Abstract
The addition of a hypoxic stimulus during resistance training is suggested to increase the metabolic responses, enhancing hypertrophy and muscle strength. The purpose of this study was to investigate the effects of resistance training performed at submaximal intensities combined with normobaric hypoxia on muscular performance, body composition and haematological parameters. Thirty-two untrained subjects participated in this study (weight: 74.68±12.89 kg; height: 175±0.08 cm; BMI: 24.28±3.80 kg/m2). They were randomized to two groups: hypoxia (FiO2 = 13%) or normoxia (FiO2 = 20.9%). The training programme lasted 7 weeks (3 d/w) and several muscle groups were exercised (3 sets x 65−80% 1RM to failure). Measurements were taken before, after the training and after a 3-week detraining period. Body composition and muscle mass were assessed through skinfolds and muscle girths. Muscle strength was evaluated by the 1RM estimated test. Finally, haemoglobin and haematocrit were taken from the antecubital vein. Both groups improved their strength performance and muscle perimeters, but the hypoxia group obtained a greater increase in muscle mass (hypoxia: +1.80% vs. normoxia: +0.38%; p<0.05) and decrease in fat mass (hypoxia: -6.83% vs. normoxia: +1.26%; p<0.05) compared to the normoxia group. Additionally, haematocrit values were also higher for the hypoxia group after the detraining period (hypoxia: +2.20% vs. normoxia: -2.22%; p<0.05). In conclusion, resistance training under hypoxic conditions could increase muscle mass and decrease fat mass more effectively than training performed in normoxia, but without contributing to greater muscle strength.
Keywords: Normobaric hypoxia, Intermittent hypoxic resistance training, IHRT, Altitude training, Haematological variables
INTRODUCTION
Hypoxic training has become available to more people in recent years through altitude chambers (hypobaric hypoxia) and hypoxic tents (normobaric hypoxia) [1]. Muscular and haematological adaptations are the main benefits of time spent at moderate altitude [2,3]. With regard to muscular adaptations, high-intensity resistance training has a potent effect in promoting increases in muscle size and strength of skeletal muscles due to improvements in muscular endurance and the promotion of angiogenesis in skeletal muscle [4]. In line with this, the addition of a hypoxic stimulus is suggested to increase the metabolic and hormonal responses to resistance exercise [5].
In addition, the combination of hypoxia and resistance training is used to increase hypertrophy and muscular strength [6,7]. Both methods, blood flow restriction (BFR) and intermittent hypoxic resistance training (IHRT) are commonly used to achieve these improvements. However, unlike BFR strategies, the use of normobaric hypoxia devices allows training the trunk musculature under hypoxic conditions with the limbs [5].
In fact, IHRT protocols are currently being investigated, using low loads [6,8], moderate loads [4,7,9–12], and high loads [13–15]. Despite there being no unanimity in the results obtained regarding strength and muscle mass, a recent meta-analysis stated that IHRT significantly improved muscle size and strength performance [16]. In line with this, Nishimura et al. [7] showed increases in the cross-sectional area of elbow flexor and extensors muscles after 6 weeks of resistance training (using loads of about 70% 1RM) under hypoxic conditions (FiO2 = 16%), but without observing differences in strength between groups. Similar results were found by Kurobe et al. [11] after 8 weeks of IHRT of unilateral elbow extension (3 sets x 10 RM) in hypoxia (FiO2 = 12.7%). In addition, Chycki et al. [12] reported important hypertrophic and anabolic hormonal responses after 6 weeks of training in normobaric hypoxia (FiO2 = 12.9%) with moderate loads (70% 1RM). Moreover, Martínez-Guardado et al. [17] reported greater increases in lean mass, as well as a significant increase in BMD for the group that performed high-intensity (6RM) circuit training in hypoxia (FiO2 = 15%) for 8 weeks. However, the strength gain was similar in both groups. Conversely, Inness et al. [12] reported a greater improvement in absolute and relative 1RM of squat in the hypoxia group after 7 weeks of IHRT (FiO2 = 14.3%) with heavy loads (75% 1RM), but no changes were found in lean mass. Moreover, Mayo et al. [13] found small improvements in upper-body strength after 3 weeks of hypoxic training (FiO2 = 14.4%) with loads between 85 and 92.5% of 1RM. However, both studies were the only ones to include highly trained individuals in their sample. Therefore, it is possible that the initial training status of the participants directly conditions the responses to hypoxia training [16].
Despite this, the aforementioned studies established a fixed number of repetitions per set. In contrast, the present study proposed an IHRT protocol to muscle failure in each set. This approach has been carried out only by a previous acute study that reported increases in metabolic stress and rating of perceived exertion (RPE) [18]. However, to the best of our knowledge, there are no studies that analyse the effect of this type of protocol to muscle failure over several weeks of IHRT in hypoxia on performance variables. In addition, none of the previous studies carried out an assessment of the potential benefits of the IHRT after a short-term detraining period. Detraining is the cessation of training volume, intensity, or frequency resulting in reduced performance [19]. It appears that the duration of the detraining period is important for the magnitude of changes, and may occur during short periods (<4 weeks) or long periods (>4 weeks) [20]. However, there are no previous references to establish possible adaptations caused by this detraining period.
Regarding haematological adaptations, deprivation of oxygen supply caused by hypoxia is counteracted by the organism through: stimulation of red corpuscles [21]; increased myo-globin content [22]; mitochondrial activity [23]; or improved mechanical efficiency [24]. In addition, when the amount of tissue oxygen in the body decreases due to hypoxia, the secretion of transcription factor hypoxia-inducible factor (HIF-1α) increases exponentially, and this fact has been associated with an increases in EPO secretion [25]. However, several studies have shown that intermittent training in hypoxia may not be useful in enhancing erythropoiesis [26,27]. Conversely, Camacho-Cardenosa et al. [28] reported higher values of haemoglobin (Hb) and haematocrit (Hct) in active participants in the detraining period (3 weeks) after eight repeated sprint training sessions in normobaric hypoxia. Despite this, with regard to resistance training, only Kon et al. [4] and Álvarez-Herms et al. [29] reported the effects of training under normobaric hypoxia and hypobaric hypoxia, respectively, on Hb concentration and Hct mass. However, these studies failed to find differences for either of the two values when comparing the two training groups.
Therefore, the aim of this study was to analyse the effect of 7 weeks of resistance training to muscle failure combined with normobaric hypoxia on muscular performance, body composition and haematological parameters. Our hypothesis was that hypoxic training would improve body composition through lean body mass increase and decrease body fat. We additionally hypothesized that the addition of hypoxia to the training programme would increase muscular performance and haematological variables.
MATERIALS AND METHODS
Participants
Thirty-two untrained male subjects (age: 25.7±6.42 years; height: 176±0.07 cm; weight: 78.41±12.07 kg) volunteered to participate in this study and were assigned to hypoxia (HYP) and normoxia (NOR) groups (n = 16 per group). All the participants were sea-level residents and had not been exposed to an altitude or hypoxia environment of more than 1 800 m within the 4 months before participation. We define the participants as totally untrained due to none of them having been involved in a regular strength training programme within 1 year before at the beginning of the study. Each participant was informed of the procedure to be followed during the study and they all provided written consent. This study was approved by the Institutional Science Ethic Committee that conforms to the 2008 Helsinki Declaration for Human Research Ethics.
DESIGN AND PROCEDURES
Experimental design
To test the effects of 7 weeks of an IHRT protocol in normoxia and hypoxia on body composition, muscle performance and haematological variables, a single-blinded randomized controlled trial with pre-, post- and detraining tests was developed. Thirty-two untrained university students were randomly assigned to 2 experimental groups [hypoxia group (HYP; n = 16) and normoxia group (NOR; n = 16)] who finished the same resistance training protocol (7 weeks) under normobaric hypoxia (FiO2 = 13%) or normoxia (FiO2 = 21%), respectively, in a single-blinded manner. The week prior to starting the training sessions all participants visited the laboratory to familiarize themselves with testing and training procedures. Maximal dynamic strength (1RM) was estimated in all exercises that were part of the training circuit. In addition, anthropometry, body composition, haemoglobin and haematocrit were measured under normoxic conditions. These measurements were also performed after the training and after a three-week detraining period. The detraining period was carried out under normoxia conditions for all participants. In addition, participants were instructed to maintain their regular dietary consumption and not practise any intense exercises during the entire measurement and training phases.
Systemic hypoxia exposure
Both groups performed the training sessions in a normobaric chamber (CAT 430, Colorado Altitude Training, USA) where oxygen-depleted air was pumped from a hypoxia generator (CAT-12, Colorado Altitude Training, USA) to create simulated hypoxic conditions (FiO2 = 13%, equivalent to 4 300 m altitude) where barometric pressure in the tent was equivalent to sea level. Ambient O2 was continuously monitored by a digital controller (Handi+, Maxtec, USA) to maintain the hypoxic conditions in the chamber. For training in normoxic conditions, the ambient FiO2 was not manipulated at a natural altitude of about 440 m.
Blood sampling
The testing sessions began upon arrival in the morning. A 2-ml blood sample was taken from the antecubital vein with the subjects seated for an immediate Hct and Hb concentration assessment. To determine Hct, 100 uL of whole blood was collected in a heparinized glass capillary tube (Brand GMBh+CO KG, Wertheim, Germany) and centrifuged during 5 min at 13.000 rpm (Zipocrit, LW Scientific, Ga, USA). Upon completion, the sample was removed and assessed using a Hawksley Hct reader for the determination of cellular volume and plasma volume. Data were expressed as a percentage of cells to total volume. Hb concentration was assessed with a photometry device (Hemocue 201, Angeholm, Sweden). This photometry device is factory-calibrated and should not be recalibrated. The calibration was checked daily according to the manufacturer’s recommendation and it was stable during our study period.
Body composition
Several anthropometric measurements were taken at the beginning of the experiment in the first session to characterize the individuals, at the end of the programme and after a detraining period. Body height was measured using a portable stadiometer (Seca 213, Leicester Height Measure, Hamburg, Germany). The body weight (Tanita BC-1500, Tanita Corporation of America, Illinois, USA), body mass index (weight/height2), three girths (Seca 201, Hamburg, Germany) and six skinfolds (Harpenden, British, Indicators, Burgess Hill, UK) were evaluated according to International Society for the Advancement of Kinanthropometry (ISAK) recommendations [30]. The girths of the upper arm, leg (measurement of the widest point of the calf) and thigh (measurement of the mid-thigh position), as well as skinfolds of the abdomen, iliac crest, subscapular, triceps, medial calf and front thigh, were measured in order to calculate skeletal muscle. Skinfolds and girths were measured in duplicate or triplicate if the difference between the first two measures was greater than 1.0 mm in skinfolds and 1.0 cm in girths. Hydration prior to body composition testing was not allowed. For the calculation of the fat weight and its percentage, the equation of Siri [31] was used. Muscle mass and percentage were calculated from the Lee equation [32]. Finally, bone mass and percentage were calculated using the Von Döbeln equation [33] modified by Rocha [34].
Strength test
Maximal dynamic strength was calculated in the first session, after resistance training and after the three-week detraining period. Initially, all subjects carried out a familiarization session, during which they were familiarized with the proper technique of all the exercises that were part of the circuit training. Approximately 2–3 days after the familiarization, the 1RM of bench press, EZ - Bar biceps curl, lying EZ - Bar French press, Pendlay row and half squat was estimated from a 3RM in normoxia using the predictive equation described by Brzycki [35] as a measure of maximal dynamic upper and lower-body strength and to determine the training load for all participants in the training intervention. Prior to testing, subjects warmed up on a stationary bicycle for 5 min at 75 W. Afterwards, subjects performed ten repetitions at 50% of the estimated 1RM. Then, each subject carried out 3–4 attempts with progressively increasing weights to achieve their 3RM, with a 3 min rest period between attempts. 1RM was estimated again under normoxic conditions after 7 weeks of resistance training and after a three-week detraining period at the same time of day as the baseline performance in each subject.
Resistance training programme
The resistance training programme lasted for 7 weeks and was the same for both the HYP and NOR groups. The training was carried out three times per week (Monday, Wednesday and Friday) for a total of twenty-one training sessions. The rest period was the same for both groups and there were approximately 48 h of rest between each training session. In hypoxic conditions, the subjects entered the chamber and were seated for 10 minutes to adapt the training environment. During all training sessions subjects started with a general warm-up, which involved sub-maximal cycling on a stationary bike for 5 min at 75 W while maintaining 75–100 rpm, followed by 5 min of active stretching of all major muscle groups, performed before the workout. Also, a specific warm-up consisting of 1 set of 10 repetition at 50% 1RM with 1 min rest in all exercises was completed. Subjects then performed 3 sets of all the exercises with a load of 65% 1RM to failure with 90 s of rest between sets during the first seven training sessions. The order of the exercises was: barbell bench press, EZ-Bar biceps curl, lying EZ-Bar French press, Pendlay row and half squat. Training loads were increased individually each 7 training sessions in both groups (1–7 = 65%; 8–14 = 75%; 15–21 = 80%). The total volume of repetitions was calculated as a control variable without observing significant differences between interventions (HYP: 52.895 reps vs. NOR: 54.863 reps). In addition, the average duration for each of the twenty-one sessions in the HYP group was 60 ± 5 min (total altitude exposure over 7 weeks = 21 hours) and the mean simulated altitude was 4 300 ± 50 m.
Statistical analysis
The statistical analysis was carried out with SPSS 22.0 computer software for Windows. The Shapiro-Wilk test was applied in order to verify a normal distribution of data and Levene´s test was used to assess the homogeneity of variance. A two-way analysis of variance with repeated measures and Bonferroni post hoc was used to investigate differences in variables in the two conditions (normoxia vs. hypoxia). The significance level was set at p ≤ 0.05, with a confidence level of 95%. Mean and standard deviations (SD) were used as descriptive statistics. A two-way fixed intraclass correlations (ICCs) reliability model, with 95% confidence intervals, was calculated on the total score to assess the reliability for body composition, haematological and strength testing across the three time points (Initial, Final and Detraining) [36]. Moreover, standard error of measurement (% SEM) was analysed to determine how much difference must exist to detect a real variation in the variables over time [36]. The statistical power was calculated at posteriori. The sample size (n=32) was large enough to obtain a statistical power greater than 0.8, based on the effect size [37] in the following variables under hypoxia: arm girth (ES: 0.49); bench press (ES: 1.06); biceps curl (ES: 1.91); French press (ES: 1.76); Pendlay row (ES: 1.57); half squat (ES: 2.14), and normoxia: bench press (ES: 1.30); biceps curl (ES: 0.56); French press (ES: 1.23); Pendlay row (ES: 2.20); half squat (ES: 2.27), with an alpha significance level of 0.05.
RESULTS
Values of body composition, anthropometry and haematological variables are shown in Table 1 for hypoxia and normoxia groups, respectively. There was a main effect of time on muscle percentage (F = 4.689; p = 0.036), fat percentage (F = 5.117; p = 0.012), arm girth (F = 25.057; p = 0.001), leg girth (F = 27.484; p = 0.001) and thigh girth (F = 56.889; p = 0.001).
TABLE 1.
Body composition, anthropometric and parameters for both groups of training
| Hypoxia Group | Normoxia Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Det | ICC | % SEM | Initial | Final | Det | ICC | % SEM | |
| Weight (Kg) | 74.68±12.89 | 74.94±12.88 | 75.01±11.89 | 0.99 | 1.67 | 81.14±11.72 | 81.94±10.70 | 81.90±10.75 | 0.99 | 1.35 |
| BMI (Kg/m2) | 24.28±3.80 | 24.36±3.85 | 24.41±3.66 | 0.99 | 1.54 | 26.65±4.45 | 26.56±4.00 | 26.56±4.09 | 0.99 | 1.57 |
| % muscle | 47.31±2.23 | 48.16±2.08a* | 47.53±2.43 | 0.95 | 1.00 | 47.02±2.31 | 47.20±2.28* | 46.80±2.65 | 0.95 | 1.08 |
| % fat | 12.88±4.09 | 12.00±3.68a* | 13.02±4.02 | 0.97 | 5.56 | 14.97±2.46 | 15.16±2.18* | 15.04±2.27 | 0.97 | 2.73 |
| % bone | 15.53±2.35 | 15.69±2.29 | 15.33±2.06 | 0.96 | 2.65 | 13.71±3.04 | 13.70±3.12 | 14.05±3.50 | 0.96 | 4.29 |
| Arm girth (cm) | 28.84±2.40 | 30.00±2.38a | 29.71±2.33b,c | 0.99 | 0.80 | 31.66±3.45 | 33.01±3.43a | 32.53±3.35b | 0.99 | 1.05 |
| Leg girth (cm) | 36.90±3.00 | 37.37±2.87a | 36.93±2.98c | 0.99 | 0.79 | 38.32±3.34 | 39.12±3.22a | 38.75±3.19b | 0.99 | 0.83 |
| Thigh girth (cm) | 49.21±5.25 | 50.34±5.26a | 50.00±5.36b,c | 0.99 | 1.06 | 55.16±6.04 | 56.21±5.85a | 55.56±5.97c | 0.99 | 1.07 |
| Haemoglobin (g/Dl) | 15.11±1.00 | 15.26±1.03 | 15.37±0.88 | 0.83 | 2.62 | 15.06±1.16 | 15.11±0.92 | 14.75±1.04 | 0.83 | 2.86 |
| Haematocrit (%) | 44.98±2.67 | 44.64±2.71 | 45.97±2.47* | 0.74 | 2.93 | 44.96±3.14 | 44.70±2.14 | 43.96±2.61* | 0.74 | 2.99 |
Significant difference (p< 0.05) between initial and final measurement. Results are means ±SD
Significant difference (p< 0.05) between initial and detraining measurement. Results are means ±SD
Significant difference (p< 0.05) between final and detraining measurement. Results are means ± SD
Significant differences (p< 0.05) between groups
ICC: interclass correlation coefficient; %SEM: standard error of measurement percentage.
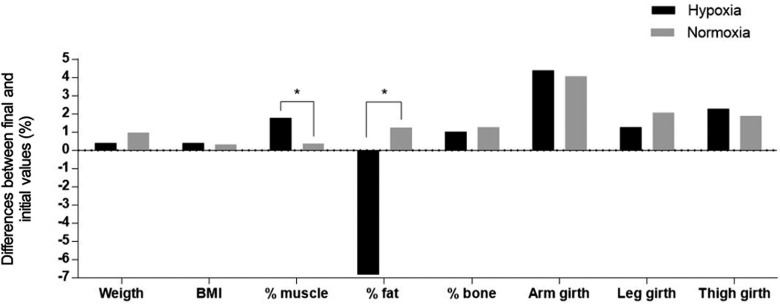
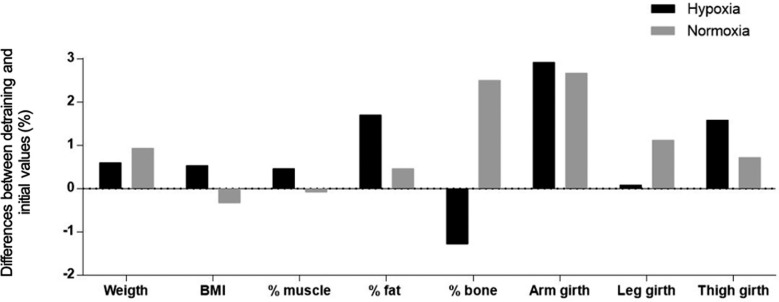
In addition, interaction effects of training group x time on muscle percentage (F = 3.455; p = 0.038; Δ1.80%; ICC = 0.95; %SEM = 1.00) and fat percentage (F = 5.931; p = 0.021; Δ-6.83%; ICC = 0.97; %SEM = 5.56) were found in the hypoxia group compared to the normoxia group (Figure 1). However, no significant changes were observed between groups or when comparing initial and detraining values (Figure 2).
FIG. 1.
Pooled data for percentage of change on body composition and anthropometrics values in both groups comparing final and initial measurements.
FIG. 2.
Pooled data for percentage of change on body composition and anthropometrics values in both groups comparing detraining and initial measurements.
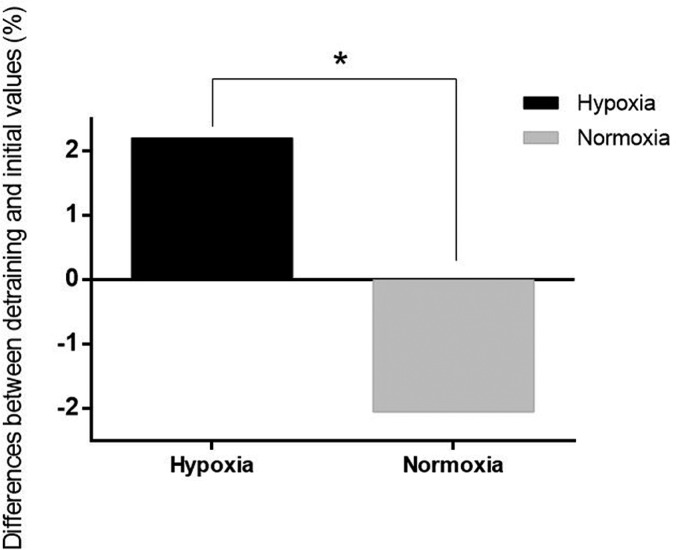
Regarding Hb values, no significant differences were observed in either group after the training and detraining periods. Nevertheless, an interaction effect of training group x time was observed in the hypoxia group for Hct values during the detraining period (F = 3.839; p = 0.027; Δ2.20%; ICC = 0.74; %SEM = 2.93) compared with the normoxia group (Δ-2.22%; ICC = 0.74; %SEM = 2.99) (Figure 3).
FIG. 3.
Pooled data for percentage of change on haematocrit in both groups comparing detraining and initial measurements.
Muscular performance was evaluated by 3RM estimated tests. Muscular performance is shown in Table 2. It increased for all of the exercises after training in both groups (p<0.05). However, an interaction effect of training group x time was observed in the hypoxia group for biceps curl (F = 8.173; p = 0.008; Δ25.79%; ICC = 0.97; %SEM = 2.25) compared to the normoxia group (Δ11.12%; ICC = 0.97; %SEM = 3.38) after training and detraining periods.
TABLE 2.
1RM values for both groups of training based on initial differences.
| Hypoxia Group | Normoxia Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Det | ICC | % SEM | Initial | Final | Det | ICC | % SEM | |
| 1RM bench pres (Kg) | 63.18±10.36 | 76.37±14.44a | 71.37±13.49bc | 0.96 | 3.39 | 62.25±12.51 | 80.12±14.89a | 74.37±14.40bc | 0.96 | 3.60 |
| 1RM biceps curl (Kg) | 35.12±3.96* | 44.18±5.54a* | 40.43±4.93b | 0.97 | 2.25 | 43.87±7.73* | 48.75±9.60a* | 44.62±7.5c | 0.97 | 3.38 |
| 1RM French press (Kg) | 30.43±5.54 | 39.68±4.96a | 37.75±5.24bc | 0.90 | 5.91 | 33.81±8.60 | 43.87±7.73a | 41.87±6.79bc | 0.84 | 7.83 |
| 1RM pendlay row (Kg) | 74.93±7.56 | 86.87±7.66a | 81.43±5.35bc | 0.84 | 3.33 | 82.06±6.73 | 92.50±2.75a | 87.16±5.73bc | 0.84 | 2.28 |
| 1RM half squat (Kg) | 108.43±7.42 | 139.37±21.47a | 125.31±13.53bc | 0.86 | 4.29 | 112.31±16.77 | 146.06±13.03a | 126.87±14.50bc | 0.86 | 4.44 |
Significant difference (p< 0.05) between initial and final measurement. Results are means ±SD
Significant difference (p< 0.05) between initial and detraining measurement. Results are means ±SD
Significant difference (p< 0.05) between final and detraining measurement. Results are means ± SD
Significant differences (p< 0.05) between groups
ICC: interclass correlation coefficient; %SEM: standard error of measurement percentage.
DISCUSSION
The present study examines the physical performance, body composition and haematological responses after 7 weeks of an IHRT. In addition, to the best of our knowledge, this is the first study that has included a detraining period of 3 weeks under normoxic conditions after hypoxia exposure.
Regarding body composition, the hypoxia group improved both the body composition values and muscle perimeters. In contrast, the normoxia group only increased the values of muscle perimeters. Along these same lines, Kurobe et al. [11] reported greater changes in triceps brachii thickness in normoxia and hypoxia groups after 8 weeks of unilateral arm training. Furthermore, Kon et al. [4] reported a significant increase in body mass, lean body mass and a reduction in body fat after 8 weeks of resistance training in normoxia and hypoxia groups. This appears logical because, as has been established, muscle hypertrophy becomes evident within a short period of resistance training (i.e. 4−8 weeks of training) [38].
Compared to the differences between groups, greater improvements in lean body mass and loss in fat mass were observed after IHRT compared to normoxia training. In line with this, Yan et al. [9] reported a greater increase in lean body mass with a high level (FiO2 = 12.6%) and moderate level of hypoxia (FiO2 = 16%) after 5 weeks’ resistance training of barbell back squat compared to the normoxia group. In addition, Nishimura et al. [7] reported a significant increase in the muscle cross-sectional area (CSA), which was only observed in the hypoxia group after 6 weeks of bilateral elbow resistance training. Moreover, Chycki et al. [12] observed significantly increased lean mass and reduction in fat mass in the hypoxia group when compared to the same training in normoxia. In addition, this study reported an increase in the secretion of insulin growth factor (IGF-1). In line with this, it has been established that secretion of IGF-1 after strength training stimulates the proliferation of satellite cells located in muscle fibres [39]. Moreover, it has been previously shown that training in normobaric hypoxia conditions does not cause a decrease in the concentration of nitric oxide in plasma [40]. Thus, nitric oxide is a potent reactive species that mediates the activation of skeletal muscle satellite cells, which contributes directly to the mechanisms that generate hypertrophy [41]. In our study, direct measurement of anabolic hormonal responses was not carried out. However, several studies have shown that hypoxic environments increase the hormonal secretion more than normoxic environments [4,12,42], so we can speculate that the low partial fraction of oxygen used (FiO2 = 13%) caused greater secretion of circulating anabolic hormones in the hypoxia group, increasing the muscle percentage after 7 weeks of training.
With regard to the fat loss observed in the present study, recent studies show that strength exercises with loads can be more effective than aerobic exercise [43], particularly in reducing body fat [44]. Moreover, the addition of a hypoxic stimulus to strength training induces the activation of HIF-1α, which causes changes in cell metabolism and the activation of different peripheral pathways in conjunction with the central nervous system, leading to greater metabolic and endocrine responses that could affect energy expenditure and energy balance [13,44]. In line with this, it has been shown that HIF-1α can modulate responses to fat loss by increasing lipid metabolism [45]. Despite this, Ho et al. [10] did not observe significant changes in body weight, lean body mass or fat mass after 6 weeks of resistance training under normobaric hypoxia. Similarly, Kurobe et al. [11] reported the same decrease in fat mass for both experimental groups after 8 weeks of unilateral arm training under normobaric hypoxia. However, these studies have used different training protocols in terms of the number of series and repetitions to be performed, training intensity and the number of exercises proposed. In contrast, in our study, a full-body workout routine of five exercises was performed. It has been previously reported that the amount of muscle mass involved in a training programme significantly affects acute metabolic demands and anabolic hormonal response, directly influencing the increase in lean body mass and reductions in body fat [46]. Furthermore, it is possible that the type of muscle trained and protocol used condition the response in the reduction of fat mass, due to the fact that each muscle group is unique in its capacity for trainability and adaptability [47].
In relation to muscle strength, resistance training is a potent factor for increasing muscular strength and promoting muscle hypertrophy [48]. Both training protocols, regardless of the oxygenation condition, achieved improvements in the 1RM in all exercises, suggesting that loads over 65% 1RM were sufficient to induce muscular strength gains after long-term resistance exercise [46]. However, with the exception of strength in the biceps curl, no significant inter-group difference was observed. Although Kon et al. [4,49] have reported that hypoxia is a potent factor for the enhancement of GH responses to moderate intensity resistance exercise, other studies have not found greater increases in muscle strength after performing the training under hypoxic conditions [7,10,11,50]. These results may indicate that other training variables (such as intensity, recovery times, training status or muscle group involved) could play a more important role than hypoxia in achieving improvements in muscle strength.
In terms of inter-set rest periods, Scott et al. [51] hypothesized that low and moderate-load IHRT might only provide added benefit when relatively brief inter-set rest periods are used (30−60 s). The inter-set rest period time in the present study (90 s between sets) could explain the lack of improvement in muscle strength of the majority of the muscle groups investigated, as in previous studies [4,10]. However, the training protocols of all the previous studies established a fixed number of repetitions per set. In contrast, in our training protocol we reached muscle failure in each series. Consequently, we established a relatively short inter-set rest period in order to increase intramuscular metabolic stress, because the training focused on hypertrophic responses [52]. Nonetheless, it is possible that this recovery between sets during this moderate-load training will limit the accumulation of metabolites, attenuating any hypoxia-mediated benefits during this type of training [51], not allowing high levels of tension in successive series, and so the training stimulus would not be effective enough to trigger the desired adaptations [11,24]. In agreement with these explanations, the gain in strength in the biceps curl could be attributed to the fact that the initial levels of kilograms raised in the biceps curl were lower in the hypoxia group. This experimental group could have had a greater room for improvement throughout the protocol, or the gain in muscle strength could even be related to the individual trainability and adaptability of each muscle group [21]. On the other hand, the level of hypoxia used in IHRT protocols can also influence long-term muscle adaptations and gains in muscle strength [9].
According to haematological variables, haemoglobin values did not change significantly with either training protocol. This is in agreement with previous studies that did not find changes in this variable after a resistance training programme under normobaric [4] and hypobaric [29] hypoxia. It is possible that only 3 hours a week of training for 7 weeks in normobaric hypoxia is not a sufficient stimulus to stimulate erythropoiesis. In addition, the haematocrit followed the same trend as haemoglobin values. However, our results showed that haematocrit values remained elevated (Δ 2.20%) in the hypoxia group as compared to the normoxia group after the detraining period. Although Bonetti et al. [54] established that the haematocrit needs a period of time to form once the hypoxia training sessions have finished, this slight increase may not be useful in practical terms because the haematocrit values of the normoxia group decreased after detraining. We could establish that the differences in this parameter were due to the fact that the hypoxia group suffered an increase in haematocrit through dehydration. This dehydration may be a consequence of haemoconcentration, which has been associated with high-intensity exercise [53].
Regarding the detraining period, none of the previous studies that have carried out several weeks of IHRT have evaluated the influence of this period on performance. In this context, it has been established that a detraining period reduces the adaptions induced by training [54]. However, the detraining period applied in the current study provoked a non-significant reduction in body composition after 3 weeks without training. In line with this, Hortobágyi et al. [55] observed a significant decrease in size of fast-twitch muscle fibres after only 2 weeks of detraining in power lifters and highly trained soccer players. Comparing both experimental groups, the muscle percentage decreased to a lesser extent in the hypoxia group, which experienced greater gains after 7 weeks of training. It is possible that the greater metabolic stress, induced by the addiction of a hypoxic stimulus to strength training [18,56], allowed the muscle mass gains experienced by this group to be maintained. In addition, an increase in the fat percentage was observed with respect to the end of the training in the hypoxia group. Similarly, Häkkinen et al. [57] reported a slight increase in body fat percentage after 12 weeks of detraining in highly strength-trained subjects. Even though appetite loss (anorexia phenomenon) has long been recognized as a side effect of high altitude [58], this effect remains unclear under normobaric hypoxic conditions as yet. However, Wasse et al. [59] reported a significant reduction in energy intake at a buffet meal during normobaric hypoxia exposure (FiO2 = 12.7%) compared with normoxia. Additionally, they observed that short-term hypoxic exposure suppressed the plasma acylated ghrelin concentrations, which are directly implicated in the anorexia phenomenon. Nonetheless, an increase in fat percentage in our study due to the increase in appetite when hypoxic stimulus finished could be very speculative due to no marker having been measured; nor was caloric intake monitored throughout the study.
In the present study, the detraining period decreased the performance in all the exercises of the training programme, although the strength gains remained elevated with respect to the initial levels. By comparison, Kraemer et al. [60] observed maintenance of the maximum dynamic strength in individuals trained after 6 weeks of detraining. However, Häkkinen et al. [57] reported a decrease in 1RM squat in Olympic lifters after 4 weeks of detraining. It is possible that the magnitude of maintenance or loss of performance after a period of detraining may be influenced by the level or initial physical condition of the athlete. However, further research to elucidate this point is needed.
Limitations
Several limitations of this study need to be addressed. The first is the impossibility of using dual X-ray absorptiometry (DEXA) for measuring changes in body composition. Second, we could not measure the hormonal response to resistance training in hypoxia, which has been established to influence directly the hypertrophic and strength responses. Third, the caloric intake of participants was not checked during the 8 weeks of training, which may have affected the results.
CONCLUSIONS
In this study, 7 weeks of IHRT under high-level hypoxia (FiO2 = 13%) caused a greater increase in muscle mass and a greater reduction of the fat mass in untrained subjects. Furthermore, IHRT improved strength performance after 7 weeks of training in untrained participants, but without inter-group differences. These findings may be of interest to coaches and other researchers for optimizing performance through improvements of body composition in men previously untrained in resistance exercise.
Acknowledgment
The authors would like to thank the Council of Extremadura (Fundings for research groups GR18003). No conflicts of interest, financial or otherwise, are declared by the authors. The experiments comply with the current laws of the country in which they were performed.
Declarations of interest
none
REFERENCES
- 1.Álvarez-Herms J, Julià-Sánchez S, Corbi F, et al. Changes in heart rate recovery index after a programme of strength/endurance training in hypoxia. Apunt Med l’Esport. 2012;47:23–29. [Google Scholar]
- 2.Burtscher M, Nachbauer W, Baumgartl P, et al. Benefits of training at moderate altitude versus sea level training in amateur runners. Eur J Appl Physiol Occup. Physiol. 1996;74:558–563. doi: 10.1007/BF02376773. [DOI] [PubMed] [Google Scholar]
- 3.Levine BD, Stray-Gundersen J. “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol. 1997;83:102–112. doi: 10.1152/jappl.1997.83.1.102. [DOI] [PubMed] [Google Scholar]
- 4.Kon M, Ohiwa N, Honda A, et al. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiol Rep. 2014;2:1–13. doi: 10.14814/phy2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott BR, Slattery KM, Sculley D V, et al. Hypoxia and resistance exercise: A comparison of localized and systemic methods. Sport Med. 2014;44:1037–1054. doi: 10.1007/s40279-014-0177-7. [DOI] [PubMed] [Google Scholar]
- 6.Manimmanakorn A, Hamlin MJ, Ross JJ, et al. Effects of low-load resistance training combined with blood flow restriction or hypoxia on muscle function and performance in netball athletes. J Sci Med Sport. 2013;16:337–342. doi: 10.1016/j.jsams.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura A, Sugita M, Kato K, et al. Hypoxia increases muscle hypertrophy induced by resistance training. Int J Sport Physiol Perform. 2010;5:497–508. doi: 10.1123/ijspp.5.4.497. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann B, Kinscherf R, Borisch S, et al. Effects of low-resistance/high-repetition strength training in hypoxia on muscle structure and gene expression. Pflügers Arch. 2003;446:742–751. doi: 10.1007/s00424-003-1133-9. [DOI] [PubMed] [Google Scholar]
- 9.Yan B, Lai X, Yi L, et al. Effects of Five-Week Resistance Training in Hypoxia on Hormones and Muscle Strength. J strength Cond Res. 2016;30:184–193. doi: 10.1519/JSC.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 10.Ho JY, Kuo TY, Liu KL, et al. Combining normobaric hypoxia with short-term resistance training has no additive beneficial effect on muscular performance and body composition. J Strength Cond Res. 2014;28:935–941. doi: 10.1519/JSC.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 11.Kurobe K, Huang Z, Nishiwaki M, et al. Effects of resistance training under hypoxic conditions on muscle hypertrophy and strength. Clin Physiol Funct Imaging. 2015;35:197–202. doi: 10.1111/cpf.12147. [DOI] [PubMed] [Google Scholar]
- 12.Chycki J, Czuba M, Gołaś A, et al. Neuroendocrine Responses and Body Composition Changes Following Resistance Training under Normobaric Hypoxia. J Hum Kinet. 2016;53:91–98. doi: 10.1515/hukin-2016-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inness MW, Billaut F, Walker EJ, et al. Heavy Resistance Training in Hypoxia Enhances 1RM Squat Performance. Front Physiol. 2016;7:502. doi: 10.3389/fphys.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayo B, Miles C, Sims S, et al. The Effect of Resistance Training in a Hypoxic Chamber on Physical Performance in Elite Rugby Athletes. High Alt Med Biol. 2017;19:28–34. doi: 10.1089/ham.2017.0099. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Campo DJ, Rubio-Arias JÁ, Freitas TT, et al. Acute Physiological and Performance Responses to High-Intensity Resistance Circuit Training in Hypoxic and Normoxic Conditions. J Strength Cond Res. 2017;31:1040–1047. doi: 10.1519/JSC.0000000000001572. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Campo DJ, Scott BR, Alcaraz PE, et al. The efficacy of resistance training in hypoxia to enhance strength and muscle growth: A systematic review and meta-analysis. Eur J Sport Sci. 2018;18:92–103. doi: 10.1080/17461391.2017.1388850. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Guardado I, Ramos-Campo DJ, Olcina GJ, et al. Effects of high-intensity resistance circuit-based training in hypoxia on body composition and strength performance. Eur J Sport Sci. 2019:1–11. doi: 10.1080/17461391.2018.1564796. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Guardado I, Sánchez-Ureña B, Olcina G, et al. Bench press performance during an intermittent hypoxic resistance training to muscle failure. J Sports Med Phys Fitness. 2019:59. doi: 10.23736/S0022-4707.18.08940-5. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer WJ, Ratamess NA. Endocrine Responses and Adaptations to Strength and Power Training. Strength Power Sport. 2003:361–386. [Google Scholar]
- 20.Mujika I, Padilla S. Detraining: Loss of training-induced physiological and performance adaptations. Part II: Long term insufficient training stimulus. Sport Med. 2000;30:145–154. doi: 10.2165/00007256-200030030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Dick FW. Training at altitude in practice. Int J Sports Med. 1992:p. S203–6. doi: 10.1055/s-2007-1024640. [DOI] [PubMed] [Google Scholar]
- 22.Vogt M, Puntschart A, Geiser J, et al. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol. 2001;91:173–182. doi: 10.1152/jappl.2001.91.1.173. [DOI] [PubMed] [Google Scholar]
- 23.Hoppeler H, Vogt M, Weibel ER, et al. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- 24.Morton JP, Cable NT. Effects of intermittent hypoxic training on aerobic and anaerobic performance. Ergonomics. 2005;48:1535–1546. doi: 10.1080/00140130500100959. [DOI] [PubMed] [Google Scholar]
- 25.Knaupp W, Khilnani S, Sherwood J, et al. Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol. 1992;73:837–840. doi: 10.1152/jappl.1992.73.3.837. [DOI] [PubMed] [Google Scholar]
- 26.Gore CJ, Clark SA, Saunders PU. Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc. 2007:p. 1600–1609. doi: 10.1249/mss.0b013e3180de49d3. [DOI] [PubMed] [Google Scholar]
- 27.Geiser J, Vogt M, Billeter R, et al. Training high – Living low: Changes of aerobic performance and muscle structure with training at simulated altitude. Int J Sports Med. 2001;22:579–585. doi: 10.1055/s-2001-18521. [DOI] [PubMed] [Google Scholar]
- 28.Camacho-Cardenosa M, Camacho-Cardenosa A, Martinez Guardado I, et al. A new dose of maximal-intensity interval training in hypoxia to improve body composition and hemoglobin and hematocrit levels: a pilot study. J Sports Med Phys Fitness. 2017;57:60–69. doi: 10.23736/S0022-4707.16.06549-X. [DOI] [PubMed] [Google Scholar]
- 29.Álvarez-Herms J, Julià-Sánchez S, Corbi F, et al. A program of circuit resistance training under hypobaric hypoxia conditions improves the anaerobic performance of athletes. Sci Sport. 2016;31:78–87. [Google Scholar]
- 30.Stewart A, Marfell-Jones M, Olds T, et al. International standards for anthropometric assessment. Wellington, New Zealand: International Society for the Advancement of Kinanthropometry;; 2011. [Google Scholar]
- 31.Siri WE. Techniques for Measuring Body Composition. Washington, DC: National Academy of Science;; 1961. Body composition from fluid spaces and density: analysis of methods; pp. 223–244. [Google Scholar]
- 32.Lee RC, Wang Z, Heo M, et al. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 33.Von Döbeln W. Symposia of the Swedish nutrition foundation. Occurrence, causes and prevention of overnutrition. Upsala: Almquist and Wilksell;; 1964. Determination of body constitutions. [Google Scholar]
- 34.Rocha MS. Peso ósseo do brasileiro de ambos os sexos de 17 a 25 años. Arq Anat Antropol. 1975;1:445–451. [Google Scholar]
- 35.Brzycki M. Strength Testing-Predicting a One-Rep Max from Reps-to-Fatigue. JOPERD. 1993;64:88–90. [Google Scholar]
- 36.Weir JP. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J Strength Cond Res. 2005;19:231. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Quantitative Methods in Psychology: A Power Primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Phillips SM. Short-Term Training: When Do Repeated Bouts of Resistance Exercise Become Training? Can J Appl Physiol. 2000;25:185–193. doi: 10.1139/h00-014. [DOI] [PubMed] [Google Scholar]
- 39.Hawke TJ. Muscle stem cells and exercise training. Exerc Sport Sci Rev. 2005:63–68. doi: 10.1097/00003677-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Faiss R, Pialoux V, Sartori C, et al. Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia. Med Sci Sports Exerc. 2013;45:253–260. doi: 10.1249/MSS.0b013e31826d5aa2. [DOI] [PubMed] [Google Scholar]
- 41.Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 42.Yan B, Lai X, Yi L, Wang Y, Hu Y. The effects of 5-week resistance training in hypoxia on hormones and muscle strength. J Strength Cond Res. 2016;30:184–193. doi: 10.1519/JSC.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 43.Wernbom M, Augustsson J, Thome R. The Influence of Frequency, Intensity, Volume and Mode of Strength Training on Whole Muscle Cross-Sectional Area in Humans. Training. 2007;37:225–264. doi: 10.2165/00007256-200737030-00004. [DOI] [PubMed] [Google Scholar]
- 44.McInnis KJ, Franklin BA, Rippe JM. Counseling for physical activity in overweight and obese patients. Am Fam Physician. 2003;67:1249–1256. [PubMed] [Google Scholar]
- 45.Workman C, Basset FA. Post-metabolic response to passive normobaric hypoxic exposure in sedendary overweight males: A pilot study. Nutr Metab. 2012:9. doi: 10.1186/1743-7075-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraemer WJ, Ratamess NA. Fundamentals of Resistance Training: Progression and Exercise Prescription. Med Sci Sports Exerc. 2004;36:674–688. doi: 10.1249/01.mss.0000121945.36635.61. [DOI] [PubMed] [Google Scholar]
- 47.Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Med Sci Sport Exerc. 1999:p. 38–45. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Staron RS, Karapondo DL, Kraemer WJ, et al. Skeletal-muscle adaptations during early phase of heavy resistance training in men and women. J Appl Physiol. 1994;76:1247–1255. doi: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 49.Kon M, Ikeda T, Homma T, et al. Effects of low-intensity resistance exercise under acute systemic hypoxia on hormonal responses. J Strength Cond Res. 2012;26:611–617. doi: 10.1519/JSC.0b013e3182281c69. [DOI] [PubMed] [Google Scholar]
- 50.Friedmann B, Kinscherf R, Borisch S, et al. Effects of low-resistance/high-repetition strength training in hypoxia on muscle structure and gene expression. Pflügers Arch Eur J Physiol. 2003;446:742–751. doi: 10.1007/s00424-003-1133-9. [DOI] [PubMed] [Google Scholar]
- 51.Scott BR, Slattery KM, Dascombe BJ. Intermittent hypoxic resistance training: Is metabolic stress the key moderator? Med Hypotheses. 2015;84:145–149. doi: 10.1016/j.mehy.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Bird SP, Tarpenning KM, Marino FE. Designing resistance training programmes to enhance muscular fitness: A review of the acute programme variables. Sport Med. 2005;35:841–851. doi: 10.2165/00007256-200535100-00002. [DOI] [PubMed] [Google Scholar]
- 53.Van Beaumont W, Underkofler S, Van Beaumont S. Erythrocyte volume, plasma volume, and acid-base changes in exercise and heat dehydration. J Appl Physiol Respir Environ Exerc Physiol. 1981;50:1255–1262. doi: 10.1152/jappl.1981.50.6.1255. [DOI] [PubMed] [Google Scholar]
- 54.Mujika I, Padilla S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part I: Short term insufficient training stimulus. Sport Med. 2000;30:145–154. doi: 10.2165/00007256-200030030-00001. [DOI] [PubMed] [Google Scholar]
- 55.Hortobágyi T, Houmard JA, Stevenson JR, et al. The effects of detraining on power athletes. Med Sci Sport Exerc. 1993;25:929–935. [PubMed] [Google Scholar]
- 56.Ramos-Campo DJ, Rubio-Arias JA, Dufour S, et al. Biochemical responses and physical performance during high-intensity resistance circuit training in hypoxia and normoxia. Eur J Appl Physiol. 2017;117:809–818. doi: 10.1007/s00421-017-3571-7. [DOI] [PubMed] [Google Scholar]
- 57.Häkkinen K, Alen M, Komi P V. Changes in isometric force-and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol Scand. 1985;125:573–585. doi: 10.1111/j.1748-1716.1985.tb07760.x. [DOI] [PubMed] [Google Scholar]
- 58.Yingzhong Y, Droma Y, Rili G, et al. Regulation of body weight by leptin, with special reference to hypoxia-induced regulation. Intern Med. 2006;45:941–946. doi: 10.2169/internalmedicine.45.1733. [DOI] [PubMed] [Google Scholar]
- 59.Wasse LK, Sunderland C, King JA, et al. Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J Appl Physiol. 2012;112:552–559. doi: 10.1152/japplphysiol.00090.2011. [DOI] [PubMed] [Google Scholar]
- 60.Kraemer WJ, Koziris LP, Ratamess NA, et al. Detraining produces minimal changes in physical performance and hormonal variables in recreationally strength-trained men. J Strength Cond Res. 2002;16:373–382. [PubMed] [Google Scholar]