Abstract
Diet, especially seafood, is the main source of arsenic exposure for humans. The total arsenic content of a diet offers inadequate information for assessment of the toxicological consequences of arsenic intake, which has impeded progress in the establishment of regulatory limits for arsenic in food. Toxicity assessments are mainly based on inorganic arsenic, a well-characterized carcinogen, and arsenobetaine, the main organoarsenical in seafood. Scarcity of toxicity data for organoarsenicals, and the predominance of arsenobetaine as an organic arsenic species in seafood, has led to the assumption of their nontoxicity. Recent toxicokinetic studies show that some organoarsenicals are bioaccessible and cytotoxic with demonstrated toxicities like that of pernicious trivalent inorganic arsenic, underpinning the need for speciation analysis. The need to investigate and compare the bioavailability, metabolic transformation, and elimination from the body of organoarsenicals to the well-established physiological consequences of inorganic arsenic and arsenobetaine exposure is apparent. This review provides an overview of the occurrence and assessment of human exposure to arsenic toxicity associated with the consumption of seafood.
Keywords: arsenic, speciation, toxicity, carcinogen, exposure, seafood, organoarsenicals
1. INTRODUCTION
It is estimated that the ocean is inhabited by more than 1000 species of crustaceans and 50000 species of mollusks, aside from the 13000 species of finfish.1 Marine species including seafood and seaweed are deemed as essential portions of healthy diets as they comprise various nutrients linked with beneficial health effects2 and are widely used as dietary supplements. Human consumption of seafood has been increasing steadily mainly because of the reports of health benefits associated with their consumption.2–4
Like all mammals, humans lack enzymes for the synthesis of omega-3 (ω-3) and omega-6 (ω-6) precursors of DHA and EPA, which are therefore essential fatty acids and need to be provided by dietary sources.5 Fish is the best dietary source of protein, long-chain omega-3 polyunsaturated fatty acids (ω-3 LCPUFAs) and other nutrients which are linked to a range of health benefits.6 Long-chain omega-3 polyunsaturated fatty acids (ω-3 LCPUFAs), particularly eicosapentanoic acids (EPA, 20:5ω-3) and decosahexanoic acid (DHA, 22:6ω-3), have been demonstrated to have antiatherosclerotic and antithrombotic effects3 and are linked to reduced risk of cardiovascular disease (CVD).7 Benefits on visual acuity and cognitive development have been largely established in term8,9 and preterm10,11 infants fed ω-3 LCPUFAs-supplemented formula.12
Typically, shellfish contain substantial quantities of digestible proteins, essential amino acids, bioactive peptides, ω-3 LCPUFAs, astaxanthin and other carotenoids, vitamin B12 and other vitamins, minerals, including copper, zinc, inorganic phosphate, sodium, potassium, selenium, iodine, and other nutrients, which offer a variety of health benefits to the consumer.13,14 Seafood is low in calories compared with other animal foods. For example, a 100 g serving of shrimp provides approximately 106 kcal of energy, whereas the same amount of fish provides 110–150 kcal, lean beef 250 kcal, and roasted chicken 200 kcal of energy.15
Seaweed has been part of the human diet, especially in Asia, for centuries and has gained popularity in western countries.16,17 Seaweeds are extensively utilized in the food industry and are becoming increasingly commercially available because of their properties as food additives,18 their nutritional values,18,19 and implied medical applications.19,20 Seaweed intake has been linked with reduced risk of breast and colorectal cancers, perhaps owing to the extraordinary fiber and vitamin content.21 Both kelp and laver contain a large amount of iodine, a vital element for thyroid health.17 Laver is an excellent source of vitamins A and C, while kelp is a good source of folic acid.17 Human exposure to arsenosugars (AsSugars) is fairly high in Asia owing to a diet rich in seaweed.22 Fortunately, there are no indication for acute and chronic toxicity related to seaweed ingestion from epidemio-logical studies.21
According to the State of World Fisheries and Aquaculture, published by the United Nation’s Food and Agriculture Organization (FAO), in 2014, an amount of 167.2 million metric tons (MMT) of seafood was globally available, with landings of shrimp, American lobsters, and cephalopods at 3.5, 0.16, and 4.3 MMT, respectively.23 Total fish production in 2016 reached an all-time high of 171 MMT, of which 88% was utilized for direct human consumption with per capita consumption of 20.3 kg and an export value of U.S. $143 billion.24 An estimated 38% of the 23.8 MMT of seaweeds in the 2012 global harvest was eaten by humans.25 The global harvest of seaweeds in 2013 was estimated at U.S. $6.7 billion.26
Consumer demand for shellfish and other seafood has resulted in a significant rise in their wild catch and aquaculture in fresh, brackish, and marine waters, with a total production of 73.8 MMT in 2014, at an estimated value of U.S. $160 billion. This included 16.1 MMT of mollusks composed of 104 species valued at U.S. $19 billion and 6.9 MMT of crustacean valued at U.S. $36.2 billion.23 Aquaculture is projected to grow at almost 39% annually, with an estimated production of about 102 MMT in 2025.23 Shellfish formed 38% of total seafood traded in 2013, with shrimp being the most popular shellfish.27
In the United States, where the per capita shrimp consumption of about 1.73 kg was recorded in 2012, almost 90% of the shrimp consumed came from imports.28 The per capita shellfish consumption in 2013 was 4.9 kg, subdivided into 1.8 kg of crustaceans, 0.5 kg of cephalopods, and 2.6 kg of other mollusks.23 In 2014, an amount of 146.3 MMT of seafood was used as human food, giving a global per capita seafood consumption of 20.1 kg and contributing to about 20% of total average per capita intake of animal protein.13 Per capita seafood consumptions of 25.8 and 35 kg were also reported in 2017 in the European Union (E.U.) and southern Europe, respectively.13
Evidently, seafood is a highly consumed and traded commodity, and therefore, intentional or unintentional contamination with toxic elements like arsenic may become technical barriers to trade. For example, in December 2013 China imposed a ban on all U.S. imports of geoduck clams (Panacea generosa), a large edible saltwater clam found along the Pacific Northwest extending from northern California to southeastern Alaska, citing high levels of arsenic contamination. Revenue from U.S. exports of geoduck clams are upward of $80 million annually with about 90% of all exports going to China.29
Jennings et al. acknowledged the need for seafood supply to not only meet the needs and preferences of consumers but also for it to be sustainable and provide nutritional benefits while posing minimal health risks.30 Despite the many health benefits of seafood, they are unfortunately inherently contaminated with arsenic, especially organic arsenic species.31 It is therefore critical from a risk-based approach to conduct arsenic speciation analysis in order to determine the arsenic species present and their relative proportions consumed by humans and thus enable more accurate risk assessment.32–34
Seafood is the major dietary source of total arsenic in humans,35 excluding regions with widespread elevated drinking water contamination.36–39 Organic arsenic predominates in seafood; however, this is not always the case, as there are reported cases of elevated inorganic arsenic levels in seafood such as in edible seaweed Hijiki (60–150 μg g−1, iAs),40 fish from Thailand,41 and mussel from Norway.42 Marine algae and shellfish are the seafood exposure sources with the greatest diversity of arsenicals.43 Among these arsenicals the potential for biotransformation upon ingestion varies considerably.44 Arsenic speciation (Figure 1) is complicated, and diverse arsenic species display great difference in toxicities.45
Figure 1.
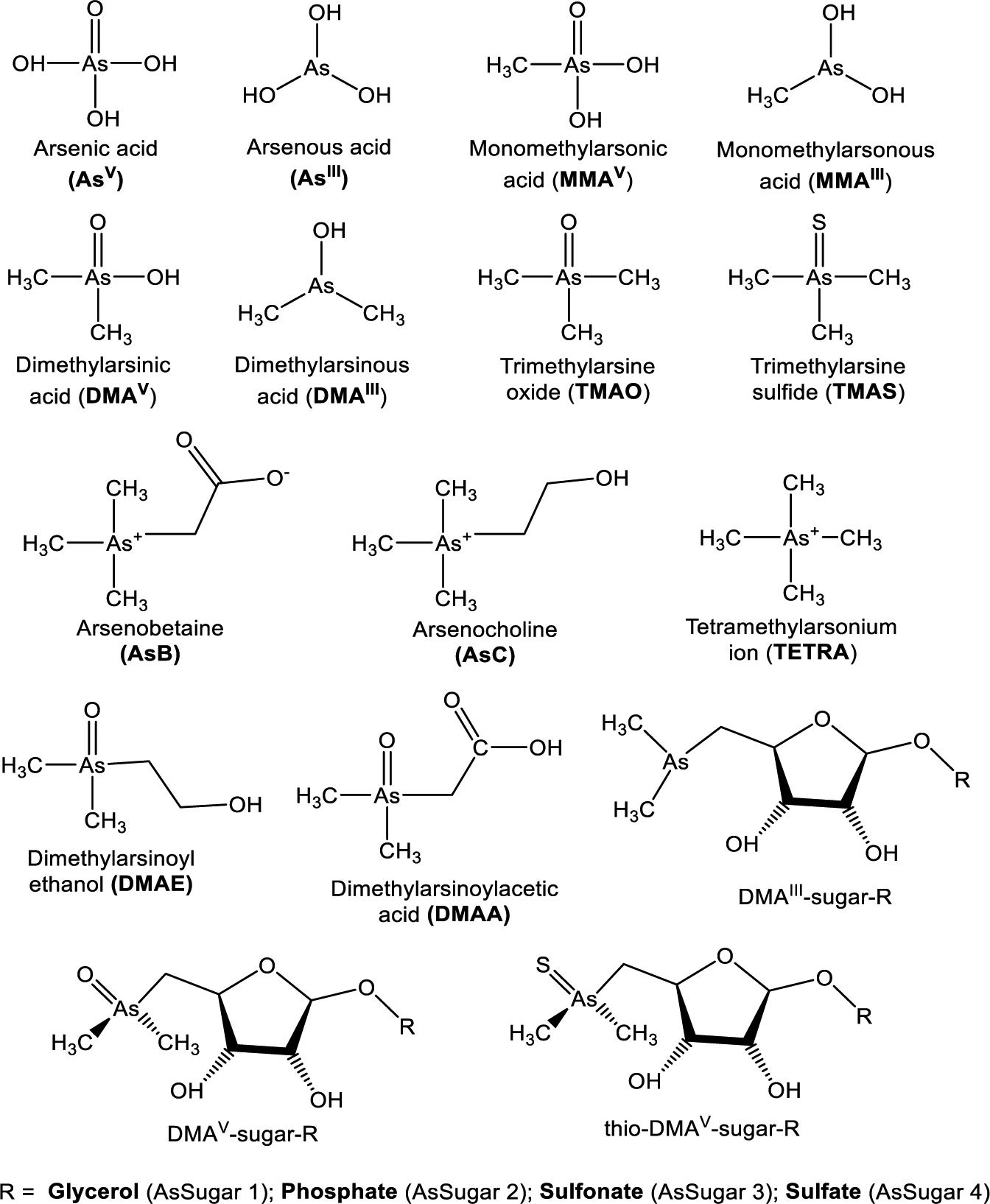
Structures of common arsenic compounds.
The chemical state of arsenic influences its bioavailability, mobility, and toxicity, among other properties.46 Arsenic exists in four oxidation states in the inorganic form as trivalent arsenite (iAsIII) and thermodynamically stable pentavalent arsenate (iAsV).47 Elemental arsenic (As0) and arsine, H3As (As−III), exist in strongly reducing conditions.48–50 Arsenic exists in organic form where arsenic is bonded to carbon as low molecular weight compounds like monomethylarsonate (MMA), dimethylarsenate (DMA), arsenobetaine (AsB), arsenocholine (AsC), trimethylarsine oxide (TMAO), and tetramethylarsonium ion (TETRA); high molecular weight arsenosugars (AsSugar) and arsenolipids (AsLipids); and in complexed form as arsenopeptides (glutathione (As-GSH), and phytochelatins (AsPC)).46,51,52
Arsenobetaine (AsB) is the main arsenical in seafood, commonly comprising in excess of 90% of the total arsenic in fish.53–56 AsB is the major polar arsenical, which together with AsSugars and a range of other lipophilic arsenicals account for over 70 naturally occurring organoarsenicals found in seafood.34,37 There is great diversity in the level of arsenic in seafood, but arsenic in most samples falls within the mass fraction range of about 5 to 100 μg g−1 dry mass.34 Sirot et al. reported the levels of inorganic arsenic (iAs) in 30 fish species collected in France as 0.005–0.073 μg g−1 on wet mass basis. This clearly demonstrated that the proportion of iAs to total arsenic content, mostly AsB, was 100-fold lower.57
The aim of this paper is to give a comprehensive summary of the current state of understanding of the various aspects of organoarsenical formation in the marine food chain in terms of occurrence, exposure, metabolic transformation, and toxicity of smaller-molecule oxo-arsenicals, arsenosugars, arsenolipids, and thio-arsenicals. A brief discussion about the mechanism of toxicity of inorganic arsenic is also presented in the paper because it is the arsenic species with well-understood adverse effects. This may seem out of place but is important for the understanding of the general behavior of organoarsenicals. Where the term arsenic is used, it means inorganic arsenic (iAs).
2. METABOLIC TRANSFORMATION AND TOXICITY OF ARSENIC
An enormous assortment of biologically relevant arsenic species has been characterized in samples of dietary sources.16,34,55,58–60 Arsenic toxicity, bioaccumulation, and mobility are greatly dependent on the chemical state in which the element appears and the extent of methylation through the metabolism process.38,43,61,62 iAsV and iAsIII are categorized as nonthreshold Class I carcinogens,63 with acute toxicity of LD50 = 15–42 mg/kg body mass, while simple methylated arsenicals are deemed to pose intermediary toxicity (LD50 = 890–10600 mg/kg body weight), and the tetraalkylated compound AsB, present in fish and the principal dietary source of arsenic exposure for humans, is nontoxic with LD50 = >10000 mg/kg body weight and is primarily eliminated intact by humans in the urine.56,64
Typically the lower the oxidation number is, the higher the toxicity is, and the higher the methylation is, the lower the toxicity is, thus producing the following order of toxicity for arsenic species in human cell lines: monomethylarsonous acid (MMAIII) > dimethylarsinous acid (DMAIII) > arsenite (AsIII) > arsenate (AsV) > trimethylarsine (TMA+) > dimethylarsinic acid (DMAV) = monomethylarsonic acid (MMAV) > trimethylarsine oxide (TMAO) > arsenocholine (AsC) > arsenobetaine (AsB).65–68 Moreover, AsB, AsC, TMAO, AsSugars, AsLipids, and other organoarsenicals are usually mildly toxic compared to iAs species.34,45,69
Due to the extreme toxicity of iAs, microalgae and other living organisms may undergo dissimilar processes to lessen the toxic effects, including cell surface adsorption, arsenite oxidation and arsenate reduction,70,71 methylation,72 conversion to AsSugars or AsLipids,73 chelation of iAsIII with glutathione and phytochelatins,74,75 and elimination from cells.45 Arsenic detoxification occurs through a series of biotransformations in biotic systems which produce a wide range of organoarsenicals, whereby arsenic is covalently bonded with one or more carbon atoms containing functional groups.75,76 Toxicity is instituted once liver methylation ability is impeded or surpassed.77–79
It is important to note that discussions on arsenic toxicity are mainly in reference to inorganic arsenic and methylated arsenicals (MMA and DMA) since their toxicity mechanism is well established and understood unlike for AsSugars and AsLipids which are yet to be fully elucidated. Organoarsenicals have not demonstrated acute toxicity; therefore, their toxicity may arise from metabolic transformations that lead to formation of highly toxic metabolites.52,58,64 For example, the toxicity of AsSugar and AsLipids may arise from their metabolic breakdown to other arsenicals such as DMAV, which is also the metabolite of iAs found in urine and is a known tumor promoter and confirmed carcinogen in experimental animals.80,81
Liver, kidneys, heart, and lungs are main repository organs of arsenic, with slight buildup in brain and muscle tissues.62 This buildup is linked with a variety of ailments including diabetes, hepatotoxicity, cancer, and nephrotoxicity. Arsenic can cause thiamine deficiency by lowering its accessibility, leading to lactic acidosis by enhancing lactic acid concentration.62 In addition, arsenic may cause genotoxicity by impeding DNA repair mechanism and further stimulates oxidative stress by producing reactive nitrogen species (RNS) and reactive oxygen species (ROS).62,82,83
In biological systems, arsenate can replace phosphate and form esters that resemble phosphate esters, which are abundant in biomolecules, from the sugar phosphates of intermediary metabolism, to membrane phospholipids, to the phosphate backbone of genetic materials like deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).84 However, these compounds are less stable because they are more delicate than the subsequent phosphate esters, which is partly attributed to the bond lengths and bond angles.85 The P-O bond length in phosphates is almost 1.5 Å, whereas the bond length of As-O in arsenates is roughly 1.6 Å to 2.0 Å. Arsenic usually form lengthier bonds than phosphorus (Figure 2).85 In addition, arsenic angles are considerably less obtuse than the phosphorus angles,84 i.e., the O-P-O bond angle is about 117°, whereas the O-As-O bond angle is roughly 100°, with minor variabilities in diverse compounds. Therefore, since longer bonds are fragile, arsenate esters disintegrate more easily than phosphate esters.
Figure 2.
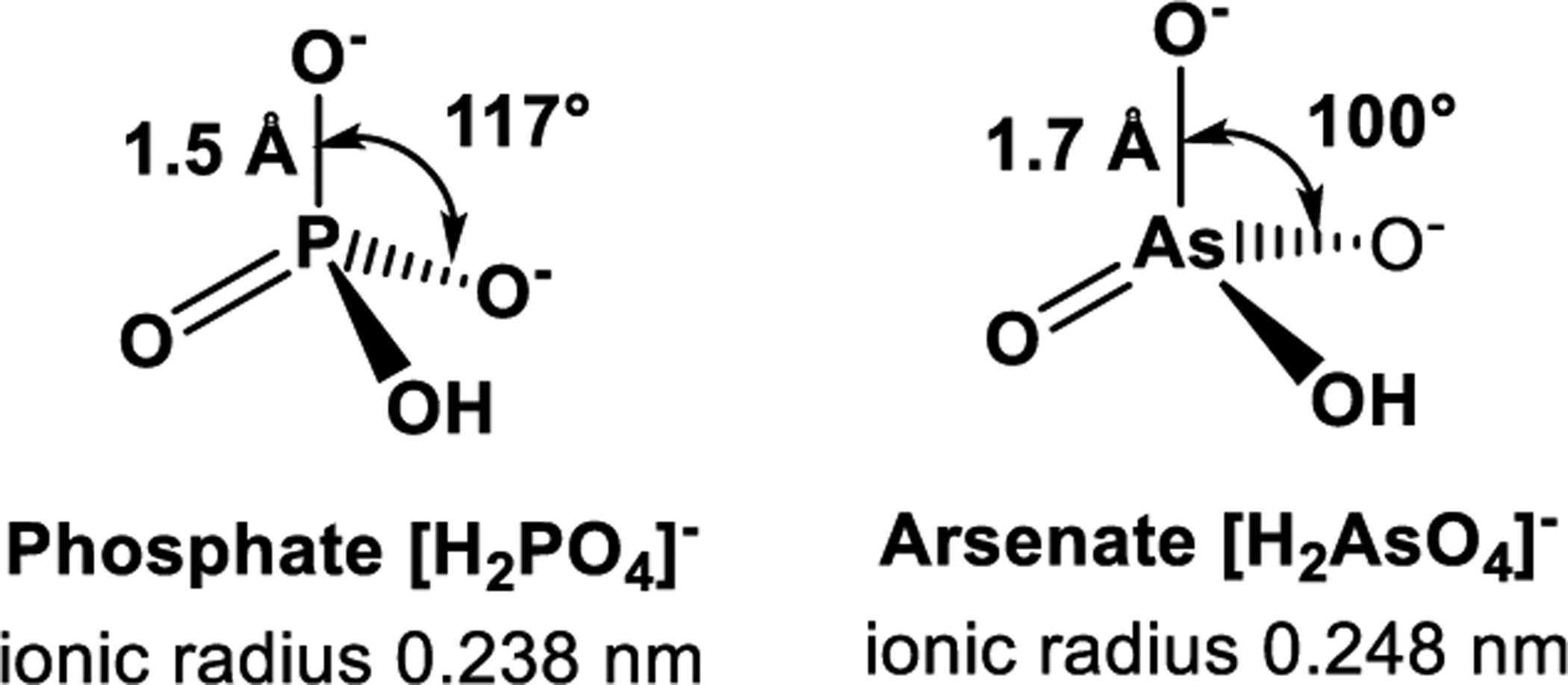
Structures of deprotonated oxo-anions of arsenate and phosphate.
Within the cell, metabolic enzymes which utilize phosphate may also incorporate arsenate in alkylation, acylation, or phosphorylation reactions because of their isosteric and isoelectronic nature.86 For example, arsenate inhibits the formation of ATP as a result of generation of delicate anhydrides and also uncouples ATP synthesis during oxidative phosphorylation by coupling with adenosine diphosphate (ADP) in the presence of succinate in the mitochondria to form ADP-arsenate rather than ATP.87–89 This disrupts phosphorus metabolism since ADP-arsenate may be utilized as a substrate for hexokinase, which under normal conditions produces glucose-6-phosphate, the first intermediary in the glycolytic pathway.87 This inhibits hexokinase by negative feedback. The glucose-6-arsenate produced by hexokinase is then transformed to glucose-1-arsenate by phosphoglucomutase, proving that intermediary metabolism can produce arsenicals.86 The rate constant for spontaneous breakdown of glucose-6-arsenate has been shown to be 4 × 10−4 s−1, compared to 4 × 10−9 s−1 for glucose-6-phosphate. Therefore, the short half-life of arsenate ester causes it to spontaneously hydrolyze 105 times faster than the phosphate ester, which is the major cause of decoupling action of arsenate in oxidative phosphorylation,87 and therefore cells essentially starve for scarcity of metabolic intermediates.45,75,84,86,90
The other factor that potentially contributes to the instability of arsenicals is the fact that iAsV is swiftly reduced to iAsIII within the cells.84 The biological half-life of As is roughly 4 days, which is dependent on the manner of exposure as iAsIII is considered to have a shorter half-life in comparison to iAsV.91 iAsIII is the most hazardous and toxic form of inorganic arsenic, which block sulfhydryl (−SH) groups by creating robust bonds of metallic nature with thiols in proteins and small molecules.84 iAsIII toxicity is due to its high affinity for thiol groups, causing allosteric inhibition of respiration by binding to vicinal thiols in pyruvate dehydrogenase (PDH) and 2-oxoglutarate dehydrogenase,90 with resultant membrane destruction and cell death by generating reactive oxygen species (ROS).45,92 The allosteric inhibition of PDH, a vital precursor of acetyl-CoA, not only restricts the generation of ATP in the electron transport chain but also impedes the formation of gluconeogenesis intermediaries.93–96 Moreover, research findings indicate that arsenite is able to traverse across the blood-brain barrier.97–99
Due to scarcity of toxicity and long-term exposure data for organoarsenicals in humans or other mammals, health hazards from exposure to organoarsenicals are challenging to evaluate. Most of the injurious effects of inorganic arsenic have been documented, but the uncertainty concerning the threat to the people exposed to organic arsenic, especially from seafood, and the dosage needed to trigger these effects still linger. Numerous investigations have contemplated the latent carcinogenicity from the formation of the metabolite DMAV,100–104 centered on elevated dose exposure studies in rats to DMA in water81 or diet.105 There is, however, compelling indication that the rat model is invalid for human exposure to DMA, because of the difference in the metabolic pathways of rats and humans and also because these studies are not capable of evaluating the pathway to DMA (from iAs or orgAs compounds) and the intermediary effects.106
On the basis of projected exposure level and expected metabolism, it seems unlikely that arsenic in seafood can significantly promote arsenic-associated carcinogenic effects.100 The bulk of arsenic in seafood exist as AsB, which is benign and is rapidly excreted from the body intact. Amounts of TETRA resulting from dry cooking or AsB-containing fish are unlikely to reach toxic levels. In addition, levels of iAs and methylarsenicals in seafood are relatively low to allay suspicions of their potential detrimental effects in seafood consumers.100,107
Whereas no deductions can be made regarding the effects of organoarsenicals, proof of toxicity from AsLipids and organoarsenic metabolic intermediates from in vitro assessment confirms the necessity for animal and human research to assess probable health impact of arsenic in seafood.108,109 The majority of research on natural AsLipids has focused on the polar lipids, leading to the characterization of arsenic-containing fatty acids (AsFAs) and arsenic-containing hydrocarbons (AsHCs). Profound discernment of arsenic biochemistry may perhaps be garnered from characterization of the additional lipid fractions.110
2.1. Methylated Arsenicals.
Methylarsenicals are present in marine food as MMA and DMA at low levels. Other methylated arsenicals include TMAO, AsB, AsC, and TETRA. Methylarsenicals in marine ecosystem are generated by phytoplankton, bacteria, and microbial breakdown of botanical matter from iAs and are introduced into the seafood food chain.70,111 Phytoplankton absorb iAsV in the euphotic surface waters and successively transform it to DMA and MMA.112–116 Anaerobic members of archaea and bacteria are known to biotransform iAs into both volatile (methylarsines) and nonvolatile (MMA and DMA) species.117–120
Three metabolic pathways have been proposed for arsenic biotransformation.112,121–123iAs undergoes enzymatically biotransformation into several methylated metabolites following the classical Challenger’s metabolic pathway as follows: [iAsV] → [iAsIII] → [MMAV] → [MMAIII] → [DMAV].121,122,124–126 DMAV can be reduced to DMAIII and further methylated to trimethylarsine (TMAIII) via TMAO intermediate.127 Mammalian systems do not subsequently produce arsines except under extraordinary conditions.128,129
The Challenger pathway illustrates the reduction of pentavalent iAsV and MMAV to their trivalent species, iAsIII and MMAIII, followed by an oxidative methylation phase where S-adenosylmethionine (SAM) acts as the methyl donor, producing MMAV and DMAV as major metabolites. Although the precise pathway in humans has never been entirely understood,62,130 it has for long been deemed as a detoxification process.131–133 Biotransformation on iAs results in the production of MMAIII and DMAIII,134,135 which are more toxic than iAs, and therefore, biotransformation of iAs should be generalized as a detoxification process in micro-organisms.93 The Challenger pathway is coherent with the distribution of arsenicals in urine and can be entirely verified using (CH3) 3S+ as a CH3+ donor and SO2 as the reducing agent in likeness with the Meyer reaction, which is an uncatalyzed oxidative addition reaction employed in the preparation of MMAV from iAsIII and methyl halide.91,121,126,127
Other pathways proposing glutathione- or protein-conjugated intermediaries have been advanced by Hayakawa et al.126 in 2005 and Naranmandura et al.125 in 2006, respectively, though their chemical basis is questionable because they involve accepting CH3− group.136 They, however, appear to be in agreement with the belief that trivalent arsenicals were confirmed to be readily absorbed by the organs/tissue and linked to cellular proteins, as a substitute to elimination.137 The two pathways propose that DMAV and MMAV ought to be final products (instead of intermediaries) of arsenic biotransformation,138 because trivalent arsenic, whether in glutathione or protein complex states, is subjected to reductive methylation without being oxidized.91 MMAIII that has long been considered as an transitory intermediate in the methylation pathway is rather a stable metabolite of iAs and has been detected in appreciable (e.g., effect levels) concentrations in hamster liver, rat bile, and human urine following exposure to iAs.127,139
Production of methylarsenicals is linked to the growth phase or phytoplankton nutritional state.112 Production of DMAV increases gradually, while DMAIII and MMAIII remain fairly constant during the lag phase of phytoplankton growth. Formation of DMAV is elevated when the proportion of phosphate-to-arsenate declines implying enhancement of production at phosphate-replete conditions.112,140
MMA and DMA are generally available in trace amounts or are not present in seafood.100 Measurable amounts have mainly been detected in fatty types of fish.141 Extremely low amounts (i.e., μg As/kg) of DMA have been detected in mackerel and herring and in prawns but were not measurable in cod, dab, haddock, sole, plaice, tuna, or whiting.100,141–143 DMA has also been detected in seaweed like kelp.
The International Agency for Research on Cancer (IARC) classification of the carcinogenicity of arsenic species categorizes MMA and DMA as Group 2B (possible carcinogen to humans).63 In vivo studies have demonstrated that methylated arsenic metabolites can traverse the placental barrier, although the methylation capacity is enhanced during gestation as a protective measure for the developing fetus.134
It has been suggested that MMAIII is more hazardous and toxic than iAs to the liver, skin, and lung cells.144 In addition, DMAIII is more toxic than DMAV and iAs145 because DMAIII has neutral charge and can readily permeate cells (up to 16%), but DMAV which has a negative charge can scarcely enter cells (0% to 2%).146 The methylated trivalent arsenicals, MMAIII and DMAIII, have higher cytotoxicity than AsIII and AsV, which are more cytotoxic than the methylated pentavalent arsenicals, MMAV and DMAV.91 The adverse effects of arsenic are therefore intimately connected to its metabolism and is significantly reliant on the methylation level and the valence state of the metabolites.147
2.2. Trimethylarsine Oxide (TMAO).
TMAO has been isolated in various species of aquatic organisms as a minor arsenic species, seldom identified except in miniscule amounts.51,148 Quantities are much lower in stored, frozen fish than in fresh fish, likely due to post-mortem degradation, but dietary ingestion of TMAO is most likely small.51,100,149 TMAO is fundamentally benign, with an acute oral LD50 for arsenic in mice of 5500 mg/kg.100,150
2.3. Arsenocholine (AsC).
Arsenocholine is a metabolic predecessor for AsB in aquatic animals.100,151,148 After inoculation of labeled AsC, it is swiftly taken up and converted to AsB with minimum breakdown to iAs, MMA, or DMA.100,151,152 Even though findings on AsC toxicity are scanty, it is deemed to be benign.100 The acute oral LD50 for arsenocholine in mice was 6500 mg/kg, whereas the acute intravenous LD50 was 187 mg/kg.100,152
2.4. Arsenobetaine (AsB).
Seawater contains traces of arsenic (~2 μg/L), which is bioaccumulated by aquatic organisms by up to 5 orders of magnitude.51 The bulk of arsenic in aquatic organisms exist as AsB, mainly found in fish and shrimp, and as AsSugars, mainly found in marine algae.21 AsB is an arsenic analogue of the amino acid derivative, glycine betaine, and it is extremely stable to hydrolysis or metabolism.43 The source of AsB in the food web is vague, though various speculations concerning its biosynthetic pathway have been proposed.153,154 Arsenic, in AsB, is oxidized with four stable carbon bonds which are enzymatically or thermally recalcitrant. Even though AsB can be degraded by microflora existing in human gut, their incubation time (7 days)155 is lengthier than practical gut retention, and this metabolic pathway has not been witnessed in vivo; thus, AsB is usually not converted in humans and other mammals.43,51,100
This makes AsB biochemically quasi-inert, which may explain why this species, though readily accessible, does not convert into other metabolites when consumed by humans and is swiftly eliminated from the mammalian body intact.54 The postulation that the four stable As-C bonds account for the innocuous nature of AsB earns credence from the fact that tetramethylarsonium ion (TETRA) and arsenocholine (AsC), both of which are structurally analogous to AsB, also display no indication of toxicity (Table 1, Figure 1).54,69 AsB is nonmutagenic and does not show cytotoxicity, immunotoxicity, or biotransformation in mammalian cells. The acute oral LD50 of AsB in mice is more than 10000 mg As/kg.64
Table 1.
Experimental Biological Activities of Different Arsenicals
| no. | arsenic species | LD50 (mg kg−1)a |
animal | ref |
|---|---|---|---|---|
| 1 | MMAIII | 2 | mice | Petrick et al.158 |
| 2 | MMAV | 916 | mice | Kaise et al.150 |
| 3 | DMAV | 648 | mice | Kaise et al.150 |
| 4 | AsC | 6500 | mice | Kaise et al.152 |
| 5 | AsB | > 10000 | mice | Kaise et al.64 |
| 6 | TMAO | 5500 | mice | Kaise et al.150 |
| 7 | TETRA as TMA-chloride | 890 | mice | Shiomi et al.159 |
| 8 | TETRA as TMA-hydroxide | no toxicity | mice | Sakurai et al.160 |
| 9 | DMAsIII-sugar-glycerol | 6.56 × 10−2b | human UROtsa | Andrewes et al.21 |
| 10 | DMAsV-sugar-glycerol | 1.968b | human UROtsa | Andrewes et al.21 |
| 11 | AsHC 332 | 3.25 × 10−3b | human UROtsa | Meyer et al.61 |
| 12 | AsHC 360 | 1.73 × 10−3b | human UROtsa | Meyer et al.61 |
| 13 | AsHC 444 | 2.31 × 10−3b | human UROtsa | Meyer et al.61 |
LD50: Concentration at which 50% of a population dies.
IC50: Concentration at which the cell viability is reduced by 50%.
There is adequate proof that higher aquatic animals do not produce AsB, but the complete description of its formation remains unclear.148 Experimentally, AsB has been proven to be efficiently assimilated from seawater by mussels, whereas shrimp and fish accumulate AsB efficiently only from food, which includes phytoplankton, among others.156,157 In mussels, retention of AsB is dependent on the salt content of their ambient water, which supports the notion that AsB can mimic an osmolyte.43 Likewise, the tendency to increase total arsenic with salinity was witnessed among three species of pelagic fish, where AsB is presumed to be the main arsenic species, also implying AsB absorption and retention is linked to salinity.43,157 This experiment gains credence from data that show a positive correlation between arsenic content and salinity of mussels kept in aquatic environment of changing salinities.156
2.5. Tetramethyl Arsonium Ion (TETRA).
TETRA is a minor arsenical in finfish but the main species in various mollusks.51,148,161 Amounts ranging from 0.2 to 16 μg/g were reported in different organs of a few clams.148 Concentrations of TETRA can be enriched by freezing or dry cooking (grilling, roasting, and baking) at temperatures above 160 °C, particularly in burnt meat, possibly owing to thermal decarboxylation of AsB.162 Consequently, TETRA concentrations above 1.0 μg/g dry mass have been documented in cooked fish where TETRA was not present before cooking.100 The halogenated TETRA has substantial acute toxicity; in mice, the acute oral LD50 of TETRA-chloride was 890 μg/g. Conversely, such toxicity may arise from the halogen anion and not TETRA as analyses of synthetic TETRA-hydroxide have revealed nontoxicity.160 Because of the reduced amounts of TETRA in ingested fish, acute toxicity by TETRA is improbable; the highest documented amounts in grilled or roasted fish are 0.571 μg/g wet basis and 1.79 μg/g dry basis.100,162,163
2.6. Thioarsenicals.
Thio-arsenicals and oxo-arsenicals are structurally similar with sulfur replacing oxygen and are produced when oxo-arsenicals are exposed to hydrogen sulfide (H2S).164 Knowledge of thio-organoarsenicals is fairly recent, and few studies have examined their production processes and detection techniques.165 The main focus of arsenic speciation has been on oxo-arsenicals, owing to their abundance in nature.166 Speciation analysis of thio-arsenicals is a challenging task, and there is need to exercise caution during sample storage, pretreatment, extraction, separation of arsenic species, and detection.91
Despite the latest advances in characterization and detection of thioarsenicals, there are lingering intricacies in their analysis in biological matrices, especially seafood and seaweed, owing to the complexity of arsenic metabolism, the homophyly of oxo-arsenicals and thio-arsenicals,166 and lack of standards for thio-arsenicals.91 Many thio-arsenical standards must be produced in specific laboratories, and in certain instances, the production is grueling owing to the instability of species, like DMMTAIII.91 Figure 3 enumerates the names, abbreviations, and chemical structures of the main trivalent and pentavalent arsenicals involved in arsenic metabolism.91
Figure 3.
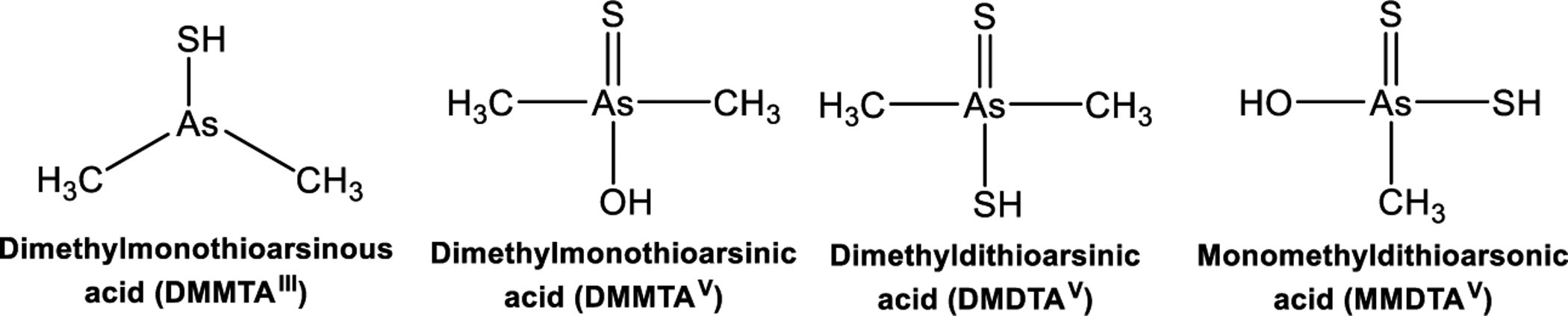
Structures, names, and abbreviations of methylated thioarsenical compounds.
Presence of dimethylmonothioarsinic acid (DMMTAV) and dimethyldithioarsinic acid (DMDTAV) in human and animal urine, which may cause interferences with metabolic processes, after ingestion of AsSugars has led to the recent upsurge in the research on thio-arsenic species.165,166 Methylated thio-arsenicals have been identified in urine samples following long-term ingestion of iAs-contaminated drinking water or intake of AsSugars.133 Following intake of AsSugars, DMMTAV appeared at trace levels in the urine of Japanese males.167,168 In sheep consuming algae with elevated AsSugars content, 2-dimethylarsinothioyl acetic acid (thio-DMAA) was identified in urine.166 Trace amounts of thio-DMAA and thio-DMAE were detected in the serum following intake of an oxo-AsSugar.169 Wang et al. reported the presence of thio-DMAA in human saliva samples following ingestion of Chinese seaweed, with the usual excretion profile observed in urine.170
The toxicological significance of thio-arsenicals to organisms is still uncertain, but there are indications that methylated thio-arsenicals have appreciable toxicity compared to their oxo-anion counterparts.171 As regards human epidermoid carcinoma, DMMTAV has higher cytotoxicity than DMAV (LD50 = 10.7 and 843 μmol/L, respectively).172 DMDTAV has injurious consequences in culture cells that result in DNA impairment.173 Thio-DMAV has been observed as a product of both iAs133 and AsSugar metabolism.168,169 Considerable toxicity from thio-DMAV has been witnessed in skin, bladder, liver, and lung cells, which is partly linked to its extreme cellular bioavailability.174–179 Thio-DMAV has been proven to generate ROS in healthy cells174–176 and to disturb cellular stress response, even at picomolar levels, in oxidatively stressed cells.101–103 Thio-DMAV exhibited no genotoxic mode of action in lung cells,177 but DNA injury and alterations in gene expression were witnessed in bladder cells exposed to such species.176 Epigenetic effects from long-term exposure to thio-DMAV have also been detected at low picomolar levels.180
2.7. Arsenosugars (AsSugars).
AsSugars is a general expression that jointly denotes ribofuranosides containing arsenic.21 There are at least 20 known AsSugars in different molecular forms, of which four (AsSugars-OH, PO4, SO3, and SO4) are the most commonly occurring in aquatic systems.21,43,54,100 Arsenosugars are the predominant arsenicals present in macroalgae159,181–193 and have also been reported in clam,194,195 gastropods,196 shrimp,197 mollusks,198 and oyster tissue.199
Most AsSugars contain a dimethylarsinoyl group, where arsenic is pentavalent and connects to two methyl moieties, at C5 of the ribose ring and to oxygen, with a variety of substituents at the C1 position of the sugar backbone shown in Figure 4. The dimethylarsinoyl moiety of the oxo-AsSugars is prone to be protonated at low pH (below 3), thus bestowing a cationic and polar nature to the molecule. However, this property is countered by the aglycone moiety if it holds an acidic moiety because the acid-base characteristics witnessed are essentially regulated by the aglycone.16 With the exception of AsSugar-glycerol, the other oxo-AsSugars with widespread occurrence possess a strongly acidic group. The acidic potency intensifies from the phosphoric acid ester, followed by the sulfuric acid ester through to the sulfonic acid ester.200,201
Figure 4.
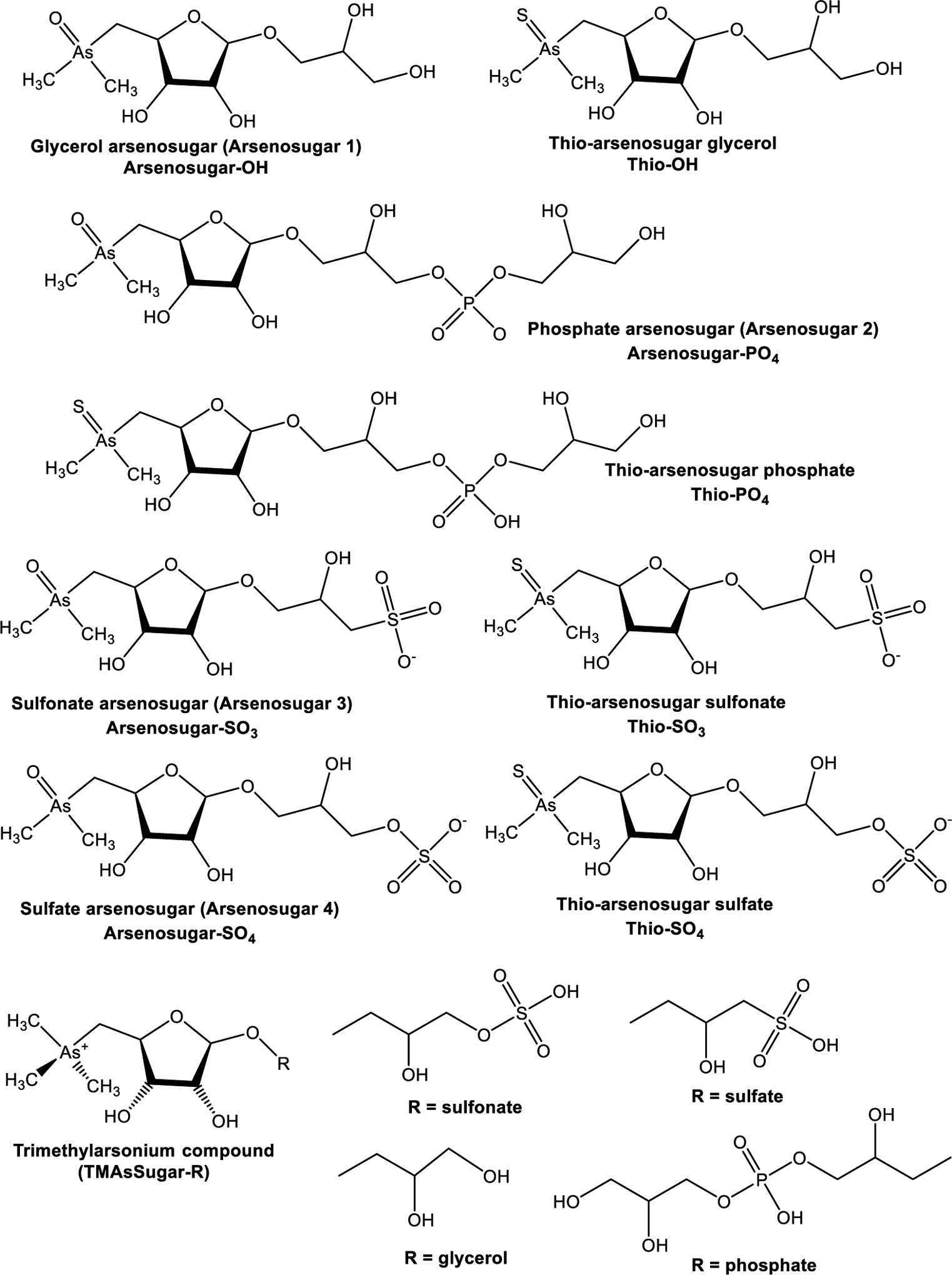
Structures, names, and abbreviations of arsenosugars.
AsSugars may occur as thio-AsSugar where the oxygen is replaced by sulfur and as trimethylarsonium compounds, as shown in Figure 4.21,54 Naturally, corresponding oxo and thio analogues are present, though the pentavalent dimethylated oxo species predominate.16 Most AsSugars are polar. There are several other lipophilic arsenic-containing oxo-ribofuranoside. The molecular structure of the first arsenosugar phospholipid (AsPL) identified by Morita et al. in 1988 is shown in Figure 5.183 Garćia-Salgado et al. identified additional AsPLs from two species of brown macroalgae in 2012.184
Figure 5.
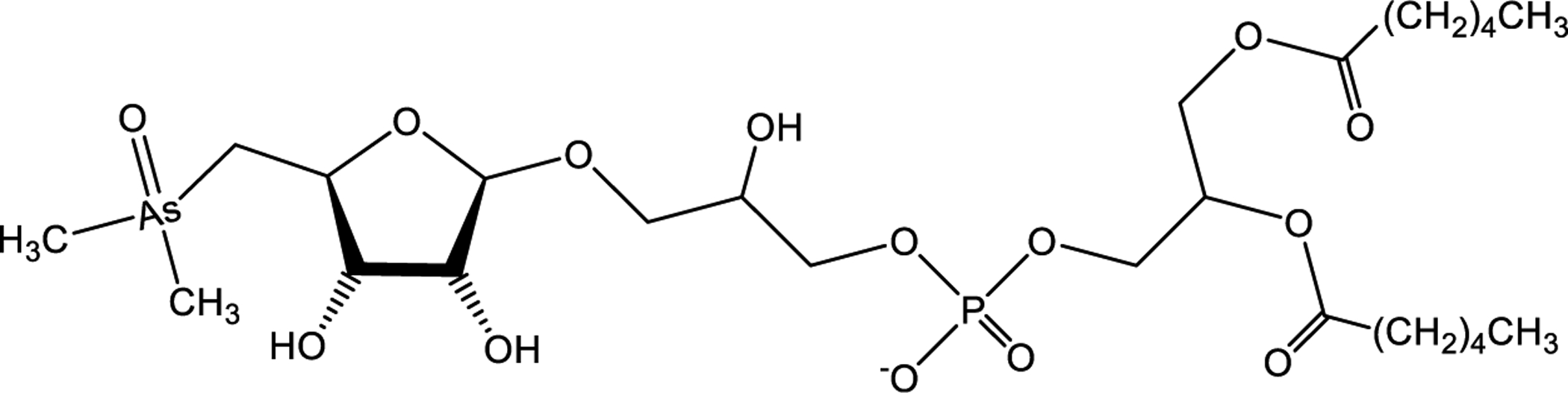
Chemical structure of lipophilic oxoribofuranoside extracted from the brown algae U. pinnatifida (Wakame).
The polarity of AsSugars, which is based on their behavior in reversed-phase (RP) chromatography, shows that oxo-AsSugars are more polar than their thio analogues, with the AsSugar-glycerol being the species with the highest polarity.16,168 The presence of diastereomeric sulfonate and carboxylate oxo-AsSugar species in natural samples was revealed following NMR studies.16,19
AsSugars are substantially more chemically labile than AsB, and biodegradation is probable when they are exposed to an acid or base hydrolysis or modeled gastric-type environment.202–204 However, degradation of AsSugars is not entirely stimulated by the chemical conditions and may be initiated by enzymatic and/or microbial activity.205 Only limited information is available with regards to temperature stability of AsSugars which are neither decomposed by cooking of seaweed nor by stomach acid digestion, which suggests that the occurrence of DMAV after AsSugars ingestion is attributable to either enzymatic or microbial activity in the human body.202,206 Oxo-AsSugars stability persisted during transitory heating to 100 °C for 10 min even though this does not demonstrate normal cooking conditions. At elevated temperatures and under acidic conditions, some AsSugars undergo acid hydrolysis to yield the disintegration product AsSugar 254 (DMAsSugarHydroxy) shown in Figure 6.205
Figure 6.
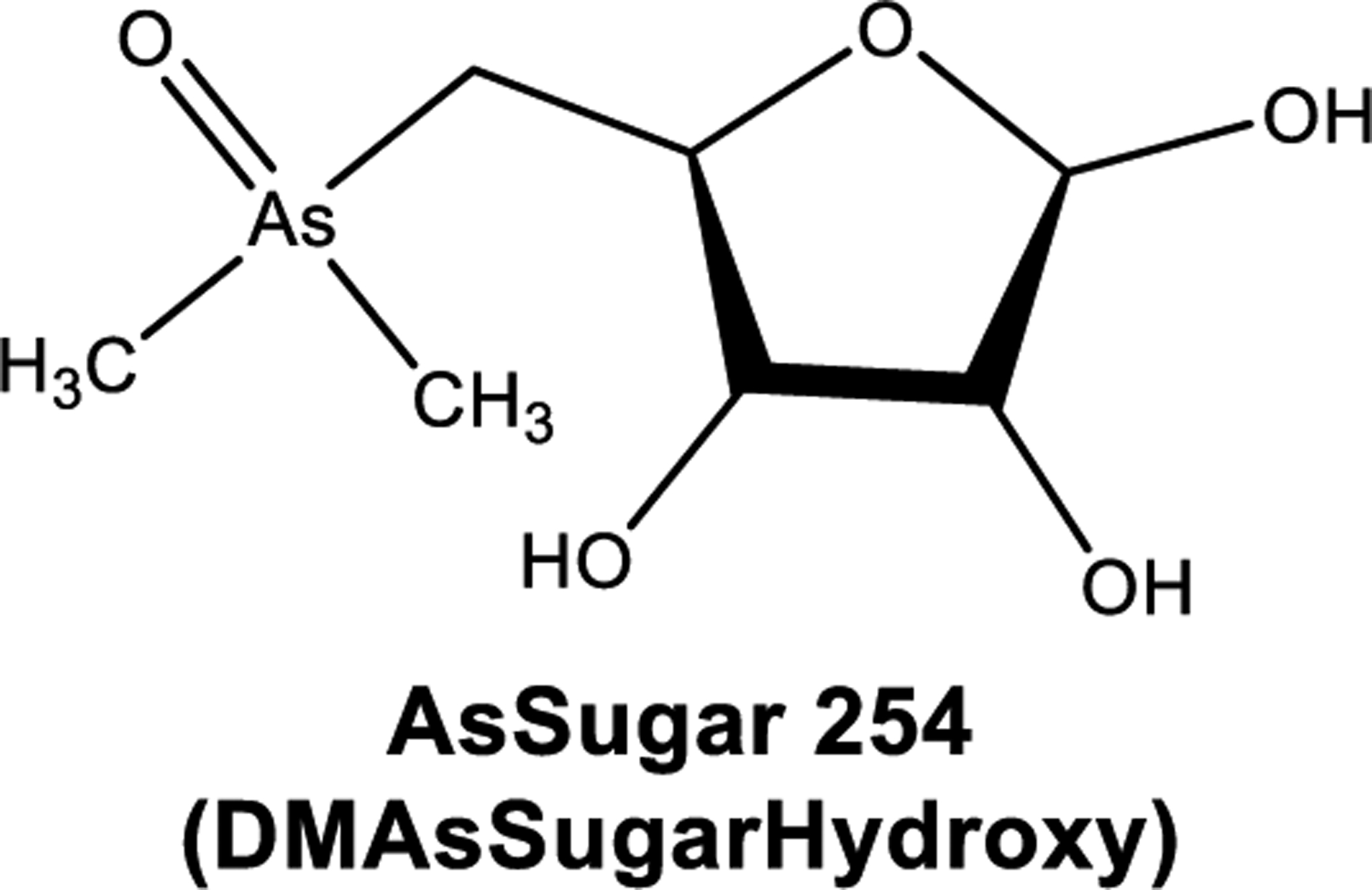
Structure of AsSugar 254, DMAsSugarHydroxy.
Not much is known concerning the source or potential function of AsSugars in biological systems, though it is assumed to be produced in organisms because of detoxification and excretion after ingestion of iAsV naturally occurring in seawater.207,208 Probable pathways for their formation and conversion have been illustrated in literature.21,207,209 AsSugars are not just the major arsenicals in seaweed but also exist in an enormous quantity in filter feeding herbivorous mollusks and gastropods from consuming algae or phytoplankton.16,43,100,210 The focus of research has been on the characterization and quantitation of AsSugars present in diverse aquatic macroalgae, regularly referred to as seaweed or kelp, and commercially accessible algae products, whose intake is promoted for their health benefits but may be a cause of exposure to arsenic that is intrinsically present in them.16,211,212 Aquatic organisms have higher levels of AsSugars compared to the freshwater and terrestrial organisms where the top-most detected amounts in sea algae and commercial kelp powders were 10–40 μg As/g (dry weight).18
In cells of mammals, generated AsSugar was not cytotoxic at micromolar levels.36 Of the four AsSugars with widespread occurrence in seaweed, only two (AsSugar-OH101,102,21,213 and AsSugar-SO3 101–103) have been assessed and demonstrated significantly reduced cytotoxicity in comparison to iAs. However, a trivalent derivative of AsSugar-OH (DMAIII-AsSugar-OH) displayed substantial toxicity to the cell, but this arsenic species has at no time been witnessed in biological systems.21 DMAV-AsSugar-OH has been assessed with cell cultures and presented no cytotoxicity at the micromolar level, indicating that the AsSugars in their native state in dietary sources have extremely decreased toxicity and are likened to AsB, though this conclusion was made upon the evaluation of a single AsSugar.54
AsSugars that were previously reported in literature as not exhibiting cytotoxic or mutagenic activities187 have demonstrated bioaccessibility following metabolism within the human body.202 The high intake of AsSugar and the resemblance they share with iAs with regards to metabolism and accumulation suggest that AsSugar may display more toxicity than earlier assumed.21 For example, trivalent AsSugar is more cytotoxic (IC50 = 200 μmol/L, 48 h exposure) than the corresponding pentavalent species (IC50 > 6000 μmol/L, 48 h exposure) in typical human epidermal keratinocytes. AsSugar metabolites may also occur in trivalent forms, which have thus far not been identified in biota, likely as a result of their reactivity, but have demonstrated elevated cytotoxicity while directly linked to plasmid DNA at the μmolar level.21 Reduction of DMAV-AsSugars is envisaged to happen promptly in vivo via reaction with thiol compounds, thus making these AsSugars hazardous to human health.21,214,215
AsSugars are metabolized and biodegraded to various minor compounds after retention in the body.202 Assimilation and elimination of AsSugars167,202,210 is much slower than AsB or AsLipids216,217 and is highly variable between individuals.202 Volunteers from single consumption studies of seaweed showed either no buildup or just a slight elevation in urinary arsenic content, while others eliminated up to 95% of the consumed arsenic.,167,169,202,203,210,218 The same consumption experiment was repeated with volunteers who showed the least (4%) and the most (95%) recovery of the consumed AsSugars, and consistent outcomes were reported.169 Difference in metabolism by gut microflora, permeation past intestinal barriers, and conversion within the liver may provide explanations for the retention variabilities between individuals.43,219
DMAV is the main metabolite for AsSugar in human urine, although the transformation sites are yet to be confirmed.21 Feldmann et al. studied sheep, which perennially fed on seaweed and therefore were chronically exposed to AsSugars, to provide further insight into the metabolism of AsSugars because of their metabolic similarity with humans.214 Elevated urinary arsenic concentration peaked about 20 h after ingestion and were reported in all 12 sheep in the study.215 However, tissue arsenic concentrations were not significantly elevated,214 and only 4–20% of consumed arsenic was detected in feces, indicating that most arsenic was eliminated via urine.215 Assuming that this interpretation is accurate, the results could not be verified due to challenges in acquiring 24 h urine samples from the sheep.215 Unfortunately, sheep that were used as proxies to study human susceptibility to cancer risks have a limited lifespan of four to six years, which is not sufficient to assess the long-term exposure effects. However, the sheep study underscores the need for thorough investigation of foodstuff that contains arsenic in a state that is metabolized, but whose toxicological latency is unknown.54
2.8. Arsenolipids (AsLipids).
Human exposure to AsLipids arises from ingestion of seafood for example fatty fish,56 algae,184,220 and crustaceans.51,221 However, there is scarce knowledge with regard to abundance, identity, and toxicity of these compounds.56,222 AsLipids are an emerging species of interest that have relatively high natural levels in marine animals and algae.223 In seafood, AsLipids comprise up to 70% of the total arsenic content,224 with amounts between 0.3–3.6 mg As/kg dry weight.225 The greatest quantities are obtained in fatty fish like herring and mackerels.56 AsLipids are believed to move upward in the food chain, starting with algae to higher organisms such as fish, with the possibility of endogenous production in the organism since the detected arsenic-containing fatty acids (AsFAs) show similarities to common fatty acids found in aquatic organisms.56,108 Arsenic-containing hydrocarbons (AsHCs) are present in various aquatic systems, such as herring,225 tuna,226 cod,227 fish oils,224,228,229 and edible brown algae.220,223,230
AsLipids occur as derivatives of fatty acids (AsFAs),37,110,220,225,228,229,231–237 hydrocarbons (AsHC),37,110,220,225,228,229,231,233–238 phosphatidylethanolamine (AsPE),239,240 phosphatidylcholine (AsPC),239–241 fatty alcohols,231 and AsSugar-PLs.184,220 The existence of arsenic in lipid extracts of fish and algae was originally documented by Lunde in 1968,242 but their structures remained unknown.234 Several molecular structures of AsLipids have been elucidated based on their exact mass and their product ion mass spectra, demonstrating the chemical complexity of these compounds in seafood.37,184,220,232,235 Subsequent biochemical studies showed that AsLipids in unicellular algae involved three main lipid types,243 and similar species were also detected in clam tissues resulting from algal-clam symbiosis.234,244
Morita and Shibata in 1988 successfully purified and determined the structure of the main AsLipids in the brown macroalga, Wakame (Undaria pinnatifida), which was found to be a phospholipid with an AsSugar headgroup (Figure 4).183 Garcia-Salgado et al.184 and Raab et al.220 were able to extract AsPLs and other arsenicals from brown macroalgae species. AsLipids were first detected and identified in fish oils in 2008, where arsenic was directly linked to either a long chain fatty acid232 or a hydrocarbon224 (Figure 7). Characterization of AsLipids is work in progress with more than 50 known AsLipids.43
Figure 7.
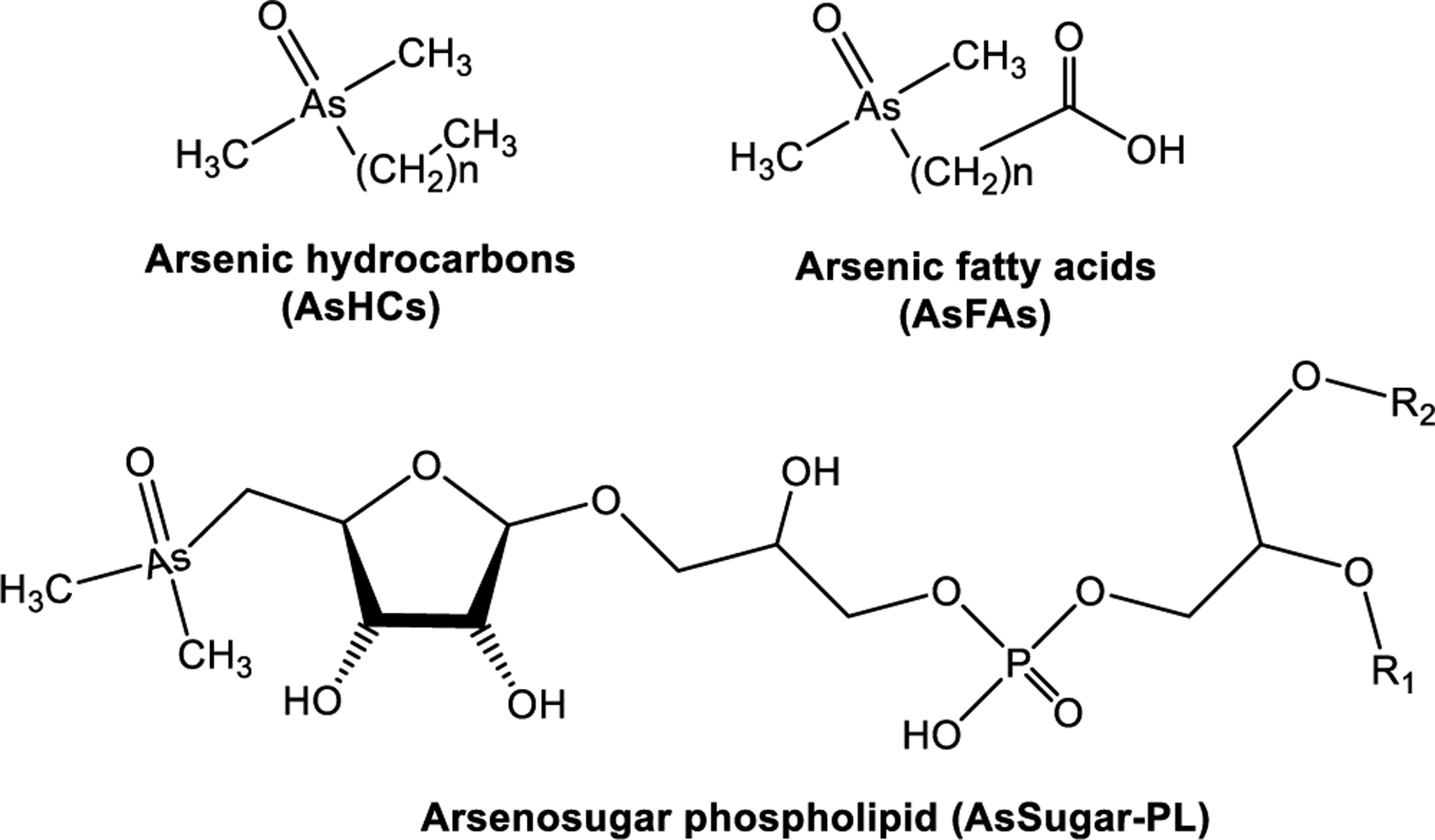
Structures, names, and abbreviations of arsenolipids.
AsLipids primarily elicited research interest due to their novel structures, their likely role in membrane biochemistry, and since they exist in ordinary seafood with potential health concerns based on arsenic toxicity.34 Advances in the biochemistry and toxicology of AsLipids has been impeded by the difficulty related to separation and analysis of AsLipids,245 trace amounts of AsLipids in marine samples compared to polar arsenicals,56 limited knowledge on the stability of the analytes in the course of ordinary sample preparation steps,246 and absence of standards and quantitative analytical methods.223
The identification and quantification of AsLipids has been made possible by concurrent analysis using LC-ICP-MS for element specific detection and ESI-MS for structural determination.233–238 Several AsHCs and AsFAs have been synthesized for confirmation of identity.234,247–249 However, there are still no standards or reference materials accessible for AsLipids and AsSugar phospholipids (AsSugar-PLs). For example, standards for unsaturated AsLipids are not available, which incidentally are difficult to synthesize and therefore must be isolated from fish muscle.225
Lack of standards is a progressive challenge because as more compounds get identified there is a higher demand for new standards. Pure compounds are necessary for accurate quantification and to aid in the investigation of their metabolic transformation and potential toxicities.46 Improved knowledge with regards to chemical structures, amounts, bioavailability, and toxicity of the specific AsLipids is necessary for a more comprehensive risk assessment of arsenicals found in food and feed.43
AsLipids are of toxicological concern because their metabolites are similar to iAsIII a well-characterized carcinogen.217,222 However, the molecular modes of action regarding their toxicity as well as their metabolism in liver remain unclear.250 AsHC 332, AsHC 360, and AsHC 444 have recently shown substantial toxicity in various in vitro and in vivo systems.61 In an in vitro blood-brain barrier model, it was shown that AsHC 360 [1-(dimethylarsinyl)heptadecane] was up to 5-fold more cytotoxic than iAsIII, followed by AsHC 332 [1-(dimethylarsinyl)pentadecane)] and AsHC 444 [1-(dimethylarsinyl)tricosane], which were 3.7-fold and 1.8-fold more cytotoxic than iAsIII, respectively.222 AsHC 332 and AsHC 360 are effective permeability enhancers that would allow other food borne toxicants easy access to the brain.222 Cytotoxic latency of AsFAs and their water-soluble metabolites were much lower in comparison to iAsIII and AsHCs. No substantial cytotoxicities were detected for AsFA 362 [15-(dimethylarsinyl)pentadecanoic acid] or AsFA 388 [17-dimethylarsinyl-9-heptadecenoic acid].222,251
Elevated cellular bioavailability in human differentiated neurons, liver, and bladder cells,61,252 together with the detected increased intestinal bioavailability in the Caco-2 intestinal barrier model,251 implies that AsHCs appear to effortlessly permeate cell membranes, along with other physiological membranes, which explains how AsHC 332 is able to transport into the brain of Drosophila melanogaster;109 this suggests that these compounds are bioaccessible to the brain.43,248,253 In the same way, iAsIII and DMAV have been demonstrated to traverse the blood-brain barrier in mice and rats.97–99
Due to their amphiphilic structure, intact AsLipids are seemingly able to substantially transfer across physiological barriers, such as intestinal barriers251 and the blood-brain barrier.253 The blood-brain barrier is a physiological barrier comprising capillary endothelial cells that separates the circulatory system from the brain parenchyma.222 Interestingly, AsHCs were neither metabolized in the in vitro blood-brain barrier model222 nor in the in vitro intestinal barrier model.251 In contrast, arsenolipids present in cod liver oil ingested by humans were biotransformed to small arsenic-containing fatty acids and DMA, which is a similar metabolite for iAsIII.217 AsHCs have been shown to disturb mitochondrial function, leading to decreased cellular ATP levels in fruit flies;61 while in humans, AsHCs not only reduced the mitochondrial membrane potential but also induced apoptosis, and such effects were not observed with iAsIII.108,254
A small number of studies have looked into bioaccessibility and metabolism of AsLipids, and the data suggest that AsLipids are swiftly taken in via the gut and, as opposed to AsB, are metabolized before excretion in urine within 6–15 h of consumption.43 Following ingestion of cod liver oil mainly consisting of AsLipids by two human subjects, more than 85% of the ingested arsenic was eliminated after 2 days. DMAV constituted up to 70% of the arsenic excreted in urine with no intact AsLipids detected.216,217 Other metabolites included short-chain AsFAs derivatives like dimethylarsenopropanoic acid (DMAPr), dimethylarsenobutanoic acid (DMAB), and their thio-analogues (thio-DMAPr and DMAB).108,216,217 DMAPr and thio-DMAPr did not trigger any adverse effects in human liver cells (HepG2), human bladder cells (UROtsa), or differentiated neurons.108,251,252 The fact that AsLipids can occur at high levels in food and have been shown to be bioavailable to humans and extensively degraded to small arsenic species justifies the great interest on the possible toxicities of these compounds.234
The latest study performed by Al Amin et al. projected the daily consumption of AsLipids in the Japanese population conducted on 17 food composites, and the results showed that the population is exposed to AsHCs, AsFAs, and AsSugar-PLs.255 AsLipids were identified mainly in algae, fish, and shellfish of the 17 dietary composites with amounts between 4.4 and 233 ng As/g fresh weight (fw).255 Of concern was that two AsLipids, AsHC332 and AsHC360, with known cytotoxic effects were identified in algae, fish, and shellfish in amounts in the range of 33–40 ng As/g fw and with an approximated mean daily consumption of about 3000–360 ng As/person/ day or 50–6.0 ng As/kg bw/day. From these findings, it is apparent that diet contributes to the daily intake of toxic AsHC; fortunately, the margin of exposure does not seem to present a health risk with respect to the IC50 values of 3.05 and 1.73 μg As/g for human liver and bladder cells exposed to AsHC 332 or AsHC 360, respectively.61,255
3. CONSIDERATIONS FOR RISK ASSESSMENT
Seafood is a highly traded and consumed product with implications on commerce and food safety. There is need for regulatory limits based on speciated data, especially for seafood, because data based on total arsenic only as an indicator for risk assessment is inadequate. Detection of arsenic species that exceed regulatory limits can become a technical barrier to trade as was witnessed in 2013 when China imposed a ban on all U.S. imports of geoduck clams (Panacea generosa) with an annual value of $80 million.
The European Food Safety Agency (EFSA) provided a risk profile for arsenicals in diet that highlighted the need to legislate in relation to toxic arsenic in food and to generate more speciated arsenic data. Currently, there is no tolerable intake level set for As, since the latest EFSA scientific opinion on As concluded that the previous provisional tolerable weekly intake (PTWI) of 15 μg kg−1 bw was no longer appropriate. The EFSA review was an important instrument that stimulated appropriate allocation of resources to more detailed scientific assessments that led to the generation of relevant information. However, this information needs to be systematically collated and evaluated with the objective of establishing regulatory limits, especially for organoarsenicals in seafood, which do not currently have set limits.
The review article by Feldmann et al. highlighted lack of structural and toxicity data on organic arsenic species and suggested the categorization of food samples into three fractions based on the International Agency for Research on Cancer (IARC) classification that classifies all organoarsenicals as potentially toxic. New information has emerged since then with the identification and characterization of some novel organoarsenicals having toxicities like iAsIII, a known carcinogen. The molecular structures of more than 50 lipophilic organoarsenicals have been assigned, and new analytical methods have been developed. This information has changed the landscape of risk assessment with respect to organoarsenicals in food, especially seafood.
Risk assessment is a scientifically based process consisting of four main stages, namely, hazard identification, hazard characterization, exposure assessment, and risk characterization. Hazard identification entails the screening process with the purpose of ascertaining the presence of a hazard. For the purpose of this review, a hazard will be defined as a biological or chemical agent capable of causing an adverse health effect and that may be present in a food or group of foods. In the case of arsenic, iAsIII is a well-characterized carcinogen. Other toxic arsenicals include arsenic-containing hydrocarbons like AsHC 332, AsHC 360, and AsHC 444, arsenic-containing fatty acids like AsFA 362 and AsFA 388, and methylated trivalent arsenicals like MMAIII and DMAIII. DMAV is also a known tumor promotor in rat liver.
There is need for more toxicity studies on the new organoarsenicals to identify all the potential sources of hazards. Toxicity studies aim at establishing the severity and frequency of the associated adverse health effect (response). Toxicity studies should not only be limited to the organoarsenicals but also should be extended to their metabolites, because it has been established that most arsenicals are not acutely toxic but that toxicity usually emanates from metabolic transformations. For example, AsSugars have metabolites similar to iAsIII, which is a known carcinogen. There are still many organoarsenicals that have not been tested for toxicity and are assumed to be nontoxic because of the benign nature of some of the known organoarsenicals such as AsB. However, these compounds are not known to be nontoxic. It is therefore imperative that toxicity studies are performed for these recently identified organoarsenicals.
Most arsenic toxicity information was garnered from studies using laboratory animals. This information is important but not necessarily useful in its current form for establishment and/or accurate simulations of regulatory limits. It is therefore imperative that the LD50 values obtained from laboratory animals are converted to enforceable limits for human subjects. This call for additional data and information that has been systematically obtained to enable the setting of exposure metrics that can practically be implemented in regulatory practices.
In order to appreciate the exposure level to organoarsenicals, there is need to conduct exposure assessment studies, which entail dietary studies considering the potential sources of exposure with regards to seafood. Exposure assessment requires data from the number of servings of potentially dangerous food ingested (provided from dietary studies) and the level of contamination (provided by information from arsenic speciation analysis), which determines the magnitude of exposure (dose). A few dietary studies have been performed targeting certain foods in different countries. Dose-response assessment links the amount of the hazard ingested (dose) with the chance of developing adverse health effects and the severity of the same. These studies enable the establishment of exposure metrics like PTWI and allowable dietary intake (ADI) which are important in establishing regulatory limits.
The current identification of organoarsenicals have been based on expensive instrumental setups that are beyond the reach of many laboratories. It would be beneficial to have access to an instrument that can concurrently identify and quantify novel organoarsenicals without the need for standards. Even with standards available there is still the challenge of coelutions and isobaric interferences that significantly affect quantification by LC-ICP-MS. There is therefore great need for an element-sensitive detector with a high resolving power and mass accuracy. This will hopefully enable the concurrent identification and detection of organoarsenicals. Such an instrument should be affordable, robust, and with high sensitivity.
The identification of arsenic-containing hydrocarbons (AsHCs), arsenic-containing fatty acids (AsFAs), arsenic-containing fatty alcohols (AsFOHs), and arsenosugar phospholipids (AsPLs) has been aided by high-resolution mass spectrometers with high resolving power and mass accuracy. Mass spectrometers such as the quadrupole ion trap (QITMS), quadrupole time-of-flight (Q-ToFMS), Orbi-trapMS, and Fourier transform ion cyclotron mass spectrometers (FT-ICT-MS) have been used. These instruments are not affordable and require a high level of expertise for operation, which makes them beyond the reach of many laboratories. In addition, the structural information for the identified organoarsenicals need to be confirmed, which requires the use of techniques like nuclear magnetic resonance (NMR) spectroscopy. Additional information can be provided by X-ray crystallography, X-ray absorption near-edge spectroscopy (XANES) or infrared spectroscopy, and mass spectrometry. Unfortunately, these techniques require analytes at high concentrations, yet the amounts of AsLipids present in the seafood samples are usually very low. Therefore, the arsenicals must be synthesized and characterized in the laboratory for synthetic protocols and characterization of the synthetic products.
It is almost impossible to synthesize standards for all known arsenicals. A pragmatic approach would be to synthesize the standards for arsenicals with known toxicities and to also synthesize their labeled analogues. Due to the monoisotopic nature of arsenic, it is impossible to find labeled As standards. However, for organoarsenicals, the heteroatoms of the synthetic standards can be labeled with 13C or 2H, thus enabling their use as internal standards for identification and quantification of analytes of interest. Concurrent use of these synthetic standards can also be useful in overcoming coelutions and isobaric and polyatomic interferences associated with quantification of organoarsenicals using ICPMS.
There is currently no agreement on a method that is internationally acceptable for arsenic speciation analysis. There is an urgent need for higher-order analytical protocols for the detection and structural assignment, especially for the novel organoarsenicals. There are currently no certified reference materials (CRMs) for the new organoarsenicals. Access to CRMs can be helpful in the validation of analytical methods. Interlaboratory comparisons like proficiency testing (PT) schemes can also provide an additional level of confidence in the measurement results as a tool for assessing the robustness of the validated analytical protocols and for evaluating the equivalence of measurement results.
The concurrent use of the synthetic standards and labeled synthetic standards as internal standard in combination with reliable and robust analytical method for exact quantification of selected arsenicals will play an important role in the establishment of regulatory limits for the toxic organoarsenicals. With reliable analytical methods and availability of standards, development of matrix-matched CRMs will then become a reality.
As an initial step, further effort should be expended in the characterization of lipophilic organoarsenicals in order to obtain a more exhaustive list that can then be tested for toxicity. There is still a lot more to be learnt from the hexane extracts of fatty and oily fish that is mostly discarded because of the high matrix effect. Improvement in the sample cleanup techniques may also allow access to more information from the nonpolar portions of the samples. Another area that might require attention is analysis of samples with high organic content because analysis of intact AsLipids requires them to be dissolved in organic solvents that are not amenable with ICP-MS. The importance of arsenic speciation data cannot be overemphasized. The current approaches for simultaneous identification and quantification will play a critical role in arsenic speciation. Analytical instruments have enabled species identification and structural elucidation; however, there is still need for standards and certified reference materials. These materials will be used for the identification and quantification of arsenolipids in food samples.
Once the hazard identification, hazard characterization, and exposure assessment have been concluded, then the information thereof is used as an input for risk characterization, which is simply the estimation of risk that informs the setting of regulatory limits. Some of the implementation consid-erations include availability of standards, certified reference materials, validated analytical methods, and established regulatory limits.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Caleb Luvonga, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland, and University of Maryland, College Park, Maryland.
Catherine A. Rimmer, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland
Lee L. Yu, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland
Sang B. Lee, University of Maryland, College Park, Maryland
REFERENCES
- (1).Nybakken JW Marine Biology: An Ecological Approach, 4th ed.; Addison-Wessley Publishing: Boston, MA, 2001. [Google Scholar]
- (2).Larsen R; Eilertsen KE; Elvevoll EO Health Benefits of Marine Foods and Ingredients. Biotechnol. Adv 2011, 29, 508–518. [DOI] [PubMed] [Google Scholar]
- (3).Larsson SC; Orsini N Fish Consumption and the Risk of Stroke a Dose-Response Meta-Analysis. Stroke 2011, 42, 3621–3623. [DOI] [PubMed] [Google Scholar]
- (4).Krauss RM; Eckel RH; Howard B; Appel LJ; Daniels SR; Deckelbaum RJ; Erdman JW; Kris-Etherton P; Goldberg IJ; Kotchen TA; Lichtenstein AH; Mitch WE; Mullis R; Robinson K; Wylie-Rosett J; St. Jeor S; Suttie J; Tribble DL; Bazzarre TL; et al. AHA Dietary Guidelines. Circulation 2000, 2284–2299. [DOI] [PubMed] [Google Scholar]
- (5).Uauy R; Calderon F; Mena P Essential Fatty Acids in Somatic Growth and Brain Development. World Rev. Nutr. Diet 2001, 89, 134–160. [DOI] [PubMed] [Google Scholar]
- (6).Christenson JK; O’Kane GM; Farmery AK; McManus A The Barriers and Drivers of Seafood Consumption in Australia: A Narrative Literature Review. Int. J. Consum. Stud 2017, 41, 299–311. [Google Scholar]
- (7).Hu FB; Manson JAE; Willett WC Types of Dietary Fat and Risk of Coronary Heart Disease: A Critical Review. J. Am. Coll. Nutr 2001, 20, 5–19. [DOI] [PubMed] [Google Scholar]
- (8).Agostoni C; Trojan S; Bellù R; Riva E; Giovannini M Neurodevelopmental Quotient of Healthy Term Infants at 4 Months and Feeding Practice: The Role of Long-Chain Polyunsaturated Fatty Acids. Pediatr. Res 1995, 38, 262–266. [DOI] [PubMed] [Google Scholar]
- (9).Makrides M; Neumann M; Simmer K; Gibson R; Pater J Are Long-Chain Polyunsaturated Fatty Acids Essential Nutrients in Infancy? Lancet 1995, 345, 1463–1468. [DOI] [PubMed] [Google Scholar]
- (10).O’Connor DL; Hall R; Adamkin D; Auestad N; Castillo M; Connor WE; Connor SL; Fitzgerald K; Groh-Wargo S; Hartmann EE; et al. Growth and Development in Preterm Infants Fed Long-Chain Polyunsaturated Fatty Acids: A Prospective, Randomized Controlled Trial. Pediatrics 2001, 108, 359–371. [DOI] [PubMed] [Google Scholar]
- (11).Leaf A; Gosbell A; McKenzie L; Sinclair A; Favilla I Visual Evoked Potentials and Dietary Long Chain Polyunsaturated Fatty Acids in Preterm Infants. Early Hum. Dev 1996, 45, 35–53. [DOI] [PubMed] [Google Scholar]
- (12).Cimatti AG; Martini S; Munarini A; Zioutas M; Vitali F; Aceti A; Mantovani V; Faldella G; Corvaglia L Maternal Supplementation with Krill Oil during Breastfeeding and Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) Composition of Human Milk: A Feasibility Study. Front. Pediatr 2018, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Venugopal V; Gopakumar K Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf 2017, 16, 1219–1242. [DOI] [PubMed] [Google Scholar]
- (14).Alves RN; Maulvault AL; Barbosa VL; Fernandez-Tejedor M; Tediosi A; Kotterman M; van den Heuvel FHM; Robbens J; Fernandes JO; Romme Rasmussen R; et al. Oral Bioaccessibility of Toxic and Essential Elements in Raw and Cooked Commercial Seafood Species Available in European Markets. Food Chem 2018, 267, 15–27. [DOI] [PubMed] [Google Scholar]
- (15).Tou JC; Jaczynski J; Chen Y-C Krill for Human Consumption. Nutr. Rev 2007, 65, 63–77. [DOI] [PubMed] [Google Scholar]
- (16).Niegel C; Matysik FM Analytical Methods for the Determination of Arsenosugars-A Review of Recent Trends and Developments. Anal. Chim. Acta 2010, 657, 83–99. [DOI] [PubMed] [Google Scholar]
- (17).Yu LL; Wei C; Zeisler R; Tong J; Oflaz R; Bao H; Wang J An Approach for Identification and Determination of Arsenic Species in the Extract of Kelp. Anal. Bioanal. Chem 2015, 407, 3517–3524. [DOI] [PubMed] [Google Scholar]
- (18).Pergantis SA; Francesconi KA; Goessler W; Thomas-Oates JE Characterization of Arsenosugars of Biological Origin Using Fast Atom Bombardment Tandem Mass Spectrometry. Anal. Chem 1997, 69, 4931–4937. [DOI] [PubMed] [Google Scholar]
- (19).Morita M; Shibata Y Chemical Forms of Arsenic in Marine Macroalgae. Appl. Organomet. Chem 1990, 4, 181–190. [Google Scholar]
- (20).Van Hulle M; Zhang C; Zhang X; Cornelis R Arsenic Speciation in Chinese Seaweeds Using HPLC-ICP-MS and HPLC-ES-MS. Analyst 2002, 127, 634–640. [DOI] [PubMed] [Google Scholar]
- (21).Andrewes P; Demarini DM; Funasaka K; Wallace K; Lai VWM; Sun H; Cullen WR; Kitchin KT Do Arsenosugars Pose a Risk to Human Health? The Comparative Toxicities of a Trivalent and Pentavalent Arsenosugar. Environ. Sci. Technol 2004, 38, 4140–4148. [DOI] [PubMed] [Google Scholar]
- (22).Wells ML; Potin P; Craigie JS; Raven JA; Merchant SS; Helliwell KE; Smith AG; Camire ME; Brawley SH Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol 2017, 29, 949–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).FAO. The State of World Fisheries and Aquaculture: Contributing to Food Security and Nutrition for All; Food and Agriculture Oraganization of the United Nations: Rome, 2016; pp 50. [Google Scholar]
- (24).FAO. The State of the World Fisheries and Aquaculture: Meeting the Sustainable Development Goals; Food and Agriculture Oraganization of the United Nations: 2018; pp 35. [Google Scholar]
- (25).FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Oraganization of the United Nations: Rome, 2014; pp 35. [Google Scholar]
- (26).FAO. FAO Global Aquaculture Production Statistics Database Updated to 2013: Summary Information; Food and Agriculture Oraganization of the United Nations: 2015. [Google Scholar]
- (27).Parisenti J; Beirao LH; Tramonte VLCG; Ourique F; da Silveira Brito CC; Moreira CC Preference Ranking of Colour in Raw and Cooked Shrimps. Int. J. Food Sci. Technol 2011, 46, 2558–2561. [Google Scholar]
- (28).Reed BK; Royales S Shrimp Disease in Asia Resulting in High U.S. Import Prices. Beyond the Numbers 2014, 3 (14), 1–8. [Google Scholar]
- (29).Washington Sea Grant. Final Report: Geoduck Aquaculture Research Program Report to the Washington State Legislature; Washington Sea Grant: 2013. [Google Scholar]
- (30).Jennings S; Stentiford GD; Leocadio AM; Jeffery KR; Metcalfe JD; Katsiadaki I; Auchterlonie NA; Mangi SC; Pinnegar JK; Ellis T; et al. Aquatic Food Security: Insights into Challenges and Solutions from an Analysis of Interactions between Fisheries, Aquaculture, Food Safety, Human Health, Fish and Human Welfare, Economy and Environment. Fish Fish 2016, 17, 893–938. [Google Scholar]
- (31).Maulvault AL; Anacleto P; Barbosa V; Sloth JJ; Rasmussen RR; Tediosi A; Fernandez-Tejedor M; van den Heuvel FHM; Kotterman M; Marques A Toxic Elements and Speciation in Seafood Samples from Different Contaminated Sites in Europe. Environ. Res 2015, 143, 72–81. [DOI] [PubMed] [Google Scholar]
- (32).Kalantzi I; Mylona K; Sofoulaki K; Tsapakis M; Pergantis SA Arsenic Speciation in Fish from Greek Coastal Areas. J. Environ. Sci. (Beijing, China) 2017, 56, 300–312. [DOI] [PubMed] [Google Scholar]
- (33).Moreda-Piñeiro J; Alonso-Rodríguez E; Romarís-Hortas V; Moreda-Piñeiro A; Lopez-Mahía P; Muniategui-Lorenzo S; Prada-Rodríguez D; Bermejo-Barrera P Assessment of the Bioavailability of Toxic and Non-Toxic Arsenic Species in Seafood Samples. Food Chem 2012, 130, 552–560. [Google Scholar]
- (34).Francesconi KA Arsenic Species in Seafood: Origin and Human Health Implications. Pure Appl. Chem 2010, 82, 373–381. [Google Scholar]
- (35).Schoof RA; Yost LJ; Eickhoff J; Crecelius EA; Cragin DW; Meacher DM; Menzel DB A Market Basket Survey of Inorganic Arsenic in Food. Food Chem. Toxicol 1999, 37, 839–846. [DOI] [PubMed] [Google Scholar]
- (36).Baeyens W; Gao Y; de Galan S; Bilau M; van Larebeke N; Leermakers M Dietary Exposure to Total and Toxic Arsenic in Belgium: Importance of Arsenic Speciation in North Sea Fish. Mol. Nutr. Food Res 2009, 53, 558–565. [DOI] [PubMed] [Google Scholar]
- (37).Sele V; Sloth JJ; Holmelid B; Valdersnes S; Skov K; Amlund H Arsenic-Containing Fatty Acids and Hydrocarbons in Marine Oils - Determination Using Reversed-Phase HPLC-ICP-MS and HPLC-QTOF-MS. Talanta 2014, 121, 89–96. [DOI] [PubMed] [Google Scholar]
- (38).Molin M; Ulven SM; Meltzer HM; Alexander J Arsenic in the Human Food Chain, Biotransformation and Toxicology - Review Focusing on Seafood Arsenic. J. Trace Elem. Med. Biol 2015, 31, 249–259. [DOI] [PubMed] [Google Scholar]
- (39).Thomas DJ; Bradham K Role of Complex Organic Arsenicals in Food in Aggregate Exposure to Arsenic. J. Environ. Sci. (Beijing, China) 2016, 49, 86–96. [DOI] [PubMed] [Google Scholar]
- (40).Hanaoka K; Yosida K; Tamano M; Kuroiwa T; Kaise T; Maeda S Arsenic in the Prepared Edible Brown Alga Hijiki, Hizikia Fusiforme. Appl. Organomet. Chem 2001, 15, 561–565. [Google Scholar]
- (41).Jankong P; Chalhoub C; Kienzl N; Goessler W; Francesconi KA; Visoottiviseth P Arsenic Accumulation and Speciation in Freshwater Fish Living in Arsenic-Contaminated Waters. Environ. Chem 2007, 4, 11–17. [Google Scholar]
- (42).Sloth JJ; Julshamn K Survey of Total and Inorganic Arsenic Content in Blue Mussels (Mytilus Edulis L.) from Norwegian Fiords: Revelation of Unusual High Levels of Inorganic Arsenic. J. Agric. Food Chem 2008, 56, 1269–1273. [DOI] [PubMed] [Google Scholar]
- (43).Taylor V; Goodale B; Raab A; Schwerdtle T; Reimer K; Conklin S; Karagas MR; Francesconi KA Human Exposure to Organic Arsenic Species from Seafood. Sci. Total Environ 2017, 580, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Conklin SD; Creed PA; Creed JT Detection and Quantification of a Thio-Arsenosugar in Marine Molluscs by IC-ICP-MS with an Emphasis on the Interaction of Arsenosugars with Sulfide as a Function of PH. J. Anal. At. Spectrom 2006, 21, 869–875. [Google Scholar]
- (45).Wang YY; Wang S; Xu P; Liu C; Liu M; Wang YY; Wang C; Zhang C; Ge Y Review of Arsenic Speciation, Toxicity and Metabolism in Microalgae. Rev. Environ. Sci. Bio/Technol 2015, 14, 427–451. [Google Scholar]
- (46).Maher WA; Ellwood MJ; Krikowa F; Raber G; Foster S Measurement of arsenic species in environmental, biological fluids and food samples by HPLC-ICPMS and HPLC-HG-AFS. J. Anal. At. Spectrom 2015, 30, 2129–2183. [Google Scholar]
- (47).Smith E; Naidu R; Alston AM Arsenic in the Soil Environment - A Review. Adv. Agron 1998, 64, 149–195. [Google Scholar]
- (48).Sharma VK; Sohn M Aquatic Arsenic: Toxicity, Speciation, Transformations, and Remediation. Environ. Int 2009, 35, 743–759. [DOI] [PubMed] [Google Scholar]
- (49).Tamaki S; Frankenberger WT Environmental Biochemistry of Arsenic. Reviews of Environmental Contamination and Toxicology 1992, 79–110. [DOI] [PubMed] [Google Scholar]
- (50).Mandal BK; Suzuki KT Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [PubMed] [Google Scholar]
- (51).Cullen WR; Reimer KJ Arsenic Speciation in the Environment and Humans. Chem. Rev 1989, 89, 713–764. [Google Scholar]
- (52).Ng JC Environmental Contamination of Arsenic and Its Toxicological Impact on Humans. Environ. Chem 2005, 2, 146–160. [Google Scholar]
- (53).Amlund H; Ingebrigtsen K; Hylland K; Ruus A; Eriksen DØ; Berntssen MHG Disposition of Arsenobetaine in Two Marine Fish Species Following Administration of a Single Oral Dose of [14C]Arsenobetaine. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol 2006, 143, 171–178. [DOI] [PubMed] [Google Scholar]
- (54).Feldmann J; Krupp EM Critical Review or Scientific Opinion Paper: Arsenosugars-a Class of Benign Arsenic Species or Justification for Developing Partly Speciated Arsenic Fractionation in Foodstuffs? Anal. Bioanal. Chem 2011, 399, 1735–1741. [DOI] [PubMed] [Google Scholar]
- (55).Wolle MM; Conklin SD Speciation Analysis of Arsenic in Seafood and Seaweed: Part I — Evaluation and Optimization of Methods. Anal. Bioanal. Chem 2018, 410, 5675–5687. [DOI] [PubMed] [Google Scholar]
- (56).Sele V; Sloth JJ; Lundebye AK; Larsen EH; Berntssen MHG; Amlund H Arsenolipids in Marine Oils and Fats: A Review of Occurrence, Chemistry and Future Research Needs. Food Chem 2012, 133, 618–630. [Google Scholar]
- (57).Sirot V; Guérin T; Volatier JL; Leblanc JC Dietary Exposure and Biomarkers of Arsenic in Consumers of Fish and Shellfish from France. Sci. Total Environ 2009, 407, 1875–1885. [DOI] [PubMed] [Google Scholar]
- (58).Alexander J; Benford DJ; Boobis A; Ceccatelli S; Cravedi J-P; Di Domenico A; Doerge D; Dogliotti E; Edler LFM Scientific Opinion on Arsenic in Food: EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 2009, 7, 1351. [Google Scholar]
- (59).Navas-Acien A; Francesconi KA; Silbergeld EK; Guallar E Seafood Intake and Urine Concentrations of Total Arsenic, Dimethylarsinate and Arsenobetaine in the US Population. Environ. Res 2011, 111, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Petursdottir AH; Sloth JJ; Feldmann J Introduction of Regulations for Arsenic in Feed and Food with Emphasis on Inorganic Arsenic, and Implications for Analytical Chemistry. Anal. Bioanal. Chem 2015, 407, 8385–8396. [DOI] [PubMed] [Google Scholar]
- (61).Meyer S; Matissek M; Muller SM; Taleshi MS; Ebert F; Francesconi KA; Schwerdtle T In Vitro Toxicological Characterisation of Three Arsenic-Containing Hydrocarbons. Metallomics 2014, 6, 1023–1033. [DOI] [PubMed] [Google Scholar]
- (62).Sattar A; Xie S; Hafeez MA; Wang X; Hussain HI; Iqbal Z; Pan Y; Iqbal M; Shabbir MA; Yuan Z Metabolism and Toxicity of Arsenicals in Mammals. Environ. Toxicol. Pharmacol 2016, 48, 214–224. [DOI] [PubMed] [Google Scholar]
- (63).International Agency for Research on Cancer (IARC). A Review of Human Carcinogens: Arsenic, Metals, Fibres and Dusts IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C; International Agency for Research on Cancer (IARC): Lyon, France, 2012; Vol. 100. [PMC free article] [PubMed] [Google Scholar]
- (64).Kaise T; Watanabe S; Itoh K The Acute Toxicity of Arsenobetaine. Chemosphere 1985, 14, 1327–1332. [Google Scholar]
- (65).Styblo; Del Razo ML; Vega L; Germolec DR; Lecluyse EL; Hamilton GA; Reed W; Wang C; Cullen WR; Thomas DJ Comparative Toxicity of Trivalent and Pentavalent Inorganic and Methylated Arsenicals in Rat and Human Cells. Arch. Toxicol 2000, 74, 289–299. [DOI] [PubMed] [Google Scholar]
- (66).Szramek K; Walter LM; Mccall P Arsenic Mobility in Groundwater/Surface Water Systems in Carbonate-Rich Pleistocene Glacial Drift Aquifers (Michigan). Appl. Geochem 2004, 19, 1137–1155. [Google Scholar]
- (67).Shraim A; Sekaran NC; Anuradha CD; Hirano S Speciation of Arsenic in Tube-Well Water Samples Collected from West Bengal, India, by High-Performance Liquid Chromatography - Inductively Coupled Plasma Mass. Appl. Organomet. Chem 2002, 16, 202–209. [Google Scholar]
- (68).Lin T-H; Huang Y-L; Wang M-Y Arsenic Species in Drinking Water, Hair, Fingernails, and Urine of Patients with Blackfoot Disease. J. Toxicol. Environ. Health, Part A 1998, 53, 85–93. [DOI] [PubMed] [Google Scholar]
- (69).Vega L; Styblo M; Patterson R; Cullen W; Wang C; Germolec D Differential Effects of Trivalent and Pentavalent Arsenicals on Cell Proliferation and Cytokine Secretion in Normal Human Epidermal Keratinocytes. Toxicol. Appl. Pharmacol 2001, 172, 225–232. [DOI] [PubMed] [Google Scholar]
- (70).Oremland RS; Stoltz JF The Ecology of Arsenic. Science (Washington, DC, U. S.) 2003, 300, 939–945. [DOI] [PubMed] [Google Scholar]
- (71).Rahman MAM; Hogan B; Duncan E; Doyle C; Krassoi R; Rahman MAM; Naidu R; Lim RP; Maher W; Hassler C Toxicity of Arsenic Species to Three Freshwater Organisms and Biotransformation of Inorganic Arsenic by Freshwater Phytoplankton (Chlorella Sp. CE-35). Ecotoxicol. Environ. Saf 2014, 106, 126–135. [DOI] [PubMed] [Google Scholar]
- (72).Stolz JF; Basu P; Santini JM; Oremland RS Arsenic and Selenium in Microbial Metabolism. Annu. Rev. Microbiol 2006, 60, 107–130. [DOI] [PubMed] [Google Scholar]
- (73).Murray LA; Raab A; Marr IL; Feldmann J Biotransformation of Arsenate to Arsenosugars by Chlorella Vulgaris. Appl. Organomet. Chem 2003, 17, 669–674. [Google Scholar]
- (74).Jiang Y; Purchase D; Jones H; Garelick H Technical Note: Effects of Arsenate (As5+) on Growth and Production of Glutathione (GSH) and Phytochelatins (PCS) in Chlorella Vulgaris. Int. J. Phytorem 2011, 13, 834–844. [DOI] [PubMed] [Google Scholar]
- (75).Levy JL; Stauber JL; Adams MS; Maher WA; Kirby JK; Jolley DF Toxicity, Biotransformation, and Mode of Action of Arsenic in Two Freshwater Microalgae (Chlorella Sp. and Monoraphidium Arcuatum). Environ. Toxicol. Chem 2005, 24, 2630–2639. [DOI] [PubMed] [Google Scholar]
- (76).Button M; Moriarty MM; Watts MJ; Zhang J; Koch I; Reimer KJ Arsenic Speciation in Field-Collected and Laboratory-Exposed Earthworms Lumbricus Terrestris. Chemosphere 2011, 85, 1277–1283. [DOI] [PubMed] [Google Scholar]
- (77).Vahter M Species Differences in the Metabolism of Arsenic Compounds. Appl. Organomet. Chem 1994, 8, 175–182. [Google Scholar]
- (78).Concha G; Vogler G; Nermell B; Vahter M Intra-Individual Variation in the Metabolism of Inorganic Arsenic. Int. Arch. Occup. Environ. Health 2002, 75, 576–580. [DOI] [PubMed] [Google Scholar]
- (79).Sakurai T Biomethylation of Arsenic Is Essentially Detoxicating Event. J. Health Sci 2003, 49, 171–178. [Google Scholar]
- (80).Kenyon EM; Hughes MF A Concise Review of the Toxicity and Carcinogenicity of Dimethylarsinic Acid. Toxicology 2001, 160, 227–236. [DOI] [PubMed] [Google Scholar]
- (81).Wei M; Wanibuchi H; Morimura K; Iwai S; Yoshida K; Endo G; Nakae D; Fukushima S Carcinogenesis 2002, 23, 1387–1397. [DOI] [PubMed] [Google Scholar]
- (82).Hunt KM; Srivastava RK; Elmets CA; Athar M The Mechanistic Basis of Arsenicosis: Pathogenesis of Skin Cancer. Cancer Lett 2014, 354, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Jomova K; Jenisova Z; Feszterova M; Baros S; Liska J; Hudecova D; Rhodes CJ; Valko M Arsenic: Toxicity, Oxidative Stress and Human Disease. J. Appl. Toxicol 2011, 31, 95–107. [DOI] [PubMed] [Google Scholar]
- (84).Rosen BP; Ajees AA; Mcdermott TR Life and Death with Arsenic. BioEssays 2011, 33, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Harkins PC; Petersson G; Haake P Distortion of O - P - O Bond Angles in Phosphorus Monoanions: Ab Initio Studies. J. Inorg. Biochem 1996, 61, 25–41. [Google Scholar]
- (86).Long JW; Ray WJ Jr. Kinetics and Thermodynamics of the Formation of Glucose Arsenate. Reaction of Glucose Arsenate with Phosphoglucomutaset. Biochemistry 1973, 12, 3932–3937. [DOI] [PubMed] [Google Scholar]
- (87).Moore SA; Moennich DM; Gresser MJ Synthesis and Hydrolysis of ADP-Arsenate by Beef Heart Submitochondrial Particles. J. Biol. Chem 1983, 258 (10), 6266–6271. [PubMed] [Google Scholar]
- (88).Gresser MJ ADP-Arsenate: Formation by Submitochondrial Particles under Phosphorylating Conditions. J. Biol. Chem 1981, 256 (12), 5981–5983. [PubMed] [Google Scholar]
- (89).Chan TL; Thomas BR; Wadkins CL The Formation and Isolation of an Arsenylated Component of Rat Liver Mitochondria. J. J. Biol. Chem 1969, 244 (11), 2883–2390. [PubMed] [Google Scholar]
- (90).Lloyd JR; Oremland RS Microbial Transformations of Arsenic in the Environment: From Soda Lakes to Aquifers. Elements 2006, 2, 85–90. [Google Scholar]
- (91).Sun Y; Liu G; Cai Y Thiolated Arsenicals in Arsenic Metabolism: Occurrence, Formation, and Biological Implications. J. Environ. Sci. (Beijing, China) 2016, 49, 59–73. [DOI] [PubMed] [Google Scholar]
- (92).Finnegan PM; Chen W Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol 2012, 3, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Hughes MF Arsenic Toxicity and Potential Mechanisms of Action. Toxicol. Lett 2002, 133, 1–16. [DOI] [PubMed] [Google Scholar]
- (94).Styblo M; Drobná Z; Jaspers I; Lin S; Thomas DJ The Role of Biomethylation in Toxicity and Carcinogenicity of Arsenic: A Research Update. Environ. Health Perspect 2002, 110, 767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Reichl F-X; Szinicz L; Kreppel H; Forth W Effect of Arsenic on Carbohydrate Metabolism after Single or Repeated Injection in Guinea Pigs. Arch. Toxicol 1988, 62, 473–475. [DOI] [PubMed] [Google Scholar]
- (96).Szinicz L; Forth W Effect of As2O3 on Gluconeogenesis. Arch. Toxicol 1988, 61, 444–449. [DOI] [PubMed] [Google Scholar]
- (97).Rodriguez VM; Del Razo LM; Limon-Pacheco JH; Giordano M; Sanchez-Penna LC; Uribe-Querol E; Gutierrez-Ospina G; Gonsebatt ME Glutathione Reductase Inhibition and Methylated Arsenic Distribution in Cd1Mice Brain and Liver. Toxicol. Sci 2005, 84, 157–166. [DOI] [PubMed] [Google Scholar]
- (98).Su C-K; Yang C-H; Lin C-H; Sun Y-C In-Vivo Evaluation of the Permeability of the Blood - Brain Barrier to Arsenicals, Molybdate, and Methylmercury by Use of Online Microdialysis - Packed Minicolumn - Inductively Coupled Plasma Mass Spectrometry. Anal. Bioanal. Chem 2014, 406, 239–247. [DOI] [PubMed] [Google Scholar]
- (99).Xi S; Sun W; Wang F; Jin Y; Sun G Transplacental and Early Life Exposure to Inorganic Arsenic Affected Development and Behavior in Offspring Rats. Arch. Toxicol 2009, 83, 549–556. [DOI] [PubMed] [Google Scholar]
- (100).Borak J; Hosgood HD Seafood Arsenic: Implications for Human Risk Assessment. Regul. Toxicol. Pharmacol 2007, 47, 204–212. [DOI] [PubMed] [Google Scholar]
- (101).Leffers L; Ebert F; Taleshi MS; Francesconi KA; Schwerdtle T In Vitro Toxicological Characterization of Two Arsenosugars and Their Metabolites. Mol. Nutr. Food Res 2013, 57, 1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Leffers L; Unterberg M; Bartel M; Hoppe C; Pieper I; Stertmann J; Ebert F; Humpf HU; Schwerdtle T In Vitro Toxicological Characterisation of the S-Containing Arsenic Metabolites Thio-Dimethylarsinic Acid and Dimethylarsinic Glutathione. Toxicology 2013, 305, 109–119. [DOI] [PubMed] [Google Scholar]
- (103).Leffers L; Wehe CA; Huwel S; Bartel M; Ebert F; Taleshi MS; Galla H-J; Karst U; Francesconi KA; Schwerdtle T Metallomics Metabolites and Presystemic Metabolism of Thio-Dimethylarsinic Acid in Caco-2 Cells. Metallomics 2013, 5, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Chen BC; Chou WC; Chen WY; Liao CM Assessing the Cancer Risk Associated with Arsenic-Contaminated Seafood. J. Hazard. Mater 2010, 181, 161–169. [DOI] [PubMed] [Google Scholar]
- (105).Arnold LL; Cano M; St John M; Eldan M; van Gemert M; Cohen SM Effects of Dietary Dimethylarsinic Acid on the Urine and Urothelium of Rats. Carcinogenesis 1999, 20, 2171–2179. [DOI] [PubMed] [Google Scholar]
- (106).Cohen SM; Arnold LL; Eldan M; Lewis AS; Beck BD Crit. Rev. Toxicol 2006, 36, 99–133. [DOI] [PubMed] [Google Scholar]
- (107).Donohue JM; Abernathy CO Exposure to Inorganic Arsenic from Fish and Shellfish. Arsenic Exposure and Health Effects 1999, 89–98. [Google Scholar]
- (108).Witt B; Meyer S; Ebert F; Francesconi KA; Schwerdtle T Toxicity of Two Classes of Arsenolipids and Their Water-Soluble Metabolites in Human Differentiated Neurons. Arch. Toxicol 2017, 91, 3121–3134. [DOI] [PubMed] [Google Scholar]
- (109).Niehoff AC; Schulz J; Soltwisch J; Meyer S; Kettling H; Sperling M; Jeibmann A; Dreisewerd K; Francesconi KA; Schwerdtle T; et al. Imaging by Elemental and Molecular Mass Spectrometry Reveals the Uptake of an Arsenolipid in the Brain of Drosophila Melanogaster. Anal. Chem 2016, 88, 5258–5263. [DOI] [PubMed] [Google Scholar]
- (110).Taleshi MS; Raber G; Edmonds JS; Jensen KB; Francesconi KA Arsenolipids in Oil from Blue Whiting Micromesistius Poutassou - Evidence for Arsenic-Containing Esters. Sci. Rep 2015, 4, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Suhendrayatna; Ohki A; Kuroiwa T; Maeda S Arsenic Compounds in the Freshwater Green Microalga Chlorella Vulgaris after Exposure to Arsenite. Appl. Organomet. Chem 1999, 13, 127–133. [Google Scholar]
- (112).Hasegawa H; Sohrin Y; Seki K; Sato M; Norisuye K; Naito K; Matsui M Biosynthesis and Release of Methylarsenic Compounds during the Growth of Freshwater Algae. Chemosphere 2001, 43, 265–272. [DOI] [PubMed] [Google Scholar]
- (113).Howard AG; Comber SDW; Kifle D; Antai EE; Purdie DA Arsenic Speciation and Seasonal Changes in Nutrient Availability and Micro-Plankton Abundance in Southampton Water, U. K. Estuarine, Coastal Shelf Sci 1995, 40, 435–450. [Google Scholar]
- (114).Hasegawa H; Rahman MA; Matsuda T; Kitahara T; Maki T; Ueda K Effect of Eutrophication on the Distribution of Arsenic Species in Eutrophic and Mesotrophic Lakes. Sci. Total Environ 2009, 407, 1418–1425. [DOI] [PubMed] [Google Scholar]
- (115).Hasegawa H; Matsui M; Okamura S; Hojo M; Iwasaki N; Sohrin Y Arsenic Speciation Including ` Hidden ’ Arsenic in Natural Waters. Appl. Organomet. Chem 1999, 13, 113–119. [Google Scholar]
- (116).Sohrin Y; Matsui M; Kawashima M; Hojo M; Hasegawa H Arsenic Biogeochemistry Affected by Eutrophication in Lake Biwa, Japan. Environ. Sci. Technol 1997, 31, 2712–2720. [Google Scholar]
- (117).Azizur Rahman M; Hasegawa H; Peter Lim R Bioaccumulation, Biotransformation and Tropic Transfer of Arsenic in Aquatic Food Chain: Review. Environ. Res 2012, 116, 118–135. [DOI] [PubMed] [Google Scholar]
- (118).Meyer J; Schmidt A; Michalke K; Hensel R Volatilisation of Metals and Metalloids by the Microbial Population of an Alluvial Soil. Syst. Appl. Microbiol 2007, 30, 229–238. [DOI] [PubMed] [Google Scholar]
- (119).Bentley R; Chasteen TG Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth. Microbiol. Mol. Biol. Rev 2002, 66, 250–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Meyer J; Michalke K; Kouril T; Hensel R Volatilisation of Metals and Metalloids: An Inherent Feature of Methanoarchaea? Syst. Appl. Microbiol 2008, 31, 81–87. [DOI] [PubMed] [Google Scholar]
- (121).Cullen WR Chemical Mechanism of Arsenic Biomethylation. Chem. Res. Toxicol 2014, 27, 457–461. [DOI] [PubMed] [Google Scholar]
- (122).Challenger F Chem. Rev 1945, 36, 315–361. [Google Scholar]
- (123).Rehman K; Naranmandura H Arsenic Metabolism and Thioarsenicals. Metallomics 2012, 4, 881–892. [DOI] [PubMed] [Google Scholar]
- (124).Suzuki KT; Mandal BK; Katagiri A; Sakuma Y; Kawakami A; Ogra Y; Yamaguchi K; Sei Y; Yamanaka K; Anzai K; et al. Dimethylthioarsenicals as Arsenic Metabolites and Their Chemical Preparations. Chem. Res. Toxicol 2004, 17, 914–921. [DOI] [PubMed] [Google Scholar]
- (125).Naranmandura H; Suzuki N; Suzuki KT Trivalent Arsenicals Are Bound to Proteins during Reductive Methylation. Chem. Res. Toxicol 2006, 19, 1010–1018. [DOI] [PubMed] [Google Scholar]
- (126).Hayakawa T; Kobayashi Y; Cui X; Hirano S A New Metabolic Pathway of Arsenite: Arsenic - Glutathione Complexes Are Substrates for Human Arsenic Methyltransferase Cyt19. Arch. Toxicol 2005, 79, 183–191. [DOI] [PubMed] [Google Scholar]
- (127).Le XC; Lu X; Ma M; Cullen WR; Aposhian HV; Zheng B Speciation of Key Arsenic Metabolic Intermediates in Human Urine Routine Arsenic Speciation Analysis Required for Toxicologi-. Anal. Chem 2000, 72, 5172–5177. [DOI] [PubMed] [Google Scholar]
- (128).Cohen SM; Arnold LL; Beck BD; Lewis AS; Eldan M Evaluation of the Carcinogenicity of Inorganic Arsenic. Crit. Rev. Toxicol 2013, 43, 711–752. [DOI] [PubMed] [Google Scholar]
- (129).Cohen SM; Chowdhury A; Arnold LL Inorganic Arsenic: A Non-Genotoxic Carcinogen. J. Environ. Sci. (Beijing, China) 2016, 49, 28–37. [DOI] [PubMed] [Google Scholar]
- (130).Aposhian HV Enzymatic Methylation of Arsenic Spicies and Other New Approaches to Arsenic Toxicity. Annu. Rev. Pharmacol. Toxicol 1997, 37, 397–419. [DOI] [PubMed] [Google Scholar]
- (131).Vahter M Mechanisms of Arsenic Biotransformation. Toxicology 2002, 181, 211. [DOI] [PubMed] [Google Scholar]
- (132).Vahter M Methylation of Inorganic Arsenic in Different Mammalian Species and Population Groups. Sci. Prog 1999, 82, 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Raml R; Rumpler A; Goessler W; Vahter M; Li L; Ochi T; Francesconi KA Thio-Dimethylarsinate Is a Common Metabolite in Urine Samples from Arsenic-Exposed Women in Bangladesh. Toxicol. Appl. Pharmacol 2007, 222, 374–380. [DOI] [PubMed] [Google Scholar]
- (134).Petrick JS; Ayala-Fierro F; Cullen WR; Carter DE; Vasken Aposhian H Monomethylarsonous Acid (MMA III) Is More Toxic Than Arsenite in Chang Human Hepatocytes. Toxicol. Appl. Pharmacol 2000, 163, 203–207. [DOI] [PubMed] [Google Scholar]
- (135).Dopp E; Kligerman AD; Diaz-Bone RA Organoarsenicals. Uptake, Metabolism and Toxicity. Organometallics in Environment and Toxicology: Metal Ions in Life Sciences 2010, 7, 231–265. [DOI] [PubMed] [Google Scholar]
- (136).Moe B; Peng H; Lu X; Chen B; Chen LWL; Gabos S; Li XF; Le XC Comparative Cytotoxicity of Fourteen Trivalent and Pentavalent Arsenic Species Determined Using Real-Time Cell Sensing. J. Environ. Sci. (Beijing, China) 2016, 49, 113–124. [DOI] [PubMed] [Google Scholar]
- (137).Hippler J; Zdrenka R; Reichel RAD; Weber DG; Rozynek P; Johnen G; Dopp E; Hirner AV Intracellular, Time-Resolved Speciation and Quantification of Arsenic Compounds in Human Urothelial and Hepatoma Cells. J. Anal. At. Spectrom 2011, 26, 2396–2403. [Google Scholar]
- (138).Naranmandura H; Suzuki N; Iwata K; Hirano S; Suzuki KT Arsenic Metabolism and Thioarsenicals in Hamsters and Rats. Chem. Res. Toxicol 2007, 20, 616–624. [DOI] [PubMed] [Google Scholar]
- (139).Shaw JR; Glaholt SP; Greenberg NS; Sierra-Alvarez R; Folt CL Acute Toxicity of Arsenic to Daphnia Pulex: Influence of Organic Functional Groups and Oxidation State. Environ. Toxicol. Chem 2007, 26, 1532–1537. [DOI] [PubMed] [Google Scholar]
- (140).Hellweger FL; Lall U Modeling the Effect of Algal Dynamics on Arsenic Speciation in Lake Biwa. Environ. Sci. Technol 2004, 38, 6716–6723. [DOI] [PubMed] [Google Scholar]
- (141).Arbouine MW; Wilson HK The Effect of Seafood Consumption on the Assessment of Occupational Exposure to Arsenic by Urinary Arsenic Speciation Measurements. J. Trace Elem. Electrolytes Health Dis 1992, 6 (3), 153–160. [PubMed] [Google Scholar]
- (142).Heinrich-Ramm R; Mindt-Prufert S; Szadkowski D Arsenic Species Excretion after Controlled Seafood Consumption. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 2002, 778, 263–273. [DOI] [PubMed] [Google Scholar]
- (143).Branch S; Ebdon L; O’Neill P Determination of Arsenic Species in Fish by Directly Coupled High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom 1994, 9, 33–37. [Google Scholar]
- (144).Jin Y; Xi S; Li X; Lu C; Li G; Xu Y; Qu C; Niu Y; Sun G Arsenic Speciation Transported through the Placenta from Mother Mice to Their Newborn Pups. Environ. Res 2006, 101, 349–355. [DOI] [PubMed] [Google Scholar]
- (145).Bredfeldt TG; Jagadish B; Eblin KE; Mash EA; Gandolfi AJ Monomethylarsonous Acid Induces Transformation of Human Bladder Cells. Toxicol. Appl. Pharmacol 2006, 216, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Dopp E; Hartmann LM; von Recklinghausen U; Florea AM; Rabieh S; Zimmermann U; Shokouhi B; Yadav S; Hirner AV; Rettenmeier AW Forced Uptake of Trivalent and Pentavalent Methylated and Inorganic Arsenic and Its Cyto-/Genotoxicity in Fibroblasts and Hepatoma Cells. Toxicol. Sci 2005, 87, 46–56. [DOI] [PubMed] [Google Scholar]
- (147).Tseng CH A Review on Environmental Factors Regulating Arsenic Methylation in Humans. Toxicol. Appl. Pharmacol 2009, 235, 338–350. [DOI] [PubMed] [Google Scholar]
- (148).Francesconi KA; Edmonds JS; Morita M Biotransformation of Arsenic in Marine Environment. Adv. Environ. Sci. Technol 1994, 26, 221–261. [Google Scholar]
- (149).Yamauchi H; Fowler BA Toxicity and Metabolism or Inorganic and Methylated Arsenicals. Adv. Environ. Sci. Technol 1994, 27, 33–53. [Google Scholar]
- (150).Kaise T; Yamauchi H; Horiguchi Y; Tani T; Watanabe S; Hirayama T; Fukui S A Comparative Study on Acute Toxicity of Methylarsonic Acid, Dimethylarsinic Acid and Trimethylarsine Oxide in Mice. Appl. Organomet. Chem 1989, 3, 273–277. [Google Scholar]
- (151).Marafante E; Vahter M; Dencker L Metabolism of Arsenocholine in Mice, Rats and Rabbits. Sci. Total Environ 1984, 34, 223–240. [DOI] [PubMed] [Google Scholar]
- (152).Kaise T; Horiguchi Y; Fukui S; Shiomi K; Chino M; Kikuchi T Acute Toxicity and Metabolism of Arsenocholine in Mice. Appl. Organomet. Chem 1992, 6, 369–373. [Google Scholar]
- (153).Caumette G; Koch I; Reimer KJ Arsenobetaine Formation in Plankton: A Review of Studies at the Base of the Aquatic Food Chain. J. Environ. Monit 2012, 14, 2841–2853. [DOI] [PubMed] [Google Scholar]
- (154).Ciardullo S; Aureli F; Raggi A; Cubadda F Arsenic Speciation in Freshwater Fish: Focus on Extraction and Mass Balance. Talanta 2010, 81, 213–221. [DOI] [PubMed] [Google Scholar]
- (155).Harrington CF; Brima EI; Jenkins RO Biotransformation of Arsenobetaine by Microorganisms from the Human Gastrointestinal Tract. Chem. Speciation Bioavailability 2008, 20, 173–180. [Google Scholar]
- (156).Clowes AL; Francesconi KA Uptake and Elimination of Arsenobetaine by the Mussel Mytilus Edulis Is Related to Salinity. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol 2004, 137, 35–42. [DOI] [PubMed] [Google Scholar]
- (157).Larsen EH; Francesconi KA Arsenic Concentrations Correlate with Salinity for Fish Taken from the North Sea and Baltic Waters. J. Mar. Biol. Assoc. U. K 2003, 83, 283–284. [Google Scholar]
- (158).Petrick JS; Jagadish B; Mash EA; Aposhian HV Monomethylarsonous Acid (MMA III) and Arsenite: LD50 in Hamsters and In Vitro Inhibition of Pyruvate Dehydrogenase. Chem. Res. Toxicol 2001, 14, 651–656. [DOI] [PubMed] [Google Scholar]
- (159).Shiomi K Arsenic in Marine Organisms: Chemical Forms and Toxicological Aspects In Arsenic in the Environment, Part II: Human Health and Ecosystem Effects; Nriagu JO, Ed.; John Wiley: New York, 1994; pp 261–282. [Google Scholar]
- (160).Sakurai T Biological Effects of Organic Arsenic Compounds in Seafood. Appl. Organomet. Chem 2002, 16, 401–405. [Google Scholar]
- (161).Francesconi KA; Edmonds JS; Hatcher BG Examination of the Arsenic Constituents of the Herbivorous Marine Gastropod Tectus Pyramis: Isolation of Tetramethylarsonium Ion. Comp. Biochem. Physiol., C: Comp. Pharmacol 1988, 90, 313–316. [Google Scholar]
- (162).Devesa V; Súñer MA; Algora S; Vélez D; Montoro R; Jalon M; Urieta I; Macho ML Organoarsenical Species Contents in Cooked Seafood. J. Agric. Food Chem 2005, 53, 8813–8819. [DOI] [PubMed] [Google Scholar]
- (163).Devesa V; Martínez A; Súñer MA; Vélez D; Almela C; Montoro R Effect of Cooking Temperatures on Chemical Changes in Species of Organic Arsenic in Seafood. J. Agric. Food Chem 2001, 49, 2272–2276. [DOI] [PubMed] [Google Scholar]
- (164).Fricke MW; Zeller M; Sun H; Lai VWM; Cullen WR; Shoemaker JA; Witkowski MR; Creed JT Chromatographic Separation and Identification of Products from the Reaction of Dimethylarsinic Acid with Hydrogen Sulfide. Chem. Res. Toxicol 2005, 18, 1821–1829. [DOI] [PubMed] [Google Scholar]
- (165).Jeong S; Lee H; Kim YT; Yoon HO Development of a Simultaneous Analytical Method to Determine Arsenic Speciation Using HPLC-ICP-MS: Arsenate, Arsenite, Monomethylarsonic Acid, Dimethylarsinic Acid, Dimethyldithioarsinic Acid, and Dimethylmonothioarsinic Acid. Microchem. J 2017, 134, 295–300. [Google Scholar]
- (166).Hansen HR; Pickford R; Thomas-Oates J; Jaspars M; Feldmann J 2-Dimethylarsinothioyl Acetic Acid Identified in a Biological Sample: The First Occurrence of a Mammalian Arsinothioyl Metabolite. Angew. Chem 2004, 116, 341–344. [DOI] [PubMed] [Google Scholar]
- (167).Raml R; Goessler W; Traar P; Ochi T; Francesconi KA Novel Thioarsenic Metabolites in Human Urine after Ingestion of an Arsenosugar, 2’,3′-Dihydroxypropyl 5-Deoxy-5-Dimethylarsinoyl-β-D-Riboside. Chem. Res. Toxicol 2005, 18, 1444–1450. [DOI] [PubMed] [Google Scholar]
- (168).Raml R; Goessler W; Francesconi KA Improved Chromatographic Separation of Thio-Arsenic Compounds by Reversed-Phase High Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2006, 1128, 164–170. [DOI] [PubMed] [Google Scholar]
- (169).Raml R; Raber G; Rumpler A; Bauernhofer T; Goessler W; Francesconi KA Individual Variability in the Human Metabolism of an 5-Deoxy-5-Dimethylarsinoyl-β-D-Riboside, a Naturally Occurring Arsenical in Seafood. Chem. Res. Toxicol 2009, 22, 1534–1540. [DOI] [PubMed] [Google Scholar]
- (170).Wang D; Shimoda Y; Kurosawa H; Liu J; Xu X; Liu X; Jin H; Tong J; Yamanaka K; An Y Excretion Patterns of Arsenic and Its Metabolites Inhuman Saliva and Urine after Ingestion of Chinese Seaweed. Int. J. Environ. Anal. Chem 2015, 95, 379–389. [Google Scholar]
- (171).Styblo M; Serves SV; Cullen WR; Thomas DJ Comparative Inhibition of Yeast Glutathione Reductase by Arsenicals and Arsenothiols. Chem. Res. Toxicol 1997, 10, 27–33. [DOI] [PubMed] [Google Scholar]
- (172).Wang QQ; Thomas DJ; Naranmandura H Importance of Being Thiomethylated: Formation, Fate, and Effects of Methylated Thioarsenicals. Chem. Res. Toxicol 2015, 28, 281–289. [DOI] [PubMed] [Google Scholar]
- (173).Chen R; Smith BW; Winefordner JD; Tu MS; Kertulis G; Ma LQ Arsenic Speciation in Chinese Brake Fern by Ion-Pair High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectroscopy. Anal. Chim. Acta 2004, 504, 199–207. [Google Scholar]
- (174).Naranmandura H; Ibata K; Suzuki KT Toxicity of Dimethylmonothioarsinic Acid toward Human Epidermoid Carcinoma A431 Cells. Chem. Res. Toxicol 2007, 20, 1120–1125. [DOI] [PubMed] [Google Scholar]
- (175).Naranmandura H; Ogra Y; Iwata K; Lee J; Suzuki KT; Weinfeld M; Le XC Evidence for Toxicity Differences between Inorganic Arsenite and Thioarsenicals in Human Bladder Cancer Cells. Toxicol. Appl. Pharmacol 2009, 238, 133–140. [DOI] [PubMed] [Google Scholar]
- (176).Naranmandura H; Carew MW; Xu S; Lee J; Leslie EM; Weinfeld M; Le XC Comparative Toxicity of Arsenic Metabolites in Human Bladder Cancer EJ-1 Cells. Chem. Res. Toxicol 2011, 24, 1586–1596. [DOI] [PubMed] [Google Scholar]
- (177).Bartel M; Ebert F; Leffers L; Karst U; Schwerdtle T Toxicological Characterization of the Inorganic and Organic Arsenic Metabolite Thio-DMAV in Cultured Human Lung Cells. J. Toxicol 2011, 2011, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Ebert F; Leffers L; Weber T; Berndt S; Mangerich A; Beneke S; Burkle A; Schwerdtle T Toxicological Properties of the Thiolated Inorganic Arsenic and Arsenosugar Metabolite Thio-Dimethylarsinic Acid in Human Bladder Cells. J. Trace Elem. Med. Biol 2014, 28, 138–146. [DOI] [PubMed] [Google Scholar]
- (179).Ochi T; Kita K; Suzuki T; Rumpler A; Goessler W; Francesconi KA Cytotoxic, Genotoxic and Cell-Cycle Disruptive Effects of Thio-Dimethylarsinate in Cultured Human Cells and the Role of Glutathione. Toxicol. Appl. Pharmacol 2008, 228, 59–67. [DOI] [PubMed] [Google Scholar]
- (180).Unterberg M; Leffers L; Hubner F; Humpf HU; Lepikhov K; Walter J; Ebert F; Schwerdtle T Toxicity of Arsenite and Thio-DMAVafter Long-Term (21 Days) Incubation of Human Urothelial Cells: Cytotoxicity, Genotoxicity and Epigenetics. Toxicol. Res. (Cambridge, U. K.) 2014, 3, 456–464. [Google Scholar]
- (181).Miguens-Rodriguez M; Pickford R; Thomas-Oates JE; Pergantis SA Arsenosugar Identification in Seaweed Extracts Using High-Performance Liquid Chromatography/Electrospray Ion Trap Mass Spectrometry. Rapid Commun. Mass Spectrom 2002, 16, 323–331. [DOI] [PubMed] [Google Scholar]
- (182).Almela C; Laparra JM; Vélez D; Barberá R; Farré R; Montoro R Arsenosugars in Raw and Cooked Edible Seaweed: Characterization and Bioaccessibility. J. Agric. Food Chem 2005, 53, 7344–7351. [DOI] [PubMed] [Google Scholar]
- (183).Morita M; Shibata Y Isolation and Identification of Arseno-Lipid from a Brown Alga, Undaria Pinnatifida (Wakame). Chemosphere 1988, 17, 1147–1152. [Google Scholar]
- (184).García-Salgado S; Raber G; Raml R; Magnes C; Francesconi KA Arsenosugar Phospholipids and Arsenic Hydrocarbons in Two Species of Brown Macroalgae 2012, 9, 63–66. [Google Scholar]
- (185).Francesconi KA; Edmonds JS; Stick RV; Skelton BW; White AH Arsenic-Containing Ribosides from the Brown Alga Sargassum Lacerifolium: X-Ray Molecular Structure of 2-Amino-3-[5′-Deoxy-5′-(Dimethylarsinoyl) - Ri Bosyloxy] Propane-I -Sulphonic Acid. J. Chem. Soc., Perkin Trans. 1 1991, 1, 2707–2716. [Google Scholar]
- (186).Foster S; Maher W Arsenobetaine and Thio-Arsenic Species in Marine Macroalgae and Herbivorous Animals: Accumulated through Trophic Transfer or Produced in Situ? J. Environ. Sci. (Beijing, China) 2016, 49, 131–139. [DOI] [PubMed] [Google Scholar]
- (187).Shibata Y; Jin K; Morita M Arsenic Compounds in the Edible Red Alga, Porphyra Tenera, and in Nori and Yakinori, Food Items Produced from Red Algae. Appl. Organomet. Chem 1990, 4, 255–260. [Google Scholar]
- (188).Shibata Y; Morita M A Novel, Trimethylated Arseno-Sugar Isolated from the Brown Alga Sargassum thunbergii. Agric. Biol. Chem 1988, 52, 1087–1089. [Google Scholar]
- (189).Shibata Y; Morita M; Edmonds JS Purification and Identification of Arsenic-Containing Ribofuranosides from the Edible Brown Seaweed, Laminaria Japonica (Makonbu). Agric. Biol. Chem 1987, 51, 391–398. [Google Scholar]
- (190).McSheehy S; Szpunar J Speciation of Arsenic in Edible Algae by Bi-Dimensional Size-Exclusion Anion Exchange HPLC with Dual ICP-MS and Electrospray MS/MS Detection. J. Anal. At. Spectrom 2000, 15, 79–87. [Google Scholar]
- (191).McSheehy S; Marcinek M; Chassaigne H; Szpunar J Identification of Dimethylarsinoyl-Riboside Derivatives in Seaweed by Pneumatically Assisted Electrospray Tandem Mass Spectrometry. Anal. Chim. Acta 2000, 410, 71–84. [Google Scholar]
- (192).McSheehy S; Pohl P; Łobiński R; Szpunar J Complementarity of Multidimensional HPLC-ICP-MS and Electrospray MS-MS for Speciation Analysis of Arsenic in Algae. Anal. Chim. Acta 2001, 440, 3–16. [DOI] [PubMed] [Google Scholar]
- (193).McSheehy S; Pohl P; Vélez D; Szpunar J Multidimensional Liquid Chromatography with Parallel ICP MS and Electrospray MS/MS Detection as a Tool for the Characterization of Arsenic Species in Algae. Anal. Bioanal. Chem 2002, 372, 457–466. [DOI] [PubMed] [Google Scholar]
- (194).Francesconi KA; Edmonds JS; Stick RV Arsenic Compounds from the Kidney of the Giant Clam Tridacna Maxima: Isolation and Identification of an Arsenic-Containing Nucleoside. J. Chem. Soc., Perkin Trans. 1 1992, 1, 1349–1357. [Google Scholar]
- (195).McSheehy S; Szpunar J; Lobinski R; Haldys V; Tortajada J; Edmonds JS Characterization of Arsenic Species in Kidney of the Clam Tridacna Derasa by Multidimensional Liquid Chromatography-ICPMS and Electrospray Time-of-Flight Tandem Mass Spectrometry. Anal. Chem 2002, 74, 2370–2378. [DOI] [PubMed] [Google Scholar]
- (196).Francesconi KA; Goessler W; Panutrakul S; Irgolic KJ A Novel Arsenic Containing Riboside (Arsenosugar) in Three Species of Gastropod. Sci. Total Environ 1998, 221, 139–148. [Google Scholar]
- (197).Francesconi KA; Hunter DA; Bachmann B; Raber G; Goessler W Uptake and transformation of arsenosugars in the shrimp Crangon crangon. Appl. Organomet. Chem 1999, 13, 669–679. [Google Scholar]
- (198).Fricke MW; Creed PA; Parks AN; Shoemaker JA; Schwegel CA; Creed JT Extraction and Detection of a New Arsine Sulfide Containing Arsenosugar in Molluscs by IC-ICP-MS and IC-ESI-MS/MS. J. Anal. At. Spectrom 2004, 19, 1454–1459. [Google Scholar]
- (199).McSheehy S; Pohl P; Lobiński R; Szpunar J Investigation of Arsenic Speciation in Oyster Test Reference Material by Multidimensional HPLC-ICP-MS and Electrospray Tandem Mass Spectrometry (ES-MS-MS). Analyst 2001, 126, 1055–1062. [DOI] [PubMed] [Google Scholar]
- (200).Madsen AD; Goessler W; Pedersen SN; Francesconi KA Characterization of an Algal Extract by HPLC-ICP-MS and LC-Electrospray MS for Use in Arsenosugar Speciation Studies. J. Anal. At. Spectrom 2000, 15, 657–662. [Google Scholar]
- (201).van Elteren JT; Slejkovec Z; Kahn M; Goessler W A Systematic Study on the Extractability of Arsenic Species from Algal Certified Reference Material IAEA-140/TM (Fucus Sp., Sea Plant Homogenate) Using Methanol/Water Extractant Mixtures. Anal. Chim. Acta 2007, 585, 24–31. [DOI] [PubMed] [Google Scholar]
- (202).Le XC; Cullen WR; Reimer KJ Human Urinary Arsenic Excretion after One-Time Ingestion of Seaweed, Crab and Shrimp. Clin. Chem 1994, 40, 617–624. [PubMed] [Google Scholar]
- (203).Ma MS; Le XC Effect of Arsenosugar Ingestion on Urinary Arsenic Speciation. Clin. Chem 1998, 44 (3), 539–550. [PubMed] [Google Scholar]
- (204).Edmonds JS; Francesconi KA Arseno-Sugars from Brown Kelp (Ecklonia Radiata) as Intermediates in Cycling of Arsenic in a Marine Ecosystem. Nature 1981, 289, 602–604. [Google Scholar]
- (205).Gamble BM; Gallagher PA; Shoemaker JA; Wei X; Schwegel CA; Creed JT An Investigation of the Chemical Stability of Arsenosugars in Simulated Gastric Juice and Acidic Environments Using IC-ICP-MS and IC-ESI-MS/MS. Analyst 2002, 127, 781–785. [DOI] [PubMed] [Google Scholar]
- (206).García Sartal C; d. C. Barciela-Alonso M; Bermejo-Barrera P Effect of the Cooking Procedure on the Arsenic Speciation in the Bioavailable (Dialyzable) Fraction from Seaweed. Microchem. J 2012, 105, 65–71. [Google Scholar]
- (207).Edmonds JS; Francesconi KA Arsenic in Seafoods: Human Health Aspects and Regulations. Mar. Pollut. Bull 1993, 26, 665–674. [Google Scholar]
- (208).Geiszinger A; Goessler W; Pedersen SN; Francesconi KA Arsenic Biotransformation by the Brown Macroalga Fucus serratus. Environ. Toxicol. Chem 2001, 20, 2255–2262. [DOI] [PubMed] [Google Scholar]
- (209).Edmonds JS; Francesconi KA; Hansen JA Dimethyloxarsylethanol from Anaerobic Decomposition of Brown Kelp (Ecklonia Radiata) - a Likely Precursor of Arsenobetaine in Marine Fauna. Experientia 1982, 38, 643–644. [Google Scholar]
- (210).Francesconi KA; Tanggaar R; McKenzie CJ; Goessler W Arsenic Metabolites in Human Urine after Ingestion of an Arsenosugar. Clin. Chem 2002, 48 (1), 92–101. [PubMed] [Google Scholar]
- (211).Lai VW-M; Cullen WR; Harrington CF; Reimer KJ The characterization of arsenosugars in commercially available algal products including a Nostoc species of terrestrial origin. Appl. Organomet. Chem 1997, 11, 797–803. [Google Scholar]
- (212).Nischwitz V; Kanaki K; Pergantis SA Mass Spectrometric Identification of Novel Arsinothioyl-Sugars in Marine Bivalves and Algae. J. Anal. At. Spectrom 2006, 21, 33–40. [Google Scholar]
- (213).Sakurai T; Kaise T; Ochi T; Saitoh T; Matsubara C Study of in Vitro Cytotoxicity of a Water Soluble Organic Arsenic Compound, Arsenosugar, in Seaweed. Toxicology 1997, 122, 205–212. [DOI] [PubMed] [Google Scholar]
- (214).Feldmann J; John K; Pengprecha P Arsenic Metabolism in Seaweed-Eating Sheep from Northern Scotland. Fresenius’ J. Anal. Chem 2000, 368, 116–121. [DOI] [PubMed] [Google Scholar]
- (215).Hansen HR; Raab A; Francesconi KA; Feldmann J Metabolism of Arsenic by Sheep Chronically Exposed to Arsenosugars as a Normal Part of Their Diet. 1. Quantitative Intake, Uptake, and Excretion. Environ. Sci. Technol 2003, 37, 845–851. [DOI] [PubMed] [Google Scholar]
- (216).Schmeisser E; Rumpler A; Kollroser M; Rechberger G; Goessler W; Francesconi KA Arsenic Fatty Acids Are Human Urinary Metabolites of Arsenolipids Present in Cod Liver. Angew. Chem., Int. Ed 2006, 45, 150–154. [DOI] [PubMed] [Google Scholar]
- (217).Schmeisser E; Goessler W; Francesconi KA Human Metabolism of Arsenolipids Present in Cod Liver. Anal. Bioanal. Chem 2006, 385, 367–376. [DOI] [PubMed] [Google Scholar]
- (218).Van Hulle M; Zhang C; Schotte B; Mees L; Vanhaecke F; Vanholder R; Zhang XR; Cornelis R Identification of Some Arsenic Species in Human Urine and Blood after Ingestion of Chinese Seaweed Laminaria. J. Anal. At. Spectrom 2004, 19, 58–64. [Google Scholar]
- (219).Taylor VF; Li Z; Sayarath V; Palys TJ; Morse KR; Scholz-Bright RA; Karagas MR Distinct Arsenic Metabolites Following Seaweed Consumption in Humans. Sci. Rep 2017, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Raab A; Newcombe C; Pitton D; Ebel R; Feldmann J Comprehensive Analysis of Lipophilic Arsenic Species in a Brown Alga (Saccharina Latissima). Anal. Chem 2013, 85, 2817–2824. [DOI] [PubMed] [Google Scholar]
- (221).Francesconi KA; Edmonds JS Arsenic and Marine Organisms. Adv. Inorg. Chem 1996, 44, 147–189. [Google Scholar]
- (222).Müller SM; Ebert F; Raber G; Meyer S; Bornhorst J; Hüwel S; Galla HJ; Francesconi KA; Schwerdtle T Effects of Arsenolipids on in Vitro Blood-Brain Barrier Model. Arch. Toxicol 2018, 92, 823–832. [DOI] [PubMed] [Google Scholar]
- (223).Glabonjat RA; Raber G; Jensen KB; Ehgartner J; Francesconi KA Quantification of Arsenolipids in the Certified Reference Material NMIJ 7405-a (Hijiki) Using HPLC/Mass Spectrometry after Chemical Derivatization. Anal. Chem 2014, 86, 10282–10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Taleshi MS; Jensen KB; Raber G; Edmonds JS; Gunnlaugsdottir H; Francesconi KA Arsenic-Containing Hydrocarbons: Natural Compounds in Oil from the Fish Capelin, Mallotus Villosus. Chem. Commun 2008, 39, 4706–4707. [DOI] [PubMed] [Google Scholar]
- (225).Lischka S; Arroyo-Abad U; Mattusch J; Kuhn A; Piechotta C The High Diversity of Arsenolipids in Herring Fillet (Clupea Harengus). Talanta 2013, 110, 144–152. [DOI] [PubMed] [Google Scholar]
- (226).Taleshi MS; Edmonds JS; Goessler W; Ruiz-Chancho MJ; Raber G; Jensen KB; Francesconi KA Arsenic-Containing Lipids Are Natural Constituents of Sashimi Tuna. Environ. Sci. Technol 2010, 44, 1478–1483. [DOI] [PubMed] [Google Scholar]
- (227).Arroyo-Abad U; Mattusch J; Mothes S; Moder M; Wennrich R; Elizalde-González MP; Matysik FM Detection of Arsenic-Containing Hydrocarbons in Canned Cod Liver Tissue. Talanta 2010, 82, 38–43. [DOI] [PubMed] [Google Scholar]
- (228).Amayo KO; Petursdottir A; Newcombe C; Gunnlaugsdottir H; Raab A; Krupp EM; Feldmann J Identification and Quantification of Arsenolipids Using Reversed-Phase HPLC Coupled Simultaneously to High-Resolution ICPMS and High-Resolution Electrospray MS without Species-Specific Standards. Anal. Chem 2011, 83, 3589–3595. [DOI] [PubMed] [Google Scholar]
- (229).Amayo KO; Raab A; Krupp EM; Gunnlaugsdottir H; Feldmann J Novel Identification of Arsenolipids Using Chemical Derivatizations in Conjunction with RP-HPLC-ICPMS/ESMS. Anal. Chem 2013, 85, 9321–9327. [DOI] [PubMed] [Google Scholar]
- (230).García-Salgado S; Quijano MA; Bonilla MM Arsenic Speciation in Edible Alga Samples by Microwave-Assisted Extraction and High Performance Liquid Chromatography Coupled to Atomic Fluorescence Spectrometry. Anal. Chim. Acta 2012, 714, 38–46. [DOI] [PubMed] [Google Scholar]
- (231).Amayo KO; Raab A; Krupp EM; Marschall T; Horsfall M; Feldmann J Arsenolipids Show Different Profiles in Muscle Tissues of Four Commercial Fish Species. J. Trace Elem. Med. Biol 2014, 28, 131–137. [DOI] [PubMed] [Google Scholar]
- (232).Rumpler A; Edmonds JS; Katsu M; Jensen KB; Goessler W; Raber G; Gunnlaugsdottir H; Francesconi KA Arsenic-Containing Long-Chain Fatty Acids in Cod-Liver Oil: A Result of Biosynthetic Infidelity? Angew. Chem 2008, 120, 2705–2707. [DOI] [PubMed] [Google Scholar]
- (233).Arroyo-Abad U; Lischka S; Piechotta C; Mattusch J; Reemtsma T Determination and Identification of Hydrophilic and Hydrophobic Arsenic Species in Methanol Extract of Fresh Cod Liver by RP-HPLC with Simultaneous ICP-MS and ESI-Q-TOF-MS Detection. Food Chem 2013, 141, 3093–3102. [DOI] [PubMed] [Google Scholar]
- (234).Taleshi MS; Seidler-Egdal RK; Jensen KB; Schwerdtle T; Francesconi KA Synthesis and Characterization of Arsenolipids: Naturally Occurring Arsenic Compounds in Fish and Algae. Organometallics 2014, 33, 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Arroyo-Abad U; Hu Z; Findeisen MM; Pfeifer D; Mattusch J; Reemtsma T; Piechotta C Synthesis of Two New Arsenolipids and Their Identification in Fish. Eur. J. Lipid Sci. Technol 2016, 118, 445–452. [Google Scholar]
- (236).Ruiz-Chancho MJ; Taleshi MS; Goessler W; Francesconi KA A Method for Screening Arsenolipids in Fish Oils by HPLC-ICPMS. J. Anal. At. Spectrom 2012, 27, 501–504. [Google Scholar]
- (237).Arroyo-Abad U; Pfeifer M; Mothes S; Stark HJ; Piechotta C; Mattusch J; Reemtsma T Determination of Moderately Polar Arsenolipids and Mercury Speciation in Freshwater Fish of the River Elbe (Saxony, Germany). Environ. Pollut 2016, 208, 458–466. [DOI] [PubMed] [Google Scholar]
- (238).Raber G; Khoomrung S; Taleshi MS; Edmonds JS; Francesconi KA Identification of Arsenolipids with GC/MS. Talanta 2009, 78, 1215–1218. [DOI] [PubMed] [Google Scholar]
- (239).Francesconi KA; Schwerdtle T Fat-Soluble Arsenic - New Lipids with a Sting in Their Tail. Lipid Technol 2016, 28, 96–98. [Google Scholar]
- (240).Viczek SA; Jensen KB; Francesconi KA Arsenic-Containing Phosphatidylcholines: A New Group of Arsenolipids Discovered in Herring Caviar. Angew. Chem., Int. Ed 2016, 55, 5259–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).Fukuda S; Terasawa M; Shiomi K Phosphatidylarsenocholine, One of the Major Arsenolipids in Marine Organisms: Synthesis and Metabolism in Mice. Food Chem. Toxicol 2011, 49, 1598–1603. [DOI] [PubMed] [Google Scholar]
- (242).Lunde G Analysis of Arsenic in Marine Oil by Neutron Activation: Evidence of Arseno Organic Compounds. J. Am. Oil Chem. Soc 1968, 45, 331–332. [DOI] [PubMed] [Google Scholar]
- (243).Cooney RV; Mumma RO; Benson AA Arsoniumphospholipid in Algae. Proc. Natl. Acad. Sci. U. S. A 1978, 75, 4262–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Benson AA; Summons RE The American Association for the Advancement of Science. Science (Washington, DC, U. S.) 1981, 211, 482–483. [DOI] [PubMed] [Google Scholar]
- (245).Schmeisser E; Goessler W; Kienzl N; Francesconi KA Direct Measurement of Lipid-Soluble Arsenic Species in Biological Samples with HPLC-ICPMS. Analyst 2005, 130, 948–955. [DOI] [PubMed] [Google Scholar]
- (246).Khan M; Francesconi KA Preliminary Studies on the Stability of Arsenolipids: Implications for Sample Handling and Analysis. J. Environ. Sci. (Beijing, China) 2016, 49, 97–103. [DOI] [PubMed] [Google Scholar]
- (247).Guttenberger N; Glabonjat RA; Jensen KB; Zangger K; Francesconi KA Synthesis of Two Arsenic-Containing Cyclic Ethers: Model Compounds for a Novel Group of Naturally-Occurring Arsenolipids. Tetrahedron Lett 2016, 57, 4578–4580. [Google Scholar]
- (248).Guttenberger N; Glabonjat RA; Tassoti S; Francesconi KA Synthetic Access to Arsenic-Containing Phosphatidylcholines. Tetrahedron Lett 2017, 58, 2651–2653. [Google Scholar]
- (249).Guttenberger N; Sagmeister P; Glabonjat RA; Hirner S; Francesconi KA Facile Access to Arsenic-Containing Triacylglycerides. Tetrahedron Lett 2017, 58, 362–364. [Google Scholar]
- (250).Muller SM; Finke H; Ebert F; Kopp JF; Schumacher F; Kleuser B; Francesconi KA; Raber G; Schwerdtle T Arsenic-Containing Hydrocarbons: Effects on Gene Expression, Epigenetics, and Biotransformation in HepG2 Cells. Arch. Toxicol 2018, 92, 1751–1765. [DOI] [PubMed] [Google Scholar]
- (251).Meyer S; Raber G; Ebert F; Taleshi MS; Francesconi KA; Schwerdtle T Arsenic-Containing Hydrocarbons and Arsenic-Containing Fatty Acids: Transfer across and Presystemic Metabolism in the Caco-2 Intestinal Barrier Model. Mol. Nutr. Food Res 2015, 59, 2044–2056. [DOI] [PubMed] [Google Scholar]
- (252).Meyer S; Raber G; Ebert F; Leffers L; Muller SM; Taleshi MS; Francesconi KA; Schwerdtle T In Vitro Toxicological Characterisation of Arsenic-Containing Fatty Acids and Three of Their Metabolites. Toxicol. Res. (Cambridge, U. K.) 2015, 4, 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Meyer S; Schulz J; Jeibmann A; Taleshi MS; Ebert F; Francesconi KA; Schwerdtle T Arsenic-Containing Hydrocarbons Are Toxic in the in Vivo Model Drosophila Melanogaster. Metallomics 2014, 6, 2010–2014. [DOI] [PubMed] [Google Scholar]
- (254).Witt B; Ebert F; Meyer S; Francesconi KA; Schwerdtle T Assessing Neurodevelopmental Effects of Arsenolipids in Pre-Differentiated Human Neurons. Mol. Nutr. Food Res 2017, 61, 1700199. [DOI] [PubMed] [Google Scholar]
- (255).Al Amin MH; Xiong C; Glabonjat RA; Francesconi KA; Oguri T; Yoshinaga J Estimation of Daily Intake of Arsenolipids in Japan Based on a Market Basket Survey. Food Chem. Toxicol 2018, 118, 245–251. [DOI] [PubMed] [Google Scholar]


