Abstract
Self-expandable metallic stent (SEMS) placement is widely used for relieving symptoms in malignant gastric outlet obstruction (MGOO). This study aimed to evaluate the efficacy and safety of multiple gastroduodenal stent placement using the stent-in-stent technique and to identify factors predictive of stent patency.
We retrospectively analyzed data from 170 patients with GOO receiving SEMS using the stent-in-stent technique between July 2006 and July 2018. Of these, 90 had been treated with SEMS placement for MGOO. Technical and clinical success rates were evaluated. Clinical outcomes and predictors of stent patency were also analyzed.
Second SEMS placement was used in 34.4% of cases and 9.7% were treated with third SEMS placement because of prior stent dysfunction. Median stent patency time was 15.7 weeks for the first SEMS, 10.4 weeks for the second, and 11.3 weeks for the third. The technical and clinical success rates were 100% and 97.8% for the first SEMS, 100% and 90.3% for the second, respectively, and both 100% for the third. Multivariable analysis showed that use of covered SEMS and chemotherapy after first and second SEMS placement was significant predictors of stent patency. Serious complications such as bleeding or perforation did not occur in any patient.
Second and third gastroduodenal SEMS placement using the stent-in-stent technique is safe and effective for management of first stent dysfunction in MGOO. Stent patency is significantly associated with the use of covered SEMS and chemotherapy after SEMS placement.
Keywords: malignant gastric outlet obstruction, predictive factor, self-expandable metallic stent, stent patency, stent-in-stent technique
1. Introduction
Malignant gastric outlet obstruction (MGOO) is a late complication of advanced gastrointestinal or pancreatobiliary malignancies.[1] Prognoses of these advanced cancers are still poor and the median overall survival is approximately 1 year.[2–4] MGOO dramatically reduces the quality of life in patients with limited life expectancy. Patients have nausea, vomiting, poor appetite, intolerance to oral feeding, and weight loss.[5] It is important to alleviate obstructive symptoms to improve the quality of life in terminal patients.
Self-expandable metallic stent (SEMS) placement has been widely used to relieve obstructive symptoms of MGOO and is considered an alternative to surgical bypass such as gastrojejunostomy, especially in patients with a limited life span or those in poor general condition.[6–8] The placement of a SEMS has several advantages compared to surgical bypass, including early time to oral intake, faster symptom relief, lower morbidity and mortality, shorter hospital stay, and decreased cost.[7,9–11] As a result of recent advances in cancer treatment, patients treated with SEMS for MGOO can live longer than expected.[5] In these cases, the SEMS is often clogged and requires second SEMS placement.
Multiple SEMS placement is usually performed using a stent-in-stent technique, which involves the insertion of a stent into the stenotic portion of the prior stent.[12] Previous reports on multiple gastroduodenal SEMS placement after first stent dysfunction are limited. Therefore, our aim was to assess the efficacy and safety of multiple gastroduodenal SEMS placement using the stent-in-stent technique and to identify factors predictive of stent patency.
2. Materials and methods
2.1. Patient population
Between July 2006 and July 2018, 170 patients with gastric outlet obstruction (GOO) underwent gastroduodenal SEMS placement using a stent-in-stent technique. Of these, 90 had been treated with gastroduodenal SEMS placement for MGOO. Gastroduodenal SEMS placement was performed at Gangnam Severance Hospital in Seoul, Korea. Dysfunction of a prior stent was confirmed endoscopically. Contraindications to multiple gastroduodenal SEMS placement were inability to tolerate the endoscopic procedure due to poor general condition. Peritoneal carcinomatosis and massive ascites were not considered contraindications to multiple gastroduodenal SEMS placement. Informed consent was obtained from all patients before the procedure. The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Gangnam Severance Hospital (IRB No: 3-2018-0365).
2.2. Self-expandable metallic stent placement and follow-up
Computed tomography (CT) and upper endoscopy were performed to evaluate the obstruction site and stricture length before SEMS placement. All SEMS placement was performed using upper endoscopy through the working channels under fluoroscopic guidance. The endoscope was carefully inserted near the obstruction site and the causes of prior stent dysfunction were evaluated endoscopically. A guidewire was passed through the obstruction site and water-soluble radiographic contrast was injected to identify the length and location of the obstruction. The length of SEMS was determined by the stricture length, with an additional 2 to 3 cm on each side to ensure adequate margins after placement.
Abdominal X-ray images were routinely taken after SEMS placement to monitor SEMS expansion. Oral liquid intake was allowed after the procedure and soft solids were allowed later. Patients were permitted to eat a full diet as tolerated. Chemotherapy including oral anticancer drug regimens and radiotherapy were allowed after SEMS placement if the patients remained in good general condition. If obstructive symptoms recurred during follow-up, upper endoscopy and CT were performed to evaluate the cause of obstruction. The next SEMS was inserted to relieve obstructive symptoms using the stent-in-stent technique.
2.3. Evaluation of the degree of gastric outlet obstruction
The degree of obstruction was assessed using the gastric outlet obstruction scoring system (GOOSS).[13] Scoring is based on the level of oral intake. For example, 0: no oral intake, 1: liquids only, 3: low-residue or full diet.
2.4. Definitions
Clinical outcomes of multiple gastroduodenal SEMS placement were evaluated according to the following criteria:
-
(1)
technical success,
-
(2)
clinical success,
-
(3)
status of oral intake evaluated with GOOSS,
-
(4)
stent patency time,
-
(5)
stent dysfunction,
-
(6)
reintervention rate, and
-
(7)
complications.
Technical success was defined as precise SEMS placement at the obstruction site and adequate SEMS expansion. Clinical success was defined as improvement in GOOSS score after SEMS placement. Stent patency time was defined as the period between SEMS insertion and SEMS restenosis. Stent dysfunction was defined as recurrence of obstructive symptoms and failure to resume oral intake. Causes of obstruction were classified as ingrowth, overgrowth, fracture, or extrinsic obstruction on upper endoscopic findings or fluoroscopic imaging. Complications were monitored after SEMS placement.
2.5. Data collection
All data, including radiologic reports, procedure reports, and blood biochemistry results, were obtained from medical records. The retrospectively collected data included baseline characteristics, primary cancer site, cancer stage, the presence of peritoneal dissemination and ascites, chemotherapy/radiotherapy treatment, GOOSS score, adverse events, and stent patency time. This study was approved by the Institutional Review Board of the Gangnam Severance Hospital.
2.6. Statistical analysis
Categorical variables were presented as a number (percentage). The stent patency time was expressed as median ± Interquartile Range (IQR) or actual range. Univariate analysis of stent patency was analyzed with simple linear regression. Variables with P values < .05 in univariate analysis were evaluated subsequently with multivariate analysis. The multivariate analysis was analyzed using multiple linear regression and the chi-squared test. Statistical significance was defined as a P value < .05. An improvement in GOOSS score after SEMS placement was analyzed with the paired t test. SPSS ver. 23.0 for Windows (SPSS, Chicago, IL) was used for all statistical analyses.
3. Results
3.1. Patient characteristics
Baseline characteristics are summarized in Table 1. The mean age in our study group was 72.1 years, and 59 were males (65.6%). Gastric cancer (73.3%) predominated, followed by pancreatic cancer (12.2%) and cholangiocarcinoma (7.8%). These patients were inoperable, with 82 (91.1%) already at stage IV. Peritoneal carcinomatosis was present in 53 patients (58.9%) before the first gastroduodenal SEMS placement and 27 (50.9%) had ascites. Among these patients, 17 (18.9%) failed to resume any oral intake.
Table 1.
Baseline patient characteristics.

3.2. Clinical outcomes
Table 2 shows the details after first SEMS placement. The pylorus (54.4%) was the most common obstruction site in the stomach and an uncovered SEMS (67.8%) was used more often than a covered SEMS for initial placement. An 8 cm long SEMS (33.3%) was most frequently inserted in this population and the median patency time for the first SEMS was 15.7 weeks (IQR 3–21 weeks). Technical success was achieved in all patients and clinical success was attained in 88 (97.8%). After first SEMS placement, 48 patients (53.3%) were able to take a low-residue or full diet, and the median GOOSS score was significantly improved from 1.06 to 2.54 (P < .001). Systemic chemotherapy was performed in 42 patients (46.7%) and 65 (72.2%) had peritoneal carcinomatosis.
Table 2.
Clinical outcomes after first self-expandable metallic stent (SEMS) placement.
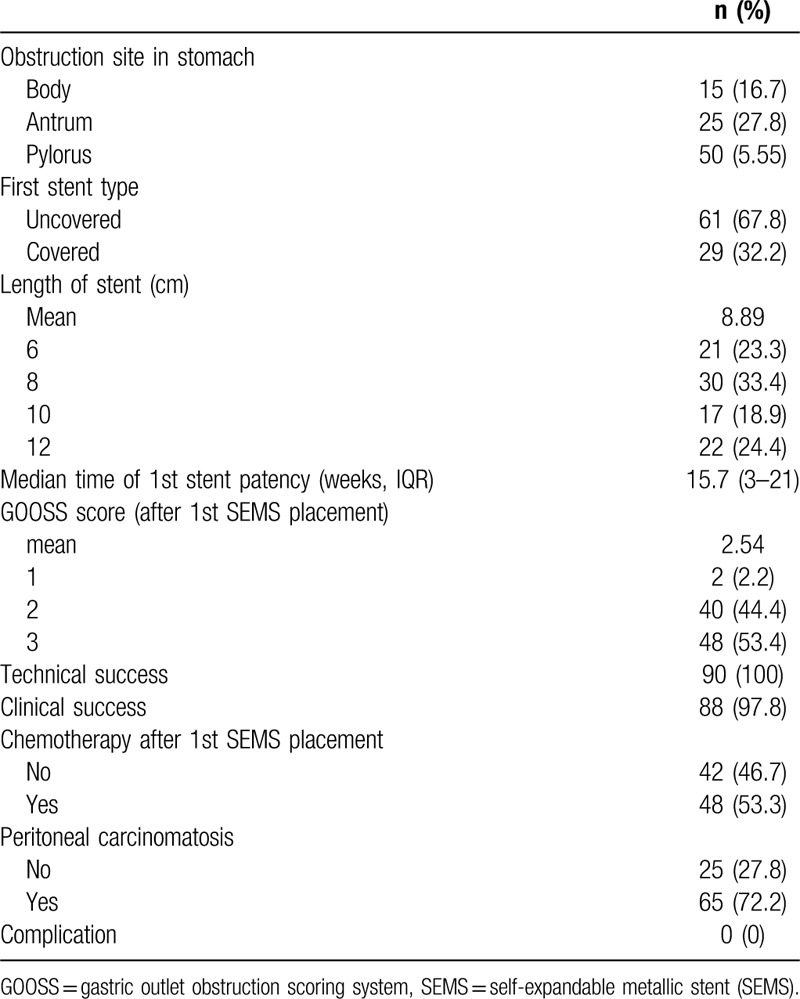
Table 3 shows the details of second SEMS placement. First, stent dysfunction was observed in 31 patients (34.4%). The major indication was stent occlusion caused by tumor ingrowth (77.4%) or overgrowth (12.9%). A covered SEMS (54.8%) was used more often than an uncovered SEMS for second placement, and the median patency time for the second SEMS was 10.4 weeks (IQR 1–13 weeks). Technical success was achieved in all patients, with clinical success in 28 (90.3%). After second SEMS placement, 18 patients (58.1%) were able to take a low-residue or full diet, and the median GOOSS score was significantly improved from 1.0 to 2.48 (P < .001). Systemic chemotherapy was performed in 21 patients (67.7%).
Table 3.
Clinical outcomes after second self-expandable metallic stent (SEMS) placement.
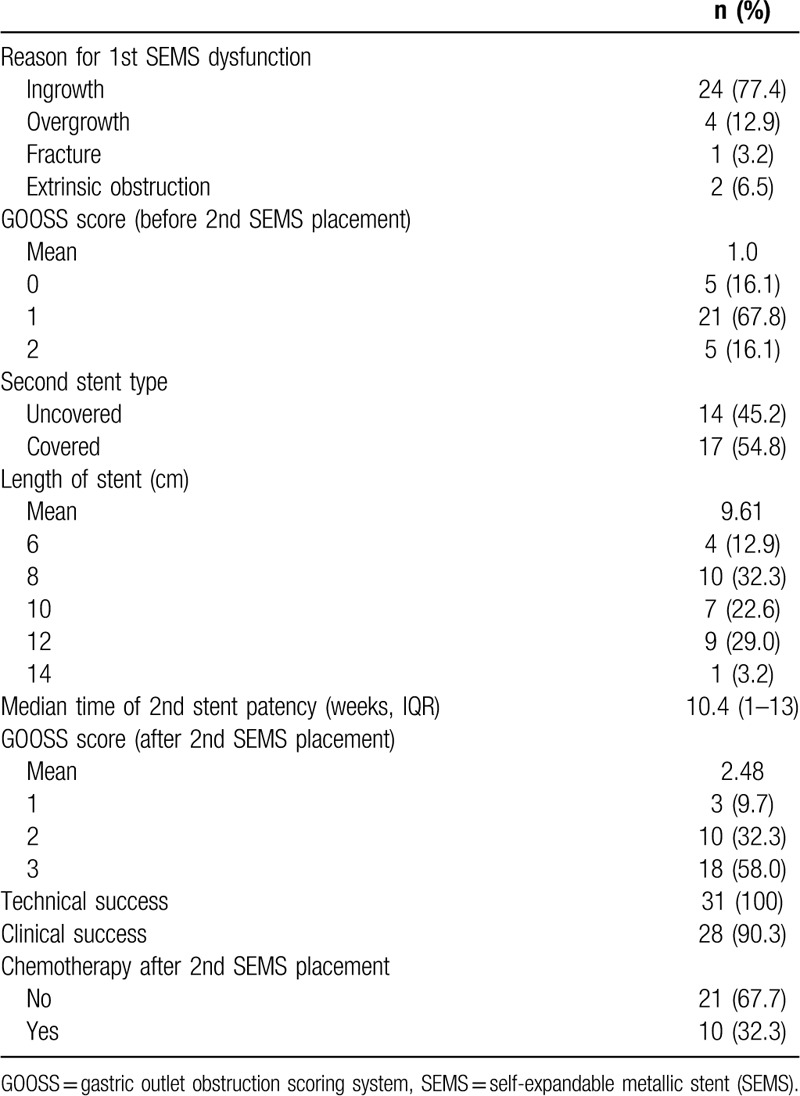
3.3. Comparison of clinical outcomes after first, second, and third SEMS placement
Comparison of clinical outcomes after first, second, and third SEMS placement is shown in Table 4. Among 90 patients who received first SEMS placement, 31 (34.4%) underwent second SEMS placement for symptom relief. Among 31 patients who received second SEMS placement, 3 (9.7%) underwent third SEMS placement. Median stent patency time was 15.7 weeks for the first SEMS, 10.4 weeks for the second, and 11.3 weeks for the third. Technical success was achieved in all patients (100%) and the clinical success rate was 97.8% for first SEMS, 90.3% for second SEMS, and 100% for third SEMS. The mean GOOSS scores were improved after first, second, and third SEMS placement. Figure 1 shows endoscopic and fluoroscopic imaging of patient who underwent first (A), second (B), and third (C) SEMS placement. This 52-year-old male patient diagnosed with advanced gastric cancer with partial GOO.
Table 4.
Comparison of clinical outcomes after first, second, and third self-expandable metallic stent (SEMS) placement.
Figure 1.

Endoscopic and fluoroscopic imaging of patient who underwent first (A), second (B), and third (C) SEMS placement for MGOO. MGOO = malignant gastric outlet obstruction, SEMS = self-expandable metallic stent.
3.4. Factors predictive of stent patency
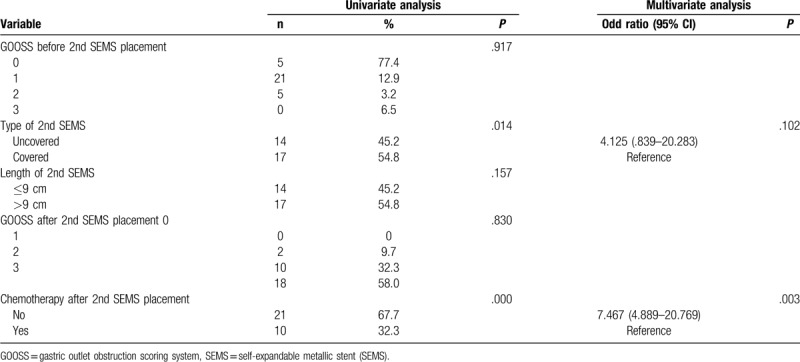
Univariate analysis showed that tumor origin and type of first SEMS, chemotherapy after first SEMS placement, and complications were factors predictive of patency. However, in multivariate analysis, only the type of SEMS and chemotherapy after first SEMS placement were independent predictors (Table 5). Univariate analysis showed that the type of second SEMS and chemotherapy after second SEMS placement were predictive of patency. Multivariate analysis showed that only chemotherapy after second SEMS placement was significantly associated with stent patency (Table 6).
Table 5.
Univariate and multivariate analysis of first self-expandable metallic stent (SEMS) patency.
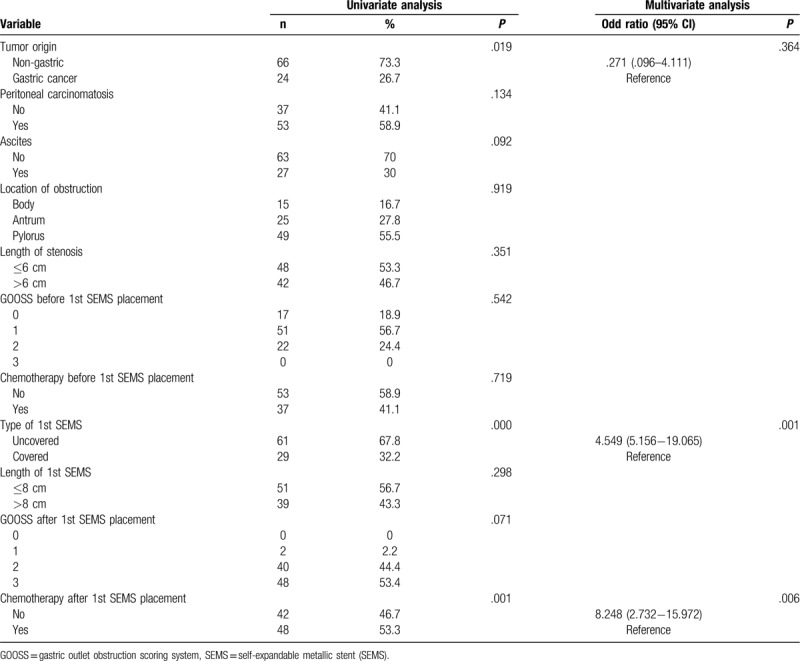
Table 6.
Univariate and multivariate analysis of second SEMS patency.
4. Discussion
Endoscopic SEMS placement has been accepted as a safe and effective palliative measure for MGOO that enables oral intake.[14–16] SEMS placement has gradually replaced surgical gastrojejunostomy for the treatment of MGOO.[17] A meta-analysis of treatment using stents vs surgical gastrojejunostomy reported in 2010 by Ly et al[18] showed that endoscopic stenting was associated with increased tolerance of oral intake, shorter time to initiation of oral intake, and shorter hospital stay after the procedure.
However, obstructive symptoms can be problematic with first stent dysfunction. Re-obstruction occurred in 13% to 26% of patients who underwent SEMS placement because of stent dysfunction caused by tumor progression.[15,19] For patients who experience stent dysfunction, additional SEMS placement is usually required to correct for the loss of first stent patency.[20] Second and third gastroduodenal SEMS placement is usually performed using a stent-in-stent technique, which involves the insertion of a stent into the stenotic portion of the prior stent. Stent-in-stent technique is an effective treatment for patients with MGOO who have occluded first stents.[12]
We analyzed the clinical outcomes of multiple gastroduodenal SEMS placement. Among the 170 patients, 34.4% underwent second SEMS placement and 9.7% underwent third SEMS placement because of prior stent dysfunction. In this study, multiple SEMS placement was safe and effective for obstructive symptoms caused by dysfunction of first SEMS. The technical and clinical success rates were 100% and 97.8% for the first SEMS, 100% and 90.3% for the second, respectively, and both 100% for the third. These results were similar to those of previous studies that reported technical success rates of 92% to 100% and clinical success rates of 75% to 92% with palliative SEMS placement in MGOO.[21–25] The mean GOOSS scores increased after first SEMS placement as well as additional SEMS placement. The median stent patency time was 15.7 weeks for the first SEMS, 10.4 weeks for the second, and 11.3 weeks for the third. These results showed that the efficacy and safety of second and third gastroduodenal SEMS placement were similar to those of first SEMS placement. Sasaki et al[5] reported that the perforation rate was higher after second stent placement than after first stent placement (13.8% vs 0%; P = .02). However, there were no procedure-related adverse events such as bleeding or perforation after first, second, or third SEMS placement in our study.
We also identified factors predictive of stent patency. A few studies of factors affecting stent patency have been published. Telford et al reported that chemotherapy after stent insertion was significantly associated with prolongation of oral intake.[26] Their study was the first to assess the factors predictive of stent patency in the treatment of inoperable MGOO through stent insertion. Since then, other studies have reported similar results following the use of chemotherapy.[23,27,28] Kim et al reported chemotherapy to be a significant protective factor against restenosis.[28] In addition to chemotherapy, the stent type and type of malignant obstructive lesion have been proposed as predictive of stent patency.[23,29] However, other studies have reported that stent type and the type of malignant obstructive lesion have no significant association with stent patency.[30–32] Consequently, this is an important subject for further investigation.[17]
In our multivariate analysis, covered SEMS and chemotherapy after first and second SEMS placement were significant predictors of stent patency. Performing chemotherapy after SEMS placement may reduce or stabilize the tumor burden, diminish tumor growth through the stent mesh, or overgrowth at the stent edges, and prolong the duration of oral intake.[17,20] The patients who received chemotherapy had better performance status and longer stent patency than those who did not. A covered SEMS may have a more favorable outcome than an uncovered stent in the treatment of GOO caused by primary gastrointestinal cancer.[23] Moreover, a covered SEMS can extend stent patency by reducing the risk of stent obstruction due to tumor ingrowth or mucosal hyperplasia.[33] However, there is no consensus regarding the use of covered SEMS to treat GOO because only a few prospective randomized comparison studies have been reported. Therefore, the choice of covered or uncovered SEMS depends on the operator's preference or lesion characteristics.[25]
The first stent patency was related to both covered SEMS and chemotherapy after placement, but the second stent patency was related only to chemotherapy after SEMS placement. This suggests that it may be important to reduce the tumor burden with chemotherapy rather than changing the stent type as the number of stents increases. This is because stent patency becomes more consistent with survival over time.
Our study had several limitations. First, this was a single-center study with a small sample size. Second, this study was conducted in a retrospective manner, and SEMS patency was assessed with GOOSS instead of second-look endoscopy, which was only performed for selected patients who experienced stent dysfunction. Third, SEMS placements were performed by several different endoscopists. Fourth, various malignancies with different prognoses may influence SEMS patency, particularly with addition of chemotherapy.
In conclusion, second and third gastroduodenal SEMS placement using the stent-in-stent technique is safe and effective for first SEMS dysfunction in MGOO. Patency is significantly associated with the use of covered SEMS and chemotherapy after SEMS placement.
Author contributions
Young Min Kim and Jin Won Mo: Study concept and design, acquisition, analysis, and interpretation of data, statistical analysis, and drafting of manuscript.
Seung Yong Shin, Young Hoon Youn, and Hyojin Park: Important intellectual content and study supervision.
Jie-Hyun Kim: Study concept and design, analysis and interpretation of data, important intellectual content, and study supervision.
Jie-Hyun Kim orcid: 0000-0002-9198-3326.
Footnotes
Abbreviations: CT = computed tomography, GOO = gastric outlet obstruction, GOOSS = gastric outlet obstruction scoring system, IQR = interquartile range, MGOO = malignant gastric outlet obstruction, SEMS = self-expandable metallic stent.
How to cite this article: Mo JW, Kim YM, Kim JH, Shin SY, Youn YH, Park H. Clinical outcomes after multiple self-expandable metallic stent placement using stent-in-stent technique for malignant gastric outlet obstruction. Medicine. 2020;99:21(e19432).
JWM and YMK contributed equally to this work.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2015R1C1A2A01053924).
The authors declare that they have no conflicts of interest.
References
- [1].Ye BW, Chou CK, Hsieh YC, et al. Metallic stent expansion rate at day one predicts stent patency in patients with gastric outlet obstruction. Dig Dis Sci 2017;62:1286–94. [DOI] [PubMed] [Google Scholar]
- [2].Park SC, Chun HJ. Chemotherapy for advanced gastric cancer: review and update of current practices. Gut Liver 2013;7:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tada M, Nakai Y, Sasaki T, et al. Recent progress and limitations of chemotherapy for pancreatic and biliary tract cancers. World J Clin Oncol 2011;2:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sasaki T, Isayama H, Nakai Y, et al. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J Intern Med 2013;28:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sasaki T, Isayama H, Nakai Y, et al. Clinical outcomes of secondary gastroduodenal self-expandable metallic stent placement by stent-in-stent technique for malignant gastric outlet obstruction. Dig Endosc 2015;27:37–43. [DOI] [PubMed] [Google Scholar]
- [6].Maetani I, Tada T, Ukita T, et al. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy 2004;36:73–8. [DOI] [PubMed] [Google Scholar]
- [7].Hosono S, Ohtani H, Arimoto Y, et al. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007;42:283–90. [DOI] [PubMed] [Google Scholar]
- [8].Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc 2010;71:490–9. [DOI] [PubMed] [Google Scholar]
- [9].Del Piano M, Ballare M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005;61:421–6. [DOI] [PubMed] [Google Scholar]
- [10].Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc 2006;20:1083–7. [DOI] [PubMed] [Google Scholar]
- [11].Roy A, Kim M, Christein J, et al. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012;26:3114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim CG, Choi IJ, Lee JY, et al. Outcomes of second self-expandable metallic stent insertion for malignant gastric outlet obstruction. Surg Endosc 2014;28:281–8. [DOI] [PubMed] [Google Scholar]
- [13].Piesman M, Kozarek RA, Brandabur JJ, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol 2009;104:2404–11. [DOI] [PubMed] [Google Scholar]
- [14].Kazi HA, O’Reilly DA, Satchidanand RY, et al. Endoscopic stent insertion for the palliation of malignant gastric outlet obstruction. Dig Surg 2006;23:28–31. [DOI] [PubMed] [Google Scholar]
- [15].Sasaki T, Isayama H, Maetani I, et al. Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig Endosc 2013;25:1–6. [DOI] [PubMed] [Google Scholar]
- [16].Kang MK, Song HY, Kim JW, et al. Additional gastroduodenal stent placement: retrospective evaluation of 68 consecutive patients with malignant gastroduodenal obstruction. Acta Radiol 2013;54:944–8. [DOI] [PubMed] [Google Scholar]
- [17].Kim SH, Chun HJ, Yoo IK, et al. Predictors of the patency of self-expandable metallic stents in malignant gastroduodenal obstruction. World J Gastroenterol 2015;21:9134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ly J, O’Grady G, Mittal A, et al. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010;24:290–7. [DOI] [PubMed] [Google Scholar]
- [19].Caglar E, Dobrucali A. Self-expandable metallic stent placement in the palliative treatment of malignant obstruction of gastric outlet and duodenum. Clin Endosc 2013;46:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jin EH, Kim SG, Seo JY, et al. Clinical outcomes of re-stenting in patients with stent malfunction in malignant gastric outlet obstruction. Surg Endosc 2016;30:1372–9. [DOI] [PubMed] [Google Scholar]
- [21].Mosler P, Mergener KD, Brandabur JJ, et al. Palliation of gastric outlet obstruction and proximal small bowel obstruction with self-expandable metal stents: a single center series. J Clin Gastroenterol 2005;39:124–8. [PubMed] [Google Scholar]
- [22].Nassif T, Prat F, Meduri B, et al. Endoscopic palliation of malignant gastric outlet obstruction using self-expandable metallic stents: results of a multicenter study. Endoscopy 2003;35:483–9. [DOI] [PubMed] [Google Scholar]
- [23].Cho YK, Kim SW, Hur WH, et al. Clinical outcomes of self-expandable metal stent and prognostic factors for stent patency in gastric outlet obstruction caused by gastric cancer. Dig Dis Sci 2010;55:668–74. [DOI] [PubMed] [Google Scholar]
- [24].Maetani I, Ukita T, Tada T, et al. Metallic stents for gastric outlet obstruction: reintervention rate is lower with uncovered versus covered stents, despite similar outcomes. Gastrointest Endosc 2009;69:806–12. [DOI] [PubMed] [Google Scholar]
- [25].Im JP, Kang JM, Kim SG, et al. Clinical outcomes and patency of self-expanding metal stents in patients with malignant upper gastrointestinal obstruction. Dig Dis Sci 2008;53:938–45. [DOI] [PubMed] [Google Scholar]
- [26].Telford JJ, Carr-Locke DL, Baron TH, et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc 2004;60:916–20. [DOI] [PubMed] [Google Scholar]
- [27].Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc 2007;66:256–64. [DOI] [PubMed] [Google Scholar]
- [28].Kim CG, Park SR, Choi IJ, et al. Effect of chemotherapy on the outcome of self-expandable metallic stents in gastric cancer patients with malignant outlet obstruction. Endoscopy 2012;44:807–12. [DOI] [PubMed] [Google Scholar]
- [29].Canena JM, Lagos AC, Marques IN, et al. Oral intake throughout the patients’ lives after palliative metallic stent placement for malignant gastroduodenal obstruction: a retrospective multicentre study. Eur J Gastroenterol Hepatol 2012;24:747–55. [DOI] [PubMed] [Google Scholar]
- [30].Kim CG, Choi IJ, Lee JY, et al. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc 2010;72:25–32. [DOI] [PubMed] [Google Scholar]
- [31].Cheng HT, Lee CS, Lin CH, et al. Treatment of malignant gastric outlet obstruction with metallic stents: assessment of whether gastrointestinal position alters efficacy. J Investig Med 2012;60:1027–32. [DOI] [PubMed] [Google Scholar]
- [32].Kim YW, Choi CW, Kang DH, et al. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci 2011;56:2030–6. [DOI] [PubMed] [Google Scholar]
- [33].Maetani I. Self-expandable metallic stent placement for palliation in gastric outlet obstruction. Ann Palliat Med 2014;3:54–64. [DOI] [PubMed] [Google Scholar]


