Abstract
We show that third harmonic generation (THG) microscopy using a 1-MHz train of 1,300-nm femtosecond duration laser pulses enabled visualization of the structure and quantification of flow speed in the cortical microvascular network of mice to a depth of > 1 mm. Simultaneous three-photon imaging of an intravascular fluorescent tracer enabled us to quantify the cell free layer thickness. Using the label-free imaging capability of THG, we measured flow speed in different types of vessels with and without the presence of an intravascular tracer conjugated to a high molecular weight dextran (2 MDa FITC-dextran, 5% w/v in saline, 100 µl). We found a ∼20% decrease in flow speeds in arterioles and venules due to the dextran-conjugated FITC, which we confirmed with Doppler optical coherence tomography. Capillary flow speeds did not change, although we saw a ∼7% decrease in red blood cell flux with dextran-conjugated FITC injection.
1. Introduction
Nonlinear microscopy is widely used in biomedical research to study the function and dynamic behavior of cells in both healthy and disease states [1–3]. It has enabled researchers to visualize cell and tissue structure with subcellular resolution in highly scattering tissue. Third harmonic generation (THG) is a non-linear optical process in which the incoming energy of three photons is combined to generate an outgoing photon with a wavelength of one third the original excitation wavelength. THG produced on either side of a laser focus is out of phase and cancels in the far field, but this cancelation becomes imperfect if there is an optical interface in the focal volume [4–6]. THG microscopy thus provides a 3D imaging modality that highlights optical interfaces. This THG signal is generated in the forward direction, so when imaging in scattering samples the epi-detected THG signal is the result of light that scatters back out of the sample. In addition, the THG signal can be resonantly enhanced by the presence of electronic transitions at one, two, or three times the photon energy [7,8]. When used in vivo, THG has been shown to provide high contrast imaging of a number of tissue structures, including myelinated axons in the brain [9] and spinal cord [10], lipid deposits in atherosclerotic plaques in aorta [11], muscle fiber sarcomeres [12], red blood cells (RBCs) in vessels of the ear in mice [13] and white blood cells in human skin [14]. THG can be resonantly enhanced in RBCs by the Soret band transitions in hemoglobin around 425 nm when using excitation wavelengths around 1275 nm (3 photon resonant enhancement) [8] or around 850 nm (2 photon resonant enhancement) [7,15]. When imaging the mouse cortex in vivo, the two dominant sources of THG contrast appear to be from myelinated axons [10] and RBCs in the vessels.
Here, we used an energetic 1,300-nm excitation source to image THG signals from the cortex of live, anesthetized mice, focusing on the signals from RBCs and blood vessels. Conventionally, 3D nonlinear imaging of vascular structure and blood flow required a fluorescent dye to be injected into the blood stream to label the blood plasma, which was then imaged with two-photon excited fluorescence [16–19]. In order to prevent this fluorescent tracer from being rapidly filtered out of the blood by the kidneys, a high molecular weight dextran with conjugated fluorescent molecules was used [20]. However, the impact of this high molecular weight dextran on blood flow, especially at the microvascular level, remains understudied. In this work, we took advantage of the ability to visualize RBCs label free using THG to characterize cortical vessel structure and a number of hemodynamic parameters in cortical microvessels in mice, including blood flow speeds, RBC flux, and cell-free layer (CFL) thickness. We also examined the impact of the injection of fluorophore-conjugated high molecular weight dextran on these parameters. Finally, we used Doppler optical coherence tomography (OCT) to corroborate our findings on the impact of fluorophore-conjugated dextran injection on flow in cortical venules.
2. Materials and methods
2.1. Animals
Experiments were performed in 3-5 months old wild-type mice (C57BL/6J). The THG imaging experiments used two male and two female mice, with weights of 20-30 g, while the OCT studies used 3 female mice, with weights of 20-25 g. Surgeries and experiments were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by National Institutes of Health. Animal procedures and experiments using multiphoton microscopy were approved by the Cornell University Institutional Animal Care and Use Committee (protocol number 2015-0029). OCT experiments and animal procedures were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (protocol number 2007N000050). No animals dropped out of this study due to mortality or morbidity after surgery or imaging sessions.
2.2. Chronic cranial window preparation
Optical access to the brain was achieved via a cranial window, as described previously [21]. For THG imaging, animals were anesthetized using isoflurane (1.5–2% in oxygen) and placed on a feedback-controlled heating pad that maintained body temperature at 37°C (50-7053P; Harvard Apparatus). Mice were given atropine sulfate (0.005 mg/100 g animal weight; subcutaneously; 54925-063-10, Med-Pharmex Inc.) to reduce buildup of lung secretions. Animals were also given dexamethasone (0.025 mg/100 g; subcutaneously; 07-808-8194, Phoenix Pharm Inc.) and ketoprofen (0.5 mg/100 g; subcutaneously; Zoetis Inc.) to reduce post-surgical inflammation and pain, respectively. A 6-mm diameter bilateral craniotomy was performed over parietal cortex using a dental drill. The exposed brain was covered with sterile saline and sealed with an 8-mm diameter glass coverslip using cyanoacrylate glue (Loctite 495; Henkel), tissue adhesive (70200742529, 3M™), and dental cement (Co-Oral-Ite Dental Mfg Co.). Animals were given a single subcutaneous injection of 5% weight/volume (w/v) glucose in saline at the conclusion of surgery (1 ml/100 g). Following surgery, mice were given at least 21 days to recover before in vivo imaging.
For OCT experiments, chronic cranial windows were prepared following the procedures described in Goldey, et al. [22]. Body temperature was maintained at 37 °C during all surgical and experimental procedures. Briefly, a custom-made head-post [23] was glued to the skull, overlaying the right hemisphere, and a craniotomy (round, 3-mm diameter) was performed over the left hemisphere, centered approximately over the E1 whisker barrel. The dura was left intact. The cranial window was subsequently sealed with a glass plug [24] and dental acrylic. After surgery, mice were given 1 month to recover before experiments.
2.3. Multi channel in vivo multiphoton microscopy
A tunable Ti:Sapphire laser (Vision II, Coherent), set to a wavelength of 800 nm, was used for 2PEF imaging (used only for low-magnification imaging to create a map of the cortical surface vasculature to facilitate navigation; data not shown). For generating 1300-nm laser light to induce 3PEF and THG, an optical parametric amplifier (OPA) (Opera-F, Coherent) was seeded by a diode-pumped femtosecond laser (40 µJ/pulse at 1 MHz; Monaco, Coherent), producing pulses at 1-MHz repetition rate with ∼1.3-µJ energy. The bandwidth of the laser supports a pulse duration as short as ∼50 fs. Dispersion for the 1,300-nm excitation light was compensated with an SF11 prism pair with a separation that was adjusted to achieve the highest 3PEF signal through the microscope objective of our locally-built laser scanning microscope. For imaging, the excitation laser was scanned with a line rate of ∼1 kHz with galvanometric scanners (bi-directional; Cambridge Tech.), with a 1-µs pixel clock. We used achromatic lenses for the scan lens (80-mm focal length; AC508-080-C, Thor Labs) and tube lens (300-mm focal length; AC508-300-C, Thor Labs), which delivered the laser light to the back aperture of the microscope objective. With this laser repetition rate and pixel rate, we have one laser pulse per image pixel, on average. We delivered a maximal power of ∼85 mW at 1,300 nm through the objective for in vivo experiments. Two-channel imaging was performed using the following emission filters (center wavelength/bandwidth): 417/60 nm (for THG), 550/49 nm (for FITC fluorescence) separated by a long-pass dichroic with a cutoff wavelength of 458 nm. Fluorescent light collected by the microscope objective was separated by a long pass dichroic with a 720 nm cutoff wavelength (Semrock) and was directed through a four lens optical system that could collect all the light emerging from the back aperture of the microscope objective and efficiently deliver it to the 5-mm diameter active area of the detectors. The details of these detection optics are described in Tsai, et al. [25]. Optical signals were detected using GaAsP photomultiplier tubes (PMTs) with a quantum efficiency of ∼30% around 420 nm (H7422P-40, Hammamatsu). The PMT electronic signals were amplified with a pre-amplifier with a gain of 20 and a 10-MHz bandwidth and were then low pass filtered with a cutoff frequency of ∼0.5 MHz. This low pass filtering slightly blurred pixels into each other along the fast scan direction (x-axis) and provided some averaging to enable reliable imaging with just one incident laser pulse per image pixel and no synchronization of the laser pulse train with the pixel clock. All images were acquired with 1024 X 1024 pixels in the x-y plane using ScanImage software (version 3.8, Vidrio Technologies) [26] to control sample movement, laser scanning, laser power, and signal detection. Image data was continuously acquired during laser scanning, with no significant delay between the acquisition of image frames.
2.4. Repeated imaging with and without dextran-conjugated fluorescent dyes
Mice were anesthetized with isofluorane (1.5–2% in oxygen) and placed in a stereotaxic apparatus equipped with a feedback-controlled heating pad. In imaging sessions with dextran dyes, blood plasma was labeled with a high concentration of high molecular weight dextran conjugated with FITC (2 MDa, 52471, Sigma) diluted in sterile saline (5% w/v) and retro-orbitally injected (100 µl) prior to imaging. Respiratory rate was monitored throughout the imaging session and the isoflurane level was adjusted to maintain a steady respiratory rate of ∼1 Hz. During the imaging session, mice received an hourly dose of 5% w/v glucose in saline (1 ml/100 g; subcutaneously) and atropine (0.001 mg/100 g; subcutaneously). During the very first imaging session of each mouse, a shallow (300 µm deep) 2PEF wide-field map of the cortical vasculature in the cranial window was taken using a 4x objective (0.28 NA, Olympus). This map was then used to reference the relative location of all future measurements. All line scan data, z-stacks through measured vessels, and high-resolution z-stacks from the surface of the brain into the deep cortex were taken through a higher magnification 25x, water-immersion objective (XLPlan N 1.05 NA, Olympus) using 1,300-nm excitation. The 1,300-nm excitation drove both 3PEF of the FITC as well as THG. We took 1-µm spaced image stacks, beginning with a laser power of ∼4 mW near the brain surface and manually increasing this power with greater imaging depths to maintain image contrast, until ∼85 mW was used at the maximum imaging depth of ∼1 mm. We took several steps to reduce the risk of tissue damage with the higher laser pulse energies used for 3PEF and THG imaging. First, we blocked the edges of the scan during line-scans (details in Section 2.5) and second, we were careful to ensure that the laser power was never too high for the imaging depth. We determined the in vivo resolution performance of the system by fitting the 3PEF-FITC signal at the edges of blood vessels to an error function. We sampled three different vessels, in both the lateral and axial directions, at depth intervals of 100 µm to a maximum depth of ∼850 µm. We selected blood vessels for blood flow speed measurements in two ways. First, we used the direction of blood flow identified from the angle of the streaks formed by moving RBCs to identify penetrating arterioles (blood flow into the cortex) and ascending venules (blood flow out of the cortex) [27]. We then took line scan and vessel diameter data from penetrating arterioles, their upstream surface arteriole segments, and from downstream capillary branches, going 3-4 branches into the capillary bed. We similarly took measurements in ascending venules, their downstream surface venules, and their upstream capillary branches, also 3-4 branches into the capillary bed. We followed 1-2 sets of capillaries starting with both arterioles and venules in each mouse. Second, we randomly selected two capillary segments at 100 µm depth intervals going as deep into the brain as the signal to noise of the imaging signal allowed in each mouse. After taking all data with intravenously injected dextran dye, mice recovered for at least two days to allow the fluorophore-conjugated dextran to be completely cleared out from the circulatory system. In the subsequent imaging session we used only THG signals for identifying and measuring blood flow in all previously examined vessels, with no intravascular dextran present. Multiphoton image stacks were processed and analyzed using FIJI, except the renderings in Fig. 2(A), which were done using Imaris (Bitplane). The images of individual vessels represent an average axial maximum intensity projection of a 1-µm spaced image stack taken from just above to just below the vessel. Image manipulations were limited to linear adjustments of image contrast.
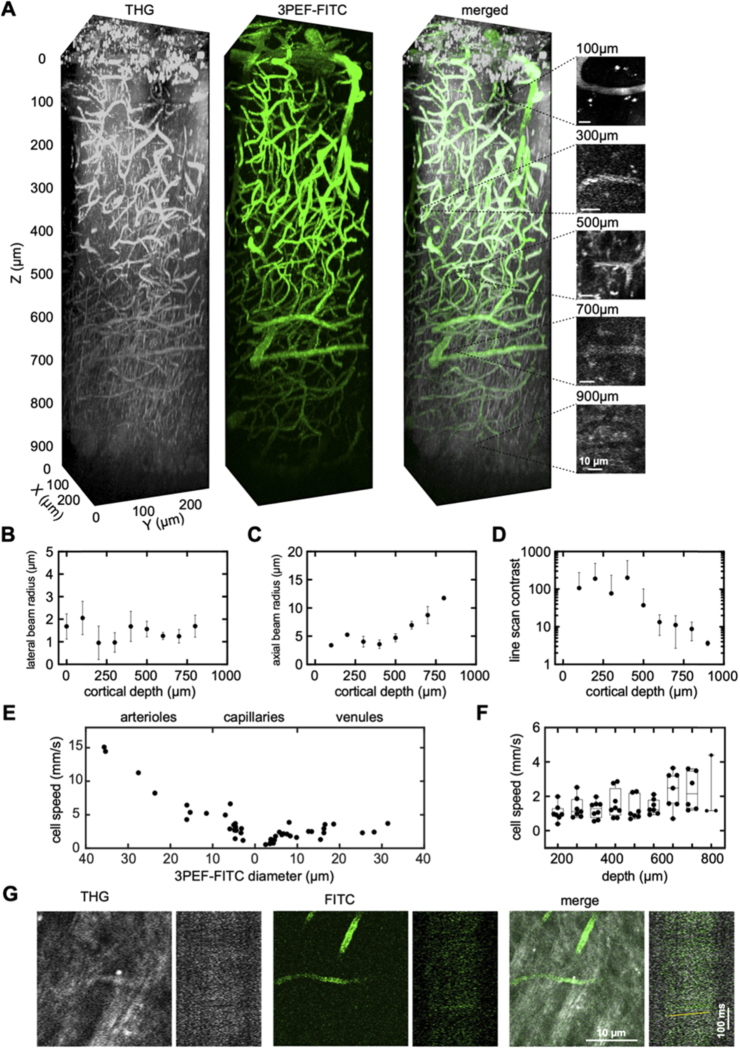
Fig. 2.
THG imaging of RBC motion enabled quantification of blood flow speed in vessels from the brain surface to the white matter. (A) 3D renderings of image stacks taken to a depth of 1 mm into the cortex of a live, anesthetized mouse using THG (left, greyscale), 3PEF-FITC (middle, green), and merged (right). The small images on the right show representative capillaries at different depths together with space-time images of line scan data, both using THG. (B) Lateral and (C) axial 1/e beam radius, measured by fitting an error function to the rise in 3PEF-FITC signal at the edge of a vessel, shown as a function of depth below the cortical surface. Three capillaries were measured at each depth. Error bars show the SD. (D) Average contrast of the capillary line scans, defined as the difference in brightness of the brightest and dimmest 10% of pixels, plotted as function of depth, showing mean and SD (n=70 capillaries from 4 mice; contrast was measured in some deep vessels for which we were unable to extract a flow speed). (E) RBC flow speed as a function of vessel diameter from THG line scan data. Data for arterioles are displayed on the left with diameter decreasing to the right, while venules are displayed on the right with diameter increasing to the right. Capillaries are displayed toward the middle, and are located on the arteriole or venule side based on their topological proximity to the nearest penetrating arteriole or ascending venule. (F) Box plot of RBC flow speed in capillaries at different cortical depths, measured using THG line scans (e.g. images on right in A) (n=60 capillaries from 4 mice). (G) THG (left, greyscale), 3PEF-FITC (middle, green), and merged (right) average projection images and line scan data from a capillary in the corpus callosum, at a depth of 1,060 µm. The yellow line in the merged line scan indicates the angle of the streaks formed by moving RBCs. Scale bars are 10 µm (horizontal) and 100 ms (vertical) in both A and E.
2.5. Multiphoton determination of flow speed and RBC flux
We tracked blood cell displacement along the vessel lumen by repetitively scanning a bi-directional line across the full frame (1024 pixels) along the central axis of single vessels at a line rate of 1.7 kHz for at least 35 s (note the line rate is slightly faster than what we used when taking image frames; for capillaries deeper than ∼800 µm beneath the cortical surface, we used a line rate of 0.85 kHz to increase signal strength, which was still fast enough to quantify RBC flow speed). To reduce the risk of tissue damage during line scans, we placed an iris in the plane conjugate to the imaging plane between the scan and tube lenses in the microscope. This iris was manually closed to block the beam at both edges of the scan during line scans. This reduced the repetitive exposure of tissue at the edges of the line scan (often outside the measured vessel). The intravenously injected fluorescent dye labels only the blood plasma, while THG is strongly produced by RBCs within the vessel lumen. The space-time images produced by the line scan thus contained diagonal dark streaks in the FITC image and alternatively matching diagonal bright streaks in the THG image, both formed by moving RBCs. The slope of these streaks is inversely proportional to the centerline blood cell speed, and analyzing either set of stripes to determine RBC speed yielded the same results (less than 1% difference). All blood cell speeds reported in this paper were determined from the THG signal. All line scans were analyzed using custom MATLAB code that utilized a Radon transform-based algorithm that computes line integrals of the 2D image [g(x,y)] at different angles [16],
We then found the angle, θ, that had the greatest variance along the distance, ρ. This angle is orthogonal to the slope of the streaks formed by moving RBCs, and the speed was thus proportional to tan-1θ. Volumetric blood flow, F, was calculated as
where is the centerline RBC speed as determined from the THG line scan, and r corresponds to the vessel radius, which was calculated only from the FITC image, because the THG image underestimates the full lumen size due to the cell free layer. All the line scans shown represent a single, un-averaged space time image.
The RBC flux was determined by manually counting the number of RBCs from the THG line scan data in twelve to fifteen 0.6-s long time segments spaced by 3 s throughout the full duration of each line scan measurement. Each cell was represented by a bright streak in the THG space time image. In some capillaries, the streaks from the RBCs were too dense to enable reliable counting and these vessels were excluded from the analysis. We were able to determine RBC flux both with and without circulating dextran in 54 out of the 77 capillaries measured in this study.
2.6. Measurement of blood flow using Doppler OCT
Animals were anesthetized using isoflurane and given a subcutaneous dose of glucose in saline before the experiment (1 ml/100 g of 5% w/v in saline). During the entire imaging session, respiratory rate was monitored and the anesthesia level (1.5-2% isoflurane in a mixture of air and oxygen) was adjusted to maintain a steady respiratory rate of ∼1 Hz. Doppler OCT imaging was first conducted without administration of dextran-conjugated FITC. Volumetric Doppler OCT scans were performed over a 1 × 1 mm2 lateral field of view, with scan steps of 0.26 µm and 1.95 µm along the fast and slow scanning directions, respectively. The axial resolution, pixel size and imaging range along the axial direction were estimated as 3.5 µm, 2.8 µm and 1.6 mm in biological tissue, respectively. Subsequently, 100 µl of 2 MDa dextran-conjugated FITC (5% w/v, Sigma 52471) was administered retro-orbitally into the blood, and Doppler OCT imaging was repeated using the same acquisition parameters and over the same region of interest. The two imaging sessions for each mouse were completed within one hour. The axial flow speed was determined directly from the Doppler OCT signal. The maximal flow speed for each vessel was determined from a small region of interest at the vessel center (small black circle in Fig. 4(A)). The volumetric flow was determined by integrating the axial flow speed data over a manually drawn region of interest that fully enclosed the vessel cross section (larger white circle in Fig. 4(A)) [28]. The background Doppler signal was estimated from the nearby parenchymal signal (region outlined with a green line in Fig. 4(A)) and was subtracted from all measurements.
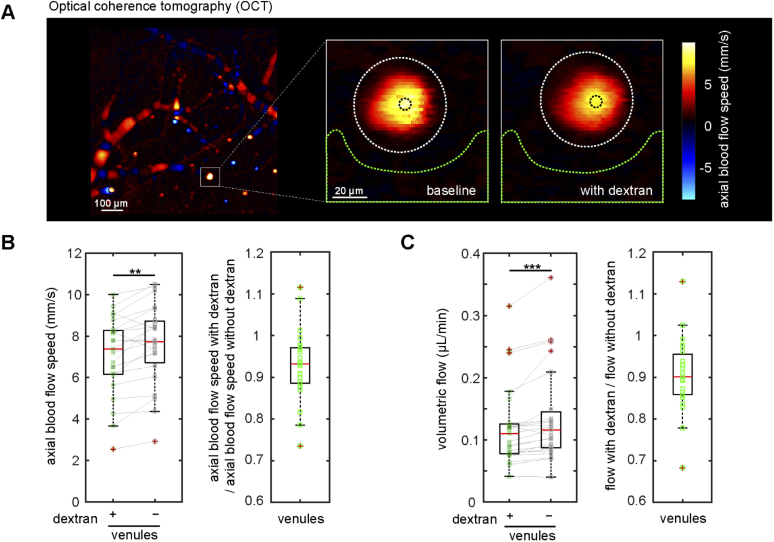
Fig. 4.
Doppler optical coherence tomography confirmed that intravascular high molecular weight dextran slows blood flow in cortical ascending venules. (A) Doppler OCT image of the cortical surface (left, scale bar is 100 µm), and zoomed-in views of the ascending venule indicated by the white square taken at baseline and shortly after intravascular injection of high molecular weight dextran (right, scale bar is 20 µm). Colors correspond to axial blood flow speeds, as indicated with the color bar. (B) Axial blood flow speed from ascending venules at baseline and after dextran injection (left). Measurements from same venule are connected with a solid line. Ratio of axial flow speed with and without dextran (right) (23 venules from 3 mice). (C) Volumetric blood flow with and without dextran (left) and the corresponding ratio (right). ** p<0.01, *** p<0.001. Data were normally distributed for flow speed (panel B) so a paired t-test was used. Data were not normally distributed for volumetric flow (panel C) so Wilcoxon tests were used.
2.7. Data visualization and statistical analysis
Much of our data is shown using box plots, where the box contains the data lying between the 25th and 75th percentiles of the data. The whiskers extend 1.5 times the interquartile range and the horizontal bar inside the box represents the median. All individual data points are overlaid. All statistical analysis was performed with GraphPad Prism software. We first tested if data sets followed a normal distribution using the D’Agostino and Pearson normality test. Depending on the result, we used either paired t-test or Wilcoxon test at 95% significance. We compared hemodynamic parameters measured with and without dextran injection in the same vessel segments. We expected and observed a blood flow reduction after dextran injection so we tested for statistical significance using one-tailed paired tests. Statistical significance is indicated in plots using the following convention: * p<0.05; ** p<0.01; *** p<0.001.
3. Results
In the first imaging session, the blood plasma was labeled by an intravenous injection of high molecular weight dextran with conjugated FITC (5% w/v 2 MDa FITC-Dextran in sterile saline, 100 µl). Using 1,300-nm excitation, 3PEF images of FITC showed the cortical vascular network with high contrast. Several sources of THG contrast were evident, including from RBCs inside blood vessels, nerve fibers in the cortex, the myelinated fiber tracts in the subcortical white matter, as well as some un-identified punctate round objects near the cortical surface (Fig. 1(A)).
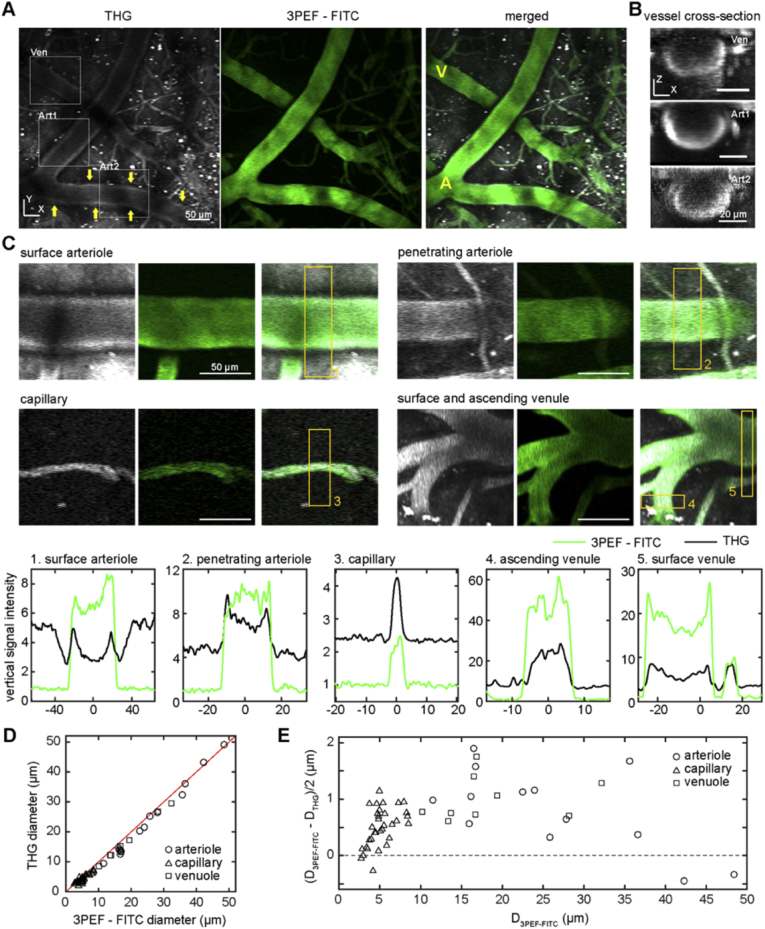
Fig. 1.
Imaging of RBCs using THG highlighted blood vessels and, when combined with 3PEF of intravascular FITC, enabled measurement of the CFL thickness. (A) Average projections of 30-µm deep image stack using THG (left, greyscale), 3PEF of intravascular FITC-dextran (middle, green), and the merged images (right) taken from the cortical surface in the XY plane in a live, anesthetized mouse. The yellow A and V in the merged image label an arteriole and venule, respectively. Yellow arrows in the THG image point to THG signal arising from the vessel wall of an arteriole. Boxes identify vessels shown in XZ projections (B) from venules (Ven) and arterioles (Art1 and Art2, respectively). The XZ projections were projected along the axis of the vessel. (C) Average projections of THG (left, greyscale), 3PEF-FITC (middle, green), and merged (right) from different classes of surface and sub-surface blood vessels. Projections were from just above to just below each vessel. The plots (below) show the transverse intensity profiles for the THG (black) and 3PEF-FITC (green) signals, taken at the location of the correspondingly numbered yellow rectangles in the images. (D) Plot of the full width at half maximum (FWHM) of the THG signal (THG diameter, DTHG) versus the FWHM of 3PEF-FITC signal (vessel diameter, D3PEF-FITC) for all vessels measured, distinguishing arterioles, capillaries, and venules (n=52 vessels across 4 mice). (E) Cell free layer thickness, defined as half the difference between the 3PEF-FITC diameter and the THG diameter, for all vessels measured plotted as a function of vessel diameter. Scale bar in A and B is 50 µm.
The cortical vascular network consists of several different types of blood vessels. Surface arterioles, fed by the main cerebral arteries, form an interconnected network on the brain surface that branches out to smaller penetrating arterioles that plunge into the brain and further divide into a highly-interconnected capillary network. This capillary network, in turn, drains into ascending venules that bring blood back to the cortical surface and then merge into a network of surface venules that drain out of the brain. We distinguished penetrating arterioles and ascending venules from each other based on the directionality of blood flow (e.g. flow into the brain implied a penetrating arteriole) and traced forward and backward through the network from these vessels to topologically classify surface vessels and capillaries [27]. We could readily identify surface and penetrating arterioles, capillaries, and ascending and surface venules in both the 3PEF – FITC and THG images (Fig. 1(A)). In larger diameter vessels, the THG signal at the center of the vessel was lower than at the edges, and was brightest from the bottom of the vessel (Fig. 1(B)). This variation in THG signal strength across the vessel cross section is likely due to absorption of the ∼433-nm THG light by hemoglobin. The THG signal is generated primarily in the forward direction and must propagate into the tissue and be scattered back to the detector. The absorption length of the THG light is about 30 (90) µm for deoxygenated (oxygenated) blood, so the THG generated from RBCs near the top of the vessel can be significantly attenuated during propagation through the blood in vessels with diameters of even a few tens of micrometers. Because the 3PEF – FITC signal comes from the blood plasma, but the THG signal comes from RBCs and several subtypes of leukocytes [14], the difference between the vessel diameter determined from these two images (Figs. 1(C) and 1(D)) gives a measure of the thickness of the cell free layer at the vessel wall. We measured the thickness of the CFL in arterioles, capillaries and venules (Fig. 1(E)) and found that it increased with increasing vessel diameter in capillaries, reaching a thickness of about 1 µm for vessels with ∼8-µm diameter. For larger diameter arterioles and venules, the CFL thickness remained at around 1 µm (Fig. 1(E)). For some measurements in larger-diameter arterioles, signal in the THG channel that was produced by a structure within the vessel wall confounded the measurement of the CFL because the border of the signal from RBCs could not be readily identified (see arrows in THG image in Fig. 1(A)). This effect is responsible for the two large-diameter arterioles with a negative thickness in our measurement of the CFL in Fig. 1(E).
Both the 3PEF-FITC and THG signals enabled imaging of the capillary network deep into the cortex, to a depth of about 1 mm (Fig. 2(A)). From these image stacks, we estimated the point spread function of the laser focus by fitting the 3PEF-FITC signal transition at blood vessel boundaries in the lateral and axial directions, assuming a Gaussian beam focus. We found that the lateral beam radius was relatively constant with imaging depth and averaged to ∼1.4 µm (Fig. 2(B)), while the axial beam radius increased with imaging depth, consistent with the effects of spherical aberration (Fig. 2(C)). We measured blood flow speed in individual vessels by taking line scans along the vessel axis. RBCs show up as a dark streak or bright line in the resulting 3PEF-FITC or THG space-time image data, respectively (see vessel images and corresponding THG line scan data on the right in Fig. 2(A)). The slope of these streaks is inversely proportional to the blood flow speed. The contrast in the THG line scan measurement was high and nearly constant to a depth of about 500 µm and then decreased with depth (Fig. 2(D)). Blood flow speed, determined from the THG line scan data, decreased with decreasing diameter in arterioles, was slowest in capillaries, and increased slightly with vessel diameter in venules (Fig. 2(E)). Capillary blood flow speeds were relatively constant as a function of cortical depth (Fig. 2(F)). There was an apparent increase in capillary flow speeds at the deepest depths. This finding is consistent with recently published work by Li, B. et al. [18], where they saw increased average capillary RBC flux and a trend toward increased average capillary RBC speed in the white matter (0.90 to 1.05 mm depth from the surface) compared to the measurements made in the gray matter (0.2 - 0.8 mm depth). The deepest capillary in which we were able to measure blood flow speed was in the sub-cortical white matter at a depth of 1,060 µm (Fig. 2(G)).
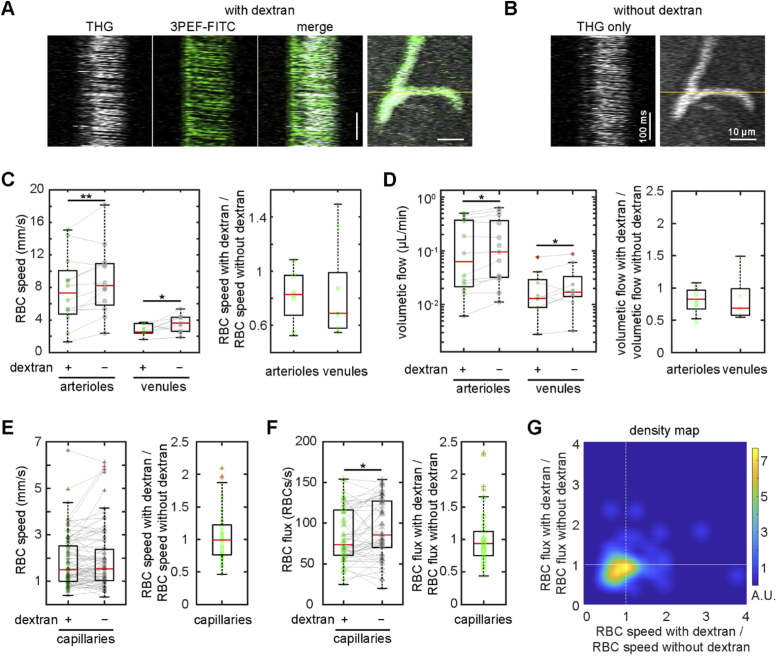
We reimaged the same mice two days after the initial imaging session, but without injecting FITC-labeled dextran intravenously. Even without a fluorescent tracer in the blood plasma, we were able to visualize the vascular network from the THG signal alone and repeated measurements of blood flow speed in as many of the same arterioles, capillaries, and venules from the first imaging session that we could identify (Fig. 3(A) and (B)). By measuring the same vessels both with and without the intravascular dextran present, we found that dextran administration decreased average RBC speed in both arterioles (14% +/- 16% decrease (mean +/- SD), p = 0.0053) and venules (21% +/- 35% decrease, p = 0.047) (Fig. 3(C)). A corresponding decrease was also found in average volumetric blood flow in arterioles (13% +/- 24% decrease, p = 0.034) and venules (19% +/- 30% decrease, p = 0.049) (Fig. 3(D)). In contrast, mean capillary RBC speeds were unchanged by the presence of dextran (Fig. 3(E)). We did find that the mean RBC flux in capillaries decreased by 7% +/- 30% after dextran injection (p = 0.025) (Fig. 3(F)). Given the large natural variations in RBC speed and hematocrit in capillaries, we examined the distribution of RBC flux and speed changes after dextran injection for all capillaries measured (Fig. 3(G)). In about half of the capillaries flow speed slowed, while in about two-thirds of capillaries the RBC flux was reduced, and the overall center of mass of the distribution was shifted toward lower RBC flux and speed.
Fig. 3.
Intravenous injection of high-molecular weight dextran slowed RBC flow speed in arterioles and venules, but decreased RBC flux rather than speed in capillaries. (A) Representative line scan data taken using THG (left, greyscale), 3PEF-FITC (middle, green), and merged (right), and an average projection of a merged THG and 3PEF-FITC image stack for a cortical capillary. The yellow line indicates the line scan path. (B) Line scan and average projection of the same capillary taken a few days later using THG, now without the intravenous FITC-dextran present. (C) RBC speed measured by THG line scans in cortical arterioles or venules taken first with intravenous high molecular weight dextran present, and then repeated two days later without the dextran (left) (12 arterioles and 9 venules from 4 mice). Measurements from the same vessels are linked with a solid line. RBC speed with dextran normalized to the speed without dextran for arterioles and venules (right). (D) Volumetric flow for arterioles and venules taken with and without dextran (left). We used the measurement of vessel diameter taken with 3PEF-FITC, when dextran was present, for both estimates of volumetric flow. Ratio of volumetric flow with dextran to that without dextran for arterioles and venules (right). (E) RBC speed in capillaries with and without dextran dye (left) and the corresponding RBC speed ratios (right) (77 capillaries from 4 mice). Two outliers were excluded from the RBC speed ratio plot: 2.9 and 3.8. (F) RBC flux with and without dextran (left) and the corresponding RBC flux ratios (right). (G) Density map of measured capillaries, plotted as function of the ratios of RBC flux and RBC speed with and without dextran. The density map was created by smoothing a scatter plot of the data from individual capillaries with a gaussian smoothing kernel that had a standard deviation of 1.5 along both axes. Scale bars in A and B are 100 ms and 10 µm. * p<0.05, ** p<0.01. Data were normally distributed for RBC speed (panel C) and arteriolar volumetric flow (panel D) so paired t-tests were used. Venular volumetric flow (panel D), RBC speed (panel E), RBC flux (panel F) were not normally distributed so the Wilcoxon test was used.
Finally, to verify that the reduction in blood flow speed we observed was solely due to dextran administration, we performed a similar experiment using Doppler optical coherence tomography (OCT) (Fig. 4(A)). Doppler OCT measurements were taken before and after injection of the same concentration and volume of FITC conjugated dextran used in the multiphoton imaging described above. Flow speeds were calculated in large diameter ascending venules, as the Doppler OCT signals from these vessels were typically less noisy and phase unwrapping was not necessary for the analysis. We found that dextran injection, on average, caused a significant reduction in both maximum blood flow speed (7% +/- 8% decrease, p = 0.0003) and in volumetric blood flow (10% +/- 13% decrease, p < 0.0001).
4. Discussion
In this work, we explored the use of THG for studying the structure and function of the cortical microvasculature in mice. In the cortex, the two major sources of THG signal came from RBCs in blood vessels and from myelinated nerve fiber tracts. In addition, we observed some un-identified punctate round objects near the cortical surface that produced strong THG, perhaps intracellular compartments in leukocytes (Fig. 1(A)). Some larger surface arterioles also exhibited signal in the THG channel from a structure in the vessel wall. Using 1,300-nm excitation light led to strong THG from RBCs, likely due to the resonant enhancement of THG by the Soret-band transitions of oxy- and deoxy-hemoglobin at 415 and 430 nm, respectively [7,8]. This strong THG from RBCs allowed us to visualize the vasculature, label free, and to quantify blood flow speeds by tracking the motion of individual RBCs in vessels to a depth of ∼1 mm beneath the cortical surface. While producing a glass-covered cranial window is standard procedure for brain imaging studies, the THG from RBCs is bright enough to enable through-skull imaging. Indeed, recent work by Wang et al. demonstrated THG imaging of RBCs using 1300-nm excitation through the intact skull and up to 500 µm into the cortex [29]. Larger diameter vessels showed a dimmer THG signal from the center of the vessel as compared to the edges, likely due to the absorption of the THG light by hemoglobin before it could propagate out of the vessel. Vessels with diameters of a few tens of micrometers displayed the brightest THG signal near the bottom edge of the vessel, where the THG produced had the least remaining blood in the vessel to propagate through before having an opportunity to scatter back out of the brain and be detected. Although we could visualize microvessels using the THG signal alone, there are several challenges with using this signal for label-free studies of hemodynamics. First, we would tend to miss even seeing capillaries with insufficient cellular flow due to temporary stalls [30,31] in them. The capillary wall does not generate a significant THG signal, thus visualization of a capillary with THG relies solely on the signal that comes from the blood cells inside the vessel lumen. Second, once imaging at depths of greater than about 900 µm, where white matter tracts are prominent, it can become difficult to distinguish blood vessels due to the strong THG from myelinated axon fibers [32]. Nonetheless, the THG from RBCs with 1,300-nm excitation light is a robust imaging signal that can be used alone or in combination with other optical labels to explore functional properties of the vasculature. We used this signal to characterize the CFL and to explore the impact of high molecular weight dextran injection on cortical blood flow.
The reduced cellular density near the wall of flowing blood vessels, called the cell free layer, plays an important role in the hemodynamic properties of microvascular networks and in molecular signaling important for vascular function (e.g. NO production and scavenging) [33,34]. Measurement of the thickness of the CFL and how it varies across a vessel network or in response to perturbations requires a technique that can visualize the vessel wall as well as the location of RBCs. This has dominantly been done using linear optical absorption microscopy with wavelengths of light strongly absorbed by RBCs together with manual identification of the vessel wall in the images [35–37]. This approach is fundamentally limited to measurements in two-dimensional vascular beds, such as that in the externalized cremaster muscle, and is poorly suited to examining the smallest of blood vessels. Here, we showed that combining THG from RBCs with 3PEF imaging of a fluorescent blood plasma label enables quantification of the thickness of the CFL in cortical microvessels. Because we identify the location of the vessel lumen and of RBCs using nonlinear optical signals, this approach works well in complex, three-dimensional vascular beds. We defined the CFL thickness as the difference of the FWHM intensity of 3PEF from a 2-MDa fluorescently-labeled dextran and the THG signal from flowing RBCs, and found that CFL increased rapidly with vessel diameter in capillaries and then stabilized around 1 µm in vessels with diameter larger than ∼10 µm. Using this definition of the CFL, we did not see continued increase in CFL thickness with vessel diameter as has been observed previously [35]. This may be due to confounding effects of signal in the THG channel we saw from a structure in the vessel wall in some larger diameter arterioles and perhaps due to the fact that this high molecular weight dextran does not fully penetrate the glycocalyx [38] and thus we may underestimate the full CFL thickness. The CFL quantified in Fig. 1(E) may better represent the physical distance between RBCs in the vessel lumen and the start of the glycocalyx layer or a portion of glycocalyx layer [38,39]. Using fluorescent labels that bind specifically to the glycocalyx (e.g. fluorescently conjugated wheat germ agglutinin) or to the vessel wall, instead of labeling the blood plasma, could enable more precise CFL measurements using THG and 3PEF. Using a smaller molecular weight dextran that more readily penetrated the glycocalyx could also help [38].
Blood plasma labeling with fluorescently-labeled high molecular weight dextran has been widely used to study hemodynamics [17,18,40,41]. A high molecular weight dextran is conjugated to the fluorescent tracer because it is slowly cleared from the blood, enabling several hours-long imaging of the vasculature. However, it remains poorly understood how the presence of this tracer alters the hemodynamic properties of the network, especially at the microvascular level. The THG contrast generated by RBCs allowed us to directly measure the blood flow speed in individual vessel segments with and without dextran administration, and we saw significant slowing of flow speed in both arterioles and venules, consistent with previous findings in venules [42] and validated with OCT imaging in this study. We found a smaller blood flow reduction after dextran injection using OCT than with THG imaging, perhaps due to the different size of venules measured with each modality (mean venule diameter for THG imaging was 14 µm, for OCT it was 27 µm). The increase in blood viscosity due to the injection of the dextran likely underlies this slowing of flow speeds in larger arterioles and venules. In capillaries, we observed a small decrease in RBC flux across capillaries due to dextran administration, but no reduction in blood flow speed, on average. The decrease in capillary RBC flux may be due to an enhancement of the Faehraeus effect – the reduction of hematocrit with vessel diameter – due to increased RBC aggregation caused by the dextran [43,44]. It is also possible to have RBC trapping in other organs that could be dependent on dextran administration, such as trapping in the spleen due to RBC aggregation [45]. This would be leading to a depletion of circulating RBCs, thus decreasing the number of RBCs in vessels. The concentration (100 µl of 5% w/v in saline) and the size (2 MDa) of dextran we used was a dose that lies near the high range of what has been used in hemodynamic studies in the past. It is almost certain that using smaller fluorescent dextran and using less volume will have lesser impacts on flow speed and flux.
Funding
National Institute of Neurological Disorders and Stroke10.13039/100000065 (NS100447, NS104350); American Heart Association10.13039/100000968 (16PRE27600010); Chan Zuckerberg Initiative10.13039/100014989.
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Centonze V. E., White J. G., “Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging,” Biophys. J. 75(4), 2015–2024 (1998). 10.1016/S0006-3495(98)77643-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipfel W. R., Williams R. M., Webb W. W., “Nonlinear magic: multiphoton microscopy in the biosciences,” Nat. Biotechnol. 21(11), 1369–1377 (2003). 10.1038/nbt899 [DOI] [PubMed] [Google Scholar]
- 3.Kobat D., Durst M. E., Nishimura N., Wong A. W., Schaffer C. B., Xu C., “Deep tissue multiphoton microscopy using longer wavelength excitation,” Opt. Express 17(16), 13354–13364 (2009). 10.1364/OE.17.013354 [DOI] [PubMed] [Google Scholar]
- 4.Squier J. A., Müller M., Brakenhoff G. J., Wilson K. R., “Third harmonic generation microscopy,” Opt. Express 3(9), 315–324 (1998). 10.1364/OE.3.000315 [DOI] [PubMed] [Google Scholar]
- 5.Yelin D., Silberberg Y., “Laser scanning third-harmonic-generation microscopy in biology,” Opt. Express 5(8), 169–175 (1999). 10.1364/OE.5.000169 [DOI] [PubMed] [Google Scholar]
- 6.Weigelin B., Bakker G.-J., Friedl P., “Third harmonic generation microscopy of cells and tissue organization,” J. Cell Sci. 129(2), 245–255 (2016). 10.1242/jcs.152272 [DOI] [PubMed] [Google Scholar]
- 7.Clay G. O., Millard A. C., Schaffer C. B., Aus-der-Au J., Tsai P. S., Squier J. A., Kleinfeld D., “Spectroscopy of third-harmonic generation: evidence for resonances in model compounds and ligated hemoglobin,” J. Opt. Soc. Am. B 23(5), 932–950 (2006). 10.1364/JOSAB.23.000932 [DOI] [Google Scholar]
- 8.Chang C. F., Yu C. H., Sun C. K., “Multi-photon resonance enhancement of third harmonic generation in human oxyhemoglobin and deoxyhemoglobin,” J. Biophotonics 3(10-11), 678–685 (2010). 10.1002/jbio.201000045 [DOI] [PubMed] [Google Scholar]
- 9.Xia F., Wu C., Sinefeld D., Li B., Qin Y., Xu C., “In vivo label-free confocal imaging of the deep mouse brain with long-wavelength illumination,” Biomed. Opt. Express 9(12), 6545–6555 (2018). 10.1364/BOE.9.006545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar M. J., Wise F. W., Fetcho J. R., Schaffer C. B., “In Vivo Imaging of Myelin in the Vertebrate Central Nervous System Using Third Harmonic Generation Microscopy,” Biophys. J. 100(5), 1362–1371 (2011). 10.1016/j.bpj.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Small D. M., Jones J. S., Tendler I. I., Miller P. E., Ghetti A., Nishimura N., “Label-free imaging of atherosclerotic plaques using third-harmonic generation microscopy,” Biomed. Opt. Express 9(1), 214–229 (2018). 10.1364/BOE.9.000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehberg M., Krombach F., Pohl U., Dietzel S., “Label-Free 3D Visualization of Cellular and Tissue Structures in Intact Muscle with Second and Third Harmonic Generation Microscopy,” PLoS One 6(11), e28237 (2011). 10.1371/journal.pone.0028237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietzel S., Pircher J., Nekolla A. K., Gull M., Brandli A. W., Pohl U., Rehberg M., “Label-free determination of hemodynamic parameters in the microcirculaton with third harmonic generation microscopy,” PLoS One 9(6), e99615 (2014). 10.1371/journal.pone.0099615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C. H., Wang T. D., Hsieh C. H., Huang S. H., Lin J. W., Hsu S. C., Wu H. T., Wu Y. M., Liu T. M., “Imaging Cytometry of Human Leukocytes with Third Harmonic Generation Microscopy,” Sci. Rep. 6(1), 37210 (2016). 10.1038/srep37210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saytashev I., Glenn R., Murashova G. A., Osseiran S., Spence D., Evans C. L., Dantus M., “Multiphoton excited hemoglobin fluorescence and third harmonic generation for non-invasive microscopy of stored blood,” Biomed. Opt. Express 7(9), 3449–3460 (2016). 10.1364/BOE.7.003449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santisakultarm T. P., Cornelius N. R., Nishimura N., Schafer A. I., Silver R. T., Doerschuk P. C., Olbricht W. L., Schaffer C. B., “In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in mice,” Am. J. Physiol-Heart C 302(7), H1367–H1377 (2012). 10.1152/ajpheart.00417.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih A. Y., Ruhlmann C., Blinder P., Devor A., Drew P. J., Friedman B., Knutsen P. M., Lyden P. D., Mateo C., Mellander L., Nishimura N., Schaffer C. B., Tsai P. S., Kleinfeld D., “Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture,” Microcirculation 22(3), 204–218 (2015). 10.1111/micc.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B., Ohtomo R., Thunemann M., Adams S. R., Yang J., Fu B., Yaseen M. A., Ran C., Polimeni J. R., Boas D. A., Devor A., Lo E. H., Arai K., Sakadzic S., “Two-photon microscopic imaging of capillary red blood cell flux in mouse brain reveals vulnerability of cerebral white matter to hypoperfusion,” J. Cereb. Blood Flow Metab. 40(3), 501–512 (2020). 10.1177/0271678X19831016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinfeld D., Mitra P. P., Helmchen F., Denk W., “Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex,” Proc. Natl. Acad. Sci. U. S. A. 95(26), 15741–15746 (1998). 10.1073/pnas.95.26.15741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotb A. M., Muller T., Xie J., Anand-Apte B., Endlich K., Endlich N., “Simultaneous assessment of glomerular filtration and barrier function in live zebrafish,” Am. J. Physiol-Renal 307(12), F1427–F1434 (2014). 10.1152/ajprenal.00029.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtmaat A., Bonhoeffer T., Chow D. K., Chuckowree J., De Paola V., Hofer S. B., Hubener M., Keck T., Knott G., Lee W. C., Mostany R., Mrsic-Flogel T. D., Nedivi E., Portera-Cailliau C., Svoboda K., Trachtenberg J. T., Wilbrecht L., “Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window,” Nat. Protoc. 4(8), 1128–1144 (2009). 10.1038/nprot.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldey G. J., Roumis D. K., Glickfeld L. L., Kerlin A. M., Reid R. C., Bonin V., Schafer D. P., Andermann M. L., “Removable cranial windows for long-term imaging in awake mice,” Nat. Protoc. 9(11), 2515–2538 (2014). 10.1038/nprot.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateo C., Avermann M., Gentet L. J., Zhang F., Deisseroth K., Petersen C. C., “In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition,” Curr. Biol. 21(19), 1593–1602 (2011). 10.1016/j.cub.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 24.Komiyama T., Sato T. R., O’Connor D. H., Zhang Y. X., Huber D., Hooks B. M., Gabitto M., Svoboda K., “Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice,” Nature 464(7292), 1182–1186 (2010). 10.1038/nature08897 [DOI] [PubMed] [Google Scholar]
- 25.Tsai P. S., Mateo C., Field J. J., Schaffer C. B., Anderson M. E., Kleinfeld D., “Ultra-large field-of-view two-photon microscopy,” Opt. Express 23(11), 13833–47 (2015). 10.1364/OE.23.013833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pologruto T. A., Sabatini B. L., Svoboda K., “ScanImage: Flexible software for operating laser scanning microscopes,” BioMed. Eng. OnLine 2(1), 13 (2003). 10.1186/1475-925X-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura N., Schaffer C. B., Friedman B., Lyden P. D., Kleinfeld D., “Penetrating arterioles are a bottleneck in the perfusion of neocortex,” Proc. Natl. Acad. Sci. 104(1), 365–370 (2007). 10.1073/pnas.0609551104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasan V. J., Atochin D. N., Radhakrishnan H., Jiang J. Y., Ruvinskaya S., Wu W., Barry S., Cable A. E., Ayata C., Huang P. L., Boas D. A., “Optical coherence tomography for the quantitative study of cerebrovascular physiology,” J. Cereb. Blood Flow Metab. 31(6), 1339–1345 (2011). 10.1038/jcbfm.2011.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T., Ouzounov D. G., Wu C., Horton N. G., Zhang B., Wu C. H., Zhang Y., Schnitzer M. J., Xu C., “Three-photon imaging of mouse brain structure and function through the intact skull,” Nat. Methods 15(10), 789–792 (2018). 10.1038/s41592-018-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz Hernández J. C., Bracko O., Kersbergen C. J., Muse V., Haft-Javaherian M., Berg M., Park L., Vinarcsik L. K., Ivasyk I., Rivera D. A., Kang Y., Cortes-Canteli M., Peyrounette M., Doyeux V., Smith A., Zhou J., Otte G., Beverly J. D., Davenport E., Davit Y., Lin C. P., Strickland S., Iadecola C., Lorthois S., Nishimura N., Schaffer C. B., “Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models,” Nat. Neurosci. 22(3), 413–420 (2019). 10.1038/s41593-018-0329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdener S. E., Tang J., Sajjadi A., Kilic K., Kura S., Schaffer C. B., Boas D. A., “Spatio-temporal dynamics of cerebral capillary segments with stalling red blood cells,” J. Cereb. Blood Flow Metab. 39(5), 886–900 (2019). 10.1177/0271678X17743877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koizumi K., Hattori Y., Ahn S. J., Buendia I., Ciacciarelli A., Uekawa K., Wang G., Hiller A., Zhao L., Voss H. U., Paul S. M., Schaffer C., Park L., Iadecola C., “Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function,” Nat. Commun. 9(1), 3816 (2018). 10.1038/s41467-018-06301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makena Hightower C., Salazar Vázquez B. Y., Woo Park S., Sriram K., Martini J., Yalcin O., Tsai A. G., Cabrales P., Tartakovsky D. M., Johnson P. C., Intaglietta M., “Integration of cardiovascular regulation by the blood/endothelium cell-free layer,” WIREs Syst. Biol. Med. 3(4), 458–470 (2011). 10.1002/wsbm.150 [DOI] [PubMed] [Google Scholar]
- 34.Ong P. K., Jain S., Kim S., “Temporal variations of the cell-free layer width may enhance NO bioavailability in small arterioles: Effects of erythrocyte aggregation,” Microvasc. Res. 81(3), 303–312 (2011). 10.1016/j.mvr.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 35.Kim S., Kong R. L., Popel A. S., Intaglietta M., Johnson P. C., “Temporal and spatial variations of cell-free layer width in arterioles,” Am. J. Physiol-Heart C 293(3), H1526–H1535 (2007). 10.1152/ajpheart.01090.2006 [DOI] [PubMed] [Google Scholar]
- 36.Namgung B., Kim S., “Effect of uneven red cell influx on formation of cell-free layer in small venules,” Microvasc. Res. 92, 19–24 (2014). 10.1016/j.mvr.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 37.Yalcin O., Jani V. P., Johnson P. C., Cabrales P., “Implications Enzymatic Degradation of the Endothelial Glycocalyx on the Microvascular Hemodynamics and the Arteriolar Red Cell Free Layer of the Rat Cremaster Muscle,” Front. Physiol. 9, 168 (2018). 10.3389/fphys.2018.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutuzov N., Flyvbjerg H., Lauritzen M., “Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier,” Proc. Natl. Acad. Sci. U. S. A. 115(40), E9429–E9438 (2018). 10.1073/pnas.1802155115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelm I., Nyul-Toth A., Suciu M., Hermenean A., Krizbai I. A., “Heterogeneity of the blood-brain barrier,” Tissue Barriers 4(1), e1143544 (2016). 10.1080/21688370.2016.1143544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davalos D., Kyu Ryu J., Merlini M., Baeten K. M., Le Moan N., Petersen M. A., Deerinck T. J., Smirnoff D. S., Bedard C., Hakozaki H., Gonias Murray S., Ling J. B., Lassmann H., Degen J. L., Ellisman M. H., Akassoglou K., “Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation,” Nat. Commun. 3(1), 1227 (2012). 10.1038/ncomms2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill R. A., Tong L., Yuan P., Murikinati S., Gupta S., Grutzendler J., “Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes,” Neuron 87(1), 95–110 (2015). 10.1016/j.neuron.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Namgung B., Ng Y. C., Nam J., Leo H. L., Kim S., “Alteration of Blood Flow in a Venular Network by Infusion of Dextran 500: Evaluation with a Laser Speckle Contrast Imaging System,” PLoS One 10(10), e0140038 (2015). 10.1371/journal.pone.0140038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop J. J., Popel A. S., Intaglietta M., Johnson P. C., “Effect of aggregation and shear rate on the dispersion of red blood cells flowing in venules,” Am. J. Physiol-Heart C 283(5), H1985–H1996 (2002). 10.1152/ajpheart.00888.2001 [DOI] [PubMed] [Google Scholar]
- 44.Pribush A., Zilberman-Kravits D., Meyerstein N., “The mechanism of the dextran-induced red blood cell aggregation,” Eur. Biophys. J. 36(2), 85–94 (2007). 10.1007/s00249-006-0107-1 [DOI] [PubMed] [Google Scholar]
- 45.Lipowsky H. H., “Microvascular rheology and hemodynamics,” Microcirculation 12(1), 5–15 (2005). 10.1080/10739680590894966 [DOI] [PubMed] [Google Scholar]