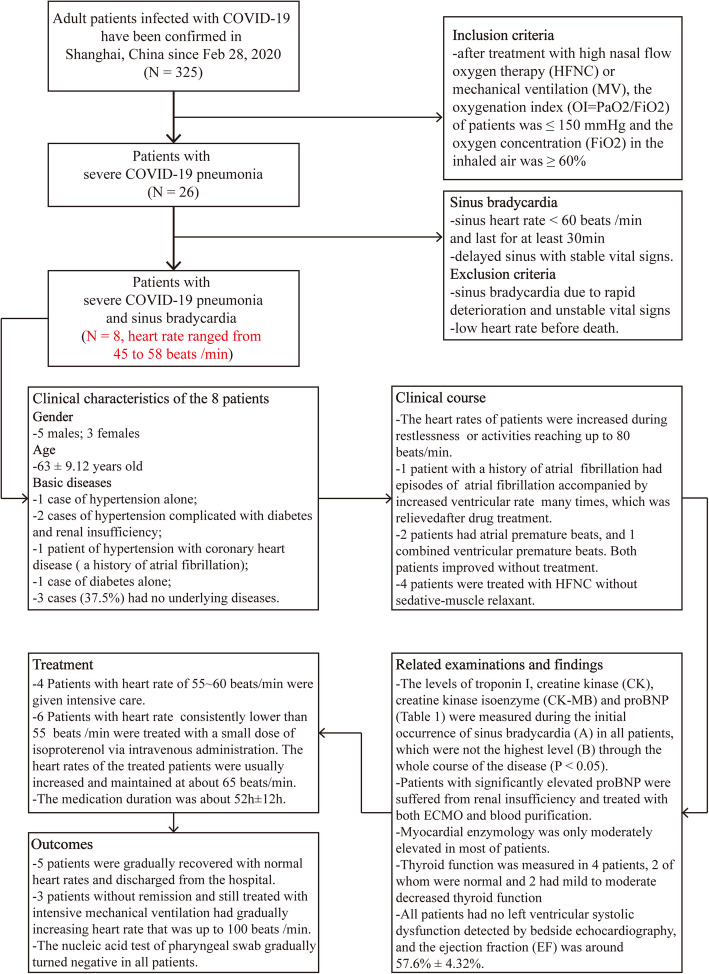
There were cases of sudden death in some patients infected with COVID-19 including a few of young physicians, which had a huge impact on medical community and society [1]. The unexpected phenomenon lets us think about the underlying problems that caused the sudden death and some issues maybe ignored and that should be appropriately resolved. The initial manifestation of severe COVID-19 pneumonia patients was hypoxemic respiratory failure, accompanied by rapid increased reactive heart rate and susceptibility to supraventricular arrhythmia [2]. It is notable that a proportion of these patients developed sinus bradycardia, which was significantly different from other patients with multiple types of respiratory failure.
In addition to lung injury, cardiac injury has often been reported in patients with COVID-19 [2]. Some experts believed that the virus invasion into myocardium led to severe myocarditis or the severe “cytokine storm”-induced acute myocardial injury may explain the sudden death in some affected patients [3]. It is noteworthy that about 1/3 of the patients with severe illness in our study developed sinus bradycardia (Fig. 1). The troponin and proBNP were basically normal among these patients except for those with renal failure (Table 1). The clinical characteristics of explosive myocarditis and myocardial infarction were not presented among these patients, suggesting these are not the cause of sinus bradycardia in these patients. It was previously reported that no pathological evidence of myocarditis or myocardial microinfarction was observed in the heart of suffered patients [4], consisting with our results. Therefore, we speculated that sudden death among some severe patients with improved symptoms post-treatment may be caused by severe arrhythmia such as ventricular fibrillation induced by severe sinus delay.
Fig. 1.
Flowchart of the screening and clinical features of severe COVID-19 pneumonia patients with sinus bradycardia involved in the study
Table 1.
The related indexes of 8 severe COVID-19 pneumonia patients with sinus bradycardia
| Troponin I (ng/ml) | CK (U/L) | CK-MB (U/L) | proBNP (ng/ml) | ||||
|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B |
| 0.01 | 0.05 | 416 | 1154 | 14 | 34 | 128 | 160 |
| 0.02 | 0.03 | 30 | 98 | 9 | 11 | 449 | 896 |
| 0.03 | 0.15 | 21 | 445 | 8 | 15 | 200 | 354 |
| 0.07 | 0.26 | 29 | 76 | 18 | 16 | 909 | 6312* |
| 0.04 | 0.23 | 57 | 568 | 12 | 81 | 780 | 4048* |
| 0.02 | 0.22 | 34 | 291 | 17 | 28 | 149 | 275 |
| 0.02 | 0.03 | 500 | 506 | 19 | 19 | 38 | 38 |
| 0.23 | 0.06 | 46 | 568 | 11 | 34 | 449 | 4048* |
| Paired t tests | 1.64 | Paired t tests | 3.39 | Z value | 2.11 | Z value | 2.46 |
| P | 0.146 | P | 0.012 | P | 0.035 | P | 0.014 |
Stata 14.0 software was used for the statistical analysis of these data. Paired t test or Wilcoxon’s paired rank sum test was used to calculate the corresponding P value. Difference is considered statistically significant when P < 0.05
CK creatine jubase, CK-MB creatine kinase isoenzyme MB
*Patients had renal insufficiency and treated with both ECMO and hemodialysis
We found that sinus bradycardia often occurred during sleep. So, deep sleep or sedation may be an important risk factor for sinus bradycardia. A few patients had mild to moderate decreased thyroid function, which was consistent with secondary pathological thyroid syndrome and may also be one of the causes of sinus bradycardia. When viral nucleic acid tests gradually turned negative, the heart rate returned to normal no matter whether the patient’s condition improved or worsened and the uses of catecholamine were gradually discontinued. According to the results, we speculated that the inhibitory effect of virus on sinus node activity was the main cause of sinus bradycardia in these patients.
Previous study indicated that COVID-19 invaded host cells via the receptor angiotensin-converting enzyme 2 (ACE2) [5]. Zou et al. identified specific cell types including myocardial cells which were vulnerable to COVID-19 infection through scRNA-seq data analyses [5]. However, there was no severe myocardial damage or cardiac insufficiency in our patients with sinus bradycardia. We referred the gene ontology (GO) enrichment analysis for ACE2 gene in GeneCards Database (https://www.genecards.org/). Biological processes (BP) for ACE2 gene showed that it not only promoted the contraction of cardiac muscle, but also regulated the cardiac conduction. Donoghue et al. demonstrated that cardiac ACE2 overexpression in transgenic mice caused sudden death in a gene dose-dependent fashion; they also found that increased ACE2 expression led to progressive conduction and rhythm disturbances with lethal ventricular arrhythmias via detailed electrophysiology [6]. In light of those evidences, it may be speculated that the toxic role of virus on cardiac conduction system instead of that generated myocardial damage resulted in a sudden death of patients infected with COVID-19.
Taken together, heart rate monitoring of severe COVID-19 pneumonia patients should be strengthened during treatment, and catecholamines should be appropriately applied when necessary. Moreover, a possible inhibitory influence of the virus on activity of cardiac nervous conduction system including sinus node via ACE2 should not be ignored when studying the pathogenic mechanisms among these patients.
Acknowledgements
Not applicable
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- BP
Biological processes
- CK
Creatine kinase
- CK-MB
Creatine kinase isoenzyme
- EF
Ejection fraction
- FiO2
Oxygen concentration
- GO
Gene ontology
- HFNC
High nasal flow oxygen therapy
- MV
Mechanical ventilation
- OI
Oxygenation index
- SARS-CoV
Severe acute respiratory syndrome coronavirus
Authors’ contributions
LZ and YZZ conceived the study idea. LZ, YZZ, LJH, and LJG participated in the study design. ZLJ and QBW gathered the data and performed the data analyses. All authors interpreted the data analyses. All authors co-wrote and revised the manuscript for intellectual content. LZ and YZZ read and approved the final manuscript.
Funding
The study was financially supported by the National Natural Science Foundation of China (grant/award number: no. 81873420) and the Shanghai Science and Technology Committee (grant/award number: STCSM, nos. 19411969700 and 18411966800). Special fund for clinical research of Zhongshan Hospital, Fudan University, Grant/Award Number: (No. 2016ZSLC11), Sponsor: Lei Zhu.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics approval and consent to participate
Ethical approval for this study was reviewed and approved by the Ethics Committee of Zhongshan Hospital, Fudan University (Shanghai, China).
Consent for publication
Not applicable
Competing interests
The authors declare no conflict of interest. All the authors listed have approved the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lijuan Hu and Linjing Gong contributed equally to this work.
Contributor Information
Yunzeng Zou, Email: zou.yunzeng@zs-hospital.sh.cn.
Lei Zhu, Email: tfzhu@126.com.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arabi Yaseen M., Fowler Robert, Hayden Frederick G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Medicine. 2020;46(2):315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Zhe, Shi Lei, Wang Yijin, Zhang Jiyuan, Huang Lei, Zhang Chao, Liu Shuhong, Zhao Peng, Liu Hongxia, Zhu Li, Tai Yanhong, Bai Changqing, Gao Tingting, Song Jinwen, Xia Peng, Dong Jinghui, Zhao Jingmin, Wang Fu-Sheng. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Xin, Chen Ke, Zou Jiawei, Han Peiyi, Hao Jie, Han Zeguang. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Frontiers of Medicine. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donoghue M. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35(9):1043–1053. doi: 10.1016/S0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.