Dear Sirs,
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak emerged in December 2019 in South-Eastern China and rapidly spreaded throughout the globe. Lombardy (and the city of Milan) is one of the hardest-hit regions of Italy. Although fever and respiratory symptoms are the core presenting features of coronavirus disease 2019 (COVID-19), there is an increasing awareness of neurological manifestations as an important factor for the prognosis [1]. We report a patient with a post-infectious Guillain–Barré syndrome (GBS) associated with SARS-CoV-2 infection.
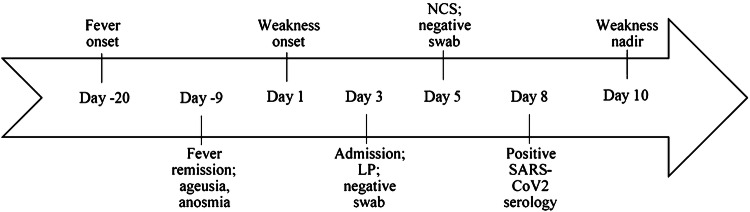
On 13 April 2020, a man in his sixties, living in the urban area of Milan, referred to our emergency department complaining a three-day history of progressive limb weakness and distal paresthesia at four-limbs. His past medical history was unremarkable. Twenty days before he had developed fever (37.7–38.5 °C), headache and myalgia followed by anosmia and ageusia (Fig. 1). He was, therefore, prescribed a home self-isolation protocol until symptom resolution. He recovered within about ten days, except for the persistence of mild ageusia. At admission, vital signs and general examination were normal. Neurological examination disclosed moderate proximal muscle weakness (Medical Research Council Grade 3–4/5) and severe vibratory sensation and proprioception deficit at lower limbs. Deep tendon reflexes were absent. Cell blood count, C-reactive protein, creatine phosphokinase, arterial blood gases, renal and hepatic function tests were normal. Anti-ganglioside antibodies tested negative. Serum levels of interleukin-6 [93.1 pg/ml; (reference 0–7 pg/ml)], ferritin [1040 ng/ml (30–400 ng/ml)], lactic dehydrogenase [281 U/l (125–220 U/l)] and fibrinogen [525 mg/dl (150–400 mg/dl)] were elevated. Chest CT scan showed bilateral ground-glass opacities, consistent with COVID-19 pneumonia. Two nasal swabs tested negative for SARS-CoV-2. Cervical spine MRI ruled out lesions of the cervical cord [2]. CSF testing (day 3) showed normal cell count and protein levels. Polymerase-chain reaction for EBV, CMV, VZV, HSV 1–2, HIV and SARS-CoV2 on CSF tested negative. Nerve conduction studies (day 5) showed reduced conduction velocities, reduced sensory action potential and compound motor action potential (cMAP) amplitudes with sural nerve sparing and abnormal temporal dispersion of peroneal nerves cMAP, indicating an acute inflammatory demyelinating polyneuropathy (Table 1) [3]. We started a 5-day course of intravenous immunoglobulin at 0.4 g/kg daily. A thorough serological analysis ruled out other causes of atypical pneumonia. As soon as available, SARS-CoV-2 IgG tested positive [DiaSorin LIAISON system: 81.2 AU/ml (< 12 AU/ml)]. Muscle weakness worsened and rapidly spread distally and to thoracic and cranial nerves causing facial diplegia, hypophonia and dysarthria. The time from symptoms onset to nadir was 10 days, followed by a slow improvement; no ventilation or feeding tube support was required.
Fig. 1.
Timeline of symptom progression. Timeline showing GBS symptom progression and key points in the diagnostic process. LP lumbar puncture, NCS nerve conduction studies
Table 1.
Neurophysiological features
| Test | Tibial | Peroneal | Median | Ulnar | Sural | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Motor | ||||||||||
| DML (ms) | n.d | 5.6 | 6.6 | 5.6 | 18.9 | n.d | 2.7 | n.d | ||
| CV (m/s) | n.d | 36.6 | 31.7 | 36.6 | 17.9 | n.d | 49.0 | n.d | ||
| cMAPampl (mV) | ||||||||||
| Dist | n.d | 5.2 | 3.2 | 5.2 | 1.0 | n.d | 7.0 | n.d | ||
| Prox | n.d | 1.9 | 0.5 | 1.9 | 0.1 | n.d | 6.5 | n.d | ||
| F wave | ||||||||||
| Latmin (ms) | n.d | n.e | n.e | 56.5 | n.e | n.d | 33.7 | n.d | ||
| Persistence (%) | 10 | 80 | ||||||||
| Sensory | ||||||||||
| CV (m/s) | n.e | n.d | n.e | n.d | 57.1 | 52.0 | ||||
| SAPampl (mcV) | n.e | n.d | n.e | n.d | 19.8 | 14.7 | ||||
DML distal motor latency, CV conduction velocity, cMAPampl compound motor action potential amplitude, Latmin F wave minimal latency, persistence ratio between number of recorded F waves and delivered stimuli, SAPampl sensory action potential amplitude, ms millisecond, m/s meters per second, mV millivolt, mcV microvolt, n.d. not done, n.e. not excitable
Few cases of GBS have already been reported in concomitance with SARS-CoV-2 infection [4–6]. While epidemiological data and radiological findings guided our presumptive diagnosis of SARS-CoV2, serological tests proved essential for its confirmation. Therefore, despite the negativity of two repeated nasal swabs, we were able to implement appropriate isolation precautions. The timing of the onset of neurological symptoms, together with the negativity of oropharyngeal swabs and the demonstration of a SARS-CoV2 IgG response, are all in support of a post-infective immune-mediated disease mechanism. Recent evidence suggests that although the immune response seems crucial to control and resolve the viral infection, an excessive, dysregulated inflammatory response may also be detrimental. Increased levels of IL-6, as detected in our patient, and other pro-inflammatory cytokines, referred to as “cytokine storm”, are considered a hallmark of this aberrant reaction [7]. GBS has a recognized dysimmune pathogenesis, mediated by the antibody response. Therefore, we might speculate that also other neurological or extra-neurological SARS-CoV-2-related manifestations might share similar pathogenic mechanisms, suggesting tailored therapeutic approaches for subgroup of patients.
Funding
The authors did not receive any compensation for the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
We have obtained the patient’s informed consent and permission for the publishing of his information.
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riva N, Cerri F, Butera C, et al. Churg strauss syndrome presenting as acute neuropathy resembling Guillain Barre syndrome: case report. J Neurol. 2008;255:1843–1844. doi: 10.1007/s00415-008-0035-3. [DOI] [PubMed] [Google Scholar]
- 3.Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat Rev Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barre syndrome following COVID-19: new infection, old complication? J Neurol. 2020 doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou G, Chen S, Chen Z. Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front Med. 2020;14:117–125. doi: 10.1007/s11684-020-0773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]