Abstract
The present paper represents the fifth contribution in the Genera of Fungi series, linking type species of fungal genera to their morphology and DNA sequence data. This paper focuses on 11 genera of microfungi, for seven of which the type species are neo- or epitypified here: Arthrinium (Arthrinium caricicola; Apiosporaceae, Xylariales, Sordariomycetes), Ceratosphaeria (Ceratosphaeria lampadophora; Magnaporthaceae, Magnaporthales, Sordariomycetes), Dimerosporiopsis (Dimerosporiopsis engleriana; Venturiaceae, Venturiales, Dothideomycetes), Hormodochis (Hormodochis melanochlora; Stictidaceae, Ostropales, Ostropomycetidae, OSLEUM clade, Lecanoromycetes), Lecanostictopsis (Lecanostictopsis kamatii; Mycosphaerellaceae, Capnodiales, Dothideomycetes), Lembosina (Lembosina aulographoides; Lembosinaceae fam. nov., Lembosinales ord. nov., Dothideomycetes), Neomelanconium (Neomelanconium gelatosporum; Cenangiaceae, Helotiales, Leotiomycetes), Phragmotrichum (Phragmotrichum chailletii; Melanommataceae, Pleosporales, Pleosporomycetidae, Dothideomycetes), Pseudomelanconium gen. nov. (Pseudomelanconium spartii; incertae sedis, Pezizomycotina), Rutola (Rutola graminis; Torulaceae, Pleosporales, Pleosporomycetidae, Dothideomycetes), and Trullula (Trullula oreoselini; incertae sedis, Pezizomycotina).
Keywords: biodiversity, ITS barcodes, multi-gene phylogeny, new taxa, systematics, typification
INTRODUCTION
This paper represents the fifth contribution to the Genera of Fungi (GoF) project (www.GeneraOfFungi.org; Crous et al. 2014), which has the aim to revise the generic names of fungi. The 11 genera treated are supplemented with recently collected specimens, with those designated as epi- or neotypes registered in MycoBank (Robert et al. 2013). Furthermore, in keeping with the one fungus = one name initiative for fungi (Hawksworth et al. 2011, Crous et al. 2015), a single morph is indicated for each genus. Mycologists and other researchers wishing to contribute to future issues of GoF are encouraged to contact Pedro Crous (p.crous@wi.knaw.nl).
MATERIALS AND METHODS
Isolates
Freshly collected twigs were placed in damp chambers, and incubated at room temperature (ca. 20 °C) for 1–2 d. Single conidial colonies were grown from sporulating sporocarps in Petri dishes containing 2 % malt extract agar (MEA) as described by Crous et al. (1991). Leaf and stem tissues bearing ascomata were soaked in water for approximately 2 h, after which they were attached to the undersides of the lids of Petri dishes containing MEA. Ejected ascospore germination patterns were determined on MEA after 24 h, and single ascospore or conidial cultures were established following the method described by (Crous 1998). Colonies were sub-cultured on 2 % potato-dextrose agar (PDA), oatmeal agar (OA), MEA (Crous et al. 2019), autoclaved pine needles on 2 % tap water agar (PNA) (Smith et al. 1996), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Reference strains and specimens of the studied fungi are maintained in the CBS culture collection (CBS) of the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands.
DNA extraction, amplification (PCR) and phylogeny
Fungal mycelium (Table 1) was scraped from the agar surface of cultures with a sterile scalpel and the genomic DNA was isolated using the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) following the manufacturers’ protocols. Seven loci were amplified following previously published protocols. First, the 28S nrRNA gene (LSU) and internal transcribed spacer regions with intervening 5.8S nrRNA gene (ITS) of the nrDNA operon were sequenced for all the isolates included in this study (for amplification conditions, see Fan et al. 2018). Other loci were sequenced for various species or genera using primers and conditions specific for those groups of fungi (Table 1). Amplification of the partial DNA-directed RNA polymerase II second largest subunit gene (rpb2), the partial translation elongation factor 1-alpha gene (tef1) and the partial beta-tubulin gene (tub2) followed Braun et al. (2018), while the amplification of the partial actin gene (act) followed Videira et al. (2016) and the partial DNA-directed RNA polymerase II largest subunit gene (rpb1) followed Klaubauf et al. (2014). The resulting fragments were sequenced in both directions using the respective PCR primers and the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA); DNA sequencing amplicons were purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences were analysed and consensus sequences were computed using SeqMan Pro v. 13 (DNASTAR, Madison, WI, USA).
Table 1.
Collection details and GenBank accession numbers of species treated or newly sequenced in the present study.
| Species | Strain(s)1 | Country | Substrate/Host | Collector and Collection date | GenBank accession numbers2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tefl | Other loci | |||||
| Arthrinium caricicola | ALV16691 | Germany | Carex ericetorum | R. Jarling, 23 Jun. 2018 | MK014871.1 | MK014838.1 | – | MK017948.1 | tub2: MK017977.1 |
| CPC 33297 = CBS 145903, ex-epitype | Germany | Carex ericetorum, dead and attached leaves | R.K. Schumacher, 4 Apr. 2017 | MN313782.1 | MN317266.1 | – | – | tub2: MN313861.1 | |
| CPC 33299 = CBS 144986 | Germany | Carex ericetorum, dead and attached leaves | R.K. Schumacher, 4 Apr. 2017 | MN313783.1 | MN317267.1 | – | - | tub2: MN313862.1 | |
| CPC 33368 | Germany | Carex ericetorum, dead and attached leaves | R.K. Schumacher, 4 Apr. 2017 | MN313784.1 | MN317268.1 | – | - | – | |
| Ceratosphaeria lampadophora | CBS 117555 | France | Populus tremula, decorticated wood | J. Fournier, 11 Apr. 2001 | - | AY761084.1 | – | – | SSU: AY761088.1 |
| CBS 125415 = MR 1834 = JF 01115 | France | Populus tremula, decorticated wood | J. Fournier, 18 Jun. 2001 | MH863598.1 | MH875074.1 | – | – | - | |
| CPC 33633 = CBS 144991 | France | Populus nigra, fallen and partly decorticated stem | M. Wilhelm, 15 Mar. 2017 | MN313785.1 | MN317269.1 | – | – | act: MN313860.1 | |
| Voucher SMH4822 | France | Branch on the ground | A.N. Miller et al., 25 Sep. 2002 | – | AY346270.1 | – | – | – | |
| Dimerosporiopsis engleriana | HPC 2591, epitype specimen | South Africa | Erica sp., stems | A. Wood, 24 Sep. 2014 | MN313786.1 | MN317270.1 | – | – | – |
| Hormodochis aggregata, sp. nov. | CPC 24027 | Germany | Cytisus scoparius (= Sarothamnus scoparius), twig | R.K. Schumacher, 21 Dec. 2013 | MN313787.1 | MN317271.1 | MN313819.1 | – | tub2: MN313863.1 |
| CPC 24159 | Germany | Cytisus scoparius (= Sarothamnus scoparius), twig | R.K. Schumacher, 21 Dec. 2013 | MN313788.1 | MN317272.1 | MN313820.1 | – | tub2: MN313864.1 | |
| CPC 24593 | Germany | Frangula alnus, twig | R.K. Schumacher, 5 Apr. 2014 | MN313789.1 | MN317273.1 | MN313821.1 | – | tub2: MN313865.1 | |
| CPC 26669 | Germany | Ulmus laevis, twig | R.K. Schumacher, 7 Mar. 2015 | MN313790.1 | MN317274.1 | MN313822.1 | MN313842.1 | – | |
| CPC 30017 | Germany | Prunus (= Cerasus) sp., twig | R.K. Schumacher, 9 Jan. 2016 | MN313791.1 | MN317275.1 | MN313823.1 | – | – | |
| CPC 30413 | Germany | Sorbus aucuparia | R.K. Schumacher, 17 Feb. 2016 | MN313792.1 | MN317276.1 | MN313824.1 | MN313843.1 | – | |
| CPC 30453 | Germany | Sambucus racemosa, twig | R.K. Schumacher, 12 Feb. 2016 | MN313793.1 | MN317277.1 | MN313825.1 | MN313844.1 | – | |
| CPC 30528 | Germany | Berberis vulgaris, twig | R.K. Schumacher, 2 Apr. 2016 | MN313794.1 | MN317278.1 | MN313826.1 | MN313845.1 | – | |
| CPC 30530 | Germany | Sambucus nigra, twig | R.K. Schumacher, 11 Mar. 2016 | MN313795.1 | MN317279.1 | MN313827.1 | MN313846.1 | - | |
| CPC 30580 | Germany | Solanum dulcamara, twig | R.K. Schumacher, 20 Apr. 2016 | MN313796.1 | – | – | - | – | |
| CPC 30630 | Germany | Sambucus nigra, twig | R.K. Schumacher, 1 Mar. 2016 | MN313797.1 | - | - | MN313847.1 | - | |
| CPC 30683 = CBS 145904, ex-type | Germany | Sorbus aucuparia, twig and bud | R.K. Schumacher, 27 Apr. 2016 | MN313798.1 | MN317280.1 | MN313828.1 | MN313848.1 | tub2: MN313866.1 | |
| CPC 30685 | Germany | Taxus baccata, twig | R.K. Schumacher, 2 May 2016 | MN313799.1 | MN317281.1 | MN313829.1 | MN313849.1 | – | |
| CPC 30737 | Germany | Hippophae rhamnoides (= Elaeagnus rhamnoides), twig | R.K. Schumacher, 30 Apr. 2016 | MN313800.1 | MN317282.1 | MN313830.1 | MN313850.1 | – | |
| CPC 30990 | Hungary | Lycium barbarum, twig | R.K. Schumacher, 1 May 2016 | MN313801.1 | MN317283.1 | MN313831.1 | MN313851.1 | – | |
| CPC 33325 | Germany | Ulmus laevis, twig | R.K. Schumacher, 3 Feb. 2017 | MN313802.1 | – | MN313832.1 | – | tub2: MN313867.1 | |
| CPC 33331 | Germany | Platanus hispanica, twig | R.K. Schumacher, 11 Mar. 2017 | MN313803.1 | MN317284.1 | MN313833.1 | – | – | |
| CPC 33913 | Germany | Viburnum opulus, twig | R.K. Schumacher, 2 Jun. 2017 | MN313804.1 | MN317285.1 | MN313834.1 | – | – | |
| CPC 35471 = CBS 145905 | Germany | Prunus cerasifera, attached dead twig | R.K. Schumacher, 25 Apr. 2018 | MN313805.1 | MN317286.1 | MN313835.1 | MN313852.1 | tub2: MN313868.1 | |
| CPC 35475 | Germany | Viscum album on Populus alba | R.K. Schumacher, 27 Mar. 2018 | MN313806.1 | MN317287.1 | MN313836.1 | MN313853.1 | – | |
| CPC 37499 | Germany | Crataegus sp., dead, attached, twig | R.K. Schumacher, 15 Feb.2019 | MN313807.1 | MN317288.1 | MN313837.1 | MN313854.1 | – | |
| Hormodochis melanochlora | CPC 24125 = CBS 138861, ex-epitype | Germany | Cytisus scoparius (= Sarothamnus scoparius), dead twig | R.K. Schumacher, 21 Dec. 2013 | KP004459.1 | KP004487.1 | MN313838.1 | MN313855.1 | tub2: MN313869.1 |
| Lecanostictopsis syzygii | HPC 2573, epitype specimen | South Africa | Syzygium cordatum, living leaves | P.W. Crous, 11 Aug. 2018 | MN313808.1 | MN317289.1 | – | – | – |
| Lembosina aulographoides | CPC 33049 = CBS 145946, ex-epitype | Netherlands | Rhododendron sp., stems | P.W. Crous, 1 Feb. 2017 | MN313809.1 | MN317290.1 | MN313839.1 | – | – |
| Neomelanconium gelatosporum | CPC 31126 = CBS 144985, ex-epitype | Germany | Tilia platyphyllos, dead, corticated and attached twig | R.K. Schumacher, 2 May 2016 | MN313810.1 | MN317291.1 | – | MN313856.1 | rpbl: MN313870.1 |
| CPC 31127, ex-epitype | Germany | Tilia platyphyllos, dead, corticated and attached twig | R.K. Schumacher, 2 May 2016 | MN313811.1 | MN317292.1 | – | MN313857.1 | rpbl: MN313871.1 | |
| Phragmotrichum chailletii | CPC 33263 = CBS 144994, ex¬neotype | Switzerland | Picea abies, fallen cones | J.Gilgen & R.K. Schumacher, 7 Mar. 2017 | MN313812.1 | MN317293.1 | MN313840.1 | MN313858.1 | – |
| CPC 33341 = CBS 144993 | Switzerland | Picea abies, fallen cones | J. Gilgen & R.K. Schumacher, 7 Mar. 2017 | MN313813.1 | MN317294.1 | MN313841.1 | MN313859.1 | – | |
| Rutola graminis | CPC 33267 = CBS 145906, ex-epitype | Germany | Typha sp., dead and still attached leaf | R.K. Schumacher, 1 Apr. 2017 | MN313814.1 | MN317295.1 | – | – | – |
| CPC 33695 | Norway | Scirpus sylvaticus | Kaare Flomble, 3 May 2017 | MN313815.1 | MN317296.1 | – | – | – | |
| CPC 33715 | Germany | Scirpus sylvaticus, dead and still attached leaf | R.K. Schumacher, 3 May 2017 | MN313816.1 | MN317297.1 | – | – | – | |
| Torula herbarum | CPC 24114 = CBS 140066, exneotype | Netherlands | Phragmites australis, culms | W. Quaedvlieg, 24 Jan. 2014 | KR873260.1 | KR873288.1 | ‒ | ‒ | ‒ |
| CPC 24115 | Netherlands | Phragmites australis, culms | W. Quaedvlieg, 24 Jan. 2014 | MN313817.1 | ‒ | ‒ | ‒ | ‒ | |
| CPC 33688 = CBS 144995 | Germany | Deschampsia cespitosa, dead culm base and dead leaf sheath | R.K. Schumacher, 3 May 2017 | MN313818.1 | MN317298.1 | ‒ | ‒ | ‒ | |
1CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS.
2ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; act: partial actin gene; rpb1: partial DNA-directed RNA polymerase II largest subunit gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; SSU: small subunit (18S) of the nrRNA gene operon; tef1: partial translation elongation factor 1-alpha gene; tub2: partial beta-tubulin gene.
The sequences for each gene region were subjected to megablast searches (Zhang et al. 2000) to identify closely related sequences in the NCBI’s GenBank nucleotide database. The results are provided as part of the species notes or as selected phylogenetic trees where applicable. Phylogenetic trees were generated using Bayesian analyses performed with MrBayes v. 3.2.6 (Ronquist et al. 2012) for the overview trees (Figs 1–3) and Maximum Parsimony analyses performed with PAUP v. 4.0b10 (Swofford 2003) for the species tree (Fig. 4) as explained in Braun et al. (2018). All resulting trees were printed with Geneious v. 11.0.3 (http://www.geneious.com, Kearse et al. 2012) and the layout of the trees was done in Adobe Illustrator v. CC 2017. Statistical measures calculated included posterior probabilities, parsimony bootstrap values, tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC).
Fig. 1.
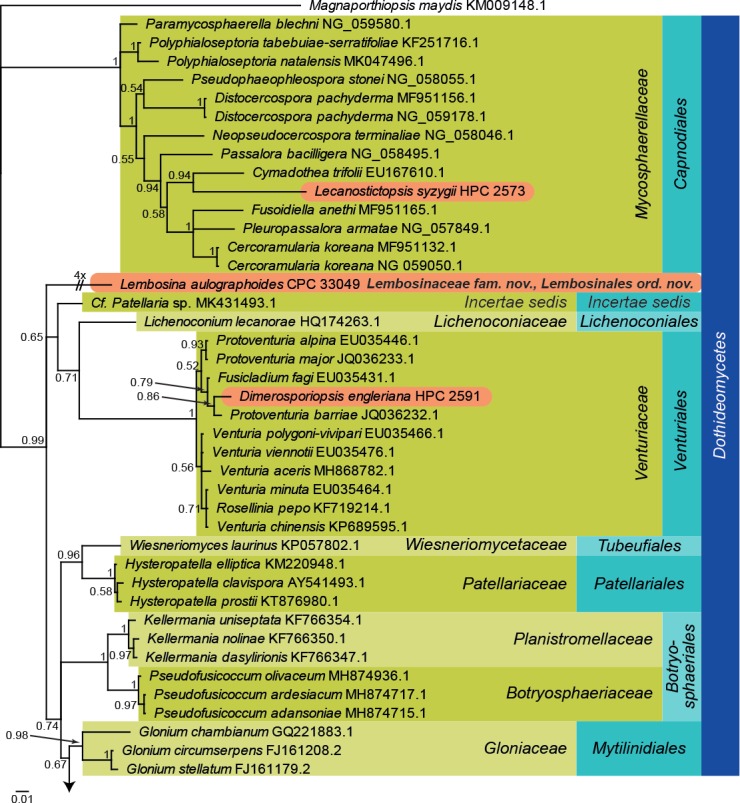
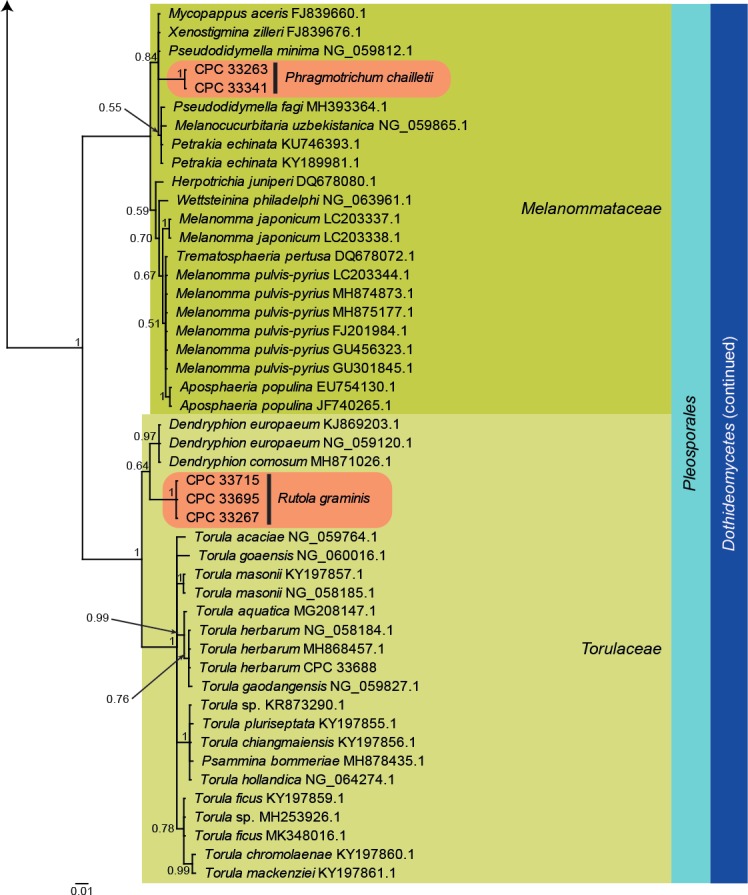
Bayesian phylogeny calculated from the Dothideomycetes LSU sequence alignment. Bayesian posterior probabilities are shown at the nodes and the scale bar represents the expected changes per site. Classes, families and orders are indicated with coloured blocks to the right of the tree. GenBank accession or culture numbers are indicated behind the species names. The tree was rooted to Magnaporthiopsis maydis (GenBank KM009148.1) and the species treated in this study for which LSU sequence data were available are indicated in coloured rounded rectangles.
Fig. 2.
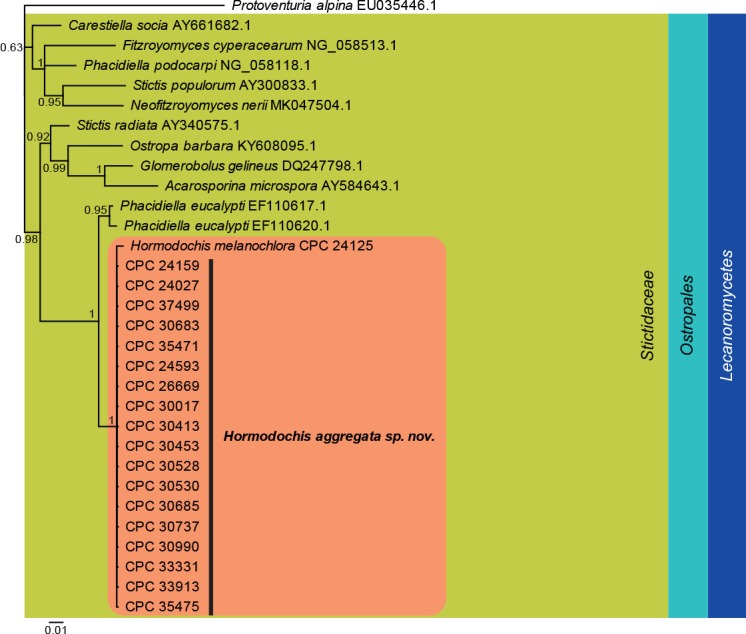
Bayesian phylogeny calculated from the Lecanoromycetes LSU sequence alignment. Bayesian posterior probabilities are shown at the nodes and the scale bar represents the expected changes per site. Classes, families and orders are indicated with coloured blocks to the right of the tree. GenBank accession or culture numbers are indicated behind the species names. The tree was rooted to Protoventuria alpina (GenBank EU035446.1) and the species treated in this study for which LSU sequence data were available are indicated in the coloured rounded rectangle.
Fig. 3.
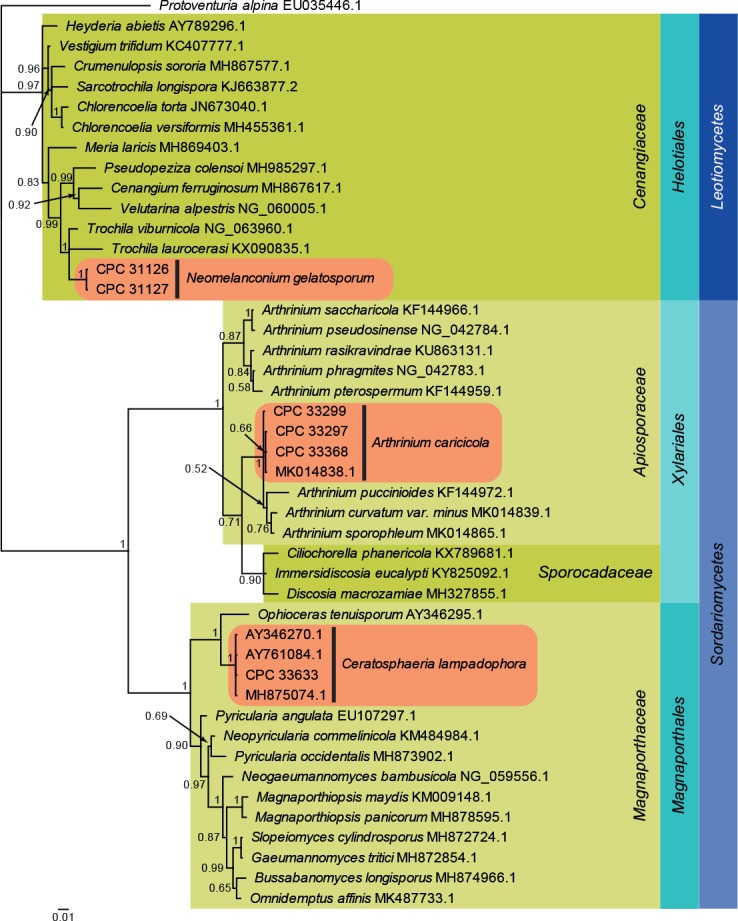
Bayesian phylogeny calculated from the Leotiomycetes and Sordariomycetes LSU sequence alignment. Bayesian posterior probabilities are shown at the nodes and the scale bar represents the expected changes per site. Classes, families and orders are indicated with coloured blocks to the right of the tree. GenBank accession or culture numbers are indicated behind the species names. The tree was rooted to Protoventuria alpina (GenBank EU035446.1) and the species treated in this study for which LSU sequence data were available are indicated in the coloured rounded rectangles.
Fig. 4.
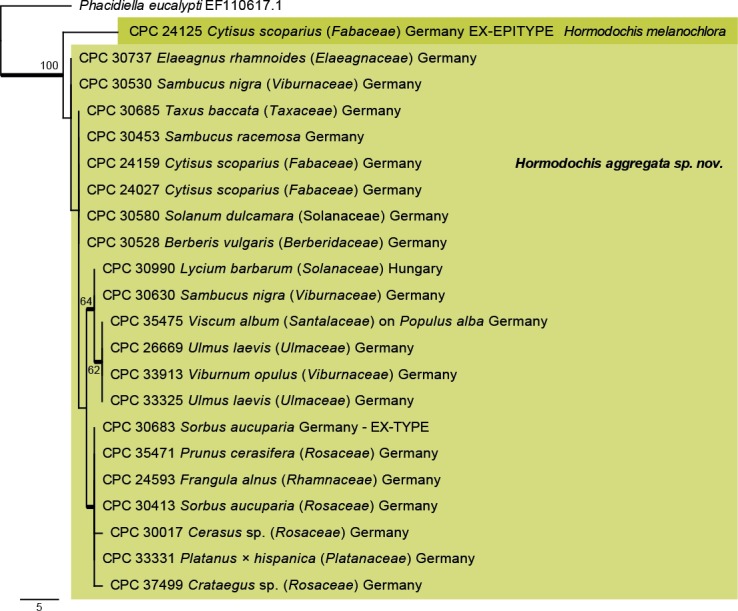
The first of two equally most parsimonious trees obtained from a phylogenetic analysis of the Hormodochis ITS alignment (23 strains including the outgroup; 491 characters analysed: 463 constant, 22 variable and parsimony-uninformative and 6 parsimony-informative). The tree was rooted to Phacidiella eucalypti (GenBank EF110617.1) and the scale bar indicates the number of changes. Bootstrap support values higher than 49 % are shown at the nodes and the species clades are highlighted with coloured boxes. Species names are indicated to the right of the tree. Strain numbers are followed by the substrate and country of origin. Branches present in the strict consensus tree are thickened. Tree statistics: TL = 32, CI = 0.938, RI = 0.923, RC = 0.865.
Morphology
Slide preparations were mounted in Shear’s mounting fluid or water, from colonies sporulating on MEA, PDA, PNA or OA. Sections through conidiomata were made by hand. Observations were made with a Nikon SMZ25 dissection-microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and images recorded on a Nikon DS-Ri2 camera with associated software. Colony characters and pigment production were noted after 2–4 wk of growth on MEA, PDA and OA (Crous et al. 2019) incubated at 25 °C. Colony colours (surface and reverse) were scored using the colour charts of Rayner (1970). Sequences derived in this study were deposited in GenBank (Table 1), the alignment in TreeBASE (www.treebase.org; study number S24788), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
RESULTS
Phylogeny
Dothideomycetes LSU phylogeny (Fig. 1): The alignment contained 88 isolates and Magnaporthiopsis maydis (strain M84, GenBank KM009148.1) which was used as outgroup. The final alignment contained a total of 757 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 297 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 30 402 trees from which 22 802 were sampled after 25 % of the trees were discarded as burn-in. The tree revealed the following associations for the species treated in this manuscript: Lecanostictopsis syzygii in Mycosphaerellaceae (Capnodiales); Dimerosporiopsis engleriana in Venturiaceae (Venturiales); Phragmotrichum chailletii and Rutola graminis in Melanommataceae and Torulaceae (both Pleosporales), respectively. Lembosina aulographoides did not cluster with any known family or order and therefore a family and order are introduced below to accommodate it.
Lecanoromycetes LSU phylogeny (Fig. 2): The alignment contained 30 isolates and Protoventuria alpina (CBS 373.53, GenBank EU035446.1) which was used as outgroup. The final alignment contained a total of 747 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 158 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 1 902 trees from which 1 428 were sampled after 25 % of the trees were discarded as burn-in. Hormodochis is shown to be a member of Stictidaceae (Ostropales).
Leotiomycetes and Sordariomycetes LSU phylogeny (Fig. 3): The alignment contained 40 isolates and Protoventuria alpina (CBS 373.53, GenBank EU035446.1) which was used as outgroup. The final alignment contained a total of 780 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total 240 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 24 702 trees from which 18 528 were sampled after 25 % of the trees were discarded as burn-in. Neomelanconium gelatosporum is placed in Cenangiaceae (Helotiales, Leotiomycetes), while Arthrinium caricicola (including GenBank MK014838.1; Pintos et al. 2019) is placed in Apiosporaceae (Xylariales, Sordariomycetes) with other species of Arthrinium and Ceratosphaeria lampadophora (including GenBank AY346270.1, AY761084.1 and MH875074.1; Huhndorf et al. 2004, Réblová 2006 and Vu et al. 2019) is placed in Magnaporthaceae (Magnaporthales, Sordariomycetes).
THE GENERA
Arthrinium Kunze, Mykologische Hefte (Leipzig) 1: 9. 1817.
Synomyms: See Crous & Groenewald (2013).
Classification: Apiosporaceae, Xylariales, Sordariomycetes.
Colonies compact, black to dark brown, superficial to erumpent. Mycelium immersed and superficial. Conidiophores arising from basal cells that are subcylindrical, subhyaline with refractive, thick transverse septa, brown to dark brown, forming conidia laterally and terminally; conidiophores frequently aggregated in a brown stroma, forming black sporodochia on the host and in culture. Setae present or absent, brown, smooth, erect, sparsely septate, tapering to subacute apex, intermingled among conidiophores. Conidiogenous cells discrete, doliiform, ampulliform to subcylindrical, subhyaline to pale brown, smooth to finely verruculose, aggregated on aerial hyphae, giving rise to clusters of conidia; at times reduced to lateral pegs on hyphae, holoblastic, proliferating sympodially (at times clearly phialidic with periclinal thickening, rarely with percurrent proliferation). Conidia aseptate, brown to dark brown, smooth to verruculose, guttulate to granular, with distinctive shape (round, curved, curved with two horns, oblong, irregular, limoniform, fusiform, navicular, dentate or lobed), at times flattened, with equatorial slit of lighter pigment. Sterile cells when formed replace conidia, usually smaller and paler than conidia, with different shape, frequently containing refractive cubical bodies. Stromata immersed in epidermis, becoming erumpent through a longitudinal split, revealing rows of densely arranged perithecial ascomata. Ascomata globose with papillate ostioles; wall composed of 6–9 layers of pseudoparenchymatous cells. Paraphyses broadly filiform, septate, deliquescing early. Asci 8-spored, unitunicate (appearing bitunicate when young), clavate to broadly cylindrical. Ascospores smooth, hyaline, bi- to tri-seriate, ellipsoid, inequilateral, tapered at both ends, apiosporous, 1-septate near the lower end, with the lower, smaller cell subglobose; ascospores with or without mucoid sheath (from Crous & Groenewald 2013).
Type species: Arthrinium caricicola Kunze
Arthrinium caricicola Kunze, Mykologische Hefte (Leipzig) 1: 9. 1817. Fig. 5.
Fig. 5.
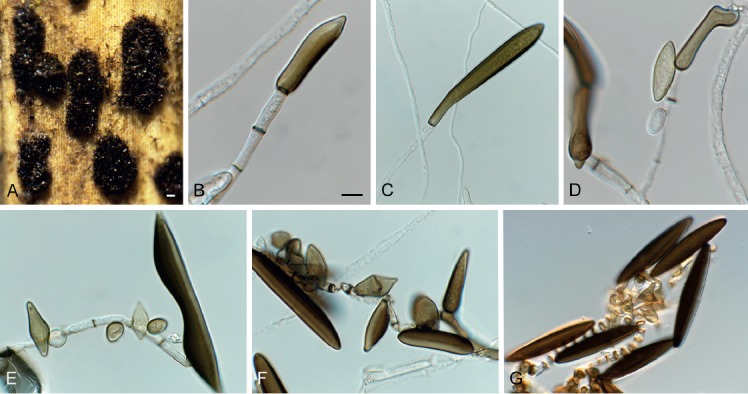
Arthrinium caricicola (CPC 33297). A. Colonies on host material. B–F. Sterile cells. G. Conidiophore mother cells ad conidia. Scale bars: A = 400 μm, B = 10 μm, applies to all others.
Colonies compact, pulvinate, 150–400 µm diam, dark blackish brown. Mycelium immersed and superficial, composed of a network of branched and anastomosing, septate, brown to dark brown, smooth-walled, 4–7 µm diam hyphae; immersed hyphae pale to dark brown, 2–6 µm diam. Conidiophore mother cells subspherical to lageniform, 5–8 × 5–7 µm. Conidiophores erect, simple, flexuous, cylindrical, pale brown or dark brown, with thick, dark brown transverse septa, smooth-walled, 20–120 µm tall, 2–5 µm diam. Conidia fusoid or cigar-shaped in face view, navicular in side view, dark brown with distinct hyaline rim, (31–)40–50(–56) × (7.5–)8–11(–15) µm. Sterile cells much smaller and paler than conidia, irregularly lobed.
Culture characteristics: Colonies covering the dish in 2 wk with moderate, fluffy aerial mycelium, and smooth, even margins. On MEA surface pale olivaceous grey, reverse umber; on PDA surface olivaceous grey, reverse isabelline; on OA surface dirty white.
Typus: Germany, Berlin, “Jungfernheide”, dead leaf of Carex ericetorum (= C. ciliata) (Cyperaceae), Apr. 1817 or before, G. Kunze (isotype BPI 422608, designated as lectotype here, MBT388205); near Berlin, on dead and attached leaves of Carex ericetorum, 4 Apr. 2017, R.K. Schumacher, RKS 86 = HPC 2039 (epitype designated here CBS H-24083, MBT388206, cultures ex-epitype CPC 33297 = CBS 145903, CPC 33298 = CBS 145903).
Additional materials examined: Germany, near Berlin, on dead and attached leaves of Carex ericetorum, 4 Apr. 2017, leg. et det. R.K. Schumacher, RKS 87 = HPC 2040, cultures PC 33299 = CBS 144986, CPC 33300; near Berlin, on dead and attached leaves of Carex ericetorum, 4 Apr. 2017, leg. et det. R.K. Schumacher, RKS 88 = HPC 2041, cultures CPC 33368, CPC 33369.
Notes: Arthrinium [sexual morph (synonym) Apiospora; Apiosporaceae] was recently treated by Crous & Groenewald (2013). Since this study, several other species have been added to the genus (Jiang et al. 2018, Wang et al. 2018, Pintos et al. 2019). The present study fixes the application of the genus Arthrinium by designating an epitype for the type species Arthrinium caricicola. Our ITS, LSU and tub2 sequences are identical to those of strain ALV16691 (Pintos et al. 2019), also from Carex ericetorum, Germany.
Ceratosphaeria Niessl, Verh. nat. Ver. Brünn 14: 203. 1876.
Classification: Magnaporthaceae, Magnaporthales, Sordariomycetes.
Ascomata perithecial, singly to densely crowded, immersed, later erumpent, more or less globose with a central and long rostrum, black, thick, rough. Peridium multi-layered, consisting of a textura prismatica. Paraphyses numerous, longer than the asci, basally moniliform, upwards tapered and filiform, unbranched, no anastomoses, hyaline. Asci 8-spored, clavate, apically conical rounded and thick-walled, otherwise thin-walled, pedicel short and furcate, apex diaporthoid, inoperculate, inamyloid, spores bi- to triseriate overlapping. Ascospores transversely septate, fusoid, slightly curved, end cells tapered, hyaline, thin-walled, smooth, septa thin-walled. Harpophora-like morph in culture. Mycelium consisting of hyaline, smooth, hyphae that become pigmented in fertile regions. Conidiophores reduced to conidiogenous cells, or septate, branched, solitary, erect. Conidiogenous cells terminal and intercalary, pale brown, smooth, subcylindrical with apical taper and flared collarette. Conidia aggregating in mucoid mass, aseptate, hyaline, smooth, curved, narrowly fusoid with obtuse ends.
Type species: Ceratosphaeria lampadophora (Berk. & Broome) Niessl
Ceratosphaeria lampadophora (Berk. & Broome) Niessl, Verh. nat. Ver. Brünn 14: 203. 1876. Fig. 6.
Fig. 6.
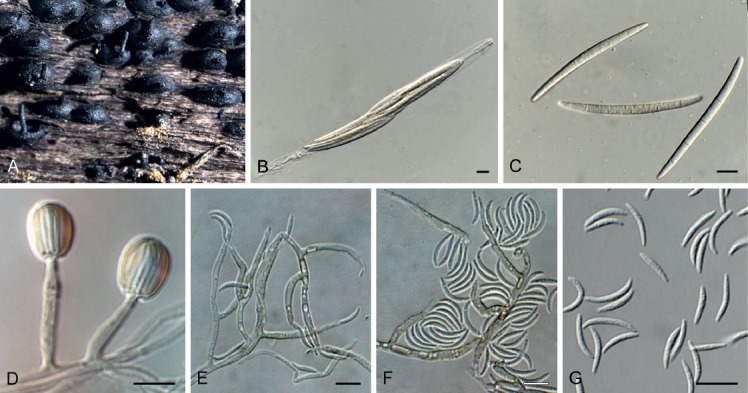
Ceratosphaeria lampadophora (CPC 33633). A. Black perithecial ascomata. B. Ascus. C. Ascospores. D–F. Conidiophores giving rise to conidia. G. Conidia. Scale bars: A = 3 mm, all others = 10 μm.
Basionym: Sphaeria lampadophora Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3 3(17): 372. 1859.
Occurring on dead twigs. Ascomata perithecial, singly to densely crowded, immersed, later erumpent, more or less globose with a central and long rostrum, black, thick, rough, up to 1 000 µm diam, 400–650 µm high. Peridium multilayered, consisting of a textura prismatica, inner layers hyaline and outer layers red-brown. Paraphyses numerous, longer than the asci, basally moniliform, upwards tapered and filiform, unbranched, no anastomoses, hyaline. Asci 8-spored, clavate, apically conical rounded and thick-walled, otherwise thin-walled, pedicel short and furcate, apex diaporthoid, inoperculate, inamyloid (water + Lugol), spores bi- to triseriate overlapping. Ascospores 5–7-septate, fusoid, slightly curved, end cells tapered, hyaline, thin-walled, smooth, septa thin-walled and smooth, one small to middle sized guttule per cell, examined in water, living and mature, (54–)60–66(–72) × (3.5–)4 µm. Harpophora-like morph in culture. Mycelium consisting of hyaline, smooth, 1.5–2 µm diam hyphae that become pigmented in fertile regions. Conidiophores reduced to conidiogenous cells, or 1-septate, branched, solitary, erect, 10–30 × 2.5–3 µm. Conidiogenous cells terminal and intercalary, pale brown, smooth, subcylindrical with apical taper and flared collarette, 10–17 × 2.5–3 µm. Conidia aggregating in mucoid mass, aseptate, hyaline, smooth, curved, narrowly fusoid with obtuse ends, (5–)8–10(–13) × 1–1.5 µm.
Culture characteristics: Colonies spreading, erumpent with smooth, lobate margins, reaching 25 mm diam after 2 wk on MEA, but covering dish on OA, with moderate aerial mycelium. On MEA surface amber, isabelline at margin, with diffuse sepia pigment, reverse sepia; on PDA surface and reverse cream, with dark sepia diffuse pigment; on OA surface honey.
Typus: UK, Combe Hay, on decayed wood, leg. C.E. Broome, Apr. 1855 (holotype K(M)82672).
Material examined: France, Kembs/Elsass, alt. 240 m a.s.l., on fallen and partly decorticated stem of Populus nigra (Salicaceae), 15 Mar. 2017, leg. M. Wilhelm, det. R.K. Schumacher, RKS 72 = HPC 2013, cultures CPC 33633 = CBS 144991, CPC 33634.
Notes: Ceratosphaeria lampadophora was originally described from decayed wood collected in the UK. A re-examination of the holotype reported ascospores to be 5–7(–8)-septate, 72–82 × 3.5–4 µm (Hyde et al. 1997), somewhat longer than observed in the present European collection. Huhndorf et al. (2008) cite a collection from France as also having smaller ascospores, namely 5–7-septate, 52–72 × 3.5–4.5 µm. The present collection is thus not designated as epitype, as it is quite possible that two cryptic species might be involved. Of interest is the harpophora-like morph observed in culture, which is typical of Magnaporthaceae. Our ITS sequence differs with an extra T nucleotide from the sequence of CBS 125415 (Vu et al. 2019), while the LSU sequence is identical to three sequences in GenBank (AY346270.1, AY761084.1 and MH875074.1; Huhndorf et al. 2004, Réblová 2006, Vu et al. 2019).
Dimerosporiopsis Henn., Fungi Europ. Extraeur. Exs., Cent. 43: no. 4260. 1901.
Classification: Venturiaceae, Venturiales, Dothideomycetes.
Caulicolous, causing some thickening and distortion of the affected parts, covering them with a dark brown to black mycelial growth. Mycelium extending through the cortex, giving rise to tufts of erect hyphae, which cover the stem with a turf-like growth. Ascomata pseudothecial, intermixed amongst the erect hyphae and attached to them at the base, globose or somewhat flattened, non-setose, collapsing and becoming cupulate when dry; wall rough externally, grossly verrucose, olivaceous, composed of several layers of irregularly polygonal cells 10–15 µm diam; without true ostiole, but with an irregular pore. Asci bitunicate, 8-spored, cylindrical, rounded at apex, tapering to a well-defined foot. Pseudoparaphyses hyaline, septate, filiform. Ascospores bi-seriate, 1-septate, pale olivaceous, clavate-ellipsoid, rounded at ends, slightly constricted at the septum, guttulate.
Type species: Dimerosporiopsis engleriana (Henn.) Henn.
Dimerosporiopsis engleriana (Henn.) Henn., Fungi Eur. Extraeur. Exs., Cent. 43: no. 4260. 1901. Fig. 7.
Fig. 7.

Dimerosporiopsis engleriana (HPC 2591). A. Branch canker on Erica sp. B. Ascomata. C–E. Asci and pseudoparaphyses. F. Ascospores. Scale bars: B = 250 μm, all others = 10 μm.
Basionym: Dimerosporium englerianum Henn., Die Pflanzenwelt Ost-Afrikas und der Nachbargebiete, Teil C: 31. 1895.
Synonymy: Dimerium englerianum (Henn.) Sacc. & P. Syd., Syll. fung. 17: 537. 1905.
Dimerosporis engleriana (Henn.) Clem., Gen. fung.: 32. 1909.
Phaeodimeriella engleriana (Henn.) Speg., Revista Mus. La Plata 15(2): 13. 1908.
Antennularia engleriana (Henn.) Höhn., Sber. Akad. Wiss. Wien. 119: 920. 1910.
Gibbera engleriana (Henn.) Van der Byl, S. African J. Sci. 25: 182. 1928.
Protoventuria engleriana (Henn.) Sivan., Trans. Brit. Mycol. Soc. 63(3): 590. 1974.
Caulicolous, causing some thickening and distortion of the affected parts, and covering them with a dark brown to black mycelial growth, which is often continuous for several centimetres. Mycelium extending through the cortex, and producing in the tissues of the host numerous small cushions, cellular in structure and irregular in form and size; these are brown, and formed of cells which may be irregularly polygonal and 5–10 µm diam, or, especially towards the periphery, with a tendency to become cubical and to develop in rows at right angles to the surface of the stem. At the surface, these cushions give rise to tufts of erect hyphae, which become so numerous as to completely clothe the stem with a turf-like growth. Erect hyphae brown, thick-walled, 5–6 µm thick, up to 400 µm high, septate; cells 20–25 µm long; sparingly branched and often tortuous and tangled. Ascomata pseudothecial, numerous, nestling amongst the erect hyphae and attached to them at the base, globose or somewhat flattened, non-setose, 220–350 µm diam, 250–300 µm high, collapsing and becoming cupulate when dry. Perithecial wall rough externally, grossly verrucose, olivaceous, composed of several layers of irregularly polygonal cells 10–15 µm diam; without true ostiole, but with a thin place at the apex which breaks down and forms an irregular pore. Asci bitunicate, 8-spored, cylindrical, rounded at apex with small apical chamber, 90–130 × 11–13 µm. Pseudoparaphyses numerous, hyaline, filiform, branched, septate, anastomosing, 2–3 µm diam. Ascospores distichous, medianly 1-septate, pale olivaceous, smooth, clavate-ellipsoid with rounded ends, constricted at septum, pluri-guttulate, (16–)17–19(–22) × 6–7 µm (up to 9 µm diam when mounted in water) (adapted from Doidge 1941).
Typus: Tanzania, on the mountain “Mawensi”, volcano “Kisimba”, on inflorescens of Erica mannii (= Ericinella mannii) (Ericaceae), Sep. 1893, G.L.A. Volkens (isotype, LE117684 ex Flora des Kilimandscharo nr. 945, selected here as lectotype, MBT388207). South Africa, Western Cape Province, Bontebok National Park, on stems of Erica sp., 24 Sep. 2014, A. Wood, HPC 2591 (epitype designated here CBS H-24086, MBT388208).
Notes: The present fungus has had a very confused history, and has been placed in numerous genera (see synonymy above). For many years it was referred to in literature as Antennularia engleriana, until Sivanesan (1974) placed it in Protoventuria. Dimerosporiopsis engleriana is a member of the Venturiaceae, and clusters close to species described in “Protoventuria”. However, the genus Protoventuria (1887) (based on Venturia rosae, CBS 312.58), clusters with P. alpina (CBS 140.83), but both are related to species of Cadophora (Helotiales), suggesting that these strains could be incorrectly identified. Several other species of Protoventuria cluster with Dimerosporiopsis, as would be expected based on their morphology. Based on the distinct lineage shown by Zhang et al. (2011), Rossman et al. (2015) concluded that the genus Ramalia Bat., 1957, is a synonym of Protoventuria, but as stated here, we suspect that these isolates have been misidentified. The best course of action would thus be to recollect Venturia rosae (on Rosa alpina, Italy), and R. veronicae (on Parasterina veronicae and Asteromella veronicae, on Veronica derwentia: New South Wales, Australia) before this matter can be resolved.
All attempts to culture D. engleriana failed, hence an ITS and LSU sequence were generated from DNA isolated directly from fungal material.
Hormodochis Clem., Gen. fung. (Minneapolis): 163. 1909.
Classification: Stictidaceae, Ostropales, Ostropomycetidae, OSLEUM clade, Lecanoromycetes.
Conidiomata erumpent, globose, brown, separate to aggregated, opening via irregular rupture wall of thin-walled brown textura angularis. Conidiophores lining the inner layer, arising from stromatic cells, hyaline, smooth, guttulate, branched below or not, cylindrical, straight or slightly curved, septate, upper cell fertile, becoming septate, and disarticulating into arthroconidia via basipetal secession, intact chains. Conidia aseptate, arranged in cylindrical chains, olivaceous brown, smooth, subcylindrical to somewhat doliiform, with truncate ends, with minute marginal frill.
Type species: Hormodochis melanochlora (Desm.) Clem.
Hormodochis aggregata Crous & R.K. Schumach., sp. nov. MycoBank MB832085. Fig. 8.
Fig. 8.
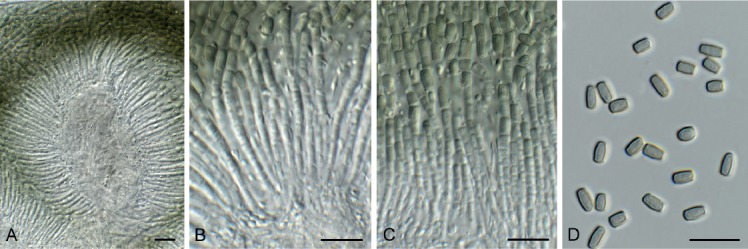
Hormodochis aggregata (CPC 30683). A. Section through conidioma. B, C. Conidiophores giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Etymology: Referring to its aggregated conidiomata.
Conidiomata erumpent, globose, brown to black, up to 300 µm diam, stromatic, multilocular, solitary to aggregated, immersed to erumpent, opening via irregular rupture wall of thin-walled brown textura angularis. Conidiophores lining the inner layer, arising from stromatic cells, hyaline, smooth, branched below or not, forming a rosette, cylindrical, straight or slightly curved, septate, 5–20 × 2–3 µm, upper cell fertile, becoming septate, and disarticulating into arthroconidia via basipetal secession, forming long intact chains. Conidia aseptate, basipetal, arranged in unbranched or branched cylindrical chains, olivaceous brown to green-brown, smooth, subcylindrical to somewhat doliiform, with truncate ends, with minute marginal frill, (4–)5–6 × 2(–2.5) µm.
Culture characteristics: Colonies flat, spreading, surface folded, with moderate aerial mycelium, and smooth, lobed margins, reaching 20 mm diam after 2 wk at 25 °C in the dark. On MEA, PDA and OA surface dirty white, reverse ochreous.
Typus: Germany, near Berlin, on twig and bud of Sorbus aucuparia (Rosaceae), 27 Apr. 2016, R.K. Schumacher RKS 0986 = HPC 1188 (holotype CBS H-24084, culture ex-type CPC 30683 = CBS 145904).
Additional material examined: Germany, near Berlin, on attached dead twig of Prunus cerasifera (Rosaceae), 25 Apr. 2018, R.K. Schumacher RKS161 = HPC 2312 = CBS H-24085, culture CPC 35471 = CBS 145905.
Notes: The two strains of H. aggregata studied here were quite variable in conidial morphology, with conidia of the ex-type strain being (3–)4–5 × 2(–2.5) µm (CPC 30683, on Sorbus aucuparia), while they were larger, (4–)5–6(–8) × (2–)2.5(–3) µm in CPC 35471, isolated from Prunus cerasifera. The genus Hormodochis appears to be quite common, and to occur on a wide host range. Although only two species are treated here (Figs 2, 4; rpb2, tef1 and tub2 data in GenBank), several others remain to be named. Although H. aggregata and H. melanochlora are highly similar on LSU [Fig. 2; 972/1 002 (97 %)–796/799 (99 %)) and ITS (Fig. 4; 622/650 (96 %)–554/573 (97 %)), the other loci are much more variable (rpb2: on average 774/864 (90 %), tef1: 546/613 (89 %)–502/550 (91 %) and tub2: 229/271 (85 %)–511/583 (88 %)].
Hormodochis melanochlora (Desm.) Clem., Gen. fung. (Minneapolis): 163. 1909. Fig. 9.
Fig. 9.
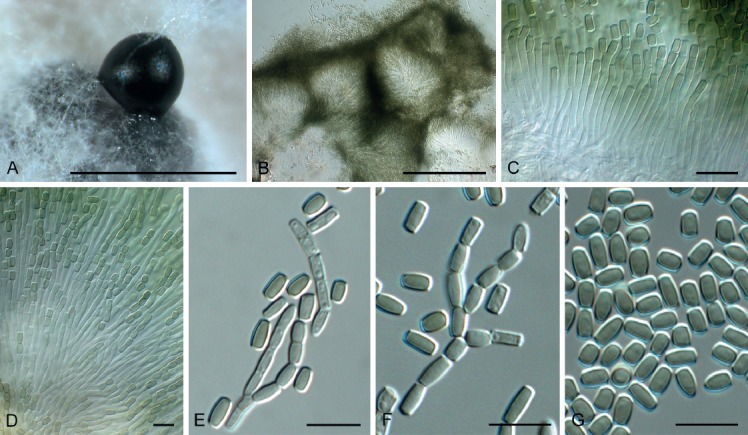
Hormodochis melanochlora (CPC 24125). A. Erumpent conidioma. B. Section through aggregated conidiomata. C–F. Conidiophores giving rise to conidia. G. Conidia. Scale bars: A, B = 300 μm, all others = 10 μm.
Basionym: Epidochium melanochlorum Desm., Annls Sci. Nat., Bot., sér. 3, 16: 327. 1851.
Synonyms: Hormodochium melanochlorum (Desm.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 120(4): 465. 1911.
Trullula melanochlora (Desm.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 124(1–2): 97. 1915.
Conidiomata erumpent, globose, brown, up to 300 µm diam, separate to aggregated, opening via irregular rupture of the wall of thin-walled brown textura angularis. Conidiophores lining the inner layer, arising from stromatic cells, hyaline, smooth, guttulate, branched below or not, cylindrical, straight or slightly curved, 1–2-septate, 5–20 × 1.5–3 µm, upper cell fertile, becoming septate, and disarticulating into arthroconidia via basipetal secession, intact chains up to 70 µm long. Conidia aseptate, arranged in cylindrical chains, olivaceous brown, smooth, subcylindrical to somewhat doliiform, with truncate ends, with minute marginal frill, (4–) 5–6(–7) × (2–)2.5–3 µm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium, and feathery, lobed margins, reaching 7 mm diam after 2 wk at 25 °C in the dark. On MEA, PDA and OA surface and reverse white.
Typus: France, no locality given, on twig of Laburnum anagyroides (= Cytisus laburnum) (Fabaceae), spring–summer 1849 or before, anonymous (isotype, Plantes cryptogames du nord de la France 1851, edit. 1, fasc. 44, nr. 2166 = K(M)249048, selected as lectotype here MBT388210). Isolectotypes: Desm., Plantes cryptogames du nord de la France 2166 (e.g., BM, FH, G, NY). Germany, near Berlin, on dead twig of Cytisus scoparius (= Sarothamnus scoparius) (Fabaceae), 21 Dec. 2013, R.K. Schumacher, RKS 0433 (epitype designated here CBS H-21993, MBT388211, culture ex-type CPC 24125 = CBS 138861).
Notes: The description of Höhnel (1911: 465) is based on the specimen nr. 2166 (publ. 1851). Pirozynski & Morgan-Jones (1968) provided a modern account of the genus under the name Trullula olivascens (conidia 3–6.5 × 1.5–2.5 µm, IMI 124616 ex K), which was followed by Sutton (1980). Our specimen chosen as epitype of Hormodochis melanochlora closely matches the lectotype in morphology. Hormodochis melanochlora is genetically distinct on all sequenced loci from the other species described here, H. aggregata (Figs 2, 4; also see species notes under H. aggregata).
Lecanostictopsis B. Sutton & Crous, Mycol. Res. 101: 215. 1997.
Classification: Mycosphaerellaceae, Capnodiales, Dothideomycetes.
Mycelium immersed, intercellular, branched, septate, dark to reddish brown. Conidiomata epidermal to subepidermal, erumpent, eustromatic, acervular to sporodochial, composed of thick-walled, dark to reddish brown textura angularis. Conidiophores dark to reddish brown, coarsely verrucose, cylindrical, unbranched, septate, formed from the upper cells of the conidiomata. Conidiogenous cells integrated, dark to reddish brown, coarsely verrucose to tuberculate, cylindrical, with several percurrent enteroblastic proliferations. Conidia holoblastic, dark to reddish brown, coarsely verrucose to tuberculate, with 0–several eusepta, straight or curved, obtuse or acute at the apex, truncate at the base, cylindrical to fusiform (adapted from Sutton & Crous 1997).
Type species: Lecanostictopsis kamatii (Ullasa) B. Sutton & Crous
Lecanostictopsis syzygii (Ciccar.) B. Sutton & Crous, Mycol. Res. 101: 218. 1997. Fig. 10.
Fig. 10.

Lecanostictopsis syzygii (HPC 2573). A. Conidiomata on leaf. B–G. Conidiogenous cells giving rise to conidia. H, I. Conidia. Scale bars: A = 1 mm, all others = 10 μm, and C applies to D–I.
Basionym: Scolicosporium syzygii Ciccar. [as “syzigii”], Mycopath. Mycol. appl. 5: 230. 1951.
Description and illustration: Sutton & Crous (1997).
Typus: Ethiopia, Uoccia, near Omo Bottego River, on living leaves of Syzygium guineense (Myrtaceae), 25 Jan. 1939, A. Ciccarone, U.S. National Fungus Collections isotype BPI 404952A (holotype of Scolecosporium syzygii, IMI 367859, associated with Micronectria syzygii). South Africa, KwaZulu-Natal Province, St. Lucia wetlands, on living leaves of Syzygium cordatum, 11 Aug. 2018, P.W. Crous, HPC 2573 (epitype designated here CBS H-24087, MBT388212).
Additional materials examined: South Africa, KwaZulu-Natal, Eshowe, on living leaves of Syzygium cordatum, Apr. 1941, A.P.D. McClean, Union Dept Agriculture, Mycological Herbarium 33092, IMI 45164a, associated with Kamatella apiospora and Mycohypallage congesta. Zambia, nr Kawambse, on living leaves of Syzygium guineenese var. macrocarpum, 7 Apr. 1961, A. Angus M1096, PPNR 3363, IMI 89987b, associated with Mycohypallage congesta, Asterina syzygii var. microspora and Microthyriella sp.
Notes: The genus Lecanostictopsis is a foliar pathogen of Syzygium (Myrtaceae). Since it was described, Lecanostictopsis was assumed to be a member of the Mycosphaerellaceae (Sutton & Crous 1997). However, all attempts to cultivate species of the genus have thus far proven unsuccessful, as conidia germinate, but die immediately post germination (irrespective of culture medium or temperature used). In the present study we have thus used selective primers (Videira et al. 2017) to amplify the ITS rDNA, which resolved Lecanostictopsis as a distinct genus in the Mycosphaerellaceae, allied to Cymadothea, confirming it as additional member of the Mycosphaerellaceae (Fig. 1).
Lembosinales Crous, ord. nov. MycoBank MB832086.
Etymology: Name reflects the genus Lembosina.
Classification: Lembosinaceae, Lembosinales, Dothideomycetes.
Ascomata thyriothecial or hysterothecial, linear, rarely y-shaped, solitary, gregarious, superficial, loose on host surface, black, opening with linear fissures. Upper wall comprising a thin layer of mostly neatly arranged dark cells, which are branched at the outer rim, base poorly developed. Hamathecium comprising, sparse, filiform pseudoparaphyses. Asci 8-spored, bitunicate, subglobose to oblong. Ascospores overlapping 2–4-seriate, 1-septate, with an upper cell slightly wider and shorter than the lower cell, hyaline, becoming brown with age, with basal protrusion.
Type family: Lembosinaceae Crous
Lembosinaceae Crous, fam. nov. MycoBank MB832087.
Etymology: Name reflects the genus Lembosina.
Ascomata thyriothecial or hysterothecial, linear, rarely y-shaped, solitary, gregarious, superficial, loose on host surface, black, opening with linear fissures. Upper wall comprising a thin layer of mostly neatly arranged dark cells, which are branched at the outer rim, base poorly developed. Hamathecium comprising, sparse, filiform pseudoparaphyses. Asci 8-spored, bitunicate, subglobose to oblong. Ascospores overlapping 2–4-seriate, 1-septate, with an upper cell slightly wider and shorter than the lower cell, hyaline, becoming brown with age, with basal protrusion.
Type genus: Lembosina Theiss.
Lembosina Theiss., Annls Mycol. 11(5): 437. 1913.
Ascomata thyriothecial or hysterothecial, linear, rarely y-shaped, solitary, gregarious, superficial, loose on host surface, black, opening with linear fissures. Upper wall comprising a thin layer of mostly neatly arranged dark cells, which are branched at the outer rim, base poorly developed. Hamathecium comprising, sparse, filiform pseudoparaphyses. Asci 8-spored, bitunicate, subglobose to oblong. Ascospores overlapping 2–4-seriate, 1-septate, with an upper cell slightly wider and shorter than the lower cell, hyaline, becoming brown with age, with basal protrusion.
Type species: Lembosina aulographoides (E. Bommer et al.) Theiss. (≡ Lembosia aulographoides E. Bommer et al.)
Lembosina aulographoides (E. Bommer et al.) Theiss., Annls mycol. 11(5): 437. 1913. Fig. 11.
Fig. 11.
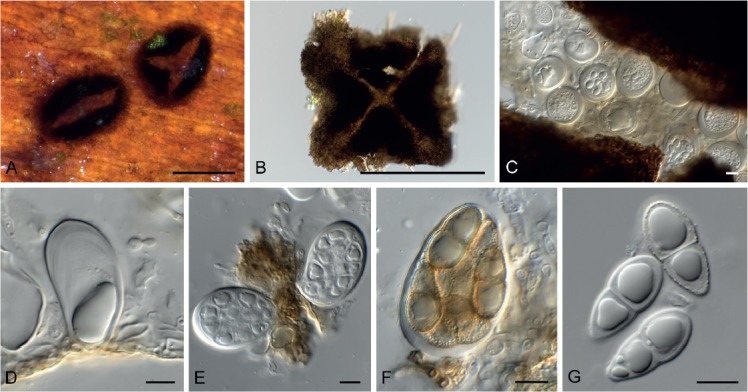
Lembosina aulographoides (CPC 33049). A. Hysterothecioid ascomata on host tissue. B. Superficial view of hysterothecium. C. Asci viewed from above. D–F. Asci. G. Ascospores. Scale bars: A, B = 250 μm, all others = 10 μm.
Basionym: Lembosia aulographoides E. Bommer et al., Bull. Soc. R. Bot. Belg. 29(1): 238. 1890.
Synonyms: Echidnodes aulographoides (E. Bommer et al.) N.F. Robertson, Trans. Br. mycol. Soc. 33: 108. 1950.
Microthyrium rhododendri Grove, J. Bot., London 71: 287. 1933.
Hypostroma well-developed, subcuticular, consisting of brown, smooth textura angularis to epidermoidea, cells compact, 6–12 µm long, 5–7 µm wide. Mycelium consisting of dark brown, verruculose, 2.5–3.5 µm diam, branched, septate hyphae, frequently covered in mucoid sheath. Hysterothecioid ascomata superficial, loose on surface, opening by central split, rarely with star-shaped central split, dark brown, 180–250 µm wide, 250–700 µm long; margin of dark brown hyphal cells, bluntly rounded, crenulate edge; surface cells 2.5–5 µm diam. Asci ovoid to obovoid or broadly ellipsoid, apex obtuse, with apical chamber, 2–3 µm diam, bitunicate, fissitunicate, wall thick, 3–5 µm in apical part, endotunica appearing multi-layered, base flat, 4–5 µm diam, attached to base of cavity, 30–50 × 20–30 µm. Pseudoparaphyses hyaline, smooth, branched, septate, hyphae-like, 2–3.5 µm diam; end cells frequently somewhat swollen. Ascospores multiseriate in asci, broadly fusoid-ellipsoid, widest in middle of apical cell, medianly 1-septate, constricted at septum, wall 1–2 µm thick, with irregular, prominent guttules, with basal mucoid appendage (plug), 5–6 µm diam, 2–8 µm long, persistent, (20–)22–23(–27) × (9–)10–12(–13) µm; ascospores turning brown and verruculose with age in asci; ascospores germinate irregularly, with germ tubes mostly from one cell, growing down into agar, brown, verruculose; ascospores not distorting upon germination.
Typus: Belgium, Tervuren, on dead branch of Rhododendron ponticum (Ericaceae), Jul. 1889, E. Bommer & M. Rousseau (holotype BR-MYC 049457,84). Netherlands, Bilthoven, on stems of Rhododendron sp., 1 Feb. 2017, P.W. Crous (epitype designated here CBS H-24088, MBT388213, culture ex-epitype CBS 145946 = CPC 33049).
Notes: Von Arx & Müller (1975) placed Lembosina in the Leptopeltidaceae, while Hawksworth et al. (1995) regarded it as a member of Asterinaceae, and Hyde et al. (2013) placed it in Aulographaceae. As shown here, Lembosina clusters in a separate clade next to Lichenoconiales (Fig. 1), an order based on Lichenoconium, which is a lichenicolous coelomycete genus with no known sexual morph (Hyde et al. 2013). A new order and family are therefore introduced to accommodate Lembosina.
Neomelanconium Petr., Annls mycol. 38(2–4): 208. 1940.
Classification: Cenangiaceae, Helotiales, Leotiomycetes.
Conidiomata acervular, intracorticolous, somewhat erumpent single, gregarious, lens-shaped with a flattened base sitting on a white to cream basal layer, exuding conidia in a black mucoid droplet. Conidiophores mostly reduced to conidiogenous cells arranged in a basal layer, hyaline, smooth, subcylindrical, proliferating percurrently at apex. Conidia aseptate, solitary, red-brown, ovoid to broadly ellipsoid, or pyriform to clavate, apex obtuse, base truncate, thick-walled, prominently coarsely guttulate, outer wall roughened, surrounded by prominent mucoid sheath.
Type species: Neomelanconium gelatosporum (H. Zimm.) Petr. (≡ Melanconium gelatosporum H. Zimm.)
Neomelanconium gelatosporum (H. Zimm.) Petr., Annls mycol. 38(2–4): 209. 1940. Fig. 12.
Fig. 12.
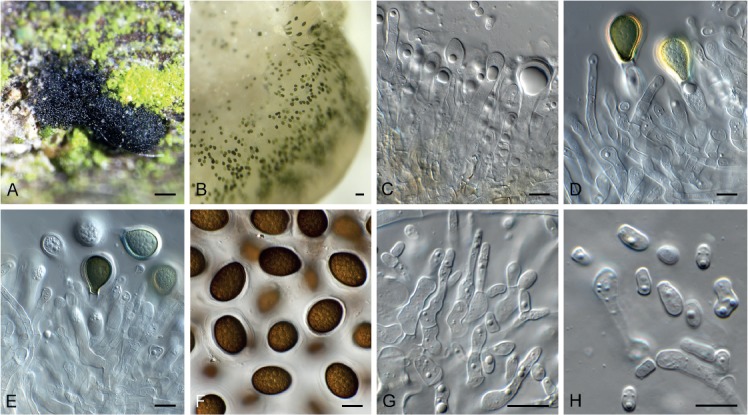
Neomelanconium gelatosporum (CPC 31126). A. Conidioma oozing conidia on host. B. Mucoid conidial droplet. C–E. Macroconidiogenous cells giving rise to macroconidia. F. Macroconidia. G, H. Microconidiogenous cells giving rise to microconidia. Scale bars: A = 250 μm, B = 30 μm, all others = 10 μm.
Basionym: Melanconium gelatosporum H. Zimm., Verh. nat. Ver. Brünn 52: 111. 1914.
Conidiomata acervular, intracorticolous, somewhat erumpent by tearing the bark, single, gregarious, lens-shaped with a flattened base sitting on a white to cream basal layer, exuding conidia in a black mucoid droplet. Macroconidiophores mostly reduced to macroconidiogenous cells arranged in a basal layer, hyaline, smooth, subcylindrical, 10–25 × 3–4 µm, proliferating percurrently at apex. Macroconidia aseptate, solitary, red-brown, ovoid to broadly ellipsoid, or pyriform to clavate, apex obtuse, base truncate, thick-walled, prominently coarsely guttulate, outer wall roughened, surrounded by prominent mucoid sheath, up to 18 µm thick (3 µm thick if dry), (22–)25–28(–35) × (15–)16–17(–18) µm. Microconidia forming in same conidioma on OA, intermingled among macroconidia. Microconidiophores aggregated, hyaline, smooth, subcylindrical, 0–2-septate, branched or not, 15–30 × 3–4 µm. Microconidiogenous cells subcylindrical, hyaline, smooth, terminal and intercalary, 10–20 × 3–4 µm, phialidic with minute periclinal thickening. Microconidia hyaline, smooth, guttulate, subcylindrical to somewhat clavate, apex obtuse, base truncate, 3–8 × 2.5–3 µm.
Culture characteristics: Colonies reaching 40 mm diam on OA after 2 wk at 25 °C, only 20 mm diam on MEA and PDA; surface folded, margin smooth, lobate. On MEA salmon to ochreous, reverse ochreous; on PDA surface and reverse ochreous, on OA cream to saffron.
Typus: Czechia, near Lednice (formerly “Eisgrub”), “Unterwald”, on dead stems and branches of Tilia sp. (Malvaceae), 15 Jan. 1913, H. Zimmermann (isotype, Petrak, Flora Bohemiae et Moraviae exsiccata, ser. 2, Abt. 1, Pilze, Lieferung 14–15 (nrs. 651–750), 1913, specimen nr. 669 in Herbarium H, Hamburg, Germany, selected here as lectotype MBT388214). Germany, near Berlin, green area, dead, corticated and attached twig of Tilia platyphyllos, 2 May 2016, R.K. Schumacher, RKS 1028 = HPC 1227 (epitype designated here CBS H-24089, MBT388215, cultures ex-epitype CPC 31126 = CBS 144985, CPC 31127).
Notes: Sutton (1980) did not see any material of Melanconium gelatosporum, and based his interpretation of Neomelanconium on N. deightonii, noting that the two species differ in the fact that N. deightonii lacks a conidial sheath. Although the phylogeny of N. deightonii remains unknown, a subsequent species, N. spartii (Wijayawardene et al. 2016), morphologically clearly represents a distinct genus from Neomelanconium, for which the name Pseudomelanconium is proposed below. No sequence data related to N. spartii were found in GenBank, nor is there any reference to such data in the original publication. A sequence-based comparison was therefore not possible. In our phylogenetic analysis, N. gelatosporum clusters in Leotiomycetes (Fig. 3). No highly similar sequences were obtained with a megablast search using the rpb1 and tef1 sequences.
Phragmotrichum Kunze, Mykologische Hefte (Leipzig) 2: 84. 1823.
Classification: Melanommataceae, Pleosporales, Pleosporomycetidae, Dothideomycetes.
Conidiomata stromatic to cupulate, immersed to erumpent, solitary to gregarious, dark brown, wall of textura angularis. Conidiophores hyaline, branched at base, septate, smooth, cylindrical, formed from inner layer of conidioma. Conidiogenous cells thallic, integrated, hyaline, smooth, cylindrical, producing unbranched, basipetal chains of conidia. Conidia brown, muriformly septate, with transverse and longitudinal septa, truncate at both ends, straight to curved, fusoid to ellipsoid, smooth-walled.
Type species: Phragmotrichum chailletii Kunze
Phragmotrichum chailletii Kunze, Mykologische Hefte (Leipzig) 2: 84. 1823. Fig. 13.
Fig. 13.
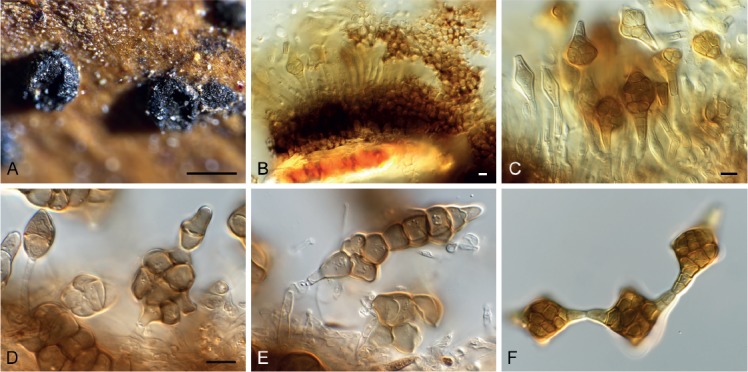
Phragmotrichum chailletii (CPC 33263). A. Cupulate conidiomata on host tissue. B. Section through conidioma. C. Conidiogenous cells giving rise to conidia. D–F. Conidial chains. Scale bars: A = 350 μm, all others = 10 μm, and D applies to E, F.
Synonym: Gymnosporangium chailletii (Kunze) Spreng., Systema Vegetabilium edit. 16, 4(1): 562. 1827.
Conidiomata 350–600 µm diam, stromatic to cupulate, immersed to erumpent, solitary to gregarious, dark brown, wall of textura angularis, up to 80 µm diam. Conidiophores hyaline, branched at base, 1–3-septate, smooth, cylindrical, formed from inner layer of conidioma, 15–30 × 3–4 µm. Conidiogenous cells thallic, integrated, hyaline, smooth, cylindrical, producing unbranched basipetal chains of conidia, 10–12 × 3–4 µm. Conidia brown, muriformly septate, with 4–5 transverse septa, and 2–3 longitudinal septa, truncate at both ends, straight to curved, fusoid to ellipsoid, smooth-walled, (25–)35–45(–50) × (18–)20–23(–25) µm (based on CPC 33263).
Culture characteristics: Colonies erumpent, spreading, covering the dish in 2 wk at 25 °C with moderate aerial mycelium and smooth, even margins. On MEA surface vinaceous buff with patches of dirty white, reverse brown vinaceous; on PDA surface dark mouse grey with patches of olivaceous grey; on OA surface sepia with patches of purplish grey.
Typus: Switzerland, near Neuchátel (“Neuenburg”), on fallen cones of “Pinus abies” (Pinaceae), 1823 or before, J.F. de Chaillet. Specimen not mentioned, and not located. Switzerland, Hindelbank, “Crajholz”, fallen cones of Picea abies (Pinaceae), 7 Mar. 2017, J. Gilgen & R.K. Schumacher, RKS 77 = HPC 2016 (neotype designated here CBS H-24090, MBT388216, cultures ex-neotype CPC 33263 = CBS 144994, CPC 33264).
Additional material examined: Switzerland, Hindelbank, “Crajholz”, fallen cones of Picea abies, 7 Mar. 2017, J. Gilgen & R.K. Schumacher, RKS 74 = HPC 2014, cultures CPC 33341, CPC 33342.
Notes: The holotype grew on fallen cones of “Pinus abies” collected in Switzerland, but could not be traced in this study, (Kunze’s herbarium at LZ was destroyed during World War II), and thus a neotype is designated. The old substrate name is a synonym of Abies alba and Picea abies. Colonies of the neotype sporulated in culture. The species is shown here to belong to Melanommataceae (Fig. 1).
Pseudomelanconium Crous & R.K. Schumach., gen. nov. MycoBank MB832088.
Classification: incertae sedis, Pezizomycotina.
Etymology: Name reflects its morphological similarity to the genus Melanconium.
Conidiomata pycnidial, immersed to subepidermal, solitary or gregarious, occasionally confluent, unilocular, globose to subglobose, black; wall multi-layered, outer layer thick, composed of brown textura angularis, inner layer thin, hyaline to pale brown. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, short, indeterminate, discrete, cylindrical, hyaline to dark brown, smooth-walled. Conidia globose, aseptate, base truncate, dark brown, thick and smooth-walled.
Type species: Pseudomelanconium spartii (Wijayaw. et al.) Crous & R.K. Schumach. (≡ Neomelanconium spartii Wijayaw. et al.)
Pseudomelanconium spartii (Wijayaw. et al.) Crous & R.K. Schumach., comb. nov. MB832089.
Basionym: Neomelanconium spartii Wijayaw. et al., Fungal Diversity 77: 182. 2016.
Description and illustration: Wijayawardene et al. (2016).
Typus: Italy, Arezzo, AR Province, Montalone, Pieve Santo Stefano, on dead branch of Spartium junceum (Fabaceae), 6 Jun. 2012, E. Camporesi, IT 404 (holotype MFLU 15-3453, isotype HKAS 92541).
Notes: Pseudomelanconium is morphologically distinct from Neomelanconium gelatosporum, lacking persistent mucoid sheaths on its conidia. Whether P. spartii is congeneric with N. deightonii, can only be resolved once DNA data become available.
Rutola J.L. Crane & Schokn., Canad. J. Bot. 55: 3015. 1978 (1977).
Classification: Torulaceae, Pleosporales, Pleosporomycetidae, Dothideomycetes.
Colonies oval, powdery, dry, black. Conidiophores appressed to substrate, micronematous, branched, septate, pale brown. Conidiogenous cells integrated, terminal or intercalary, monoblastic, pale brown. Conidia phragmosporous, composed of long, simple to branched chains of brown, verruculose acrogenous cells, constricted at septa, fragmenting into segments, 0–multiseptate.
Type species: Rutola graminis (Desm.) J.L. Crane & Schokn.
Rutola graminis (Desm. ex Fr.) J.L. Crane & Schokn., Canad. J. Bot. 55: 3015. 1978 (1977). Fig. 14.
Fig. 14.

Rutola graminis (CPC 33267). A. Sporodochia on host tissue. B. Conidiogenous cells giving rise to conidia. C, D. Conidial chains. Scale bars: A = 5 mm, all others = 10 μm.
Basionym: Torula graminis Desm., Pl. Crypt. Nord. Fr., fasc. 4 no. 169. 1826. [: Fr., Syst. mycol. (Lundae) 3(2): 502. 1832].
Synonym: Torula tritici Corda, Icon. fung. (Prague) 1: 8. 1837 (see Braun & Kirk 2019).
Colonies on stubble oval, powdery, dark brown, 0.5–2 × 0.1–0.5 mm. Mycelium consisting of branched, septate, subhyaline to pale brown, smooth, immersed to superficial, 1.5–2 µm diam hyphae. Conidiophores appressed to substrate, branched, pale brown, smooth. Conidiogenous cells integrated, terminal or intercalary, monoblastic, pale brown, smooth, 4–9 × 3–3.5 µm. Conidia phragmosporous, composed of long (13–20 conidia), simple to branched acrogenous chains, attached by narrow isthmus, constricted at septa, fragmenting into segments with one to several septa. Conidial segments dark brown, semi-spherical, broader than long, thick-walled (1–1.5 µm diam), verruculose, (3–)4–5(–6) × 4–6(–8) µm.
Culture characteristics: Colonies reaching 30 mm diam after 2 wk, with sparse to moderate aerial mycelium and smooth, lobate margins. On PDA surface pale olivaceous grey, reverse grey olivaceous; on OA surface olivaceous grey with patches of pale olivaceous grey, margins frequently olivaceous black.
Typus: France, north France, on dead leaf of an unnamed grass (family unknown), Apr. 1826 or before, J. Desmazières (isotype, L 910.267-926 = L0054599 ex Plantes cryptogames du nord de la France 1826, edit. 1, fasc. 4, nr. 169, as “Torula graminis Desm.”, selected here as lectotype, MBT388217). Isolectotypes: Desm., Plantes cryptogames du nord de la France 169 (e.g., BM, FH, G, ILLS841, NY). Germany, near Berlin, on dead and still attached leaf of Typha sp. (Typhaceae), 1 Apr. 2017, R.K. Schumacher, HPC 2021 = RKS 80 (epitype designated here CBS H-24078, MBT388218, cultures ex-epitype CPC 33267 = CBS 145906, CPC 33268).
Additional materials examined: Germany, near Berlin, on dead and still attached leaf of Scirpus sylvaticus (Cyperaceae), 3 May 2017, R.K. Schumacher, HPC 2112 = RKS 103, cultures CPC 33714, CPC 33715. Norway, Akershus, Skedsmo, Lahaug, on Scirpus sylvaticus, 3 May 2017, K. Homble, HPC 2115, culture CPC 33695.
Notes: Although Rutola was treated by Crane & Schoknecht (1977), and recently by Crous et al. (2015), the type species was not known from culture or DNA sequence data, and thus had to be recollected. As shown here, Rutola is morphologically and phylogenetically distinct from Torula, but both genera reside in the Torulaceae (Fig. 1), and T. herbarum (Fig. 15) can frequently co-occur on the same material.
Fig. 15.
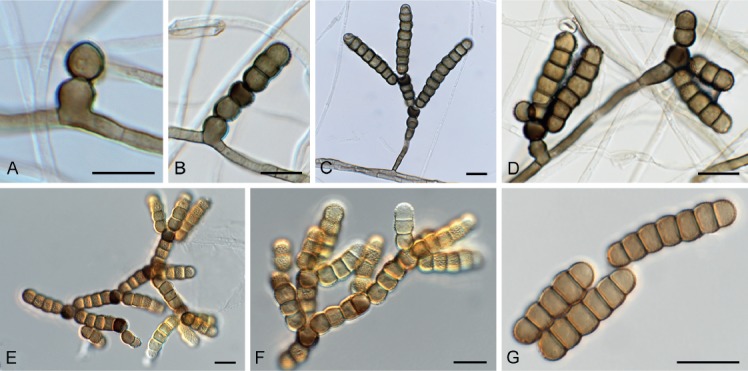
Torula herbarum (CBS 144995). A–D. Conidiogenous cells giving rise to conidia. E–G. Conidia. Scale bars = 10 μm.
Trullula Ces., in Rabenh., Klotzschii Herbarium Vivum Mycologicum, cent. 17, nr. 1660. 1852, also in Bot. Ztg. 10: 287. 1852.
Classification: incertae sedis, Pezizomycotina.
Conidiomata eustromatic, immersed, becoming erumpent, globose, cupulate, unilocular, pale brown; wall of 3–6 layers of textura angularis. Conidiophores unbranched, septate, hyaline to subhyaline, cylindrical. Conidiogenous cells phialidic, subcylindrical, terminal, determinate, hyaline with minute collarette. Conidia aseptate, in unbranched chains, subcylindrical to doliiform, straight, hyaline to subhyaline, thin-walled, apex obtuse, base truncate.
Type species: Trullula oreoselini Ces.
Trullula oreoselini Ces. in Rabenh., Klotzschii Herbarium Vivum Mycologicum, cent. 17, nr. 1660. 1852. Fig. 16.
Fig. 16.
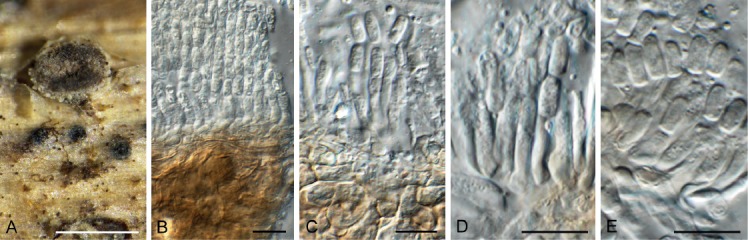
Trullula oreoselini (holotype Herbarium B). A. Semi-immersed conidioma on host. B–E. Conidiogenous cells giving rise to conidia. Scale bars: A = 350 μm, all others = 10 μm.
Conidiomata eustromatic, immersed, becoming erumpent, globose, 200–350 µm diam, cupulate, unilocular, pale brown to black olive, sometimes white bordered, soft, gelatinous; wall of 3–6 layers of textura angularis. Conidiophores unbranched, 0–1-septate, hyaline to subhyaline, cylindrical, 5–15 × 2–3 µm. Conidiogenous cells phialidic, subcylindrical, terminal, determinate, hyaline with minute collarette, 5–8 × 2–3 µm. Conidia aseptate, in unbranched chains, subcylindrical to doliiform, straight, hyaline to subhyaline to olivaceous (olivaceous in mass), thin-walled, apex obtuse, base truncate, (5–)6–7(–8) × 3(–4) µm.
Typus: Italy, Vercelli, on dead stem of Peucedanum oreoselinum (Apiaceae), winter 1852, V. de Cesati (B, s.n., ex Rabenh., Klotzschii herbarium Vivum Mycologicum 1852, Cent. 17, Nr. 1660; selected here as lectotype, MBT388219). Isolectotypes: Rabenh., Klotzschii herbarium Vivum Mycologicum 1660 (e.g., HAL).
Notes: In his treatment of Trullula, Sutton (1980) indicated that Trullula oreoselini was the correct lectotype species of Trullula. However, as he did not see the type, he based his interpretation of the genus on Epidochium melanochlorum (= T. melanochlorum), stating that if the type of T. oreoselini was ever found to deviate morphologically from that of E. melanochlorum, a new generic name would have to be chosen from among the synonyms he listed for Trullula to accommodate E. melanochlorum.
Our examination of the type specimen of T. oreoselini showed that it is not congeneric with that of E. melanochlorum, and that Trullula s.str., 1852, probably represents an older name for what is now called Sirexcipula [1907, based on S. kabatiana Bubák: Czech Republic, Turnov, on dead leaves of Hosta sieboldiana (= Funkia sieboldiana) (Asparagaceae), leg. J.E. Kabat, 12 May 1905, syntypes BPI 392537 and 392538, IMI 194246 (Kabát & Bubák, Fungi imperfect exsiccate 571, e.g., S-F58940 and WIS-F-00822942, = topotype material collected at 22 May 1905)]. However, as there are no cultures of Trullula oreoselini nor Sirexcipula kabatiana, this matter cannot be pursued further here.
ACKNOWLEDGEMENTS
We are grateful to Arien van Iperen, Diana Vos, Yda Vlug, Trix Merkx (cultures), Mieke Starink-Willemse (DNA isolation, amplification and sequencing), and Marjan Vermaas (photographic plates) for their technical assistance. We are also grateful to Robert Lucking (B) for sending us a loan of Trullula oreoselini for study, and the curators of BR for material of Lembosia aulographoides.
REFERENCES
- Braun U, Nakashima C, Crous PW, et al. (2018). Phylogeny and taxonomy of the genus Tubakia s. lat. Fungal Systematics and Evolution 1: 41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Kirk P. (2019). Reassessment of the nomenclature of some ascomycete names. Schlechtendalia 36: 87–89. [Google Scholar]
- Crane JL, Schoknecht JD. (1977) Revision of Torula species. Rutola, a new genus for Torula graminis. Canadian Journal of Botany 55: 3013–3019. [Google Scholar]
- Crous PW. (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. APS Press, St. Paul, MN, USA. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Giraldo A, Hawksworth DL, et al. (2014). The Genera of Fungi: fixing the application of type species of generic names. IMA Fungus 5: 141–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. (2013). A phylogenetic re-evaluation of Arthrinium. IMA Fungus 4: 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Hawksworth DL, Wingfield MJ. (2015). Identifying and naming plant-pathogenic fungi: past, present, and future. Annual Review of Phytopathology 53: 246–267. [DOI] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. (eds) (2019). Fungal Biodiversity. [Westerdijk Laboratory Manual Series no.1.] Utrecht: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991). Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632. [Google Scholar]
- Fan XL, Bezerra JDP, Tian CM, et al. (2018). Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Persoonia 40: 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. (2011). The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Kirk PM, Sutton BC, et al. (1995). Ainsworth & Bisby’s dictionary of the fungi, 7th edn CAB International, Wallingford. [Google Scholar]
- Huhndorf SM, Greif M, Mugambi GK, et al. (2008). Two new genera in the Magnaporthaceae, a new addition to Ceratosphaeria and two new species of Lentomitella. Mycologia 100: 940–955. [DOI] [PubMed] [Google Scholar]
- Huhndorf SM, Miller AN, Fernández FA. (2004). Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia 96: 368–387. [PubMed] [Google Scholar]
- Hyde KD, Jones EBG, Lui J-K, et al. (2013). Families of Dothideomycetes. Fungal Diversity 63: 1–313. [Google Scholar]
- Hyde KD, Read SJ, Gareth Jones EB, et al. (1997). Tropical Australian Freshwater Fungi. XII. Rivulicola incrustata gen. et sp. nov. and notes on Ceratosphaeria lampadophora. Nova Hedwigia 64: 185–196. [Google Scholar]
- Jiang N, Li J, Tian CM. (2018). Arthrinium species associated with bamboo and reed plants in China. Fungal Systematics and Evolution 2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaubauf S, Tharreau D, Fournier E, et al. (2014). Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology 79: 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintos A, Alvarado P, Planas J, et al. (2019). Six new species of Arthrinium from Europe and notes about A. caricicola and other species found in Carex spp. hosts. MycoKeys 49: 15–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozynski KA, Morgan-Jones G. (1968). Notes on Microfungi. III. Transactions of the British Mycological Society 51: 185–206. [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Réblová M. (2006). Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia 98: 68–93. [DOI] [PubMed] [Google Scholar]
- Robert V, Vu D, Amor ABH, et al. (2013). MycoBank gearing up for new horizons. IMA Fungus 4: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Crous PW, Hyde KD, et al. 2015. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 6: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan A. (1974). A new species of Protoventuria and some further combinations of Protoventuria for Antennularia species. Transactions of the British Mycological Society 63: 590–594. [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, et al. (1996) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Sutton BC. (1980). The Coelomycetes: fungi imperfecti with pycnidia, acervuli, and stromata. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Sutton BC, Crous PW. (1997). Lecanostictopsis gen. nov. and similar fungi from Syzygium species. Mycological Research 101: 215–225. [Google Scholar]
- Swofford DL. (2003). PAUP*: phylogenetic analysis using parsimony. (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland. [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. (2016). All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. (2017). Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arx JA, Müller E. (1975). A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology 9: 1–159. [Google Scholar]
- Vu D, Groenewald M, de Vries M, et al. (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tan X-M, Liu F, et al. (2018) Eight new Arthrinium species from China. MycoKeys 34: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardene NN, Hyde KD, Wanasinghe DN, et al. (2016). Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Diversity 77: 1–316. [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, et al. (2011). A molecular, morphological and ecological re-appraisal of Venturiales – a new order of Dothideomycetes. Fungal Diversity 51: 249–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, et al. (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203–214. [DOI] [PubMed] [Google Scholar]


