Abstract
Objectives:
To compare the clinical outcomes of endoscopic biliary drainage (EBD) with those of percutaneous transhepatic biliary drainage (PTBD) in patients with resectable hilar cholangiocarcinoma (HCCA) and evaluate the effect of EBD and PTBD on tumor prognosis.
Materials and methods:
PubMed, EMBASE, and Cochrane Library databases were searched for articles about the comparison between PTBD and EBD. Data were analyzed by Revman 5.3.
Results:
PTBD showed a lower risk of drainage-related complications than EBD (OR, 2.73; 95%CI, 1.52–4.91; P < .05). PTBD was also associated with lower risk of pancreatitis (OR, 8.47; 95%CI, 2.28–31.45; P < .05). The differences in preoperative cholangitis, R0 resection, blood loss and recurrence showed no statistically significance between EBD and PTBD (all P > .05). Several literatures have reported the tumor implantation metastasis after PTBD. Since no well-designed prospective randomized controlled studies have explored in this depth, this article is unable to draw conclusions on this aspect.
Conclusion:
PTBD is a reasonable choice for PBD, and EBD should only be used as preoperative drainage for HCCA by more experienced physicians. There is a greater need to design prospective randomized controlled studies to obtain high-level evidence-based medicinal proof. It is worth noting that, whether EBD or PTBD, accurate selective biliary drainage should be the trend.
Keywords: endoscopic biliary drainage, hilar cholangiocarcinoma, Klatskin tumor, percutaneous transhepatic biliary drainage, preoperative biliary drainage
1. Introduction
Hilar cholangiocarcinoma (HCCA) is a kind of malignant tumor originated from hilar cholangiocytes, which often invades the common hepatic duct and the confluence of left and right hepatic ducts.[1] Up to now, the biological characteristics and molecular pathology of HCCA are still poorly understood.[2] Surgical resection is the main method for the treatment of HCCA. Extended liver resection and vascular reconstruction are always needed in order to achieve resection with free margins (R0 resection).[3–5]
The growth of tumor in porta hepatis can easily cause obstructive jaundice. Cholestasis may lead to damage of liver function and affect the regeneration of liver tissue.[6,7] The postoperative mortality of cholestatic patients with extended liver resection can up to 18%, suggesting that the abnormal liver function caused by cholestasis was an important risk factor of surgical prognosis.[8] Biliary drainage can relieve obstruction, reduce symptoms of cholangitis and correct severe malnutrition. Therefore, it has become a routine method to treat patients with preoperative biliary drainage (PBD) during peroperative period.[9]
Techniques of PBD include endoscopic biliary drainage (EBD) and percutaneous transhepatic biliary drainage (PTBD). EBD is an internal drainage procedure via endoscopic biliary stenting (EBS) or endoscopic nasobiliary drainage (ENBD) and has the advantage of low trauma, but it seems to induce procedure-related complications more easily. For example, EBS was often associated with stent occlusion and retrograde infection of bile duct.[10] Bacterial contamination caused by EBD may induce preoperative cholangitis, which is considered to be an independent prognostic factor in surgical patients.[11,12] Therefore, PTBD was used to be the preferred method for PBD in Asia. PTBD has a high success rate in technology. The drainage catheter of PTBD can also be used for selective cholangiography to determine the extent of tumor invasion. Although recent studies have associated PTBD with tumor seeding metastasis, whether it will lead to a poor prognosis remains controversial.[13,14] So far, no consensus has been reached on the best choice of PBD.
Previous studies have been published to compare EBD and PTBD mainly in drainage-related complications.[15–17] In this study, we additionally collected data to explore the impact of PTBD and EBD on surgery. In order to analyze whether methods of PBD have influence on the long-term prognosis, we also focus on the data of tumor recurrence and the overall survival (OS) in each study. We hope to provide reference for preoperative drainage of HCCA patients.
2. Materials and methods
2.1. Ethics statement
The meta-analysis we made is a secondary study, and the original researches included have been approved by the ethics committees of the relevant units. Thus, the ethical approval of this meta-analysis was not necessary.
2.2. Search strategy and selection criteria
Literature search was performed through PubMed, EMBASE, and Cochrane Library databases (the latest date was October 10, 2019), the free-text terms and MeSH terms included “Hilar cholangiocarcinoma,” “Klatskin Tumor,” “preoperative biliary drainage,” “endoscopic biliary drainage,” and “percutaneous transhepatic biliary drainage.” We also made a manual search for potential target references, irrespective of the language restrictions.
The selection criteria were as follows:
-
1.
Randomized clinical trials (RCT), cohorts studies or case–control studies that based on EBD and PTBD in HCCA;
-
2.
Details of the drainage related complications and the prognosis should be provided;
-
3.
Reviews, case reports, and comments were excluded;
-
4.
Studies of palliative treatment were excluded.
2.3. Data extraction
The basic data included the author, enrollment period, study regions, study design, and study population. Data associated with surgery included R0 resection, intraoperative blood loss, and postoperative complications. Furthermore, we recorded the Bismuth-Corlette type of patients in each study and the prognosis of patients in each group as far as possible.
2.4. Quality evaluation
According to the selection strategy, two investigators were employed to assess the articles independently. The JADAD score standard table was adopted for the evaluation of RCT. Studies with scores below 2 were considered to be of low quality, while studies with scores above 3 were considered to be of high quality. The Newcastle-Ottawa scale (NOS) was used for the assessment of non-randomized controlled studies. Studies with a total score of 7 were defined as high quality, 4 to 6 as medium and <4 as low quality.
2.5. Statistical analysis
We used the Revman 5.3 software (Cochrane Collaboration, Oxford, UK) for meta-analysis. If continuous variables were provided in the form of median and range, they would be transformed in mean and standardized mean differences (SMD) as suggested by Wan et al.[18] The difference between groups were measured by the odds ratio (OR) and 95% confidence intervals (CIs). Heterogeneity was assessed by the chi-square test. A fixed-effects model was used when the heterogeneity is small (P > .1 or I2 < 50%), otherwise we used a random-effects model. We used Z test to calculate the P value for overall effect, and the statistical significant level was set at P < .05.
3. Results
Overall, we found 128 potential target references, of which 13 were in line with our research direction. Further by excluding studies that focus on palliation of unresectable HCCA, we identified 8 studies (with data for 1148 participants) for our meta-analysis[14,19–25] (Fig. 1). These 8 trials included one randomized clinical trial and seven retrospective cohort studies. The years of publication were ranged from 2009 to 2018. None of them were considered low quality (Table 1).
Figure 1.
Flowchart of study inclusion.
Table 1.
Quality of studies included in this work.

The characteristics of patients in each study were summarized in Table 2. There were no significant difference between the EBD group and PTBD group in sex and age. The preoperative bilirubin levels and the Bismuth-Corlette type were similar between the two groups in most studies.
Table 2.
Characteristics of studies included.

3.1. The drainage-related complications
Three studies reported the total number of drainage-related complications.[19,22,23] It was 62.7% (101 of 161) in the EBD group and 35.0% (35 of 100) in the PTBD group, suggesting a lower complications rate in PTBD group (OR, 2.73; 95%CI, 1.52–4.91; P < .05; Fig. 2).
Figure 2.

Forest plot for the total complications after PBD (EBD vs PTBD).
The incidence of cholangitis was provided in five studies,[14,19,21–23] with 23.2% (91 of 392) in the EBD group and 23.9% (61 of 255) in the PTBD group. However, the analysis showed a high heterogeneity (P < .00001, I2 = 88%, OR, 0.77; 95%CI, 0.52–1.15; Fig. 3), and the result was not statistically significant (P = .20).
Figure 3.

Forest plot for the incidence of cholangitis (EBD vs PTBD).
Other drainage-related complications included pancreatitis, tube dislocation and drain disfunction. The pancreatitis rate was reported in four studies.[19,21–23] It was significantly higher in EBD (OR, 8.47; 95%CI, 2.28–31.45; P < .05; Fig. 4). The tube dislocation or drain disfunction were not statistically significant (OR, 0.57; 95%CI, 0.28–1.17; P > .05; Fig. 5).
Figure 4.

Forest plot for the incidence of pancreatitis (EBD vs PTBD).
Figure 5.

Forest plot of tube dislocation/drain disfunction rate between EBD and PTBD.
3.2. Conversion
Three studies reported the conversion rate between the two groups.[19,22,23] It was 29.8% (48 of 161) in EBD and 4.0% (4 of 100) in PTBD, suggesting that the drainage effect of PTBD may be better than that of EBD (OR, 8.68; 95%CI, 3.02–24.96; P < .05; Fig. 6).
Figure 6.

Forest plot of conversion rate between EBD and PTBD.
3.3. Data about surgery
Rate of R0 resection were reported in four studies.[20–22,25] The total rate of EBD group and PTBD group from them are 73.8% (211/286) and 71.9% (230/320), respectively. But the results of meta-analysis were not statistically significant (OR, 1.10; 95%CI, 0.76–1.59; P = .63; Fig. 7).
Figure 7.

Forest plot of R0 resection between EBD and PTBD.
The volume of blood loss during surgery was reported in three studies.[19,24,25] The outcome showed high heterogeneity, using a random-effects model and there was no statistically significance between the two groups (P = .95, Fig. 8).
Figure 8.
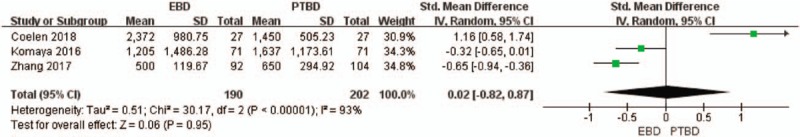
Forest plot of blood loss during surgery between EBD and PTBD.
3.4. Postoperative complications
The postoperative complications include cholangitis, haemorrhage, biliary leakage, liver failure, and so on. Five studies provided data on postoperative complications.[19–22,25] While the analysis showed no statistically significance about the complications (OR, 0.85; 95%CI, 0.62–1.16; P = .30; Fig. 9).
Figure 9.
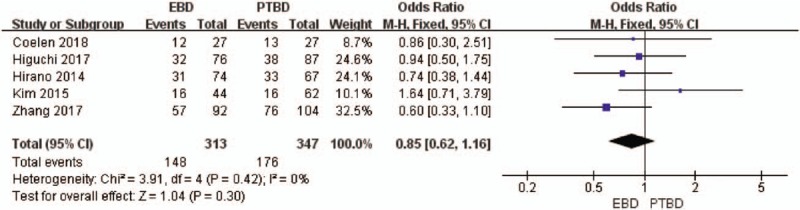
Forest plot of postoperative complications between EBD and PTBD.
3.5. Cancer recurrence
Five studies provided the rate of cancer recurrence.[14,20–22,25] The total rate of EBD group and PTBD group are 39.5% (175/443) and 49.0% (200/408), respectively. The analysis showed no statistically significance (OR, 1.10; 95%CI, 0.76–1.59; P = .63; Fig. 10).
Figure 10.

Forest plot of recurrence between EBD and PTBD.
4. Discussion
Previous studies have showed that PTBD had a lower incidence of PBD-related complications than EBD, which was consistent with our results. Preoperative obstructive jaundice and cholangitis are considered to be the risk factors for poor prognosis of HCCA. Different from the previous studies, the comparison of cholangitis rate in our analysis has a high heterogeneity, and the final results are not statistically significant. The heterogeneity may be related to technological innovation. For example, PBD group in our analysis include ENBD and EBS, technology of ENBD has been greatly improved in the past decade, while EBS has been gradually out of use. This situation was not reflected in the selected studies. In the randomized clinical trial, the incidence of cholangitis in PTBD group was higher than that in EBD group. In the PTBD procedure, catheters are punctured into the bile duct from the portal vein, which may easily cause the internal fistula of portal vein and bile duct, and the drainage time will also be prolonged (Table 2). Another concern for doctors not using EBD is the induction of pancreatitis. In recent studies, however, the severity of pancreatitis did not affect the subsequent surgery.[10,26] Several measures have been adopted to ensure the safety of EBD, including air contrast, avoidance of endoscopic sphincterotomy (EST) and the use of smaller diameter catheters. According to Kawashima et al, a large number of patients with Bismuth-type III/IV did not affect the technical success rate of ENBD, 80% of which were effective after successful insertion into future residual liver (FRL).[26] This is contradictory to the conclusion of Tang et al.[17] It is speculated that due to the immaturity of EBD technology, operators tend to use PTBD to avoid severe portal stenosis in the past decade.
EBD is considered as a kind of intracavitary drainage, which will not cause bile overflow in theory. The catheter of PTBD is placed freely through the abdominal cavity or chest cavity, so bile containing exfoliated cancer cells may overflow.[24] Thus, PTBD is considered to be a risk factor for seeding metastasis. In the studies we selected, cancer dissemination was defined as seeding metastasis, peritoneal dissemination and pleural dissemination. However, there were significant differences in the judgment of recurrence in each study. For example, Wiggers et al performed CT or MRI based on the tumor marker levels or physical examination, which may underestimate the recurrence.[14] The total recurrence rate showed a significant difference in favor of EBD. The 5-year overall survival rate in several studies also confirmed that the prognosis of PTBD was indeed worse than that of EBD.
PBD aims to relieve biliary obstruction and ensure the recovery of preoperative liver function.[27,28] However, due to inadequate preoperative drainage, patients will not benefit from PBD when the volume of FRL is greater than 50%.[7,8,29] EBD dredges the left and right hepatic ducts to achieve total biliary drainage (TBD), while PTBD uses catheter to achieve selective biliary drainage (SBD). This means PTBD could regulate the drainage of different liver segments according to the surgical plan.[30,31] De Palma et al evaluated the drainage effect of SBD and TBD in unresectable HCCA, they found that SBD is better than TBD in promoting hypertrophy of FRL.[32] Whether it is the same in resectable HCCA needs to be confirmed. In another retrospective cohort study, no increased risk of cholangitis was found in patients with SBD.[33] Studies above showed the advantages of SBD, which may indirectly explain why PTBD is more popular in the past decade.
In general, we compared the drainage effect of EBD and PTBD, their influence on prognosis were also analyzed. As a mature technique, PTBD has certain advantages in preoperatively drainage for HCCA. However, the studies we selected have a big chronological span and the technical level of EBD can be uneven, which may limit our analysis. Therefore, there is a greater need to design prospective randomized controlled studies to obtain high-level evidence-based medicinal proof. PTBD is a reasonable choice for PBD, and EBD should only be used as preoperative drainage for HCCA by more experienced physicians. Moreover, it is worth noting that, whether EBD or PTBD, accurate selective biliary drainage should be the trend.
Author contributions
Conceptualization: Guo-Feng Chen, Yu Dong Qiu.
Data curation: Guo-Feng Chen.
Formal analysis: Guo-Feng Chen.
Investigation: Wei-Di Yu, Ji-Ru Wang, Fu-Zhen Qi.
Writing – original draft: Guo-Feng Chen.
Writing – review & editing: Guo-Feng Chen, Yu Dong Qiu.
Footnotes
Abbreviations: EBD = endoscopic biliary drainage, EBS = endoscopic biliary stenting, ENBD = endoscopic nasobiliary drainage, FRL = future residual liver, HCCA = hilar cholangiocarcinoma, OR = odds ratio, OS = overall survival, PBD = preoperative biliary drainage, PTBD = percutaneous transhepatic biliary drainage, RCT = randomized clinical trials, SBD = selective biliary drainage, SMD = standardized mean differences, TBD = total biliary drainage.
How to cite this article: Chen GF, Yu WD, Wang JR, Qi FZ, Qiu YD. The methods of preoperative biliary drainage for resectable hilar cholangiocarcinoma patients: a protocol for systematic review and meta analysis. Medicine. 2020;99:21(e20237).
G-FC as the first author only.
The authors have no funding information to disclose.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are publicly available. All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
- [1].Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the Porta Hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med 1965;38:241–56. [DOI] [PubMed] [Google Scholar]
- [2].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Llado L, Ramos E, Torras J, et al. Radical resection of a hilar cholangiocarcinoma. Indications and results. Cir Esp 2008;83:139–44. [DOI] [PubMed] [Google Scholar]
- [4].van Gulik TM, Kloek JJ, Ruys AT, et al. Improved treatment results in hilar cholangiocarcinoma after transition to more extensive procedure: 20 years experience AMC. Ned Tijdschr Geneeskd 2010;154:A1815. [PubMed] [Google Scholar]
- [5].van Gulik TM, Kloek JJ, Ruys AT, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol 2011;37:65–71. [DOI] [PubMed] [Google Scholar]
- [6].Dixon JM, Armstrong CP, Duffy SW, et al. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut 1983;24:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 2013;100:274–83. [DOI] [PubMed] [Google Scholar]
- [8].Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg 2016;223:321–31. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ratti F, Cipriani F, Ferla F, et al. Hilar cholangiocarcinoma: preoperative liver optimization with multidisciplinary approach. Toward a better outcome. World J Surg 2013;37:1388–96. [DOI] [PubMed] [Google Scholar]
- [10].Kawakami H, Kuwatani M, Onodera M, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol 2011;46:242–8. [DOI] [PubMed] [Google Scholar]
- [11].Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129–40. [DOI] [PubMed] [Google Scholar]
- [12].Sakata J, Shirai Y, Tsuchiya Y, et al. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbecks Arch Surg 2009;394:1065–72. [DOI] [PubMed] [Google Scholar]
- [13].Takahashi Y, Nagino M, Nishio H, et al. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg 2010;97:1860–6. [DOI] [PubMed] [Google Scholar]
- [14].Wiggers JK, Groot Koerkamp B, Coelen RJ, et al. Percutaneous preoperative biliary drainage for resectable perihilar cholangiocarcinoma: no association with survival and no increase in seeding metastases. Ann Surg Oncol 2015;22: Suppl 3: S1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Al Mahjoub A, Menahem B, Fohlen A, et al. Preoperative biliary drainage in patients with resectable perihilar cholangiocarcinoma: is percutaneous transhepatic biliary drainage safer and more effective than endoscopic biliary drainage? A meta-analysis. J Vasc Interv Radiol 2017;28:576–82. [DOI] [PubMed] [Google Scholar]
- [16].Hameed A, Pang T, Chiou J, et al. Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma—a systematic review and meta-analysis. HPB (Oxford) 2016;18:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tang Z, Yang Y, Meng W, et al. Best option for preoperative biliary drainage in Klatskin tumor: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:pe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coelen RJS, Roos E, Wiggers JK, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol 2018;3:681–90. [DOI] [PubMed] [Google Scholar]
- [20].Higuchi R, Yazawa T, Uemura S, et al. ENBD is associated with decreased tumor dissemination compared to PTBD in perihilar cholangiocarcinoma. J Gastrointest Surg 2017;21:1506–14. [DOI] [PubMed] [Google Scholar]
- [21].Hirano S, Tanaka E, Tsuchikawa T, et al. Oncological benefit of preoperative endoscopic biliary drainage in patients with hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:533–40. [DOI] [PubMed] [Google Scholar]
- [22].Kim KM, Park JW, Lee JK, et al. A comparison of preoperative biliary drainage methods for perihilar cholangiocarcinoma: endoscopic versus percutaneous transhepatic biliary drainage. Gut Liver 2015;9:791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010;14:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Komaya K, Ebata T, Yokoyama Y, et al. Verification of the oncologic inferiority of percutaneous biliary drainage to endoscopic drainage: a propensity score matching analysis of resectable perihilar cholangiocarcinoma. Surgery 2017;161:394–404. [DOI] [PubMed] [Google Scholar]
- [25].Zhang XF, Beal EW, Merath K, et al. Oncologic effects of preoperative biliary drainage in resectable hilar cholangiocarcinoma: percutaneous biliary drainage has no adverse effects on survival. J Surg Oncol 2018;117:1267–77. [DOI] [PubMed] [Google Scholar]
- [26].Kawashima H, Itoh A, Ohno E, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg 2013;257:121–7. [DOI] [PubMed] [Google Scholar]
- [27].van der Gaag NA, Kloek JJ, de Castro SM, et al. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg 2009;13:814–20. [DOI] [PubMed] [Google Scholar]
- [28].Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg 2007;14:159–66. [DOI] [PubMed] [Google Scholar]
- [29].Ribero D, Zimmitti G, Aloia TA, et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg 2016;223:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hadjis NS, Adam A, Gibson R, et al. Nonoperative approach to hilar cancer determined by the atrophy-hypertrophy complex. Am J Surg 1989;157:395–9. [DOI] [PubMed] [Google Scholar]
- [31].Miyagawa S, Makuuchi M, Kawasaki S. Outcome of extended right hepatectomy after biliary drainage in hilar bile duct cancer. Arch Surg 1995;130:759–63. [DOI] [PubMed] [Google Scholar]
- [32].De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc 2001;53:547–53. [DOI] [PubMed] [Google Scholar]
- [33].Ishizawa T, Hasegawa K, Sano K, et al. Selective versus total biliary drainage for obstructive jaundice caused by a hepatobiliary malignancy. Am J Surg 2007;193:149–54. [DOI] [PubMed] [Google Scholar]


