Abstract
Continuous contamination of the environment with xenobiotics and related recalcitrant compounds has emerged as a serious pollution threat. Bioremediation is the key to eliminating persistent contaminants from the environment. Traditional bioremediation processes show limitations, therefore it is necessary to discover new bioremediation technologies for better results. In this review we provide an outlook of alternative strategies for bioremediation via synthetic biology, including exploring the prerequisites for analysis of research data for developing synthetic biological models of microbial bioremediation. Moreover, cell coordination in synthetic microbial community, cell signaling, and quorum sensing as engineered for enhanced bioremediation strategies are described, along with promising gene editing tools for obtaining the host with target gene sequences responsible for the degradation of recalcitrant compounds. The synthetic genetic circuit and two-component regulatory system (TCRS)-based microbial biosensors for detection and bioremediation are also briefly explained. These developments are expected to increase the efficiency of bioremediation strategies for best results.
Keywords: synthetic biology, bioremediation, xenobiotics, genetic circuit, biosensor
Introduction
The remediation processes aided by microorganisms present at the various contaminated scenarios constitute bioremediation (Basu et al., 2018; Kumar et al., 2019). Microbial remediation uses multiple metabolic pathways responsible for enzyme production (Sharma B. et al., 2018; Dangi et al., 2019). These enzymes mainly take part in the degradation pathways of xenobiotics (Junghare et al., 2019). There are different customary methods for bioremediation, primarily based on the site of bioremediation, in and ex situ (Tomei and Daugulis, 2013). In situ is applied to the site to minimize soil disturbance. This method is mostly adopted due to less expenditure from avoiding excavation and transport of contaminated soil (Khan et al., 2004). According to Khan et al., 2004 less disruption in in situ bioremediation causes less dust dispersion and hence better degradation (Joshi et al., 2016) of contaminant. Bioaugmentation, bioventing, biosparging, and engineered in situ bioremediation are main in situ bioremediation methods (Azubuike et al., 2016). Ex situ bioremediation methods are solid phase system (composting, landfarming, and biopiling) and slurry phase system (bioreactors) (Kumar et al., 2011). Transportation of soil to accelerate microbial degradation are done by solid and slurry phase systems, whereby treatments of domestic, industrial, and organic waste are done by ex situ bioremediation (Juwarkar et al., 2010). These traditional bioremediation methods take time and consume much cost expenditure, giving less result output. Traditional bioremediation (Duarte et al., 2017) processes showed the above limitations of extra time taking, less removal or dissimilation of pollutants, (Bharagava et al., 2019) disturbance to nature delicacy such as more land coverage for a long time, and a foul smell in the environment (Dangi et al., 2019; Kumar, 2019). Therefore, researchers are eager to discover new bioremediation technologies for best results. Dvoøák et al. (2017) described bioremediation via synthetic biology for boosting bioremediation strategies. This approach can catch the catabolic (Jacquiod et al., 2014) and metabolic complexities for reviewing the potential of the microbial population synthetically. The preliminary information for developing synthetic microbial models for bioremediation can be obtained by mining genes from the databases (Fajardo et al., 2019). The computer logics involvement can determine the microbial cell interactions with recalcitrant compounds (Kim et al., 2015). These strategies can together grasp the natural metabolic potential of microorganisms to transform into novel biological entities of interest (Dangi et al., 2019). Furthermore, the regulation of metabolic pathways (Alves et al., 2018) in a controlled manner can also be achieved for bioremediation processes (Rochfort, 2005). This transition via synthetic biology application (Figure 1) for remediation purposes would improve the bioremediation processes via the involvement of potent (Zhu et al., 2017) dissimilating particular contaminants (Trigo et al., 2008). Synthetic biological systems mediate cellular modulations for efficient functioning and working of existing processes. They permit the modification of cellular processes viz. metabolic pathway acting for a particular chemical compound. The advancement of synthetic biology for bioremediation of various contaminants is attaining the focus of scientists and researchers. For instance, a sustainable synthetic microbial community’s establishment for bioremediation is being investigated. Microbial interactions and quorum sensing within communities are vastly studied for application in the area of bioremediation with synthetic biology applications. Achievement of the synthetic genetic circuit of Pseudomonas putida proved to be the golden gadget for degradation studies. Besides this, genome editing by CRISPR-Cas, TALEN, and ZFNs adds knowledge for reviewing the progression in bioremediation studies. Synthetic microbial biosensors and metabolic engineering of cellular processes for utilization and detection of contaminant residues will remediate the environment from persistent recalcitrant pollutants. This review is focused on the above mentioned strategies and their elements (Figure 2) applicable for bioremediation purposes and research.
FIGURE 1.
The strategies of synthetic biology applicable for bioremediation.
FIGURE 2.
The components and their construction elements of synthetic biology for bioremediation studies.
Metabolic Reconstruction for Designing Synthetic Models
A computational platform is utilized for the reconstruction of cellular metabolism (Agapakis et al., 2012) via metabolic pathway analysis (MPA) (Banerjee et al., 2016). MPA mathematically represents the reactions of metabolism. This method is based on stoichiometric balance reactions so as to propose steady-state metabolic flux during cellular growth. The stoichiometry matrix imposes constraints of flux, making the consumption and production of the compound at a steady state (Bordbar et al., 2014). The maximum and minimum flux of a reaction can also be determined by providing the topper and least bound. This helps to define the extent of permissible flux supply (Richelle et al., 2016; Rawls et al., 2019). The next step is defining the objective according to the biological problem to be studied. This objective mathematically represents the reactions responsible for the phenotype appearance. The mathematical reactions and phenotype are combined with linear equations and solved by computational algorithms such as COBRA Toolbox11 and Matlab toolbox (Orth et al., 2010; Bordel, 2014). FBA is fundamentally simple, having immense applications in studying gaps, physiology, and genomes via systems biology approach (Kim et al., 2015; Hellweger et al., 2016). These gaps are missing metabolic reactions, making the genome partially known. FBA uses computational algorithms that can predict missing reactions viz. OptKnock and OptCom, which can knock out the genes responsible for producing the desired compound (Biggs et al., 2015). These approaches are beneficial for constructing microbial communities for bioremediation of particular contaminants (Khandelwal et al., 2013). However, MPA is the most challenging method when metabolic information is incomplete, making it difficult to obtain a real model (Covert et al., 2001). But this method shows cellular functions in the dynamic community, and thus is very useful for the prediction and exchange of metabolic flux in communities of microorganisms (Khandelwal et al., 2013). Recently, a metabolic model has been constructed by using two Geobacter species with parameterized electron transfer and metabolic exchange to characterize syntrophic growth dynamics. Such a system may have useful applications in the field of bioremediation and degradation of particular contaminants (Butler et al., 2010). A computational platform is also needed for better prediction of engineered genetic pathways for community dynamics. A graph-based tool Metabolic Tinker was developed by McClymont and Soyer to identify thermodynamically feasible biochemical routes for compounds deterioration (Johns et al., 2016). This may be applied to identify the routes for degradation of recalcitrant compounds by microbial consortia. These computational tools are utilized along with omics (Kim et al., 2014; El Amrani et al., 2015) and biological data for desired output (Berger et al., 2013) and toxicity prediction (i.e., META-CASETOX System) (Peijnenburg and Damborský, 2012). These are also applied for functional gene identification and their profile analysis, PCR analysis and drug discovery, etc (Dangi et al., 2019). Computer-aided drug discovery and development (CADDD) is used effectively with chemical and biological aspects, i.e., chemical structures accounting the biological role and its activity via ligand-based drug design, structure-based drug design, quantitative structure-property relationships, and quantitative structure-activity (Kapetanovic, 2008). Furthermore, Table 1 depicts similar methodologies applicable to bioremediation studies. De Jong (2002) analyzed the multicellular feedback control strategy in a bacterial consortium (Bruneel et al., 2011) to define the robustness conceivable
TABLE 1.
The methodologies applied for bioremediation research.
| S.no. | Purpose | Approach | Methodology | References |
| 1. | Degradation study | Functional gene identification for bioremediation | PCR (Polymerase Chain Reaction) product sequence analysis | Jagwani et al., 2018 |
| 2. | Cell behavior study | Whole-cell simulation | A computer model for bacterial cell in response to the contaminated environment | O’Brien et al., 2015; Panigrahi et al., 2019 |
| 3. | Toxicity of chemicals | Analysis of chemical and biological properties | In silico toxicology (IST) protocols for toxicity assessment; QSAR (Quantitative Structure Activity Relationship) model | Pavan and Worth, 2006; Myatt et al., 2018 |
| 4. | Identification of functional bioremediating microbe | Target identification | Protein structure prediction, Protein – protein interaction (PPIs) | Shukla, 2017 |
| 5. | Remediation of textile dyes | Interaction of protein-ligand | Molecular docking | Sridhar and Chandra, 2014; Kumar et al., 2016 |
| 6. | Bioremediation of toxic pollutants | Structure prediction | Biodegradability evaluation and simulation system | Das et al., 2016 |
under desired conditions. They utilized an ordinary differential equations (ODE)-based model and agent-based simulation on a consortium (Hawley et al., 2014; Atashgahi et al., 2018) of interacting species population for increasing the efficacy of the proposed feedback control strategy. The application of bioinformatics (Arora and Bae, 2014) resources is a prerequisite dimension for obtaining the data to begin the microbial bioremediation studies of recalcitrant compounds (Gong et al., 2012; Ofaim et al., 2019). This involves the information related to the degradation of xenobiotics by microbes and their pathways for dissimilation (Dao et al., 2019; Salam and Ishaq, 2019; Thelusmond et al., 2019; Wei et al., 2019). The data related to end products and intermediate metabolites released throughout degradation pathways can also be retrieved (Dvoøák et al., 2017). An extended information source linked to degradation is MetaRouter, allowing data (Singh and Gothalwal, 2018) for life sciences laboratories to explore degradation possibilities of recalcitrant compounds (Mohanta et al., 2015). The information on oxygenic degradation of xenobiotics can be retrieved from OxDBase, a biodegradative oxygenase database (Chakraborty et al., 2014; Shah et al., 2018). Oxygenase is a class of enzyme which transfers the oxygen molecule for oxidizing the chemical compound. They play a role in the degradation of organic compounds by aromatic ring cleavage (Jadeja et al., 2014). OxDBase is very particular in providing knowledge of oxygenases-catalyzed reactions, and is a powerful tool applicable to bioremediation studies (Singh, 2018). Bioconversion and biodegradation of persistent and toxic xenobiotics (Desai et al., 2010; Bao et al., 2017) compounds catalyzed by oxygenases decrease the compound sustainability and toxicity in the environment (Kües, 2015; Kondo, 2017). Therefore, OxDBase is very helpful in acknowledging the degradation processes involved in bioremediation (Shah et al., 2012). The transcriptional characterization of genes responsible for the biodegradation and biodissimilation of a particular compound has great significance in proposing molecular methodologies. This can be done by the Bionemo (Biodegradation Network Molecular Biology) database (Libis et al., 2016a). Bionemo contains the entries for sequences of genes coding for biodegradation (Carbajosa and Cases, 2010). It also links the gene transcription and its regulation (Libis et al., 2016b). The data retrieved from Bionemo can be used for designing cloning experiments and primers (Arora and Shi, 2010). Garg et al. (2014) used eMolecules and the EAWAG-BBD PPS database for the prediction of pathways involved in the biodegradation of 1-naphthyl-N-methyl carbamate. These above findings empower the researchers to analyze and establish what prerequisites must be fulfilled for developing synthetic bioremediation models.
Designing the Synthetic Microbial Communities
Recent advancements in the field of synthetic biology for environmental issues have shown a great impact. The use of GMOs in environmental biotechnology for remediation (Malla et al., 2018) of toxic compounds, xenobiotics, and pesticidal compounds are being done. To design a synthetic community, it is important to understand natural microbial communities (Schloss and Handelsman, 2008). In a natural community, it is difficult to find out which species are actually taking part in bioremediation (Großkopf and Soyer, 2014). Thus, a synthetic microbial community is a promising method for constructing an artificial microbial community with function-specific species for bioremediation purposes. These communities may act as a model system for the study of functional, ecological, and structural characteristics in a controlled manner. Großkopf and Soyer (2014), defined synthetic community by the culturing of two microbial species under well-defined conditions on the basis of interaction and function (Bruggeman and Westerhoff, 2007). These factors determine the dynamics and structure of the community. It is based upon the identification of processes and patterns engaged in by bacterial species. These microbial interaction patterns are metabolism-driven and responsible for community interaction (Wintermute and Silver, 2010). Social-based microbial interactions (i.e., mutualism, cooperation, and competition, etc.) and the total outcome of these interactions between two microbial populations can be +/+ and −/+ or +/−, respectively (Foster and Bell, 2012). It is said that community structure and function majorly depends on cooperation. The effect of cooperation on community dynamics is determined by engineered cooperation resulting in the synthetic community. Engineered cooperation between two microbial strains (Singh et al., 2016) can be done by manipulation of environmental conditions, i.e., knocking the genes out and in Zuroff et al. (2013). Beyond this, other interaction patterns have been analyzed with engineered microbial species in the synthetic community. Such an application of engineered interaction is highly recognizable in bioremediation strategies (Sharma, 2012). Synthetic biology provides greater potential for the sustainable existence of microorganisms (Dellagnezze et al., 2014) acting together in a large population. Thus, synthetic microbial communities are proved as a key strategy for the bioremediation of contaminants, i.e., pesticides, petroleum (Kachienga et al., 2018), oil spill, acid drainage (Serrano and Leiva, 2017), etc. For building the synthetic microbial communities, the engineered interspecies and intraspecies interactions can make cellular functions robust and enhance the capabilities of microbial consortia in various contaminated scenarios. Quorum sensing is a bacterial signaling mechanism, which is a density-dependent phenomenon via cell-cell communication and population level behavior. The signaling is done by the release and reception of chemical compounds by microbial candidates in a population. This leads to multicellular behavior (Obst, 2007), offering engineerable tasks to design function specific synthetic communities. These synthetic models can also be exploited to obtain a rational design that can lose the function when subjected to competition with other species in the natural environment. With the evolution of genomic constituents and gene transfer, the possibility of the gradual extinction of genetic circuits is present. Thus, strategies are required to maintain the robustness of the synthetic community, achieved via the synthetic models by the development of synergistic and cooperative properties that reduce instability and loss of function (Johns et al., 2016). A recent study by Coyte et al. (2015) suggests that competition among species is significant in determining the stability of communities, acting as a limiting factor in the stability of the synthetic community. Thus, these dynamics must be accelerated in order to design particular function specific synthetic communities for bioremediation purposes (Coyte et al., 2015).
Genetic and Metabolic Engineering
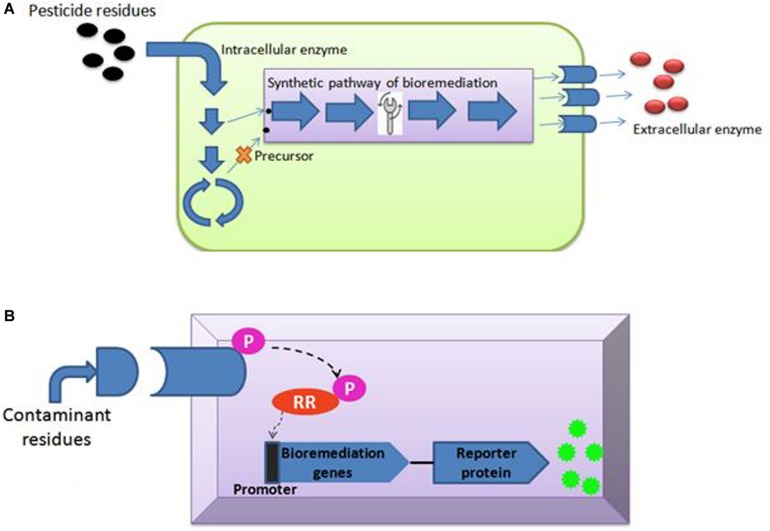
Enríquez (2016) said that genome editing is an umbrella term that refers to “scientific technological advances that enable rational genetic engineering at a local (gene) or global (genome) level to facilitate precise insertion, removal, or substitution of fragments of Deoxyribonucleic acid (“DNA”) molecules, comprising one or more nucleotides into the cell(s) of an organism’s genome.” Transcription-activators like effector nucleases (TALEN), clustered regularly interspaced short palindromic repeats (CRISPR-Cas), and zinc finger nucleases (ZFNs) are major gene editing tools used (Table 2). The most efficient and simple technique of gene editing has been described as CRISPR-Cas (Kanchiswamy et al., 2016). These tools can boost the process of bioremediation. TALEN has a DNA-binding modular which is sequence-specific for the host genome (Utturkar et al., 2013). TALEN binding to DNA creates a double stranded break (DSB) in the target sequence and leaves sticky ends for stability. Similarly, ZFNs is also a DNA-binding domain composed of 30 amino acids. It introduces DSB at the target site of the host genome by the Fok1 cleavage domain. A new sense of using hybrid nucleases containing TALENs and ZFNs nucleases came to act for solving the molecular complications. The CRISPR-Cas system, on the other hand, has unique action properties of high sequence specificity and multiplex gene editing. This unique property is derived from bacteria Streptococcus pyogenes as immunity against the virus. The CRISPR-Cas system consists of crisper derived RNA (crRNA) and trans acting antisense RNA (trcRNA) joined by guide RNA (gRNA). gRNA directs the Cas9 enzyme to introduce DSB in the target DNA sequence by recognizing it. These gene editing tools create the knock-in and knock-out and are under processing for implementation in bioremediation studies (Kumar V. et al., 2018). Recent reports indicate though that the CRISPR-Cas system is mostly adopted and performed by researchers in model organisms i.e., Pseudomonas (Karimi et al., 2015; Nogales et al., 2020) or Escherichia coli (Chen et al., 2018; Marshall et al., 2018; Pontrelli et al., 2018). Nowadays, the new insights toward CRISPR tool kits and designing gRNA for expression of function-specific genes related to remediation in non-model organisms (i.e., Rhodococcus ruber TH, Comamonas testosteroni and Achromobacter sp. HZ01) are also suggested in the field of bioremediation (Mougiakos et al., 2016; Jusiak et al., 2016; Hong et al., 2017; Stein et al., 2018; Tang et al., 2018; Liang et al., 2020). For gene editing and metabolic engineering, the contaminant-inhabited bacteria are the most suitable candidates because they are used to survive and harbor in stress conditions of various toxic, recalcitrant and non-degradable xenobiotics, as discussed above. Moreover, understanding metabolic pathways seems to be important in studying the microbial bioremediation (Plewniak et al., 2018), i.e., bioremediation of toxic pollutants by the haloalkane dehalogenases production pathway and decontamination of pyrethroid from the soil via the biodegradation pathway of fenpropathrin studied in Bacillus sp. DG-02 (Chen et al., 2014). Metabolic engineering leads to modification of the existing pathway for the betterment of the bioremediation process (Michel et al., 2007). This approach majorly covers the study of microbial enzymes, i.e., oxidases, esterases, monooxygenases, oxidoreductases, phenoloxidases involved at various degradation steps (Figure 3A; Mónica and Jaime, 2019; Mujawar et al., 2019). Enzyme-based bioremediation has many advantages because it is an eco-friendly process. The genetic approach increases the perspective of getting recombinant enzymes. There are research reports of extracellular enzymes having a role in enzymatic bioremediation. For instance, arsenic bioremediation (Andres and Bertin, 2016; Choe and Sheppard, 2016; Akhter et al., 2017; Biswas et al., 2019) (bioaccumulation and biotransformation) is achieved via arsenite oxidase coded by aioA gene of Klebsiella pneumonia (Mujawar et al., 2019); enzymes released by white rot fungi degrade PAHs (polycyclic aromatic hydrocarbon) (Zhao and Poh, 2008; Košnár et al., 2019), dyes, TNT (2,4,6- Trinitrotoluene) and PCBs (polychlorinated biphenyls) (Gupte et al., 2016; Kutateladze et al., 2018; Sadańoski et al., 2018). Esterase D enzyme acts on β-endosulfan (organochlorine pesticide), producing simpler compounds (Mehr et al., 2017; Chandra et al., 2019). LiPs encoding hemoproteins from Phanerochaete chrysosporium degrade PAHs. However, incomplete or partial degradation of contaminants lead to simpler non-toxic degradable compounds which can be consumed by microbes (Kumavath and Satyanarayana, 2014) as intermediates in biological pathways or substrate, i.e., LiP (lignin peroxidase) dissimilate benzopyrene to three compounds of quinine, namely 1,6- quinone, 6,12- quinine and 3,6- quinine (Gupta and Pathak, 2020). Furthermore, MnP (Manganese peroxidase) oxidizes organic compounds in the presence of Mn(II) (Xu et al., 2018; Singh et al., 2019). Laccase, MFO (mixed function oxidases), glutathione S transferase, cytochrome P450 also acts in biodegradation of recalcitrant compounds (Singh, 2019; Boudh et al., 2019). Catechol 1,2-dioxygenase (intracellular enzyme) from Pseudomonas NP-6 dissimilate catechol to muconate compounds (Guzik et al., 2011). Also, enzyme immobilization (Cavalca et al., 2013; Sharma B. et al., 2018) increases the half-life, stability, and enzyme activity at a notable level. The enzymatic bioremediation is an elementary, expeditious, and environmental friendly method for microbial removal and degradation of persistent xenobiotics compounds (Sharma B. et al., 2018). Isolation and characterization of microorganisms with enzymatic capabilities have been done with the limitation of less productivity (Rayu et al., 2012). Organophosphates (OP) and organochlorines (OC), major constituents of insecticides, accumulate in the agricultural soil (Panelli et al., 2017) and reach the water bodies via agricultural run-off. Effective bioremediation of γ-hexachlorocyclohexane (OC) and methyl parathion (OP) has been reported by genetically engineered microorganisms (Gong et al., 2016). Moreover, bioremediation of organophosphates and pyrethroids has been experimented with using genetically modified P. putida KT2440 (Zuo et al., 2015). With the advent of metabolic engineering, the catabolism and degradation of various persistent compounds has been reported. The degradation pathways of methyl parathion and γ-hexachlorocyclohexane in Sphingobium japonicum and Pseudomonas sp. WBC-3 witnessed the bioremediation strategy (Liu et al., 2005; Miyazaki et al., 2006). Furthermore, 1, 2, 3-trichloropropane, a persistent constituent of fumigant, is dissimilated into the environment (Techtmann and Hazen, 2016) via heterologous catabolism by the assembly of three enzymes from two different microorganisms in E. coli (Dvorak et al., 2014). A metabolic pathway (Bertin et al., 2011) degrading organophosphorus and paraoxon is engineered by inserting the organophosphorus hydrolase gene (opd) and pnp operon encoding enzymes that convert p-nitrophenol into β-ketoadipate in P. putida (de la Pena Mattozzi et al., 2006). A study showed pobA and chcpca gene clusters of Rhodococcus opacus R7 take part in the bioremediation of naphthenic acid; more specifically, expression aliA1 gene codes for fatty acid CoA ligase for degrading long chains of linear as well as alicyclic naphthenic acid (Zampolli et al., 2020). To minimize the accumulation, the above-mentioned strategy is attained using microbes for partial or complete mineralization of persistent compounds (Miyazaki et al., 2006).
TABLE 2.
Comparative features of CRISPR, TALE, and ZFNs.
| Features | Gene editing tools | References | ||
| CRISPR | TALEN | ZFNs | ||
| System | Adaptive immune system | Pathogenic Xanthomonas | Gene expression system | Kumar N. M. et al., 2018 |
| Specificity | crRNA | TALE Domain | Zn finger Domain | Jaiswal et al., 2019b |
| Cleavage | Cas9 | FokI nuclease | Nuclease | Kumar V. et al., 2018; Jaiswal et al., 2019b |
| Nucleases per target per experiment | Single or more sgRNA; singleCas9 | Single TALEN pair | Single ZFN pair | Hamilton et al., 2019 |
| Activity | High | High | Moderate | Shanmugam et al., 2019 |
| Designing and screening | Easy | Difficult | Difficult | Dangi et al., 2019 |
| Multiple gene editing | Suitable | Not suitable | Not suitable | Sinha et al., 2019 |
FIGURE 3.
Schematic presentation (A) intracellular and extracellular enzymes production; (B) TCRS based biosensor.
Synthetic Genetic Circuit and Microbial Biosensor
The synthetic genetic circuit requires chassis for implantation. The P. putida is a HVB (Host Vector Biosafety) strain recognized as safe by the Recombinant DNA Advisory Committee. It is also referred to as GRAS (Generally Recognized as Safe) to release in the environment. It is ideal for the next generation of synthetic biology chassis panel because it can withstand high intolerant changing conditions including temperature, pH, solvents, toxins, osmotic, and oxidative stress. Also, P. putida has versatile metabolism and low nutrient requirements (Pabo and Nekludova, 2000). These qualities make this organism the best bacterial model for environmental bioremediation applications (Tanveer et al., 2018). Recently, the P. putida synthetic genetic circuit has been established for the designing of promoter genes and expression of the gene responsible for the degradation of persistent compounds (Adams, 2016). An extension of synthetic biology is the integration of genome with reporter system, and synthetic promoters of P. putida may be developed for synthetic bioremediation pathways. Elmore et al. use serine integrases for synthetic genetic circuit development. Microbial cells have the advantage of a cellular system, which controls cell growth and response to external factors like temperature, light, pH, and oxygen (Tropel and Van Der Meer, 2004). The external environment of microbes inhabiting the contaminated site will respond to concentrations of various persistent compounds present (Ray et al., 2018; Antonacci and Scognamiglio, 2019). Whole cell biosensors for checking the presence, detection and biodegradation potential of xenobiotics compounds (pharmaceutical residues, pesticides, paraffin, PAHs and PCBs, etc.) present (Adhikari, 2019) in environmental samples are attaining attention (Wynn et al., 2017; Heng et al., 2018; Patel et al., 2019). The reporter proteins acting microbe makes a color signal at the detection of particular contaminants via transducer (Zhang and Liu, 2016). A biosensor aiming for detection and bioremediation purposes must have enhanced contact between microbe and contaminant (Dhar et al., 2019). This helps the bacterium to adjust their cellular pathways in response to external environmental conditions and encodes the genes for utilizing the recalcitrant compounds as substrate (Bilal and Iqbal, 2019; Skinder et al., 2020). Synthetic biology strategies are feasible for removing a particular toxic compound because the genetic circuits can be developed against the exogenous environmental toxin (Checa et al., 2012; Tay et al., 2017). The synthetic genetic circuits are assembled via a two-component regulatory system (TCRS) in bacteria (Futagami et al., 2014; Uluşeker et al., 2017). This system acts upon environmental change, and thereby, cells respond to these changes. A prokaryotic TCRS has histidine kinase (HK) and response regulator (RR). The HK is a sensor domain with an extracellular loop present as an integral membrane protein. HK also has a transmitter domain in the last cytoplasmic transmembrane, which is a highly conserved domain. Histidine phosphotransfer (DHp) and catalytic ATP-binding domain (CA) acts for molecular recognition of RR and ATP hydrolysis. The transmitter domain transmits the signal from periplasm to cytoplasm via PAS (Periodic circadian proteins, Aryl hydrocarbon nuclear translocator proteins, and single minded proteins), HAMP (HKs, Adenyltatecyclases, Methyltransferases, and Phosphodiesterases) and GAF (cGMP-specific phosphodiesterases, adenylyl cyclases, and formate hydrogenases) (Casino et al., 2010). Thus, HK senses the external environmental change and adds phosphate to conserved histidine. The HK also regulates RR by phosphorylating the aspartate residues. This promotes the promoter (Figure 3B) binding to activate the gene expression or vice-versa (Ravikumar et al., 2017). Therefore, TCRS-based synthetic biology application for biosensor development for cell-mediated detection and bioremediation can prove to be a new advancement.
Ecological Safety and Risk Assessment
The scientists and researchers are performing the experimental setup to study the bioremediation (Yergeau et al., 2012) potential against various pollutants like oil spill, plastics, synthetic dyes, organic hydrocarbons (Yadav et al., 2015), pesticides (Jaiswal et al., 2019a), heavy metals (Hemmat-Jou et al., 2018; Lebrazi and Fikri-Benbrahim, 2018), and other xenobiotics, etc (Rucká et al., 2017; Paniagua-Michel and Fathepure, 2018; Wu et al., 2020). Considering that bioremediation is performed in an open environment rather than in a closed fermentation tank, the ecological safety of bioremediation performing bacteria must be considered. Economic safety is justified by the metabolic aptness (Gillan et al., 2015) of microorganisms as compared to other traditional physical and chemical bioremediation methods. Besides, regulation for using genetically and metabolically modified bacteria is released to evaluate the possible risks (Khudur et al., 2019). The risk assessment is mainly done by regulatory agencies, i.e., Organization for Economic Cooperation and Development (OECD) at the application level for environmental safety (Russo et al., 2019; Alam and Murad, 2020; Pastor-Jáuregui et al., 2020). The possible risks are gene contamination in the native member of microbial consortium, leading to mislaying of the natural trait (Mills et al., 2019; Pineda et al., 2019; Rycroft et al., 2019). The competitiveness between natural and genetically modified species can give rise to selection pressure on non-target microflora (Kumar N. M. et al., 2018; Mohapatra et al., 2019). Moreover, environment and ecosystem risk assessments infer unpredictable and adverse effects, as discussed above (Cervelli et al., 2016; van Dorst et al., 2020). Particularly, the ecological risk assessment behind addition of GEMs (Genetically Engineered Microorganisms) (Benjamin et al., 2019; Ahankoub et al., 2020) to the native environment rather than a laboratory (Fernandez et al., 2019) is done mainly because of unnecessary delivery of antibiotic resistance marker along with recombinant genome of interest (Davison, 2002; Nora et al., 2019), and unintentional uptake or transfer of induced genes to other microorganisms (French et al., 2019; Janssen and Stucki, 2020). This phenomenon is definately disturbing the microbial native genome entity (Gangar et al., 2019; Petsas and Vagi, 2019). Another aspect come into considerance, the change in microbial metabolism (Okino-Delgado et al., 2019), will release uncertain toxic compounds for the environment and health, indirectly acting as opposition microbial candidates in this context (Myhr and Traavik, 2002). Under the TSCA (Toxic Substances Control Act) (Gardner and Gunsch, 2020), the Office of Pollution Prevention and Toxics (OPPT) programs (Pietro-Souza et al., 2020) of the United States Environmental Protection Agency (Leong and Chang, 2020; Saxena et al., 2020) moniters the environmental and health risks and releases premanufacture legal notice for the accreditation of field research outlines and prototypes (McPartland and McKernan, 2017; Khan et al., 2020). A magnificant example is given by University of Tennessee. In 1995, they gave application and suggested the risk evaluation of microbial bioremediation agents (mainly Pseudomonas fluorescens HK44) on the environment and health (Sayler et al., 1999; Khan et al., 2016; Sharma J. K. et al., 2018; El Zanfaly, 2019). Most remarkable is that the literature survey points toward a biowar weapon for humanity (Gómez-Tatay and Hernández-Andreu, 2019; Wang and Zhang, 2019), stating that gene editing tools left in bad hands could mislead ethical and moral duties (Khan, 2019; Thakur et al., 2019; Sharma et al., 2020).
Conclusion and Future Perspectives
The microbial bioremediation process for removal and detoxification of contaminants from the environment has now emerged as the best option. Synthetic biology is addressing the decontamination and remediation strategies for xenobiotics and related compounds from the environment. It has been observed that the requisite for understanding existing metabolic pathways is a must for developing synthetic models of bioremediation. Moreover, genomics reconstruction methods (Luo et al., 2014; Marco and Abram, 2019) and technologies made a solid platform for bioremediation studies. Satisfactory progress has been witnessed in the field of bioremediation among various contaminants with the role of specific genes and enzymes applicable via synthetic biology methodologies. Therefore, it is concluded that microbial synthetic biology remediation strategies not only increase the efficiency of microbial bioremediation processes for a particular contaminant, but also provide the best methodologies for researchers.
Author Contributions
SJ contributed to this article under the guidance of PS. SJ acknowledges the support and guidance of PS in pursuing a doctorate under his guidance.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The author SJ acknowledges Maharshi Dayanand University, Rohtak, India, for providing University Research Scholarship (URS). PS acknowledges the infrastructural support from Department of Science and Technology, New Delhi, Government of India, through FIST grant (Grant No. 1196 SR/FST/LS-I/2017/4).
References
- Adams B. L. (2016). The next generation of synthetic biology chassis: moving synthetic biology from the laboratory to the field. ACS Synth Biol. 5 1328–1330. 10.1021/acssynbio.6b00256 [DOI] [PubMed] [Google Scholar]
- Adhikari B. R. (2019). “Advances in the oligonucleotide-based sensor technology for detection of pharmaceutical contaminants in the environment,” in Tools, Techniques and Protocols for Monitoring Environmental Contaminants, eds Brar S. K., Hegde K., Pachapur V. L. (Amsterdam: Elsevier; ), 125–146. [Google Scholar]
- Agapakis C. M., Boyle P. M., Silver P. A. (2012). Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 8 527–535. 10.1038/nchembio.975 [DOI] [PubMed] [Google Scholar]
- Ahankoub M., Mardani G., Ghasemi-Dehkordi P., Mehri-Ghahfarrokhi A., Doosti A., Jami M. S., et al. (2020). Biodecomposition of phenanthrene and pyrene by a genetically engineered Escherichia coli. Recent Patents Biotechnol. 10.2174/1872208314666200128103513 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- Akhter M., Tasleem M., Alam M. M., Ali S. (2017). In silico approach for bioremediation of arsenic by structure prediction and docking studies of arsenite oxidase from Pseudomonas stutzeri TS44. International Biodeterioration & Biodegradation 122 82–91. [Google Scholar]
- Alam M. M., Murad M. W. (2020). The impacts of economic growth, trade openness and technological progress on renewable energy use in organization for economic co-operation and development countries. Renew. Energy 145 382–390. [Google Scholar]
- Alves L. D. F., Westmann C. A., Lovate G. L., de Siqueira G. M. V., Borelli T. C., Guazzaroni M. E. (2018). Metagenomic approaches for understanding new concepts in microbial science. Int. J. Genom. 2018:2312987. 10.1155/2018/2312987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres J., Bertin P. N. (2016). The microbial genomics of arsenic. FEMS Microbiol. Rev. 40 299–322. 10.1093/femsre/fuv050 [DOI] [PubMed] [Google Scholar]
- Antonacci A., Scognamiglio V. (2019). Biotechnological advances in the design of algae-based biosensors. Trends Biotechnol. 38 334–347. 10.1016/j.tibtech.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Arora P. K., Bae H. (2014). Integration of bioinformatics to biodegradation. Biol. Procedures Online 16:8. 10.1186/1480-9222-16-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. K., Shi W. (2010). Tools of bioinformatics in biodegradation. Rev. Environ. Sci. Bio/Technol. 9 211–213. [Google Scholar]
- Atashgahi S., Hornung B., Waals M. J., Rocha U. N., Hugenholtz F., Nijsse B., et al. (2018). A benzene-degrading nitrate-reducing microbial consortium displays aerobic and anaerobic benzene degradation pathways. Sci. Rep. 8:4490. 10.1038/s41598-018-22617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azubuike C. C., Chikere C. B., Okpokwasili G. C. (2016). Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 32:180. 10.1007/s11274-016-2137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C., Singh P. K., Shukla P. (2016). Microalgal bioengineering for sustainable energy development: recent transgenesis and metabolic engineering strategies. Biotechnol. J. 11 303–314. 10.1002/biot.201500284 [DOI] [PubMed] [Google Scholar]
- Bao Y. J., Xu Z., Li Y., Yao Z., Sun J., Song H. (2017). High-throughput metagenomic analysis of petroleum-contaminated soil microbiome reveals the versatility in xenobiotic aromatics metabolism. J. Environ. Sci. 56 25–35. 10.1016/j.jes.2016.08.022 [DOI] [PubMed] [Google Scholar]
- Basu S., Rabara R. C., Negi S., Shukla P. (2018). Engineering PGPMOs through gene editing and systems biology: a solution for phytoremediation? Trends Biotechnol. 36 499–510. 10.1016/j.tibtech.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Benjamin S. R., de Lima F., Rathoure A. K. (2019). “Genetically engineered microorganisms for bioremediation processes: GEMs for bioremediaton,” in Biotechnology: Concepts, Methodologies, Tools, and Applications, Ed. Information Resources Management Association (Pennsylvania: IGI Global; ), 1607–1634. [Google Scholar]
- Berger B., Peng J., Singh M. (2013). Computational solutions for omics data. Nat. Rev. Genet. 14:333. 10.1038/nrg3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin P. N., Heinrich-Salmeron A., Pelletier E., Goulhen-Chollet F., Arsène-Ploetze F., Gallien S., et al. (2011). Metabolic diversity among main microorganisms inside an arsenic-rich ecosystem revealed by meta-and proteo-genomics. ISME J. 5:1735. 10.1038/ismej.2011.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharagava R. N., Purchase D., Saxena G., Mulla S. I. (2019). “Applications of metagenomics in microbial bioremediation of pollutants: from genomics to environmental cleanup,” in Microbial Diversity in the Genomic Era, eds Das S., Dash H. R. (Cambridge, MA: Academic Press; ), 459–477. [Google Scholar]
- Biggs M. B., Medlock G. L., Kolling G. L., Papin J. A. (2015). Metabolic network modeling of microbial communities. Wiley Interdiscipl. Rev. 7 317–334. 10.1002/wsbm.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M., Iqbal H. M. (2019). Microbial-derived biosensors for monitoring environmental contaminants: recent advances and future outlook. Process Saf. Environ. Protect. 124 8–17. [Google Scholar]
- Biswas R., Vivekanand V., Saha A., Ghosh A., Sarkar A. (2019). Arsenite oxidation by a facultative chemolithotrophic Delftia spp. BAs29 for its potential application in groundwater arsenic bioremediation. Int. Biodeteriorat. Biodegrad. 136 55–62. [Google Scholar]
- Bordbar A., Monk J. M., King Z. A., Palsson B. O. (2014). Constraint-based models predict metabolic and associated cellular functions. Nat. Rev. Genet. 15:107. 10.1038/nrg3643 [DOI] [PubMed] [Google Scholar]
- Bordel S. (2014). “Genome-scale metabolic models of yeast, methods for their reconstruction, and other applications,” in Yeast Metabolic Engineering, ed. Mapelli V. (New York, NY: Humana Press; ), 269–279. 10.1007/978-1-4939-0563-8_16 [DOI] [PubMed] [Google Scholar]
- Boudh S., Singh J. S., Chaturvedi P. (2019). “Microbial resources mediated bioremediation of persistent organic pollutants,” in New and Future Developments in Microbial Biotechnology and Bioengineering, eds Singh J. S., Singh D. P. (Amsterdam: Elsevier; ), 283–294. [Google Scholar]
- Bruggeman F. J., Westerhoff H. V. (2007). The nature of systems biology. TRENDS Microbiol. 15 45–50. [DOI] [PubMed] [Google Scholar]
- Bruneel O., Volant A., Gallien S., Chaumande B., Casiot C., Carapito C., et al. (2011). Characterization of the active bacterial community involved in natural attenuation processes in arsenic-rich creek sediments. Microb. Ecol. 61 793–810. 10.1007/s00248-011-9808-9 [DOI] [PubMed] [Google Scholar]
- Butler J. E., Young N. D., Lovley D. R. (2010). Evolution of electron transfer out of the cell: comparative genomics of six Geobacter genomes. BMC Genomics 11:40. 10.1186/1471-2164-11-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajosa G., Cases I. (2010). “Transcriptional networks that regulate hydrocarbon biodegradation,” in Handbook of Hydrocarbon and Lipid Microbiology, eds Timmis K. N., McGenity T., van der Meer J. R., de Lorenzo V. (Berlin: Springer; ), 1399–1410. [Google Scholar]
- Casino P., Rubio V., Marina A. (2010). The mechanism of signal transduction by two-component systems. Curr. Opin. Struct. Biol 20 763–771. 10.1016/j.sbi.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Cavalca L., Corsini A., Zaccheo P., Andreoni V., Muyzer G. (2013). Microbial transformations of arsenic: perspectives for biological removal of arsenic from water. Future Microbiol. 8 753–768. 10.2217/fmb.13.38 [DOI] [PubMed] [Google Scholar]
- Cervelli E., Pindozzi S., Capolupo A., Okello C., Rigillo M., Boccia L. (2016). Ecosystem services and bioremediation of polluted areas. Ecol. Eng. 87 139–149. 10.1016/j.copbio.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Chakraborty J., Jana T., Saha S., Dutta T. K. (2014). R ing-H ydroxylating O xygenase database: a database of bacterial aromatic ring-hydroxylating oxygenases in the management of bioremediation and biocatalysis of aromatic compounds. Environ. Microbiol. Rep. 6 519–523. 10.1111/1758-2229.12182 [DOI] [PubMed] [Google Scholar]
- Chandra D., General T., Chandra S. (2019). “Microorganisms: an asset for decontamination of soil,” in Smart Bioremediation Technologies, Ed. Bhatt P. (Cambridge, MA: Academic Press; ), 319–345. [Google Scholar]
- Checa S. K., Zurbriggen M. D., Soncini F. C. (2012). Bacterial signaling systems as platforms for rational design of new generations of biosensors. Curr. Opin. Biotechnol 23 766–772. 10.1016/j.copbio.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Chen S., Chang C., Deng Y., An S., Dong Y. H., Zhou J., et al. (2014). Fenpropathrin biodegradation pathway in Bacillus sp. DG-02 and its potential for bioremediation of pyrethroid-contaminated soils. J. Agricult. Food Chem. 62 2147–2157. 10.1021/jf404908j [DOI] [PubMed] [Google Scholar]
- Chen W., Zhang Y., Zhang Y., Pi Y., Gu T., Song L., et al. (2018). CRISPR/Cas9-based genome editing in Pseudomonas aeruginosa and cytidine deaminase-mediated base editing in Pseudomonas species. IScience 6 222–231. 10.1016/j.isci.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S. I., Sheppard D. C. (2016). “Bioremediation of arsenic using an aspergillus system,” in New and Future Developments in Microbial Biotechnology and Bioengineering, ed. Gupta V. K. (Amsterdam: Elsevier Science; ), 267–274. [Google Scholar]
- Covert M. W., Schilling C. H., Famili I., Edwards J. S., Goryanin I. I., Selkov E., et al. (2001). Metabolic modeling of microbial strains in silico. Trends Biochem. Sci. 26 179–186. 10.1016/s0968-0004(00)01754-0 [DOI] [PubMed] [Google Scholar]
- Coyte K. Z., Schluter J., Foster K. R. (2015). The ecology of the microbiome: networks, competition, and stability. Science 350 663–666. 10.1126/science.aad2602 [DOI] [PubMed] [Google Scholar]
- Dangi A. K., Sharma B., Hill R. T., Shukla P. (2019). Bioremediation through microbes: systems biology and metabolic engineering approach. Crit. Rev. Biotechnol. 39 79–98. 10.1080/07388551.2018.1500997 [DOI] [PubMed] [Google Scholar]
- Dao A. T., Vonck J., Janssens T. K., Dang H. T., Brouwer A., de Boer T. E. (2019). Screening white-rot fungi for bioremediation potential of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Indus. Crops Products 128 153–161. [Google Scholar]
- Das S., Dash H. R., Chakraborty J. (2016). Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Appl. Microbiol. Biotechnol. 100 2967–2984. 10.1007/s00253-016-7364-4 [DOI] [PubMed] [Google Scholar]
- Davison J. (2002). Towards safer vectors for the field release of recombinant bacteria. Environ. Biosaf. Res. 1 9–18. 10.1051/ebr:2002001 [DOI] [PubMed] [Google Scholar]
- De Jong H. (2002). Modeling and simulation of genetic regulatory systems: a literature review. J. Comput. Biol. 9 67–103. 10.1089/10665270252833208 [DOI] [PubMed] [Google Scholar]
- de la Pena Mattozzi M., Tehara S. K., Hong T., Keasling J. D. (2006). Mineralization of paraoxon and its use as a sole C and P source by a rationally designed catabolic pathway in Pseudomonas putida. Appl. Environ. Microbiol. 72 6699–6706. 10.1128/AEM.00907-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagnezze B. M., de Sousa G. V., Martins L. L., Domingos D. F., Limache E. E., de Vasconcellos S. P., et al. (2014). Bioremediation potential of microorganisms derived from petroleum reservoirs. Mar. Pollut. Bull. 89 191–200. 10.1016/j.marpolbul.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Desai C., Pathak H., Madamwar D. (2010). Advances in molecular and “-omics” technologies to gauge microbial communities and bioremediation at xenobiotic/anthropogen contaminated sites. Bioresour. Technol. 101 1558–1569. 10.1016/j.biortech.2009.10.080 [DOI] [PubMed] [Google Scholar]
- Dhar D., Roy S., Nigam V. K. (2019). “Advances in protein/enzyme-based biosensors for the detection of pharmaceutical contaminants in the environment,” in Tools, Techniques and Protocols for Monitoring Environmental Contaminants, eds Brar S. K., Hegde K., Pachapur V. L. (Amsterdam: Elsevier; ), 207–229. [Google Scholar]
- Duarte M., Nielsen A., Camarinha-Silva A., Vilchez-Vargas R., Bruls T., Wos-Oxley M. L., et al. (2017). Functional soil metagenomics: elucidation of polycyclic aromatic hydrocarbon degradation potential following 12 years of in situ bioremediation. Environ. Microbiol. 19 2992–3011. 10.1111/1462-2920.13756 [DOI] [PubMed] [Google Scholar]
- Dvorak P., Bidmanova S., Damborsky J., Prokop Z. (2014). Immobilized synthetic pathway for biodegradation of toxic recalcitrant pollutant 1, 2, 3-trichloropropane. Environ. Sci. Technol. 48 6859–6866. 10.1021/es500396r [DOI] [PubMed] [Google Scholar]
- Dvořák P., Nikel P. I., Damborský J., de Lorenzo V. (2017). Bioremediation 3.0: engineering pollutant-removing bacteria in the times of systemic biology. Biotechnol. Adv. 35 845–866. 10.1016/j.biotechadv.2017.08.001 [DOI] [PubMed] [Google Scholar]
- El Amrani A., Dumas A. S., Wick L. Y., Yergeau E., Berthome R. (2015). Omics” insights into PAH degradation toward improved green remediation biotechnologies. Environ. Sci. Technol. 49 11281–11291. 10.1021/acs.est.5b01740 [DOI] [PubMed] [Google Scholar]
- El Zanfaly H. T. (2019). Biotechnology Contributions in Sustainable Environmental Development. Cairo: National Research Center. [Google Scholar]
- Enríquez P. (2016). Genome editing and the jurisprudence of scientific empiricism. Vand. J. Ent. & Tech. L. 19:603. [Google Scholar]
- Fajardo C., Costa G., Nande M., Botías P., García-Cantalejo J., Martín M. (2019). Pb, Cd, and Zn soil contamination: monitoring functional and structural impacts on the microbiome. Appl. Soil Ecol. 135 56–64. [Google Scholar]
- Fernandez M., Paisio C. E., Perotti R., Pereira P. P., Agostini E., González P. S. (2019). Laboratory and field microcosms as useful experimental systems to study the bioaugmentation treatment of tannery effluents. J. Environ. Manag. 234 503–511. 10.1016/j.jenvman.2019.01.019 [DOI] [PubMed] [Google Scholar]
- Foster K. R., Bell T. (2012). Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 22 1845–1850. 10.1016/j.cub.2012.08.005 [DOI] [PubMed] [Google Scholar]
- French K. E., Zhou Z., Terry N. (2019). Horizontal” gene drives” harness indigenous bacteria for bioremediation. bioRxiv [Preprint] 10.1101/735886v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagami T., Goto M., Furukawa K. (2014). “Genetic system of organohalide-respiring bacteria,” in Biodegradative Bacteria, eds Kamagata Y., Tsuda M., Nojiri H. (Tokyo: Springer; ), 59–81. [Google Scholar]
- Gangar T., Bhardwaj K. K., Gupta R. (2019). “Microbes and processes in bioremediation of soil,” in Microbes and Enzymes in Soil Health and Bioremediation, eds Kumar A., Sharma S. (Singapore: Springer; ), 11–37. [Google Scholar]
- Gardner C. M., Gunsch C. K. (2020). “Environmental microbiome analysis and manipulation,” in Women in Water Quality, Ed. O’Bannon D. J. (Cham: Springer; ), 113–133. [Google Scholar]
- Garg V., Khan S., Dutt K. (2014). Systematic Analysis of Microbial Degradation Pathway of 1-Naphthyl-N-Methyl carbamate generated by EAWAG Biocatalysis/biodegradation database-pathway prediction System. Int. J. Appl. Sci. Res. Rev. 1 049–055. [Google Scholar]
- Gillan D. C., Roosa S., Kunath B., Billon G., Wattiez R. (2015). The long-term adaptation of bacterial communities in metal-contaminated sediments: a metaproteogenomic study. Environ. Microbiol. 17 1991–2005. 10.1111/1462-2920.12627 [DOI] [PubMed] [Google Scholar]
- Gómez-Tatay L., Hernández-Andreu J. M. (2019). Biosafety and biosecurity in synthetic biology: a review. Crit. Rev. Environ. Sci. Technol. 49 1587–1621. [Google Scholar]
- Gong J., Liu X., Cao X., Diao Y., Gao D., Li H., et al. (2012). PTID: an integrated web resource and computational tool for agrochemical discovery. Bioinformatics 29 292–294. 10.1093/bioinformatics/bts651 [DOI] [PubMed] [Google Scholar]
- Gong T., Liu R., Zuo Z., Che Y., Yu H., Song C., et al. (2016). Metabolic engineering of Pseudomonas putida KT2440 for complete mineralization of methyl parathion and γ-hexachlorocyclohexane. ACS Synthetic Biol. 5 434–442. 10.1021/acssynbio.6b00025 [DOI] [PubMed] [Google Scholar]
- Großkopf T., Soyer O. S. (2014). Synthetic microbial communities. Curr. Opin. Microbiol. 18 72–77. 10.1016/j.mib.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Pathak B. (2020). “Mycoremediation of polycyclic aromatic hydrocarbons,” in Abatement of Environmental Pollutants, eds Singh P., Kumar A., Borthakur A. (Amsterdam: Elsevier; ), 127–149. [Google Scholar]
- Gupte A., Tripathi A., Patel H., Rudakiya D., Gupte S. (2016). Bioremediation of polycyclic aromatic hydrocarbon (PAHs): a perspective. Open Biotechnol. J. 10 363–378. 10.1016/j.chemosphere.2013.03.025 [DOI] [PubMed] [Google Scholar]
- Guzik U., Greń I., Hupert-Kocurek K., Wojcieszyńska D. (2011). Catechol 1, 2-dioxygenase from the new aromatic compounds–degrading Pseudomonas putida strain N6. Int. Biodeterior. Biodegrad. 65 504–512. [Google Scholar]
- Hamilton T. A., Pellegrino G. M., Therrien J. A., Ham D. T., Bartlett P. C., Karas B. J., et al. (2019). Efficient inter-species conjugative transfer of a CRISPR nuclease for targeted bacterial killing. Nat. Commun. 10 1–9. 10.1038/s41467-019-12448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley E. R., Piao H., Scott N. M., Malfatti S., Pagani I., Huntemann M., et al. (2014). Metagenomic analysis of microbial consortium from natural crude oil that seeps into the marine ecosystem offshore Southern California. Standards Genom. Sci. 9:1259. 10.4056/sigs.5029016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellweger F. L., Clegg R. J., Clark J. R., Plugge C. M., Kreft J. U. (2016). Advancing microbial sciences by individual-based modelling. Nat. Rev. Microbiol. 14:461. 10.1038/nrmicro.2016.62 [DOI] [PubMed] [Google Scholar]
- Hemmat-Jou M. H., Safari-Sinegani A. A., Mirzaie-Asl A., Tahmourespour A. (2018). Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology 27 1281–1291. 10.1007/s10646-018-1981-x [DOI] [PubMed] [Google Scholar]
- Heng L. Y., Ooi L., Mori I. C., Futra D. (2018). “Environmental toxicity and evaluation,” in Environmental Risk Analysis for Asian-Oriented, Risk-Based Watershed Management, eds Yoneda M., Mokhtar M. (Singapore: Springer; ), 71–94. [Google Scholar]
- Hong Y. H., Ye C. C., Zhou Q. Z., Wu X. Y., Yuan J. P., Peng J., et al. (2017). Genome sequencing reveals the potential of Achromobacter sp. HZ01 for bioremediation. Front. Microbiol. 8:1507. 10.3389/fmicb.2017.01507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquiod S., Demanèche S., Franqueville L., Ausec L., Xu Z., Delmont T. O., et al. (2014). Characterization of new bacterial catabolic genes and mobile genetic elements by high throughput genetic screening of a soil metagenomic library. J. Biotechnol. 190 18–29. 10.1016/j.jbiotec.2014.03.036 [DOI] [PubMed] [Google Scholar]
- Jadeja N. B, More R. P, Purohit H. J, Kapley A. (2014). Metagenomic analysis of oxygenases from activated sludge. Bioresour. Technol. 165 250–256. 10.1016/j.biortech.2014.02.045 [DOI] [PubMed] [Google Scholar]
- Jagwani J., Johnson J., Datta M., Lakshmi B. J. (2018). Bacterial community dynamics involved in Reactive Orange M2R dye degradation using a real time quantitative PCR and scale up studies using sequence batch reactor. Bioremed. J. 22 63–71. [Google Scholar]
- Jaiswal S., Sharma B., Shukla P. (2019a). Integrated approaches in microbial degradation of plastics. Environ. Technol. & Innovat. 17:100567. [Google Scholar]
- Jaiswal S., Singh D. K., Shukla P. (2019b). Gene editing and systems biology tools for pesticide bioremediation: a review. Front. Microbiol. 10:87. 10.3389/fmicb.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D. B., Stucki G. (2020). Perspectives of genetically engineered microbes for groundwater bioremediation. Environ. Sci. Process. Impacts 22 487–499. 10.1039/c9em00601j [DOI] [PubMed] [Google Scholar]
- Johns N. I., Blazejewski T., Gomes A. L., Wang H. H. (2016). Principles for designing synthetic microbial communities. Curr. Opin. Microbiol. 31 146–153. 10.1016/j.mib.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G., Schmidt R., Scow K. M., Denison M. S., Hristova K. R. (2016). Effect of benzene and ethylbenzene on the transcription of methyl-tert-butyl ether degradation genes of Methylibium petroleiphilum PM1. Microbiology 162 1563–1571. 10.1099/mic.0.000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghare M., Spiteller D., Schink B. (2019). Anaerobic degradation of xenobiotic isophthalate by the fermenting bacterium Syntrophorhabdus aromaticivorans. ISME J. 13 1252–1268. 10.1038/s41396-019-0348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusiak B., Cleto S., Perez-Pińera P., Lu T. K. (2016). Engineering synthetic gene circuits in living cells with CRISPR technology. Trends Biotechnol. 34 535–547. 10.1016/j.tibtech.2015.12.014 [DOI] [PubMed] [Google Scholar]
- Juwarkar A. A., Singh S. K., Mudhoo A. (2010). A comprehensive overview of elements in bioremediation. Rev. Environ. Sci. Bio/Technol. 9 215–288. [Google Scholar]
- Kachienga L., Jitendra K., Momba M. (2018). Metagenomic profiling for assessing microbial diversity and microbial adaptation to degradation of hydrocarbons in two South African petroleum-contaminated water aquifers. Sci. Rep. 8:7564. 10.1038/s41598-018-25961-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchiswamy C. N., Maffei M., Malnoy M., Velasco R., Kim J. S. (2016). Fine-tuning next-generation genome editing tools. Trends Biotechnol. 34 562–574. 10.1016/j.tibtech.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Kapetanovic I. M. (2008). Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem. Biol. Interact 171 165–176. 10.1016/j.cbi.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi B., Habibi M., Esvand M. (2015). Biodegradation of naphthalene using Pseudomonas aeruginosa by up flow anoxic–aerobic continuous flow combined bioreactor. J. Environ. Health Sci. Eng. 13 26. 10.1186/s40201-015-0175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F. I., Husain T., Hejazi R. (2004). An overview and analysis of site remediation technologies. J. Environ. Manag. 71 95–122. 10.1016/j.jenvman.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Khan S., Nawab J., Waqas M. (2020). “Constructed wetlands: a clean-green technology for degradation and detoxification of industrial wastewaters,” in Bioremediation of Industrial Waste for Environmental Safety, eds Bharagava R. N., Saxena G. (Singapore: Springer; ), 127–163. [Google Scholar]
- Khan S., Ullah M. W., Siddique R., Nabi G., Manan S., Yousaf M., et al. (2016). Role of recombinant DNA technology to improve life. Int. J. Genom. 2016:2405954. 10.1155/2016/2405954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. H. (2019). Genome-editing technologies: concept, pros, and cons of various genome-editing techniques and bioethical concerns for clinical application. Mol. Ther. Nucleic Acids 16:326. 10.1016/j.omtn.2019.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. A., Olivier B. G., Röling W. F., Teusink B., Bruggeman F. J. (2013). Community flux balance analysis for microbial consortia at balanced growth. PLoS One 8:e64567. 10.1371/journal.pone.0064567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudur L. S., Shahsavari E., Webster G. T., Nugegoda D., Ball A. S. (2019). The impact of lead co-contamination on ecotoxicity and the bacterial community during the bioremediation of total petroleum hydrocarbon-contaminated soils. Environ. Pollut. 253 939–948. 10.1016/j.envpol.2019.07.107 [DOI] [PubMed] [Google Scholar]
- Kim B., Kim W. J., Kim D. I., Lee S. Y. (2015). Applications of genome-scale metabolic network model in metabolic engineering. J. Indus. Microbiol. Biotechnol. 42 339–348. 10.1007/s10295-014-1554-9 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Park S. J., Cha I. T., Min D., Kim J. S., Chung W. H., et al. (2014). Metabolic versatility of toluene-degrading, iron-reducing bacteria in tidal flat sediment, characterized by stable isotope probing-based metagenomic analysis. Environ. Microbiol. 16 189–204. 10.1111/1462-2920.12277 [DOI] [PubMed] [Google Scholar]
- Kondo R. (2017). “Microbial degradation of endsulfan and endsulfan sulfate,” in Microbe-Induced Degradation of Pesticides, Ed. Singh S. N. (Cham: Springer; ), 151–166. [Google Scholar]
- Košnár Z., Èástková T., Wiesnerová L., Praus L., Jablonský I., Koudela M., et al. (2019). Comparing the removal of polycyclic aromatic hydrocarbons in soil after different bioremediation approaches in relationto the extracellular enzyme activities. J. Environ. Sci. 76 249–258. 10.1016/j.jes.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Kües U. (2015). Fungal enzymes for environmental management. Curr. Opin. Biotechnol 33 268–278. 10.1016/j.copbio.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Kumar A., Bisht B. S., Joshi V. D., Dhewa T. (2011). Review on bioremediation of polluted environment: a management tool. Int. J. Environ. Sci. 1:1079. [Google Scholar]
- Kumar M., Jaiswal S., Sodhi K. K., Shree P., Singh D. K., Agrawal P. K., et al. (2019). Antibiotics bioremediation: perspectives on its ecotoxicity and resistance. Environ. Int. 124 448–461. 10.1016/j.envint.2018.12.065 [DOI] [PubMed] [Google Scholar]
- Kumar N. M., Muthukumaran C., Sharmila G., Gurunathan B. (2018). “Genetically modified organisms and its impact on the enhancement of bioremediation,” in Bioremediation: Applications for Environmental Protection and Management, eds Varjani S. J., Agarwal A. K., Gnansounou E., Gurunathan B. (Singapore: Springer; ), 53–76. [Google Scholar]
- Kumar V., Dangi A. K., Shukla P. (2018). Engineering thermostable microbial xylanases toward its industrial applications. Mol. Biotechnol. 60 226–235. 10.1007/s12033-018-0059-6 [DOI] [PubMed] [Google Scholar]
- Kumar P. S. (2019). “Soil bioremediation techniques,” in Advanced Treatment Techniques for Industrial Wastewater, eds Athar H., Sirajuddin A. (IGI Global; ), 35–50. [Google Scholar]
- Kumar S. S., Shantkriti S., Muruganandham T., Murugesh E., Rane N., Govindwar S. P. (2016). Bioinformatics aided microbial approach for bioremediation of wastewater containing textile dyes. Ecol. Inform. 31 112–121. [Google Scholar]
- Kumavath R. N., Satyanarayana S. V. (2014). Preserving the earth’s microbes for drug discovery in the future. J. Mýcrobýol. Mýcrob. Res. 2 1–7. [Google Scholar]
- Kutateladze L., Zakariashvili N., Khokhashvili I., Jobava M., Alexidze T., Urushadze T., et al. (2018). Fungal elimination of 2, 4, 6-trinitrotoluene (TNT) from the soils. Eurobiotech J. 2 39–46. [Google Scholar]
- Lebrazi S., Fikri-Benbrahim K. (2018). “Rhizobium-Legume Symbioses: heavy metal effects and principal approaches for bioremediation of contaminated soil,” in Legumes for Soil Health and Sustainable Management, eds Meena R. S., Das A., Yadav G. S., Lal R. (Singapore: Springer; ), 205–233. [Google Scholar]
- Leong Y. K., Chang J. S. (2020). Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour. Technol. 303:122886. 10.1016/j.biortech.2020.122886 [DOI] [PubMed] [Google Scholar]
- Liang Y., Jiao S., Wang M., Yu H., Shen Z. (2020). A CRISPR/Cas9-based genome editing system for Rhodococcus ruber TH. Metab. Eng. 57 13–22. 10.1016/j.ymben.2019.10.003 [DOI] [PubMed] [Google Scholar]
- Libis V., Delepine B., Faulon J. L. (2016a). Expanding biosensing abilities through computer-aided design of metabolic pathways. ACS Synthet. Biol. 5 1076–1085. 10.1021/acssynbio.5b00225 [DOI] [PubMed] [Google Scholar]
- Libis V., Delépine B., Faulon J. L. (2016b). Sensing new chemicals with bacterial transcription factors. Curr. Opin. Microbiol. 33 105–112. 10.1016/j.mib.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang J. J., Wang S. J., Zhang X. E., Zhou N. Y. (2005). Plasmid-borne catabolism of methyl parathion and p-nitrophenol in Pseudomonas sp. strain WBC-3. Biochem. Biophys. Res. Commun. 334 1107–1114. 10.1016/j.bbrc.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Luo J., Bai Y., Liang J., Qu J. (2014). Metagenomic approach reveals variation of microbes with arsenic and antimony metabolism genes from highly contaminated soil. PLoS One 9:e108185. 10.1371/journal.pone.0108185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla M. A., Dubey A., Yadav S., Kumar A., Hashem A., Abd_Allah E. F. (2018). Understanding and designing the strategies for the microbe-mediated remediation of environmental contaminants using omics approaches. Front. Microbiol. 9:1132. 10.3389/fmicb.2018.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco D. E., Abram F. (2019). Using genomics, metagenomics and other” omics” to assess valuable microbial ecosystem services and novel biotechnological applications. Front. Microbiol. 10:151. 10.3389/fmicb.2019.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R., Maxwell C. S., Collins S. P., Jacobsen T., Luo M. L., Begemann M. B., et al. (2018). Rapid and scalable characterization of CRISPR technologies using an E. coli cell-free transcription-translation system. Mol. Cell 69 146–157. 10.1016/j.molcel.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J. M., McKernan K. J. (2017). “Contaminants of concern in cannabis: microbes, heavy metals and pesticides,” in Cannabis sativa L.-Botany and Biotechnology, eds Chandra S., Lata H., ElSohly M. A. (Cham: Springer; ), 457–474. [Google Scholar]
- Mehr M. A., Farivar T. N., Najafipour R., Peymani A., Alizadeh S. A., Johari P. (2017). Biodegradation of endosulfan as an organochlorine pesticide with Pseudomonas plecoglocissida transfected by LinA gene. Biotechnol. Health Sci. 5:e45306. [Google Scholar]
- Michel C., Jean M., Coulon S., Dictor M. C., Delorme F., Morin D., et al. (2007). Biofilms of As (III)-oxidising bacteria: formation and activity studies for bioremediation process development. Appl. Microbiol. Biotechnol. 77 457–467. 10.1007/s00253-007-1169-4 [DOI] [PubMed] [Google Scholar]
- Mills M. G., Ramsden R., Ma E. Y., Corrales J., Kristofco L. A., Steele W. B., et al. (2019). CRISPR-generated Nrf2a loss-and gain-of-function mutants facilitate mechanistic analysis of chemical oxidative stress-mediated toxicity in zebrafish. Chem. Res. Toxicol. 33 426–435. 10.1021/acs.chemrestox.9b00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki R., Sato Y., Ito M., Ohtsubo Y., Nagata Y., Tsuda M. (2006). Complete nucleotide sequence of an exogenously isolated plasmid, pLB1, involved in γ-hexachlorocyclohexane degradation. Appl. Environ. Microbiol. 72 6923–6933. 10.1128/AEM.01531-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanta T. K., Mohanta Y. K., Mohanta N. (2015). “Role of biotechnology in bioremediation,” in Handbook of Research on Uncovering New Methods for Ecosystem Management Through Bioremediation, eds Singh S. O., Srivastava K. (Pennsylvania: IGI Global; ), 399–432. [Google Scholar]
- Mohapatra B., Kazy S. K., Sar P. (2019). Comparative genome analysis of arsenic reducing, hydrocarbon metabolizing groundwater bacterium Achromobacter sp. KAs 3-5T explains its competitive edge for survival in aquifer environment. Genomics 111 1604–1619. 10.1016/j.ygeno.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Mónica M. G. A., Jaime M. Q. (2019). “Phenoloxidases of Fungi and Bioremediation,” Fungal Bioremediation: Fundamentals and Applications, eds Campocosio A. T., Santiesteban H. H. L. (Boca Raton, FL: CRC Press; ), 62–90. [Google Scholar]
- Mougiakos I., Bosma E. F., de Vos W. M., van Kranenburg R., van der Oost J. (2016). Next generation prokaryotic engineering: the CRISPR-Cas toolkit. Trends Biotechnol. 34 575–587. 10.1016/j.tibtech.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Mujawar S. Y., Shamim K., Vaigankar D. C., Dubey S. K. (2019). Arsenite biotransformation and bioaccumulation by Klebsiella pneumoniae strain SSSW7 possessing arsenite oxidase (aioA) gene. Biometals 32 65–76. 10.1007/s10534-018-0158-7 [DOI] [PubMed] [Google Scholar]
- Myatt G. J., Ahlberg E., Akahori Y., Allen D., Amberg A., Anger L. T., et al. (2018). In silico toxicology protocols. Regul. Toxicol. Pharmacol. 96 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhr A. I., Traavik T. (2002). The precautionary principle: Scientific uncertainty and omitted research in the context of GMO use and release. J. Agricult. Environ. Ethics 15 73–86. [Google Scholar]
- Nogales J., Mueller J., Gudmundsson S., Canalejo F. J., Duque E., Monk J. (2020). High-quality genome-scale metabolic modelling of Pseudomonas putida highlights its broad metabolic capabilities. Environ. Microbiol. 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora L. C., Westmann C. A., Martins-Santana L., Alves L. D. F., Monteiro L. M. O., Guazzaroni M. E., et al. (2019). The art of vector engineering: towards the construction of next-generation genetic tools. Microb. Biotechnol. 12 125–147. 10.1111/1751-7915.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E. J., Monk J. M., Palsson B. O. (2015). Using genome-scale models to predict biological capabilities. Cell 161 971–987. 10.1016/j.cell.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst U. (2007). Quorum sensing: bacterial chatting. Anal. Bioanal. Chem. 387 369–370. [DOI] [PubMed] [Google Scholar]
- Ofaim S., Zarecki R., Porob S., Gat D., Lahav T., Xu X., et al. (2019). Genome-Scale reconstruction of Paenarthrobacter aurescens TC1 metabolic model towards the study of atrazine bioremediation. bioRxiv [Preprint] 10.1101/536011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino-Delgado C. H., Zanutto-Elgui M. R., do Prado D. Z., Pereira M. S., Fleuri L. F. (2019). “Enzymatic bioremediation: current status, challenges of obtaining process, and applications,” in Microbial Metabolism of Xenobiotic Compounds, eds Arora P. K. (Singapore: Springer; ), 79–101. [Google Scholar]
- Orth J. D., Thiele I., Palsson B. Ø. (2010). What is flux balance analysis? Nat. Biotechnol. 28:245. 10.1038/nbt.1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Nekludova L. (2000). Geometric analysis and comparison of protein-DNA interfaces: why is there no simple code for recognition? 1. J. Mol. Biol. 301 597–624. 10.1006/jmbi.2000.3918 [DOI] [PubMed] [Google Scholar]
- Panelli S., Capelli E., Comandatore F., Landinez-Torres A., Granata M. U., Tosi S., et al. (2017). A metagenomic-based, cross-seasonal picture of fungal consortia associated with Italian soils subjected to different agricultural managements. Fungal Ecol. 30 1–9. [Google Scholar]
- Paniagua-Michel J., Fathepure B. Z. (2018). “Microbial consortia and biodegradation of petroleum hydrocarbons in marine environments,” in Microbial Action on Hydrocarbons, eds Kumar V., Kumar M., Prasad R. (Singapore: Springer; ), 1–20. [Google Scholar]
- Panigrahi S., Velraj P., Rao T. S. (2019). “Functional microbial diversity in contaminated environment and application in bioremediation,” in Microbial Diversity in the Genomic Era, eds Das S., Dash H. R. (Amsterdam: Academic Press; ), 359–385. [Google Scholar]
- Pastor-Jáuregui R., Paniagua-López M., Martínez-Garzón J., Martín-Peinado F., Sierra-Aragón M. (2020). Evolution of the residual pollution in soils after bioremediation treatments. Appl. Sci. 10:1006. [Google Scholar]
- Patel R., Zaveri P., Mukherjee A., Agarwal P. K., More P., Munshi N. S. (2019). Development of fluorescent protein-based biosensing strains: a new tool for the detection of aromatic hydrocarbon pollutants in the environment. Ecotoxicol. Environ. Saf. 182:109450. 10.1016/j.ecoenv.2019.109450 [DOI] [PubMed] [Google Scholar]
- Pavan M., Worth A. P. (2006). Review of QSAR Models for Ready Biodegradation. Brussels: European Commission Directorate General Joint Research Centre. [Google Scholar]
- Peijnenburg W. J., Damborský J. (eds) (2012). Biodegradability Prediction, Vol. 23 Berlin: Springer Science & Business Media. [Google Scholar]
- Petsas A. S., Vagi M. C. (2019). Trends in the bioremediation of pharmaceuticals and other organic contaminants using native or genetically modified microbial strains: a review. Curr. Pharm. Biotechnol 20 787–824. 10.2174/1389201020666190527113903 [DOI] [PubMed] [Google Scholar]
- Pietro-Souza W., de Campos Pereira F., Mello I. S., Stachack F. F. F., Terezo A. J., da Cunha C. N., et al. (2020). Mercury resistance and bioremediation mediated by endophytic fungi. Chemosphere 240:124874. 10.1016/j.chemosphere.2019.124874 [DOI] [PubMed] [Google Scholar]
- Pineda M., Lear A., Collins J. P., Kiani S. (2019). Safe CRISPR: challenges and possible solutions. Trends Biotechnol. 37 389–401. 10.1016/j.tibtech.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Plewniak F., Crognale S., Rossetti S., Bertin P. N. (2018). A genomic outlook on bioremediation: the case of arsenic removal. Front. Microbiol. 9:820. 10.3389/fmicb.2018.00820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontrelli S., Chiu T. Y., Lan E. I., Chen F. Y. H., Chang P., Liao J. C. (2018). Escherichia coli as a host for metabolic engineering. Metab. Eng. 50 16–46. 10.1016/j.ymben.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Ravikumar S., Baylon M. G., Park S. J., Choi J. I. (2017). Engineered microbial biosensors based on bacterial two-component systems as synthetic biotechnology platforms in bioremediation and biorefinery. Microb. Cell Fact. 16:62. 10.1186/s12934-017-0675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls K. D., Dougherty B. V., Blais E. M., Stancliffe E., Kolling G. L., Vinnakota K., et al. (2019). A simplified metabolic network reconstruction to promote understanding and development of flux balance analysis tools. Comput. Biol. Med. 105 64–71. 10.1016/j.compbiomed.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Ray S., Panjikar S., Anand R. (2018). Design of protein-based biosensors for selective detection of benzene groups of pollutants. ACS Sens. 3 1632–1638. 10.1021/acssensors.8b00190 [DOI] [PubMed] [Google Scholar]
- Rayu S., Karpouzas D. G., Singh B. K. (2012). Emerging technologies in bioremediation: constraints and opportunities. Biodegradation 23 917–926. 10.1007/s10532-012-9576-3 [DOI] [PubMed] [Google Scholar]
- Richelle A., Gziri K. M., Bogaerts P. (2016). A methodology for building a macroscopic FBA-based dynamical simulator of cell cultures through flux variability analysis. Biochem. Eng. J. 114 50–64. [Google Scholar]
- Rochfort S. (2005). Metabolomics reviewed: a new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 68 1813–1820. 10.1021/np050255w [DOI] [PubMed] [Google Scholar]
- Rucká L., Nešvera J., Pátek M. (2017). Biodegradation of phenol and its derivatives by engineered bacteria: current knowledge and perspectives. World J. Microbiol. Biotechnol. 33:174. 10.1007/s11274-017-2339-x [DOI] [PubMed] [Google Scholar]
- Russo F., Ceci A., Pinzari F., Siciliano A., Guida M., Malusà E., et al. (2019). Bioremediation of DDT-contaminated agricultural soils: the potential of two autochthonous saprotrophic fungal strains. Appl. Environ. Microbiol. 85 e01720–19. 10.1128/AEM.01720-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft T., Hamilton K., Haas C. N., Linkov I. (2019). A quantitative risk assessment method for synthetic biology products in the environment. Sci. Total Environ. 696:133940. 10.1016/j.scitotenv.2019.133940 [DOI] [PubMed] [Google Scholar]
- Sadańoski M. A., Velázquez J. E., Fonseca M. I., Zapata P. D., Levin L. N., Villalba L. L. (2018). Assessing the ability of white-rot fungi to tolerate polychlorinated biphenyls using predictive mycology. Mycology 9 239–249. 10.1080/21501203.2018.1481152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam L. B., Ishaq A. (2019). Biostimulation potentials of corn steep liquor in enhanced hydrocarbon degradation in chronically polluted soil. 3 Biotech 9:46. 10.1007/s13205-019-1580-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena G., Purchase D., Bharagava R. N. (2020). “Environmental hazards and toxicity profile of organic and inorganic pollutants of tannery wastewater and bioremediation approaches,” in Bioremediation of Industrial Waste for Environmental Safety, eds Bharagava R. N., Saxena G. (Singapore: Springer; ), 381–398. 10.1007/398_2015_5009 [DOI] [Google Scholar]
- Sayler G. S., Cox C. D., Burlage R., Ripp S., Nivens D. E., Werner C., et al. (1999). “Field application of a genetically engineered microorganism for polycyclic aromatic hydrocarbon bioremediation process monitoring and control,” in Novel Approaches for Bioremediation of Organic Pollution, eds Reuveny S., Flashner Y., Fass R. (Boston, MA: Springer; ), 241–254. [Google Scholar]
- Schloss P. D., Handelsman J. (2008). A statistical toolbox for metagenomics: assessing functional diversity in microbial communities. BMC Bioinformatics 9:34. 10.1186/1471-2105-9-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano J., Leiva E. (2017). Removal of arsenic using acid/metal-tolerant sulfate reducing bacteria: a new approach for bioremediation of high-arsenic acid mine waters. Water 9:994. [Google Scholar]
- Shah S. B., Ali F., Huang L., Wang W., Xu P., Tang H. (2018). Complete genome sequence of Bacillus sp. HBCD-sjtu, an efficient HBCD-degrading bacterium. 3 Biotech 8:291. 10.1007/s13205-018-1326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V., Jain K., Desai C., Madamwar D. (2012). “Molecular analyses of microbial activities involved in bioremediation,” in Microorganisms in Environmental Management, eds Prakash A., Satyanarayana T. (Dordrecht: Springer; ), 221–247. [Google Scholar]
- Shanmugam K., Ramalingam S., Venkataraman G., Hariharan G. N. (2019). The CRISPR/Cas9 system for targeted genome engineering in free-living fungi: advances and opportunities for lichenized fungi. Front. Microbiol. 10:62. 10.3389/fmicb.2019.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Gupta G., Ahmad T., Krishan K., Kaur B. (2020). “Next generation agents (synthetic agents): emerging threats and challenges in detection, protection, and decontamination,” in Handbook on Biological Warfare Preparedness, eds Flora S. J. S., Pachauri V. (Cambridge, MA: Academic Press; ), 217–256. [Google Scholar]
- Sharma B., Dangi A. K., Shukla P. (2018). Contemporary enzyme based technologies for bioremediation: a review. J. Environ. Manag. 210 10–22. 10.1016/j.jenvman.2017.12.075 [DOI] [PubMed] [Google Scholar]
- Sharma J. K., Gautam R. K., Nanekar S. V., Weber R., Singh B. K., Singh S. K., et al. (2018). Advances and perspective in bioremediation of polychlorinated biphenyl-contaminated soils. Environ. Sci. Pollut. Res. 25 16355–16375. 10.1007/s11356-017-8995-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. (2012). Bioremediation: features, strategies and applications. Asian J. Pharm. Life Sci. 2 202–213. [Google Scholar]
- Shukla N. (2017). Bioinformatics in environmental bioremediation-a review. Int. J. Sci. Res. Sci. Eng. Technol. 3 195–205. [Google Scholar]
- Singh A., Gothalwal R. (2018). A reappraisal on biodegradation of fluoride compounds: role of microbes. Water Environ. J. 32 481–487. 10.1111/wej.12322 [DOI] [Google Scholar]
- Singh N., Kumar A., Sharma B. (2019). “Role of fungal enzymes for bioremediation of hazardous chemicals,” in Recent Advancement in White Biotechnology Through Fungi, eds Yadav A. N., Mishra S., Singh S., Gupta A. (Cham: Springer; ), 237–256. [Google Scholar]
- Singh N., Srivastava S., Rathaur S., Singh N. (2016). Assessing the bioremediation potential of arsenic tolerant bacterial strains in rice rhizosphere interface. J. Environ. Sci. 48 112–119. 10.1016/j.jes.2015.12.034 [DOI] [PubMed] [Google Scholar]
- Singh S. B. (2018). “Enzyme Catalysis and Its Role in Food Processing Industries,” in Enzymes in Food Technology, Ed. Law B. A. (Singapore: Springer; ), 143–165. [Google Scholar]
- Singh V. (2019). “Bioremediation: new prospects for environmental cleaning by enzymes,” in Biotechnology: Concepts, Methodologies, Tools, and Applications, Ed. Information Resources Management Association (Pennsylvania: IGI Global; ), [Google Scholar]
- Sinha R., Sharma B., Dangi A. K., Shukla P. (2019). Recent metabolomics and gene editing approaches for synthesis of microbial secondary metabolites for drug discovery and development. World J. Microbiol. Biotechnol. 35:166. 10.1007/s11274-019-2746-2 [DOI] [PubMed] [Google Scholar]
- Skinder B. M., Uqab B., Ganai B. A. (2020). “Bioremediation: a sustainable and emerging tool for restoration of polluted aquatic ecosystem,” in Fresh Water Pollution Dynamics and Remediation, eds Qadri H., Bhat R. A., Mehmood M. A., Dar G. H. (Singapore: Springer; ), 143–165. [Google Scholar]
- Sridhar S., Chandra J. H. (2014). Involvement of Computational tools towards In Silico remediation-Synthetic textile dyes interacting with Azoreductase. Int. J. Chem. Technol. Res. 6 4412–4416. [Google Scholar]
- Stein H. P., Navajas-Pérez R., Aranda E. (2018). “Potential for CRISPR genetic engineering to increase xenobiotic degradation capacities in model fungi,” in Approaches in Bioremediation, eds Prasad R., Aranda E. (Cham: Springer; ), 61–78. [Google Scholar]
- Tang Q., Lu T., Liu S. J. (2018). Developing a synthetic biology toolkit for comamonas testosteroni, an emerging cellular chassis for bioremediation. ACS Synthetic Biol. 7 1753–1762. 10.1021/acssynbio.7b00430 [DOI] [PubMed] [Google Scholar]
- Tanveer T., Shaheen K., Parveen S., Misbah Z. T., Babar M. M., Gul A. (2018). “Omics-based bioengineering in environmental biotechnology,” in Omics Technologies and Bio-Engineering, eds Barh D., Azevedo V. (Cambridge, MA: Academic Press; ), 353–364. [Google Scholar]
- Tay P. K. R., Nguyen P. Q., Joshi N. S. (2017). A synthetic circuit for mercury bioremediation using self-assembling functional amyloids. ACS Synthetic Biol. 6 1841–1850. 10.1021/acssynbio.7b00137 [DOI] [PubMed] [Google Scholar]
- Techtmann S. M., Hazen T. C. (2016). Metagenomic applications in environmental monitoring and bioremediation. J. Indus. Microbiol. Biotechnol. 43 1345–1354. 10.1007/s10295-016-1809-8 [DOI] [PubMed] [Google Scholar]
- Thakur M., Medintz I. L., Walper S. A. (2019). Enzymatic bioremediation of organophosphate compounds-progress and remaining challenges. Front. Bioeng. Biotechnol. 7:289. 10.3389/fbioe.2019.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelusmond J. R., Strathmann T. J., Cupples A. M. (2019). Carbamazepine, triclocarban and triclosan biodegradation and the phylotypes and functional genes associated with xenobiotic degradation in four agricultural soils. Sci. Total Environ. 657 1138–1149. 10.1016/j.scitotenv.2018.12.145 [DOI] [PubMed] [Google Scholar]
- Tomei M. C., Daugulis A. J. (2013). Ex situ bioremediation of contaminated soils: an overview of conventional and innovative technologies. Crit. Revi. Environ. Sci. Technol. 43 2107–2139. [Google Scholar]
- Trigo A., Valencia A., Cases I. (2008). Systemic approaches to biodegradation. FEMS Microbiol. Rev. 33 98–108. [DOI] [PubMed] [Google Scholar]
- Tropel D., Van Der Meer J. R. (2004). Bacterial transcriptional regulators for degradation pathways of aromatic compounds. Microbiol. Mol. Biol. Rev. 68 474–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uluşeker C., Torres J., García J. L., Hanczyc M. M., Nogales J., Kahramanoğullarý O. (2017). “September. a dynamic model of the phosphate response system with synthetic promoters in Escherichia coli,” in Proceedings of the Artificial Life Conference, Vol. 14 (Cambridge, MA: MIT Press; ), 412–419. [Google Scholar]
- Utturkar S. M., Bollmann A., Brzoska R. M., Klingeman D. M., Epstein S. E., Palumbo A. V., et al. (2013). Draft genome sequence for Ralstonia sp. strain OR214, a bacterium with potential for bioremediation. Genome Announc. 1:e00321-13. 10.1128/genomeA.00321-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorst J., Wilkins D., King C. K., Spedding T., Hince G., Zhang E., et al. (2020). Applying microbial indicators of hydrocarbon toxicity to contaminated sites undergoing bioremediation on subantarctic Macquarie Island. Environ. Pollut. 259:113780. 10.1016/j.envpol.2019.113780 [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang W. (2019). Syntheticbiology: recent progress, biosafety and biosecurity concerns, and possible solutions. J. Biosaf. Biosecur. 1 22–30. [Google Scholar]
- Wei K., Yin H., Peng H., Lu G., Dang Z. (2019). Bioremediation of triphenyl phosphate in river water microcosms: proteome alteration of Brevibacillus brevis and cytotoxicity assessments. Sci. Total Environ. 649 563–570. 10.1016/j.scitotenv.2018.08.342 [DOI] [PubMed] [Google Scholar]
- Wintermute E. H., Silver P. A. (2010). Dynamics in the mixed microbial concourse. Genes Dev. 24 2603–2614. 10.1101/gad.1985210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen Y., Wei N. (2020). Biocatalytic properties of cell surface display laccase for degradation of emerging contaminant acetaminophen in water reclamation. Biotechnol. Bioeng. 117 342–353. 10.1002/bit.27214 [DOI] [PubMed] [Google Scholar]
- Wynn D., Deo S., Daunert S. (2017). “Engineering rugged field assays to detect hazardous chemicals using spore-based bacterial biosensors,” in Methods in Enzymology, Vol. 589 eds Abelson J., Simon M., Verdine G., Pyle A. (Cambridge, MA: Academic Press; ), 51–85. [DOI] [PubMed] [Google Scholar]
- Xu P., Lai C., Zeng G., Huang D., Chen M., Song B., et al. (2018). Enhanced bioremediation of 4-nonylphenol and cadmium co-contaminated sediment by composting with Phanerochaete chrysosporium inocula. Bioresour. Technol. 250 625–634. 10.1016/j.biortech.2017.11.069 [DOI] [PubMed] [Google Scholar]
- Yadav T. C., Pal R. R., Shastri S., Jadeja N. B., Kapley A. (2015). Comparative metagenomics demonstrating different degradative capacity of activated biomass treating hydrocarbon contaminated wastewater. Bioresour. Technol. 188 24–32. 10.1016/j.biortech.2015.01.141 [DOI] [PubMed] [Google Scholar]
- Yergeau E., Sanschagrin S., Beaumier D., Greer C. W. (2012). Metagenomic analysis of the bioremediation of diesel-contaminated Canadian high arctic soils. PLoS One 7:e30058. 10.1371/journal.pone.0030058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampolli J., Di Canito A., Cappelletti M., Collina E., Lasagni M., Di Gennaro P. (2020). Biodegradation of naphthenic acids: identification of Rhodococcus opacus R7 genes as molecular markers for environmental monitoring and their application in slurry microcosms. Appl. Microbiol. Biotechnol. 104 2675–2689. 10.1007/s00253-020-10378-5 [DOI] [PubMed] [Google Scholar]
- Zhang D., Liu Q. (2016). Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 75 273–284. 10.1016/j.bios.2015.08.037 [DOI] [PubMed] [Google Scholar]
- Zhao B., Poh C. L. (2008). Insights into environmental bioremediation by microorganisms through functional genomics and proteomics. Proteomics 8 874–881. 10.1002/pmic.200701005 [DOI] [PubMed] [Google Scholar]
- Zhu Y. G., Xue X. M., Kappler A., Rosen B. P., Meharg A. A. (2017). Linking genes to microbial biogeochemical cycling: lessons from arsenic. Environ. Sci. Technol. 51 7326–7339. 10.1021/acs.est.7b00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z., Gong T., Che Y., Liu R., Xu P., Jiang H., et al. (2015). Engineering Pseudomonas putida KT2440 for simultaneous degradation of organophosphates and pyrethroids and its application in bioremediation of soil. Biodegradation 26 223–233. 10.1007/s10532-015-9729-2 [DOI] [PubMed] [Google Scholar]
- Zuroff T. R., Xiques S. B., Curtis W. R. (2013). Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture. Biotechnol. Biofuels 6:59. 10.1186/1754-6834-6-59 [DOI] [PMC free article] [PubMed] [Google Scholar]