Abstract
Introduction:
This phase I trial was conducted to determine the safety, maximum tolerated dose (MTD)/recommended phase II dose, and efficacy of crizotinib plus erlotinib in patients with advanced NSCLC.
Methods:
Patients with NSCLC and an Eastern Cooperative Oncology Group performance status of 0 to 2 after failure of one or two prior chemotherapy regimens were eligible. Erlotinib, 100 mg, was given continuously once daily starting between day 14 and 7; crizotinib, 200 mg twice daily (dose level 1) or 150 mg twice daily (dose level 1), was added continuously beginning on day 1 of treatment cycle 1. Potential pharmacokinetic interactions between crizotinib and erlotinib were evaluated.
Results:
Twenty-seven patients received treatment; 26 received crizotinib plus erlotinib. Frequent adverse events were diarrhea, rash, decreased appetite, and fatigue. Dose-limiting toxicities were dehydration, diarrhea, dry eye, dysphagia, dyspepsia, esophagitis and vomiting. The MTD was crizotinib, 150 mg twice daily, with erlotinib, 100 mg once daily. Crizotinib increased the erlotinib area under the concentration-time curve 1.5-fold (dose level 1) and 1.8-fold (dose level 1). The plasma level of crizotinib appeared to be unaffected by coadministration of erlotinib. Two patients whose tumors harbored activating EGFR mutations achieved confirmed partial responses, one at each crizotinib dose level.
Conclusions:
The MTD of the combination of crizotinib and erlotinib in patients with advanced NSCLC was crizotinib, 150 mg twice daily, with erlotinib, 100 mg once daily, which is less than the approved dose of either agent. The phase II portion of the study was not initiated.
Keywords: Crizotinib, Erlotinib, Phase I combination trial, MET inhibitor, EGFR inhibitor
Introduction
Secondary MNNG HOS Transforming gene (MET) amplification is one of the mechanisms of resistance to EGFR tyrosine kinase inhibitor (TKI) treatment in patients with NSCLC having activating EGFR mutations.1 Combination of EGFR TKIs and mesenchymal-epithelial transition (MET) inhibitors is a rational approach to potentially delay the emergence of resistance to EGFR TKIs in TKI-naive patients with EGFR-positive NSCLC or to overcome resistance in patients with EGFR-positive NSCLC who progress while receiving single-agent EGFR TKIs. Crizotinib is approved for the treatment of advanced anaplastic lymphoma kinase (ALK)-rearranged or ROS1-rearranged NSCLC,2,3 but it was initially developed as a MET inhibitor and has shown clinical activity in NSCLC that harbors MET amplification or MET exon 14 skipping alterations.4–6 Herein we report the results from a phase I study combining crizotinib with erlotinib in patients with advanced nonsquamous NSCLC (NCT00965731).
Patients and Methods
Study Design and Eligibility Criteria
This was a single-arm phase I study of crizotinib plus erlotinib, to be followed by a planned randomized phase II portion comparing the efficacy of crizotinib plus erlotinib versus erlotinib alone in patients with chemotherapy-refractory locally advanced/metastatic nonsquamous NSCLC.
The primary end point was to determine the maximum tolerated dose (MTD) of the combination of crizotinib and erlotinib. Secondary end points included evaluating the effect of crizotinib on erlotinib pharma-cokinetics (PK) (as crizotinib is a moderate cytochrome P450 family 3 subfamily A member 4 [CYP3A4] inhibitor,7 whereas erlotinib is metabolized by CYP3A48) and documenting any antitumor activity.
A standard 3 plus 3 dose escalation/deescalation design for phase I studies was used; the trial schema is shown in Supplementary Figure 1. The study protocol was approved by the institutional review board at each clinical site.
Patients with histologically proven, locally advanced/ metastatic (stage IIIB/IV) nonsquamous NSCLC who were aged 18 years or older, had progressed after one or two chemotherapy regimens for advanced disease, had an Eastern Cooperative Oncology Group performance status of 0 to 2, had measurable disease (defined by Response Evaluation Criteria in Solid Tumors, version 1.1), and had adequate organ function were eligible for inclusion in this study. Patients with brain metastasis were eligible if appropriately treated and neurologically stable for at least 4 weeks. No prior crizotinib or EGFR TKIs were allowed. A subsequent protocol amendment allowed patients with prior EGFR TKIs onto the phase I portion of the trial. Patients with interstitial lung fibrosis or interstitial lung disease were also excluded. All patients provided signed informed consent before study participation.
Treatment and Pharmacokinectic Evaluations
There was a 7- to 14-day lead-in period of erlotinib, once daily alone continuously, to determine the steady-state PK of erlotinib, which was evaluated on day 1 of cycle 1. The starting doses were crizotinib 200 mg twice daily, and erlotinib, 100 mg once daily (dose level 1). The starting dose of crizotinib was chosen to exceed the predicted level necessary for MET and anaplastic lymphoma kinase inhibition.7,9 The starting dose of erlotinib was chosen on the basis of the predicted effect of crizotinib on erlotinib exposure.10 Crizotinib and erlotinib were then given concomitantly on a continuous schedule; cycle 1 was 28 days in length, and subsequent cycles were 21 days long.
Blood samples (3 mL) were collected at 0 (predose) and 0.5, 1, 2, 3, 4, 5, 6, 8, and 12 hours (for determining both crizotinib and erlotinib PK) and at 24 hours for determining erlotinib PK only after morning dosing on days 1 and 15 of cycle 1. PK parameters, including area under the concentration-time curve over the dosing interval (AUCτ), were obtained by noncompartmental analysis. The effect of continuous daily crizotinib dosing on erlotinib PK was evaluated by using the AUCτ of erlotinib on both day 1 and day 15 of cycle 1.
Toxicity and Response Evaluation
Toxicities were graded using the Common Terminology Criteria for Adverse Events, version 4.0. Dose-limiting toxicities (DLTs) were defined as treatment-related grade 4 or higher hematologic toxicities (excluding lymphopenia in the absence of other DLTs), grade 3 or higher treatment-related febrile neutropenia, treatment-related grade 3 or higher nonhematologic toxicities, diagnosis of interstitial lung disease, or inability to receive at least 80% of the planned crizotinib or erlotinib doses during cycle 1 on account of possible treatment-related adverse events (AEs). All patients had an ophthalmologic examination at baseline, which was repeated during the study if clinically indicated. Response evaluation is described in Supplementary Methods.
Statistical Analysis
Patients who received at least one dose of crizotinib and erlotinib were included in the safety analysis for the combination period and were evaluated for antitumor activity (those who had an adequate baseline tumor assessment). The PK analysis was performed in patients who received at least one dose of the study drug and had at least one PK assessment.
Descriptive statistics were used to summarize safety, antitumor activity, and PK variables. To assess the effect of repeated crizotinib dosing on erlotinib PK, AUCτ was log-transformed and analyzed using a mixed effects model with treatment as a fixed effect and patient as a random effect. The 90% confidence interval (CI) for the ratio of the geometric mean of AUC for crizotinib and erlotinib to erlotinib alone was calculated.
For the evaluation of a potential drug-drug interaction between crizotinib and erlotinib, a sample size of 16 patients provided more than an 80% probability that the 90% CI for the ratio of the AUC of crizotinib and erlotinib to erlotinib alone was within 0.8 to 2.15 if the true ratio was 1.5, assuming a level of intrapatient variability for the erlotinib AUC of 38.5%.
Results
Patient Characteristics
Between January 2010 and December 2011, 27 patients received at least one dose of erlotinib during the lead-in phase. Twenty-six patients received at least one dose of crizotinib and erlotinib. Baseline clinicopathologic characteristics are listed in Table 1. At the time of the database snapshot (August 21, 2012), one patient (who withdrew from the study in January 2014 after disease progression in December 2013) was still receiving treatment (crizotinib, 150 mg twice daily, and erlotinib, 100 mg once daily). Data (duration of treatment and duration of response) from this last patient was captured in the database (locked on April 17, 2014) and included in this report. All other data in this manuscript were based on the database snapshot of August 21, 2012.
Table 1.
Patient Characteristics by Dose Level
| Characteristics | Dose Level 1 (n = 7) | Dose Level –1 (n = 20) | All Patients (N = 27) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 1 (14) | 7 (35) | 8 (30) |
| Female | 6 (86) | 13 (65) | 19 (70) |
| Median age (range), y | 55 (43–68) | 63 (47–79) | 60 (43–79) |
| Mean weight (SD), kg | 69 (7) | 65 (13) | 66 (11) |
| Mean height (SD), cm | 164 (8) | 163 (11) | 163 (10) |
| Race, n (%) | |||
| White | 4 (57) | 13 (65) | 17 (63) |
| Asian | 1 (14) | 4 (20) | 5 (19) |
| Black | 2 (29) | 1 (5) | 3 (11) |
| Other | 0 | 2 (10) | 2 (7) |
| ECOG PS, n (%) | |||
| 0 | 3 (43) | 6 (30) | 9 (33) |
| 1 | 4 (57) | 13 (65) | 17 (63) |
| 2 | 0 | 1 (5) | 1 (4) |
| Smoking status, n (%) | |||
| Never | 2 (29) | 12 (60) | 14 (52) |
| Former | 5 (71) | 8 (40) | 13 (48) |
| Histologic type, n (%) | |||
| Adenocarcinoma | 7 (100) | 14 (70) | 21 (78) |
| Bronchioloalveolar carcinoma | 0 | 5 (25) | 5 (19) |
| Papillary adenocarcinoma | 0 | 1 (5) | 1 (4) |
| Stage, n (%) | |||
| Locally advanced | 1 (14) | 1 (5) | 2 (7) |
| Metastatic | 6 (86) | 19 (95) | 25 (93) |
| Number of prior systemic therapies, n (%) | |||
| 1 | 5 (71) | 9 (45) | 14 (52) |
| 2 | 2 (29) | 10 (50) | 12 (44) |
| 3 | 0 | 1 (5) | 1 (4) |
| Prior EGFR TKI, n (%) | 0 | 7 (35) | 7 (26) |
Note: Dose level of 1: crizotinib, 150 mg twice daily, plus erlotinib, 100 mg once daily. Dose level of 1: crizotinib, 200 mg twice daily, plus erlotinib, 100 mg once daily.
ECOG PS, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor.
DLTs
Three patients had DLTs: grade 3 esophagitis (n = 1) and dry eye (n = 1) at dose level 1 and grade 2 esophagitis, dysphagia, and dyspepsia precluding receipt of at least 80% of the cycle 1 doses at dose level 1 (n = 1). The MTDs were determined to be crizotinib, 150 mg twice daily, and erlotinib, 100 mg once daily. Enrollment was expanded to a total of 20 patients at the MTD. DLTs developed in two additional patients: grade 3 diarrhea and dehydration (n 1) and grade 2 vomiting that rendered the patient unable to receive 80% of the cycle 1 doses on account of possible treatment-related AEs (n=1). All DLTs resolved after the study medication was stopped.
AEs (Combination Treatment)
All but one patient (96%) had at least one treatment-related AE during the combination treatment period; however, these were predominantly grade 1 or 2 in severity (Table 2). There were no grade 4 or 5 treatment-related AEs.
Table 2.
Treatment–Related Adverse Events in More Than 15% of Patients Receiving Crizotinib and Erlotinib during Combination Treatment
| n (%) | ||
|---|---|---|
| Adverse Eventa | Any Grade | Grade 3b |
| Dose level 1 (n = 7) | ||
| Diarrhea | 5 (71) | 0 |
| Dry skin | 5 (71) | 0 |
| Fatigue | 3 (43) | 0 |
| Rash | 3 (43) | 1 (14) |
| Vomiting | 3 (43) | 0 |
| Decreased appetite | 2 (29) | 0 |
| Hypokalemia | 2 (29) | 1 (14) |
| Muscle spasm | 2 (29) | 0 |
| Nausea | 2 (29) | 0 |
| Pustular rash | 2 (29) | 0 |
| Dose level −1 (n = 19) | ||
| Diarrhea | 14 (74) | 4 (21) |
| Rash | 13 (68) | 0 |
| Decreased appetite | 10 (53) | 0 |
| Fatigue | 9 (47) | 1 (5) |
| Nausea | 9 (47) | 0 |
| Vomiting | 6 (32) | 1 (5) |
| Dehydration | 4 (21) | 1 (5) |
| Anemia | 3 (16) | 0 |
| Dry skin | 3 (16) | 0 |
| Dyspepsia | 3 (16) | 0 |
| Hypoalbuminemia | 3 (16) | 0 |
| Upper abdominal pain | 3 (16) | 0 |
| Visual impairment | 3 (16) | 0 |
| Weight decreased | 3 (16) | 0 |
Note: Dose level of 1: crizotinib 150 mg twice daily, plus erlotinib, 100 mg once daily. Dose level of 1: crizotinib, 200 mg twice daily, plus erlotinib, 100 mg once daily.
Dose-limiting toxicities comprised grade 3 esophagitis (n = 1); dry eye (n = 1); and grade 2 esophagitis, dysphagia. and dyspepsia (n = 1).
There were no treatment-related grade 4 or 5 adverse events.
PK Evaluations
At dose level 1 (crizotinib, 150 twice daily, and erlotinib, 100 mg once daily), the ratios of geometric means for erlotinib exposure (cycle 1, day 15) compared with erlotinib administered alone (cycle 1, day —1) were 149% (90% CI: 126–177) for AUCτ (Fig. 1A) and 135% (90% CI: 115–157) for the maximum plasma concen-tration (Cmax) (Table 3). At dose level 1 (crizotinib, 200 twice daily, and erlotinib, 100 mg once daily), the ratios of geometric means for erlotinib exposure (cycle 1, day 15) compared with erlotinib administered alone (cycle 1, day —1) were 185% (90% CI:150–228) for AUCτ (Fig. 1A) and 160% (90% CI: 121–212) for Cmax (Table 3). Compared with historical PK data, crizotinib exposure appeared to be unaffected by coadministration with erlotinib (Fig. 1B).
Figure 1.
Pharmacokinetics of erlotinib and crizotinib administered in combination during cycle 1 of treatment. (A) Erlotinib exposure (by patient and geometric mean [geomean]) when administered alone (day 1) or in combination with crizotinib (days 1 and 15). (B) Crizotinib exposure on day 15. Circles indicate individual values, horizontal white lines indicate medians, boxes indicate 25th to 75th percentiles, and whiskers indicate 1.5 × interquartile range. AUCτ, area under the concentration– time curve over the dosing interval (crizotinib, 12 hours; erlotinib, 24 hours). aPfizer Inc., data on file.
Table 3.
Pharmacokinetics of Erlotinib and Crizotinib When Given in Combination at Two Crizotinib Dose Levels
| Geometric Mean (% CV) | ||||||
|---|---|---|---|---|---|---|
| Pharmacokinentic Parameter | Dose Level 1 (n = 7) |
Dose Level −1 (n = 19) |
||||
| Day −1a | Cycle 1, Day 1 | Cycle 1, Day 15b | Day −1a | Cycle 1, Day 1 | Cycle 1, Day 15c | |
| Erlotinib | ||||||
| Tmax, d h | 2.0 (1.0–5.0) | 4.0 (0.7–12.0) | 3.1 (1.0–6.1) | 3.0 (0.5–5.0) | 4.0 (0.6–12.0) | 3.0 (2.0–25.2) |
| Cmax, ng/mL | 1593 (26) | 1452 (21) | 2546 (24) | 1797 (36) | 1723 (38) | 2346 (43) |
| AUCτ, ng·h/mL | 23,490 (31) | 26,520 (25) | 41,770 (27) | 26,880 (39) | 30,040 (39) | 38,910 (49) |
| Crizotinib | ||||||
| Tmax d h | NA | 3.5 (1.0–6.0) | 2.0 (0.6–8.0) | NA | 4.0 (0.6–8.1) | 4.5 (0.5–6.0) |
| Cmax, ng/mL | NA | 86.8 (37) | 251.0 (46) | NA | 65.3 (50) | 185.9 (40) |
| AUCτ, ng·h/mL | NA | 581.9 (49) | 2274 (43) | NA | 400.3 (40) | 1720 (40) |
Note: Dose level of 1: crizotinib, 150 mg twice daily, plus erlotinib, 100 mg once daily. Dose level of 1: crizotinib, 200 mg twice daily, plus erlotinib, 100 mg once daily.
Erlotinib administered alone.
n = 5.
n = 14.
Presented as median (range).
CV, coefficient of variation; Tmax, time to maximum plasma concentration; Cmax, maximum concentration; AUCτ, area under the concentration-time curve over the dosing interval (crizotinib, 12 hours; erlotinib, 24 hours); NA, not applicable.
Treatment Activity
Median duration of treatment with crizotinib and erlotinib in the combination period was 7 weeks for the overall patient cohort (n = 26) (range <1 to 78 weeks). Twenty-five of the 26 patients who received at least one dose of crizotinib and erlotinib had a baseline assessment evaluable for antitumor activity (Table 4).
Table 4.
Response Based on Investigator Assessment
| Objective Response/Response Duration | n (%) |
||
|---|---|---|---|
| Dose Level 1 (n = 7) | Dose Level −1 (n = 18) | All Patients (N = 25) | |
| Partial response | 1 (14) | 1 (6) | 2(8) |
| Stable disease | 2 (29) | 6 (33) | 8(32) |
| Objective progression | 4(57) | 6 (33) | 10 (40) |
| Early death | 0 | 2(11) | 2(8) |
| Indeterminatea | 0 | 3 (17) | 3 (12) |
| Objective response rate, % (95% exact CI) | 14 (<1–58) | 6 (<1 −27) | 8 (1–26) |
| Duration of stable diseaseb | |||
| 0 to <3 months | 1 (50) | 0 | 1 (13) |
| 3 to <6 months | 0 | 6 (100) | 6(75) |
| 6 to <9 months | 0 | 0 | 0 |
| 9 to <12 months | 0 | 0 | 0 |
| >12 months | 1 (50) | 0 | 1 (13) |
Note: Dose level of 1: crizotinib, 150 mg twice daily, plus erlotinib, 100 mg daily. Dose level of 1: crizotinib, 200 mg twice daily, plus erlotinib, 100 mg daily.
Withdrawn from study before first scan during treatment.
In patients with stable disease.
CI, confidence interval.
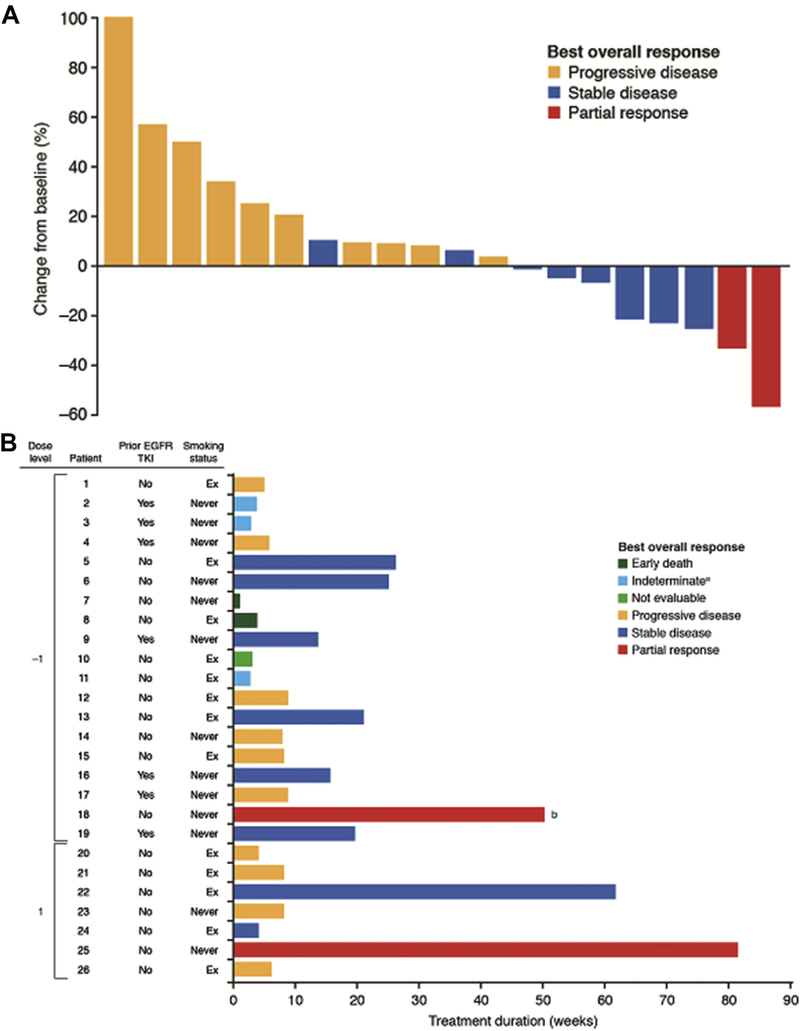
A waterfall plot among the 20 response-evaluable patients reporting at least one follow-up scan is presented in Figure 2A. The treatment duration, best response, smoking status, and prior EGFR TKI treatment for each patient are shown in Figure 2B. Both patients who achieved a partial response had tumors with EGFR exon 19 deletion mutations.
Figure 2.
Best overall response in patients who received at least one dose of crizotinib and erlotinib. (A) Waterfall plot of best percent change from baseline in size of target lesions in patients who had at least one follow-up scan (n = 20). (B) Treatment duration, best response, smoking status, and prior EGFR tyrosine kinase inhibitor (TKI) treatment in patients grouped by dose level. aWithdrawn from the study before first scan during treatment. bDuration of treatment at data cutoff (August 2012); this patient ultimately continued treatment for a total of 28 months.
Discussion
The MTD from the phase I portion of this study was crizotinib, 150 mg twice daily, and erlotinib, 100 mg once daily. No unexpected AEs were observed and the safety profile of the combination was consistent with those of erlotinib and crizotinib administered as single agents. The combination of crizotinib and erlotinib was feasible and generally well tolerated, albeit at doses less than those approved for single-agent use in patients with advanced nonsquamous NSCLC, with two of 25 patients achieving a partial response. PK data demonstrated that crizotinib at 150 or 200 mg twice daily increased erlotinib exposure (AUC) by 1.5- and 1.8-fold, respectively, on account of crizotinib inhibiting CYP3A4-mediated metabolism of erlotinib. Thus, when erlotinib was administered at 100 mg once daily in combination with either dose of crizotinib, its plasma levels were similar to those of single-agent erlotinib dosed at 150 mg once daily according to historical data. Plasma concentrations of crizotinib did not appear to be affected by the coadministration of erlotinib. The planned phase II portion was not initiated.
Supplementary Material
Acknowledgments
This study was sponsored by Pfizer Inc., which participated in the trial design and managed all operational aspects of the study, including monitoring data collection, statistical analyses, and writing of the report. We would like to thank all of the participating patients and their families, as well as the investigators, research nurses, study coordinators, and operations staff. This study was sponsored by Pfizer Inc. Medical writing and editorial support was provided by Wendy Sacks at ACUMED (New York, NY), an Ashfield Company that is part of UDG Healthcare plc, and by Jade Drummond at inScience Communications (Chester, UK) and was funded by Pfizer Inc.
Dr. Ou has received lecture and consulting honoraria from Pfizer. Dr.Govindan has received lecture and consulting honoraria from Pfizer, Merck, Boehringer-Ingelheim, Clovis, Helsinn Healthcare, Genetech, AbbVie, GlaxoSmithKline, and Celgene. Drs. Eaton and Otterson have received research funding from Pfizer. Drs. Brega, Tan, and Ho and Ms. Usari are employees of Pfizer and hold Pfizer stock.
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at http://dx.doi.org/10.1016/j.jtho.2016.09.131 .
Disclosure: The remaining authors delcare no conflict of interest.
References
- 1.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;23(3):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19:e5–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazandjian D, Blumenthal GM, Luo L, et al. Benefit-risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive, metastatic non-small cell lung cancer. Oncologist. 2016;21: 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6: 942–946. [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Ou S-HI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2014;32(suppl 5):8001. [Google Scholar]
- 6.Drilon AE, Camidge DR, Ou S-HI, et al. Efficacy and safety of crizotinib in patients (pts) with advanced MET exon 14-altered non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2016;34(suppl):108. [Google Scholar]
- 7.Tan W, Wilner KD, Bang Y, et al. Pharmacokinetics (PK) of PF-02341066, a dual ALK/MET inhibitor after multiple oral doses to advanced cancer patients [abstract]. J Clin Oncol. 2010;28(suppl 15):2596. [Google Scholar]
- 8.Rakhit A, Pantze MP, Fettner S, et al. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. Eur J Clin Pharmacol. 2008;64: 31–41. [DOI] [PubMed] [Google Scholar]
- 9.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small cell lung cancer. N Engl J Med. 2010;363:1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki S, Vicini P, Shen Z, et al. Pharmacokinetic/ pharmacodynamic modeling of crizotinib for anaplastic lymphoma kinase inhibition and antitumor efficacy in human tumor xenograft mouse models. J Pharmacol Exp Ther. 2012;340:549–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.