Abstract
To investigate the different expression of epidermal growth factor receptor 1 (EGFR) and human epidermal growth factor receptor 2 (HER2) in gastric cancer based on tumor locations and its impact on patients survival.
Gastric cancer is heterogeneous disease, recent years have established a molecular classification and described distribution of molecular subtypes in stomach. However, the difference of EGFR and HER-2 expression among tumor location is still unknown.
Between January 2010 and August 2014, 2477 consecutive patients with gastric cancer were treated in our surgery department. The tumor locations were classified into 4 groups: cardia, fundus, corpus, and antrum. Based on tumor locations, the clinicopathologic characteristics, EGFR and HER-2 expression, and follow-up data were analyzed by univariant analysis and Kaplan-Meier analysis retrospectively.
There were difference of gender, age, Borrmann type, pathological type, differentiation, T-stage, tumor size, gastrectomy method, and complications among the locations. The positive rate of EGFR expression in fundus was 18.18%, which was lower than cardia (46.21%), corpus (43.62%), and antrum (48.83%) (P < .001). The 5-year survival rate in EGFR positive patients was 50.8%, which was significantly lower than EGFR negative patients (64.0%, P = .021). The positive rate of HER-2 expression in cardia was 48.15%, which was significantly higher than fundus (37.5%), corpus (35.45%), and antrum (38.54%) (P = .009), but HER-2 expression did not correlate with 5-year survive (P = .548).
Our results suggest that there exist difference of EGFR and HER-2 expression based on tumor locations, and the distribution of EGFR impact on patients survival. Emphasizing the role of EGFR and HER-2 in the context of location contribute to make appropriate treatment strategy and improve prognosis of gastric cancer.
Keywords: clinicopathological characteristic, epidermal growth factor receptor 1, gastric cancer, human epidermal growth factor receptor 2, tumor location
1. Introduction
Gastric cancer (GC) ranks third for morbidity and second for morality worldwide. The 5-year survival was 25% to 39% in most countries.[1] The Cancer Genome Atlas project propose a molecular classification dividing gastric adenocarcinomas into 4 subtypes: tumors positive for Epstein-Barr virus; microsatellite unstable tumors; genomically stable tumors and tumors with chromosomal instability. Identification of these subtypes provides a roadmap for patient stratification and trials of targeted therapies.[2] Through the molecular and genomic basis of GC, the distribution of molecular subtypes in tumors has obtained. These advances are making it feasible to integrate genome-based and phenotype-based diagnostic and therapeutic methods, and apply them to individual GC patients.[3–5]
The molecular subtype and its different distribution in GC suggest that clinicopathologic characteristic and prognosis are closely correlate with the tumor locations. Sheikh demonstrate about one half of the GCs are located in the lower stomach, and remaining is located in the corpus and fundus of the stomach (20%), lesser curvature (20%), cardia (10%), and greater curvature (3%).[6] Cristescu has established 4 clinically relevant molecular subtypes, they find Microsatellite-unstable tumors are frequently occurred in the antrum,[7] Birkman show Epstein-Barr virus positive intestinal-type tumors are more often found in the gastric corpus and the majority of the intestinal-type tumors with TP53 aberrations are proximally located.[8] However, the distribution of GC maybe changed with time, it has reported that the proportion of cardia/fundus cancer remain stable, but that of body cancer increase in Korea, and in the distribution of disease extent, the proportion of localized disease increase, and regional and distant disease decrease in all tumor locations.[9] Thereby, the distribution of GC should be emphasized in clinic.
Epidermal growth factor receptor 1 (EGFR) and human epidermal growth factor receptor 2 (HER2) are receptors for members of the epidermal growth factor family (EGF family).[10] EGFR overexpression is associated with development, metastasis and prognosis of GC,[10–12] EGFR tyrosine kinase inhibitors has been confirmed to cure some patients with non-small cell lung cancer, which induce a paradigm shift from “empiric” treatment to what can be called an “integrated” approach in 2009.[13] HER-2 is over-expressed in approximately 7% to 34% of patients with GC.[14,15] The identification of EGFR and HER-2 as oncogenes has led to develop a series of targeting therapeutic agents, such as gefitinib,[16] cetuximab,[17] and erlotinib for EGFR, and lapatinib,[18] trastuzumab,[19] and apatinib[20] for HER2. EGFR-positive patients have shown a 60% response rate, which exceeds the response rate for conventional chemotherapy,[21] and EGFR-target therapy could benefit a group of patients with low plasma levels of EGFR.[22] Similarly, HER-2 positive GC have been proved to benefit from trastuzumab treatment,[23] and except for HER2 amplification, no biomarker is available for predicting treatment response of trastuzumab in the individual patient.[24]
However, till now, the distinct expression of EGFR and HER-2 expression among tumor locations is unknown in GC. Therefore, in this study, based on tumor location, we investigated the correlation between the expression of EGFR and HER-2 and prognosis of GC.
2. Methods
2.1. Patient enrollment
Between January 2010 and August 2014, 4420 consecutive patients with GC underwent gastrectomy by the First Department of Digestive Surgery of XiJing Hospital, Fourth Military Medical University (Xi’an, China). For this retrospective cohort study, all patient data were evaluated by 2 researchers and the patient inclusion criteria were as follows:
-
(1)
patients were diagnosed as gastric adenocarcinoma according to pathologic characteristics;
-
(2)
patients underwent gastrectomy or explorative surgery, the tumor locations were clearly recorded by surgeons;
-
(3)
tumor locations were classified as cardia, fundus, corpus, and antrum, tumor only located in 1 area were included, whereas patients with transregional tumors were excluded;
-
(4)
patients had not severe basic disease and were at the level of I or II according to the American Society Anesthesiology Physical Status Classification System.
This study was approved by the Ethics Committee of the Fourth Military Medical University. All patients received verbal and written information regarding the study and provided informed consent before surgery.
2.2. Demographic and perioperative data
Demographic data, including sex, age, symptom, positive sign, history of past illness, and preoperative data, including routine hematological, biochemical tests, and X-rays, were collected to enable subsequent analysis of the comparability of the groups. Postoperative data included pathological type, Borrmann type, grade of differentiation, and tumor size. The histological subtype and pathological stage were determined using the Union for International Cancer Control and Tumor-Lymph Node-Metastasis (TNM) classification for GC. EGFR and HER-2 were stained postoperatively by immunohistochemistry in the pathological department, and the results were judged as positive staining or negative staining. These data were evaluated by 2 pathologists and collected from pathologic records for analysis.
2.3. Immunohistochemistry
Paraffin-embedded tumor tissues were sliced into 5-μm thick sections and mounted on glass. Slides were deparaffinized and rehydrated in 10 minutes through a graded alcohol series to deionized water in 1% Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) and microwaved to enhance antigen retrieval. Tissue samples were sequentially incubated with anti-mouse immunoglobulin coupled to horseradish peroxidase. Slides were incubated with the specific primary mouse anti-human EGFR (MAB-0196; MXB, Fuzhou, China) and mouse anti-human HER-2 monoclonal antibodies with a dilution 1:200, then incubated with an horseradish peroxidase-conjugated secondary antibody (KIT-9710; MXB), and then stained with 3,3-diaminobenzidine and counterstained with hematoxylin. In addition, slides stained without primary antibody or without secondary antibody were used as the control. Two pathologists independently observed and interpreted the results of the immunohistochemical staining.
2.4. Assessment of staining
Staining of EGFR and HER-2 was evaluated according to the percentage of positive cells under an optical microscope (Leica Microsystems, GmbH, Wetzlar, Germany; magnification, ×20). Staining intensity was classified as the following: Negative (−), no immunopositive staining or <10% of positive cells observed; weak to moderate (+), 10% to 30% positive cells; high (++), 30% to 70% positive cells; and strong (+++), >70% positive cells.
2.5. Follow-up data
All patients were followed for 5 years from the beginning of operation. And at the end of follow-up, the status of patients was recorded, which included survival, death, and lost follow-up.
2.6. Statistical analysis
Statistical analysis was performed using SPSS 17 software (SPSS Inc., Chicago, IL). Differences among groups consisted of measurement data were analyzed by Student t test, and when unequal variance existed, the adjust-T test was used; Differences in expression rate among groups were analyzed by Pearson Chi-squared (χ2) test. The Fisher exact test was used to assess the difference of positive rate when the number of total cases was less than 40. P-value <.05 was considered statistically significant. Survival analysis were used by Kaplan-Meier.
3. Results
3.1. Baseline characteristics
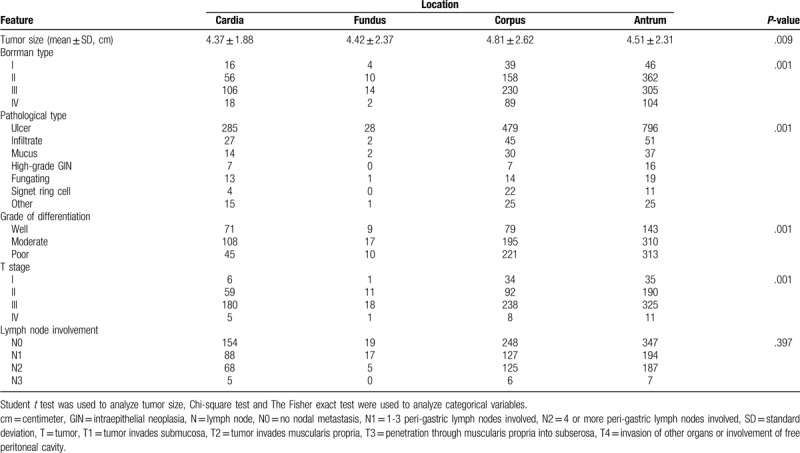
A total of 2477 cases met the inclusion criteria in this cohort study (Fig. 1), of those, 452 (18.3%), 54 (2.2%), 797 (32.2%), and 1174 (47.4%) cases were distributed in cardia, fundus, corpus, and antrum, respectively. The comparison of baseline data among the 4 locations was described in Table 1. There were significant differences among the locations regarding preoperative variables, such as age, sex, symptoms, positive sign, and blood test. The difference of pathological type, histological subtype, Borrman type, tumor differentiation, and TNM stage was also found among the 4 groups (Table 2).
Figure 1.
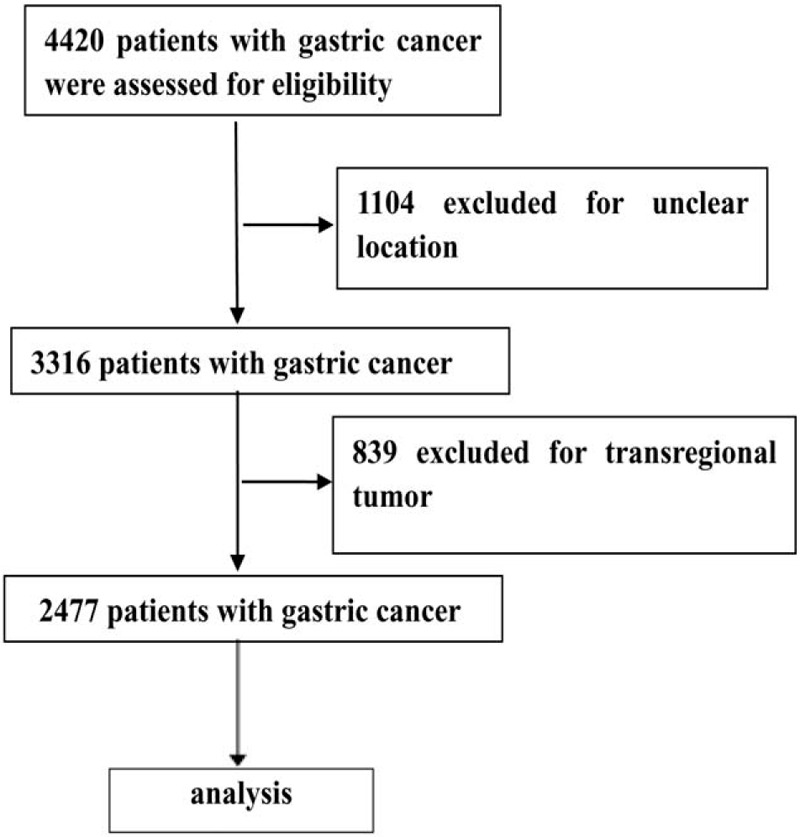
Flow diagraph of patients enrollment.
Table 1.
Characteristics of patients with gastric cancer according to tumor locations.
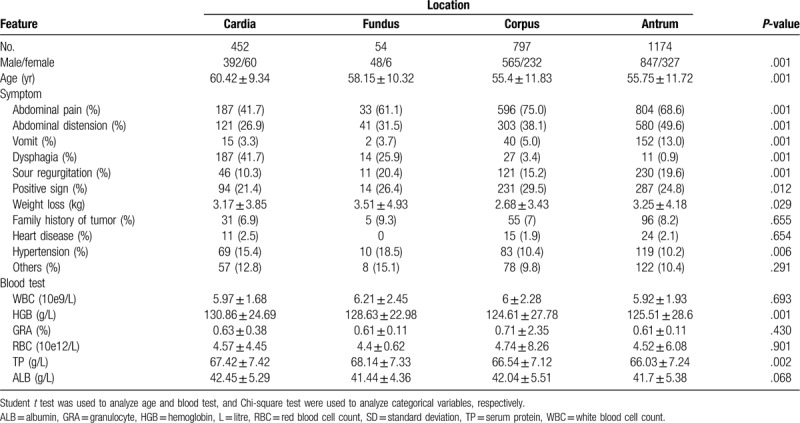
Table 2.
Pathological characteristics of patients with gastric cancer according to locations.
3.2. The EGFR and HER-2 expression in different location
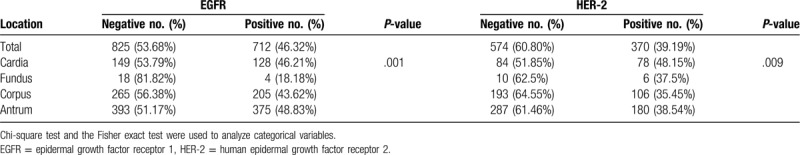
The staining levels of EGFR and HER-2 was shown in Figure 2. We found the positive rate of EGFR expression in fundus was 18.18%, which was significantly lower than that in cardia (46.21%), corpus (43.62%), and antrum (48.83%) (P = .001). The positive rate of HER-2 expression in cardia was 48.15%, which was significantly higher than that in fundus (37.5%), corpus (35.45%), and antrum (38.54%) (P = .009) (Table 3). By correlation analysis, we found the EGFR and HER-2 expression was closely correlated. R2 = 0.02, P = .001.
Figure 2.
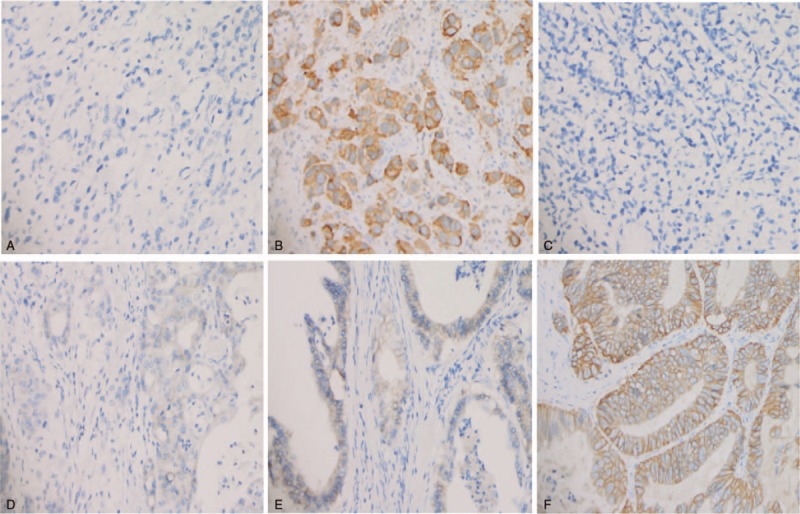
Immunohistochemical analysis of expression of EGFR and HER-2 in GC tissues. (A) Negative expression of EGFR; (B) Positive expression of EGFR was observed in GC. The main staining site was located in cytoplasm; (C) Negative expression of HER-2 in GC; (D) Weak to moderate positive expression of HER-2 in GC; (E) High expression of HER-2 in GC; (F) Strong expression of HER-2 in GC, The main staining site was located in membrane. The cells with brown yellow staining were positive (magnification, ×20). EGFR = epidermal growth factor receptor, GC = gastric cancer, HER-2 = human epidermal growth factor receptor 2.
Table 3.
The expression of EGFR and HER-2 in different locations.
3.3. Five-year survival rate of GC in different location
A total of 2145 cases had complete follow-up data, and average flow-up time was 27.28 ± 17.48 months (ranged from 0.3 to 66.73 months). We found the 5-year survival rate of GC in fundus was 73.7% ± 0.11%, higher than in cardia (61.1% ± 0.04%), corpus (56.3% ± 0.03%), and antrum (58.1% ± 0.02%), but the difference was not statically significant (P = .323) by Kaplan-Meier analysis (Fig. 3A and B).
Figure 3.
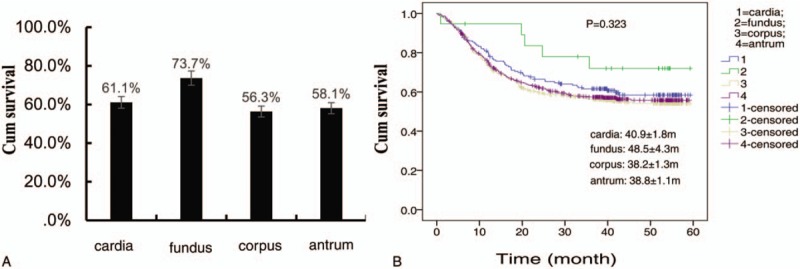
The survival difference among tumor locations in patients with gastric cancer. (A) The 5-survival rate was not statically different among tumor locations. (B) 5-yr survival curve of patients with gastric cancer in different tumor locations.
3.4. The correlation between the expression of EGFR and HER-2 and prognosis of GC
We also found EGFR expression was negatively correlated with 5-year survival, the survival rate in patients with EGFR positive was 50.8% ± 0.06%, which was significantly lower than that in patients with EGFR negative (64.0% ± 0.03%, P = .002) (Fig. 4A and B). But we did not find HER-2 expression correlated with 5-year survive, the survival rate in patients with HER-2 positive was 63.1% ± 0.06%, which was similar to that in patients with HER-2 negative (62.9% ± 0.03%, P = .548) by Kaplan-Meier analysis (Fig. 4A and C).
Figure 4.
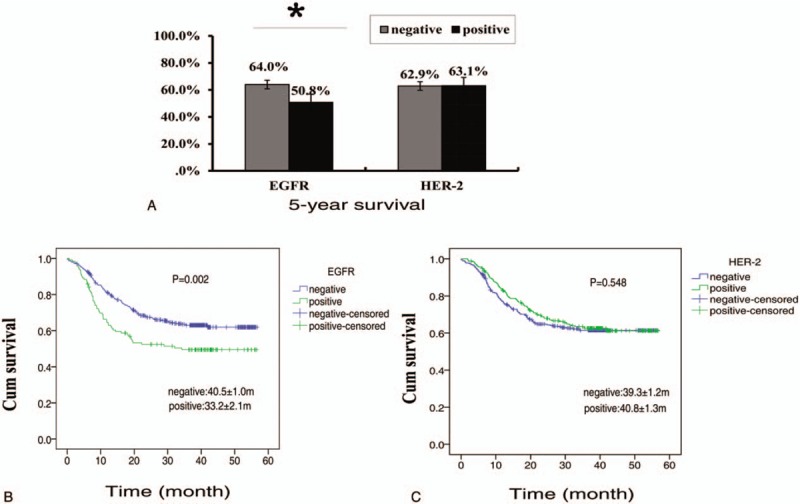
The effect of EGFR and HER-2 expression on prognosis of gastric cancer. (A). the difference of 5-survival rate between EGFR-positive group and EGFR-negative group, and between HER-2 positive group and HER-2 group. ∗ Denoted there was a statistically difference between the 2 groups, P-value < .05. (B) 5-yr survival curve of patients with EGFR-positive and with EGFR-negative. (C) 5-yr survival curve of patients with HER-2 positive and with HER-2 negative. EGFR = epidermal growth factor receptor 1, HER-2 = human epidermal growth factor receptor 2.
3.5. Combined effect of tumor location with EGFR expression on 5-year survival rate of GC
Combined analysis of location and EGFR expression, we found the 5-year survival rate was 37.9% in patients with tumor located in non-cardia and with positive EGFR expression. The prognosis of the patients was significantly worse than those patients with tumor in other locations (P = .002) (Fig. 5A and B).
Figure 5.
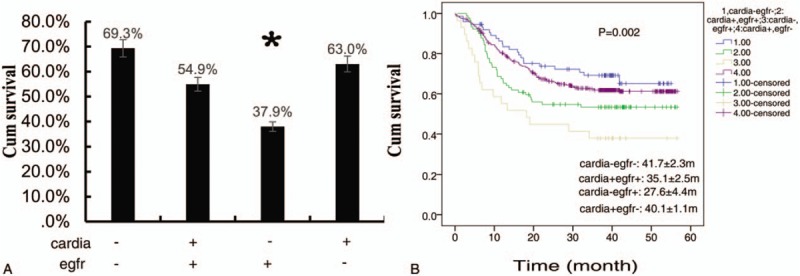
Combined effect of tumor location with EGFR expression on 5-yr survival rate of gastric cancer. (A) Combined analysis of the effect of tumor location and EGFR expression, the difference of 5-yr survival rate of gastric cancer was statically significant. ∗ Denoted there was a statistically difference between the 2 groups, P-value < .05. “+” mean with; “−” mean without; (B) 5-yr survival curve of 4 combinations of location and EGFR expression in patients with gastric cancer. EGFR = epidermal growth factor receptor 1.
4. Discussion
The aim of the present study was to investigate the difference of clinicopathologic and prognosis of GC according to the expression of EGFR and HER-2 in different tumor locations. We found EGFR and HER-2 expression level were significantly different according to tumor locations, and the 5-year survival was correlated with EGFR expression and the interaction between tumor location and EGFR expression.
It has reported the positive expression of EGFR and HER-2 was associated with male gender, older age, intestinal type, Borrmann classification of 0 or 1 tumors, and higher stage.[23,25–27] In our study, EGFR and HER-2 expression were found statistically different among locations. Our results showed a decreasing expression rate of EGFR in fundus (18.18%) and an increasing expression rate of HER-2 in cardia (48.15%). The result of HER-2 expression in this study was higher than the result reported by other investigators, who demonstrate the overexpression of HER-2 in gastroesophageal junction adenocarcinomas is 12.5%,[28] the divergence of HER-2 expression may attribute to the different positive judgement standard and detective procedure.[29,30] Our results supplemented for the distribution of EGFR and HER-2 in GC.
Several studies have reported that the prognosis of GC were influenced by tumor size, depth of invasion, lymph node metastasis, early detection, chemotherapy, and radical resection.[31,32] In this study, we found the 5-year survival in EGFR negative group was significantly higher than it in EGFR positive group, our result was consistence with the previous report that the patients with EGFR positive GC had an unfavorable prognosis,[25] but was distinct from a study demonstrated EGFR family had not prognostic influence in GC according to analysis by a parametric model.[33] Our data provided powerful evidence for the prognostic value of EGFR and supported it as a therapeutic target in GC.[34]
We did not find the difference of 5-year survival rate according to tumor locations. However, we found the patients with tumor located in non-cardia and with positive EGFR expression had the worst prognosis. In addition, we found the lowest rate of EGFR expression and the best prognosis in fundus. Our results were similar to the report showed microsatellite-unstable tumors are hyper-mutated intestinal-subtype tumors occurring in the antrum, and the tumors have the best overall prognosis and the lowest frequency of recurrence.[7] Combined with molecular classification of GC, our result underscored that the interaction between tumor locations and EGFR expression play an important role of in the prognosis of GC, and suggested tumor location may represent relevant molecular subtypes.
HER-2 is one of the most effective targets for its outstanding performance in prognosis.[35] Combined with standard first-line chemotherapy, trastuzumab significantly improves the prognosis.[36] However, we did not find the correlation between HER-2 and prognosis of GC, our results was consistent with the study showed only EGFR amplification had a prognostic impact, while HER-2 amplification was not prognostically relevant in advanced GC.[11] But a close correlation between EGFR and HER-2 was also found in this study, suggested that HER-2 did not affect prognosis directly. Our results supported the view that HER-2 alteration is not an independent prognostic factor for curatively resectable GC.[25]
In conclusion, our results revealed there exist difference of EGFR and HER-2 expression based on tumor locations. These findings supplemented for the distributing characteristics of GC and underscored the need for further studies on the influence of biomarker expression in the context of location, as this may influence treatment strategy as well as prognosis.
Acknowledgments
The authors would like to thank Dr Man Guo and Dr Xiao Lian for their help in the design, data collection, and analysis.
Author contributions
Li GC: conceiving and designing the study, and writing the manuscript; Jia XC: Immunohistochemical analysis; Zhang HW and Zhao QC: providing critical revisions; Yang P: analyzing and interpreting the data; Pang FN, Xu LL and Sun JB: collecting the literatures; All authors approved the final version of the manuscript.
Footnotes
Abbreviations: EGFR = epidermal growth factor receptor 1, GC = gastric cancer, HER-2 = human epidermal growth factor receptor 2.
How to cite this article: Li GC, Jia XC, Zhao QC, Zhang HW, Yang P, Xu LL, Pang FN, Sun JB. The expression of epidermal growth factor receptor 1 and human epidermal growth factor receptor 2 based on tumor location affect survival in gastric cancer. Medicine. 2020;99:21(e20460).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu X, Meltzer SJ. Gastric cancer in the era of precision medicine. Cell Mol Gastroenterol Hepatol 2017;3:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim HJ, Kang SK, Kwon WS, et al. Forty-nine gastric cancer cell lines with integrative genomic profiling for development of c-MET inhibitor. Int J Cancer 2018;143:151–9. [DOI] [PubMed] [Google Scholar]
- [5].Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol 2015;1:23–32. [DOI] [PubMed] [Google Scholar]
- [6].Sheikh IA, Mirza Z, Ali A, et al. A proteomics based approach for the identification of gastric cancer related markers. Curr Pharm Des 2016;22:804–11. [DOI] [PubMed] [Google Scholar]
- [7].Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–56. [DOI] [PubMed] [Google Scholar]
- [8].Birkman EM, Mansuri N, Kurki S, et al. Gastric cancer: immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch 2018;472:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eom BW, Jung KW, Won YJ, et al. Trends in gastric cancer incidence according to the clinicopathological characteristics in Korea, 1999-2014. Cancer Res Treat 2018;50:1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004;59: 2 Suppl: 21–6. [DOI] [PubMed] [Google Scholar]
- [11].Cavanna L, Bodini FC, Stroppa EM, et al. Advanced gastric cancer with liver and lymph node metastases successfully resected after induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil. Chemotherapy 2014;60:224–7. [DOI] [PubMed] [Google Scholar]
- [12].Kurokawa Y, Matsuura N, Kawabata R, et al. Prognostic impact of major receptor tyrosine kinase expression in gastric cancer. Ann Surg Oncol 2014;21: Suppl 4: S584–90. [DOI] [PubMed] [Google Scholar]
- [13].Gandara DR. Personalizing therapy of lung cancer: a paradigm shift from empiric to integrated decision-making. J of Thoracic Oncol 2009;4: 9 Supplement 1: S5–6. [DOI] [PubMed] [Google Scholar]
- [14].Venkatesh T, Shetty A, Chakraborti S, et al. PTPH1 immunohistochemical expression and promoter methylation in breast cancer patients from India: a retrospective study. J Cell Physiol 2019;234:1071–9. [DOI] [PubMed] [Google Scholar]
- [15].Meza-Junco J, Au HJ, Sawyer MB. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. Cancer Manag Res 2011;3:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. [DOI] [PubMed] [Google Scholar]
- [17].Yan L, Beckman R. Pharmacogenetics and pharmacogenomics in oncology therapeutic antibody development. Biotechniques 2005;39: 10 Suppl: S565–8. [DOI] [PubMed] [Google Scholar]
- [18].Yu Y, Yu X, Liu H, et al. miR494 inhibits cancerinitiating cell phenotypes and reverses resistance to lapatinib by downregulating FGFR2 in HER2positive gastric cancer. Int J Mol Med 2018;42:998–1007. [DOI] [PubMed] [Google Scholar]
- [19].Xiong J, Han S, Ding S, et al. Antibody-nanoparticle conjugate constructed with trastuzumab and nanoparticle albumin-bound paclitaxel for targeted therapy of human epidermal growth factor receptor 2-positive gastric cancer. Oncol Rep 2018;39:1396–404. [DOI] [PubMed] [Google Scholar]
- [20].Geng R, Song L, Li J, et al. The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf 2018;17:1145–50. [DOI] [PubMed] [Google Scholar]
- [21].Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stahl M, Maderer A, Lordick F, et al. Perioperative chemotherapy with or without epidermal growth factor receptor blockade in unselected patients with locally advanced oesophagogastric adenocarcinoma: randomized phase II study with advanced biomarker program of the German Cancer Society (AIO/CAO STO-0801). Eur J Cancer 2018;93:119–26. [DOI] [PubMed] [Google Scholar]
- [23].Wang HB, Liao XF, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: a meta-analysis. Medicine (Baltimore) 2017;96:e8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kankeu Fonkoua L, Yee NS. Molecular characterization of gastric carcinoma: therapeutic implications for biomarkers and targets. Biomedicines 2018;6:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oh HS, Eom DW, Kang GH, et al. Prognostic implications of EGFR and HER-2 alteration assessed by immunohistochemistry and silver in situ hybridization in gastric cancer patients following curative resection. Gastric Cancer 2014;17:402–11. [DOI] [PubMed] [Google Scholar]
- [26].Qiu MZ, Shi SM, Chen M, et al. Comparison of HER2 and Lauren classification between biopsy and surgical resection samples, primary and metastatic samples of gastric cancer. J Cancer 2017;8:3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Narita Y, Kadowaki S, Oze I, et al. Establishment and validation of prognostic nomograms in first-line metastatic gastric cancer patients. J Gastrointest Oncol 2018;9:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dominguez C, Rosa M, George TB, et al. Evaluation of expression of human epidermal growth factor receptor 2 (HER2) in gastric and gastroesophageal junction adenocarcinoma using IHC and dual-ISH. Anticancer Res 2018;38:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wong N, Amary F, Butler R, et al. HER2 testing of gastro-oesophageal adenocarcinoma: a commentary and guidance document from the Association of Clinical Pathologists Molecular Pathology and Diagnostics Committee. J Clin Pathol 2018;71:388–94. [DOI] [PubMed] [Google Scholar]
- [30].Wang L, Zhang Q, Ni S, et al. Programmed death-ligand 1 expression in gastric cancer: correlation with mismatch repair deficiency and HER2-negative status. Cancer Med 2018;7:2612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhu YL, Yang L, Sui ZQ, et al. Clinicopathological features and prognosis of Borrmann type IV gastric cancer. J BUON 2016;21:1471–5. [PubMed] [Google Scholar]
- [32].Kang WM, Meng QB, Yu JC, et al. Factors associated with early recurrence after curative surgery for gastric cancer. World J Gastroenterol 2015;21:5934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jacome AA, Wohnrath DR, Scapulatempo Neto C, et al. Prognostic value of epidermal growth factor receptors in gastric cancer: a survival analysis by Weibull model incorporating long-term survivors. Gastric Cancer 2014;17:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aratani K, Komatsu S, Ichikawa D, et al. Overexpression of EGFR as an independent prognostic factor in adenocarcinoma of the esophagogastric junction. Anticancer Res 2017;37:3129–35. [DOI] [PubMed] [Google Scholar]
- [35].Ren Z, Sun J, Sun X, et al. Efficacy and safety of different molecular targeted agents based on chemotherapy for gastric cancer patients treatment: a network meta-analysis. Oncotarget 2017;8:48253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen ZL, Zhao A, Li P, et al. Clinical use of trastuzumab combined with different chemotherapy regimens in multi-line treatment of advanced human epidermal growth factor receptor 2-positive gastric cancer: a case report. Oncol Lett 2018;16:4614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


