Abstract
Setting regulatory limits for arsenic in food is complicated, owing to the enormous diversity of arsenic metabolism in humans, lack of knowledge about the toxicity of these chemicals, and lack of accurate arsenic speciation data on foodstuffs. Identification and quantification of the toxic arsenic compounds are imperative to understanding the risk associated with exposure to arsenic from dietary intake, which, in turn, underscores the need for speciation analysis of the food. Arsenic speciation in seafood is challenging, owing to its existence in myriads of chemical forms and oxidation states. Interconversions occurring between chemical forms, matrix complexity, lack of standards and certified reference materials, and lack of widely accepted measurement protocols present additional challenges. This review covers the current analytical techniques for diverse arsenic species. The requirement for high-quality arsenic speciation data that is essential for establishing legislation and setting regulatory limits for arsenic in food is explored.
Keywords: arsenic, speciation, toxicity, seafood, organoarsenicals, standards
1. INTRODUCTION
Marine organisms are sources of staple and functional food. However, they are also the main dietary source of total arsenic exposure in humans, excluding regions with widespread elevated drinking water contamination.1 Arsenic occurs naturally in seafood in a variety of organic chemical forms. There are hydrophilic arsenicals, such as arsenobetaine (AsB), arsenic-containing ribofuranosides commonly referred to as arsenosugars (AsSugar), and also lipophilic arsenicals, like arsenolipids (AsLipids), besides inorganic arsenic (iAs) that is known to be toxic. Total arsenic as an indicator for dietary risk is inadequate,2 and an accurate account for the myriads of arsenic species in seafood presents a considerable challenge for food safety regulatory authorities.3-5
Current regulations for arsenic exposure focus mainly on iAs, a well-characterized Class A carcinogen.6 Setting of standards for arsenic in food is complicated, owing to the enormous metabolic diversity of organoarsenicals in humans and lack of reliable speciation data on dietary sources.2 Furthermore, the regulatory limits for iAs are derived from studies of high exposure from regions with endemic contamination of drinking water.7,8 iAs is 100% bioavailable in drinking water. Therefore, the mode of action and exposure levels invalidate the significance of these risk assessments with regard to seafood as a source of arsenic exposure.9 Additionally, a lack of data on arsenic toxicity in humans and other mammals from intake of significant amounts of seafood10 provides supporting evidence against arsenic acute toxicity.9 However, because consumption of seafood may result in production of metabolites that are important in arsenic-induced carcinogenicity, it may be prudent to assess the effects of chronic exposure to arsenic in seafood and the contribution to long-term cancer risk.9
The intricate distribution of arsenic in marine organisms implies that evaluation of risk focusing primarily on iAs may provide a warped outlook.2 In addition, myriads of compounds where arsenic is attached to an organic group have been detected in seafood, besides toxic iAs. These organoarsenic compounds constitute more than 85% of the total arsenic content in most seafood, especially fish.9,11,12 Therefore, considering only the iAs fraction in determining toxicity might underestimate the risk, because the major fraction containing arsenic may be present in a form with potential and unknown toxicity.5 This would contravene the precautionary principle of risk assessment that errs on the side of caution, because focusing only on iAs, especially for seaweed, where arsenosugars with unknown toxicities predominate, would misrepresent the level of potential toxicity.2
Seafood is considered safe owing to the benign nature of AsB that predominates and the low levels of iAs. Knowledge of arsenic speciation is the key because the chemical form of arsenic controls its bioavailability, mobility, and toxicity.13 The need for speciation data to fully assess the environmental, biological, or toxicological role of elements has been embraced by the scientific community.5 The challenge remains how to practically implement these ideas into the food regulatory framework, with the case of arsenic being particularly complex, owing to its presence in foods in a myriad of chemical forms and as a result of the scarcity of information on the toxicity and metabolism of such arsenicals.5 The requirement to establish regulations in relation to toxic arsenic in food and to collate additional arsenic speciation data was underscored by the European Food Safety Authority (EFSA) in their 2009 review.14
Speciation analysis of seafood samples requires analytical methods that can quantitatively characterize diverse forms of arsenic from dietary sources; however, matrix complexity and the general dominance of AsB are an impediment.15-18 It is also imperative that the arsenic species in the sample are maintained in the form that they naturally occur in food.19 This requires analytical methods that prevent species interconversions. Unfortunately, there is currently no robust, simple, and affordable method available.2,20 Systematic assessment of methods is essential for reliable arsenic speciation in seafood.21 A practical approach in arsenic speciation analysis comprises four main aspects: extraction, separation, detection, and characterization (identification).6 Every analytical step must be optimized with special attention to interferants, including doubly charged species, isobaric polyatomic species, and organic signal enhancers.13
Organic arsenic species display great diversity, and therefore, it becomes almost impossible to routinely identify and determine their relative composition in seafood in the conformity assessment scheme, where regulatory limits are established. This motivated the suggestion by Feldmann et al. to develop a routine analytical approach that categorizes arsenic species into three fractions based on the International Agency for Research on Cancer (IARC) classification of the carcinogenicity of arsenic species.2 The three categories are (1) toxic inorganic arsenic fraction, which is determined as arsenate after oxidation, (2) AsB, which is established as non-toxic, and (3) leftover arsenicals, which may contain arsenosugars and other non-water extractable, lipophilic arsenicals with potential and unknown acute toxicity.2
AsSugars and AsLipids have attracted a lot of interest because little is known about their toxicity to humans. AsSugars occur in high concentrations (10–40 μg g−1 of dry weight)22 in marine organisms, including those used as human food, and there is considerable interest regarding their toxicological behavior.23 Studies suggest that AsSugars exhibit no acute cytotoxicity or mutagenicity; however, these compounds may be metabolized within humans to form toxic metabolites.24 Dimethylarsinic acid (DMA), a known tumor promotor and metabolite of iAs,25,26 is also a metabolite of AsSugars, and studies have revealed elevated levels of DMA in human urine after consumption of seafood containing high levels of AsSugars.27,28
The cellular toxicity of three arsenic-containing hydrocarbons (AsHC), namely, AsHC 332, AsHC 360, and AsHC 444, was investigated in cultured human bladder (UROtsa) and liver (HepG2) cells.29 The three AsHC showed toxicity comparable to that of arsenite.29-31 Similar studies were performed on two arsenic-containing fatty acids (AsFA), namely, AsFA 362 and AsFA 388,30 which were found to be less toxic than AsHC and arsenite, although they demonstrated significant effects at the micromolar level.29-31
These latest findings underscore the urgent and dire need for more speciation and toxicological information on organoarsenic compounds and drive the development of robust methods for routine analysis that will support the establishment and implementation of regulatory requirements in food, especially seafood.2,4 A risk-based approach should be adapted in the development of a new regulatory framework targeting the known toxic arsenicals, i.e., iAs, AsHC 332, AsHC 360, AsHC 444, AsFA 362, and AsFA 388. This new approach should also pay attention to the neglected area of arsenic speciation research, enhancement of extraction efficiency.6,17,32 The proportion of non-extractable arsenicals is considerable, especially in seafoods, and this portion may contain potentially toxic forms of arsenic with unknown identities.2
Arsenic speciation analysis should be performed with the objective of understanding the presence and proportions of the various arsenicals from dietary sources.21 However, the major challenge, especially with respect to organoarsenicals, is the availability of standards and certified reference materials (CRMs). Therefore, owing to the general variabilities in toxicities based on the chemical forms present, significant risk assessment related to arsenic must exploit speciation data, which entails the deployment of reliable, robust, and widely accepted analytical methods.33
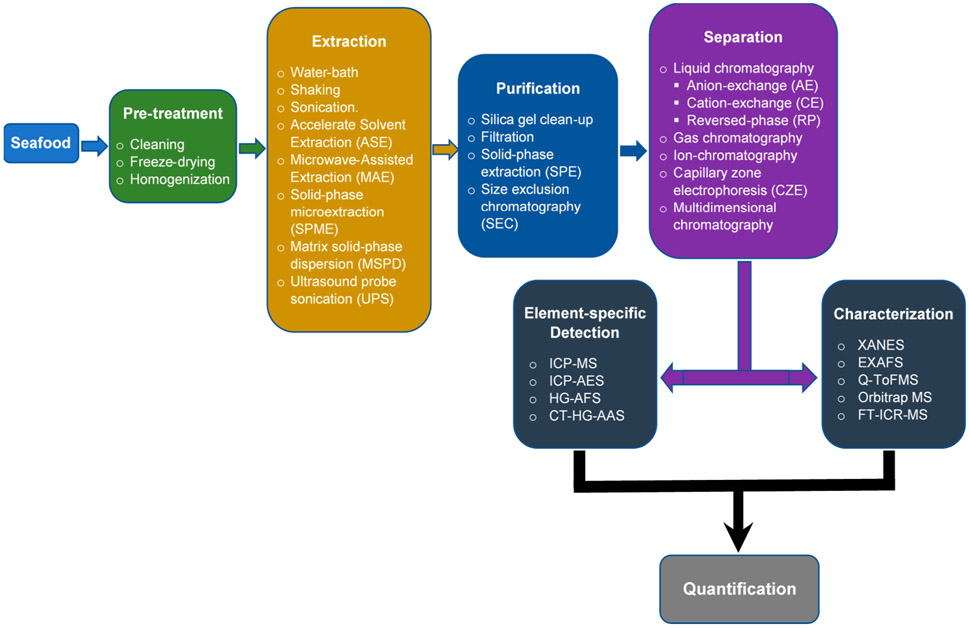
This review paper aims to provide an overview of the current state of practice in arsenic speciation analysis of edible marine species, which include seafood and seaweed from extraction to detection, quantitation, and characterization (Figure 1), while highlighting the general analytical considerations. Thioarsenicals are not covered in this review because they have been discussed in other reviews.34,35 Discussions in this review will focus on hydrophilic organoarsenicals, like methylated arsenicals and arsenic-containing ribofuranosides, and lipophilic organoarsenicals, like arsenic-containing hydrocarbons (As-HCs), arsenic-containing fatty acids (As-FAs), and arsenosugar phospholipids (As-PLs), collectively called arsenolipids.
Figure 1.
Analytical procedure for arsenic speciation in seafood.
2. SAMPLE HANDLING AND PRETREATMENT
Sample pretreatment is a crucial step in speciation analysis because of the complex nature of seafood matrices and the low limits of detection required. Sample handling and pretreatment are critically important in arsenic speciation and need to be carefully optimized to guarantee species integrity.15 Factors such as pH, temperature, light, microbial activity, and container material are critical for sample handling and have a direct effect on species stability.36 Procedures such as freezing, cooling, acidification, sterilization, deaeration, and storage in the dark have been recommended for preservation of species integrity.37
2.1. Cleaning.
Macroalgae often need to be cleaned to remove surface material and epiphytes before analysis, because they may contain arsenic species that can bias speciation results.13 Cleaning of seaweeds may be achieved using a dilute saline solution that does not disrupt cells as a result of osmotic pressure differences.38 It is however impossible to completely eliminate contaminating organisms in some samples. For example, seaweeds contain symbiotic fungi that are part of the plant matrix and cannot be removed by washing or scrapping.39 AsB is an arsenic species not known to be produced by seaweeds but likely to be produced by associated epiphytes, yet several studies have reported its presence in seaweeds.38,40
Marine animals need to be depurated before analysis because their digestive systems may contain sediment particles or undigested plant and animal tissues, which can contain arsenic species that can bias speciation results. For example, many fish that fed on seagrass epiphytes contain undigested seagrass blades, which contain mostly iAs,39 and if the seagrass material is not depurated, elevated levels of iAs will be reported in marine animals.
2.2. Freeze Drying.
For marine samples where bacteria naturally exist, storage at low temperatures or even lyophilization may be required to prevent biological activity that could modify the nature of the sample.41 Other key issues that must be considered and which have implications on the speciation pattern observed include storage conditions and storage time.15 Total arsenic and oxo-methylated arsenic species have been shown to be stable for long periods in frozen and freeze-dried tissues of marine animals and marine algae.42-44 However, Dahl et al. demonstrated that a decrease in the arsenic concentration may occur in blue mussels that are frozen for periods greater than 1–3 months.43
Some degradation of arsenic species in unfrozen samples may occur as a result of microbial action. For example, the production of (CH3)3As as a degradation product has been reported in fish.45 Freeze-dried samples are unlikely to result in arsenic speciation alteration because microbial or chemical conversion cannot occur in dried samples as long as they are desiccated.13 However, although most arsenic species are stable in frozen samples, freezing and thawing of seaweed samples before drying and extraction may result in the loss of organoarsenicals. Therefore, seaweed samples should not be frozen and thawed before freeze drying.13
2.3. Homogenization.
Most seafood samples are often cleaned, freeze-dried, and homogenized by cryogenic grinding. However, the particle size is rarely specified or characterized.13 The particle size is critical because it determines the extraction efficiency based on sample wetting, which is directly proportional to the surface area of the sample that comes into contact with the extractant. For example, Narukawa et al.,46 showed that the extraction efficiency of arsenic from rice with deionized water decreased by 10–30% when the particle size increased from <150 to 500 mm. Similarly, Alava et al.47 showed that extractable arsenic from rice increased from 70% for whole grain to about 80% for the particle size below 1 mm, to 90% for the particle size below 0.5 mm, and to 100% when the particle size was reduced to a powder. Even though the studies were performed on rice, which is not a seafood, the importance of the particle size on arsenic extraction was demonstrated.
3. EXTRACTION
Extraction is the key analytical step in arsenic speciation analysis because it releases the target analytes from the matrix to a solvent that must be amenable with the detection method of choice.48 Regardless of the effectiveness of separation techniques or the sensitivity of the detectors, the limiting step that determines the quality of the analytical results is sample preparation, in particular, extraction and sample cleanup.49 Extraction methods have been developed focusing on enhanced efficiency with reduced extraction solvent volumes and reduced extraction times.41 Arsenicals are difficult to extract in solid tissues, making optimization of methods for each matrix type a necessity.
High extraction efficiencies are desirable and are not only dependent upon the species and type of tissue examined but may show variability for different species of the same family.50,51 For example, fish tissues gave 90–100% extraction,52-55 while oyster and red and brown algae allowed 85–100% extraction with a water/methanol mixture.24,56 Quantitative extraction is possible in a few cases, but complete extraction of arsenic in seaweed or fatty seafood is mostly challenging.41 Extraction techniques should be designed with the aim of achieving the highest recovery, maintaining the integrity of analyte species, and ensuring that the composition of the extractants are compatible with the intended separation and detection methods.6,57,58
3.1. Extraction Techniques.
There are many approaches and techniques employed in extraction of arsenic species from marine dietary sources. Environmental considerations, such as low toxicity of the extractants and low waste generation, have been the driving force toward improving the classical extraction techniques to obtain faster, more reliable, and environmentally friendly methods.59 Different procedures are used, including optimization of solvent polarity, sample acidification16,60 to enhance recoveries of the species, enzymatic hydrolysis (EH),61,62 and use of chelating agents.63-66
The classic sample extraction technique is solvent extraction using different solvents and/or solvent mixtures, with microwave,67 magnetic stirring,68 sonication,69,70 heating,71 or physical shaking16,60 applied to aid in solvent extraction.72 Solvent extraction as a stand-alone technique is less used because it is characterized with long extraction times, use of large volumes of toxic solvents, and low preconcentration factors.73 Modern extraction techniques tend to reduce solvent consumption, e.g., by supercritical fluid extraction (SFE),74,75 accelerated solvent extraction (ASE) or pressurized liquid extraction (PLE),61,76-78 and microwave-assisted extraction (MAE),57,79-82 and make use of solvent-free methods, such as solid-phase microextraction (SPME),83 or sorbent extraction phases, which is the case for matrix solid-phase dispersion (MSPD),59 and physical treatments, as in ultrasound probe sonication (UPS).84 Most of the modern extraction techniques have demonstrated higher capabilities for organic analyte extraction from complex matrices, like seafood, by simultaneously performing both the extraction and cleanup stages.59
3.1.1. Solvent Extraction.
Solvent extraction by mechanical shaking or magnetic stirring68,85,86 or assisted procedures by ultrasound water baths87,88 has widely been used in the quantitative extraction of polar arsenicals in seafood and marine-based products; however, the procedure is laborious, time-consuming, and less efficient for lipophilic samples.73,89 Marine samples are complex, and there is no single method or extractant that exists with the capability to extract all of the arsenic species.80,90 Common extraction solvents for marine samples include ultrapure water,32,53,91-93 methanol–water,53,93-100 hexane,101-105 dilute acids,53,106 and chloroform.93,107
A number of approaches, which involve a combination of several polar and nonpolar organic solvents as extractants, have been reported to achieve successful extraction of arsenic species from seafood.93,101,104,108,109 For example, a mixture of methanol/dichloromethane (DCM)101,104,110 and methanol/chloroform93,111-113 mixtures have been separately employed to extract AsLipids from fish. Other commonly used solvents include the following:
3.1.1.1. Methanol–Water Mixture.
This is the most commonly used solvent mixture in arsenic speciation. Ultrapure water is environmentally friendly and by far the best extractant for speciation analysis as a result of the polar nature of most arsenic species, although as a soft extractant water cannot extract all arsenic as a result of the presence of lipophilic arsenicals in seafood.106 Methanol is also extensively employed as an extractant for seafood, owing to its limited co-extraction of non-arsenicals and ease of removal by evaporation.114 The combination of the individual superior qualities of water and methanol as extractants has motivated their wide application in arsenic speciation analysis.53,93-100 The methanol–water mixture, therefore, affords a fitting balance between arsenic solubility and simplicity of solvent elimination because the majority of naturally occurring arsenicals in seafood are polar and water-soluble.15
Aqueous extraction is suitable for polar arsenicals and, more importantly, preserves them in their innate chemical form.2 Subsequent analysis of the aqueous extract is therefore less challenging because it does not involve any complex sample manipulation other than filtration and dilution. This strategy is convenient and gives a clearer outlook of the distribution of marine arsenicals rather than applying harsh conditions in an attempt to extract all arsenicals simultaneously.21 However, when a lipophilic matrix common in seafood samples is extracted, a sequential extraction procedure is recommended because it aids in fractionating arsenicals on the basis of their polarities to realize adequate extraction efficiencies.33,115
Owing to the great diversity of arsenic species in marine samples, each arsenical should be extracted using customized extraction methods.106,114 For example, physical extraction techniques, like mechanical agitation and sonication, have been coupled with methanol as an extractant to enhance the extraction efficiency. However, low recoveries of arsenicals have been reported for seaweed, even with repeated (3 or 4) extractions,80,96,116,117 and for oily seafood having high proportions of nonpolar arsenicals.118,119
3.1.1.2. Acidic Extraction Conditions.
Acidic extraction conditions are reported to improve extraction efficiencies of AsSugars120,121 as a result of acid hydrolysis that releases arsenical degradation products in the lipid and protein fractions.6 However, these harsh conditions are also believed to be responsible for the production of a single riboside species observed from the degradation of different AsSugars.94,120 Severe degradation is usually experienced when high temperatures and high acid concentrations are employed over lengthy periods.35
3.1.1.3. Basic Extraction Conditions.
Tetramethylammonium hydroxide (TMAH) has been used by Gamble et al.122 for extraction of AsSugars, which are difficult to extract in oysters and shellfish and reported improved extraction efficiencies. However, at high concentrations, degradation of AsSugars was observed on the basis of a SN2 mechanism. Ackley et al. also reported highest recoveries using 5% TMAH on spiny dogfish muscle (DORM-2).57 Neutralized TMAH extracts shifted peak retention times when injected on a C18 column.2 Therefore, regardless of the extraction technique or choice of extractant combinations, especially for AsSugars, species integrity is usually compromised in pursuit of higher extraction efficiencies.
3.1.1.4. Enzymatic Hydrolysis (EH).
Extraction of proteins, lipids, and sugars present in marine samples may require more aggressive leaching or solubilization methods, with the potential to cause species interconversion.73 Enzymes have been used in speciation analysis because of their ability to break down specific bonds in the substrate under mild pH and temperature conditions, thus allowing for selective release of the analytes from the sample matrix without species transformation.61,123,124 For example, enzymes, such as trypsin, pancreatin, and phospholipase D, have been used for arsenic speciation extraction.113,125,126 Trypsin, a proteolytic enzyme, has been used in arsenic speciation studies on fish species, such as cod, dab, haddock, mackerel, plaice, and whiting.93,94
Enzymes can be used to determine the bioavailable fraction of species by mimicking living environments, e.g., gastric digestion processes.73 Artificial gastric juice has been demonstrated to have higher extraction efficiency for arsenic species compared to commonly used extractants, such as ultrapure water, methanol–water, and 0.15 M HNO3.127 Therefore, the artificial gastric juice extraction could be used to simulate the dissolution procedure of arsenic species in the human body. Many factors could influence the efficiency of enzyme-assisted extraction, including the enzyme dosage, pH value, extraction temperature, and incubation time.124
A general drawback of conventional enzymatic hydrolysis is the long incubation time, typically from 12 to 24 h, the need for incubation in a bath at 37 °C, and the relatively high cost of the reagents, which limit its applicability in speciation analysis.62 However, a combination of MAE, PLE, or UPS to EH significantly reduces the extraction time from several hours to 30 min,83,128 10 min,23 and from 30 s to 2 min,129,130 respectively. Ultrasonication provides effective disruption of the cell walls, thus facilitating enzyme interaction with liberated cytosolic components.131 Improvements on the enzymatic hydrolysis under microwaves are attributed to pressure effects on the enzyme and/or the substrate–enzyme interaction and conformational changes in the protein that permit exposure of the new cleavage sites to enzymatic hydrolysis,131,132 which lead to efficient contact between the solvent molecules and the solid particles.
3.1.2. Supercritical Fluid Extraction (SFE).
SFE uses CO2 as an extractant virtually exclusively; thus, its scope is restricted to nonpolar analytes.133 Therefore, the application of this technique in speciation analysis is rare, owing to it low extraction efficiency for highly polar or ionic compounds, which form the bulk of organoarsenicals. Wenclawiak et al. were able to extract DMA, monomethylarsonic acid (MMA), AsIII, and AsV from spiked samples with CO2 in the presence of thioglycolic acid methyl ester.134 The derivatization reaction was carried out in supercritical CO2, leading to the formation of derivatives that were determined reproducibly by gas chromatography, reporting recoveries from 90 to 103% for MMA and DMA under the optimum extraction conditions compared to the liquid-solvent extraction technique. See Table 1 for comparison of extraction techniques, along with their advantages and disadvantages.
Table 1.
Comparison of Sample Extraction Techniques
| advantages | disadvantages |
|---|---|
| Solvent Extraction | |
| 1 relatively robust extraction procedure that allows for efficient transfer of analytes into the extraction solvent | 1 time consuming; long procedure for sample extraction, with extraction times between 8 and 48 h |
| 2 applicable to complex sample matrices, like seafood | 2 uses a lot of solvent, of which most is toxic, and, thus, generates a lot of hazardous waste |
| 3 can be directly applied to unfiltered samples | 3 has a low preconcentration factor |
| Supercritical Fluid Extraction (SFE) | |
| 1 simple, faster, and high-precision extraction compared to conventional solvent extraction methods | 1 because it uses CO2 as an extractant, the scope of application is limited to nonpolar analytes |
| 2 uses CO2, an environmentally friendly extractant, that reduces the need for consumption of and exposure to toxic organic solvents; CO2 is non-toxic, non-flammable, and relatively cheap | 2 application in arsenic speciation is rare as a result of low extraction efficiency for highly polar or ionic compounds, which form the majority of the organoarsenicals |
| 3 low viscosity and diffusion coefficient, contributing to rapid mass transfer of solutions and enhanced interactions at the molecular level, which favors the solubilization process | 3 poor selectivity, which requires advanced optimization |
| 4 capable of extracting thermolabile species, owing to the use of CO2 as an extractant, which has a low critical temperature that allows for extractions under mild conditions, and, thus, suitable for speciation analysis | |
| Accelerated Solvent Extraction (ASE) | |
| 1 analyte- and matrix-independent technique | 1 extraction efficiency asymptotically reaches a maximum, at which point the quantitative nature of extraction becomes matrix-dependent |
| 2 provides cleaner extracts than conventional extraction procedures | 2 no exact volume control of solvent used for extraction is provided |
| 3 applicable for extraction of analytes in complex matrices | 3 only fixed and relatively high-volume extraction cells are commercially available |
| 4 relatively short sample extraction time | 4 limited application in speciation analysis |
| Microwave-Assisted Extraction (MAE) | |
| 1 highly efficient extraction method for a wide range of sample matrices; solubility and not solvent diffusion is the only critical parameter to obtain good recovery | 1 extraction medium, microwave power, and exposure time must be carefully optimized to avoid species losses or transformation |
| 2 capable of hyphenation to chromatographic and spectroanalytical techniques | 2 requires polar solvents |
| 3 suitable for extraction of thermolabile species | |
| 4 supports derivatization reactions | |
| 5 fast and effective extraction method | |
| 6 environmentally friendly because of reduced solvent waste | |
| Ultrasound Probe Sonication (UPS) | |
| 1 simple extraction procedure with fewer operations and, thus, less prone to contamination | 1 being a batch system, the solvent cannot be renewed during the process; therefore, its efficiency is a function of the partitioning coefficient |
| 2 cavitation increases the polarity of the system, including extractants, analytes, and matrices, which increases the extraction efficiency | 2 the need for filtration and rinsing after extraction lengthens the overall duration of the process and increases solvent consumption and the risk of losses or contamination |
| 3 allows for addition of a co-extractant to further increase the polarity of the liquid phase | 3 particle size is a critical factor |
| 4 allows for the extraction of thermolabile analytes, which are altered when using conventional extraction techniques | 4 less robust because the extraction efficiency can be altered as the surface of the ultrasonic probe ages |
| 5 allows for extraction of a wide variety of compounds with various polarities and, therefore, can be used with any solvent | 5 lower precision, resulting from the use of an ultrasonic bath, in which energy distribution is not uniform and ultrasound energy is wasted |
| 6 generally an expeditious, inexpensive, and effective alternative to other extraction techniques, with the possibility of full automation | 6 not reproducible |
| 7 safer for acid digestion because it does not require high pressure or temperature | |
| Matrix Solid-Phase Dispersion (MSPD) | |
| 1 mild extraction technique that maintains species integrity | |
| 2 suitable for speciation analysis | |
| 3 allows for simultaneous extraction and cleanup of samples | |
| 4 high capability for organic analyte extraction from complex matrices | |
3.1.3. Accelerated Solvent Extraction (ASE).
ASE, also known as pressurized liquid extraction (PLE), is an analyte- and matrix-independent technique that provides cleaner extracts than the time-consuming classical procedures used for extraction of compounds from complex matrices.61,76,77,78 The process is based on applying increased temperatures, accelerating the extraction kinetics and elevated pressure, keeping the solvent below its boiling point, and, thus, enabling safe and rapid extractions. Solvent composition and solvent temperature are the parameters that produce the most dramatic increase in extraction efficiency, and hence, they must be optimized.76 Since the first instruments became commercially available in the mid 1990s, this technique gained widespread acceptance for extraction of organics. However, applications in speciation analysis are rare. For example, ASE has been used in the extraction of organoarsenicals from ribbon kelp.76
3.1.4. Microwave-Assisted Extraction (MAE).
MAE is an alternative to conventional solvent extraction, where microwave energy is used to heat solvents that are in contact with solid samples, thus enhancing their penetration into the sample to facilitate the partitioning of the analytes of interest from the sample into the solvent.135 A low-power focused-microwave field, typically 20–90 W, can be employed to accelerate leaching of arsenic species without affecting carbon–arsenic bonds while working at atmospheric pressure.73
Microwave heating is currently extensively used in the extraction of arsenicals from seafood and seaweed, with significant improvements shown in the extraction efficiencies when compared to shaking and sonication.48,57,67,80 Low-power microwaves, employed to decrease extraction times while maintaining efficiency, have been applied to edible marine algae,112 oysters,136 mussels,137,138 and fish tissues,55 allowing for 98, 97, 85, and 95% extraction efficiencies, respectively. Online procedures can be easily implemented using flow injection for hyphenation to chromatographic and spectroanalytical techniques.73 It is suitable for extraction of labile species and also supports derivatization reactions.139
3.1.5. Ultrasound-Assisted Extraction (UAE) and Ultrasound Probe Sonication (UPS).
UAE has been demonstrated to significantly speed up the extraction procedure and increase the extraction efficiency.84,140,141 UAE can accelerate the permeability of the solvent and increase the dissolution rate of extracted components by sonoporation.124 The thermal and mechanical effects of ultrasound accelerate the diffusion of the extracted components and facilitate their extraction. In comparison to MAE, UAE can achieve extraction efficiency in minutes without destructing the components by high temperature and pressure.124
UPS is a fast, relatively cheap, and effective alternative to other extraction techniques. The driving force of sonochemical action in UPS is the acoustic cavitation, provoked by bubbles formed by the soundwave in a liquid that continuously compresses and decompresses.142 This results in extreme local temperatures and pressures generated in the liquid as well as solute thermolysis and formation of hydroxyl radicals and hydrogen peroxide,142 with the latter in case of aqueous solvents. Consequently, when a solid is present in a solvent, compounds present in the solid may be partially or totally extracted into the liquid medium faster than other classical methods.62 These features have made the use of focused UPS one of the upcoming approaches in sample treatment.84
3.1.6. Solid-Phase Microextraction (SPME).
SPME is based on the partition equilibrium of target analytes between a polymeric stationary phase attached onto a fiber and the sample matrix, combining analyte extraction and preconcentration into a single step.73 The analyte is then desorbed from the fiber at a high temperature into an appropriate separation and detection system, usually gas chromatography (GC). Because the extracting phase is non-volatile, only extracted analytes are introduced into the instrument. In the great majority of the cases, extraction of metal species has been carried out using the commercial 100 μm polydimethylsiloxane (PDMS)-coated fiber.73 Volatile organometallic compounds can be collected by SPME from the sample headspace or liquid phase, directly or after derivatization. The suitability of this technique for speciation purposes is fairly limited by the range of characteristics of commercially available stationary phases, although an increasing number of tailor-made coatings is presented in a review by Diezt et al.143 Non-volatile analyte species can be collected from the sample liquid phase and separated by liquid chromatography (LC) or high-performance liquid chromatography (HPLC); this has been performed for arsenic speciation83 using inductively coupled plasma mass spectrometry (ICP–MS) detection. The application of SPME in the field of trace metal speciation is discussed in the review by Mester et al.144
3.1.7. Matrix Solid-Phase Dispersion (MSPD).
The MSPD technique was introduced by Barker et al. in 1989 as an extraction method for organic compounds.145 MSDP disrupts the sample by mechanical blending with a solid-support-bonded phase to provide a material suitable for extraction.146 The shearing forces generated by the blending process disrupt the sample architecture and provide a more finely divided material suitable for extraction by sequential elution using different solvents.147 Therefore, as a result of the disruption of the sample as a consequence of dispersion, analytes are weakly bonded to the newly formed solid-support/sample matrix and analyte extraction is possible using dilute and less toxic reagents.148 In this way, MSDP reduces solvent consumption, amount of sample, and time required for analysis.145,149,150 Detailed theoretical aspects about the MSDP technique are available in the literature.146,149,150 MSPD is therefore a mild extraction technique that is suitable for arsenic speciation analysis in various matrices, including seafood.59,148,151 This fact together with the possibility of performing a cleanup step simultaneously or just before extraction makes the MSPD technique a potential frontrunner in modern speciation analysis.
3.2. Extraction Efficiency and Arsenic Species Transformation.
A neglected area of arsenic speciation research is dealing with the “non-extractable” fraction, which is thought to comprise “protein-bound” arsenic and/or “lipid arsenic”.6,17,32 Quantitative extraction must therefore overcome interactions between analytes and the matrix, which partly depend upon the matrix composition.142 A lack of quantitative extraction is a common challenge associated with the inadequate release of analyte from insoluble constituents of the sample matrix (e.g., protein, lipids, and cells)48 as a result of entrapment or strong physicochemical binding.152 Research in these areas is currently hindered by a lack of suitable extraction techniques capable of quantitative extraction without arsenic species interconversion, especially for arsenolipids and arsenopeptides. Aggressive methods are therefore employed to improve the extraction efficiency of organoarsenicals from seafood, which may affect the integrity of the arsenic species.153,154 For example, the use of severe extraction conditions153 and the application of a high temperature82 could extract all of the arsenic species and accelerate the extraction process; however, this may lead to species interconversion and, at times, degradation of species, like arsenosugars.106
There is no universal procedure that ensures species integrity during extraction and analysis because the arsenic species stability depends upon the sample matrix, the concentration level, and the sample extraction procedure.155 Therefore, a delicate balance must be maintained between achieving higher extraction efficiencies and preserving species integrity.6,94 Some of the modern approaches to overcome this challenge are by combining enzymatic treatment with UPS.156 For example, the approach was developed for various sample matrices, where optimum extraction efficiencies achieved for both total arsenic and species were 70–109 and 86–91%, respectively.129 Stability studies are therefore necessary to ensure that there is no species transformation during sample extraction and analysis.36 Simulated gastric juice has been used as an extractant to aid in understanding “bioavailability” and the balance between quantitative extraction and arsenic species-specific integrity.94
4. SAMPLE CLEANUP PROCEDURES
Extraction is rarely selective. The raw extract may contain the analytes of interest and also co-extracted compounds, most of which interfere with the analytical process.142 Therefore, adequate removal of the matrix is necessary to improve the sensitivity and reliability of instrumental analysis and decrease interferences in chromatographic separation related to the matrix as well as analyte detection.15 For example, the presence of arsenic-free carbohydrates may hinder purification.157 However, the choice for further purification is strongly dependent upon the nature of the sample and the separation and/or detection methods to be employed.15,76
4.1. Silica Gel Cleanup.
In the analysis of AsLipids, the lipid extracts are characterized with high normal lipid matrix interference.158 Silica gel fractionation has been used to remove normal lipid matrix interferences from hexane extracts of fish oil, thus simplifying the analysis.104,105,158 AsLipids are separated from normal lipid in the sample matrix because of their profound affinity for silica, because most of them contain the dimethylarsinoyl, (CH3)2OAs,158 moiety, which interacts with acidic silica, causing them to be strongly adsorbed on the column, while normal lipids are eluted with low to moderate polarity solvents.108 AsLipids have such a high affinity for silica that copious amounts of highly polar solvents, like methanol, are required to elute them.158 Caution, however, must be taken to avoid quantitative transesterification of the less polar arsenic-containing fatty acids (AsFAs) that may form fatty acid methyl esters (FAMEs) in the column.
Silica gel has also been used for the cleanup of MeOH/DCM extracts of marine algae to improve chromatographic separation.6,108 Studies of the silica chromatography cleanup procedure by Glabonjat et al. demonstrate that the technique is effective for arsenic-containing hydrocarbons (AsHCs) and arsenosugar phospholipids (AsSugar-PL) with apparent negligible losses.108 However, when the procedure was applied on lipids in fish oil containing high amounts of AsFA conjugates, most of the initial compounds were altered by the procedure.19,159 Therefore, washing the column with a H2S/acetone mixture that converts oxo-AsLipids to their thio analogues that are less polar and readily elute from the column may address this challenge.158
4.2. Solid-Phase Extraction (SPE).
The principle of SPE is like that of solvent extraction, involving a partitioning of solutes between two phases. However, instead of two immiscible liquid phases, partitioning between a liquid (sample matrix) and a solid (sorbent) phase is exploited.160 The basic approach involves passing the liquid sample through a column, cartridge, tube, or disk containing an adsorbent that retains the analytes and subsequent recovery upon elution with an appropriate solvent. The mechanism of retention depends upon the nature of the sorbent and may include simple adsorption, chelation, ion-exchange, or ion-pair solid-phase extraction.160 Main advantages of the SPE approach are the possible integration of columns and cartridges in online flow injection systems, less solvent consumption, ease of use, and possible application as a species storage device for field sampling.73 Ionic compounds may selectively be preconcentrated using anionic or cationic cartridges, besides avoiding possible signal overlapping with related species in complex matrices when using atomic detectors. In that way, the AsIII interference on AsB in arsenic speciation could be avoided by placing an anionic cartridge before the separation column, leading to retention of arsenite, arsenate, MMA, and DMA.161,162
4.3. Size-Exclusion Chromatography (SEC).
SEC is a molecular-weight-based separation technique that is not used as an analytical technique but as a cleanup technique.163 SEC is important in purifying complex seafood matrices characterized with complex biopolymers, like sugars, lipids, and proteins,164,165 that may bind to the stationary phase or co-elute with analytes of interest.15 Rigorous cleanup of extracts is necessary to prolong the lifetime of LC columns, minimize matrix interferences during detection, reduce deterioration of chromatographic resolution, and fractionate highly concentrated extracts before separation of AsLipids in lipid fractions, which will ensure achievement of good separation of arsenic by reversed-phase liquid chromatography (RP-LC) on C8 or C18 columns.103,104,111,164
Secondary mechanisms, such as adsorption and ion-exchange effects, may impact the retention of analytes.15 Therefore, the arsenic charge state should be considered when determining SEC conditions to avoid retention based on factors other than size and molecular weight.15 Strong electrostatic attractions are induced when charged groups of the stationary phase material interact with quaternary arsenic of TMAsSugars in the presence of water as an eluent, which may result in inhibition of their elution, especially in the absence of an acidic group in the aglycone moiety in the C1 position.166
While investigating the effects of mobile phase composition and flow rate on SEC for AsSugars cleanup, McSheehy et al. chose not to use a buffer to enhance the non-specific interactions of AsSugars with the column and to minimize the salts in the collected and lyophilized fractions.97 Under the same conditions, the dimethylarsinoyl moiety of the AsSugars was preferentially protonated, which resulted in less retention of the AsSugars with acidic aglycone.15 Electrostatic repulsion between the anionic stationary phase and the anionic functional groups of the AsSugars may explain why AsSugars act as though they were ejected from the pores.97 On the basis of these conditions, McSheehy et al. succeeded in fractionating the acidic AsSugars from AsSugar-OH that lacks an acidic group in aglycone in the C1 position, which was voided in the dead volume with other cationic species and required additional purification steps.15,97
5. SEPARATION
There are numerous separation techniques available for arsenic speciation analysis, which includes capillary zone electrophoresis (CZE), GC, and LC. The choice of separation technique depends upon, among others, the sample matrix, extraction techniques, analyte stability, and detection systems. The advantages of CZE are low running costs, low sample volumes for analysis, fast analysis, and simultaneous separation of anionic and cationic species with high resolution.124 However, CZE application is mainly limited to pure standard solutions or simple matrices and has poor sensitivity,167,168 and interfacing with various detection systems is difficult.124 The analytes that are to be separated by CZE must carry an ionic charge.169 Not all arsenicals, especially AsLipids, are ionic in nature, which greatly limits the application of CZE in arsenic speciation analysis.
GC can provide excellent separation of volatile arsenic species and can be easily interfaced with various element-specific detection systems, like electrothermal atomic absorption spectrometry (ET–AAS), inductively coupled plasma atomic emission spectrometry (ICP–AES), hydride generation atomic fluorescence spectrometry (HG–AFS), and inductively coupled plasma mass spectrometry (ICP–MS). However, it is not widely used in arsenic speciation analysis because most organoarsenicals are non-volatile and thermolabile.114 Derivatization (hydride generation) is therefore required to form arsines; however, most of the organoarsenicals do not form arsines, which further limits the scope of application of GC in arsenic speciation analysis.
LC is often preferred over GC or CZE because it is generally more versatile, capable of being applied to a wide range of sample matrices, and can analyze non-volatile polar and lipophilic organoarsenicals.170 Ion chromatography (IC) is predominantly used for arsenic speciation in one of the three separation modes: ion-pairing,69,70 ion-exclusion, or ion-exchange chromatography.170-172 For neutral arsenic species, especially for AsLipids analysis, RP-LC is employed.68 LC can also be interfaced with numerous detection systems, including ICP–-MS, HG–AFS, and tandem mass spectrometry, making it the most suitable separation technique for arsenic speciation. The major drawback for LC is post-column dispersion, co-elution of species with similar physicochemical properties, and the need for standards for retention time matching and species identification.124,173,174
5.1. Capillary Zone Electrophoresis (CZE).
CZE separations arise as a result of differences in electrophoretic mobilities of arsenic species in an electrolyte buffer under the influence of an electric field based on their charge-to-size ratio, which can be carefully controlled by an appropriate choice of buffer constituents and pH adjustments.169 CZE offers high separation efficiency, rapid analysis, and chemical simplicity.133 CZE boasts of better resolution than chromatographic separations and has been applied to the separation of organoarsenic species,169,175 but because of matrix interference, the analysis of real samples has proven to be challenging.167,168 Moreover, its sensitivity is relatively poor, and its connection to various detection systems is difficult because of buffer incompatibilities with the ionization process.169
Currently, the majority of CZE separations of arsenic have been limited to pure standard solution or simple matrices. Sample stacking, an on-column preconcentration technique with a column-switching facility, was introduced to improve CZE detection sensitivity with respect to arsenic species.41 The coupling of CZE to mass spectrometry (MS) combines the advantages of CZE and MS, so that information on both high separation efficiency and molecular masses and/or fragmentation can be obtained in one analysis, which has great potential for arsenic speciation in marine samples.168
CZE separations occur in the liquid phase, while MS detection is a gas-phase process; therefore, electrospray ionization (ESI) is the suitable interfacing technique because this atmospheric pressure ionization (API) technique produces gas-phase analyte ions directly from solution.176,177 In this regard, the ESI interface with a coaxial sheath liquid arrangement is considered highly effective.178,179 However, challenges encountered when combining CZE and MS online include the following: (i) CZE background electrolytes are limited to volatile compounds;180 (ii) migration times of analytes in a CZE–ESI–MS separation can be affected by the sheath liquid composition;181 and (iii) CZE–ESI–MS has limited absolute concentration sensitivity.167,168
5.2. Gas Chromatography (GC).
GC has successfully been used for the analysis of arsenic-containing hydrocarbons in capelin oil,182 using GC–MS, and in canned cod liver183 and commercial fish oils,184 using GC–ICP–MS. GC has the advantage of overcoming the challenges associated with the introduction of organic solvents to ICP–MS associated with RP-HPLC.184 Organic solvents destabilize the argon plasma in ICP–MS, requiring several modifications of ICP–MS, including low flow and oxygen addition,185 for analysis of lipophilic samples using the LC–ICP–MS approach. However, the plasma of ICP–MS will not be affected by organic solvents when using GC. Additionally, GC is commonly used for the separation of fatty acids and other lipids that can be volatilized be derivatization.184 Despite this, only few studies have, thus far, focused on the use of GC for analysis of arsenicals in lipophilic marine samples.
5.3. Liquid Chromatography (LC).
Hydrophilic arsenic species appear in diverse ionic forms that are pH-dependent, which makes it difficult to find a single scheme capable of separating all of the common water-soluble arsenicals.13 Lipophilic species are mostly neutral with hydrophobic properties, owing to the alkyl groups.68 Chromatographic approaches, like ion-pairing reversed-phase, ion-exchange, ion-exclusion, and reversed-phase chromatographies, are reported to facilitate speciation of arsenicals in marine sample extracts, owing to variabilities in their physicochemical properties.13,124 For example, at pH 7, MMA at pKa = 2.6 and DMA at pKa = 6.1 are anions and arsenocholine (AsC), tetramethylarsonium ion (TETRA), and trimethylarsine oxide (TMAO) at pKa = 3.6 are cations, while arsenobetaine (AsB) at pKa = 2.18 is zwitterionic.186,187 Methylated arsenicals and AsSugars have been successfully isolated on anion-exchange (AE) columns,69,70 while cation-exchange (CE) columns provide effective isolations for AsB, AsC, DMA, TMAO, TETRA, and DMAA.116,188 AsLipids, however, require additional separation, using RP-LC typically with a C8 or C18 column.101,108,189
5.3.1. Ion-Exchange Chromatography.
Ion-exchange chromatography has been used for the separation of ionic and ionizable arsenic species by employing the mechanism of exchange equilibria between a stationary phase, which contains surface ions, and oppositely charged ions in the mobile phase.190,191 Ion-exchange chromatography may be used in either of the two separation modes: AE or CE. AE is used to determine anionic arsenic species, where the arsenic species are initially retained on the column by AE and, subsequently, eluted by a competitive anion included in the mobile phase.170-172 AE chromatography enjoys significant application for arsenic speciation analysis.15 Over 50 water-soluble arsenicals have been identified in marine biota extracts using AE and CE chromatographies.13 The factors that influence the separation and retention of analytes in ion-exchange chromatography include the ionic strength of the solute, the pH of the mobile phase, the ionic strength and concentration of the buffer, the temperature, the flow rate, and the introduction of organic modifiers into the mobile phase.170
5.3.1.1. Eluent Systems.
In AE chromatography, the retention time of the analyte is dependent upon eluent composition, including the nature of the competing ions, eluent concentration, and pH.64-66
5.3.1.1.1. Competing Ions.
The nature of competing ion is the main parameter that determines whether target ions are eluted from the column by competitive ion exchange with the eluent anion.192 To obtain arsenic speciation within a reasonable retention time, it is necessary to optimize the eluting system. Generally, phosphate is a weak competing ion.193 However, changes in the eluent strength and pH can significantly improve both the resolution and retention.192 For example, when an eluent of 5 mM NH4H2PO4 at pH 4.6 was used, there was poor resolution between AsIII and DMA and it took more than 20 min for the target ions to be eluted. However, when the pH was increased to 7.9, there was a significant improvement in the resolution and a decrease in the retention time.192
In general, an increase in eluent pH reduces the retention time; however, at higher eluent pH, metal ions present in the matrix may begin to precipitate.192 In addition, the higher the concentration of the competing ions in the eluent, the more effectively the eluent displaces target ions from the stationary phase, the faster the elution of target ions from the column, and therefore, the shorter the retention time. However, a loss of resolution occurs with increasing the eluent concentration.
5.3.1.1.2. Effect of pH on Apparent Charge (Qapp) of Arsenic Species.
The retention of organoarsenic species by ion exchangers relies mainly on their electrostatic interactions with the cationic or anionic sites of the stationary phase and, therefore, their apparent charges (Qapp), which are dependent upon the pH.194 Other factors, such as hydrophobic interactions of the various species with the polymeric stationary phase, may also influence the retention, especially for methylated arsenicals and AsSugars. Methylated arsenicals and AsSugars contain acidic moieties in their chemical structure; thus, their apparent charge (Qapp) depends upon the pH of the mobile phase.195 Elution of organoarsenic species electrostatically retained by the mobile phase at a given pH depends mainly upon the apparent charge of the mobile phase (ACMP) ions.194 The buffer concentration also has a significant influence.
Guerin et al.194 established the pH domains, in which a good separation of arsenic species may be achievable (i.e., domains in which Qapp values differ sufficiently) by plotting Qapp as a function of pH for each species. They found that, in the pH ranges of 4–6 and 9–10, the differences between Qapp values for some arsenic species were quite low and the optimal overall conditions were established to be in the reduced pH domain of 6–9. The aglycone functionality of AsSugars results in different retention characteristics that are dependent upon the pH of the mobile phase that affects Qapp.15 For example, at pH below 5, the apparent negative charge of AsSugar-PO4, AsSugar-SO4, and AsSugar-SO3 may decline, resulting in decreased retention.187 At the pH range of 3.8–9, AsSugar-OH does not dissociate and cannot be retained in the AE column and, therefore, is eluted with or close to the solvent front because it has no charge.187 Identification and verification of AsSugar-OH is therefore performed using the CE column.15 Tukai et al.80 observed that AsSugar-OH was eluted after the void volume if the pH was adjusted from 5.6 to 9.2, which also helped reduce peak broadening for AsSugar-SO3.
5.3.1.1.3. Effect of pH on Apparent Charges of Mobile Phase (ACMP) Ions.
In aqueous solution, the arsenical compounds protonate to an extent determined by the pH of the mobile phase and their dissociation constants.196 The mobile phase acidity will determine Qapp of the arsenicals and, consequently, the composition of the mobile phase. For example, changes in the pH of the 5 mM NH4H2PO4 eluent from 4.6 to 7.9 directly influenced Qapp of competing and solute ions; in this case, Qapp of the phosphate ion changed from −1 (H2PO4−) to −2 (H2PO42−), resulting in an increase in eluting power.197 Similarly, Qapp values of DMA (pKa, 6.1) and MMA (pKa1, 2.6; pKa2, 8.2) were influenced by the eluent pH; however, it was only DMA that was influenced by the pH change because its pKa is 6.1, which resulted in dissociation to its ionic form, causing it to elute after MMA.
ACMP should be carefully optimized to obtain good separation of the various arsenic species, because high ACMP will lead to a reduced resolution, while low ACMP will considerably increase the retention times and eventually lead to poor elution of the most strongly retained species.194 The retention time of a given analyte depends mainly upon both the Qapp value at a given pH and the ACMP value at the same pH. Secondary effects, such as hydrophobic interactions, may alter this prediction to a certain extent,198 but major information can nevertheless be obtained from Qapp/ACMP plots as a function of pH. A plot of Qapp/ACMP as a function of pH revealed that the optimum separation conditions for arsenic species using acetate buffers covered the pH range of 4–6, using phosphate buffers covered the pH range of 6–9, and using carbonate buffers covered the pH range of 8–10.194
5.3.1.2. Gradient Elution.
Ordinarily, AE is performed in isocratic mode, mainly using aqueous mobile phases consisting of buffer salts with pH ranges covering neutral, basic, and acidic conditions.15 Gradient elution can shorten the analysis time without compromising resolutions of target analytes, allows for a broad range of retention, has a high peak capacity capable of handling a complex matrix, like seafood, and can overcome the general issues associated with elution.186,199 Gradient elution protocols are rarely used200 because they cause baseline instabilities that could lead to inaccurate quantification and generally require extra instrument maintenance in comparison to isocratic elution.96,201
5.3.1.3. Chloride Interferences.
In AE, where mass detection is used, separation of the arsenic species often suffers from chloride interference in the matrix as a result of the formation of 40Ar35Cl+ and 38Ar37Cl+ polyatomic species both of nominal m/z 75, which can interfere with detection of monoisotopic 75As+ using ICP–MS.170,171 This problem can be chromatographically solved using an eluent system that can separate chloride from the arsenic species and, therefore, avoid the chloride interference.192,202 Such eluent systems include ammonium phosphate,193,203-205 sodium phosphate,206,207 ammonium carbonate,57,208-211 tartaric acid,212 nitric acid,213 TMAH,214 sodium hydroxide,215 phthalic acid,216 and formate.217 Cations are eliminated in the void volume from the anion column, which aids in reducing matrix interference.13 Unretained cations, like sodium and potassium, elute in the solvent front in AE chromatography, with potential interference in AsSugar-OH determination.118
5.3.2. Reversed-Phase Liquid Chromatography (RP-LC).
RP-LC is based primarily on partitioning and is used mostly for analysis of intact arsenolipids. RP chromatography coupled to ICP–MS has been employed to identify different arsenolipids in cod liver oil,102,103 capelin oil,218 fish,104,111,158,219,220 macroalgae,221 fish meal from capelin,101,105 and cod liver.222,183,223 In contrast to ion-exchange chromatography, RP chromatography is more prone to matrix and pH effects.190,224,225
Sodium and potassium phosphate buffer mobile phase systems are often used in AE and RP-LC analyses when ultraviolet–visible (UV–vis) detectors are used because these phosphate buffers are UV-transparent.210 However, these buffer mobile phases are not amenable with MS detectors because they leave non-volatile buffer salts on the lenses and skimmer cones, resulting in signal drift and a high level of maintenance for cleaning the inner surfaces of the MS detector. In addition, a high concentration of sodium decreases plasma stability because sodium is readily ionizable.210 Ammonium salts of organic acids as well as ammonium carbonate are amenable to ICP–MS because, at 5000 to 10 000 K, plasma leaves little other than NH3, CO2, and H2O.170 Phthalate, formate, and TMAH buffers have the same advantages with an ICP–MS detector.170
5.3.3. Ion-Pair Chromatography.
RP-LC uses aqueous solutions as the mobile phase, which may contain a portion of organic modifiers that aids in the separation of analytes on the stationary phase that is less polar than the mobile phase. In reversed-phase ion-pair chromatography, a counterion is added to the mobile phase and a secondary chemical equilibrium of the ion-pair formation is used to control retention and selectivity.170,190,191 Ion-pair chromatography has the advantage of separating both ionic species as well as uncharged molecular species; therefore, it is has great utility in arsenic speciation analysis. Common ion-pair reagents are long-chain alkyl ions, such as heptanesulfonate anions and tetraalkylammonium salts.13,27 Ion-pair reagents are usually maintained at low concentrations in the mobile phase, typically 20 mM or less.170 The main challenge of using counterions is that they are non-selective and can also pair with matrix components, hence altering the retention times.226
Aqueous solutions with organic modifiers, usually methanol, are used to achieve elution and separation when using ICP–MS detection for arsenic speciation analysis. The selectivity of chromatographic separation of analytes in ion-pair chromatography is influenced by several factors, including the hydrophobicity of the counterion, the concentration of the ion-pair reagent, the buffer concentration, the pH and ionic strength of the mobile phase, and the properties of the stationary phase.13 For example, switching from TMAH to tetrabutylammonium hydroxide (TBAH) lengthens the retention times of arsenicals as a result of reduced polarity.190 When using less polar TBAH, the separation mechanism changes from counterion formation to dynamic ion exchange. In essence, the solute ions are bound to counterions, which are now attached to the stationary phase, as a result of their increased hydrophobicity.13
5.3.4. Micellar Liquid Chromatography (MLC).
MLC is a variant of RP-LC that has been used in arsenic speciation analysis.227 In MLC, a relatively high concentration of surface-active agents (surfactants) is used as counterions and the formation of “micelles” occurs. ICP–MS is usually used for detection. MLC offers advantages over RP-LC, such as concurrent separation of both ionic and non-ionic analytes, faster analysis times, and improved detection sensitivity and selectivity,228 which arise from its unique three-way equilibrium mechanism, where micelles acts as a pseudo-phase in addition to the mobile and stationary phases.227
5.4. Multidimensional Chromatographic Techniques.
Chromatographic separations based on a single interaction mechanism show limited selectivity in the presence of a variety of species,229 especially in complex matrices, like seafood.15 Therefore, different chromatographic approaches using AE, CE, RP, and ion-pairing chromatographies may be used individually or in combination to separate arsenic species.124 Simultaneous use of multiple complementary separation techniques, like AE, CE, and RP chromatographies, facilitates the complete separation of arsenic species while reducing chances of co-elution and, thus, enhances the reliability of analytical results.15,118,230
The general approach involves the use two or more orthogonal chromatographic separations to isolate arsenic species with varying chemical properties, resulting in efficient resolution and high arsenic species retention capacity.231 The sequential application of orthogonal isolation techniques may enhance chromatographic separation efficiency and, particularly, helps in obtaining baseline resolution.163,232 Reversed-phase ion-pairing chromatography is an ideal alternative for simultaneous separation of neutral and ionic species.124 TMAH, TBAH, and tetraethylammonium hydroxide (TEAH) are commonly used cationic pair reagents for separation of arsenic species.233 Various alkyl sulfonates have been used as anion pair reagents, while sodium 1-butanesulfonate, malonic acid, and TMAH have been used as chelating agents to increase the retention capacity of arsenic species on a C18 column.124
6. DETECTION TECHNIQUES
Atomic spectroscopy and molecular mass spectrometry are the main detection techniques used for speciation analysis.15 While techniques such as hydride generation atomic absorption spectrometry (HG–AAS), HG–AFS, ICP–AES, and ICP–MS are element-specific with high sensitivity, mass spectrometry (MS) provides additional information on the structure of the analyte based on fragmentation patterns.234,235
6.1. Atomic Absorption and Atomic Fluorescence Spectrometries.
Atomic absorption spectrometry (AAS) and atomic fluorescence spectrometry (AFS) have traditionally been the most widely used detection techniques in arsenic speciation because of their sensitivity, simplicity, and precision at low parts per billion levels.58 While using AAS and AFS, often hydrides have to be generated to enable analysis of arsenic. HG, as a means of sample introduction, can provide unique benefits for arsenic speciation analysis, including separation and enrichment of analytes from the matrix, high sample introduction efficiency, and significant elimination of spectroscopic or matrix interferences from samples with high salt and acid concentrations.124
HPLC coupled with HG–AAS or HG–AFS is a simple and convenient method for simultaneous separation and determination of arsenic species in marine products. The method combines the high separation efficiency of HPLC, the unique gas–liquid separation techniques of chemical vapor generation, and the efficient post-column online derivatization.124 However, the efficiency of HG is affected by the chemical forms and valence states of the analytes, with trivalent arsenic species readily undergoing HG compared to their pentavalent counterparts, resulting in lower detection sensitivity. In addition, there are limitations for the number of organoarsenic species capable of generating hydrides with chemical reagents;68,236 therefore, a chromatographic eluent is often irradiated with UV rays37,136,236 or microwave digested55 to change the inactive species into active species prior to analysis by post-column derivatization.41
6.2. ICP–MS.
ICP–MS is the most widely used analytical technique for detection of arsenic species since its introduction in the 1980s as a result of its amenability to front-end separations and sample introduction strategies, high element selective limit of detection, high sensitivity, accurate isotope ratio determination, wide linear dynamic range, and multi-elemental detection at low concentration levels (1 ng L−1) with minimal sample preparation constraints.6,91,237-239 ICP–MS is quite robust and less susceptible to matrix effects.15 High sampling and data acquisition rates of ICP–MS enable baseline separation of neighboring peaks and quantification without the loss of peak resolution.13 Dependent upon the physics of mass analysis, analyzers could be a quadrupole, magnetic sector, ion trap, time-of-flight (TOF), or Fourier transform (FT).240
6.2.1. ICP–MS Signal Interferences.
Because the resolution of a single quadrupole mass spectrometer is not high enough (about 0.75 amu), it cannot eliminate spectroscopic interferences.124 ICP–MS is therefore affected by polyatomic interference from marine samples in the form of molecular isobars 40Ar35Cl+ and 38Ar37Cl+, which have the same mass-to-charge ratio (m/z) as 75As+. In addition, high concentrations of rare earth elements, like samarium (Sm) with m/z 150, in extracts are an important source of doubly charged interferences because they have low ionization potentials (11–12 eV) and readily form doubly charged ions, 75Sm2+. Several approaches can effectively overcome these interferences caused by polyatomic ions.
Interference by chloride-forming polyatomic species are seldom a challenge in practice because they can be chromatographically isolated from arsenic species.13 These interferences can also be alleviated using inductively coupled plasma triple quadrupole mass spectrometry (ICP–MS/MS) using H2 or He collision cell and O2 reaction cell technologies to detect As at m/z 75 and 91, respectively via chemical reaction.69,70 In oxygen reaction mode, the quadrupole mass filters can be set to only allow ions with m/z 75 (75As+, 40Ar35Cl+, and 75Sm2+) to pass through the first quadrupole (Q1) to the reaction cell, 75As+ is easily oxidized to form 75As16O+ with m/z 91 in the reaction cell, but 40Ar35Cl+ and 75Sm2+ do not react with oxygen gas to form ionic species with m/z 91 and are, therefore, rejected and filtered out in the third quadrupole (Q3).
Alternatively, As can be detected in high-resolution mode (m/Δm >10 000) by resolving 40Ar35Cl+ interferences spectroscopically.6,241 The mass-to-charge (m/z) ratio of 77 must be monitored during method development, to establish whether the presence of ArCl+ would be a source of interference for the detection of arsenic, because about 25% of all ArCl+ is expected to have m/z 77, owing to the 40Ar37Cl+ contribution.57 For ion chromatographic separations, sodium ions arising from a high concentration of NaOH in the mobile phase must be removed because they suppress the arsenic signal35,242 and cause severe baseline drift.13 A self-regenerating suppressor may be installed to remove Na+, while OH− ions are electrochemically converted to water before sample introduction to ICP–MS.13,243
The main shortcoming in testing AsLipids is associated with incompatibility between ICP–MS and organic solvents.69,70,185 Organic solvents are necessary for analysis of lipophilic compounds, which has hampered advances of AsLipids research until recently.239 Mobile phases with high organic content may trigger arsenic signal enhancement or extinguish the plasma,244 which may require the addition of oxygen to the plasma to help in the removal of carbon that builds up on the sampling cones of the interface as a result of incomplete combustion.6,41,101 This may impact analytical performance, resulting in the loss of the analyte or reduction in signal intensity.15,245
These issues are overcome by employing a specifically designed interface, e.g., cooled spray chamber, membrane desolvator,246 or post-column dilution, using microbore LC columns,245 allowing low solvent flow, adding oxygen to the plasma gas, or including a post-column flow split.41,247,248 Grotti et al. used small-bore columns in conjunction with low dead volume interfaces to overcome challenges associated with conventional LC–ICP–MS, because these conditions provided faster separation and a lower flow rate of the mobile phase, which lead to the reduction of the matrix plasma load, reagent consumption, and waste generated.249
6.2.2. Sensitivity Improvement by Hydride Generation.
The sensitivity of LC–ICP–MS can be considerably amplified by the integration of a hydride generation (HG) system post-chromatographic separation of As species.13 A HG system is typical for operation of AFS. Many arsenic species form volatile hydrides that allow for approximately 70–80% of arsenic that is converted to hydride gas to reach the ICP–MS plasma, as opposed to only about 1% of the arsenic species that reach the ICP–MS plasma via the spray chamber.13 NaBH4 is a typical reductant for As–hydride generation,250 which is dependent upon the arsenic species and sample matrix.13 For example, AsB and AsC do not produce volatile hydride species, while AsSugars form hydride species but with a very low efficiency (~5%) or 21–28% when using an optimized HG system.251
The use of HG eliminates the overestimation of iAs, especially when the concentration is used to satisfy food regulation requirements, by eliminating the signal of the other arsenic species that do not generate hydrides.252 However, if the organoarsenical content is obligatory, a post-column reactor is essential to convert these compounds to hydride-forming species in a process that involves UV photolysis in the presence of an oxidant, which converts benign arsenic species, such as AsC and AsB, to AsV before HG.37,253 Cysteine is used to reduce AsV to AsIII254 before the formation of arsine (AsH3).13 Kumar et al. proposed that the As–cysteine complexes, where oxygen is replaced by a thioalkyl (SR) group, readily react with less sterically hindered BH− compared to AsIII255 Cysteine permits lower acid concentrations to be used256 to obtain the same response for As111, AsV, MMA, and DMA. Pohl and Prusisz reported that the use of charged surfactants enhances the generation of As–hydride complexes,257 while Karadjova et al. reported the suppression of hydride generation by some organic solvents, like ethanol.258
6.3. Hydride Generation Atomic Fluorescence Spectrometry (HG–AFS).
LC–ICP–MS is the hyphenated analytical technique of choice for arsenic speciation.80,152,187,188,259 However, HG–AFS coupled to HPLC offers an alternative to this technique.38,72,86,112,236,253,260 HG–AFS has been reported to be similar to ICP–MS with regard to sensitivity and linear calibration range, although it has other beneficial qualities, like simplicity of use and lower acquisition and running costs for arsenic speciation analysis.37,261,262 As a result of the low efficiency in generating volatile hydrides, the destruction of the organic part of organoarsenicals is necessary for their determination by HG–AFS.263 The organic arsenic species are usually converted to inorganic species subsequent to chromatographic separation by photo-oxidation using a strong oxidant in basic media and UV radiation before HG–AFS detection.112
The utility of HG–AFS has been successfully demonstrated in diverse seafood samples. For example, Slejkovec et al. analyzed six CRMs of marine origin (dogfish muscle and liver, lobster hepatopancreas, oyster tissue, brown algae, and scallop) by LC–(UV)–HG–AFS, using both AE and CE chromatographies. They identified AsB, DMA, TETRA, AsC, TMAO, AsV, MMA, and two AsSug, together with four unidentifiable compounds.236 Sánchez-Rodas et al. applied a similar analytical technique for the analysis of aqueous extracts of oysters.253 The two research groups highlighted the possibility of using LC–(UV)–HG–AFS as an alternative technique to LC–ESI–MS for the detection of arsenosugars in crude extracts, because the signal response in HG–AFS is less susceptible to the matrix effect compared to LC–ESI–MS analysis that requires additional sample cleanup steps.
In a separate experiment, Slejkovec et al. applied the LC–(UV)–HG–AFS technique in the determination of arsenicals in 10 different marine algae (red, green, and brown) from the littoral zone along the Adriatic Sea coast of Slovenia. They were able to identify arsenosugars as the predominant arsenicals in most of the analyzed algae samples, together with AsB, AsIII, AsV, and DMA.38 Schaeffer et al. were able to determine 12 arsenicals in mussels, anchovies, seabreams, sea bass, and sardines by LC–(UV)–HG–AFS, using both AE and CE chromatographies. AsB was predominantly detected in all of the samples, with trace levels of AsIII, DMA, and AsC, whereas AsSugars were detected only in mussel samples.86 Geng et al. analyzed six seafood samples and four seaweed samples using cryogenic trap hydride generation atomic absorption spectrometry (CT–HG–AAS) following alkaline digestion for arsenic speciation and found that the results were comparable to those obtained using LC–(UV)–HG–AFS.72
7. CHARACTERIZATION
The monoisotopic nature of arsenic means that it lacks a distinctive isotope pattern; therefore, it is challenging to find authentic isotopically labeled standards and CRMs for identification, quantitation, and method validation.99 Standards are mandatory for accurate and reliable identification and quantification of arsenicals using LC–ICP–MS; however, in the absence of standards, as is the case with organoarsenicals in seafood, indirect or complementary approaches may be employed.101
Identification of organoarsenicals in seafood requires either fractionation and cleanup of analytes, followed by a fragmentation experiment using high-resolution tandem mass spectrometry for identification and structural assignment of analytes,209 or indirect confirmation of structures by comparison to synthesized arsenicals that have been fully characterized by molecular mass spectrometry.159 However, the analytical workup schemes in these methods are extensive, and identification of potentially co-eluting lipophilic organoarsenicals in seafood, which are in low concentrations, is very challenging.
The main techniques capable of addressing the analytical limitations of LC–ICP–MS are X-ray absorption spectroscopy (XAS) for in situ identification174 and molecular mass spectrometry with a soft ionization technique and mass analyzers with high resolving power for structural elucidation.105,222,231
7.1. Identification Using LC–ICP–MS Coupled to X-ray Absorption Spectroscopy (XAS).
XAS, which includes X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), is an element-specific spectroscopic analytical synchrotron technique that uses the principle of X-ray fluorescence to probe the changes in the chemical environment of metal centers by means of X-rays.264,265 XAS enables the elemental characterization in terms of oxidation states, site ligation, and coordination.265 The analyses of the absorption spectra, their features, and the underlying principles are detailed in the review by Nearing et al.174
XAS presents unique capabilities over other arsenic speciation techniques by allowing in situ arsenic speciation analysis in nearly all types of sample matrices, including crude extracts, frozen hydrated samples, freeze-dried samples, and subcellular compartments independent of their actual physical state (whether solid, liquid, or gaseous), which is not possible with conventional techniques,174 and thus reducing sample preparation steps that might modify the elemental species present265 while giving detection limits of about 1–10 μg g−1, depending upon the experimental conditions.174
XAS is compatible with LC–ICP–MS, which it can be coupled with structural elucidation of novel compounds, like arsenosugars and arsenolipids, in their native state in seafood, of which some of the reported structures have been postulated from those of known fatty acids or hydrocarbons, owing to the lack of identification methods and standards.101,102 The unique speciation analysis capability offered by XAS is also key to understanding the cellular mechanisms of arsenic biotransformation in toxicity studies.265
Some of the disadvantages of XAS are that XAS uses hard X-ray beams with high energy that can potentially damage the sample; the absorption edges for some arsenic and selenium compounds may be very close or even identical, which makes the use of standards mandatory. However, most standards are not available for organoarsenicals of interest, like arsenosugars and arsenolipids. XAS is less sensitive to metals bound to the lighter elements, like O, P, N, and S functional groups. Arsenic compounds with similar nearest-neighbor environments have similar white line energies and may be misidentified in XAS without comparison to LC–ICP–MS. For example, arsenobetaine (AsB), arsenocholine (AsC), and tetramethylarsonium ion (TETRA) have the same white line energy (11 872.6 eV).174 Finally, XAS requires a high level of skill to operate and interpret because the resulting spectrum is the weighted sum of all species present in the analyzed volume, which may be very difficult to interpret in a complex matrix, like seafood.
7.2. Simultaneous Identification and Quantification Using LC–ICP–MS Coupled to High-Resolution Mass Spectrometry (HR-MS).
ICP–MS is a widely accepted analytical technique in elemental analysis at trace levels because of its high sensitivity and element selective detection;239 however, it has a number of limitations: its reliance on the extraction step usually from the solid matrix that may be incomplete or result in species interconversion, risk of co-elution of arsenic species, impossibility of species identification in the absence of well-characterized standards, lack of molecular information, and risk of species misidentification based on the retention time matching with standards.124,173 These challenges highlight the need to adapt complementary analytical techniques to gain a better understanding of the arsenic chemistry in seafood samples.
Marine samples have numerous arsenic species with similar physicochemical properties, and the insufficient chromatographic separation efficiency of a single separation technique makes co-elution of similar species practically inevitable.187 This challenge can partly be addressed by careful optimization of the separation conditions266 or running the same sample using orthogonal separation techniques, sequentially97,163 or concurrently101,105,164,222 for simultaneous quantification and confirmation.
Earlier arsenic speciation analyses were performed by first acquiring the “sample profile”, which required element-specific detection, with the most commonly used being LC–ICP–MS.81,267-269 Other element-specific detection techniques used included LC–(UV)–HG–AFS,13,86,112,236,253 CT–HG–AAS,72 and graphite furnace atomic absorption spectrometry (GF–AAS).270 This was followed by determination of the molecular mass profile of the intact molecules that required soft ionization and high-resolution mass analyzers.102
This opened the possibility for structural assignment of unknown arsenicals by either fraction collection from natural sources,209 off-line identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI–TOF–MS),102 electrospray mass spectrometry (ES–MS),101,102,105,159,222 or indirectly by comparison to synthesized arsenolipids, which had been fully characterized by molecular mass spectrometry.159 Structural assignment for unknown species has been realized by HR-MS, an approach that was successfully applied by Taleshi et al. while characterizing arsenolipids in multiple matrices159 and Nischwitz et al. in characterization of AsSugars.245 Miguens-Rodriguez et al. demonstrated the utility of electrospray ionization ion trap multistage mass spectrometry (ESI–IT–MSn)232,271 in generating distinctive product ions that enabled the rapid screening and sensitive characterization of four AsSugars in unrefined seaweed extracts with minimal sample preparation.271
The recent trend is toward concurrent sample analysis using LC–ICP–MS with another detection method that is not necessarily element-specific, like tandem mass spectrometry, from the same chromatographic run by splitting the effluent flow from the HPLC column between the two mass spectrometers.272 The high-resolving powers of various mass analyzers (see Table 2 for figures of merit) provide accurate mass data that are essential to distinguish the different isobaric and isomeric arsenicals with high precision and, thus, aid in the elucidation of molecular formulas and provide structural information. Concurrent use of LC–ICP–MS and molecular mass spectrometry with high mass accuracy offered by the high resolving powers of mass analyzers, like quadrupole time-of-flight (Q-TOF), Orbitrap, magnetic sector, and Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry, provide an additional separation dimension for isobaric and co-eluting analytes.101,105
Table 2.
Figures of Merit for Common Mass Analyzers
| mass analyzer | resolving power (fwhm) | mass accuracy (ppm) |
sensitivity (g) |
advantages | disadvantages |
|---|---|---|---|---|---|
| quadrupole | unit-mass resolution; modern instruments can reach up to 5000 | 50 | 10−15 (SRM) |
|
|
| quadrupole ion trap | 10000 | 50 | 10−15 |
|
|
| time of flight | ≥100000 (at m/z 400, fwhm) | <3 | 10−12 (full scan) |
|
|
| magnetic sector | ≤60000 | ~1 | 10−12 |
|
|
| Orbitrap | 280000 (at m/z 200, fwhm) | ~1 | 10−15 (full scan) |
|
|
| MegaOrbitrap | 1000000 (at m/z 200, fwhm) | ~1 | 10−15 (full scan) |
|
|
| FT-ICR | 1000000 (at m/z 400, fwhm) | ~1 | 10−12 (full scan) |
|
|
7.2.1. Quadrupole Time-of-Flight (Q-TOF) Mass Spectrometry.
A Q-TOF mass analyzer is a hybrid instrument that operates in both negative and positive ionization modes with high mass accuracies at ultratrace levels.23,99,226 Q-TOF has mass accuracy of 2 ppm and may be limited in its ability to provide indisputable identification of unknown analytes; however, it provides sufficient information for the determination of the exact empirical formula of analytes, therefore reducing the potential structures to a realistic number.99 Isobaric matrix interference with similar retention times overlapping with organoarsenical peaks of interest can be resolved using a high-resolution instrument like FT-ICR–MS.99 Some of the applications of Q-TOF–MS in elucidation of the structure for organoarsenicals include work by McSheehy et al. in the characterization of arsenic species in the kidney of a clam,226 Sele et al. in the identification of arsenicals in marine oils,189 and Arroyo-Abad et al. in the identification and determination of arsenolipids in fresh cod liver oil.222 fish,219,220 and canned cod liver.223
7.2.2. Orbitrap Mass Spectrometry.
Orbitrap is an accurate and compact Fourier transform mass analyzer that was first commercialized in 2005 by Thermo Electron as a hybrid instrument (LTQ-Orbitrap) featuring a linear ion trap front-end.240 The Orbitrap mass analyzer employs electrostatic trapping rather than the magnetostatic characteristic for FT-ICR.273 This precursor instrument has undergone a number of iterative improvements incorporating features like image current detection from FT-ICR–MS, use of ion trapping in precisely defined electrode structures, from the radio-frequency (RF) ion trap, and pulsed injection and use of electrostatic fields, from the TOF analyzers.240 All of these features enabled Orbitrap to overcome the major limitations experienced by the other techniques, like the necessity for a superconducting magnet in FT-ICR–MS, severe limitations on the space charge in the RF ion trap, and severe limitations on the dynamic range of detection in TOF analyzers.240 The latest version of the Orbitrap mass analyzer, MegaOrbitrap, has ultrahigh resolving power in excess of 1 000 000 within 3 s detection time, making it compatible with chromatographic separations.273 Amayo et al. concurrently used LC–ICP–MS and an Orbitrap mass analyzer for structural assignment of arsenolipids in fish meal,101 fish oil,103,105 and fish tissue.104
7.2.3. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR–MS).
FT-ICR–MS offers a resolving power upward of 106 [at m/z 200, full width at half maximum (fwhm)] and mass accuracy (<1 ppm), which is the highest of all existing mass spectrometry techniques.274 Ions are trapped in a strong magnetic field combined with a weak electric field generating an image current from coherently excited trapped ions that are detected, digitized, and converted using Fourier transform into the frequency domain and then mass spectra.275 The inherent stability and field uniformity of superconducting magnets that work in synergy with the very high accuracy and dynamic range of frequency measurements has made FT-ICR–MS an ultimate frontrunner in mass resolving power and mass accuracy.273
Despite the monoisotopic nature of arsenic, it has a distinct mass defect that FT-ICR–MS is capable of resolving and, thus, ensuring unequivocal identification of arsenicals.99 This is particularly helpful in resolving co-eluting analytes with isobaric interference, which is common in lipophilic organoarsenicals. Pickford et al. demonstrated the utility of FT-ICR–MS in the analysis of crudely purified kelp extract,99 while Rumpler et al. used FT-ICR–MS and Q-TOF–MS in structural elucidation of AsLipids in cod liver oil.102
7.3. Identification by Fragmentation Experiments Using Tandem Mass Spectrometry.
Organoarsenicals, like arsenolipids and arsenosugars, have distinct fragmentation patterns that can be used together with the high resolving power and accurate mass offered by high-resolution mass analyzers for identification and structural assignment of unknowns. For a successful fragmentation experiment, there are a set of conditions that must be fulfilled: First, there must be chromatographic separation; second, there must be a soft ionization source; and third, there must be a high-resolution mass analyzer with high resolving power and mass accuracy. Soft ionization enables mass spectrometry to be used as a separation tool to resolve arsenical peaks by molecular weight. Electrospray ionization (ESI)101,102,105,159,222 is typically used, although there are other ionization techniques, like matrix-assisted laser desorption/ionization (MALDI)102 and fast atom bombardment (FAB).22
The ESI technique coupled with HR-MS (triple quadrupole mass spectrometry233 and Q-TOF),183,219,220,222,223 Orbitrap,101,103-105 and FT-ICR–MS99 can provide excellent separation of co-eluted components in HPLC and can be used for identification of unknown species.104,174,276 In recent years, ESI–MS/MS has been applied to speciation analysis of methylarsenicals, AsSugars, AsLipids, and many other arsenic species.
Selection of distinct precursor ion/product ion transitions enables tandem mass spectrometry methods based on selected reaction monitoring (SRM), also known as parallel reaction monitoring (PRM), to minimize background noise and the matrix effect, therefore ensuring ultrafast scan rates and highly sensitive and highly selective detection methods for arsenic speciation. Characteristic fragments for the four common AsSugars detected in positive MS/MS mode are shown in Figure 2. The m/z 237 fragment is formed from the loss of aglycone at C1, and the m/z 97 fragment results from additional elimination of the dimethylarsinoyl group alongside a water molecule.23,22
Figure 2.
Typical AsSugar and thio-AsSugar fragments detected in tandem mass spectrometry.
Thio-AsSugars display fragmentation patterns similar to their oxo analogues, yielding a structurally diagnostic fragment at m/z 253 (Figure 2) corresponding to the loss of aglycone245,251,277 and m/z 97 as a result of the loss of the dimethylthionyl group alongside a water molecule.245,277 The fragment at m/z 97 for the sulfate thio-AsSugar may also emanate from the −OSO3H moiety.100,245,278 Two additional unique product ions for thio-AsSugars are observed at m/z 107 correlating to AsS+117,245 and m/z 91 presumed to result from AsO+ as a result of trace amounts of oxygen in the nitrogen drying gas.117,251,279 Thio-AsSugars exhibit a distinctive sulfur isotope pattern (32S/34S), with a mass peak appearing 2 amu higher than the 32S-containing ion and an expected ratio of approximately 23:1.15
It is important that peak assignments are verified to eliminate false positives emanating from matrix components with SRM transitions similar to the organoarsenicals.90,231 A common quality control tool employed is a comparison of the standard and analyte peak retention times by selecting a set of unique transitions for the qualifier and quantifier ions for each analyte and comparing the intensity ratios for the standard and the analyte present in the sample.90,280 Other quality control methods include establishment of an accurate mass of the molecular ion using high-resolution mass spectrometers.231 Several useful tools for SRM optimization exist, which may be considered virtual standards that are derived from numerous fragmentation experiments for purified standards from natural samples.277,280-283
The need for complete chromatographic separation is diminished in the absence of a critical signal masked by matrix components when using highly unique multiple reaction monitoring (MRM) transitions. However, sufficient chromatographic resolution is required, when DMAsSugar-SO4, and DMthio-AsSugar, which have a similar molecular mass and fragmentation pattern, are present.281 In addition, in-source dissociation of DMAsSugar-SO4,50,284,231 DMAsSugar-PO4, and DMThioAsSugar-SO4231,245 to the corresponding AsSugar-OH has been recorded even at mild cone voltage, leading to AsSugar-OH signal contribution at their corresponding retention times.50,231 The in-source fragmentation of DMAsSugar-PO4 occurs marginally, resulting in ion formation at m/z 409, in likeness to the protonated molecular ion of the sulfate oxo-sugar.50 Similar fragmentation patterns for oxo- and thio-AsSugars need to be considered.231,245
8. STANDARDS AND REFERENCE MATERIALS
The number of organoarsenicals discovered in seafood continues to increase in tandem with advances in instrumentation and analytical protocols. Significant advances have been made in designing highly sensitive and selective separation and detection methods for the explicit characterization of known and unknown organoarsenicals.15 Characterization and quantification of organoarsenicals in seafood is impeded by a lack of widely accepted analytical protocols and well-characterized and commercially available standards to be used as calibrants and CRMs for method validation.285,286
Several attempts have been made to indirectly identify and quantify organoarsenicals in marine samples in the absence of standards.104,174,276 Yu et al. were able to analyze arsenosugars in kelp by fractionation and analysis of individual fractions, using LC–ICP–MS and instrumental neutron activation analysis (INAA).209 Fractionation is a tedious and time-consuming procedure that requires voluminous extraction of natural materials116,287-289 to yield miniscule quantities of pure extracts,23,97,163,290,291 which may still need to be further characterized.
RP-HPLC coupled simultaneously to ICP–MS (element-specific detection) and HR-MS (molecular structure detection) has successfully been applied in the identification of arsenolipids in marine samples.102,105,183,184,189,218,223,284 AsSugars have been extracted from various algae sources, followed by several purification procedures including SEC and AE chromatography.163,287-289,292 Characterization and structural determination were performed mainly using one- (or two-)89,287 dimensional 1H and 13C nuclear magnetic resonance (NMR).85,288,289,292,293 Additional information for structure determination was obtained using X-ray crystallography,288,294 XANES or infrared spectroscopy,292 and mass spectrometry.15 All of these approaches have been instrumental in gaining a better understanding of the nature and proportions of organoarsenicals present in marine diets. However, the need for well-characterized standards still endures, especially as a prerequisite for establishment and implementation of regulations. The more practical approach is to synthesize analyte standards, which has successfully been performed for several arsenicals mostly for the purpose of confirmation of their identities.159,219,290
There are almost a hundred organoarsenicals identified in marine dietary sources, and it is practically impossible to attempt to develop standards for all of the currently known arsenical analytes. There is need for prioritization in the development of standards using a risk-based approach, especially for organoarsenicals with confirmed toxicities, like AsHC 332, AsHC 360, and AsHC 44429,31,295,296 and AsFA 362 and AsFA 388.30,297 These standards should be synthesized for quantification of organoarsenicals and to support the precise assessment of their toxicity mechanisms and fate in living organisms.
Synthesis of AsSugar-OH, AsSugar-SO4, and AsSugar-SO3 and their corresponding thio-analogues has been documented in the literature.290,298-303 The synthetic procedure for AsSugar-OH containing the trimethylated arsonium moiety is lengthy and challenging, involving 6–10 reaction steps.290,299,301,302 Thio-AsSugars and trimethylated291 AsSugars can be quantitatively semi-synthesized using synthetic materials or extracted and purified from naturally occurring oxo-analogues as preparatory materials. The synthesis of AsLipids is complex, and a number of studies have been published for the preparation of a limited number of AsHCs and AsFAs.29,30,159,189,219,221,291,294,304,305
Because arsenic in monoisotopic, the heteroatoms of the synthesized organoarsenicals can be labeled, using, for example, 13C, 2H, etc., to facilitate confirmation of their identity in seafood and for further studies on the biotransformation processes under physiological conditions. They can also be used as an internal standard in combination with a reliable and robust analytical method for exact quantification of selected arsenicals and, thus, play an important role in the establishment of regulatory limits for the toxic organoarsenicals. With reliable analytical methods and availability of standards, development of CRMs will then become a reality.
The presence of arsenolipids has been reported in fish oils,102,105,184,189,218,223 fish tissue,183,222,306 fish meal,101 and commercial canned fish liver.183,223 Arsenosugar phospholipids have been detected in macroalgae,221,307 while arsenosugars have been detected mainly in oyster,284 clam,226 and edible macroalgae.22,23,163,308 These materials provide a good starting point for the development of CRMs. At the time of this review, only six organoarsenicals were commercially available, with less than 10 represented in reference materials. There are at least six seafood matrix reference materials currently available that give values for at least one organoarsenic species. Five of the six are only certified for AsB (NMIJ CRM 7402-a, NMIJ 7403-a, DORM-4, TORT-3, and NIES-15), and BCR-627 is only certified for AsB and DMA. NIST SRM 2669 and NIST SRM 3669 are urine reference materials certified for MMA, DMA, TMAO, AsB, and AsC and for MMA, DMA, and AsB, respectively. Newly released NIST SRM 3232 for kelp provides values for DMA as well as three AsSugars: AsSugar-OH, AsSugar-PO4, and AsSugar-SO3.209
Footnotes
The authors declare no competing financial interest.
Contributor Information
Caleb Luvonga, Analytical Chemistry Division, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland 20899, United States; Department of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, United States.
Catherine A. Rimmer, Analytical Chemistry Division, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland 20899, United States
Lee L. Yu, Analytical Chemistry Division, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland 20899, United States
Sang Bok Lee, Department of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, United States.
REFERENCES
- (1).Baeyens W; Gao Y; de Galan S; Bilau M; van Larebeke N; Leermakers M Dietary Exposure to Total and Toxic Arsenic in Belgium: Importance of Arsenic Speciation in North Sea Fish. Mol. Nutr. Food Res 2009, 53, 558–565. [DOI] [PubMed] [Google Scholar]
- (2).Feldmann J; Krupp EM Critical Review or Scientific Opinion Paper: Arsenosugars-a Class of Benign Arsenic Species or Justification for Developing Partly Speciated Arsenic Fractionation in Foodstuffs? Anal. Bioanal. Chem 2011, 399, 1735–1741. [DOI] [PubMed] [Google Scholar]
- (3).Nachman KE; Ginsberg GL; Miller MD; Murray CJ; Nigra AE; Pendergrast CB Mitigating Dietary Arsenic Exposure: Current Status in the United States and Recommendations for an Improved Path Forward. Sci. Total Environ 2017, 581–582, 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Petursdottir AH; Sloth JJ; Feldmann J Introduction of Regulations for Arsenic in Feed and Food with Emphasis on Inorganic Arsenic, and Implications for Analytical Chemistry. Anal. Bioanal. Chem 2015, 407, 8385–8396. [DOI] [PubMed] [Google Scholar]
- (5).Francesconi KA Toxic Metal Species and Food Regulations—Making a Healthy Choice. Analyst 2007, 132, 17–20. [DOI] [PubMed] [Google Scholar]
- (6).Taylor V; Goodale B; Raab A; Schwerdtle T; Reimer K; Conklin S; Karagas MR; Francesconi KA Human Exposure to Organic Arsenic Species from Seafood. Sci. Total Environ 2017, 580, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tseng WP Prevalence of Skin Cancer in an Endemic Area of Chronic Arsenicism in Taiwan. J. Natl. Cancer Inst 1968, 40, 453–463. [PubMed] [Google Scholar]
- (8).Tseng WP Effects and Dose Response Relationships of Skin Cancer and Blackfoot Disease with Arsenic. Environ. Health Perspect. 1977, 19, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Borak J; Hosgood HD Seafood Arsenic: Implications for Human Risk Assessment. Regul. Toxicol. Pharmacol 2007, 47, 204–212. [DOI] [PubMed] [Google Scholar]
- (10).Edmonds JS; Francesconi KA Arsenic in Seafoods: Human Health Aspects and Regulations. Mar. Pollut. Bull 1993, 26, 665–674. [Google Scholar]
- (11).Wells ML; Potin P; Craigie JS; Raven JA; Merchant SS; Helliwell KE; Smith AG; Camire ME; Brawley SH Algae as Nutritional and Functional Food Sources : Revisiting Our Understanding. J. Appl. Phycol 2017, 29, 949–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Navas-acien A; Francesconi KA; Silbergeld EK; Guallar E Seafood Intake and Urine Concentrations of Total Arsenic, Dimethylarsinate and Arsenobetaine in the US Population. Environ. Res. 2011, 111, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Maher WA; Ellwood MJ; Krikowa F; Raber G; Foster S Measurement of Arsenic Species in Environmental, Biological Fluids and Food Samples by HPLC-ICPMS and HPLC-HG-AFS. J. Anal. At. Spectrom 2015, 30, 2129–2183. [Google Scholar]
- (14).Alexander J; Benford DJ; Boobis A; Ceccatelli S; Cravedi J-P; Di Domenico A; Doerge D; Dogliotti E; Edler L; Farmer P; Filipič M; Fink-Gremmels J; Fürst P; Guerin T; Knutsen HK; Machala M; Mutti A; Schlatter J; van Leeuwen R; Verger P Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar]
- (15).Niegel C; Matysik FM Analytical Methods for the Determination of Arsenosugars-A Review of Recent Trends and Developments. Anal. Chim. Acta 2010, 657, 83–99. [DOI] [PubMed] [Google Scholar]
- (16).Soeroes C; Goessler W; Francesconi KA; Kienzl N; Schaeffer R; Fodor P; Kuehnelt D Arsenic Speciation in Farmed Hungarian Freshwater Fish. J. Agric. Food Chem 2005, 53, 9238–9243. [DOI] [PubMed] [Google Scholar]
- (17).Schaeffer R; Francesconi KA; Kienzl N; Soeroes C; Fodor P; Váradi L; Raml R; Goessler W; Kuehnelt D Arsenic Speciation in Freshwater Organisms from the River Danube in Hungary. Talanta 2006, 69, 856–865. [DOI] [PubMed] [Google Scholar]
- (18).Miyashita S; Shimoya M; Kamidate Y; Kuroiwa T; Shikino O; Fujiwara S; Francesconi KA; Kaise T Rapid Determination of Arsenic Species in Freshwater Organisms from the Arsenic-Rich Hayakawa River in Japan Using HPLC-ICP-MS. Chemosphere 2009, 75, 1065–1073. [DOI] [PubMed] [Google Scholar]
- (19).Khan M; Francesconi KA Preliminary Studies on the Stability of Arsenolipids: Implications for Sample Handling and Analysis. J. Environ. Sci. (Beijing, China) 2016, 49, 97–103. [DOI] [PubMed] [Google Scholar]
- (20).Francesconi KA Arsenic Species in Seafood: Origin and Human Health Implications. Pure Appl. Chem. 2010, 82, 373–381. [Google Scholar]
- (21).Wolle MM; Conklin SD Speciation Analysis of Arsenic in Seafood and Seaweed : Part I—Evaluation and Optimization of Methods. Anal. Bioanal. Chem 2018, 410, 5675–5687. [DOI] [PubMed] [Google Scholar]
- (22).Pergantis SA; Francesconi KA; Goessler W; Thomas-Oates JE Characterization of Arsenosugars of Biological Origin Using Fast Atom Bombardment Tandem Mass Spectrometry. Anal. Chem 1997, 69, 4931–4937. [DOI] [PubMed] [Google Scholar]
- (23).Pergantis SA; Wangkarn S; Francesconi KA; Thomas-Oates JE Identification of Arsenosugars at the Picogram Level Using Nanoelectrospray Quadrupole Time-of-Flight Mass Spectrometry. Anal. Chem 2000, 72, 357–366. [DOI] [PubMed] [Google Scholar]
- (24).Shibata Y; Jin K; Morita M Arsenic Compounds in the Edible Red Alga, Porphyra tenera, and in Nori and Yakinori, Food Items Produced from Red Algae. Appl. Organomet. Chem 1990, 4, 255–260. [Google Scholar]
- (25).Cohen SM; Arnold LL; Eldan M; Lewis AS; Beck BD Methylated Arsenicals: The Implications of Metabolism and Carcinogenicity Studies in Rodents to Human Risk Assessment. Crit. Rev. Toxicol. 2006, 36, 99–133. [DOI] [PubMed] [Google Scholar]
- (26).Wei M; Wanibuchi H; Morimura K; Iwai S; Yoshida K; Endo G; Nakae D; Fukushima S Carcinogenicity of Dimethylarsinic Acid in Male F344 Rats and Genetic Alterations in Induced Urinary. Carcinogenesis 2002, 23, 1387–1397. [DOI] [PubMed] [Google Scholar]
- (27).Le XC; Ma M; Wong NA Speciation of Arsenic Compounds Using High-Performance Liquid Chromatography at Elevated Temperature and Selective Hydride Generation Atomic Fluorescence Detection. Anal. Chem 1996, 68, 4501–4506. [Google Scholar]
- (28).Le XC; Cullen WR; Reimer KJ Human Urinary Arsenic Excretion after One-Time Ingestion of Seaweed, Crab and Shrimp. Clin. Chem 1994, 40, 617–624. [PubMed] [Google Scholar]
- (29).Meyer S; Matissek M; Müller SM; Taleshi MS; Ebert F; Francesconi KA; Schwerdtle T In Vitro Toxicological Characterisation of Three Arsenic-Containing Hydrocarbons. Metallomics 2014, 6, 1023–1033. [DOI] [PubMed] [Google Scholar]
- (30).Meyer S; Raber G; Ebert F; Leffers L; Müller SM; Taleshi MS; Francesconi KA; Schwerdtle T In Vitro Toxicological Characterisation of Arsenic-Containing Fatty Acids and Three of Their Metabolites. Toxicol. Res. (Cambridge, U. K.) 2015, 4, 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Meyer S; Schulz J; Jeibmann A; Taleshi MS; Ebert F; Francesconi KA; Schwerdtle T Arsenic-Containing Hydrocarbons Are Toxic in the in Vivo Model Drosophila Melanogaster. Metallomics 2014, 6, 2010–2014. [DOI] [PubMed] [Google Scholar]
- (32).Krupp E; Feldmann J; Pétursdóttir ÁH; Küpper FC; Gunnlaugsdóttir H; Fletcher K Environmental Effects on Arsenosugars and Arsenolipids in Ectocarpus (Phaeophyta). Environ. Chem 2016, 13, 21. [Google Scholar]
- (33).Wolle MM; Conklin SD Speciation Analysis of Arsenic in Seafood and Seaweed: Part II—Single Laboratory Validation of Method. Anal. Bioanal. Chem. 2018, 410, 5689–5702. [DOI] [PubMed] [Google Scholar]
- (34).Sun Y; Liu G; Cai Y Thiolated Arsenicals in Arsenic Metabolism: Occurrence, Formation, and Biological Implications. J. Environ. Sci. (Beijing, China) 2016, 49, 59–73. [DOI] [PubMed] [Google Scholar]
- (35).Maher WA; Foster S; Krikowa F; Duncan E; St John A; Hug K; Moreau JW Thio Arsenic Species Measurements in Marine Organisms and Geothermal Waters. Microchem. J 2013, 111, 82–90. [Google Scholar]
- (36).García-Salgado S; Quijano MÁ Stability of Toxic Arsenic Species and Arsenosugars Found in the Dry Alga Hijiki and Its Water Extracts. Talanta 2014, 128, 83–91. [DOI] [PubMed] [Google Scholar]
- (37).Gómez-Ariza JL; Sánchez-Rodas D; Giráldez I; Morales E A Comparison between ICP-MS and AFS Detection for Arsenic Speciation in Environmental Samples. Talanta 2000, 51, 257–268. [PubMed] [Google Scholar]
- (38).Šlejkovec Z; Kápolna E; Ipolyi I; van Elteren JT Arsenosugars and Other Arsenic Compounds in Littoral Zone Algae from the Adriatic Sea. Chemosphere 2006, 63, 1098–1105. [DOI] [PubMed] [Google Scholar]
- (39).Maher WA; Foster SD; Taylor AM; Krikowa F; Duncan EG; Chariton AA Arsenic Distribution and Species in Two Zostera Capricorni Seagrass Ecosystems, New South Wales, Australia. Environ. Chem 2011, 8, 9–18. [Google Scholar]
- (40).Thomson D; Maher W; Foster S Arsenic and Selected Elements in Marine Angiosperms, South-East Coast, NSW, Australia. Appl. Organomet. Chem 2007, 21, 381–395. [Google Scholar]
- (41).McSheehy S; Szpunar J; Morabito R; Quevauviller P The Speciation of Arsenic in Biological Tissues and the Certification of Reference Materials for Quality Control. TrAC, Trends Anal. Chem 2003, 22, 191–209. [Google Scholar]
- (42).Lewis J; Stokes P; Brereton N; Baxter M; Macarthur R Stability of Arsenic Speciation in Fish Muscle Samples, under Different Storage and Sample Preparation Conditions. Microchem. J 2012, 105, 56–59. [Google Scholar]
- (43).Dahl L; Molin M; Amlund H; Meltzer HM; Julshamn K; Alexander J; Sloth JJ Stability of Arsenic Compounds in Seafood Samples during Processing and Storage by Freezing. Food Chem. 2010, 123, 720–727. [Google Scholar]
- (44).García Salgado S; Quijano Nieto MA; Bonilla Simón MM Assessment of Total Arsenic and Arsenic Species Stability in Alga Samples and Their Aqueous Extracts. Talanta 2008, 75, 897–903. [DOI] [PubMed] [Google Scholar]
- (45).Whitfield FB; Freeman DJ; Shaw KJ Trimethylarsine: An Important off-Flavour Component in Some Prawn Species. Chem. Ind 1983, 20, 786–787. [Google Scholar]
- (46).Narukawa T; Chiba K Heat-Assisted Aqueous Extraction of Rice Flour for Arsenic Speciation Analysis. J. Agric. Food Chem. 2010, 58, 8183–8188. [DOI] [PubMed] [Google Scholar]
- (47).Alava P; Van De Wiele T; Tack F; Du Laing G Extensive Grinding and Pressurized Extraction with Water Are Key Points for Effective and Species Preserving Extraction of Arsenic from Rice. Anal. Methods 2012, 4, 1237–1243. [Google Scholar]
- (48).van Elteren JT; Šlejkovec Z; Kahn M; Goessler W A Systematic Study on the Extractability of Arsenic Species from Algal Certified Reference Material IAEA-140/TM (Fucus Sp., Sea Plant Homogenate) Using Methanol/Water Extractant Mixtures. Anal. Chim. Acta 2007, 585, 24–31. [DOI] [PubMed] [Google Scholar]
- (49).Majors RE Sample Preparation Perspectives. LCGC North Am. 1991, 9, 16–20. [Google Scholar]
- (50).Van Hulle M; Zhang C; Zhang X; Cornelis R Arsenic Speciation in Chinese Seaweeds Using HPLC-ICP-MS and HPLC-ES-MS. Analyst 2002, 127, 634–640. [DOI] [PubMed] [Google Scholar]
- (51).Kohlmeyer U; Jantzen E; Kuballa J; Jakubik S Benefits of High Resolution IC-ICP-MS for the Routine Analysis of Inorganic and Organic Arsenic Species in Food Products of Marine and Terrestrial Origin. Anal. Bioanal. Chem 2003, 377, 6–13. [DOI] [PubMed] [Google Scholar]
- (52).Beauchemin D; Bednas ME; Berman SS; McLaren JW; Siu KWM; Sturgeon RE Exchange of Comments on Identification and Quantitation of Arsenic Species in a Dogfish Muscle Reference Material for Trace Elements. Reply to Comments. Anal. Chem 1988, 60, 2209–2212. [DOI] [PubMed] [Google Scholar]
- (53).Kuehnelt D; Irgolic KJ; Goessler W Comparison of Three Methods for the Extraction of Arsenic Compounds from the NRCC Standard Reference Material DORM-2 and the Brown Alga Hijiki Fuziforme. Appl. Organomet. Chem 2001, 15, 445–456. [Google Scholar]
- (54).Goessler W; Kuehnelt D; Schlagenhaufen C; Šlejkovec Z; Irgolic KJ Arsenobetaine and Other Arsenic Compounds in the National Research Council of Canada Certified Reference Materials DORM 1 and DORM 2. J. Anal. At. Spectrom 1998, 13, 183–187. [Google Scholar]
- (55).Villa-Lojo MC; Alonso-Rodríguez E; López-Mahía P; Muniategui-Lorenzo S; Prada-Rodríguez D Coupled High Performance Liquid Chromatography-Microwave Digestion-Hydride Generation-Atomic Absorption Spectrometry for Inorganic and Organic Arsenic Speciation in Fish Tissue. Talanta 2002, 57, 741–750. [DOI] [PubMed] [Google Scholar]
- (56).Thomas DJ; Styblo M; Lin S The Cellular Metabolism and Systemic Toxicity of Arsenic. Toxicol. Appl. Pharmacol. 2001, 176, 127–144. [DOI] [PubMed] [Google Scholar]
- (57).Ackley KL; B’Hymer C; Sutton KL; Caruso JA Speciation of Arsenic in Fish Tissue Using Microwave-Assisted Extraction Followed by HPLC-ICP-MS. J. Anal. At. Spectrom 1999, 14, 845–850. [Google Scholar]
- (58).Akter K; Naidu R Arsenic Speciation in the Environment. In Managing Arsenic in the Environment: From Soil to Human Health; Naidu R, Smith E, Owens G, Bhattacharya P, Nadebaum P, Eds.; CSIRO Publishing: Collingwood, Victoria, Australia, 2006; Part B: Analytical Tools for Assessing the Dynamics of Arsenic in Environmental Samples, Chapter 3, pp 61–74. [Google Scholar]
- (59).Moreda-Piñeiro A; Peña-Vázquez E; Hermelo-Herbello P; Bermejo-Barrera P; Moreda-Piñeiro J; Alonso-Rodríguez E; Muniategui-Lorenzo S; López-Mahía P; Prada-Rodríguez D Matrix Solid-Phase Dispersion as a Sample Pretreatment for the Speciation of Arsenic in Seafood Products. Anal. Chem 2008, 80, 9272–9278. [DOI] [PubMed] [Google Scholar]
- (60).Soeroes C; Goessler W; Francesconi KA; Schmeisser E; Raml R; Kienzl N; Kahn M; Fodor P; Kuehnelt D Thio Arsenosugars in Freshwater Mussels from the Danube in Hungary. J. Environ. Monit 2005, 7, 688–692. [DOI] [PubMed] [Google Scholar]
- (61).Moreda-Piñeiro J; Alonso-Rodríguez E; Moreda-Piñeiro A; Moscoso-Pérez C; Muniategui-Lorenzo S; López-Mahía P; Prada-Rodríguez D; Bermejo-Barrera P Simultaneous Pressurized Enzymatic Hydrolysis Extraction and Clean up for Arsenic Speciation in Seafood Samples before High Performance Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometry Determination. Anal. Chim. Acta 2010, 679, 63–73. [DOI] [PubMed] [Google Scholar]
- (62).Bermejo P; Capelo JL; Mota A; Madrid Y; Cámara C Enzymatic Digestion and Ultrasonication: A Powerful Combination in Analytical Chemistry. TrAC, Trends Anal. Chem 2004, 23, 654–663. [Google Scholar]
- (63).Sakai T Separation and Analysis of Toxic Arsenic Species, Using LC-ICP-MS; Agilent Technologies: Santa Clara, CA, 2000; Agilent Technologies; Application Note 5980–0262E. [Google Scholar]
- (64).Sarzanini C Liquid Chromatography: A Tool for the Analysis of Metal Species. J. Chromatogr. A 1999, 850, 213–228. [DOI] [PubMed] [Google Scholar]
- (65).Sarzanini C; Bruzzoniti MC Metal Species Determination by Ion Chromatography. TrAC, Trends Anal. Chem 2001, 20, 304–310. [Google Scholar]
- (66).Montes-Bayón M; DeNicola K; Caruso JA Liquid Chromatography–Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2003, 1000, 457–476. [DOI] [PubMed] [Google Scholar]
- (67).Santos CMM; Nunes MAG; Barbosa IS; Santos GL; Peso-Aguiar MC; Korn MGA; Flores EMM; Dressler VL Evaluation of Microwave and Ultrasound Extraction Procedures for Arsenic Speciation in Bivalve Mollusks by Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometry. Spectrochim. Acta, Part B 2013, 86, 108–114. [Google Scholar]
- (68).Simon S; Lobos G; Pannier F; De Gregori I; Pinochet H; Potin-Gautier M Speciation Analysis of Organoarsenical Compounds in Biological Matrices by Coupling Ion Chromatography to Atomic Fluorescence Spectrometry with On-Line Photooxidation and Hydride Generation. Anal. Chim. Acta 2004, 521, 99–108. [Google Scholar]
- (69).Braeuer S; Borovička J; Goessler W A Unique Arsenic Speciation Profile in Elaphomyces Spp. (“Deer Truffles”)—Trimethylarsine Oxide and Methylarsonous Acid as Significant Arsenic Compounds. Anal. Bioanal. Chem 2018, 410, 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Braeuer S; Goessler W Arsenic Species in Mushrooms, with a Focus on Analytical Methods for Their Determination—A Critical Review. Anal. Chim. Acta 2019, 1073, 1–21. [DOI] [PubMed] [Google Scholar]
- (71).Gao Y; Baisch P; Mirlean N; Rodrigues da Silva Júnior FM; Van Larebeke N; Baeyens W; Leermakers M Arsenic Speciation in Fish and Shellfish from the North Sea (Southern Bight) and Açu Port Area (Brazil) and Health Risks Related to Seafood Consumption. Chemosphere 2018, 191, 89–96. [DOI] [PubMed] [Google Scholar]
- (72).Geng W; Komine R; Ohta T; Nakajima T; Takanashi H; Ohki A Arsenic Speciation in Marine Product Samples: Comparison of Extraction–HPLC Method and Digestion–Cryogenic Trap Method. Talanta 2009, 79, 369–375. [DOI] [PubMed] [Google Scholar]
- (73).Dietz C; Sanz J; Sanz E; Muñoz-Olivas R; Cámara C Current Perspectives in Analyte Extraction Strategies for Tin and Arsenic Speciation. J. Chromatogr. A 2007, 1153, 114–129. [DOI] [PubMed] [Google Scholar]
- (74).Bayona JM Supercritical Fluid Extraction in Speciation Studies. TrAC, Trends Anal. Chem 2000, 19, 107–112. [Google Scholar]
- (75).Wang ZF; Cui ZJ Supercritical Fluid Extraction and Gas Chromatography Analysis of Arsenic Species from Solid Matrices. Chin. Chem. Lett 2016, 27, 241–246. [Google Scholar]
- (76).Gallagher PA; Shoemaker JA; Wei X; Brockhoff-Schwegel CA; Creed JT Extraction and Detection of Arsenicals in Seaweed via Accelerated Solvent Extraction with Ion Chromatographic Separation and ICP-MS Detection. Fresenius' J. Anal. Chem 2001, 369, 71–80. [DOI] [PubMed] [Google Scholar]
- (77).Wahlen R Fast and Accurate Determination of Arsenobetaine in Fish Tissues Using Accelerated Solvent Extraction and HPLC-ICP-MS Determination. J. Chromatogr. Sci 2004, 42, 217–222. [DOI] [PubMed] [Google Scholar]
- (78).Carabias-Martínez R; Rodríguez-Gonzalo E; Revilla-Ruiz P; Hernández-Méndez J Pressurized Liquid Extraction in the Analysis of Food and Biological Samples. J. Chromatogr. A 2005, 1089, 1–17. [DOI] [PubMed] [Google Scholar]
- (79).Leufroy A; Noël L; Dufailly V; Beauchemin D; Guérin T Determination of Seven Arsenic Species in Seafood by Ion Exchange Chromatography Coupled to Inductively Coupled Plasma-Mass Spectrometry Following Microwave Assisted Extraction: Method Validation and Occurrence Data. Talanta 2011, 83, 770–779. [DOI] [PubMed] [Google Scholar]
- (80).Tukai R; Maher WA; McNaught IJ; Ellwood MJ Measurement of Arsenic Species in Marine Macroalgae by Microwave-Assisted Extraction and High Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2002, 457, 173–185. [Google Scholar]
- (81).Kirby J; Maher W; Ellwood M; Krikowa F Arsenic Species Determination in Biological Tissues by HPLC-ICP-MS and HPLC-HG-ICP-MS. Aust. J. Chem 2004, 57, 957–966. [Google Scholar]
- (82).Han C; Cao X; Yu JJ; Wang XR; Shen Y Arsenic Speciation in Sargassum Fusiforme by Microwave-Assisted Extraction and LC-ICP-MS. Chromatographia 2009, 69, 587–591. [Google Scholar]
- (83).Wu J; Mester Z; Pawliszyn J Speciation of Organoarsenic Compounds by Polypyrrole-Coated Capillary in-Tube Solid Phase Microextraction Coupled with Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Anal. Chim. Acta 2000, 424, 211–222. [Google Scholar]
- (84).Luque-García JL; Luque De Castro MD Ultrasound: A Powerful Tool for Leaching. TrAC, Trends Anal. Chem 2003, 22, 41–47. [Google Scholar]
- (85).Šlejkovec Z; Bajc Z; Doganoc DZ Arsenic Speciation Patterns in Freshwater Fish. Talanta 2004, 62, 931–936. [DOI] [PubMed] [Google Scholar]
- (86).Schaeffer R; Soeroes C; Ipolyi I; Fodor P; Thomaidis NS Determination of Arsenic Species in Seafood Samples from the Aegean Sea by Liquid Chromatography-(Photo-Oxidation)-Hydride Generation-Atomic Fluorescence Spectrometry. Anal. Chim. Acta 2005, 547, 109–118. [Google Scholar]
- (87).Ochsenkühn-Petropulu M; Varsamis J; Parissakis G Speciation of Arsenobetaine in Marine Organisms Using a Selective Leaching/Digestion Procedure and Hydride Generation Atomic Absorption Spectrometry. Anal. Chim. Acta 1997, 337, 323–327. [Google Scholar]
- (88).Pizarro I; Gómez M; Cámara C; Palacios MA Arsenic Speciation in Environmental and Biological Samples: Extraction and Stability Studies. Anal. Chim. Acta 2003, 495, 85–98. [Google Scholar]
- (89).Amran MB; Lagarde F; Leroy MJF Determination of Arsenic Species in Marine Organisms by HPLC-ICP-OES and HPLC-HG-QFAAS. Microchim. Acta 1997, 127, 195–202. [Google Scholar]
- (90).Nischwitz V; Pergantis SA Improved Arsenic Speciation Analysis for Extracts of Commercially Available Edible Marine Algae Using HPLC-ES-MS/MS. J. Agric. Food Chem 2006, 54, 6507–6519. [DOI] [PubMed] [Google Scholar]
- (91).Pedersen SN; Francesconi KA Liquid Chromatography Electrospray Mass Spectrometry with Variable Fragmentor Voltages Gives Simultaneous Elemental and Molecular Detection of Arsenic Compounds. Rapid Commun. Mass Spectrom 2000, 14, 641–645. [DOI] [PubMed] [Google Scholar]
- (92).Francesconi KA; Edmonds JS A Novel Arsenical in Clam Kidney Identified by Liquid Chromatography/Electrospray Ionisation Mass Spectrometry. Rapid Commun. Mass Spectrom 2001, 15, 1641–1646. [DOI] [PubMed] [Google Scholar]
- (93).Branch S; Ebdon L; O’Neill P Determination of Arsenic Species in Fish by Directly Coupled High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom 1994, 9, 33–37. [Google Scholar]
- (94).Gamble BM; Gallagher PA; Shoemaker JA; Wei X; Schwegel CA; Creed JT An Investigation of the Chemical Stability of Arsenosugars in Simulated Gastric Juice and Acidic Environments Using IC-ICP-MS and IC-ESI-MS/MS. Analyst 2002, 127, 781–785. [DOI] [PubMed] [Google Scholar]
- (95).Mandal BK; Ogra Y; Anzai K; Suzuki KT Speciation of Arsenic in Biological Samples. Toxicol. Appl. Pharmacol 2004, 198, 307–318. [DOI] [PubMed] [Google Scholar]
- (96).Lai VW-M; Cullen WR; Harrington CF; Reimer KJ The Characterization of Arsenosugars in Commercially Available Algal Products Including A. Appl. Organomet. Chem 1997, 11, 797–803. [Google Scholar]
- (97).McSheehy S; Marcinek M; Chassaigne H; Szpunar J Identification of Dimethylarsinoyl-Riboside Derivatives in Seaweed by Pneumatically Assisted Electrospray Tandem Mass Spectrometry. Anal. Chim. Acta 2000, 410, 71–84. [Google Scholar]
- (98).Wangkarn S; Pergantis SA High-Speed Separation of Arsenic Compounds Using Narrow-Bore High-Performance Liquid Chromatography on-Line with Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom 2000, 15, 627–633. [Google Scholar]
- (99).Pickford R; Miguens-Rodriguez M; Afzaal S; Speir P; Pergantis SA; Thomas-Oates JE Application of the High Mass Accuracy Capabilities of FT-ICR-MS and Q-ToF-MS to the Characterisation of Arsenic Compounds in Complex Biological Matrices. J. Anal. At. Spectrom 2002, 17, 173–176. [Google Scholar]
- (100).Gallagher PA; Wei X; Shoemaker JA; Brockhoff CA; Creed JT Detection of Arsenosugars from Kelp Extracts via IC-Electrospray Ionization-MS-MS and IC Membrane Hydride Generation ICP-MS. J. Anal. At. Spectrom 1999, 14, 1829–1834. [Google Scholar]
- (101).Amayo KO; Petursdottir A; Newcombe C; Gunnlaugsdottir H; Raab A; Krupp EM; Feldmann J Identification and Quantification of Arsenolipids Using Reversed-Phase HPLC Coupled Simultaneously to High-Resolution ICPMS and High-Resolution Electrospray MS without Species-Specific Standards. Anal. Chem 2011, 83, 3589–3595. [DOI] [PubMed] [Google Scholar]
- (102).Rumpler A; Edmonds JS; Katsu M; Jensen KB; Goessler W; Raber G; Gunnlaugsdottir H; Francesconi KA Arsenic-Containing Long-Chain Fatty Acids in Cod-Liver Oil: A Result of Biosynthetic Infidelity? Angew. Chem 2008, 120, 2705–2707. [DOI] [PubMed] [Google Scholar]
- (103).Amayo KO; Raab A; Krupp EM; Feldmann J Identification of Arsenolipids and Their Degradation Products in Cod-Liver Oil. Talanta 2014, 118, 217–223. [DOI] [PubMed] [Google Scholar]
- (104).Amayo KO; Raab A; Krupp EM; Marschall T; Horsfall M; Feldmann J Arsenolipids Show Different Profiles in Muscle Tissues of Four Commercial Fish Species. J. Trace Elem. Med. Biol 2014, 28, 131–137. [DOI] [PubMed] [Google Scholar]
- (105).Amayo KO; Raab A; Krupp EM; Gunnlaugsdottir H; Feldmann J Novel Identification of Arsenolipids Using Chemical Derivatizations in Conjunction with RP-HPLC-ICPMS/ESMS. Anal. Chem 2013, 85, 9321–9327. [DOI] [PubMed] [Google Scholar]
- (106).Reis VAT; Duarte AC Analytical Methodologies for Arsenic Speciation in Macroalgae: A Critical Review. TrAC, Trends Anal. Chem 2018, 102, 170–184. [Google Scholar]
- (107).Alberti J; Rubio R; Rauret G Extraction Method for Arsenic Speciation in Marine Organisms. Fresenius' J. Anal. Chem 1995, 351, 420–425. [Google Scholar]
- (108).Glabonjat RA; Raber G; Jensen KB; Ehgartner J; Francesconi KA Quantification of Arsenolipids in the Certified Reference Material NMIJ. 7405-a (Hijiki) Using HPLC/Mass Spectrometry after Chemical Derivatization. Anal. Chem 2014, 86, 10282–10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Sele V; Sloth JJ; Julshamn K; Skov K; Amlund H A Study of Lipid- and Water-Soluble Arsenic Species in Liver of Northeast Arctic Cod (Gadus Morhua) Containing High Levels of Total Arsenic. J. Trace Elem. Med. Biol 2015, 30, 171–179. [DOI] [PubMed] [Google Scholar]
- (110).Hanaoka K; Yosida K; Tamano M; Kuroiwa T; Kaise T; Maeda S Arsenic in the Prepared Edible Brown Alga Hijiki, Hizikia Fusiforme. Appl. Organomet. Chem 2001, 15, 561–565. [Google Scholar]
- (111).Taleshi MS; Edmonds JS; Goessler W; Ruiz-Chancho MJ; Raber G; Jensen KB; Francesconi KA Arsenic-Containing Lipids Are Natural Constituents of Sashimi Tuna. Environ. Sci. Technol 2010, 44, 1478–1483. [DOI] [PubMed] [Google Scholar]
- (112).García-Salgado S; Quijano MA; Bonilla MM Arsenic Speciation in Edible Alga Samples by Microwave-Assisted Extraction and High Performance Liquid Chromatography Coupled to Atomic Fluorescence Spectrometry. Anal. Chim. Acta 2012, 714, 38–46. [DOI] [PubMed] [Google Scholar]
- (113).Devalla S; Feldmann J Determination of Lipid-Soluble Arsenic Species in Seaweed-Eating Sheep from Orkney. Appl. Organomet. Chem 2003, 17, 906–912. [Google Scholar]
- (114).Francesconi KA; Kuehnelt D Determination of Arsenic Species: A Critical Review of Methods and Applications. Analyst 2004, 129, 373–395. [DOI] [PubMed] [Google Scholar]
- (115).Cao X; Hao C; Wang G; Yang H; Chen D; Wang X Sequential Extraction Combined with HPLC-ICP-MS for As Speciation in Dry Seafood Products. Food Chem. 2009, 113, 720–726. [Google Scholar]
- (116).Madsen AD; Goessler W; Pedersen SN; Francesconi KA Characterization of an Algal Extract by HPLC-ICP-MS and LC-Electrospray MS for Use in Arsenosugar Speciation Studies. J. Anal. At. Spectrom 2000, 15, 657–662. [Google Scholar]
- (117).Kahn M; Raml R; Schmeisser E; Vallant B; Francesconi KA; Goessler W Two Novel Thio-Arsenosugars in Scallops Identified with HPLC-ICPMS and HPLC-ESMS. Environ. Chem 2005, 2, 171–176. [Google Scholar]
- (118).Ciardullo S; Aureli F; Raggi A; Cubadda F Arsenic Speciation in Freshwater Fish: Focus on Extraction and Mass Balance. Talanta 2010, 81, 213–221. [DOI] [PubMed] [Google Scholar]
- (119).Ruttens A; Blanpain AC; De Temmerman L; Waegeneers N Arsenic Speciation in Food in Belgium. Part 1: Fish, Molluscs and Crustaceans. J. Geochem. Explor 2012, 121, 55–61. [Google Scholar]
- (120).Foster S; Maher W; Krikowa F; Apte S A Microwave-Assisted Sequential Extraction of Water and Dilute Acid Soluble Arsenic Species from Marine Plant and Animal Tissues. Talanta 2007, 71, 537–549. [DOI] [PubMed] [Google Scholar]
- (121).Sadee BA; Foulkes ME; Hill SJ An Evaluation of Extraction Techniques for Arsenic in Staple Diets (Fish and Rice) Utilising Both Classical and Enzymatic Extraction Methods. Food Addit. Contam., Part A 2016, 33, 433–441. [DOI] [PubMed] [Google Scholar]
- (122).Gamble BM; Gallagher PA; Shoemaker JA; Parks AN; Freeman DM; Schwegel CA; Creed JT An Investigation of the Chemical Stability of Arsenosugars in Basic Environments Using IC-ICP-MS and IC-ESI-MS/MS. Analyst 2003, 128, 1458–1461. [DOI] [PubMed] [Google Scholar]
- (123).Moreda-Piñeiro A; Moreda-Piñeiro J; Herbello-Hermelo P; Bermejo-Barrera P; Muniategui-Lorenzo S; López-Mahía P; Prada-Rodríguez D Application of Fast Ultrasound Water-Bath Assisted Enzymatic Hydrolysis—High Performance Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometry Procedures for Arsenic Speciation in Seafood Materials. J. Chromatogr. A 2011, 1218, 6970–6980. [DOI] [PubMed] [Google Scholar]
- (124).Zou H; Zhou C; Li Y; Yang X; Wen J; Hu X; Sun C Occurrence, Toxicity, and Speciation Analysis of Arsenic in Edible Mushrooms. Food Chem. 2019, 281, 269–284. [DOI] [PubMed] [Google Scholar]
- (125).Pardo-Martínez M; Viñas P; Fisher A; Hill SJ Comparison of Enzymatic Extraction Procedures for Use with Directly Coupled High Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry for the Speciation of Arsenic in Baby Foods. Anal. Chim. Acta 2001, 441, 29–36. [Google Scholar]
- (126).Fitzpatrick S; Ebdon L; Foulkes ME Separation and Detection of Arsenic and Selenium Species in Environmental Samples by HPLC-ICP-MS. Int. J. Environ. Anal. Chem 2002, 82, 835–841. [Google Scholar]
- (127).Chen S; Guo Q; Liu L Determination of Arsenic Species in Edible Mushrooms by High-Performance Liquid Chromatography Coupled to Inductively Coupled Plasma Mass Spectrometry. Food Anal. Methods 2017, 10, 740–748. [Google Scholar]
- (128).Reyes LH; Mar JLG; Rahman GMM; Seybert B; Fahrenholz T; Kingston HMS Simultaneous Determination of Arsenic and Selenium Species in Fish Tissues Using Microwave-Assisted Enzymatic Extraction and Ion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Talanta 2009, 78, 983–990. [DOI] [PubMed] [Google Scholar]
- (129).Sanz E; Muñoz-Olivas R; Cámara C Evaluation of a Focused Sonication Probe for Arsenic Speciation in Environmental and Biological Samples. J. Chromatogr. A 2005, 1097, 1–8. [DOI] [PubMed] [Google Scholar]
- (130).Sanz E; Muñoz-Olivas R; Dietz C; Sanz J; Cámara C Alternative Extraction Methods for Arsenic Speciation in Hair Using Ultrasound Probe Sonication and Pressurised Liquid Extraction. J. Anal. At. Spectrom 2007, 22, 131–139. [Google Scholar]
- (131).Peña-Farfal C; Moreda-Piñeiro A; Bermejo-Barrera A; Bermejo-Barrera P; Pinochet-Cancino H; De Gregori-Henríquez I Ultrasound Bath-Assisted Enzymatic Hydrolysis Procedures as Sample Pretreatment for the Multielement Determination in Mussels by Inductively Coupled Plasma Atomic Emission Spectrometry. Anal. Chem 2004, 76, 3541–3547. [DOI] [PubMed] [Google Scholar]
- (132).Bonomi F; Fiocchi A; Frøkiær H; Gaiaschi A; Iametti S; Poiesi C; Rasmussen P; Restani P; Rovere P Reduction of Immunoreactivity of Bovine β-Lactoglobulin upon Combined Physical and Proteolytic Treatment. J. Dairy Res 2003, 70, 51–59. [DOI] [PubMed] [Google Scholar]
- (133).Zoorob GK; McKiernan JW; Caruso JA ICP-MS for Elemental Speciation Studies. Microchim. Acta 1998, 128, 145–168. [Google Scholar]
- (134).Wenclawiak BW; Krah M Reactive Supercritical Fluid Extraction and Chromatography of Arsenic Species. Fresenius' J. Anal. Chem 1995, 351, 134–138. [Google Scholar]
- (135).Microwave-Enhanced Chemistry: Fundamentals, Sample Preparation, and Applications; Kingston HM, Haswell SJ, Eds.; American Chemical Society (ACS): Washington, D.C., 1997. [Google Scholar]
- (136).Vilanó M; Rubio R Determination of Arsenic Species in Oyster Tissue by Microwave-Assisted Extraction and Liquid Chromatography–Atomic Fluorescence Detection. Appl. Organomet. Chem 2001, 15, 658–666. [Google Scholar]
- (137).Dagnac T; Padró A; Rubio R; Rauret G Speciation of Arsenic in Mussels by the Coupled System Liquid Chromatography–UV Irradiation–Hydride Generation-Inductively Coupled Plasma Mass Spectrometry. Talanta 1999, 48, 763–772. [DOI] [PubMed] [Google Scholar]
- (138).Dagnac T; Padró A; Rubio R; Rauret G Optimisation of the Extraction of Arsenic Species from Mussels with Low Power Focused Microwaves by Applying a Doehlert Design. Anal. Chim. Acta 1998, 364, 19–30. [Google Scholar]
- (139).Nobrega JA; Trevizan LC; Araujo GCL; Nogueira ARA Focused-Microwave-Assisted Strategies for Sample Preparation. Spectrochim. Acta, Part B 2002, 57, 1855–1876. [Google Scholar]
- (140).Chemat F; Zill-E-Huma; Khan MK Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem 2011, 18, 813–835. [DOI] [PubMed] [Google Scholar]
- (141).Chemat F; Rombaut N; Sicaire A-G; Meullemiestre A; Fabiano-Tixier A-S; Abert-Vian M Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [DOI] [PubMed] [Google Scholar]
- (142).Letellier M; Budzinski H Microwave Assisted Extraction of Organic Compounds. Analusis 1999, 27, 259–271. [Google Scholar]
- (143).Dietz C; Sanz J; Camara C Recent Developments in Solid-Phase Microextractioncoatings and Related Techniques. J. Chromatogr. A 2006, 1103, 183–192. [DOI] [PubMed] [Google Scholar]
- (144).Mester Z; Sturgeon R Solid Phase Microextraction as a Tool for Trace Element Determination. Compr. Anal. Chem 2003, 41, 371–391. [Google Scholar]
- (145).Barker SA; Long AR; Short CR Isolation of Drug Residues from Tissues by Solid Phase Dispersion. J. Chromatogr. A 1989, 475, 353–361. [DOI] [PubMed] [Google Scholar]
- (146).Kristenson EM; Ramos L; Brinkman UAT Recent Advances in Matrix Solid-Phase Dispersion. TrAC, Trends Anal. Chem 2006, 25, 96–111. [Google Scholar]
- (147).Ramos L; Ramos JJ; Brinkman UAT Miniaturization in Sample Treatment for Environmental Analysis. Anal. Bioanal. Chem 2005, 381, 119–140. [DOI] [PubMed] [Google Scholar]
- (148).Moreda-Piñeiro J; Alonso-Rodríguez E; López-Mahía P; Muniategui-Lorenzo S; Prada-Rodríguez D; López-Mahía P; Prada-Rodríguez D; Romarís-Hortas V; Míguez-Framil M; Moreda-Piñeiro A Matrix Solid-Phase Dispersion of Organic Compounds and Its Feasibility for Extracting Inorganic and Organometallic Compounds. TrAC, Trends Anal. Chem 2009, 28, 110–116. [Google Scholar]
- (149).Barker SA Matrix Solid-Phase Dispersion. J. Chromatogr. A 2000, 885, 115–127. [DOI] [PubMed] [Google Scholar]
- (150).Barker SA Matrix Solid Phase Dispersion (MSPD). J. Biochem. Biophys. Methods 2007, 70, 151–162. [DOI] [PubMed] [Google Scholar]
- (151).Duncan EG; Maher WA; Foster SD Contribution of Arsenic Species in Unicellular Algae to the Cycling of Arsenic in Marine Ecosystems. Environ. Sci. Technol 2015, 49, 33–50. [DOI] [PubMed] [Google Scholar]
- (152).Kuehnelt D; Irgolic KJ; Goessler W Comparison of Three Methods for the Extraction of Arsenic Compounds from the NRCC Standard Reference Material DORM-2 and the Brown Alga Hijiki Fuziforme. Appl. Organomet. Chem 2001, 15, 445–456. [Google Scholar]
- (153).Francesconi KA Complete Extraction of Arsenic Species: A Worthwhile Goal? Appl. Organomet. Chem 2003, 17, 682–683. [Google Scholar]
- (154).Fricke MW; Creed PA; Parks AN; Shoemaker JA; Schwegel CA; Creed JT Extraction and Detection of a New Arsine Sulfide Containing Arsenosugar in Molluscs by IC-ICP-MS and IC-ESI-MS/MS. J. Anal. At. Spectrom 2004, 19, 1454–1459. [Google Scholar]
- (155).Leermakers M; Baeyens W; De Gieter M; Smedts B; Meert C; De Bisschop HC; Morabito R; Quevauviller P Toxic Arsenic Compounds in Environmental Samples: Speciation and Validation. TrAC, Trends Anal. Chem 2006, 25, 1–10. [Google Scholar]
- (156).Sanz E; Muñoz-Olivas R; Cámara C A Rapid and Novel Alternative to Conventional Sample Treatment for Arsenic Speciation in Rice Using Enzymatic Ultrasonic Probe. Anal. Chim. Acta 2005, 535, 227–235. [Google Scholar]
- (157).Harrington CF; Ojo AA; Lai VWM; Reimer KJ; Cullen WR The Identification of Some Water-Soluble Arsenic Species in the Marine Brown Algae Fucus Distichus. Appl. Organomet. Chem 1997, 11, 931–940. [Google Scholar]
- (158).Taleshi MS; Raber G; Edmonds JS; Jensen KB; Francesconi KA Arsenolipids in Oil from Blue Whiting Micromesistius poutassou–Evidence for Arsenic-Containing Esters. Sci. Rep 2015, 4, 7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Taleshi MS; Seidler-Egdal RK; Jensen KB; Schwerdtle T; Francesconi KA Synthesis and Characterization of Arsenolipids: Naturally Occurring Arsenic Compounds in Fish and Algae. Organometallics 2014, 33, 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Camel V Solid Phase Extraction of Trace Elements. Spectrochim. Acta, Part B 2003, 58, 1177–1233. [Google Scholar]
- (161).López-Gonzálvez MA; Gómez MM; Cámara C; Palacios MA On-Line Microwave Oxidation for the Determination of Organoarsenic Compounds by High-Performance Liquid Chromatography-Hydride Generation Atomic Absorption Spectrometry. J. Anal. At. Spectrom 1994, 9, 291–295. [Google Scholar]
- (162).Gómez M; Cámara C; Palacios MA; López-Gonzálvez A Anionic Cartridge Preconcentrators for Inorganic Arsenic, Monomethylarsonate and Dimethylarsinate Determination by on-Line HPLC-HG-AAS. Fresenius' J.Anal Chem 1997, 357, 844–849. [Google Scholar]
- (163).McSheehy S; Szpunar J Speciation of Arsenic in Edible Algae by Bi-Dimensional Size-Exclusion Anion Exchange HPLC with Dual ICP-MS and Electrospray MS/MS Detection. J. Anal. At. Spectrom 2000, 15, 79–87. [Google Scholar]
- (164).McSheehy S; Pohl P; Vélez D; Szpunar J Multidimensional Liquid Chromatography with Parallel ICP MS and Electrospray MS/MS Detection as a Tool for the Characterization of Arsenic Species in Algae. Anal. Bioanal. Chem 2002, 372, 457–466. [DOI] [PubMed] [Google Scholar]
- (165).Modolon SDM; Felippe AC; Fizon TE; Da Silva L; Da Silva Paula MM; Dal-Bó AG Self-Association of Sodium Deoxycholate with EHEC Cellulose Cooperatively Induced by Sodium Dodecanoate. Carbohydr. Polym 2014, 111, 425–432. [DOI] [PubMed] [Google Scholar]
- (166).Francesconi KA; Edmonds JS; Morita M Biotransformation of Arsenic in Marine Environment.. Adv. Environ. Sci. Technol 1994, 26, 221–261. [Google Scholar]
- (167).Schramel O; Michalke B; Kettrup A Application of Capillary Electrophoresis-Electrospray Ionisation Mass Spectrometry to Arsenic Speciation. J. Anal. At. Spectrom 1999, 14, 1339–1342. [Google Scholar]
- (168).Schramel O; Michalke B; Kettrup A Analysis of Metal Species by Using Electrospray Ionization Mass Spectrometry and Capillary Electrophoresis-Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 1998, 819, 231–242. [Google Scholar]
- (169).Corr JJ; Anacleto JF Analysis of Inorganic Species by Capillary Electrophoresis-Mass Spectrometry and Ion Exchange Chromatography-Mass Spectrometry Using an Ion Spray Source. Anal. Chem 1996, 68, 2155–2163. [DOI] [PubMed] [Google Scholar]
- (170).B’Hymer C; Caruso JA Arsenic and Its Speciation Analysis Using High-Performance Liquid Chromatography and Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2004, 1045, 1–13. [DOI] [PubMed] [Google Scholar]
- (171).Gong Z; Lu X; Ma M; Watt C; Le XC Arsenic Speciation Analysis. Talanta 2002, 58, 77–96. [DOI] [PubMed] [Google Scholar]
- (172).Michalke B The Coupling of LC to ICP-MS in Element Speciation—Part II: Recent Trends in Application. TrAC, Trends Anal. Chem 2002, 21, 154–165. [Google Scholar]
- (173).Arslan Y; Yildirim E; Gholami M; Bakirdere S Lower Limits of Detection in Speciation Analysis by Coupling High-Performance Liquid Chromatography and Chemical-Vapor Generation. TrAC, Trends Anal. Chem 2011, 30, 569–585. [Google Scholar]
- (174).Nearing MM; Koch I; Reimer KJ Complementary Arsenic Speciation Methods: A Review. Spectrochim. Acta, Part B 2014, 99, 150–162. [Google Scholar]
- (175).Debusschere L; Demesmay C; Rocca JL Arsenic Speciation by Coupling Capillary Zone Electrophoresis with Mass Spectrometry. Chromatographia 2000, 51, 262–268. [Google Scholar]
- (176).Abian J The Coupling of Gas and Liquid Chromatography with Mass Spectrometry. J. Mass Spectrom 1999, 34, 157–168. [Google Scholar]
- (177).Fenn JB Ion Formation from Charged Droplets: Roles of Geometry, Energy, and Time. J. Am. Soc. Mass Spectrom 1993, 4, 524–535. [DOI] [PubMed] [Google Scholar]
- (178).Gale DC; Smith RD Small Volume and Low Flow-rate Electrospray Lonization Mass Spectrometry of Aqueous Samples. Rapid Commun. Mass Spectrom 1993, 7, 1017–1021. [Google Scholar]
- (179).Moseley MA; Jorgenson JW; Shabanowitz J; Hunt DF; Tomer KB Optimization of Capillary Zone Electrophoresis/ Electrospray Ionization Parameters for the Mass Spectrometry and Tandem Mass Spectrometry Analysis of Peptides. J. Am. Soc. Mass Spectrom 1992, 3, 289–300. [DOI] [PubMed] [Google Scholar]
- (180).Takada Y; Sakairi M; Koizumi H Atmospheric Pressure Chemical Ionization Interface for Capillary Electrophoresis/Mass Spectrometry. Anal. Chem 1995, 67, 1474–1476. [Google Scholar]
- (181).Cai J; Henion J Capillary Electrophoresis–Mass Spectrometry—Review. J. Chromatogr. A 1995, 703, 667–692. [Google Scholar]
- (182).Raber G; Khoomrung S; Taleshi MS; Edmonds JS; Francesconi KA Identification of Arsenolipids with GC/MS. Talanta 2009, 78, 1215–1218. [DOI] [PubMed] [Google Scholar]
- (183).Arroyo-Abad U; Mattusch J; Mothes S; Móder M; Wennrich R; Elizalde-González MP; Matysik FM Detection of Arsenic-Containing Hydrocarbons in Canned Cod Liver Tissue. Talanta 2010, 82, 38–43. [DOI] [PubMed] [Google Scholar]
- (184).Sele V; Amlund H; Berntssen MHG; Berntsen JA; Skov K; Sloth JJ Detection of Arsenic-Containing Hydrocarbons in a Range of Commercial Fish Oils by GC-ICPMS Analysis. Anal. Bioanal. Chem 2013, 405, 5179–5190. [DOI] [PubMed] [Google Scholar]
- (185).Kovačevič M; Leber R; Kohlwein SD; Goessler W Application of Inductively Coupled Plasma Mass Spectrometry to Phospholipid Analysis. J. Anal. At. Spectrom 2004, 19, 80–84. [Google Scholar]
- (186).Truei Y; Gu T; Tsai G; Tsao GT Large-Scale Gradient Elution Chromatography. Adv. Biochem. Eng./Biotechnol 1992, 47, 1–44. [Google Scholar]
- (187).Raber G; Francesconi KA; Irgolic KJ; Goessler W Determination of “arsenosugars” in Algae with Anion-Exchange Chromatography and an Inductively Coupled Plasma Mass Spectrometer as Element-Specific Detector. Fresenius' J. Anal. Chem 2000, 367, 181–188. [DOI] [PubMed] [Google Scholar]
- (188).Sloth JJ; Larsen EH; Julshamn K Determination of Organoarsenic Species in Marine Samples Using Gradient Elution Cation Exchange HPLC-ICP-MS. J. Anal. At. Spectrom 2003, 18, 452–459. [Google Scholar]
- (189).Sele V; Sloth JJ; Holmelid B; Valdersnes S; Skov K; Amlund H Arsenic-Containing Fatty Acids and Hydrocarbons in Marine Oils—Determination Using Reversed-Phase HPLC-ICP-MS and HPLC-QTOF-MS. Talanta 2014, 121, 89–96. [DOI] [PubMed] [Google Scholar]
- (190).Guerin T; Astruc A; Astruc M Speciation of Arsenic and Selenium Compounds by HPLC Hyphenated to Specific Detectors: A Review of the Main Separation Techniques. Talanta 1999, 50, 1–24. [DOI] [PubMed] [Google Scholar]
- (191).Do B; Robinet S; Pradeau D; Guyon F Speciation of Arsenic and Selenium Compounds by Ion-Pair Reversed-Phase Chromatography with Electrothermic Atomic Absorption Spectrometry—Application of Experimental Design for Chromatographic Optimisation. J. Chromatogr. A 2001, 918, 87–98. [DOI] [PubMed] [Google Scholar]
- (192).Chen Z; Akter KF; Rahman MM; Naidu R Speciation of Arsenic by Ion Chromatography Inductively Coupled Plasma Mass Spectrometry Using Ammonium Eluents. J. Sep. Sci 2006, 29, 2671–2676. [DOI] [PubMed] [Google Scholar]
- (193).Falk K; Emons H Speciation of Arsenic Compounds by Ion-Exchange HPLC-ICP-MS with Different Nebulizers. J. Anal. At. Spectrom 2000, 15, 643–649. [Google Scholar]
- (194).Guérin T; Astruc A; Astruc M; Batel A; Borsier M Chromatographic Ion-Exchange Simultaneous Separation of Arsenic and Selenium Species with Inductively Coupled Plasma-Mass Spectrometry On-Line Detection. J. Chromatogr. Sci 1997, 35, 213–220. [Google Scholar]
- (195).Nakazato T; Tao H; Taniguchi T; Isshiki K Determination of Arsenite, Arsenate, and Monomethylarsonic Acid in Seawater by Ion-Exclusion Chromatography Combined with Inductively Coupled Plasma Mass Spectrometry Using Reaction Cell and Hydride Generation Techniques. Talanta 2002, 58, 121–132. [DOI] [PubMed] [Google Scholar]
- (196).Morin P; Amran MB; Favier S; Heimburger R; Leroy M Speciation of Arsenical Species by Anion-Exchange and Ion-Pair Reversed-Phase Liquid Chromatography. Fresenius' J. Anal. Chem 1991, 339, 504–509. [Google Scholar]
- (197).Ammann AA Determination of Strong Binding Chelators and Their Metal Complexes by Anion-Exchange Chromatography and Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2002, 947, 205–216. [DOI] [PubMed] [Google Scholar]
- (198).Gailer J; Irgolic KJ The Ion-Chromatographic Behavior of Arsenite, Arsenate, Methylarsonic Acid and Dimethylarsinic Acid on the Hamilton PRP-X100 Anion-Exchange Column. Appl. Organomet. Chem 1994, 8, 129–140. [Google Scholar]
- (199).Horváth CG; Lipsky SR Peak Capacity in Chromatography. Anal. Chem. 1967, 39, 1893. [DOI] [PubMed] [Google Scholar]
- (200).Kanaki K; Pergantis SA Precursor Ion Scanning for the Non-Targeted Detection of Individual Arsenosugars in Extracts of Marine Organisms. Rapid Commun. Mass Spectrom 2006, 20, 1925–1931. [DOI] [PubMed] [Google Scholar]
- (201).Schellinger AP; Carr PW Isocratic and Gradient Elution Chromatography: A Comparison in Terms of Speed, Retention Reproducibility and Quantitation. J. Chromatogr. A 2006, 1109, 253–266. [DOI] [PubMed] [Google Scholar]
- (202).Ritsema R; Dukan L; Roig T; Navarro I; Van Leeuwen W; Oliveira N; Wolfs P; Lebret E Speciation of Arsenic Compounds in Urine by LC-ICP MS. Appl. Organomet. Chem 1998, 12, 591–599. [Google Scholar]
- (203).Saverwyns S; Zhang X; Vanhaecke F; Cornelis R; Moens L; Dams R Speciation of Six Arsenic Compounds Using High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry with Sample Introduction by Thermospray Nebulization. J. Anal. At. Spectrom 1997, 12, 1047–1052. [Google Scholar]
- (204).Caruso JA; Heitkemper DT; B’Hymer C An Evaluation of Extraction Techniques for Arsenic Species from Freeze-Dried Apple Samples. Analyst 2001, 126, 136–140. [DOI] [PubMed] [Google Scholar]
- (205).Heitkemper D; Creed J; Caruso J; Fricke FL Speciation of Arsenic in Urine Using High-Performance Liquid Chromatography with Inductively Coupled Plasma Mass Spectrometric Detection. J. Anal. At. Spectrom 1989, 4, 279–284. [Google Scholar]
- (206).Zheng J; Hintelmann H; Dimock B; Dzurko MS Speciation of Arsenic in Water, Sediment, and Plants of the Moira Watershed, Canada, Using HPLC Coupled to High Resolution ICP-MS. Anal. Bioanal. Chem 2003, 377, 14–24. [DOI] [PubMed] [Google Scholar]
- (207).Day JA; Montes-Bayón M; Vonderheide AP; Caruso JA A Study of Method Robustness for Arsenic Speciation in Drinking Water Samples by Anion Exchange HPLC-ICP-MS. Anal. Bioanal. Chem 2002, 373, 664–668. [DOI] [PubMed] [Google Scholar]
- (208).Yu LL; Stanoyevitch RC; Zeisler R SI Traceable Determination of Arsenic Species in Kelp (Thallus Laminariae). Anal. Methods 2017, 9, 4267–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Yu LL; Wei C; Zeisler R; Tong J; Oflaz R; Bao H; Wang J An Approach for Identification and Determination ofArsenic Species in the Extract of Kelp. Anal. Bioanal. Chem 2015, 407, 3517–3524. [DOI] [PubMed] [Google Scholar]
- (210).B’Hymer C; Caruso JA Evaluation of HPLC Systems for the Separation and Quantification of Arsenic Compounds from Apple Extracts. J. Liq. Chromatogr. Relat. Technol 2002, 25, 639–653. [Google Scholar]
- (211).Sheppard BS; Caruso JA; Heitkemper DT; Wolnik KA Arsenic Speciation by Ion Chromatography with Inductively Coupled Plasma Mass Spectrometric Detection. Analyst 1992, 117, 971–975. [DOI] [PubMed] [Google Scholar]
- (212).Vassileva E; Becker A; Broekaert JAC Determination of Arsenic and Selenium Species in Groundwater and Soil Extracts by Ion Chromatography Coupled to Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2001, 441, 135–146. [Google Scholar]
- (213).Mattusch J; Wennrich R; Schmidt AC; Reisser W Determination of Arsenic Species in Water, Soils and Plants. Fresenius' J. Anal. Chem 2000, 366, 200–203. [DOI] [PubMed] [Google Scholar]
- (214).Lintschinger J; Schramel P; Hatalak-Rauscher A; Wendler I; Michalke B A New Method for the Analysis of Arsenic Species in Urine by Using HPLC-ICP-MS. Fresenius' J. Anal. Chem 1998, 362, 313–318. [Google Scholar]
- (215).Bissen M; Frimmel FH Speciation of As(III), As(V), MMA and DMA in Contaminated Soil Extracts by HPLC-ICP/MS. Fresenius' J. Anal. Chem 2000, 367, 51–55. [DOI] [PubMed] [Google Scholar]
- (216).Chen ZL; Khan NI; Owens G; Naidu R Elimination of Chloride Interference on Arsenic Speciation in Ion Chromatography Inductively Coupled Mass Spectrometry Using an Octopole Collision/Reaction System. Microchem. J 2007, 87, 87–90. [Google Scholar]
- (217).Suzuki KT; Tomita T; Ogra Y; Ohmichi M Glutathione-Conjugated Arsenics in the Potential Hepato-Enteric Circulation in Rats. Chem. Res. Toxicol 2001, 14, 1604–1611. [DOI] [PubMed] [Google Scholar]
- (218).Taleshi MS; Jensen KB; Raber G; Edmonds JS; Gunnlaugsdottir H; Francesconi KA Arsenic-Containing Hydrocarbons: Natural Compounds in Oil from the Fish Capelin, Mallotus Villosus. Chem. Commun 2008, 39, 4706–4707. [DOI] [PubMed] [Google Scholar]
- (219).Arroyo-Abad U; Hu Z; Findeisen MM; Pfeifer D; Mattusch J; Reemtsma T; Piechotta C Synthesis of Two New Arsenolipids and Their Identification in Fish. Eur. J. Lipid Sci. Technol 2016, 118, 445–452. [Google Scholar]
- (220).Arroyo-Abad U; Pfeifer M; Mothes S; Stärk HJ; Piechotta C; Mattusch J; Reemtsma T Determination of Moderately Polar Arsenolipids and Mercury Speciation in Freshwater Fish of the River Elbe (Saxony, Germany). Environ. Pollut 2016, 208, 458–466. [DOI] [PubMed] [Google Scholar]
- (221).Morita M; Shibata Y Isolation and Identification of Arseno-Lipid from a Brown Alga, Undaria Pinnatifida (Wakame). Chemosphere 1988, 17, 1147–1152. [Google Scholar]
- (222).Arroyo-Abad U; Lischka S; Piechotta C; Mattusch J; Reemtsma T Determination and Identification of Hydrophilic and Hydrophobic Arsenic Species in Methanol Extract of Fresh Cod Liver by RP-HPLC with Simultaneous ICP-MS and ESI-Q-TOF-MS Detection. Food Chem. 2013, 141, 3093–3102. [DOI] [PubMed] [Google Scholar]
- (223).Arroyo-Abad U; Mattusch J; Reemtsma T; Piechotta C Arsenolipids in Commercial Canned Cod Liver: An Occurrence and Distribution Study. Eur. J. Lipid Sci Technol 2014, 116, 1381–1387. [Google Scholar]
- (224).Beauchemin D; Siu KWM; McLaren JW; Berman SS Determination of Arsenic Species by High-Performance Liquid Chromatography–Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom 1989, 4, 285–289. [Google Scholar]
- (225).Terlecka E Arsenic Speciation Analysis in Water Samples: A Review of the Hyphenated Techniques. Environ. Monit. Assess 2005, 107, 259–284. [DOI] [PubMed] [Google Scholar]
- (226).McSheehy S; Szpunar J; Lobinski R; Haldys V; Tortajada J; Edmonds JS Characterization of Arsenic Species in Kidney of the Clam Tridacna Derasa by Multidimensional Liquid Chromatography-ICPMS and Electrospray Time-of-Flight Tandem Mass Spectrometry. Anal. Chem 2002, 74, 2370–2378. [DOI] [PubMed] [Google Scholar]
- (227).Ding H; Wang J; Dorsey JG; Caruso JA Arsenic Speciation by Micellar Liquid Chromatogrpahy with Inductively Coupled Plasma Mass Spectrometric Detection. J. Chromatogr. A 1995, 694, 425–431. [DOI] [PubMed] [Google Scholar]
- (228).Armstrong DW; Nome F Partitioning Behavior of Solutes Eluted with Micellar Mobile Phases in Liquid Chromatography. Anal. Chem 1981, 53, 1662–1666. [Google Scholar]
- (229).Sakai T; Inoue Y; Date Y; Aoyama T; Yoshida K; Endo G Simultaneous Determination of Neutral, Anionic and Cationic Compounds within One Chromatographic Run Using an Inductively Coupled Plasma Mass Spectrometer as Element-Specific Detector. Appl. Organomet. Chem 2001, 15, 285–290. [Google Scholar]
- (230).Wuilloud RG; Altamirano JC; Smichowski PN; Heitkemper DT Investigation of Arsenic Speciation in Algae of the Antarctic Region by HPLC-ICP-MS and HPLC-ESI-Ion Trap MS. J. Anal. At. Spectrom 2006, 21, 1214–1223. [Google Scholar]
- (231).Schaeffer R; Fodor P; Soeroes C Development of a Liquid Chromatography/Electrospray Selected Reaction Monitoring Method for the Determination of Organoarsenic Species in Marine and Freshwater Samples. Rapid Commun. Mass Spectrom 2006, 20, 2979–2989. [DOI] [PubMed] [Google Scholar]
- (232).Mcsheehy S; Mester Z Arsenic Speciation in Marine Certified Reference Materials Part 1. Identification of Water-Soluble Arsenic Species Using Multidimensional Liquid Chromatography Combined with Inductively Coupled Plasma, Electrospray and Electrospray High-Field Asymmetric W. J. Anal. At. Spectrom 2004, 19, 373–380. [Google Scholar]
- (233).Lu X; Nguyen N; Gabos S; Le XC Arsenic Speciation in Cattail (Typha Latifolia) Using Chromatography and Mass Spectrometry. Mol. Nutr. Food Res 2009, 53, 566–571. [DOI] [PubMed] [Google Scholar]
- (234).Mandal BK; Ogra Y; Suzuki KT Identification of Dimethylarsinous and Monomethylarsonous Acids in Human Urine of the Arsenic-Affected Areas in West Bengal, India. Chem. Res. Toxicol 2001, 14, 371–378. [DOI] [PubMed] [Google Scholar]
- (235).Xie R; Johnson W; Spayd S; Hall GS; Buckley B Arsenic Speciation Analysis of Human Urine Using Ion Exchange Chromatography Coupled to Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2006, 578, 186–194. [DOI] [PubMed] [Google Scholar]
- (236).Šlejkovec Z; Van Elteren JT; Byrne AR Determination of Arsenic Compounds in Reference Materials by HPLC-(UV)-HG-AFS. Talanta 1999, 49, 619–627. [DOI] [PubMed] [Google Scholar]
- (237).Burger M; Schwarz G; Gundlach-Graham A; Käser D; Hattendorf B; Günther D Capabilities of Laser Ablation Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. J. Anal. At. Spectrom 2017, 32, 1946–1959. [Google Scholar]
- (238).Pröfrock D; Prange A Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) for Quantitative Analysis in Environmental and Life Sciences: A Review of Challenges, Solutions, and Trends. Appl. Spectrosc 2012, 66, 843–868. [DOI] [PubMed] [Google Scholar]
- (239).Sele V; Sloth JJ; Lundebye AK; Larsen EH; Berntssen MHG; Amlund H Arsenolipids in Marine Oils and Fats: A Review of Occurrence, Chemistry and Future Research Needs. Food Chem. 2012, 133, 618–630. [Google Scholar]
- (240).Zubarev RA; Makarov A Orbitrap Mass Spectrometry. Anal. Chem 2013, 85, 5288–5296. [DOI] [PubMed] [Google Scholar]
- (241).Maher W; Krikowa F; Kirby J; Townsend AT; Snitch P Measurement of Trace Elements in Marine Environmental Samples Using Solution ICPMS. Current and Future Application. Aust. J. Chem 2003, 56, 103–116. [Google Scholar]
- (242).Chiba K; Narukawa T The Effect of Plasma Reactions on Arsenic Measurement by ICP Spectrometry. Anal. Sci 2014, 30, 175–181. [DOI] [PubMed] [Google Scholar]
- (243).Wallschläger D; Stadey CJ Determination of (Oxy) Thioarsenates in Sulfidic Waters. Anal. Chem 2007, 79, 3873–3880. [DOI] [PubMed] [Google Scholar]
- (244).Nam SH; Oh HJ; Min HS; Lee JH A Study on the Extraction and Quantitation of Total Arsenic and Arsenic Species in Seafood by HPLC-ICP-MS. Microchem. J 2010, 95, 20–24. [Google Scholar]
- (245).Nischwitz V; Kanaki K; Pergantis SA Mass Spectrometric Identification of Novel Arsinothioyl-Sugars in Marine Bivalves and Algae. J. Anal. At. Spectrom 2006, 21, 33–40. [Google Scholar]
- (246).Axelsson BO; Jörnten-Karlsson M; Michelsen P; Abou-Shakra F The Potential of Inductively Coupled Plasma Mass Spectrometry Detection for High-Performance Liquid Chromatography Combined with Accurate Mass Measurement of Organic Pharmaceutical Compounds. Rapid Commun. Mass Spectrom 2001, 15, 375–385. [DOI] [PubMed] [Google Scholar]
- (247).Kovačevič M; Leber R; Kohlwein SD; Goessler W Application of Inductively Coupled Plasma Mass Spectrometry to Phospholipid Analysiš Evic. J. Anal. At. Spectrom 2004, 19, 80–84. [Google Scholar]
- (248).Meermann B; Kießhauer M Development of an Oxygen-Gradient System to Overcome Plasma Instabilities during HPLC/ICP-MS Measurements Using Gradient Elution. J. Anal. At. Spectrom 2011, 26, 2069–2075. [Google Scholar]
- (249).Grotti M; Terol A; Todolí JL Speciation Analysis by Small-Bore HPLC Coupled to ICP-MS. TrAC, Trends Anal. Chem 2014, 61, 92–106. [Google Scholar]
- (250).Kirby J; Maher W; Ellwood M; Krikowa F Arsenic Species Determination in Biological Tissues by HPLC–ICP–MS and HPLC–HG–ICP–MS. Aust. J. Chem 2004, 57, 957–966. [Google Scholar]
- (251).Schmeisser E; Raml R; Francesconi KA; Kuehnelt D; Lindberg AL; Sörös C; Goessler W Thio Arsenosugars Identified as Natural Constituents of Mussels by Liquid Chromatography-Mass Spectrometry. Chem. Commun 2004, 16, 1824–1825. [DOI] [PubMed] [Google Scholar]
- (252).Pétursdóttir ÁH; Gunnlaugsdóttir H; Jörundsdóttir H; Mestrot A; Krupp EM; Feldmann J HPLC-HG-ICP-MS: A Sensitive and Selective Method for Inorganic Arsenic in Seafood. Anal. Bioanal. Chem 2012, 404, 2185–2191. [DOI] [PubMed] [Google Scholar]
- (253).Sánchez-Rodas D; Geiszinger A; Gómez-Ariza JL; Francesconi KA Determination of an Arsenosugar in Oyster Extracts by Liquid Chromatography-Electrospray Mass Spectrometry and Liquid Chromatography-Ultraviolet Photo-Oxidation-Hydride Generation Atomic Fluorescence Spectrometry. Analyst 2002, 127, 60–65. [DOI] [PubMed] [Google Scholar]
- (254).Howard AG; Salou C Cysteine Enhancement of the Cryogenic Trap Hydride AAS Determination of Dissolved Arsenic Species. Anal. Chim. Acta 1996, 333, 89–96. [Google Scholar]
- (255).Ramesh Kumar A; Riyazuddin P Mechanism of Volatile Hydride Formation and Their Atomization in Hydride Generation Atomic Absorption Spectrometry. Anal. Sci 2005, 21, 1401–1410. [DOI] [PubMed] [Google Scholar]
- (256).Carrero P; Malavé A; Burguera JL; Burguera M; Rondón C Determination of Various Arsenic Species by Flow Injection Hydride Generation Atomic Absorption Spectrometry: Investigation of the Effects of the Acid Concentration of Different Reaction Media on the Generation of Arsines. Anal. Chim. Acta 2001, 438, 195–204. [Google Scholar]
- (257).Pohl P; Prusisz B Ion-Exchange Column Chromatography—An Attempt to Speciate Arsenic. TrAC, Trends Anal. Chem 2004, 23, 63–69. [Google Scholar]
- (258).Karadjova IB; Lampugnani L; Onor M; D’Ulivo A; Tsalev DL Continuous Flow Hydride Generation-Atomic Fluorescence Spectrometric Determination and Speciation of Arsenic in Wine. Spectrochim. Acta, Part B 2005, 60, 816–823. [Google Scholar]
- (259).Llorente-Mirandes T; Ruiz-Chancho MJ; Barbero M; Rubio R; López-Sánchez JF Measurement of Arsenic Compounds in Littoral Zone Algae from the Western Mediterranean Sea. Occurrence of Arsenobetaine. Chemosphere 2010, 81, 867–875. [DOI] [PubMed] [Google Scholar]
- (260).Chen BC; Chou WC; Chen WY; Liao CM Assessing the Cancer Risk Associated with Arsenic-Contaminated Seafood. J. Hazard. Mater 2010, 181, 161–169. [DOI] [PubMed] [Google Scholar]
- (261).Gomez-Ariza JL; Sanchez-Rodas D; Beltran R; Corns W; Stockwel P Evaluation of Atomic Fluorescence Spectrometry as a Sensitive Detection Technique for Arsenic Speciation. Appl. Organomet Chem 1998, 12, 439–447. [Google Scholar]
- (262).Lindberg AL; Goessler W; Grandér M; Nermell B; Vahter M Evaluation of the Three Most Commonly Used Analytical Methods for Determination of Inorganic Arsenic and Its Metabolites in Urine. Toxicol. Lett 2007, 168, 310–318. [DOI] [PubMed] [Google Scholar]
- (263).Schmeisser E; Goessler W; Kienzl N; Francesconi KA Volatile Analytes Formed from Arsenosugars: Determination by HPLC-HG-ICPMS and Implications for Arsenic Speciation Analyses. Anal. Chem 2004, 76, 418–423. [DOI] [PubMed] [Google Scholar]
- (264).Hummer AA; Rompel A The Use of X-Ray Absorption and Synchrotron Based Micro-X-Ray Fluorescence Spectroscopy to Investigate Anti-Cancer Metal Compounds in Vivo and in Vitro. Metallomics 2013, 5, 597–614. [DOI] [PubMed] [Google Scholar]
- (265).Ortega R Direct Speciation Analysis of Inorganic Elements in Single Cells Using X-Ray Absorption Spectroscopy. J. Anal. At. Spectrom 2011, 26, 23–29. [Google Scholar]
- (266).Larsen EH Method Optimization and Quality Assurance in Speciation Analysis Using High Performance Liquid Chromatography with Detection by Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta, Part B 1998, 53, 253–265. [Google Scholar]
- (267).Kohlmeyer U; Kuballa J; Jantzen E Simultaneous Separation of 17 Inorganic and Organic Arsenic Compounds in Marine Biota by Means of High-Performance Liquid Chromatography/Inductively Coupled Plasma Mass Spectrometry. Rapid Commun. Mass Spectrom 2002, 16, 965–974. [DOI] [PubMed] [Google Scholar]
- (268).Ruiz-Chancho MJ; Taleshi MS; Goessler W; Francesconi KA A Method for Screening Arsenolipids in Fish Oils by HPLC-ICPMS. J. Anal. At. Spectrom 2012, 27, 501–504. [Google Scholar]
- (269).Khan M; Jensen KB; Francesconi KA A Method for Determining Arsenolipids in Seawater by HPLC-High Resolution Mass Spectrometry. Talanta 2016, 153, 301–305. [DOI] [PubMed] [Google Scholar]
- (270).Serpe FP; Russo R; Gallo P; Severino L Method for Speciation of Organoarsenic in Mussels by Liquid Chromatography Coupled to Electrospray Ionization and QTRAP Tandem Mass Spectrometry. J. Food Prot 2013, 76, 1293–1299. [DOI] [PubMed] [Google Scholar]
- (271).Miguens-Rodriguez M; Pickford R; Thomas-Oates JE; Pergantis SA Arsenosugar Identification in Seaweed Extracts Using High-Performance Liquid Chromatography/Electrospray Ion Trap Mass Spectrometry. Rapid Commun. Mass Spectrom 2002, 16, 323–331. [DOI] [PubMed] [Google Scholar]
- (272).Sadee B; Foulkes ME; Hill SJ Coupled Techniques for Arsenic Speciation in Food and Drinking Water: A Review. J. Anal. At. Spectrom 2015, 30, 102–118. [Google Scholar]
- (273).Denisov E; Damoc E; Lange O; Makarov A Orbitrap Mass Spectrometry with Resolving Powers above 1,000,000. Int. J. Mass Spectrom 2012, 325–327, 80–85. [Google Scholar]
- (274).Marshall AG Milestones in Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Technique Development. Int. J. Mass Spectrom 2000, 200, 331–356. [Google Scholar]
- (275).Marshall AG; Verdun FR Fourier Transform Ion Cyclotron Resonance Mass Spectrometry In Fourier Transforms in NMR, Optical, and Mass Spectrometry: A User’s Handbook; Elsevier Science Publishers B.V.: Amsterdam, Netherlands, 1990; Chapter 7, pp 225–278, DOI: 10.1016/B978-0-444-87360-6.50012-1. [DOI] [Google Scholar]
- (276).Hsieh YJ; Jiang SJ Application of HPLC-ICP-MS and HPLC-ESI-MS Procedures for Arsenic Speciation in Seaweeds. J. Agric. Food Chem 2012, 60, 2083–2089. [DOI] [PubMed] [Google Scholar]
- (277).Nischwitz V; Pergantis SA Identification of the Novel Thio-Arsenosugars DMThioAsSugarCarboxyl, DMThioAsSugarCarbamate and DMThioAsSugarAdenine in Extracts of Giant Clam Tissues by High-Performance Liquid Chromatography Online with Electrospray Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom 2006, 20, 3579–3585. [DOI] [PubMed] [Google Scholar]
- (278).Corr JJ; Larsen EH Arsenic Speciation by Liquid Chromatography Coupled with Ionspray Tandem Mass Spectrometry. J. Anal. At. Spectrom 1996, 11, 1215–1224. [Google Scholar]
- (279).Kuehnelt D; Goessler W; Francesconi KA Nitrogen Purity Influences the Occurrence of As+ions in High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometric Analysis of Four Common Arsenosugars. Rapid Commun. Mass Spectrom 2003, 17, 654–659. [DOI] [PubMed] [Google Scholar]
- (280).Nischwitz V; Pergantis SA Liquid Chromatography Online with Selected Reaction Monitoring Electrospray Mass Spectrometry for the Determination of Organoarsenic Species in Crude Extracts of Marine Reference Materials. Anal. Chem 2005, 77, 5551–5563. [DOI] [PubMed] [Google Scholar]
- (281).Nischwitz V; Pergantis SA Optimisation of an HPLC Selected Reaction Monitoring Electrospray Tandem Mass Spectrometry Method for the Detection of 50 Arsenic Species. J. Anal. At. Spectrom 2006, 21, 1277–1286. [Google Scholar]
- (282).Nischwitz V; Pergantis SA Mapping of Arsenic Species and Identification of a Novel Arsenosugar in Giant Clams Tridacna Maxima and Tridacna Derasa Using Advanced Mass Spectrometric Techniques. Environ. Chem 2007, 4, 187–196. [Google Scholar]
- (283).Kanaki K; Pergantis SA Development of Mass Spectrometric Methods for Detecting Arsenic-Glutathione Complexes. J. Am. Soc. Mass Spectrom 2008, 19, 1559–1567. [DOI] [PubMed] [Google Scholar]
- (284).McSheehy S; Pohl P; Lobiński R; Szpunar J Investigation of Arsenic Speciation in Oyster Test Reference Material by Multidimensional HPLC-ICP-MS and Electrospray Tandem Mass Spectrometry (ES-MS-MS). Analyst 2001, 126, 1055–1062. [DOI] [PubMed] [Google Scholar]
- (285).Grotti M; Ardini F; Terol A; Magi E; Todolí JL Influence of Chemical Species on the Determination of Arsenic Using Inductively Coupled Plasma Mass Spectrometry at a Low Liquid Flow Rate. J. Anal. At. Spectrom 2013, 28, 1718–1724. [Google Scholar]
- (286).Yu LL; Butler TA; Turk GC Effect of Valence State on ICP-OES Value Assignment of SRM 3103a Arsenic Spectrometric Solution. Anal. Chem 2006, 78, 1651–1656. [DOI] [PubMed] [Google Scholar]
- (287).Shibata Y; Morita M A Novel, Trimethylated Arseno-Sugar Isolated from the Brown Alga Sargassum thunbergii. Agric. Biol. Chem 1988, 52, 1087–1089. [Google Scholar]
- (288).Francesconi KA; Edmonds JS; Stick RV; Skelton BW; White AH Arsenic-Containing Ribosides from the Brown Alga Sargassum lacerifolium: X-ray Molecular Structure of 2-Amino-3-[5′-deoxy-5′-(dimethylarsinoyl)ribosyloxy]propane-I-sulphonic Acid. J. Chem. Soc., Perkin Trans. 1 1991, 1, 2707–2716. [Google Scholar]
- (289).Shibata Y; Morita M; Edmonds JS Purification and Identification of Arsenic-Containing Ribofuranosides from the Edible Brown Seaweed, Laminaria Japonica (Makonbu). Agric. Biol. Chem 1987, 51, 391–398. [Google Scholar]
- (290).McAdam DP; Perera AMA; Stick RV The Synthesis of (R)-2′,3′-Dihydroxypropyl 5-deoxy-5-dimethylarsinyl-β-d-riboside, a Naturally Occurring Arsenic-Containing Carbohydrate. Aust. J. Chem 1987, 40, 1901–1908. [Google Scholar]
- (291).Fukuda S; Terasawa M; Shiomi K Phosphatidylarsenocholine, One of the Major Arsenolipids in Marine Organisms: Synthesis and Metabolism in Mice. Food Chem. Toxicol 2011, 49, 1598–1603. [DOI] [PubMed] [Google Scholar]
- (292).Edmonds JS; Francesconi KA Arseno-Sugars from Brown Kelp (Ecklonia Radiata) as Intermediates in Cycling of Arsenic in a Marine Ecosystem. Nature 1981, 289, 602–604. [Google Scholar]
- (293).Francesconi KA; Edmonds JS; Stick RV Arsenic Compounds from the Kidney of the Giant Clam Tridacna Maxima: Isolation and Identification of an Arsenic-Containing Nucleoside. J. Chem. Soc., Perkin Trans. 1 1992, 1, 1349–1357. [Google Scholar]
- (294).Edmonds JS; Francesconi KA; Stick RV Arsenic Compounds from Marine Organisms. Nat. Prod. Rep 1993, 10, 421–428. [Google Scholar]
- (295).Müller SM; Ebert F; Raber G; Meyer S; Bornhorst J; Hüwel S; Galla HJ; Francesconi KA; Schwerdtle T Effects of Arsenolipids on in Vitro Blood-Brain Barrier Model. Arch. Toxicol 2018, 92, 823–832. [DOI] [PubMed] [Google Scholar]
- (296).Müller SM; Finke H; Ebert F; Kopp JF; Schumacher F; Kleuser B; Francesconi KA; Raber G; Schwerdtle T Arsenic-Containing Hydrocarbons: Effects on Gene Expression, Epigenetics, and Biotransformation in HepG2 Cells. Arch. Toxicol 2018, 92, 1751–1765. [DOI] [PubMed] [Google Scholar]
- (297).Meyer S; Raber G; Ebert F; Taleshi MS; Francesconi KA; Schwerdtle T Arsenic-Containing Hydrocarbons and Arsenic-Containing Fatty Acids: Transfer across and Presystemic Metabolism in the Caco-2 Intestinal Barrier Model. Mol. Nutr. Food Res 2015, 59, 2044–2056. [DOI] [PubMed] [Google Scholar]
- (298).Traar P; Rumpler A; Madl T; Saischek G; Francesconi KA Synthesis of Naturally Occurring Arsenic-Containing Carbohydrates. Aust. J. Chem 2009, 62, 538–545. [Google Scholar]
- (299).Traar P; Francesconi KA Synthetic Routes for Naturally-Occurring Arsenic-Containing Ribosides. Tetrahedron Lett. 2006, 47, 5293–5296. [Google Scholar]
- (300).Edmonds JS; Shibata Y; Yang F; Morita M Isolation and Synthesis of 1-Deoxy-1-Dimethylarsinoylribitol-5-Sulfate, a Natural Constituent of Chondria Crassicaulis and Other Red Algae. Tetrahedron Lett. 1997, 38, 5819–5820. [Google Scholar]
- (301).McAdam DP; Stick RV The Synthesis of (R)-2′,3′-Dihydroxypropyl 5-Deoxy-5-dimethylarsinoyl-β-d-riboside, a Naturally-Occurring, Arsenic-Containing Carbohydrate. Tetrahedron Lett. 1986, 27, 251–254. [Google Scholar]
- (302).Liu J; O’Brien DH; Irgolic KJ Synthesis of 1-O-(2′,3′-Dihydroxypropyl)5-deoxy-β-d-ribofuranosides with (CH3)2As, (CH3)2As═S or (CH3)3As+ Groups as Substituents at the 5-Position. Appl. Organomet. Chem 1996, 10, 13–22. [Google Scholar]
- (303).Stick RV; Stubbs KA; Tilbrook DMGMG An Improved Synthesis of (R)-2,3-Dihydroxypropyl 5-Deoxy-5-Dimethylarsinyl-β-D-Riboside, a Common Marine Arsenical. Aust. J. Chem 2001, 54, 181–183. [Google Scholar]
- (304).Guttenberger N; Glabonjat RA; Tassoti S; Francesconi KA Synthetic Access to Arsenic-Containing Phosphatidylcholines. Tetrahedron Lett. 2017, 58, 2651–2653. [Google Scholar]
- (305).Guttenberger N; Glabonjat RA; Jensen KB; Zangger K; Francesconi KA Synthesis of Two Arsenic-Containing Cyclic Ethers: Model Compounds for a Novel Group of Naturally-Occurring Arsenolipids. Tetrahedron Lett. 2016, 57, 4578–4580. [Google Scholar]
- (306).Lischka S; Arroyo-Abad U; Mattusch J; Kühn A; Piechotta C The High Diversity of Arsenolipids in Herring Fillet (Clupea Harengus). Talanta 2013, 110, 144–152. [DOI] [PubMed] [Google Scholar]
- (307).García-Salgado S; Raber G; Raml R; Magnes C; Francesconi KA Arsenosugar Phospholipids and Arsenic Hydrocarbons in Two Species of Brown Macroalgae. Environ. Chem 2012, 9, 63–66. [Google Scholar]
- (308).McSheehy S; Pohl P; Łobiński R; Szpunar J Complementarity of Multidimensional HPLC-ICP-MS and Electrospray MS-MS for Speciation Analysis of Arsenic in Algae. Anal. Chim. Acta 2001, 440, 3–16. [DOI] [PubMed] [Google Scholar]