Abstract
Introduction
In addition to well-established links with cardiovascular and respiratory diseases, cigarette smoking may affect skeletal muscle; however, associations with quadriceps atrophy, density, and function are unknown. This study explored the associations of current and former smoking with quadriceps muscle area and attenuation as well as muscle force (assessed as knee extension peak torque) and rate of torque development—a measure of muscle power in older adults.
Methods
Data from 4469 older adults, aged 66–95 years at baseline in the Age, Gene/Environment Susceptibility-Reykjavik Study with measurements of thigh computed tomography, isometric knee extension testing, self-reported smoking history, and potential covariates were analyzed.
Results
Sex differences were observed in these data; therefore, our final analyses are stratified by sex. In men, both former smokers and current smokers had lower muscle area (with β= –0.10, 95% confidence interval [CI] = –0.17 to –0.03 and β = –0.19, 95% CI = –0.33 to –0.05, respectively) and lower muscle attenuation (ie, higher fat infiltration, β = –0.08, 95% CI = –0.16 to –0.01 and β = –0.17, 95% CI = –0.34 to –0.01, respectively) when compared with never smokers. Smoking status was not associated with male peak torque or rate of torque development. In women, current smoking was associated with lower muscle attenuation (β = –0.24, 95% CI = –0.34 to –0.13) compared to never smoking. Among female smokers (current and former), muscle attenuation and peak torque were lower with increasing pack-years.
Conclusions
Results suggest that cigarette smoking is related to multiple muscle properties at older age and that these relationships may be different among men and women.
Implications
This article presents novel data, as it examined for the first time the relationship between smoking and computed tomography-derived quadriceps muscle size (cross-sectional area) and attenuation. This study suggests that current cigarette smoking is related to higher muscle fat infiltration, which may have significant health implications for the older population, because of its known association with poor physical function, falls, and hip fractures.
Introduction
Smoking remains one of the most common risk behaviors worldwide. Previous epidemiological studies have examined the link between cigarette smoking and the age-related loss of muscle mass and strength,1,2 but findings are mixed and had limitations, notably the common use of dual X-ray absorptiometry or bioelectric impedance analysis to assess lean mass. Computed tomography (CT) is a gold-standard technique for measures of muscle cross-sectional area and muscle attenuation (a noninvasive indicator of fat accumulation that has been linked to reduced strength and power).3 However, the association of smoking with these CT-derived muscle outcomes is unknown.
Moreover, the leg extensors are critical muscles for the fundamental activities of daily living,4 and impaired quadriceps strength and/or quality has been associated with increased risk of fracture,5 mortality,6 hospitalization, and lower gait speed.7 Understanding the relationship of cigarette smoking with muscle strength and power can help to identify high-risk populations to efficiently prevent and delay the progress of muscle dysfunction. Previous research has examined associations between smoking and muscle decline in relatively small, select samples with highly exposed groups.8–10 Analyses of muscle measurements from datasets that more closely reflect a population distribution of smoking exposure in older adults are scarce.
To address this knowledge gap, we examined the relationships between cigarette smoking and four indicators of muscle health—quadriceps area and attenuation (assessed by CT), and peak torque and rate of torque development (RTD), which represent two valid measures of quadriceps muscle force and power measured during isometric muscle contractions—in a well-described population-based cohort of older men and women.
Methods
Study Population
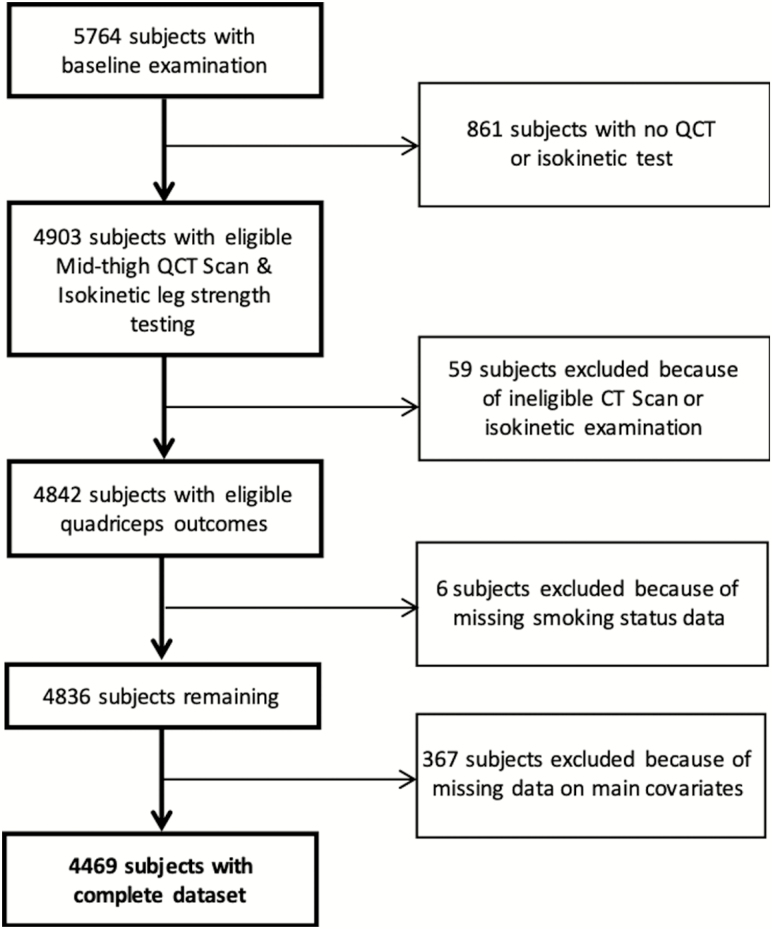
The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study is a single‐center prospective population study of adults aged 66 years and older representing the general population of Iceland. Details of the design and recruitment methods have been published.11 Our analytical sample included 4469 participants (1888 men and 2581 women) who had completed data for all included variables (Figure 1). Written informed consent was obtained from all participants, and the study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the institutional review board of the Intramural Research Program of the National Institute on Aging.
Figure 1.
Study population flow diagram.
Measures
Smoking Behavior
Information on cigarette smoking was self-reported at baseline through a standardized questionnaire. Participants reported whether they currently smoked; if they answered yes, they were asked the number of cigarettes they smoke on average per day and the age of smoking initiation. If participants reported that they were not current smokers, they were asked if they smoked in the past, the number of cigarettes they smoked on average per day when they smoked, the age of smoking initiation, and the age at which they stopped smoking.
On the basis of these data, we defined smoking status (never, former, current smokers). For each of the former smoking and current smoking groups, we computed for each subject the number of pack-years by multiplying the number of cigarettes smoked per day by the number of years of smoking, which was computed as (current age—age at smoking initiation) for current smokers, and as (age at smoking cessation—age at smoking initiation) for former smokers. Each pack-year represents exposure to 7300 cigarettes (1 year × 365 days × 1 pack/day × 20 cigarettes/pack]. We also examined age at smoking initiation and years since smoking cessation (computed for former smokers as [current age—age at smoking cessation]).
Quadriceps Cross-Sectional Area and Attenuation
CT measurements in the mid-thigh were performed using a 4-detector CT system (Sensation 4, Siemens Medical Systems, Erlangen, Germany) as described previously.6,12 In brief, a single 10-mm thick axial image (120 kVp, 200–250 mA) was obtained at the mid-thigh, and the quadriceps muscle was contoured along with the deep fascial plane. Muscle cross-sectional area was calculated in centimeter square within the muscle contour and attenuation (measured in Hounsfield units [HU]) as the mean value from all pixels within the range of 0–100 HU within the quadriceps contour. Lower HU indicates greater fat infiltration. Muscle cross-sectional area and attenuation were measured in the same leg used for knee extension testing.
Quadriceps Peak Torque and RTD
Isometric knee extension was tested with a dynamometer chair (Good Strength, Mettitur Ltd, Palokka, Finland) as described previously in detail.3 Peak torque (Nm) was derived as the product of the maximum value (N) of all possible filter windows and lower leg length (m). RTD (Nm/s; an isometric measure of power) was the product of the maximum derivative (N/s) of the force–time curve before the peak torque and lower leg length (m). For each outcome, the trial with the maximum value was analyzed.
Covariates
Several potential confounding covariates were taken into account in the analyses. These were selected from an extensive list of variables available in the AGES dataset and thought to be associated with muscle properties and smoking, based on biological plausibility or previous literature findings. These variables were age, physical activity, health status, impaired mobility, alcohol consumption, and history of chronic obstructive pulmonary disease (COPD), all measured using structured questionnaires. Body mass index, percent weight change from age 50, quadriceps intramuscular adipose tissue area, depressive symptoms, and cognitive function were also considered.
The body mass index was calculated as weight (kg) divided by height (m) squared. Percent weight change was calculated using midlife weight data from the Reykjavik Study11 and baseline AGES-Reykjavik weight measurements as follows: (baseline weight – midlife weight/midlife weight) × 100. Participants were categorized as moderate/high physically active or occasionally physically active at most, as described previously.13 Impaired mobility was defined as having much difficulty or unable to walk 500 m and/or climb 10 steps. Self-reported health status was recorded as “poor” for fair or poor responses and “good” for excellent/very good/good responses. Alcohol intake (defined as current drinker—if answered yes to the question “Do you drink alcoholic beverages now?” or non-drinker, if answered no) measured by questionnaire. Cognitive status was determined by professional consensus after reviewing results of cognitive examinations as described previously11,14 and categorized as normal or impaired. Depressive symptoms were measured by the 15-item Geriatric Depression Scale. Education was dichotomized as high (defined as >12 years of education, indicating participants completed college or more) or low (≤12). Total 25-hydroxyvitamin D and high-sensitivity C-reactive protein (above 3.0 mg/L) were measured in the Icelandic Heart Association (IHA) laboratory, using blood samples drawn after overnight fasting. Coffee intake was measured by questionnaire and dichotomized as high (defined as ≥3 cups/day) or low (<3 cups/day). On the basis of the HU values corresponding to the upper and lower adipose tissue thresholds (between −190 and −30 HU) the quadriceps intramuscular adipose tissue area was derived3,12 and expressed as a percentage of the total area of the quadriceps. Intramuscular adipose tissue area represents the visible adipose tissue lying interior to the deep fascial plane surrounding the muscle. COPD was determined (yes/no) by self-report or medication use.
Statistical Analysis
Differences in the distributions of baseline characteristics between never, former, and current smokers were assessed using analysis of variance followed by Tukey’s post hoc test, and by the chi-squared test.
Given reported sex differences in smoking and muscle outcomes,15 and because we found some indication of differences in smoking–muscle associations between men and women in our sample, all analyses were stratified by sex. Multivariable regression models were used to examine the associations between smoking variables and the z-scores of the muscle measures as dependent variables. Models were adjusted for potential confounding variables that were found to be associated with both smoking status and muscle-related variables in our sample (Model 1). In sensitivity analyses, we also adjusted for education, total 25-hydroxyvitamin D, high-sensitivity C-reactive protein, and coffee consumption that were associated with either smoking or muscle outcomes. In additional analyses, we restricted the sample to non-COPD participants to examine whether the associations are observed in healthy subjects. We evaluated the relationship of pack-years and age at smoking initiation with muscle outcomes in ever-smokers (because the examined associations were not systematically different between current smokers and former smokers). We also used the duration of smoking exposure and number of cigarettes smoked per day to further test the possible role of exposure time and intensity in our analytical sample. For former smokers, we examined the association between years since smoking cessation and muscle outcomes. Analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY: IBM Corp). Statistical significance was set at p value of less than .05.
Results
The study analytical sample consisted of 1888 men and 2581 women aged 66–95 years (mean age = 76.4 ± 5.5 years). The proportion of never, former, and current smokers was significantly different between men and women (38.9%, 55.3%, and 5.8% in men and 52.8%, 34.9%, and 12.3% in women, respectively). Baseline characteristics of participants according to smoking status are shown in Table 1.
Table 1.
Characteristics of Analytic Sample in Relation to Gender and Smoking Status (n = 4469)
| Mean ± SD | Men (n = 1888) | Women (n = 2581) | ||||||
|---|---|---|---|---|---|---|---|---|
| Never smokers (n = 735) | Former smokers (n = 1044) | Current smokers (n = 109) | Never smokers (n = 1362) | Former smokers (n = 901) | Current smokers (n = 318) | |||
| p Value | p Value | |||||||
| Age, yrs | 76.8 ± 5.3 | 76.5 ± 5.3 | 74.7 ± 5.0a,b | .001 | 76.9 ± 5.5 | 75.9 ± 5.6a | 74.6 ± 5.2 a,b | <.001 |
| Body mass index, kg/m2 | 26.6 ± 3.6 | 27.3 ± 3.9a | 24.8 ± 4.0a,b | <.001 | 27.2 ± 4.8 | 27.6 ± 4.5 | 26.1 ± 4.8a,b | <.001 |
| Weight change from age 50, % | 1.2 ± 9.7 | 3.9 ± 11.9a | –1.1 ± 12.7b | <.001 | 3.7 ± 13.0 | 8.3 ± 14.5a | 4.3 ± 15.3b | <.001 |
| 25OHD, nmol/L | 58.5 ± 24.1 | 56.9 ± 24.7 | 49.1 ± 27.1a,b | .001 | 52.4 ± 22.9 | 51.2 ± 24.3 | 47.3 ± 23.0a,b | .003 |
| IMAT, % | 5.7 ± 2.8 | 6.0 ± 2.7 | 5.4 ± 2.5 | .017 | 9.1 ± 3.1 | 9.1 ± 3.1 | 9.2 ± 3.3 | 0.745 |
| Muscle area, cm2 | 61.2 ± 9.6 | 60.9 ± 10.1 | 57.1 ± 9.5a,b | <.001 | 42.4 ± 7.0 | 43.0 ± 7.2 | 41.7 ± 7.4b | .012 |
| Muscle attenuation, HU | 46.6 ± 4.9 | 45.9 ± 4.6a | 46.4 ± 4.2 | .010 | 43.1 ± 4.7 | 43.4 ± 4.5 | 42.5 ± 4.5b | .012 |
| Peak torque, N-m | 170.4 ± 47.5 | 170.5 ± 46.4 | 168.3 ± 42.7 | .889 | 96.3 ± 29.2 | 99.5 ± 30.2a | 95.0 ± 29.4b | .014 |
| Maximum RTD, N-m/s | 584.7 ± 289.2 | 575.2 ± 285.6 | 567.5 ± 278.9 | .726 | 312.3 ± 179.6 | 329.0 ± 190.4 | 309.9 ± 177.5 | .075 |
| Smoking characteristics | ||||||||
| Pack-years | — | 23.4 ± 19.7 | 42.3 ± 25.4 | <.001 | — | 16.8 ± 16.1 | 30.3 ± 19.6 | <.001 |
| Age at smoking initiation | — | 19.2 ± 5.2 | 20.0 ± 7.6 | .311 | — | 22.8 ± 7.7 | 24.8 ± 8.9 | <.001 |
| Years since smoking cessation | — | 30.7 ± 14.7 | — | — | 25.7 ± 14.7 | — | ||
| % (N) | ||||||||
| Low Physical activity level | 78.9 (580) | 78.4 (819) | 78.0 (85) | .960 | 84.6 (1152) | 84.6 (762) | 90.3 (287) | .028 |
| Impaired cognitive function | 16.6 (122) | 16.3 (170) | 18.3 (20) | .857 | 13.2 (180) | 11.5 (104) | 13.8 (44) | .410 |
| Poor health status | 23.8 (175) | 28.8 (301) | 34.9 (38) | .012 | 33.0 (449) | 36.1 (325) | 38.7 (123) | .092 |
| Impaired mobility | 6.1 (45) | 6.2 (65) | 8.3 (9) | .685 | 11.2 (152) | 13.2 (119) | 17.0 (54) | .015 |
| Alcohol drinker | 66.3 (487) | 75.5 (788) | 76.1 (83) | <.001 | 51.0 (695) | 69.9 (630) | 69.5 (221) | <.001 |
| Depressive symptoms | 8.6 (63) | 12.5 (131) | 11.9 (13) | .029 | 10.6 (145) | 12.7 (114) | 17.6 (56) | <.001 |
| High hsCRP levels | 25.9 (190) | 29.9 (312) | 41.3 (45) | .003 | 30.3 (412) | 37.5 (338) | 41.8 (133) | <.001 |
| Low education | 61.6 (453) | 72.2 (754) | 80.7 (88) | <.001 | 74.8 (1019) | 75.9 (684) | 82.1 (261) | .024 |
| High coffee consumption | 48.6 (357) | 58.8 (612) | 72.5 (79) | <.001 | 45.4 (616) | 53.7 (482) | 76.7 (243) | <.001 |
| COPD | 6.0 (44) | 11.2 (117) | 17.4 (19) | <.001 | 7.9 (107) | 12.8 (115) | 16.7 (53) | <.001 |
25OHD = total 25-hydroxyvitamin D, COPD = chronic obstructive pulmonary disease, IMAT= intramuscular adipose tissue area, hsCRP= high-sensitivity C-reactive Protein, RTD = rate of torque development; High coffee intake was defined as ≥3 cups/day; low education was defined as ≤12 years of education; high hsCRP was defined as > 3.0 mg/L.
aSignificantly different from never smokers;
bSignificantly different from former smokers.
Smoking Status
In men, regression models adjusted for age, body mass index, % weight change from age 50, physical activity level, impaired mobility, health status, alcohol consumption, cognitive function, and depressive symptoms showed that both former smoking and current smoking were negatively associated with lower quadriceps area and attenuation, and the magnitude of the association for current smoking was almost twice as large compared to former smoking (Table 2). Results were similar in non-COPD subjects (Supplementary Table 1), and only the association between former smoking and quadriceps attenuation was attenuated and not statistically significant (p = .14).
Table 2.
Associations Between Smoking Status and Quadriceps Properties
| Men (n = 1888) | Women (n = 2581) | All sample (n = 4469) | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Coef. | 95% CI | p Value | Coef. | 95% CI | p Value | Coef. (95% CI) | p Value for sex interaction |
| Muscle CSAa, cm2 | ||||||||
| Never smokers | Ref. | Ref. | Ref. | |||||
| Former smokers | –0.10 | –0.17 to –0.03 | .003 | –0.04 | –0.11 to 0.03 | .26 | –0.10 (–0.15 to –0.05) | .39 |
| Current smokers | –0.19 | –0.33 to –0.05 | .009 | –0.07 | –0.17 to 0.03 | .18 | –0.09 (–0.17 to –0.01) | .003 |
| Muscle attenuationb, HU | ||||||||
| Never smokers | Ref. | Ref. | Ref. | |||||
| Former smokers | –0.08 | –0.16 to –0.01 | .038 | 0.02 | –0.05 to 0.10 | .54 | –0.09 (–0.14 to –0.04) | .031 |
| Current smokers | –0.17 | –0.34 to –0.01 | .045 | –0.24 | –0.34 to –0.13 | <.001 | –0.23 (–0.32 to –0.14) | .42 |
| Peak torqueb, Nm | ||||||||
| Never smokers | Ref. | Ref. | Ref. | |||||
| Former smokers | 0.02 | –0.05 to 0.09 | .59 | 0.05 | –0.03 to 0.12 | .22 | –0.06 (–0.11 to –0.01) | .73 |
| Current smokers | 0.08 | –0.08 to 0.24 | .31 | –0.06 | –0.16 to 0.04 | .25 | –0.04 (–0.13, to 0.05) | .08 |
| Maximum RTDb, Nm/s | ||||||||
| Never smokers | Ref. | Ref. | Ref. | |||||
| Former smokers | –0.02 | –0.11 to 0.07 | .67 | 0.03 | –0.05 to 0.11 | .42 | –0.03 (–0.09 to 0.03) | .27 |
| Current smokers | –0.06 | –0.25 to 0.14 | .58 | –0.04 | –0.16 to 0.08 | .49 | –0.05 (–0.16 to 0.05) | .90 |
Models were adjusted for age, BMI, % weight change from age 50, physical activity level, impaired mobility, health status, alcohol consumption, cognitive function, and depressive symptoms. BMI = body mass index; CSA = cross-sectional area; Coef.= coefficient; CI = confidence interval; RTD = rate of torque development.
aAdditionally adjusted to % IMAT (intramuscular adipose tissue) area calculated as quadriceps fat area (cm2)/total quadriceps cross-sectional area (cm2) ×100, and muscle attenuation;
bAdditionally adjusted to % IMAT, and muscle area. Outcomes were expressed in z-scores.
Only current smokers had lower muscle attenuation (coefficient = –0.24; 95% confidence interval [CI] = –0.34 to –0.13; Table 2) when compared to never-smoking women. Restricting the sample to non-COPD participants did not change this association (Supplementary Table 1).
In both men and women, we found that quadriceps peak torque and RTD were not different in former or current smokers, compared with never smokers.
Additional adjustment of these models for education level, total 25-hydroxyvitamin D, high-sensitivity C-reactive protein, and coffee consumption did not substantially change our results (data not shown).
Associations of Smoking Characteristics in Ever Smokers With Muscle Measures
Higher pack-years were not related to poorer muscle outcomes in male ever smokers, except for a trend of lower muscle area with increasing pack-years (coefficient = –0.002; 95% CI = –0.004 to 0.0001; p = .057). Pack-years was negatively associated with muscle attenuation (coefficient = –0.005; 95% CI = –0.007 to –0.002; Table 3) and peak torque (coefficient = –0.003; 95% CI = –0.006 to –0.0005; Table 3) in female ever smokers. Among male and female ever smokers, an older age at smoking initiation was associated with poorer peak torque. Duration of smoking cessation was not associated with any muscle outcomes (Table 3). In sensitivity analyses, using the duration of smoking exposure and number of cigarettes smoked per day (smoking intensity) as predictors did not alter these results. Also, adjusting these models for additional covariates did not change the results. Restricting the sample to non-COPD subjects only resulted in a weaker association (p = .11) between age at smoking initiation and peak torque in women (data not shown).
Table 3.
Associations Between Smoking Characteristics and Quadriceps Properties Among Ever Smokers (Former or Current Smokers)
| Parameter | Men | Women | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p Value | Coefficient (95% CI) | p Value | |
| Muscle areaa | ||||
| Pack-years1 | –0.002 (–0.004 to 0.0001) | .057 | 0.001 (–0.002 to 0.003) | .697 |
| Age at smoking initiation2 | –0.002 (–0.009 to 0.006) | .645 | 0.002 (–0.004 to 0.007) | .570 |
| Years since smoking cessation3 | 0.002 (–0.001 to 0.005) | .173 | 0.001 (–0.002 to 0.005) | .501 |
| Muscle attenuationb | ||||
| Pack-years1 | –0.001 (–0.003 to 0.001) | .394 | –0.005 (–0.007 to –0.002) | .001 |
| Age at smoking initiation2 | 0.007 (–0.001 to 0.016) | .100 | –0.003 (–0.009 to 0.003) | .313 |
| Years since smoking cessation3 | 0.0003 (–0.003 to 0.004) | .852 | 0.002 (–0.002 to 0.006) | .235 |
| Peak Torqueb | ||||
| Pack-years1 | 0.001 (–0.001 to 0.003) | .289 | –0.003 (–0.006 to –0.0005) | .020 |
| Age at smoking initiation2 | –0.009 (–0.017 to –0.001) | .033 | –0.007 (–0.013 to –0.001) | .024 |
| Years since smoking cessation3 | –0.001 (–0.004 to 0.002) | .492 | 0.004 (–0.0002 to 0.008) | .065 |
| Maximum RTDb | ||||
| Pack-years1 | –0.001 (–0.003 to 0.002) | .562 | –0.001 (–0.004 to 0.002) | .696 |
| Age at smoking initiation2 | –0.006 (–0.016 to 0.004) | .250 | –0.002 (–0.009 to 0.004) | .489 |
| Years since smoking cessation3 | 0.003 (–0.001 to 0.007) | .213 | 0.002 (–0.002 to 0.007) | .336 |
Models were adjusted for age, BMI, % weight change from age 50, physical activity level, impaired mobility, health status, alcohol consumption, cognitive function, and depressive symptoms. BMI = body mass index; CI = confidence interval; HU = Hounsfield units; RTD = rate of torque development.
aModels were additionally adjusted to fat area (cm2)/total thigh area (cm2) × 100 and quadriceps muscle attenuation (HU).
bAdditionally adjusted to fat area (cm2)/total quadriceps area (cm2) × 100 and quadriceps lean area (cm2); outcomes were expressed in z-scores.
1Six (men) and ten (women) observations missing data.
2Two (men) and one (woman) observations missing data.
3Four (men) and three (women) observations missing data.
Threshold value of statistical significance p < 0.05.
Discussion
We show for the first time that in older men, both former and current cigarette use were related to lower muscle mass and muscle attenuation (ie, higher fat infiltration). In women, current smokers had lower muscle attenuation compared to never smokers, and among the group of ever-smoking women, higher pack-years were associated with lower muscle attenuation. Smoking status was not associated with muscle peak torque or muscle power. However, among women who ever smoked, the extent of smoking (higher the pack-years) was related to lower peak torque. There were also associations between later age of smoking initiation and lower torque in both men and women ever smokers.
Previous observational studies reported associations between cigarette use and bioelectric impedance analysis15 or dual X-ray absorptiometry-derived lower muscle mass.16 One earlier study10 found no association between smoking history and quadriceps area measured with magnetic resonance imaging, in both men and women, aged 18–73 years. Our findings show a negative relationship between smoking and quadriceps area in men, but not in women. The reasons for this divergent finding are not clear. One explanation could be the larger extent of smoking in men as their average pack-years (25.2 ± 21.1) was significantly higher than women (20.4 ± 18.1). However, there were no systematic associations of more pack-years or time since quitting being more harmful or beneficial for the smoking–muscle relationships. Our findings are based on cross-sectional data, however, existing data from animal models17 and clinical studies8,18 show evidence for smoking-induced skeletal muscle wasting. Several potential mechanisms have been proposed to explain the smoking-associated muscle loss and impairment; these include pathways involving compromised muscle metabolism, increased inflammation and oxidative stress, overexpression of atrophy-related genes, and activation of various intracellular signaling pathways.19 Our hypothesis of smoking-induced muscle damage is further supported by our evidence of an association between former smoking and lower quadriceps area, paired with no apparent positive association between time since smoking cessation and muscle area. Taken together, these data suggest that the muscle damage caused by chronic smoking has an irreversible, residual effect on muscle mass even after smoking cessation. This finding contradicts earlier research that suggested reversible muscle signaling alterations were previously observed for blood cells20,21 or in animal models.22
This is the first study to examine the association between cigarette smoking and skeletal muscle attenuation. Our data suggest that in both men and women, current smoking is associated with lower quadriceps muscle attenuation. This was the only muscle measure different between current smokers and never smokers in women, and the association was more pronounced than for men. Male former smokers also showed an association with lower muscle attenuation although this result was attenuated when the sample was restricted to non-COPD older men. This could suggest that mechanisms related to COPD such as protein synthesis/degradation imbalance, hypoxia, inflammation, oxidative stress, and hormonal disturbances23 could be underlying the relationship between smoking history and muscle attenuation in older men.
In our study, unlike results for muscle area and attenuation, peak torque and maximum RTD were not different across smoking groups. This is an interesting result, because of the tight relationship between muscle atrophy and decreased strength/power. However, previous studies reported conflicting findings, with some reporting a negative effect of smoking on lower limb strength,9,24 whereas others finding no association between smoking and quadriceps maximal torque capacity10 or peak torque.25 Therefore, our data suggest that the possible adverse relationship between smoking and muscle properties is mostly via modifications to muscle structure rather than muscle function. However, within women with a smoking history, those who smoked more had lower torque, suggesting some role of smoking on muscle performance. We also found that initiating smoking at an older age is associated with lower peak torque in both men and women, but no previous studies have explored whether the age of smoking initiation may be associated with muscle function. This negative trend is counterintuitive and could be a spurious result as it is not in line with the other observed associations.
The strengths of this study include a design that more closely resembles the heterogeneity of health measures in the older adult population, the largest sample size to date, examination of several indicators of cigarette smoking, and control of relevant covariates. Some limitations should be noted, however. First, a single timepoint for muscle composition and strength assessment was presently explored. Second, although we controlled for C-reactive protein, we had no data on inflammatory cytokines or hormones (such as cortisol), and COPD was asserted by self-reported data and medication. It is likely that a direct measure of pulmonary function might have resulted in a different classification.
In conclusion, our data suggest that current cigarette smoking is related to poorer muscle quality (indicative of higher fat infiltration, a factor associated with poor physical function and hip fractures) in both men and women. Cigarette smoking showed other patterns of associations, including poorer muscle quantity in men and some role for the extent and timing of exposure. These findings call for more investigations to improve our understanding of the impact of this common risky behavior on muscle physiology among men and women to better sustain muscle health at an older age.
Although radiographic imaging of muscle is not feasible in most clinical settings, this study contributed to clarifying the negative consequences of smoking on skeletal muscle composition objectively quantified by CT. From a public health perspective, our study added new evidence on the link between smoking and lower muscle quality—which is recognized as a significant health problem among older adults, with serious health consequences in terms of frailty, disability, morbidity, and mortality.
Funding
This work was supported by National Institutes of Health contract N01-AG-1-2100, the Hjartavernd (the Icelandic Heart Association); and the Althingi (the Icelandic Parliament). This research was supported in part by the Intramural Research Program of the National Institutes of Health—National Institute on Aging.
Declaration of Interests
Authors have no competing interests to declare. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The text of this article was not reviewed by the sponsor. The manuscript is not under review by another journal.
Supplementary Material
Acknowledgments
None.
References
- 1. Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stenholm S, Tiainen K, Rantanen T, et al. Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J Am Geriatr Soc. 2012;60(1):77–85. [DOI] [PubMed] [Google Scholar]
- 3. Frank-Wilson AW, Chalhoub D, Figueiredo P, et al. Associations of quadriceps torque properties with muscle size, attenuation, and intramuscular adipose tissue in older adults. J Gerontol A Biol Sci Med Sci. 2018;73:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roshanravan B, Patel KV, Fried LF, et al. ; Health ABC study Association of muscle endurance, fatigability, and strength with functional limitation and mortality in the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2017;72(2):284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pham HM, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Contribution of quadriceps weakness to fragility fracture: a prospective study. J Bone Miner Res. 2016;31(1):208–214. [DOI] [PubMed] [Google Scholar]
- 6. Reinders I, Murphy RA, Brouwer IA, et al. ; Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study Muscle quality and myosteatosis: Novel associations with mortality risk: the age, gene/environment susceptibility (AGES)-Reykjavik study. Am J Epidemiol. 2016;183(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan OY, van Houwelingen AH, Gussekloo J, Blom JW, den Elzen WP. Comparison of quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care. Age (Dordr). 2014;36(5):9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montes de Oca M, Loeb E, Torres SH, De Sanctis J, Hernandez N, Talamo C. Peripheral muscle alterations in non-COPD smokers. Chest 2008;133:13–18. [DOI] [PubMed] [Google Scholar]
- 9. Rom O, Karkabi K, Reznick AZ, Keidar Z, Aizenbud D. Relationship between history of smoking, metabolic and inflammatory markers, parameters of body composition and muscle strength. Adv Exp Med Biol. 2015;849:49–56. [DOI] [PubMed] [Google Scholar]
- 10. Wüst RC, Morse CI, de Haan A, Rittweger J, Jones DA, Degens H. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol. 2008;104(1):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, gene/environment susceptibility-Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang T, Koyama A, Li C, et al. Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone. 2008;42(4):798–805. [DOI] [PubMed] [Google Scholar]
- 13. Marques EA, Gudnason V, Sigurdsson G, et al. Are bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES-Reykjavik study. Osteoporos Int. 2016;27(5):1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vidarsdottir H, Fang F, Chang M, et al. Spousal loss and cognitive function in later life: a 25-year follow-up in the AGES-Reykjavik study. Am J Epidemiol. 2014;179(6):674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: The Rancho Bernardo study. Am J Prev Med. 2003;25(3):226–231. [DOI] [PubMed] [Google Scholar]
- 16. van den Borst B, Koster A, Yu B, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66(11):961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krüger K, Dischereit G, Seimetz M, Wilhelm J, Weissmann N, Mooren FC. Time course of cigarette smoke-induced changes of systemic inflammation and muscle structure. Am J Physiol Lung Cell Mol Physiol. 2015;309(2):L119–L128. [DOI] [PubMed] [Google Scholar]
- 18. Petersen AM, Magkos F, Atherton P, et al. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab. 2007;293(3):E843–E848. [DOI] [PubMed] [Google Scholar]
- 19. Rom O, Kaisari S, Aizenbud D, Reznick AZ. Identification of possible cigarette smoke constituents responsible for muscle catabolism. J Muscle Res Cell Motil. 2012;33(3–4):199–208. [DOI] [PubMed] [Google Scholar]
- 20. Alonso JR, Cardellach F, Casademont J, Miró O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur Respir J. 2004;23(2):214–218. [DOI] [PubMed] [Google Scholar]
- 21. Cardellach F, Alonso JR, López S, Casademont J, Miró O. Effect of smoking cessation on mitochondrial respiratory chain function. J Toxicol Clin Toxicol. 2003;41(3):223–228. [DOI] [PubMed] [Google Scholar]
- 22. Caron MA, Morissette MC, Thériault ME, Nikota JK, Stämpfli MR, Debigaré R. Alterations in skeletal muscle cell homeostasis in a mouse model of cigarette smoke exposure. PLoS One. 2013;8(6):e66433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choudhury G, Rabinovich R, MacNee W. Comorbidities and systemic effects of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):101–130. [DOI] [PubMed] [Google Scholar]
- 24. Kok MO, Hoekstra T, Twisk JW. The longitudinal relation between smoking and muscle strength in healthy adults. Eur Addict Res. 2012;18(2):70–75. [DOI] [PubMed] [Google Scholar]
- 25. Neves CD, Lacerda AC, Lage VK, et al. Oxidative stress and skeletal muscle dysfunction are present in healthy smokers. Braz J Med Biol Res. 2016;49(11):e5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.