drug treatment is aligned with the circadian appearance or exacerbation of disease symptoms, the most common of which is timing of melatonin, melatonin receptor agonist, or any sleeping pill before bed time, to treat sleep disorders. There are numerous other indications where the disease symptoms may not be exacerbated at a certain time of the day, yet optimal timing of the drug may increase its efficacy and reduce adverse side effects. Some of the most cited examples are in cancer treatment. Even before the molecular mechanism of mammalian circadian clock was known, morning or evening dosing of chemotherapeutic drugs Adriamycin and Cisplatin showed significant difference in the adverse side effects and therapeutic outcomes for the treatment of ovarian cancer [69]. Similar observations have also been made for oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer patients [70]and in other cancer types that include metastatic endometrial cancer, metastatic bladder cancer and metastatic renal cell carcinoma [71]. Other examples include diminution of cisplatin (advanced non-small cell lung cancer) and radiotherapy toxicity (breast, cervical, head and neck and prostate cancer patients [72]).
Moreover, the genome-wide circadian expression profile studies in rodents, non-human primates and in human liver and lung tissues have indicated that the targets of >80% FDA approved drugs exhibit daily rhythm in mRNA levels and many of these drug targets may also have a daily rhythm in their respective functions [6, 73]. This raises the hypothesis that timing the drug treatment to align with the innate circadian rhythm of the target gene for diseases might increase its efficacy [5, 73]. There are a few successful examples recently described in comprehensive reviews [74, 75]. Some of the potential new areas that can be explored are hypertension, anti-inflammation and glucose regulation. For rheumatoid arthritis, although the symptoms are more severe in the mornings, slow release corticosteroids taken at bedtime are more effective in reducing arthritis pain in the morning [76]. Another example of chronotherapy comes from careful analysis of the SGLT2 (sodium/glucose cotransporter 2) inhibitor used for control of glucose levels in type 2 diabetes patients. The expression of SGLT2 is usually low at night [6]. Accordingly, low doses of the drug administrated in the evenings for glucose control is more effective than administrated at other times of the day [77]. These results, along with those from chronotherapy of cancer drugs are very promising. Furthermore, since blood pressure normally reduces during night time [78] and patients lacking this nighttime reduction are at higher risk for heart attacks, evening administration of blood pressure lowering drugs may have larger benefit in preventing adverse events. As metabolic diseases and cancer are poised to afflict more than 40% of the older adults, even a small improvement in drug efficacy will have a numerically bigger impact on a larger number of people.
Despite the exuberance about chronotherapy, some guidelines on choosing the right target, right drug and the right dosing is important in extracting any benefit. It is also currently unknown if the drug target that shows daily rhythms in a normal healthy individual, still maintains the same rhythm in the diseased state. In cases where the rhythm disappears in the diseased state and the target is expressed constitutively at a high level, an inhibitor may be administrated during the time of its normal low levels to restore daily rhythms. However, if the rhythm is normal in the disease condition, targeting the peak time may be a better option. Another often overlooked aspect of chronotherapy is the reference point for drug administration. As a sizeable portion of the population have a sleep-wake cycle or feeding-fasting cycle where the awake and eating period is not restricted to the daytime alone and can extend until midnight, and given that feeding itself may influence circadian rhythms, optimal drug timing should be with reference to the wake-up time, first meal, last meal or bed time. Finally, there are many steps between administering the drug and its final engagement with the target such as absorption, transportation, conversion, and uptake of the drug by target cells. Diurnal expression profiles also show rhythms in many components that participate in these steps. Therefore, there is space for a wide range of components to consider and empirically determine the factors that can potentially lead to optimal timing of drug administration and leverage maximum benefits for the patients.
Drugging the clock.
There is an increasing interest in targeting the circadian clock components as novel therapeutic approach to treat chronic diseases. In many chronic diseases such as metabolic syndrome, cancer, and chronic inflammatory diseases multiple metabolic or signaling pathways are often disrupted and an ideal drug will either activate or repress one of these affected pathways. As the circadian clock coordinates seemingly disparate cellular pathways, targeting a clock component may yield benefits comparable to a combination therapy approach where two or more drugs target separate pathways. Targeting the clock components with a drug does not necessarily mean that restoration of daily rhythms in the function of activators and repressors of the core circadian clock is necessary for the desired outcome. Rather drugging the clock is based on the idea that the circadian rhythmic outputs maintain homeostasis by transiently repressing a pathway and thereby avoiding chronic activation that would be detrimental. For example, circadian rhythm offers an intrinsic mechanism to repress inflammation and thereby ensure resolution of inflammation without progressing towards chronic inflammation. Under chronic inflammation, targeting a clock component that can mediate suppression of inflammation is desirable. While a drug that represses inflammation and has a long half-life may impart benefit, a drug with a short half-life but dosed with optimal timing may restore daily rhythm in repression and therefore by restoring rhythmicity will be more beneficial.
As many circadian clock components are known to have natural small molecule ligands that often potentiate their functions, several drug-like compounds have been developed against a handful of clock components and their regulators (Table 1). Among these compounds, a few have good bioavailability and pharmacodynamic properties enabling pre-clinical in vivo animal studies. Most of the in vivo studies with these compounds have been focused in three broad disease groups — cancer, inflammatory diseases and metabolic diseases and drugging the clock compounds in all the three disease groups have been found to be beneficial (Table 1). Additionally, the melanopsin antagonists have also been found to be effective in alleviating photophobia [57], but further research is needed for better indication and improved pharmacodynamics of this class of drugs.
Table 1.
Circadian clock components and their cognate ligand or small molecule modulators
| DRUG NAME | CIRCADIAN CLOCK TARGET GENE | ACTIVITY | References | Effect on circadian clock assessed? Y/N |
|---|---|---|---|---|
| Nobiletin | RORs | AGONIST | [105, 106] | Y |
| SR1001 | RORs | INVERSE AGONIST | [107] | Y |
| SR1078 | ROR-α/γ | AGONIST | [108] | N |
| SR3335 | ROR-α | INVERSE AGONIST | [109] | N |
| SR2211 | ROR-γ | INVERSE AGONIST | [110] | N |
| ROR-γ | INVERSE AGONIST | [111] | N | |
| XY011/8k | ROR-γ | ANTAGONIST | [112] | N |
| GSK805 | ROR-γ | ANTAGONIST | [113] | N |
| SR 1555 | ROR-γ | INVERSE AGONIST | [114] | N |
| LYC-53772 | ROR-γ | AGONIST | [115] | N |
| LYC-54143 | ROR-γ | AGONIST | [115] | N |
| Ursolic acid | ROR-γ | INVERSE AGONIST | [116] | N |
| Digoxin | ROR-γ | INVERSE AGONIST | [117] | N |
| Compound 1a | ROR-γ | AGONIST | [118] | N |
| Compound 1b | ROR-γ | AGONIST | [118] | N |
| Compound 1c | ROR-γ | AGONIST | [118] | N |
| Inhibitor Y | ROR-γ | INVERSE AGONIST | [118] | N |
| JNJ-54271074 | ROR-γ | INVERSE AGONIST | [119] | N |
| Compound 1 | ROR-β and ROR-γ | INVERSE AGONIST | [120] | N |
| GSK2945 | REV-ERBα | AGONIST | [121] | Y |
| GSK0999 | REV-ERBα | AGONIST | [121] | Y |
| GSK5072 | REV-ERBα | AGONIST | [121] | Y |
| REV-ERBα | REV-ERBs | ANTAGONIST | [122] | Y |
| GSK2667 | REV-ERBα | AGONIST | [121] | N |
| SR9011 | REV-ERBs | AGONIST | [123] | Y |
| SR9009 | REV-ERBs | AGONIST | [123] | Y |
| GSK4112 | REV-ERBα | AGONIST | [124] | Y |
Conclusion.
The field of circadian rhythm has rapidly expanded from a pure curiosity about the molecular mechanisms of the intrinsic circadian oscillator to an emerging standard bearer of integrative physiology. While biology has long focused on spatial control of gene regulation and function, the field of circadian rhythm has brought to the forefront the significance of temporal aspect of biology beyond cell cycle or early development. The widespread effects of CRD on elevating risks for a large number of childhood and adult onset chronic diseases as well as increased susceptibility to pathogens and pollution has also made this field highly relevant to medicine.
Yet, recognizing the potential for transformation also brings to forefronts some of the key questions (see Outstanding Questions) and challenges. Further, challenges also remain in integrating the science of circadian rhythms with other aspects of healthcare, convincing pharmaceutical companies and health care providers that circadian rhythm is not a nuisance, rather an opportunity for patient-centric care. For many chronic diseases including cancer, the progress over the past 2 decades of post-genomic era has been incremental. For other diseases such as arthritis, there is a sense that we have reached a plateau for delivering care at a reasonable price, even though the disease has not been sufficiently treated. For such diseases, new (delayed release or slow-release) formulations and mode of delivery through smart pumps offer new avenues for innovation and market share. Assessing in vivo pharmacodynamic/pharmacokinetic (PD/PK) of investigational new drugs at different circadian time or in the context of timing of food, sleep, light, and activity of animal models will substantially refine phase I and later clinical trials and can boost the drug pipeline. Combining the standard nutritional recommendations with circadian lifestyles that incorporate timing of sleep, activity, nutrition, and lighting also holds potential for pre-habilitation, prognosis and rehabilitation of patients. Specifically, patients undergoing acute, yet challenging therapies such as chemotherapy, radiation therapy or surgeries may benefit from such circadianly tailored lifestyle. Overall, the exuberance over circadian rhythm and its relevance to maintaining multiple organ systems in healthy states can be codified in “Circadian theory of health”. Just like the “Germ theory of disease” had a transformational impact on reducing deaths from infectious diseases and increasing human lifespan, the “Circadian theory of health” is poised for a far-reaching impact on healthcare by striving to increase healthy lifespan of individuals.
Outstanding Questions.
How do intrinsic and extrinsic factors affect the phase and/or amplitude of circadian clocks in neural and non-neural tissues?
How do pathologic conditions such as cancer, inflammation and metabolic diseases affect the circadian clock?
What are the molecules and mechanisms by which exercise, sleep, and nutrition affect the circadian clocks in a tissue specific manner?
Can regulated feeding-fasting cycles compensate for a weak or dysfunctional circadian clock and alleviate metabolic and neurologic diseases? If so, how?
Can a behaviorally supported robust circadian clock (through feeding-fasting cycle, sleep-wake patterns, and physical activity) delay the onset or alleviate the severity of age-related diseases?
Which chronic diseases can be treated effectively by pharmacologically targeting circadian clock components?
What are ideal reporters of circadian rhythms for chronotherapy?
What reference timing should be used for chronotherapy? Timing of meal or timing of sleep? Does it differ based on the type of therapy?
As sleep-disturbances are correlated with metabolic diseases and inflammation, can sleep extension alleviate or reverse metabolic diseases and inflammation in humans?
What is the mechanism by which time restricted eating improves sleep?
Which drug classes, and at what dose, are likely to benefit from chronotherapy to improve efficacy or reduce adverse side effects?
Are therapeutic benefits of drugs targeting circadian clock components due to restored circadian timing system or a result of clock component functions that are independent of their circadian properties?
Can a simple guideline be formulated for all drug trials and vital measurements to record timing of treatment and measurements?
Can circadian optimization — sleep extension/quality improvement or time restricted eating be combined with pharmacological treatment for diseases? Will this be synergistic, neutral or adverse?
Highlights.
Although circadian rhythm disruption (CRD) was typically considered to be a risk for chronic diseases solely for shift workers (~20% of workforce), new epidemiological data suggests more than 80% of the population may be living a shift work lifestyle and thus are at elevated risk for chronic diseases.
Acute CRD compromises health with temporary physical challenges and may be a trigger for underlying latent diseases. Chronic CRD raises the risk for cancer along with a range of diseases affecting the central nervous system, endocrine functions, cardiovascular health, immune system, metabolic organs and reproductive system.
Recent progress in understanding the molecular mechanisms of circadian timing and diurnal rhythms of tissue specific gene products have generated testable hypotheses for how circadian timing system optimizes health and, conversely, how circadian disruption leads to diseases.
Leveraging circadian rhythms to prevent, manage, and treat diseases involve 3 major strategies- optimizing the circadian lifestyle (“training the clock”), optimizing timing of therapies (“clocking the drugs”), and targeting specific circadian clock components (“drugging the clock”).
Acknowledgement.
This work was supported, in part, by the NIH grants EY016807 and DK115214 to S.P., FEMA grant 2016-FP-00788 to S.P and P.R.T., World Cancer Research grant to G.S., and Hillblom foundation fellowship to E.N.C.M. We thank Dr. David O’Keefe for careful reading of the manuscript.
Glossary
- Autophagy
An intracellular self-degradation system that recycles unnecessary or defective cellular components in response to nutrient stress in order to generate metabolites and energy
- Bioavailability
The amount of an administered dose of a drug that enters the circulation when introduced into the body and is therefore able to induce an active effect.
- Cell autonomous
A phenomenon that affects only the phenotype of the cell where it occurs, i.e. a genetic alteration occurring in a given cell type which determines the phenotype of that specific cell type without affecting cells that are not carrying the specific genetic alteration.
- Chronotherapy
Optimal timing of administration of a therapeutic agent such as drug, radiation, or even surgery to reduce adverse effects or improve prognosis.
- Circadian clock
A cell autonomous mechanism composed of multiple gene products whose interactions produce a near 24-hour rhythm in the function of some of the molecular components.
- Circadian transcriptome
The ensemble of all transcripts in a given tissue or cell type that shows near 24-hour rhythm in expression.
- Entrainment
Entrainment is defined as when a self-sustaining oscillator (such as the SCN) adopts the period of a driving oscillator (such as the light/dark cycle) and follows its rhythmicity
- Melanopsin
Melanopsin is an opsin (light responsive molecule) found in a subset of ganglion cells which makes them intrinsically photosensitive. These ganglion cells send non-vision related light information to the SCN.
- Peripheral circadian clocks
Circadian oscillators present in cells or tissues outside the brain are generally referred to as peripheral circadian clocks. Disruption of these peripheral clocks do not always lead to a disruption of normal sleep-activity rhythm.
- Redox regulation
Excessive oxidative stress can be deleterious for cell viability. Redox regulation employs enzymatic and non-enzymatic antioxidant systems to maintain a safe level of reactive oxygen/nitrogen species.
- SCN
The suprachiasmatic nucleus (SCN) is located at the base of the hypothalamus and is considered the master clock in mammals because it determines the endogenous behavioral period.
- Time-restricted feeding/eating (TRF or TRE)
An eating paradigm in which animals or humans eat within a fixed interval of time every day. The interval can vary between 6 to 12 hours. Although many studies in controlled TRE and TRF do not reduce daily caloric intake, some human and animal studies involve inadvertent reduction of caloric intake.
- Xenobiotic metabolism
Xenobiotics are compounds that are not physiologically present in a given organism, such as drugs or poisons. Xenobiotic metabolism is an ensemble of metabolic pathways that through chemical reactions modifies the xenobiotics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: S.P. is the author of the book titled “The Circadian Code” for which he receives author’s royalties. The grant funders nor the publisher of the book had any influence on the content of this article
REFERENCES
- 1.Mairan J.-J.d. (1729) Observation botanique. Histoire de l’Academie Royale des Sciences. 35–36. [Google Scholar]
- 2.Mohawk JA et al. (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35, 445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatori M and Panda S (2010) The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med 16 (10), 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardin PE and Panda S (2013) Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol 23 (5), 724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R et al. (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111 (45), 16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mure LS et al. (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359 (6381) doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robles MS et al. (2014) In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet 10 (1), e1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robles MS et al. (2016) Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 25(1):118–127. [DOI] [PubMed] [Google Scholar]
- 9.Kondratov RV et al. (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20 (14), 1868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakshit K and Giebultowicz JM (2013) Cryptochrome restores dampened circadian rhythms and promotes healthspan in aging Drosophila. Aging Cell 12 (5), 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeGates TA et al. (2014) Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci 15 (7), 443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunn RM et al. (2017) Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 607–608, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohsaka A et al. (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6 (5), 414–21. [DOI] [PubMed] [Google Scholar]
- 14.Damiola F et al. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14 (23), 2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokkan KA et al. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291 (5503), 490–3. [DOI] [PubMed] [Google Scholar]
- 16.Welsh D et al. (1988) Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiol Behav 43 (6), 771–77. [DOI] [PubMed] [Google Scholar]
- 17.Ehlen JC et al. (2017) Bmal1 function in skeletal muscle regulates sleep. Elife 6 pii: e26557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh PJ et al. (2015) Sleep disorders and inflammatory disease activity: chicken or the egg? Am J Gastroenterol 110 (4), 484–8. [DOI] [PubMed] [Google Scholar]
- 19.Kettner NM et al. (2016) Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 30(6), 909–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutsson A and Boggild H (2010) Gastrointestinal disorders among shift workers. Scand J Work Environ Health 36 (2), 85–95. [DOI] [PubMed] [Google Scholar]
- 21.Puttonen S et al. (2010) Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health 36 (2), 96–108. [DOI] [PubMed] [Google Scholar]
- 22.Pan A et al. (2011) Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8 (12), e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haus EL and Smolensky MH (2013) Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17 (4), 273–84. [DOI] [PubMed] [Google Scholar]
- 24.Qian J and Scheer FA (2016) Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol Metab 27 (5), 282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller BH et al. (2004) Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14 (15), 1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Iglesia HO and Schwartz WJ (2006) Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147 (3), 1148–53. [DOI] [PubMed] [Google Scholar]
- 27.Gamble KL et al. (2013) Shift work and circadian dysregulation of reproduction. Front Endocrinol (Lausanne) 4, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azzi A et al. (2014) Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat Neurosci 17 (3), 377–82. [DOI] [PubMed] [Google Scholar]
- 29.Anderson SE et al. (2017) Self-regulation and household routines at age three and obesity at age eleven: longitudinal analysis of the UK Millennium Cohort Study. Int J Obes (Lond) 41 (10), 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronfeld-Schor N and Einat H (2012) Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology 62 (1), 101–14. [DOI] [PubMed] [Google Scholar]
- 31.Wulff K et al. (2010) Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11 (8), 589–99. [DOI] [PubMed] [Google Scholar]
- 32.Scheiermann C et al. (2013) Circadian control of the immune system. Nat Rev Immunol 13 (3), 190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbs J et al. (2014) An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20 (8), 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakazato R et al. (2017) Disruption of Bmal1 impairs blood-brain barrier integrity via pericyte dysfunction. J Neurosci. 37(42):10052–10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SL et al. (2018) A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 173 (1), 130–139 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendez-Ferrer S et al. (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452 (7186), 442–7. [DOI] [PubMed] [Google Scholar]
- 37.Druzd D et al. (2017) Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 46 (1), 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Man K et al. (2016) Immunity around the clock. Science 354 (6315), 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musiek ES et al. (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123 (12), 5389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musiek ES and Holtzman DM (2016) Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354 (6315), 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castanon-Cervantes O et al. (2010) Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185 (10), 5796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feillet C et al. (2015) Coupling between the Circadian Clock and Cell Cycle Oscillators: Implication for Healthy Cells and Malignant Growth. Front Neurol 6, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaucher J et al. (2018) Molecular Cogs: Interplay between Circadian Clock and Cell Cycle. Trends Cell Biol. (5), 368–379. [DOI] [PubMed] [Google Scholar]
- 44.Manoogian EN and Panda S (2016) Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 39:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dement WC and Vaughan CC (1999) The promise of sleep : a pioneer in sleep medicine explores the vital connection between health, happiness, and a good night’s sleep, Delacorte Press. [Google Scholar]
- 46.Liu J et al. (2016) MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol 56, 361–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin CM and Benca R (2012) Chronic insomnia. Lancet 379 (9821), 1129–41. [DOI] [PubMed] [Google Scholar]
- 48.Panda S et al. (2002) Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298 (5601), 2213–6. [DOI] [PubMed] [Google Scholar]
- 49.Ruby NF et al. (2002) Role of melanopsin in circadian responses to light. Science 298 (5601), 2211–3. [DOI] [PubMed] [Google Scholar]
- 50.Thapan K et al. (2001) An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535 (Pt 1), 261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brainard GC et al. (2001) Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21 (16), 6405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatori M et al. (2017) Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. NPJ Aging Mech Dis 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lucas RJ et al. (2014) Measuring and using light in the melanopsin age. Trends Neurosci 37 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loria K (2017) Computer glasses that claim to protect your eyes from screens are selling like crazy, but they probably aren’t doing you much good. http://www.businessinsider.com/blue-blocking-glasses-science-screens-not-destroying-vision-2017-2 (accessed 07/05/2018 2018).
- 55.LeGates TA et al. (2012) Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491 (7425), 594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen SK et al. (2011) Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476 (7358), 92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones KA et al. (2013) Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol 9 (10), 630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vollmers C et al. (2009) Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A 106 (50), 21453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaix A et al. (2014) Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab 20 (6), 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang HB et al. (2018) Time-Restricted Feeding Improves Circadian Dysfunction as well as Motor Symptoms in the Q175 Mouse Model of Huntington’s Disease. eNeuro 5 (1). 5 (1). pii: ENEURO.0431–17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cisse YM et al. (2018) Time-Restricted Feeding Alters the Innate Immune Response to Bacterial Endotoxin. J Immunol 200 (2), 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill S and Panda S (2015) A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22 (5), 789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moro T et al. (2016) Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14 (1), 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ronn T et al. (2009) A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 52 (5), 830–3. [DOI] [PubMed] [Google Scholar]
- 65.Prokopenko I et al. (2009) Variants in MTNR1B influence fasting glucose levels. Nat Genet 41 (1), 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouatia-Naji N et al. (2009) A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 41 (1), 89–94. [DOI] [PubMed] [Google Scholar]
- 67.Lyssenko V et al. (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41 (1), 82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez-Minguez J et al. (2017) Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin Nutr. 37(4):1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hrushesky WJ (1985) Circadian timing of cancer chemotherapy. Science 228 (4695), 73–5. [DOI] [PubMed] [Google Scholar]
- 70.Levi F et al. (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350 (9079), 681–6. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi M et al. (2002) Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int 19 (1), 237–51. [DOI] [PubMed] [Google Scholar]
- 72.Chan S et al. (2017) Does the Time of Radiotherapy Affect Treatment Outcomes? A Review of the Literature. Clin Oncol (R Coll Radiol) 29 (4), 231–238. [DOI] [PubMed] [Google Scholar]
- 73.Anafi RC et al. (2017) CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A 114 (20), 5312–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dallmann R et al. (2016) Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol Med. 22(5):430–445. [DOI] [PubMed] [Google Scholar]
- 75.Ballesta A et al. (2017) Systems Chronotherapeutics. Pharmacol Rev 69 (2), 161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buttgereit F et al. (2008) Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 371 (9608), 205–14. [DOI] [PubMed] [Google Scholar]
- 77.Ferrannini E et al. (2010) Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33 (10), 2217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hermida RC et al. (2011) Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens 24 (4), 383–91. [DOI] [PubMed] [Google Scholar]
- 79.Marciano DP et al. (2014) The therapeutic potential of nuclear receptor modulators for treatment of metabolic disorders: PPARgamma, RORs, and Rev-erbs. Cell Metab 19 (2), 193–208. [DOI] [PubMed] [Google Scholar]
- 80.Sato TK et al. (2004) A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43 (4), 527–37. [DOI] [PubMed] [Google Scholar]
- 81.Jones CR et al. (2013) Genetic basis of human circadian rhythm disorders. Exp Neurol 243, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi JS et al. (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9 (10), 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marcheva B et al. (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466 (7306), 627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vieira E et al. (2014) Clock genes, pancreatic function, and diabetes. Trends Mol Med 20 (12), 685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turek FW et al. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308 (5724), 1043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho H et al. (2012) Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 485 (7396), 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bugge A et al. (2012) Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev 26 (7), 657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamia KA et al. (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480 (7378), 552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gibbs JE et al. (2012) The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A 109 (2), 582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Summa KC et al. (2013) Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS One 8 (6), e67102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narasimamurthy R et al. (2012) Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A 109 (31), 12662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hand LE et al. (2016) The circadian clock regulates inflammatory arthritis. FASEB J 30 (11), 3759–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao Q et al. (2017) Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A 114 (47), 12548–12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamont EW et al. (2007) The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci 9 (3), 333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu L et al. (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111 (1), 41–50. [DOI] [PubMed] [Google Scholar]
- 96.Lee S et al. (2010) Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One 5 (6), e10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber AL et al. (2016) CRY2 and FBXL3 Cooperatively Degrade c-MYC. Mol Cell 64 (4), 774–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papagiannakopoulos T et al. (2016) Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab 24 (2), 324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ueda E et al. (2002) High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORgamma. Cancer Res 62 (3), 901–9. [PubMed] [Google Scholar]
- 100.Dubrovsky YV et al. (2010) Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2 (12), 936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan X et al. (2018) Night Shift Work Increases the Risks of Multiple Primary Cancers in Women: A Systematic Review and Meta-analysis of 61 Articles. Cancer Epidemiol Biomarkers Prev 27 (1), 25–40. [DOI] [PubMed] [Google Scholar]
- 102.McHill AW et al. (2014) Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences 111 (48), 17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chomez P et al. (2000) Increased cell death and delayed development in the cerebellum of mice lacking the rev-erbA(alpha) orphan receptor. Development 127 (7), 1489–98. [DOI] [PubMed] [Google Scholar]
- 104.Panda S (2016) Circadian physiology of metabolism. Science 354 (6315), 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He B et al. (2016) The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab 23 (4), 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murakami A et al. (2000) Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res 60 (18), 5059–66. [PubMed] [Google Scholar]
- 107.Solt LA et al. (2011) Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472 (7344), 491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y et al. (2010) Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORalpha and RORgamma. ACS Chem Biol 5 (11), 1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar N et al. (2011) Identification of SR3335 (ML-176): a synthetic RORalpha selective inverse agonist. ACS Chem Biol 6 (3), 218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumar N et al. (2012) Identification of SR2211: a potent synthetic RORgamma-selective modulator. ACS Chem Biol 7 (4), 672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J et al. (2016) ROR-gamma drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat Med 22 (5), 488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y et al. (2014) Discovery of 2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamide derivatives as new RORgamma inhibitors using virtual screening, synthesis and biological evaluation. Eur J Med Chem 78, 431–41. [DOI] [PubMed] [Google Scholar]
- 113.Xiao S et al. (2014) Small-molecule RORgammat antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40 (4), 477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Solt LA et al. (2012) Identification of a selective RORgamma ligand that suppresses T(H)17 cells and stimulates T regulatory cells. ACS Chem Biol 7 (9), 1515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu X et al. (2016) Synthetic RORgamma agonists regulate multiple pathways to enhance antitumor immunity. Oncoimmunology 5 (12), e1254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu T et al. (2011) Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem 286 (26), 22707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huh JR et al. (2011) Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 472 (7344), 486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang W et al. (2012) Increasing human Th17 differentiation through activation of orphan nuclear receptor retinoid acid-related orphan receptor gamma (RORgamma) by a class of aryl amide compounds. Mol Pharmacol 82 (4), 583–90. [DOI] [PubMed] [Google Scholar]
- 119.Xue X et al. (2016) Pharmacologic modulation of RORgammat translates to efficacy in preclinical and translational models of psoriasis and inflammatory arthritis. Sci Rep 6, 37977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patouret R et al. (2018) Identification of an aminothiazole series of RORbeta modulators. Bioorg Med Chem Lett 28 (7), 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trump RP et al. (2013) Optimized chemical probes for REV-ERBalpha. J Med Chem 56 (11), 4729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kojetin D et al. (2011) Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol 6 (2), 131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Solt LA et al. (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485 (7396), 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grant D et al. (2010) GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem Biol 5 (10), 925–32. [DOI] [PubMed] [Google Scholar]


