Abstract
This paper describes a new technology that uses 1-µm-resolution optical coherence tomography (µOCT) to obtain cross-sectional images of intracellular dynamics with dramatically enhanced image contrast. This so-called dynamic µOCT (d-µOCT) is accomplished by acquiring a time series of µOCT images and conducting power frequency analysis of the temporal fluctuations that arise from intracellular motion on a pixel-per-pixel basis. Here, we demonstrate d-µOCT imaging of freshly excised human esophageal and cervical biopsy samples. Depth-resolved d-µOCT images of intact tissue show that intracellular dynamics provides a new contrast mechanism for µOCT that highlights subcellular morphology and activity in epithelial surface maturation patterns.
1. Introduction
Despite significant advances in microscopy, the vast majority of techniques obtain static images of cells. Imaging living cells opens up a new, functional dimension of evaluation, where organelle and intracellular molecular movements inform on pathophysiology in a manner that cannot be achieved by static snapshots in time. The use of fluorescence microscopy to track either fluorescently transfected organelles [1] or actively perturbed microinjected fluorescent particles within cells [2] are examples of recent techniques that probe intracellular dynamics. In addition to perturbing the biological system by adding exogenous labels, challenges interrogating a large ensemble of cells have hindered the scaling of these methodologies for studying intact tissue samples where heterogeneous cell populations exist.
Coherence-gating imaging techniques [3,4] are microscopy approaches that overcome these limitations. They do not require labeling, as they derive their image contrast from tissue reflectivity at refractive index interfaces within the sample. Yet, small variations in refractive indices within cells cause minute reflectivity changes that are difficult to ascertain in a single image; thus, images have relatively low contrast. By taking advantage of the differences in the motion of various subcellular compartments, temporo-spatial signal analysis has recently been shown to significantly enhance intracellular visualization. This principle was elegantly demonstrated using dynamic full-field optical coherence tomography (FFOCT). [5,6] FFOCT employs a high-power microscope objective and low coherence optical interferometry to acquire high-resolution transverse (en face) images of natural tissue reflectance at a given depth. Through computation of the standard deviation or autocorrelation of time-dependent signals that arise from intracellular activity, en face dynamic FFOCT images revealed clear intracellular features that were otherwise obscured in static images. [5,6]
While dynamic FFOCT provides a new and exciting avenue for label-free cellular imaging, this technique acquires images in the transverse plane. It would be much more desirable to obtain depth-resolved, cross-sectional images, as epithelial tissues mature vertically and aberrations in this maturation process are critical for disease understanding and diagnosis. Optical coherence tomography (OCT), is a coherence-gated imaging technique that obtains cross-sectional images of tissue at a resolution typically between 5-20 µm. [7] Similar to dynamic FFOCT, dynamic OCT measures changes in cross-sectional OCT images taken at the same location over time. Dynamic OCT has shown to inform on tissue viability, mucus viscosity, cell migration, and remodeling. [8–11] Because of its relatively low spatial resolution, dynamic OCT cannot see inside individual cells.
This work describes a new technology that we term dynamic micro-optical coherence tomography (d-µOCT). µOCT is a very high-resolution form of OCT that has resolutions of approximately 2 × 2 µm (lateral) x 1 µm (axial) [12] µOCT has been shown to be capable of visualizing cross-sectional images of cells at an unprecedented level of detail [13–17]. With d-µOCT, new microscopic information and enhanced contrast emerge by conducting power frequency analysis of the temporal fluctuations that arise from intracellular motion on a pixel-per-pixel basis. Because d-µOCT has a depth scan priority, it can uniquely probe subcellular dynamics over a very wide (0-10kHz) frequency range. Here, we demonstrate d-µOCT in esophageal and cervical biopsy samples, showing that it highlights depth-resolved, intracellular dynamics of intact tissues, a potentially impactful capability for the biomedical sciences and clinical diagnosis.
2. Materials and methods
Human upper gastrointestinal biopsies (Protocol # 2015P000328) and cervical biopsies (Protocol # 2016P001988) were obtained from consenting study participants in accordance with IRB-approved protocols. Each of the biopsies was immersed in cell culture medium immediately after excision to preserve tissue viability. In preparation for d-µOCT imaging, the biopsies were placed on a glass slide and, by means of a dual-axis goniometer, the luminal side of the tissue was tilted with respect to the imaging beam to avoid specular reflection. All imaging was performed at room temperature (25 °C) and samples were kept moist over the course of an imaging session by the addition of small amounts of cell culture medium on the tissue as necessary. Despite efforts to keep the sample well-moistened, image artifacts arising from moisture evaporation were occasionally noticeable in the real-time display of the images, especially for small biopsy samples. Therefore, certain samples were performed with the luminal side of the tissue placed in contact with a glass slide to minimize moisture loss during imaging. After d-µOCT imaging, the imaged region was marked with a dye before being fixed in formalin. The samples were then processed to obtain 5 µm thick hematoxylin and eosin (H&E) stained histology slides corresponding to the region imaged by d-µOCT.
The previously described µOCT benchtop system microscope [12] provides cross-sectional images of intact tissues with a resolution of 2 × 2 µm (lateral) x 1 µm (axial) to a depth of 300 µm. Imaging was performed using an A-line (depth-dependent reflectivity profile) rate of 20.48 kHz and a 1 mm lateral scan was achieved by scanning the beam across the sample using a galvanometer mirror. A custom-written data acquisition program capable of real-time display of image contrast based on a pixel-by-pixel standard deviation approximation was used to facilitate the identification of viable portions of the tissue samples. Two different imaging schemes that probed the dynamics of distinct frequency ranges were utilized to yield d-µOCT images. To probe frequencies up to 20 Hz, the imaging beam was scanned repeatedly across a 1 mm region of interest at 40 Hz, typically over a duration of 25 s. This yielded 1000 cross-sectional images of the same location, each consisting of 512 A-lines. To probe frequencies up to 10.24 kHz, the imaging beam traversed across the same 1 mm lateral range in a stepwise fashion, stopping at 512 equally-spaced positions where 1000 A-lines were acquired at a rate of 20.48 kHz. Both scanning protocols took the same amount of time (25 s) to complete.
Even though the d-µOCT imaging time was reasonably short (25 s), images of tissue that underwent the first beam scanning scheme suffered nonlinear and spatially dependent motion drift on the order of 10’s of micrometers. Tissue motion was likely due to evaporation, thermal expansion, causing tissue settling during imaging. To compensate these motion artifacts, µOCT frames were locally normalized [18] and Gaussian filtered. Local normalization uses a moving kernel across the sample and normalizes the center pixel using the pixel values within the kernel. An elastic unwarping transformation matrix [19] was computed from these processed frames using the center frame (500) as the reference. These transformation matrices were then applied to the original µOCT data. To evaluate the effectiveness of this algorithm, we quantified the mean squared error (MSE) of the µOCT frames before and after motion correction, using the case with the most severe motion artifact (data used in Fig. 4). We quantified the error present in each frame of the µOCT video as the difference in intensity values between the frames (n = 1000) and the center frame on a pixel-by-pixel basis. The MSE of each frame was subsequently computed, with the regions outside the tissue excluded from the analysis. We found that the difference in MSE with (1084, 95% CI 1077-1092) and without (1127.2, 95% CI 1119-1135) motion correction was statistically significant (p < 0.0001 by Mann-Whitney test). Subsequently, with the assumption that the fluctuations are ergodic processes, an estimated power spectrum for each pixel in the µOCT dataset was obtained by employing a short-time Fourier transform with Welch’s method. [20] With this approach, time-dependent data was analyzed in shorter segments instead of in its entirety. Multiple segments, each a fifth in length (L = 200) as compared to the full temporal data and each overlapped by 50% with the next, were processed to derive multiple modified periodograms, Ik. For a given length of recorded data, the length of each segment and the amount of overlap determined the resultant spectral resolution and variance of the estimated power spectral density, . We employed processing steps that involved first applying a Hanning window, w, to each mean-subtracted segment before zero-padding it to achieve an array of 512 elements, Sx,y. A discrete Fourier transform (DFT) was then applied to each segment and the results were averaged to yield the power spectrum at that pixel. A hyperspectral data set with M = 256 equally-spaced frequency bins (fx,y) was obtained by performing this process for every pixel in the cross-sectional plane.
| (1) |
| (2) |
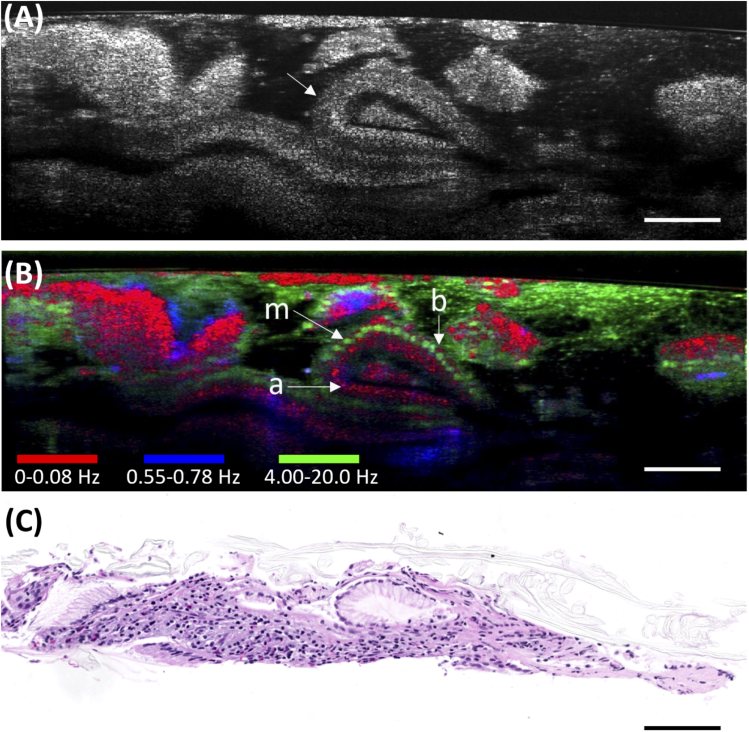
Fig. 4.
Images of a biopsy from a human gastroesophageal junction. (A) A 200-frame averaged µOCT image of the biopsy showing columnar glandular architecture (arrow). (B) Color-coded d-µOCT image demonstrating glands containing cells with low frequency regions at their base (b), high-frequency modulation in the middle (m), and low frequency apically (a). (C) A comparison with the histology of the biopsy showing mucous cells. d-µOCT color codes: Red 0-0.08 Hz, Blue 0.55-0.78 Hz, Green 4.00-20.0 Hz. Bars = 100 µm.
Based on these data acquisition parameters, the estimated power spectrum was computed from nine averages, thus effectively reducing the variance in the estimated power spectrum by that equivalent factor.
| (3) |
In both imaging schemes, similar processing methods were applied to the resultant time-dependent data to obtain 256 equally-spaced frequency bins that ranged from either 0–20 Hz or 0–10.24 kHz. The latter imaging scheme enabled the probing of cellular dynamics two orders of magnitude higher than the former, albeit at a lower frequency resolution.
To enable direct visualization of the distribution of the dominant fluctuation frequencies within the tissue, was further binned into three frequency ranges and was each assigned a color channel within an RGB image (e.g. red: 0-0.08 Hz, blue: 0.55-0.78 Hz, green: 4.00-20.00 Hz) to create a final d-µOCT image representation. In this initial study, the frequency range color assignment was selected empirically to accentuate subcellular and cellular features of the tissue and, thus varied between samples. All µOCT and d-µOCT images presented here were scaled to achieve an isotropic pixel aspect ratio, assuming a tissue refractive index of 1.4, to ensure accurate representation and to facilitate comparison with corresponding histological images. All image reformatting and analysis were conducted using ImageJ [21] and MATLAB (Mathworks Inc). Statistical analyses were performed using Prism 7.03 (GraphPad Software Inc.).
3. Results
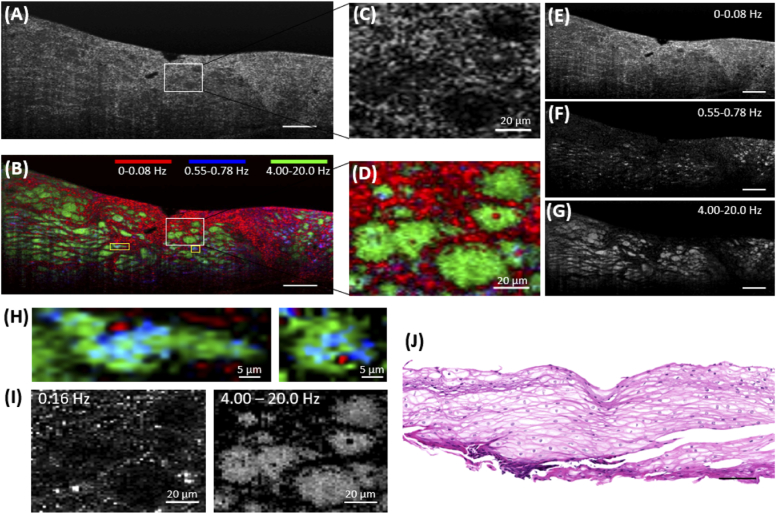
Functional imaging with d-µOCT was performed on biopsies obtained from subjects who were diagnosed with various esophageal disorders. Figure 1 shows images of an esophagus biopsy taken from a subject undergoing endoscopy for chronic gastroesophageal reflux disease (GERD). In the averaged (n = 200) standard µOCT image depicted in Fig. 1(A), some structures were discernible but not highly prominent due to insufficient refractive index contrast. Detailed visualization of cellular features was further hampered by the presence of speckle, which is a common source of noise in OCT images even after frame averaging. With d-µOCT [Fig. 1(B)], much greater cellular contrast was achieved. This increase in contrast could be explained by the differences in sub-cellular motion in separate tissue compartments, with motion of the optical scatters in the cytoplasm associated with higher frequencies than those of cell membranes and interstitial spaces. Organelles such as cell nuclei also became distinguishable using d-µOCT owing to their lower frequency content [Figs. 1(D), 1(H)]. In some cells, fluctuations over a moderate frequency range [Fig. 1(H), blue: 0.55-0.78 Hz] was seen surrounding the nucleus [Fig. 1(H), red: 0-0.08 Hz], possibly representing motion in the vicinity of perinuclear organelles. The use of power spectrum analysis and selection of suitable frequency ranges from the resultant hyperspectral data provided a means for selective visualization of different tissue structures. For instance, as illustrated in Fig. 1(I), cell membranes appeared brighter than the cytoplasm at 0.16 Hz, while the contrast was reversed at a higher frequency range of 4-20 Hz. In addition to providing detailed micro-structural information similar to the corresponding H&E stained histology [Fig. 1(J)], d-µOCT also informed on different intracellular motion rates on a single cell basis. For example, some of the suprabasal squamous cells exhibited higher frequency content than others [Figs. 2(A)–2(C)], indicating intracellular motion heterogeneity within the same cell types.
Fig. 1.
Images of a human esophageal biopsy. (A) A 200-frame averaged, standard µOCT image of the biopsy. (B) The pseudo-colored d-µOCT image shows numerous different cells exhibiting various intracellular motion-associated frequency content. (C) and (D) Magnified portions of the white ROIs shown in (A) and (B), respectively, from the same location in the sample. (E) to (G) Frequency maps of the human esophageal biopsy corresponding to the frequency ranges depicted in the red, blue, and green channels of (B), respectively. (H) Magnified views of the yellow ROIs in the d-µOCT image (B) showing cell cytoplasm (green), the nucleus (red), and a perinuclear region (blue), all exhibiting different frequency ranges. (I) d-µOCT images from the white ROI in (A) corresponding to different frequency ranges. (J) Corresponding histology image (H&E) of the biopsy sample. d-µOCT color codes: Red 0-0.08 Hz, Blue 0.55-0.78 Hz, Green 4.00-20.0 Hz. Bars = 100 µm unless indicated otherwise.
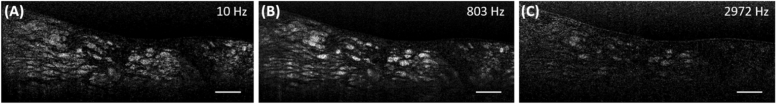
Fig. 2.
Intracellular dynamics probed over a wide frequency range. Frequency maps of the human esophageal biopsy shown in Fig. 1 at (A) 10, (B) 803, and (C) 2972 Hz. Bars = 100 µm.
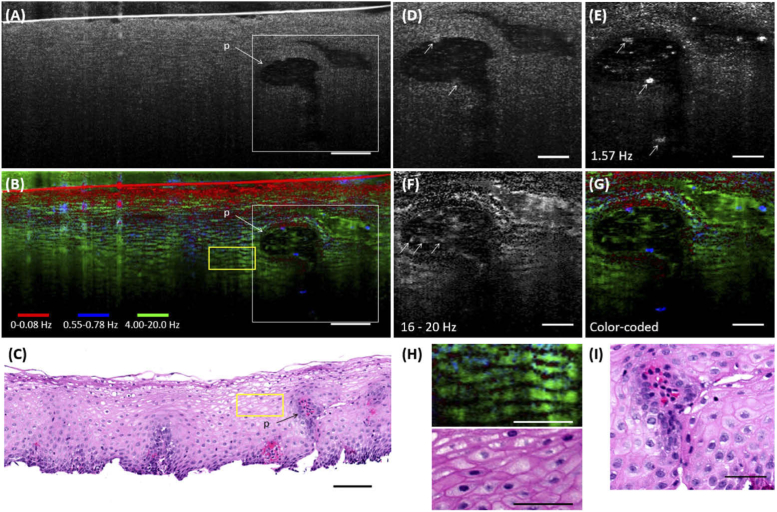
Figure 3 shows images of an esophageal biopsy. A papilla extending from the base of the biopsy [Fig. 3(A), p] can be seen in the standard µOCT image, but it otherwise was homogenous and did not reveal any clearly discernable cellular features throughout. In contrast, many additional morphological and functional details were seen in the pseudo-colored d-µOCT image [Fig. 3(B)] that were not apparent in the standard µOCT image. With d-µOCT, individual squamous cells [Figs. 3(B), 3(H)] were readily identified, which were closely matched in appearance to those in the corresponding histology [Figs. 3(C), 3(H)]. Moreover, the depth-dependent maturation was revealed in the stratification of the intracellular dynamics, where the most superficial and mature cells exhibited slow motion whereas the immature cells deeper in the biopsy showed more rapid intracellular motion. These findings are consistent with our understanding of squamous epithelial maturation, where cells divide and mature in a depth-dependent manner, from the basal layer to the surface, eventually dying and desquamating at the top. Another prominent feature that was enhanced by d-µOCT was the squamous papilla. In the standard µOCT image [Fig. 3(D)], cells at the periphery of the large papilla were sometimes faintly observed, with a brightness that was similar to that of the surrounding squamous cells. With d-µOCT, these cells were highlighted in low frequency spectral images such as in the 1.57 Hz image shown in Fig. 3(E). There were other cell types apparently within the papilla that displayed activity in the higher frequency range of 16-20 Hz [Fig. 3(F)]. The corresponding histology [Fig. 3(I)] suggests that some of these cells may be intrapapillary leukocytes and others basal epithelial cells.
Fig. 3.
Images from a human esophageal biopsy. (A) A 200-frame averaged µOCT image of the biopsy. (B) d-µOCT color-coded image. (C) Corresponding H&E stained histology of the sample. (D to G) Enlarged views of the papilla outlined in white in (A) and (B). (D) is a standard µOCT image of the papilla. (E) and (F) are d-µOCT images of the papilla at 1.57 Hz and 16-20 Hz, respectively. (G) is a d-µOCT pseudo-colored composite image of the papilla. (H) Magnified portions of the yellow ROIs in (B) and (C). (I) is a histology image of the papilla. p: papilla. d-µOCT color codes: Red 0-0.08 Hz, Blue 0.55-0.78 Hz, Green 4.00-20.0 Hz. Bars in (A) to (C) = 100 µm. Bars in (D) to (I) = 50 µm.
The ability of d-µOCT to accentuate cellular structures that were otherwise not visible with µOCT is further illustrated in an example of a biopsy taken from the gastroesophageal junction of a human subject (Fig. 4). The standard µOCT image [Fig. 4(A)] shows glandular architecture with difficult to discern intracellular contrast. With d-µOCT, low frequency basal structures consistent with nuclei [Fig. 4(B), b] and apical mucinous cytoplasm [Fig. 4(B), a] came to prominence. Interestingly, the middle of the cells showed an abundance of high frequency content [Fig. 4(B), m]. The corresponding histology [Fig. 4(C)] confirmed that these glands contained mucinous cells.
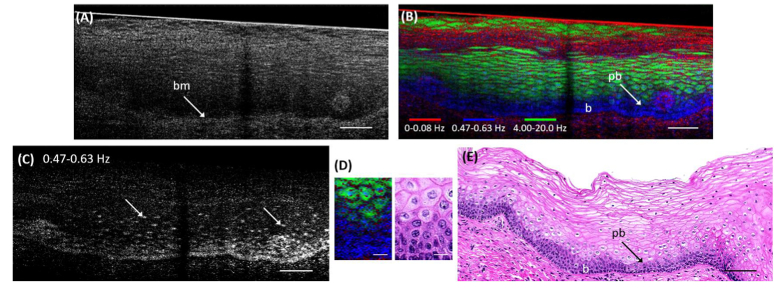
d-µOCT provided additional findings when imaging cervical squamous epithelium (Fig. 5). The basement membrane [Fig. 5(A), bm] and the outlines of a few squamous cells were observed in the frame-averaged standard µOCT image of the cervical squamous epithelium [Fig. 5(A)]. The corresponding d-µOCT image showed detailed features of most of the cells within the squamous epithelium across the entire cross-sectional image [Fig. 5(B)]. The basal and parabasal cells [Fig. 5(B), b, pb] within the lower quarter of the epithelium had a significant amount of 0.47-0.63 Hz frequency content [blue in Fig. 5(B), frequency map in 5(C)] that was present throughout the cytoplasm. The intermediate cells demonstrated this moderate-level frequency content primarily only in the center of the cells [Fig. 5(C), arrows]. Magnified portions of the d-µOCT and histology images as shown in Fig. 5(D) serve to aid visualization of these gradual depth-dependent cellular changes from the basal to parabasal layers. As the cells matured towards the surface, they became progressively flatter with less 0.47-0.63 Hz signal [Fig. 5(C)]. Low frequency content (0-0.08Hz) was predominant near the surface [Fig. 5(B), red]. These observations match well with the corresponding histology shown in Fig. 5(E) and highlight epithelial maturation features characterized by intracellular dynamics that differ in a depth-dependent manner.
Fig. 5.
Images of a human cervical biopsy. (A) A 200-frame averaged µOCT image of the cervical biopsy. The basement membrane is indicated as bm. (B) The corresponding d-µOCT image. The basal (b) and parabasal (pb) layers can be clearly delineated from the intermediate and superficial layers. (C) d-µOCT image that corresponds to the frequency range of 0.47-0.63 Hz (blue channel in (B)) showing punctate regions in a cellular distribution (arrows). (D) A magnified portion of the d-µOCT image (left) showing cellular changes that occur in a depth-dependent manner from the basal to parabasal layers. Similar depth-dependent changes could be appreciated in the histology image (right). (E) Corresponding H&E histology. d-µOCT color codes: Red 0-0.08 Hz, Blue 0.47-0.63 Hz, Green 4.00-20.0 Hz. Bars in (A, B, C, and E) = 100 µm; Bars in (D) = 20 µm.
4. Discussion
In this work, we used power spectrum analysis of temporal fluctuations found in µOCT videos to generate data that allows cross-sectional cellular dynamics in whole tissue to be studied. By appropriate binning of the power spectrum data to create pseudo-colored composite images, we demonstrated that the method enhances our ability to delineate of cellular/subcellular features in the cross-sectional imaging plane and characterize intracellular dynamics within the tissue without need for exogenous labeling. Our results also emphasize the capabilities of d-µOCT to interrogate cross-sectional subcellular structure and concomitant variations in cell dynamics and activity from the basal to superficial epithelial layers. The significance of this capability is high as depth-dependent changes in cellular and architectural maturation patterns are critical for the diagnosis of many epithelial diseases, including dysplasia and cancer.
We utilized d-µOCT in freshly excised biopsy samples that were kept alive in culture media. These biopsies were difficult to register with histology at the subcellular level and thus some of our interpretations should be considered preliminary. There is also much to learn about the mechanistic origins of the cellular motions measured by this technique. In the literature, there is evidence that supports the notion that these dynamical fluctuations, while random, are distinctly different from thermal-driven Brownian motion [1]. Instead, they are a consequence of an aggregate of ATP-dependent random forces that orchestrate cell motility, among other biomechanical effects [22,23]. Additionally, we need to gain an improved understanding of the relationship between intracellular dynamics and underlying physiological and pathobiological conditions. Numerous studies, mainly with in vitro models, have investigated the origins of intracellular dynamics [3–8,24,25]. In some of these studies, dynamic signals have been associated with contractile protein filaments in organelle transport and cell motility [3], which have shown to be modulated by pharmaceutical agents [8,25]. These results indicate that the physiological origins of the dynamical behavior being probed can be used to inform of pathologies and their response to treatment. Owing to its high resolution in all three dimensions, d-µOCT is well suited to perform these investigations. Yet, larger studies of various tissue/cell types in different states with identification/modulation of specific intracellular molecular motion-dependent mechanisms are warranted to fully understand the wealth of information that d-µOCT provides.
While intact tissue is an excellent substrate for d-µOCT, this technique could also be of great use for additional assays including two- and three-dimensional cell culture, spheroids, organoids, and organs-on-chips, among others [3,8,26–29]. The advantage of d-µOCT as a cell viability assay would be the ability to determine the metabolic or pathobiologic state of cells in these platforms without destroying the sample for viability staining or cell-type characterization. Such an application of d-µOCT could improve the efficiency of many multicellular assays being developed today.
Another promise of d-µOCT is the possibility of performing this technique in vivo. µOCT has now been demonstrated in living human patients in the nasal cavity, showing a unique capacity to interrogate ciliary and mucus dynamics. [13,17,30] d-µOCT will be more difficult to implement in vivo because of the need for patient stabilization at the subcellular level during an extended imaging period (seconds). Technologies such as tight coupling [31] of the tissue with a µOCT probe could overcome this issue, opening up a new label-free option for high cellular contrast and functionally informed optical biopsy.
Acknowledgments
We thank Catriona Grant, Anita Chung, and Anna Gao for their help with specimen collection; Jie Zhao, Neema Kuma, and Lingxian Wu from the Wellman Photopathology laboratory for histology preparation, and Brian Battersby from the Wellman Computational Core for helping with the data acquisition software.
Funding
Remondi Family Foundation; Mike and Sue Hazard Family Foundation; Massachusetts General Hospital10.13039/100005294 (Research Scholars Program).
Disclosures
G.J.T. receives sponsored research for µOCT research from CN USA Biotech Holdings and Wayvector.
References
- 1.Weber S. C., Spakowitz A. J., Theriot J. A., “Nonthermal ATP-dependent fluctuations contribute to the in vivo motion of chromosomal loci,” Proc. Natl. Acad. Sci. 109(19), 7338–7343 (2012). 10.1073/pnas.1119505109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renz M., Mackintosh F. C., Ehrlicher A. J., Jensen M. H., Lippincott-Schwartz J., Moore J. R., Goldman R. D., Weitz D. A., Guo M., “Probing the Stochastic, Motor-Driven Properties of the Cytoplasm Using Force Spectrum Microscopy,” Cell 158(4), 822–832 (2014). 10.1016/j.cell.2014.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroux C.-E., Bertillot F., Thouvenin O., Boccara A.-C., “Intracellular dynamics measurements with full field optical coherence tomography suggest hindering effect of actomyosin contractility on organelle transport,” Biomed. Opt. Express 7(11), 4501 (2016). 10.1364/BOE.7.004501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong K., Turek J. J., Nolte D. D., “Speckle fluctuation spectroscopy of intracellular motion in living tissue using coherence-domain digital holography,” J. Biomed. Opt. 15(3), 030514 (2010). 10.1117/1.3456369 [DOI] [PubMed] [Google Scholar]
- 5.Apelian C., Harms F., Thouvenin O., Boccara A. C., “Dynamic full field optical coherence tomography: subcellular metabolic contrast revealed in tissues by interferometric signals temporal analysis,” Biomed. Opt. Express 7(4), 1511 (2016). 10.1364/BOE.7.001511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholler J., Thouvenin O., Grieve K., Groux K., Boccara C., Mazlin V., Fink M., Sahel J.-A., Xiao P., “Probing dynamic processes in the eye at multiple spatial and temporal scales with multimodal full field OCT,” Biomed. Opt. Express 10(2), 731 (2019). 10.1364/BOE.10.000731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aumann S., Donner S., Fischer J., Müller F, Optical Coherence Tomography (OCT): Principle and Technical Realization. in High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics (ed. Bille J. F., 59–85 (Springer International Publishing, 2019). doi:10.1007/978-3-030-16638-0_3 [PubMed] [Google Scholar]
- 8.Yang L., Yu X., Fuller A. M., Troester M. A., Oldenburg A. L., “Characterizing optical coherence tomography speckle fluctuation spectra of mammary organoids during suppression of intracellular motility,” Quant Imaging Med Surg 10(1), 76–85 (2020). 10.21037/qims.2019.08.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackmon R. L., Kreda S. M., Sears P. R., Ostrowski L. E., Hill D. B., Chapman B. S., Tracy J. B., Oldenburg A. L., “Diffusion-sensitive optical coherence tomography for real-time monitoring of mucus thinning treatments,” in Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XX 9697, 9697: 969724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackmon R. L., Sandhu R., Chapman B. S., Casbas-Hernandez P., Tracy J. B., Troester M. A., Oldenburg A. L., “Imaging Extracellular Matrix Remodeling in Vitro by Diffusion-Sensitive Optical Coherence Tomography,” Biophys. J. 110(8), 1858–1868 (2016). 10.1016/j.bpj.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan W., Oldenburg A. L., Norman J. J., Desai T. A., Boppart S. A., “Optical coherence tomography of cell dynamics in three-dimensional engineered tissues,” Opt. Express 14(16), 7159 (2006). 10.1364/OE.14.007159 [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Gardecki J. A., Nadkarni S. K., Toussaint J. D., Yagi Y., Bouma B. E., Tearney G. J., “Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography,” Nat. Med. 17(8), 1010–1014 (2011). 10.1038/nm.2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung H. M., Birket S. E., Hyun C., Ford T. N., Cui D., Solomon G. M., Shei R.-J., Adewale A. T., Lenzie A. R., Fernandez-Petty C. M., Zheng H., Palermo J. H., Cho D.-Y., Woodworth B. A., Yonker L. M., Hurley B. P., Rowe S. M., Tearney G. J., “Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies,” Sci. Transl. Med. 11(504), eaav3505 (2019). 10.1126/scitranslmed.aav3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimiya K., Yin B., Piao Z., Ryu J., Osman H., Leung H. M., Sharma G., Liang C. P., Gardecki J. A., Zheng H., Shimokawa H., Tearney G. J., Ryu J., Leung H. M., Sharma G., Gardecki J. A., Tearney G. J., “Micro-Optical Coherence Tomography for Endothelial Cell Visualization in the Coronary Arteries,” JACC Cardiovasc. Imaging 12(9), 1878–1880 (2019). 10.1016/j.jcmg.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Xiong Q., Ge X., Bo E., Xie J., Liu X., Yu X., Wang X., Wang N., Chen S., Wu X., Liu L., “Cellular resolution corneal imaging with extended imaging range,” Opt. Express 27(2), 1298 (2019). 10.1364/OE.27.001298 [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Liu X., Wang N., Wang X., Xiong Q., Bo E., Yu X., Chen S., Liu L., “Visualizing Micro-anatomical Structures of the Posterior Cornea with Micro-optical Coherence Tomography,” Sci. Rep. 7(1), 1–10 (2017). 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz-Hildebrandt H., Pieper M., Stehmar C., Ahrens M., Idel C., Wollenberg B., König P., Hüttmann G., “Novel endoscope with increased depth of field for imaging human nasal tissue by microscopic optical coherence tomography,” Biomed. Opt. Express 9(2), 636 (2018). 10.1364/BOE.9.000636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sage D., “Local Normalization,” (2003). Available at: http://bigwww.epfl.ch/sage/soft/localnormalization/. (Accessed: 1st January 2020)
- 19.Sorzano C. Ó. S., Thévenaz P., Unser M, “Elastic registration of biological images using vector-spline regularization,” IEEE Trans. Biomed. Eng. 52(4), 652–663 (2005). 10.1109/TBME.2005.844030 [DOI] [PubMed] [Google Scholar]
- 20.Welch P. D., “Welchs Periodogram.pdf,” IEEE Trans. Audio Electroacoust. 15(2), 70–73 (1967). 10.1109/TAU.1967.1161901 [DOI] [Google Scholar]
- 21.Schneider C. A., Rasband W. S., Eliceiri K. W., “NIH Image to ImageJ: 25 years of image analysis,” Nat. Methods 9(7), 671–675 (2012). 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brangwynne C. P., Koenderink G. H., MacKintosh F. C., Weitz D. A, “Intracellular transport by active diffusion,” Trends Cell Biol. 19(9), 423–427 (2009). 10.1016/j.tcb.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Hacker C., Guimaraes S. C., Schrader M., Gurr S. J., Metz J., Ashwin P., Lin C., Schuster M., Steinberg G., “Active diffusion and microtubule-based transport oppose myosin forces to position organelles in cells,” Nat. Commun. 7(1), 11814 (2016). 10.1038/ncomms11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brazhe N. A., Brazhe A. R., Pavlov A. N., Erokhova L. A., Yusipovich A. I., Maksimov G. V., Mosekilde E., Sosnovtseva O. V., “Unraveling cell processes: Interference imaging interwoven with data analysis,” J. Biol. Phys. 32(3-4), 191–208 (2006). 10.1007/s10867-006-9012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill D., An R., Sun H., Yakubov B., Matei D., Turek J., Nolte D., “Intracellular Doppler Signatures of Platinum Sensitivity Captured by Biodynamic Profiling in Ovarian Xenografts,” Sci. Rep. 6(1), 18821 (2016). 10.1038/srep18821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins R. W., Aref A. R., Lizotte P. H., Ivanova E., Stinson S., Zhou C. W., Bowden M., Deng J., Liu H., Miao D., He M. X., Walker W., Zhang G., Tian T., Cheng C., Wei Z., Palakurthi S., Bittinger M., Vitzthum H., “Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids,” Cancer Discovery 8(2), 196–215 (2018). 10.1158/2159-8290.CD-17-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y. S., Khademhosseini A., “Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip,” Nanomedicine 10(5), 685–688 (2015). 10.2217/nnm.15.18 [DOI] [PubMed] [Google Scholar]
- 28.Biselli E., Agliari E., Barra A., Bertani F. R., Gerardino A., De Ninno A., Mencattini A., Di Giuseppe D., Mattei F., Schiavoni G., Lucarini V., Vacchelli E., Kroemer G., Di Natale C., Martinelli E., Businaro L., “Organs on chip approach: a tool to evaluate cancer -immune cells interactions,” Sci. Rep. 7(1), 12737 (2017). 10.1038/s41598-017-13070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun H., Merrill D., An R., Turek J., Matei D., Nolte D. D., “Biodynamic imaging for phenotypic profiling of three-dimensional tissue culture,” J. Biomed. Opt. 22(1), 016007 (2017). 10.1117/1.JBO.22.1.016007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeni R. Z., Yu X., Chang H., Chen P., Liu L., Xu C., “Iron Oxide Nanoparticle-Powered Micro-Optical Coherence Tomography for in Situ Imaging the Penetration and Swelling of Polymeric Microneedles in the Skin,” ACS Appl. Mater. Interfaces 9(24), 20340–20347 (2017). 10.1021/acsami.7b00481 [DOI] [PubMed] [Google Scholar]
- 31.Vinegoni C., Lee S., Aguirre A. D., Weissleder R., “New techniques for motion-artifact-free in vivo cardiac microscopy,” Front. Physiol. 6, 147 (2015). 10.3389/fphys.2015.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]