Abstract
Our understanding of the systematics of red yeasts has greatly improved with the availability of sequence data and it is now clear that the majority of these fungi belong to three different classes of Pucciniomycotina (Basidiomycota): Agaricostilbomycetes, Cystobasidiomycetes, and Microbotryomycetes. Despite improvements in phylogenetic placement, the taxonomy of these fungi has long been in need of revision and still has not been entirely resolved, partly due to missing taxa. In the present study, we present data of culture-based environmental yeast isolation, revealing several undescribed species of Symmetrospora, which was recently introduced to accommodate six species previously placed in the asexual genera Sporobolomyces and Rhodotorula in the gracilis/marina clade of Cystobasidiomycetes. Based on molecular phylogenetic analyses of three rDNA loci, morphology, and biochemical studies, we formally describe the following new species: Symmetrospora clarorosea sp. nov. from leaf surfaces in Portugal and the USA; S. pseudomarina sp. nov. from leaf surfaces in Brazil, and the USA and decaying wood in the USA; and S. suhii sp. nov. from a beetle gut in the USA, leaf surfaces in Brazil and marine water in the Taiwan and Thailand. Finally, we propose a new combination for Sporobolomyces oryzicola based on our molecular phylogenetic data, Symmetrospora oryzicola comb. nov.
Keywords: beetle gut, four new taxa, phylloplane, simple-septate basidiomycetes, systematics
INTRODUCTION
The red-pigmented yeasts are ubiquitous microbes that often occur on the surfaces of plant material (Phaff 1990, Takashima & Nakase 2000, Fell et al. 2001, Inácio et al. 2002). In the past, these were grouped artificially into asexual genera—primarily Sporobolomyces and Rhodotorula—based on morphology and physiology (Boekhout 1991, Hamamoto et al. 2011, Sampaio 2011). Although DNA sequence data have demonstrated repeatedly that these asexual genera are polyphyletic (e.g., Hamamoto & Nakase 2000, Aime et al. 2006, Wang et al. 2015a, b), taxonomic revision has been long overdue. Within Pucciniomycotina, Sporobolomyces, and Rhodotorula species have been placed in several orders within Agaricostilbomycetes, Cystobasidiomycetes, and Microbotryomycetes in the past (e.g., Fell et al. 2000, Aime et al. 2006, Bauer et al. 2006). In the seven-locus phylogeny of Wang et al. (2015a), representatives of Rhodotorula and Sporobolomyces occurred in 17 and 23 clades, respectively. Wang et al. (2015b) revised five polyphyletic genera (Bensingtonia, Rhodosporidium, Rhodotorula, Sporidiobolus and Sporobolomyces). These authors proposed new combinations for 27 species of Rhodotorula (in 15 genera) and for 40 species of Sporobolomyces (in 16 genera).
Our study focuses on one of the groups within Cystobasidiomycetes that is known as the gracilis lineage (Scorzetti et al. 2002) or marina clade (Nagahama et al. 2006, Kurtzman et al. 2011, Wang et al. 2015a). No sexual morph is known for any species in this lineage and all the species have been described as either Sporobolomyces or Rhodotorula. The gracilis/marina clade contains seven species originating from various parts of the world. The first species that was described is S. gracilis, isolated from a decaying leaf in western Europe (Derx 1930). Other known species are: S. foliicola isolated from the leaf surface of Banksia collina in Australia (Shivas & Miranda 1983); S. oryzicola from a dead Oryza sativa leaf in Japan (Nakase & Suzuki 1986); S. coprosmae from dead leaves and fruit of Coprosma tenuifolia in New Zealand (Hamamoto & Nakase 1995); S. vermiculatus from a dead leaf of Pennisetum pedicellatum in Thailand (Takashima & Nakase 2000); and S. symmetricus from a Betula platyphylla leaf in China (Wang & Bai 2004). The only species not originally described from plant material is R. marina, a yeast isolated from shrimp (Penaeus setiferus) wash water in Texas, USA (Phaff et al. 1952).
All seven species mentioned above form smooth, butyrous, somewhat shiny colonies on agar medium. The colonies produce entire margins and colony color varies from pink to brick-red. None of these species have been observed to form hyphae or pseudohyphae, but most of them do form ballistoconidia (Hamamoto et al. 2011, Sampaio 2011). These characters are shared with many other species in Cystobasidiomycetes and Microbotryomycetes, and modern generic circumscriptions of these yeasts are mainly based on molecular phylogenetic data (Wang et al. 2015b). Using phylogenetic inference analyses of a seven-locus dataset and an extended LSU rDNA locus dataset, Wang et al. (2015b) proposed the genus Symmetrospora to accommodate six of the seven species in the gracilis/marina clade. In this study, we reveal and formally describe three new species of Symmetrospora based on culture studies, physiological characterization, and rDNA sequence data (Kurtzman et al. 2011) from newly and previously collected material. In addition, according to the results of our phylogenetic reconstruction, we propose a new combination.
MATERIALS AND METHODS
Sample collection and isolations
Eighteen new yeast strains were examined during this study. Fifteen of these were obtained from various leaf surfaces in Illinois, Indiana, Louisiana, and Michigan in the USA and in Taiwan. Leaves were cut into small pieces that then were attached using Vaseline Petroleum Jelly to the inner lid of a Petri dish containing agar media. Chloramphenicol (1 mL L21) was added to the media to limit bacterial growth. One strain (BG 025-27-3-2-2) was isolated from the gut of a Staphylinidae beetle as described in Suh et al. (2005) and one strain (SA42) was isolated from a small piece of decaying wood using the spore-drop method as outlined above. Strain SA107 was obtained from indoor air by exposing a media plate on a lab bench top for 1 h.
All pure cultures were maintained on potato dextrose agar (PDA); long-term preservation of isolates was accomplished in the Aime Lab at Purdue University on PDA slants at 4 °C and as glycerol stocks at -80 °C. Holotypes are deposited at PUL (Kriebel Herbarium, Purdue University, West Lafayette, Indiana, USA) as dried inert material; ex-type and other cultures are deposited at the CBS culture collection (Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands).
Morphological and physiological characteristics
Colony morphology was described by examining 10-d-old cultures on Yeast Malt extract Agar (YMA). Corn Meal Agar (CMA) and Dalmau plates were used to test for the formation of pseudohyphae and/or true hyphae. Culture colors were described subjectively and coded from 10-d-old cultures on YMA and CMA. Color codes were assigned following the Online Auction Color Chart (Kramer 2004). Microscopic characters were examined with a Nikon Eclipse 80i microscope with standard differential interference contrast (DIC) settings and with 40× and 100× objectives. Cell measurements from 20 cells grown in YM broth for 5 d were taken with an ocular micrometer using 100× oil-immersion objective. Images were taken with Nikon Digital Sight DS-Fi1 camera setup and measurements were calibrated with a stage micrometer.
Assimilation of various single carbon sources was determined for yeast species using Biolog YT microplates (Biolog Inc., Hayward, California, USA). Two-d-old cultures on YMA were used to inoculate BUY agar plates (Biolog Inc.). After 48 h of growth, these plates were used to prepare cell suspensions for inoculating the microplates. The optical density of the cell suspension in sterile water was adjusted to 0.04 (= 91 % transmittance) and 100 μL of that suspension was transferred to each microplate well. Measurements were performed at 1, 2, 5, 10, and 14 d post inoculation (dpi) using the ELx800 Universal Microplate Reader (Bio-Tek Instruments Inc., Winooski, Vermont, USA). The turbidity in the wells of each microplate was determined separately and the well with the highest reading value per each plate was determined (considered as 100 %). The wells with turbidity values lower than 20 % of the maximum value were recorded as negative and higher than 50 % were recorded as positive. The assimilation ability of the wells that had turbidity values between 20 % and 50 % was considered uncertain. The data from each plate were used only after the turbidity values for both negative control wells of the plate remained below 20 % which in most cases was around 10 dpi.
The assimilation of nitrogen compounds, fermentation ability, and the ability to grow in highly osmotic environment were tested on agar media as described in Suh et al. (2008). The maximum growth temperature was determined on YMA plates at 30 °C, 35 °C, and 37 °C.
PCR, sequencing and phylogenetic inference
The small and large subunits (SSU, LSU) of the nuclear ribosomal DNA (rDNA) and the internal transcribed spacer (ITS) region were amplified by colony PCR. The LSU D1/D2 region is the DNA barcode for yeasts (Kurtzman & Robnett 1998), whereas the ITS is the default barcode for the Kingdom Fungi (Schoch et al. 2012). One colony of cells from a 2-d-old culture was eluted in 100 μL of sterile water, 5 μL of which was used as template for PCR. PCRs were carried out in 25 µL reactions containing 12.5 µL Apex Taq RED Master Mix (Genesee Scientific, San Diego, California, USA), 1.25 µL of each 10 µM primer, and 5 µL of ddH2O. Primer pairs were NS1/NS4 and NS3/NS8 for SSU (White et al. 1990), ITS1F/ITS4 for ITS (White et al. 1990, Gardes & Bruns 1993), and LR0R/LR6 for LSU (Vilgalys & Hester 1990, Rehner & Samuels 1994). An Eppendorf Mastercycler EP Gradient Thermal Cycler was used for amplifications. Cycling conditions for the ITS locus were initial denaturation at 95 °C for 5 min; followed by 35 cycles of denaturing at 95 °C for 30 s, annealing at 45 °C for 45 s and elongation at 72 °C for 45 s; and a final elongation step of 72 °C for 7 min. Cycling conditions were the same for SSU and LSU, except for an extended extension up to 1 min for both loci, and annealing at 55 °C for 45 s for the SSU locus. Purification of PCR products and sequencing using the same primers were outsourced to Genewiz (South Plainfield, New Jersey, USA). Generated sequences were assembled, edited and trimmed using Sequencher v. 5.2.3 (Gene Codes Corporation, Ann Arbor, Michigan, USA) and are deposited in GenBank (accession numbers in Table 1).
Table 1.
Species used in phylogenetic analysis, with strain information, type status (indicated by T), GenBank accession numbers of rDNA sequences (SSU, ITS, LSU), and references. Accession numbers of sequences generated during this study are in boldface.
A Nucleotide BLAST search (https://blast.ncbi.nlm.nih.gov/) confirmed that all strains belonged to Symmetrospora. Datasets were constructed for individual SSU, ITS, and LSU loci by downloading sequences of type strains of described Symmetrospora species (accession numbers in Table 1). Alignments were constructed using MUSCLE v. 3.7 (Edgar 2004), which is available on the CIPRES Science Gateway v. 3.3 (Miller et al. 2010). Ambiguously aligned regions and uninformative positions were removed by using trimAl v. 1.2 (Capella-Gutiérrez et al. 2009) with a gap threshold of 60 % and coverage of 50 %. We constructed a concatenated SSU+ITS+LSU dataset of 31 isolates in MEGA v. 7 (Kumar et al. 2016). Phylogenetic relationships were inferred by analyzing the combined three-locus dataset by maximum likelihood (ML). We used the command-line version of IQ-TREE (Nguyen et al. 2015) under partitioned models (Chernomor et al. 2016). Appropriate models of nucleotide substitution were selected according to the corrected Akaike Information Criterion (AICc) through the built-in ModelFinder (Kalyaanamoorthy et al. 2017). Selected models were TIM2+F+R2 (SSU, -lnL = 6946.074), TIM2+F+G4 (ITS1, -lnL = 2010.895), K3P (5.8S, -lnL = 554.742), SYM+G4 (ITS2, -lnL = 3158.263), and TIM2+F+I+G4 (LSU, -lnL = 4498.519). Ultrafast bootstrapping was done with 1 000 replicates (Hoang et al. 2018). The final tree with ML bootstrap support values (BS) was visualized in FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/) and edited in Adobe Illustrator CC 2018.
In keeping with Kurtzman & Robnett (1998), we calculated the % similarity and number of nt differences between ex-type rDNA sequences (LSU, ITS) of our new species and their closest related relatives. These numbers are given in the respective diagnoses.
RESULTS
Nucleotide alignment dataset & phylogenetic inference
During this study, we generated 47 rDNA sequences (14 SSU, 18 ITS, 15 LSU) for 18 examined strains of Symmetrospora. The SSU section of our concatenated rDNA sequence dataset comprised 1 664 characters, of which 1 523 were constant and 56 were parsimony-informative. The ITS (partitioned into ITS1, 5.8S, and ITS2) comprised 173+158+249 characters, of which 92+151+117 were constant and 49+2+73 were parsimony-informative. Finally, the LSU comprised 728 characters, of which 585 were constant and 99 were parsimony-informative. Maximum likelihood of the combined SSU+ITS+LSU dataset allowed comparisons with reference sequences from ex-type strains (Fig. 1).
Fig. 1.
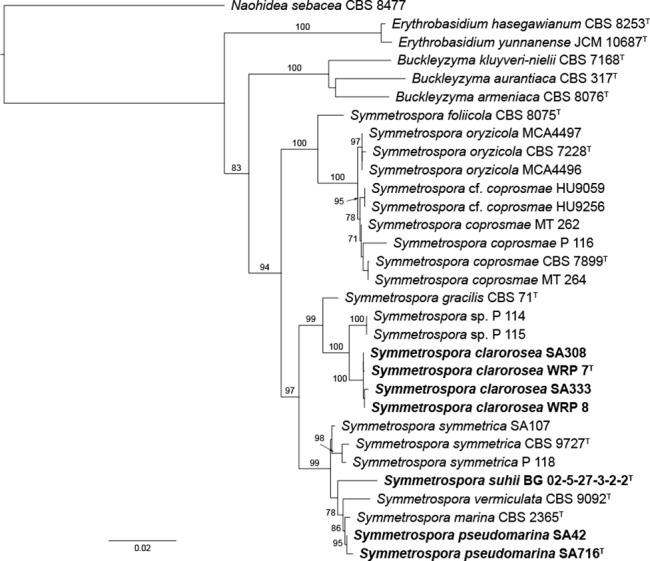
Phylogenetic placement of Symmetrospora clarorosea sp. nov., S. oryzicola comb. nov., S. pseudomarina sp. nov., and S. suhii sp. nov., reconstructed from a combined dataset of SSU, ITS, and LSU sequences. The topology is the result of maximum likelihood inference performed with IQ-TREE. For each node, ML BS support values ≥ 70 % are presented above/below the branch leading to that node.
Of the 18 strains isolated in this study, nine represented four undescribed species of which three are formally described below. The remaining nine isolates were Symmetrospora (cf.) coprosmae (MT 262, MT 264, P 116, HU9059, HU9256), S. oryzicola (MCA4496, MCA4497), and S. symmetrica (P 118, SA107). Characteristics of colony and cell morphology were not sufficient to differentiate among the new Symmetrospora species. The colony pigmentation for most of the studied strains was dark pink to orange-red, whereas the color of S. pseudomarina strains varied from salmon pink (SA716) to red (SA42). All strains had butyrous colonies with entire margins, only presenting some variation in the shiny or dull appearance. However, results from assimilation studies (Table 2) supported the delimitation of species based on our molecular phylogenetic data.
Table 2.
Physiological differences between Symmetrospora clarorosea sp. nov., S. pseudomarina sp. nov., S. suhii sp. nov., and their closest related species.
| Compound | S. clarorosea | S. gracilis | S. marina | S. pseudomarina | S. suhii | S. symmetrica | S. vermiculata |
|---|---|---|---|---|---|---|---|
| Sucrose | + | - | + | + | + | + | l |
| Melibiose | - | - | - | - | u | - | - |
| Galactose | + | - | + | u | u | - | + |
| Lactose | u | + | - | + | + | + | + |
| Trehalose | u | + | - | + | + | + | + |
| Maltose | u | - | + | - | + | - | - |
| Melezitose | + | - | w/s | + | u | + | + |
| Cellobiose | + | + | + | + | u | + | l |
| D-Xylose | + | + | s | + | + | - | + |
| L-Arabinose | + | - | s | + | u | - | + |
| L-Rhamnose | u | - | s | +a | - | - | - |
| L-Sorbose | u | - | w/s | - | - | - | l/w |
| Ribitol | + | + | w/s | + | + | + | l/w |
| Nitrate | + | - | - | u | w | - | - |
+, growth; -, no growth; l, latent; s, positive but slow; v, variable; w, weak; u, uncertain. Data for the new species taken from the ex-type strains (S. clarorosea, WRP 7; S. pseudomarina, SA716; S. suhii, BG 02-5-27-3-2-2). Data for the reference species taken from Kurtzman et al. (2011).
a The assimilation of L-rhamnose was uncertain for the ex-type strain but positive for the other strain tested (SA42).
Taxonomy
Symmetrospora Q.M. Wang et al., Stud. Mycol. 81: 175. 2015.
Type species: Symmetrospora gracilis (Derx) Q.M. Wang et al., Stud. Mycol. 81: 176. 2015.
Symmetrospora clarorosea Toome, Albu & Aime, sp. nov. MycoBank MB809695. Fig. 2A.
Fig. 2.
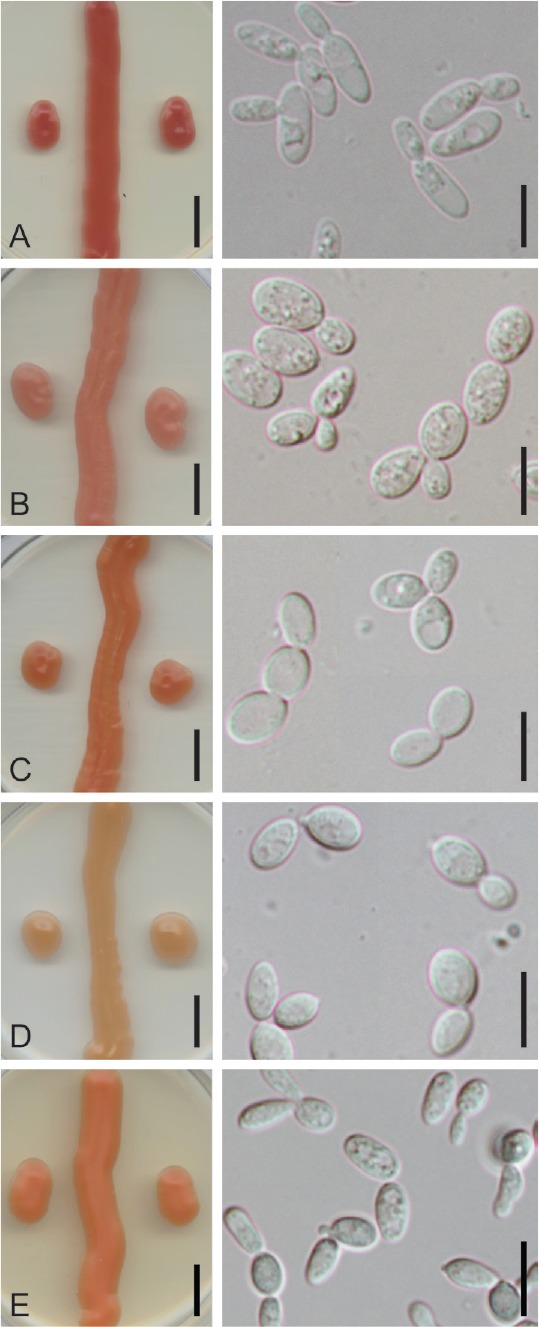
Colony and cell morphology of Symmetrospora species on YMA (left panels) and YM broth (right panels): A. Symmetrospora clarorosea strain SA308. B. Symmetrospora oryzicola strain MCA 4496. C–D. Symmetrospora pseudomarina strains SA42 (C) and SA716 (D, ex-type), showing the variation in colony color between the two strains. E. Symmetrospora suhii strain BG 02-5-27-3-2-2 (ex-type). Scale bars = 1 cm in culture images (left panels), 10 μm in cell images (right panels).
Etymology: Referring to the color of the colonies on solid media (clarus = bright, roseus = pink).
Diagnosis: LSU shares 97.84 % identity with ex-type sequence of S. gracilis (13 nt different); ITS shares 96.15 % identity with ex-type sequence of S. gracilis (23 nt different). Different from S. gracilis by the ability to assimilate sucrose, galactose, melezitose, and L-arabinose.
Typus: USA, Louisiana, Florida Parishes Region, East Baton Rouge Parish, Baton Rouge, Louisiana State University campus, 30.407817N, 91.176187W, 27 Jan. 2009, W.R. Pilcher, surface of Quercus virginiana leaf (Fagales, Fagaceae), WRP 7 (dried inert material at PUL holotype), ex-type culture at CBS (CBS 14055), GenBank accession nos. KJ701233 (SSU), KJ701231 (ITS), KJ701232 (LSU).
Description: Colonies on YMA are butyrous, smooth, with entire margins, shiny or dull, pink (oac573 on YMA but oac574 on CMA). Growth at 20–25 °C (optimal); no growth at 30 °C. Yeast cells after 5 d in YM broth ellipsoid, 2–5 × 5–10 μm (av. 3.4 × 7.2 μm), with length/width ratio of 1.4–3.5 (av. 2.2); polar budding from a narrow base, generally only one bud per cell; ballistosporic. Fermentation ability absent. Carbon compounds assimilated: D-cellobiose, gentiobiose, maltotriose, melezitose, sucrose, turanose, D-glucose, galactose, methyl-α-D-glucoside, D-mannitol, D-sorbitol, adonitol, D-arabitol, ribitol, glycerol, L-arabinose, and D-xylose. No growth on melibiose, D-glucosamine, amygdalin, and erythritol. Nitrogen compounds assimilated: nitrate, D-tryptophan, L-lysine (variable), and cadaverine (variable). Additional compounds assimilated: D-gluconic acid and 2-keto-D-gluconic acid. Osmotic stress: no growth in the presence of 10 % NaCl or 50 % glucose. No pseudohyphae or hyphae observed. Sexual morph unknown.
Additional material examined: USA, Louisiana, Florida Parishes Region, East Baton Rouge Parish, Baton Rouge, Louisiana State University campus, 30.407817N, 91.176187W, 13 Apr. 2011, S. Albu, surface of Lygopodium japonicum leaf (Schizaeales, Lygodiaceae), SA333, referred to as Sporobolomyces sp. cf. gracilis 1 in Albu (2012), culture at CBS (CBS 14085), GenBank accession nos. KJ701230 (SSU), KJ701228 (ITS), KJ701229 (LSU); Ibid., Jan. 2009, W.R. Pilcher, surface of Salix sp. leaf (Malpighiales, Salicaceae), WRP 8, culture at CBS (CBS 14093), GenBank accession nos. KJ701236 (SSU), KJ701234 (ITS), KJ701235 (LSU); Louisiana, Florida Parishes Region, East Baton Rouge Parish, Baton Rouge, Louisiana State University campus, 30.409093N, 91.176428W, 18 Mar. 2011, S. Albu, surface of Dryopteris erythrosora leaf (Polypodiales, Dryopteridaceae), SA308, referred to as Sporobolomyces sp. cf. gracilis 2 in Albu (2012), GenBank accession nos. KJ701227 (SSU), KJ701225 (ITS), KJ701226 (LSU).
Habitat and distribution: On leaf surfaces in North America (USA, Louisiana).
Notes: A Portuguese strain, CBS 10200 (Inácio et al. 2009), appears to be conspecific to S. clarorosea based on its published ITS and LSU rDNA sequences (Table 3). These sequences were submitted to GenBank as Symmetrospora sp.
Table 3.
Additional strains downloaded from NCBI GenBank of Symmetrospora clarorosea sp. nov., S. pseudomarina sp. nov., and S. suhii sp. nov., with original identification in GenBank, accession numbers for ITS and LSU, BLAST results, and references.
| Strain | ID in GenBank | Species | Origin | ITS | ITS BLAST | LSU | LSU BLAST | References |
|---|---|---|---|---|---|---|---|---|
| CBS 10200 | Symmetrospora sp. | Symmetrospora clarorosea | Portugal | EU002879 | 98.91 % | EU002821 | 99.65 % | Inácio et al. 2009 |
| A31 | Symmetrospora marina | Symmetrospora pseudomarina | Brazil | KM246155 | 98.42 % | KM246010 | 99.67 % | T.S. Leite et al. unpubl. data |
| DMKU5-4 | Symmetrospora sp. | Symmetrospora suhii | Thailand | LC216897 | 100 % | LC216897 | 99.83 % | S. Limtong & C. Kaewkrajay unpubl. data |
| IMUFRJ 52025 | Symmetrospora aff. marina | Symmetrospora suhii | Brazil | FN428894 | 99.33 % | FN428894 | 99.84 % | J.R.A. Ribeiro unpubl. data |
| IMUFRJ 52026 | Symmetrospora aff. marina | Symmetrospora suhii | Brazil | FN428925 | 99.48 % | FN428925 | 99.67 % | J.R.A. Ribeiro unpubl. data |
| SM10 | Symmetrospora sp. | Symmetrospora suhii | Taiwan | FJ515188 | 100 % | FJ515243 | 99.66 % | Chang et al. (2016) |
Symmetrospora oryzicola (Nakase & M. Suzuki) Haelew. & Aime, comb. nov. MycoBank MB833757. Fig. 2B.
Basionym: Sporobolomyces oryzicola Nakase & M. Suzuki, J. Gen. Appl. Microbiol., Tokyo 32: 152. 1986.
Description: Colonies on YMA butyrous, smooth but becoming verrucose in age, with entire margins, dull, dark pink to red (oac588 on YMA but oac577 on CMA). Growth at 20–25 °C (optimal) and at 30 °C (weak). Yeast cells after 5 d in YM broth subglobose, 4–6 × 6–8 μm (av. 4.9 × 7.2 μm), with length/width ratio of 1.2–1.75 (av. 1.5); polar budding from a narrow base; ballistosporic. No pseudohyphae or hyphae observed. Sexual morph unknown.
Materials examined: Taiwan, Southern Taiwan Region, Tainan City, Shanhua District, World Vegetable Center (Asian Vegetable Research and Development Center), 23.115782N, 120.298994E, 25 Jul. 2011, M.C. Aime, surface of Vigna sp. leaf (Fabales, Fabaceae), MCA 4496, culture at CBS (CBS 14050), GenBank accession nos. KJ701195 (SSU), KJ701193 (ITS), KJ701194 (LSU); Ibid., MCA 4497, GenBank accession nos. KJ701198 (SSU), KJ701196 (ITS), KJ701197 (LSU).
Habitat and distribution: On leaf surfaces in Asia (Japan, Taiwan).
Notes: The sister species of S. oryzicola is S. coprosmae, which is reported from various substrates in Europe (Austria, Molnár et al. 2008), North America (USA, Indiana & Michigan, this study), and Oceania (New Zealand, ex-type strain, Hamamoto & Nakase 1995). In contrast, S. oryzicola is much less commonly found; in addition to the two strains from Taiwan presented here, only the Japanese ex-type strain of S. oryzicola is known (Nakase & Suzuki 1986).
Symmetrospora pseudomarina Haelew., Albu & Aime, sp. nov. MycoBank MB809701. Fig. 2C–D.
Etymology: Referring to similarities and past confusion with S. marina.
Diagnosis: LSU shares 99.83 % identity with ex-type sequence of S. marina (1 nt different) and 99.49 % with ex-type sequence of S. vermiculata (3 nt different); ITS shares 98.24 % identity with ex-type sequence of S. marina (10 nt different) and 97.18 % with ex-type sequence of S. vermiculata (15 nt different). Different from S. marina by the ability to assimilate lactose and D-trehalose, and the inability to assimilate maltose and L-sorbose. Different from S. vermiculata by the ability to assimilate L-rhamnose and the inability to assimilate L-sorbose.
Typus: USA, Louisiana, Florida Parishes Region, East Baton Rouge Parish, Baton Rouge, Louisiana State University campus, 30.409093N, 91.176428W, 1 Nov. 2011, S. Albu, surface of Dryopteris erythrosora leaf (Polypodiales, Dryopteridaceae), SA716 (dried inert material at PUL holotype), referred to as Rhodotorula marina 3 in Albu (2012), ex-type culture at CBS (CBS 14057), GenBank accession nos. KJ701218 (SSU), KJ701216 (ITS), KJ701217 (LSU).
Description: Colonies on YMA butyrous, smooth, with entire margins, shiny, salmon pink to red (culture SA42 oac617 on YMA but oac618 on CMA; culture SA716 oac619 on YMA and CMA). Growth at 20–25 °C (optimal) and at 30 °C (variable); no growth at 35 °C. Yeast cells after five days in YM broth globose to ovoid, 3–5 × 4–8 μm (av. 3.8 × 5.5 μm), with length/width ratio of 1–2.3 (av. 1.5); polar budding from a narrow base, generally 1–2 buds per cell; ballistosporic. Fermentation ability absent. Carbon compounds assimilated: D-cellobiose, gentiobiose, melezitose, sucrose, trehalose, D-glucose, methyl-β-D-glucoside, arbutin, D-mannitol, D-sorbitol, adonitol, D-arabitol, ribitol, glycerol, D-arabinose, L-arabinose, and D-xylose. Variable growth on maltotriose, palatinose, turanose, D-psicose, and L-rhamnose. No growth on maltose, melibiose, stachylose, D-glucosamine, L-sorbose, and erythritol. Nitrogen compounds assimilated: L-lysine and D-tryptophan. Additional compounds assimilated: fumaric acid, L-malic acid, bromosuccinic acid, L-glutamic acid, and D-gluconic acid. Osmotic stress: no growth in the presence of 10 % NaCl or 50 % glucose. No pseudohyphae or hyphae observed. Sexual morph unknown.
Additional material examined: USA, Louisiana, Rapides Parish, Lena, Kisatchie National Forest, in the vicinity of 31.583217N, 92.544855W, 9 Oct. 2010, S. Albu, decaying wood, SA42, culture at CBS (CBS 14084), GenBank accession nos. KJ701215 (SSU), KJ701213 (ITS), KJ701214 (LSU).
Habitat and distribution: On leaf surfaces in North America (USA) and South America (Brazil) and on decaying wood in North America (USA).
Notes: One strain for which ITS and LSU sequences have been published (T.S. Leite et al. unpubl. data) is conspecific with S. pseudomarina. This strain, accessioned as S. marina, was isolated from Coffea arabica var. Catucaí Amarel (Gentianales, Rubiaceae) in Brazil (details in Table 3). Symmetrospora pseudomarina is distinguished from sister species S. marina by several characteristics: rDNA sequence data (Fig. 1), habitat (marine in S. marina versus phylloplane in S. pseudomarina), assimilation profiles (Atkin et al. 1970, Sampaio 2011), and colony color (pink in S. marina versus variable in S. pseudomarina).
Symmetrospora suhii Toome & Aime, sp. nov. MycoBank MB809699. Fig. 2E.
Etymology: Named after Dr. Sung-Oui Suh, scientist at the American Type Culture Collection, who isolated and partially characterized the ex-type strain of this species.
Diagnosis: LSU shares 98.67 % identity with ex-type sequence of S. marina (8 nt different), 98.47 % with ex-type sequence of S. vermiculata (9 nt different), and 98.43 % with ex-type sequence of S. pseudomarina (9 nt different); ITS shares 95.18 % identity with ex-type sequence of S. marina (28 nt different), 95.29 % with ex-type sequence of S. pseudomarina (27 nt different), and 94.74 % with ex-type sequence of S. vermiculata (29 nt different). Different from S. vermiculata by the ability to assimilate maltose and the inability to assimilate L-sorbose. Different from S. marina by the ability to assimilate lactose, trehalose, and nitrate, and the inability to assimilate L-sorbose and L-rhamnose. Different from S. pseudomarina by the ability to assimilate maltose and the inability to assimilate L-rhamnose.
Typus: USA, Louisiana, Florida Parishes Region, East Baton Rouge Parish, Baton Rouge, Rural Life Museum, 27 May 2002, S.-O. Suh, gut of staphylinid beetle (Coleoptera, Staphylinidae) collected from mushroom, BG 02-5-27-3-2-2 (dried inert material at PUL holotype), ex-type culture at CBS (CBS 14094), GenBank accession nos. AY520260 (SSU), KJ701206 (ITS), AY520389 (LSU).
Description: Colonies on YMA butyrous, smooth, with entire margins, shiny or dull, occasionally elevated in the center, red-orange (oac649 on YMA but oac650 on CMA). Growth at 20–25 °C (optimal), and at 30 °C (weak); no growth at 35 °C. Yeast cells after 5 d in YM broth ellipsoid, 3–4 × 4–7 μm (av. 3.7 × 5.4 μm), with length/width ratio of 1–1.75 (av. 1.5); polar budding from a narrow base, occasionally more than one bud per cell; ballistosporic. Fermentation ability absent. Carbon compounds assimilated: gentiobiose, maltose, palatinose, sucrose, trehalose, maltitol, D-mannitol, D-sorbitol, adonitol, D-arabitol, glycerol, D-ribose, and D-xylose. No growth on L-rhamnose, L-sorbose, and erythritol. Nitrogen compounds assimilated: ethylamine, cadaverine, creatine, D-tryptophan, and nitrate (weak). Osmotic stress: no growth in the presence of 10 % NaCl or 50 % glucose. No pseudohyphae or hyphae observed. Sexual morph unknown.
Habitat and distribution: In beetle gut in North America (USA); on leaf surfaces in South America (Brazil); in marine water in Asia (Taiwan, Thailand).
Notes: Four strains appear to be conspecific with S. suhii based on their published ITS and LSU rDNA sequences (Table 3). Their sequences were submitted to GenBank under different names. These strains are: DMKU 5–4 (from a sea sponge/marine water in Thailand, S. Limtong & C. Kaewkrajay unpubl. data); IMUFRJ 52025 and IMUFRJ 52026 (from sugarcane leaves in Brazil, as S. aff. marina, J.R.A. Ribeiro unpubl. data); SM10 (in marine water in Taiwan, Chang et al. 2016).
Additional materials examined
Symmetrospora coprosmae (Hamam. & Nakase) Q.M. Wang et al., Stud. Mycol. 81: 175. 2015.
Basionym: Bullera coprosmae Hamam. & Nakase, Antonie Leeuwenhoek 69: 281. 1996.
Materials examined: USA, Illinois, Cook County, Chicago, 18 May 2016, H. Urbina, surface of Lactuca sativa leaf (Asterales, Asteraceae), lettuce head no. L30, HU9256, GenBank accession nos. MN586904 (ITS), MN586902 (LSU), as S. cf. coprosmae; Indiana, Tippecanoe County, Wabash Township, West Lafayette, 40.455385N, 86.917498W, 5 May 2016, H. Urbina, surface of Lactuca sativa leaf, lettuce head no. L13, HU9059, GenBank accession no. MN586903 (ITS), as S. cf. coprosmae; Louisiana, Orleans Parish, New Orleans, Audubon Park, 29.924645N, 90.129245W, 13 Nov. 2010, S.L. Newerth, surface of Cyrtomium falcatum leaflet (Polypodiales, Dryopteridaceae), P 116, GenBank accession nos. KJ701204 (SSU), KJ701202 (ITS), KJ701203 (LSU); Michigan, Emet County, Cross Village Township, 45.645097N, 85.039827W, 1 Sep. 2013, M. Toome-Heller, surface of Salix sp. leaf infected with Melampsora sp. (Pucciniales, Melampsoraceae), MT 262, GenBank accession nos. KJ701201 (SSU), KJ701199 (ITS), KJ701200 (LSU); Ibid., MT 264, GenBank accession no. KJ701205 (ITS).
Symmetrospora symmetrica (F.Y. Bai & Q.M. Wang) Q.M. Wang et al., Stud. Mycol. 81: 176. 2015.
Basionym: Sporobolomyces symmetricus F.Y. Bai & Q.M. Wang, FEMS Yeast Res. 4: 584. 2004.
Materials examined: USA, Indiana, Tippecanoe County, Wabash Township, West Lafayette, Purdue University campus, 40.422300N, 86.917383W, 14 Jun. 2013, S.L. Newerth, surface of Pinus nigra leaf (Pinales, Pinaceae), P 118, culture at CBS (CBS 14058), GenBank accession nos. KJ701212 (SSU), KJ701210 (ITS), KJ701211 (LSU); Louisiana, Florida Parishes Region, East Baton Rouge Parish, Baton Rouge, Louisiana State University campus, 30.411000N, 91.177300W, 11 Nov. 2010, S. Albu, indoor air contaminant, SA107, culture at CBS (CBS 14059), GenBank accession nos. KJ701209 (SSU), KJ701207 (ITS), KJ701208 (LSU).
DISCUSSION
The asexual red-yeast genera Sporobolomyces and Rhodotorula are polyphyletic (e.g., Nakase et al. 1993, Fell et al. 2000, Hamamoto & Nakase 2000, Aime et al. 2006, Boekhout et al. 2011, Wang et al. 2015a). Wang et al. (2015b) provided a taxonomic infrastructure for the gracilis/marina clade of the Cystobasidiomycetes and introduced the genus Symmetrospora with six species. Even though Sporobolomyces oryzicola was part of the maximally supported Symmetrospora clade in their LSU rDNA phylogenetic reconstruction, the authors did not introduce the new combination. In our three-locus phylogeny, S. oryzicola was maximally supported as a sister species of S. coprosmae. Hence, we formally include it in the genus Symmetrospora.
This study reveals that Symmetrospora is more diverse and more broadly distributed than currently recognized. Our opportunistic collecting, mostly from university campuses, increased the number of known species in the group by two thirds and expanded known geographic ranges for previously described species. Our records of S. coprosmae and S. symmetrica are the first ones for the USA, and our strains of S. oryzicola represent the first report of this species outside Japan. Overall, we present the first reports of any species in this genus for North America. We also consider sequences from strains previously isolated by other researchers in Table 3, which represent additional isolates of the species described here. These isolates have identical or near-identical ITS and/or LSU sequences to our type strains. By considering these isolates, we were able to reveal broader occurrences of the new species. For example, we collected S. suhii from Louisiana, USA, but sequence data from GenBank revealed that this species is likely more broadly distributed – with isolates from South America and Asia (Chang et al. 2016, S. Limtong & C. Kaewkrajay unpubl. data, J.R.A. Ribeiro unpubl. data).
In addition to expanding both the number of species in the genus and distributional ranges, our study also reveals a diverse array of habitats for Symmetrospora species. Whereas most species were isolated from the phylloplane, several strains were from the air, marine water, the beetle gut, and a sea sponge. This is in addition to previous work, which reported the isolation of a strain of S. symmetrica from Pleurotus eryngii, causing red spot disease (Xu et al. 2014). When isolated from the phylloplane, no preference for host plants can be detected. As an example, S. clarorosea was isolated from leaves of two unrelated fern species and two unrelated species of eudicots. We also found more than one species of yeast from a single host plant; strains of both newly described species S. clarororea and S. pseudomarina were isolated from leaf surfaces of D. eyrthrosora.
We confirm previous findings that culture-based sampling from the surface of leaves, referred to as the phylloplane, effortlessly results in the discovery of undescribed species. During an ongoing study of the fungal microbiome of romaine lettuce, we sequenced the ITS region of 330 strains, resulting in 63 species of which 11 are undescribed (Urbina & Aime 2018, D. Haelewaters & M.C. Aime, unpubl. data). Two of these strains isolated from romaine lettuce leaves, HU9059 and HU9256, were identified as S. cf. coprosmae and are reported here. Whereas a number of these new species have been described over the last years (e.g., Inácio et al. 2002, 2005, Péter et al. 2007, Golubev & Scorzetti 2010, Toome et al. 2013, Wang et al. 2016, Limtong et al. 2017), many remain undescribed even though they have been recognized as new. One of such examples was a two-year survey of phylloplane yeasts at Nature Park of Arrábida in Portugal that resulted in over 850 isolates representing 70 species, half of which may be new to science (Inácio et al. 2002). Likewise, a survey targeting phylloplane-colonizing basidiomycete yeasts reported 29 potential new species from a collection of 463 isolates, including the type strain of S. pseudomarina (Albu 2012). It seems that the extent of species diversity in the leaf habitat is not fully understood yet, highlighting the importance of further studies to capture the hidden fungal biodiversity.
ACKNOWLEDGMENTS
We thank Joseph McHugh (University of Georgia, Athens, Georgia) for identifying the source beetle of strain BG 02 5-27-3-2-2; Shannon L. Newerth and John M. Cavaletto for the isolation of strains P 114, P 115, P 116, and P 118; Whitney R. Pilcher for the isolation of strains WRP 7 and WRP 8; Meredith Blackwell (Louisiana State University, Baton Rouge, Louisiana & University of South Carolina, Columbia, South Carolina) and Sung-Oui Suh (American Type Culture Collection, Manassas, Virginia) for isolating and partially characterizing strain BG 02 5-27-3-2-2; Shaun Pennycook (Landcare Research, Lincoln, New Zealand) for revising the Latin names of the new species and combination; Tom Creswell, Gail Ruhl, Anna Meier, and Chris Speers (Purdue University Plant and Pest Diagnostic Laboratory) for assistance with using microscopy facilities and the microplate reader; Paige Muse, John F. Klimek, and Ramandeep Kaur for assistance with culture maintenance at Louisiana State University and Purdue University; Prof. Shean S. Tzean and Dr. Jaw-Fen Wang for hosting M.C. Aime in Taiwan; and two anonymous reviewers for very useful suggestions to a previous version of the manuscript. D.H. is supported by the Center for Food Safety Engineering at Purdue University funded by the US Department of Agriculture (USDA), Agricultural Research Service, under project 8072-42000-077-00D. The collection of strain BG 02 5-27-3-2-2 was supported by the US National Science Foundation (DEB-0072741 and DEB-0417180 to Meredith Blackwell). The generation of sequence data was supported in part by the National Science Foundation Assembling the Fungal Tree of Life project (NSF DEB-0732968) to David Hibbett and M.C.A. Collecting on the island of Taiwan and in Louisiana was supported in part by funding from the Louisiana Board of Regents to M.C.A. This work was supported in part by the USDA National Institute of Food and Agriculture Hatch project 1010662.
Conflict of interest: The authors declare that there is no conflict of interest
REFERENCES
- Aime MC, Matheny PB, Henk DA, et al. (2006). An overview of the higher-level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences. Mycologia 98: 895–905. [DOI] [PubMed] [Google Scholar]
- Albu S. (2012). A survey of ballistosporic phylloplane yeasts in Baton Rouge, Louisiana. MSc thesis, Louisiana State University, Baton Rouge, Louisiana. [Google Scholar]
- Atkin CL, Neilands JB, Phaff HJ. (1970). Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. Journal of Bacteriology 103: 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai FY, Takashima M, Hamamoto M, et al. (2001). Sporobolomyces yunnanensis sp. nov., a Q-10 (H2)-containing yeast species with a close phylogenetic relationship to Erythrobasidium hasegawianum. International Journal of Systematic and Evolutionary Microbiology 51: 231–235. [DOI] [PubMed] [Google Scholar]
- Boekhout T. (1991). A revision of ballistoconidia-forming yeasts and fungi. Studies in Mycology 33: 1–194. [Google Scholar]
- Boekhout T, Fonseca A, Sampaio JP, et al. (2011). Chapter 100. Discussion of teleomorphic and anamorphic basidiomycetous yeasts. In: The yeasts, a taxonomic study. Volume 3. Fifth edition (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Amsterdam: 1339–1372. [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. (2009). TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Lee CF, Lin KY, et al. (2016). Diversity of yeasts associated with the sea surface microlayer and underlying water along the northern coast of Taiwan. Research in Microbiology 167: 35–45. [DOI] [PubMed] [Google Scholar]
- Chernomor O, von Haeseler A, Minh BQ. (2016). Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derx HG. (1930). Etude sur les Sporobolomycetes. Annales Mycologici 28: 1–23. [Google Scholar]
- Edgar RC. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell JW, Boekhout T, Fonseca A, et al. (2000). Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. International Journal of Systematic and Evolutionary Microbiology 50: 1351–1371. [DOI] [PubMed] [Google Scholar]
- Fonseca Á, Inácio J. (2006). Phylloplane yeasts. In: Biodiversity and ecophysiology of yeasts (Rosa C, Gábor P, eds). Springer-Verlag: 63–301. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhiza and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Golubev WI, Scorzetti G. (2010). Rhodotorula rosulata sp. nov., Rhodotorula silvestris sp. nov. and Rhodotorula straminea sp. nov., novel myo-inositolassimilating yeast species in the Microbotryomycetes. International Journal of Systematic and Evolutionary Microbiology 60: 2501–2506. [DOI] [PubMed] [Google Scholar]
- Hamamoto M, Boekhout T, Nakase T. (2011). Chapter 156. Sporobolomyces Kluyver & van Niel (1924). In: The yeasts, a taxonomic study. Volume 3. Fifth edition (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Amsterdam: 1929–1990. [Google Scholar]
- Hamamoto M, Nakase T. (1995). Ballistosporous yeasts found on the surface of plant materials collected in New Zealand. 1. Six new species in the genus Sporobolomyces. Antonie van Leeuwenhoek 67: 151–171. [DOI] [PubMed] [Google Scholar]
- Hamamoto M, Nakase T. (2000). Phylogenetic analysis of the ballistoconidium-forming yeast genus Sporobolomyces based on 18S rDNA sequences. International Journal of Systematic and Evolutionary Microbiology 50: 1373–1380. [DOI] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, et al. (2018). UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio J, Ludwig W, Spencer-Martins I, et al. (2009). Assessment of phylloplane yeasts on selected Mediterranean plants by FISH with group-and species-specific oligonucleotide probes. FEMS Microbiology Ecology 71: 61–72. [DOI] [PubMed] [Google Scholar]
- Inácio J, Pereira P, de Carvalho M, et al. (2002). Estimation and diversity of phylloplane microbiota on selected plants in a Mediterraneantype ecosystem in Portugal. Microbial Ecology 44: 344–353. [DOI] [PubMed] [Google Scholar]
- Inácio J, Portugal L, Spencer-Martins I, et al. (2005). Phylloplane yeasts from Portugal: seven novel anamorphic species in the Tremellales lineage of the Hymenomycetes (Basidiomycota) producing orangecoloured colonies. FEMS Yeast Research 5: 1167–1183. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy K, Minh BQ, Wong TKF, et al. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LA. (2004). The Online Auction Color Chart. Online Auction Color Chart Company, Stanford, 1–12. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman CP, Fell JW, Boekhout T. (2011). The yeasts, a taxonomic study. Volume 1. Fifth edition. Elsevier, Amsterdam. [Google Scholar]
- Kurtzman CP, Robnett CJ. (1998). Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 73: 331–371. [DOI] [PubMed] [Google Scholar]
- Limtong S, Polburee P, Chamnanpa T, et al. (2017). Meira siamensis sp. nov., a novel anamorphic ustilaginomycetous yeast species isolated from the vetiver grass phylloplane. International Journal of Systematic and Evolutionary Microbiology 67: 2418–2422. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Gossmann JA, Zalar P, et al. (2006). Resolving the phylogenetic position of the Wallemiomycetes: an enigmatic major lineage of Basidiomycota. Canadian Journal of Botany 84: 1794–1805. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop 14 Nov. 2010: 1–8. New Orleans, Louisiana. [Google Scholar]
- Nagahama T, Hamamoto M, Nakase T, et al. (2006). Phylogenetic relationship within the Erythrobasidium clade: molecular phylogenetics, secondary structure, and intron positions inferred from partial sequences of ribosomal RNA and elongation factor-1α genes. Journal of General and Applied Microbiology 52: 37–45. [DOI] [PubMed] [Google Scholar]
- Nakase T, Suzuki M. (1986). Bullera intermedia sp. nov. and Sporobolomyces oryzicola sp. nov. isolated from dead leaves of Oryza sativa. Journal of General and Applied Microbiology 32: 149‒155. [Google Scholar]
- Nakase T, Takematsu A, Yamada Y. (1993). Molecular approaches to the taxonomy of ballistosporous yeasts based on the analysis of the partial nucleotide sequences of 18S ribosomal ribonucleic acids. Journal of General and Applied Microbiology 39: 107–134. [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, et al. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péter G, Tornai-Lehoczki J, Dlauchy D. (2007). Ogataea allantospora sp. nov., an ascomycetous yeast species from phylloplane. Antonie van Leeuwenhoek 92:443–448. [DOI] [PubMed] [Google Scholar]
- Phaff HJ, Mrak EM, Williams OB. (1952). Yeasts isolated from shrimp. Mycologia 44: 431–451. [Google Scholar]
- Phaff HJ. (1990). Isolation of yeasts from natural sources. In: Isolation of biotechnological organisms from nature (Labeda DP, ed). McGraw-Hill Publishing Company, New York: 53–79. [Google Scholar]
- Rehner SA, Samuels GJ. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Sampaio JP. (2011). Chapter 155. Rhodotorula Harrison (1928). In: The yeasts, a taxonomic study. Volume 3 Fifth edition (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Amsterdam: 1873–1927. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the USA 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorzetti G, Fell JW, Fonseca A, et al. (2002). Systematics of basidiomycetous yeasts: a comparison of large subunit D1/D2 and internal transcribed spacer rDNA regions. FEMS Yeast Research 2: 495‒517. [DOI] [PubMed] [Google Scholar]
- Shivas RG, de Miranda LR. (1983). Two new species of the genus Sporobolomyces and a new Rhodotorula species from leaf surfaces. Antonie van Leeuwenhoek 49: 159–166. [DOI] [PubMed] [Google Scholar]
- Suh SO, McHugh JV, Pollock DD, et al. (2005). The beetle gut: a hyperdiverse source of novel yeasts. Mycological Research 109: 261‒265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SO, Sugiyama J. (1993). Phylogeny among the basidiomycetous yeasts inferred from small subunit ribosomal DNA sequence. Microbiology 139: 1595–1598. [DOI] [PubMed] [Google Scholar]
- Suh S-O, Zhang N, Nguyen N, et al. (2008). Lab manual for yeast study. Louisiana State University, Baton Rouge, Louisiana. Takashima M, Nakase T (2000). Four new species of the genus Sporobolomyces isolated from leaves in Thailand. Mycoscience 41: 357‒369. [Google Scholar]
- Toome M, Roberson RW, Aime MC. (2013). Meredithblackwellia eburnea gen. et sp. nov., Kriegeriaceae fam. nov. and Kriegeriales ord. nov. – toward resolving higher-level classification in Microbotryomycetes. Mycologia 105: 486–495. [DOI] [PubMed] [Google Scholar]
- Urbina H, Aime MC. (2018). A closer look at Sporidiobolales: Ubiquitous microbial community members of plant and food biospheres. Mycologia 110: 79–92. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, Szöke S, et al. (2016). DNA barcoding analysis of more than 9 000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Studies in Mycology 85: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q-M, Bai F-Y. (2004). Four new yeast species of the genus Sporobolomyces from plant leaves. FEMS Yeast Research 4: 579‒586. [DOI] [PubMed] [Google Scholar]
- Wang Q-M, Groenewald M, Takashima M, et al. (2015a). Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequence analyses. Studies in Mycology 81: 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q-M, Yurkov AM, Göker M, et al. (2015b). Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Studies in Mycology 81: 149–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Zhang YH, Wang B, et al. (2016). Talaromyces neofusisporus and T. qii, two new species of section Talaromyces isolated from plant leaves in Tibet, China. Scientific Reports 6: 18622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee SJ, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, et al. eds). Academic Press, San Diego: 315–322. [Google Scholar]
- Xu F, Wang SX, Liu Y, et al. (2014). First report of Sporobolomyces symmetricus induced red spot disease of Pleurotus eryngii in China. Plant Disease 98: 693. [DOI] [PubMed] [Google Scholar]


