Abstract
It is thought that most cell types of the human body share the same genetic information as that contained in the zygote from which they originate. Consistent with this view, animal cloning studies demonstrated that the intact genome of a differentiated cell can be reprogrammed to support the development of an entire organism and allow the production of pluripotent stem cells. Recent progress in reprogramming research now points to an important role for transcription factors in the establishment and the maintenance of cellular phenotypes, and to cell division as a mediator of transitions between different states of gene expression.
A central question in developmental biology asks: how do cells adopt different cell fates during development and how is it that, as cells adopt these fates, they lose their ability to give rise to other cell types? The pioneering amphibian nuclear-transfer experiments carried out more than 50 years ago by Briggs and King sought to address whether the loss of genetic information might be associated with the restricted developmental potential of differentiated cells. They reasoned that if genetic material were lost, the differentiated state would be irreversible following nuclear transfer1. Gurdon and colleagues extracted nuclei from differentiated cells. After injection into unfertilized frog eggs, these nuclei had the capacity to direct the development of the resulting embryos into tadpoles and adult, fertile frogs2. The first mammal to be cloned from an adult cell, Dolly3, and the cloning of mice from terminally differentiated cells of a defined cell type4–6 ultimately demonstrate that development is not associated with the loss of genetic information. If not by loss of genetic information, then how are genes appropriately regulated during development to result in lineage restriction, cellular differentiation and a stable cellular identity?
The identity of a cell is determined by its constituent components, such as proteins, RNA and lipids. These in turn arise from specific transcriptional programmes. Such programmes are formed by the presence of gene regulatory networks that are modulated by site- specific transcriptional regulators, non-coding RNAs, chromatin-binding proteins, DNA methyltransferases, histone-modifying enzymes and other regulators of gene expression. Unlike a genetic mutation, which is irreversible, transcriptional programmes can be flexible and reversible. The transfer of the genome of a differentiated cell into an unfertilized oocyte erases the transcriptional programme of the somatic cell and initiates an embryonic transcriptional programme7,8. How this reprogramming process occurs has remained unclear. Much of the work that has been directed towards an understanding of the establishment of transcriptional programmes during development and towards explaining nuclear reprogramming after nuclear transfer has focused on the epigenome — the post-translational modifications of histones and the methylation of DNA9–12 (BOX 1).
Box 1 |. The epigenome.
The epigenome is the information carried by the genome that is not encoded in the DNA. It usually refers to the methylation of DNA on cytosine and guanine dinucleotides9,12, to modified histones10,11,142 and to the histone variants that are incorporated into nucleosomes78,143. DNA methylation is usually repressive and inherited through the action of DNA methyltransferases.
Nucleosomes consist of an octamer of four histones, H2A, H2B, H3 and H4. Their N‑terminal tails are subject to more than 100 different post‑translational modifications, including acetylation, methylation, phosphorylation and ubiquitylation, with either activating or inhibiting effects on transcription10,11,21. Histone variants can either be associated with inactive or active chromatin. For example, the replacement histone‑3 variant H3.3 is found in active chromatin78 and MacroH2A is associated with the inactive X chromosome144.
Post‑translational modifications on histones and the methylation of DNA are reversible, as unmodified histones and nucleotides are incorporated with every replication cycle. Replication without renewal of these modifications results in their passive removal. In addition, enzymes actively remove methyl groups on histones145. DNA methylation is erased early in development13, probably in a transcription‑factor-dependent manner146. Also, in somatic cell types, in which DNA‑methylation patterns usually persist for many cell generations, active DNA demethylation has been observed147–149.
After fertilization of the oocyte by the sperm, or after nuclear transfer into an unfertilized oocyte, the DNA is globally demethylated13,14. Demethylation of the DNA and similar dynamic changes in histone modifications following fertilization or nuclear transfer 8,15 are thought to relieve the restrictive cell-type-specific transcriptional programme of germ cells or transferred somatic cells, helping to establish pluripotency12,16–21. Whether the reprogramming of these epigenetic marks is in itself causative of a change in developmental potential after nuclear transfer remains unclear.
We propose that at most stages of differentiation, the phenotype and developmental potential of a cell is primarily determined by site-specific regulators of transcription that actively propagate its gene-expression programme. Upon entry into mitosis, these transcriptional regulators dissociate from the genome and, after each cell division, reassociate with chromatin, thereby allowing gene-expression patterns to be newly established. We argue that the components of the epigenome that are stably associated with the DNA throughout the cell cycle reflect and invigorate the action of these transcriptional regulators, rather than determine the developmental state of a cell. This is an attractive model because disruption and re-establishment of transcriptional complexes with every cell division acts as an efficient mediator for the transition between different transcriptional programmes and cellular states. As we will describe, this view is supported by reprogramming experiments in which one or a combination of transcription factors are eliminated or expressed ectopically, and by the results of genome transfer between distinct cell types.
surviving genome transfer
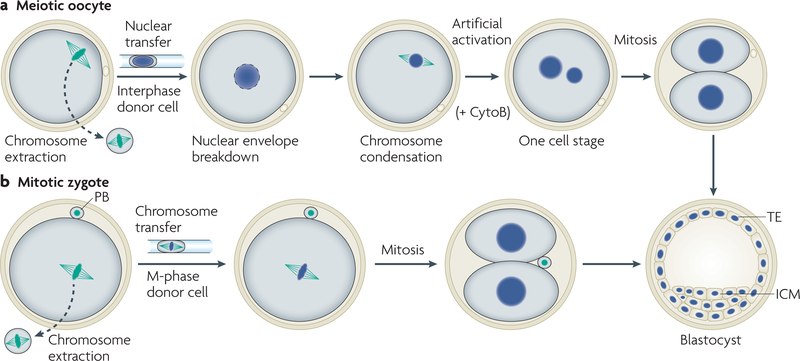
For a successful transfer of genomes, the recipient cell and the donor genome should be compatible in their cell cycle to prevent chromosomal abnormalities that lead to cell death. The standard practice in animal cloning is the transfer of an interphase nucleus into an unfertilized oocyte that has had its own genome removed (FIG. 1a). As the unfertilized oocyte is arrested at metaphase of the second meiotic division, the donor and recipient cell are asynchronous in their cell cycle. The interphase donor nucleus is ultimately synchronized after transfer by meiotic kinase activities within the oocyte, which cause nuclear-envelope breakdown and chromosome condensation. These events correlate positively with developmental potential22–24, whereas incomplete nuclear-envelope breakdown and chromosome condensation is predictive of developmental arrest after monkey nuclear transfer24. Frequent chromosomal abnormalities and inefficient development have also been observed following nuclear transfer into pre-activated oocytes (which have reduced meiotic kinase activity), which is presumably the result of less efficient nuclear-envelope breakdown and chromosome condensation25,26. To preserve chromosomal integrity and for development to occur, somatic interphase nuclei that are transferred into oocytes need to be remodelled into meiotic chromatin. We have recently shown that the transfer of a mitotic genome into a zygote in mitosis sidesteps these requirements, presumably because the donor genome is already condensed and donor and recipient cells are a priori synchronized27 (FIG. 1b).
Figure 1 |. Cell-cycle synchronization after nuclear transfer.
a | Transfer of a G1 interphase nucleus into an oocyte in meiosis results in nuclear-envelope breakdown and chromosome condensation. Chromosome condensation converts the interphase nucleus into meiotic chromatin, thereby synchronizing the cell cycle of the donor cell with the recipient oocyte. Unfertilized oocytes are naturally arrested in meiosis. To enter the cell cycle and for development to occur, an artificial activation stimulus (intracellular calcium release) is required. Cytochalasin B (CytoB) is added to prevent cytokinesis and to prevent the extrusion of the second polar body (PB), which would result in two aneuploid cells. b | Transfer of chromosomes from a mitotic somatic cell to a mitotic zygote results in development. The cell cycle of the mitotic zygote and the mitotic genome of a somatic cell are synchronized from the moment of transfer. Cell-cycle progression and cleavage leads to the formation of a two-cell stage embryo. ICM, inner cell mass; TE, trophectoderm.
Cell division reprogrammes the origins of replication.
An experiment by lemaitre and colleagues in 2005, in which erythrocyte nuclei were exposed to Xenopus egg extracts, might help to explain the importance of chromosome condensation after nuclear transfer28. The rates of DNA replication were low in erythrocyte nuclei that had been exposed to Xenopus egg S-phase extracts and the spacing of replication origins was between 30–230 kb, which is typical for erythrocyte nuclei. When the nuclei were placed in M-phase extracts in order to induce nuclear-envelope breakdown and chromosome condensation before exposure to S-phase extracts, the origin spacing on the replicating chromosomes decreased to less than 30 kb and replication rates were as efficient as in chromatin from rapidly dividing embryonic cells. Mitosis is the only time during the cell cycle when mammalian chromosomes lack functional origins of replication; replication origins are established, and their location is determined, during G1 phase29,30. origin of replication recognition complex-1 (ORC1) dissociates from chromatin during mitosis in a topoisomerase II (topo II)-dependent manner31, and re-associates with the DNA in telophase of mitosis29,32. Treatment of M-phase egg extracts with a topoisomerase-II-specific inhibitor to prevent chromosome condensation and oRC1 clearance from erythrocyte chromatin resulted in low rates of replication of erythrocyte nuclei28. The disassembly of origins of replication in cell division, and their reassembly in G1, therefore mediates the reprogramming of cell-type-specific replication patterns. These biochemical findings suggest that the absence of chromosome condensation after nuclear transfer can lead to developmental failure because DNA replication remains somatic and cannot synchronize with the first embryonic cell cycle, resulting in chromosomal abnormalities.
cell division and reprogramming
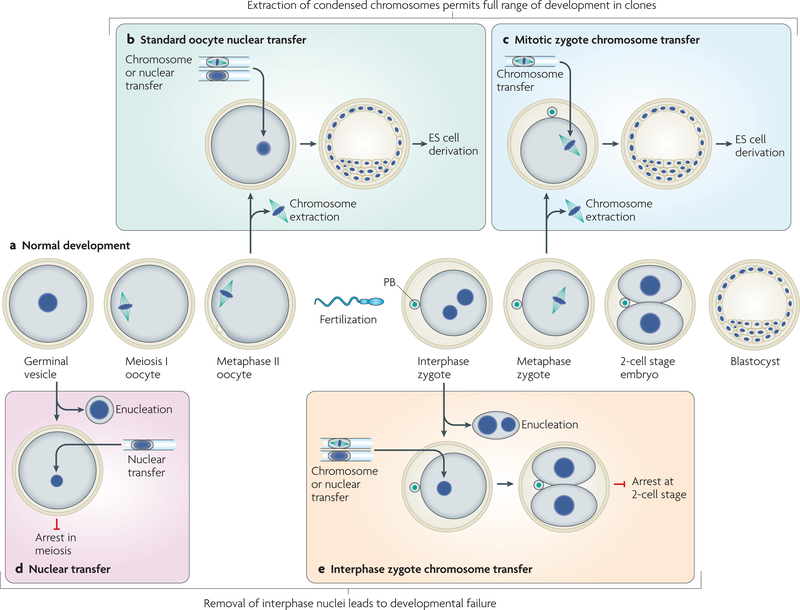
Pioneering nuclear-transfer studies in mammals used fertilized zygotes in interphase as nuclear-transfer recipients33. exchange of nuclei between different mouse zygotes resulted in proper development (FIG. 2a). However, replacement of the zygote nucleus with the nucleus of a more differentiated cell type halted development25,34–39 (FIG. 2e). By contrast, when the chromosomes of the unfertilized oocyte were replaced with the nucleus of a differentiated, somatic cell, development occurred3,40 (FIG. 2b). Following these studies, numerous species have been cloned by transferring either embryonic or adult donor nuclei into unfertilized oocytes.
Figure 2 |. Genome exchange during cell division allows development of clones.
Meiotic progression of unfertilized oocytes and embryonic development (a). The germinal vesicle (GV) is the interphase nucleus of the oocyte before entry into meiotic division. Fertilization of the metaphase II oocyte by the sperm forms the fertilized zygote. Development is shown until the blastocyst stage at which embryonic stem (ES) cells can be derived. Removal of the condensed chromosomes from the unfertilized oocyte (b) or from the zygote (c) during mitosis, followed by the transfer of a new genome, results in the development of clones, from which ES cells can be derived. Nuclei or condensed chromosomes can be transferred into the unfertilized oocyte. Replacement of the GV nucleus with the interphase nucleus of a differentiated cell results in developmental arrest41 (d). Replacement of the two haploid interphase nuclei of the zygote with the interphase nuclei (nuclear transfer) or an M-phase genome (chromosome transfer) of a diploid cell results in developmental arrest in the next interphase27 (e). PB, polar body.
Nevertheless, the ability of the oocyte cytoplast to reprogramme the genome of a differentiated cell is not exclusive. The transfer of condensed chromosomes into a zygote that has entered mitosis allows the cloning of mice and the derivation of embryonic stem-cell lines from differentiated cells27 (FIG. 2c). This indicates that reprogramming is possible when the genome of a zygote is removed in cell division, but not in interphase. A similar difference between nuclear transfer in interphase and in cell division is also seen in oocytes. When oocytes are enucleated in interphase at the germinal vesicle stage before entry into meiotic division, their ability to support development after nuclear transfer is lost41 (FIG. 2d). So, what are the differences between dividing and interphase cells that cause these different developmental outcomes? In this section, we consider the potential features of a dividing cell that might allow these manipulations and facilitate the reprogramming of gene expression.
Transcription is repressed in mitosis.
In mitosis and meiosis, the activities of the kinases cyclin B/cyclin-dependent kinase-2 (CDK2), mitogen-activated protein kinase (MAPK), polo-like kinase and Aurora B kinase drive nuclear-envelope breakdown and chromosome condensation, and cause all three RNA polymerases to cease transcription42–46 (reviewed in ReF. 47). The transcriptional silencing that occurrs in cell division might be a constraint imposed by the packaging of chromatin that is required for the mechanical separation of DNA into two cells. In addition, it might provide the cell with an opportunity to execute profound changes in gene expression.
Transcriptional activators disperse during mitosis.
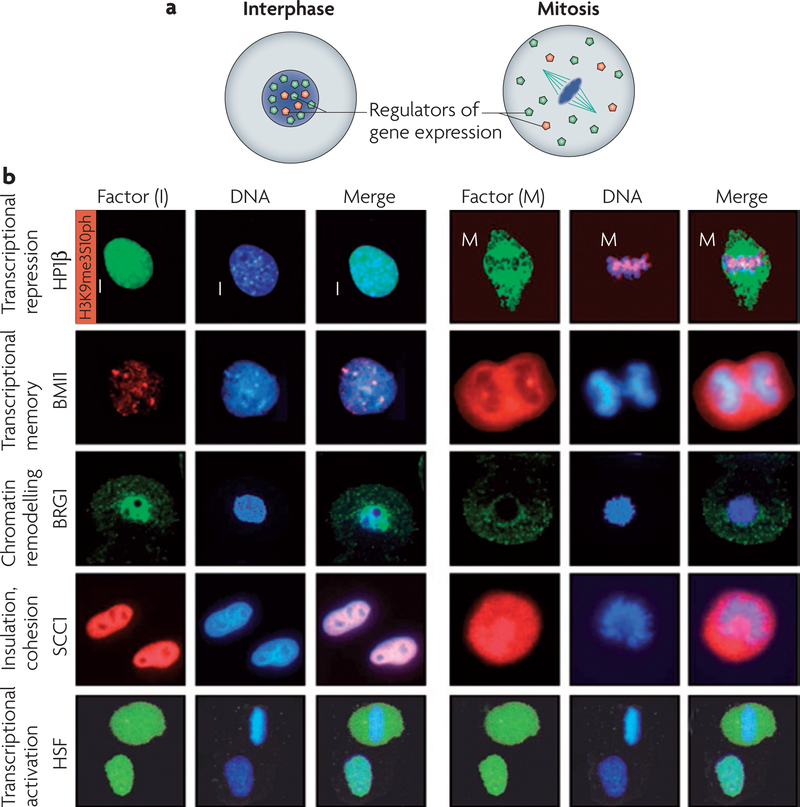
Transcriptional silencing in mitosis and meiosis is associated with the dissociation of transcription factors from condensed chromosomes and their dispersal throughout the cytoplasm48,49 (FIG. 3, Supplementary information S1 (table)). The heat-shock protein-70 (HSP70), U2, c-FOS and phosphoglycerate kinase-1 promoters, in addition to all of the RNA polymerase (pol) II promoters and the RNA pol III promoter U6, have been found to be devoid of sequence-specific transcription factors during mitosis49–52. An informative study of the human HSP70 promoter, which is ordinarily bound by activated heat-shock factor-1 (HSF1) in interphase, indicated that transcription factors, such as HSF1, are actively excluded from chromatin during mitosis49. In mitosis, despite normal DNA-binding activity of activated HSF1, this factor was dispersed throughout the mitotic cytoplasm (FIG. 3b). Controls suggested that this was not simply the result of disintegration of the nuclear envelope and the diffusion of nucleoplasmic HSF1. Heat-shock activated HSF1 was detected in interphase, but not in mitosis, on the heat-shock regulatory elements of the HSP70 promoter. It remains to be determined whether HSF1 is representative of other site-specific transcriptional regulators, the cell-cycle-dependent localization of which has not been as extensively studied.
Figure 3 |. Regulators of gene expression dissociate from mitotic chromatin.
a | In a cell in interphase, regulators of gene expression (coloured dots) associate with the genetic information in the nucleus. During mitosis, the nuclear envelope breaks down, chromosomes condense and regulators of gene expression dissociate from the genetic information and localize to the cytoplasm. b | Gene expression is regulated at multiple levels and important components of these regulatory systems dissociate from the chromatin in mitosis. Interphase (I) and mitosis (M) stages are shown. Some of the main factors that dissociate from chromatin during mitosis include the transcriptional repressor heterochromatin protein-1β (HP1β; the red signal marks the phosphorylation of Ser10 on Histone H3 that causes the dissociation of HP1β from chromatin), BMI1, the chromatin remodelling protein BRG1, sister-chromatid cohesion-1 (SCC1) and the transcriptional activator heat-shock factor (HSF). All immunocytochemistry shown is in HeLa cells, except BRG1, which is shown in 2-cell-stage mouse embryos. Cells shown are at prometaphase of mitosis, with the exception of BMI1, which shows a cell at anaphase of mitosis with the separating two groups of chromatin. HP1β images adapted, with permission, from Nature REF. 70 © (2005) Macmillan Publishers Limited. All rights reserved. BMI1 images adapted, with permission, from REF. 57 © Company of Biologists. SCC1 images adapted, with permission, from Nature REF. 160 © Macmillan Publishers Limited. All rights reserved. HSF images adapted, with permission, from REF. 49 © (1995) Cell Press.
During interphase, the nucleosome-remodelling activity of SWI/SNF (BOX 2) activates gene expression by increasing the access of transcription factors to chromatin. During mitosis, components of the SWI/SNF complexes are phosphorylated by the mitotic kinase extracellular signal-regulated kinase-1 (eRK1) and are thereby either reversibly inactivated or targeted for degradation. Phosphorylation leads to inhibition of nucleosome-remodelling activity in vitro and to the exclusion of the remodelling complex from condensed chromatin53,54 (FIG. 3b). In parallel, nucleosome positioning is lost during mitosis51. This is in contrast to interphase nuclei, in which specific positioning of nucleosomes has been observed in several eukaryotic genes and is believed to potentiate transcription by increasing accessibility to transcription-factor binding sites and by bringing regulatory sequences into proximity with their promoter elements55. During mitosis, a loss of all detectable sequence-specific binding of transcription factors in the c-FOS and U6 promoters and a disappearance of nucleosome positioning has been observed51. This suggests that the disappearance of nucleosome positioning could be caused by the inhibition of nucleosome-remodelling activities and the displacement of transcription factors from their binding sites.
Box 2 |. Polycomb and trithorax group complexes.
Polycomb and trithorax group genes mediate heritable silencing of many developmentally important genes by generating repressive chromatin (reviewed in ReFS 150–154). They were originally identified in Drosophila melanogaster, in which their mutation causes ectopic expression of homeotic genes, leading to developmental abnormalities155,156. At least two polycomb‑group complexes have been identified and characterized in flies: polycomb repressive complex‑1 (PRC1) — which consists of polycomb (PC), posterior sex combs (the mammalian homologue of which is BMI), polyhomeotic and RING — and PRC2, which consists of enhancer of zeste (E(z)), extra sex combs (ESC) and the histone‑binding protein p55. E(z) methylates H3K27; this modification mediates gene silencing. PC contains a chromodomain that specifically binds to this modification157.
The counterparts of PC repression are the trithorax group proteins that maintain the active state and prevent polycomb‑mediated silencing150,151,158,159. The trithorax group of genes encodes Brahma (BRM) and Moira (MOR), which are components of the SWI/SNF chromatin remodelling complex, and also encode the histone methyl transferases TRX and ASH, which methylate H3K4; this methylation mark is associated with active genes. The mutation of trithorax proteins in flies leads to homeotic transformation, owing to the silencing of homeotic genes.
Transcriptional repressors disperse during mitosis.
In addition to transcriptional activators, negative regulators of gene expression also dissociate from chromatin during mitosis (FIG. 3b; Supplementary information S1(table)). In Drosophila melanogaster embryos, polycomb, polyhomeotic and posterior sex comb dissociate from chromosomes in mitosis56 (BOX 2). Similarly in mammals, the polycomb protein and oncoprotein BMI1 is phosphorylated and dissociates from chromatin in mitosis and meiosis, probably in a complex with other polycomb-group proteins48,57,58 (FIG. 3b). This dissociation is not complete, as the polycomb proteins BMI1, RING-finger protein-1 (RING1) and polycomb-2 (PC2) can be detected at low levels on heterochromatin during mitosis59.
Heterochromatin protein-1 (HP1) is an essential component of heterochromatin. Through its chromodomain, HP1 binds the methylated histone H3K9 (REFS 60,61; FIG. 4a). H3K9 is methylated by the Suv39h histone methyltransferases62 (FIG. 4a). HP1 that is bound to methylated H3K9 recruits further Suv39h activity and therefore leads to further methylation of H3K9, which excludes H3K9 acetylation, thereby consolidating a repressed state. During mitosis, the different mammalian isoforms of HP1 (α, β and γ) progressively dissociate from facultative and most of the pericentric heterochromatin63–68 (FIG. 3b). This dissociation is caused by the phosphorylation of Ser10 on histone H3 by Aurora B69–72 (FIG. 4b). During mitosis, neither the levels of histone methylation nor the levels of the different HP1 isoforms are dramatically altered68,70,73. Similarly to HP1, Suv39h itself binds to methylated H3K9 during interphase but it dissociates from the chromosomal arms and localizes only to the centromeres during mitosis74.
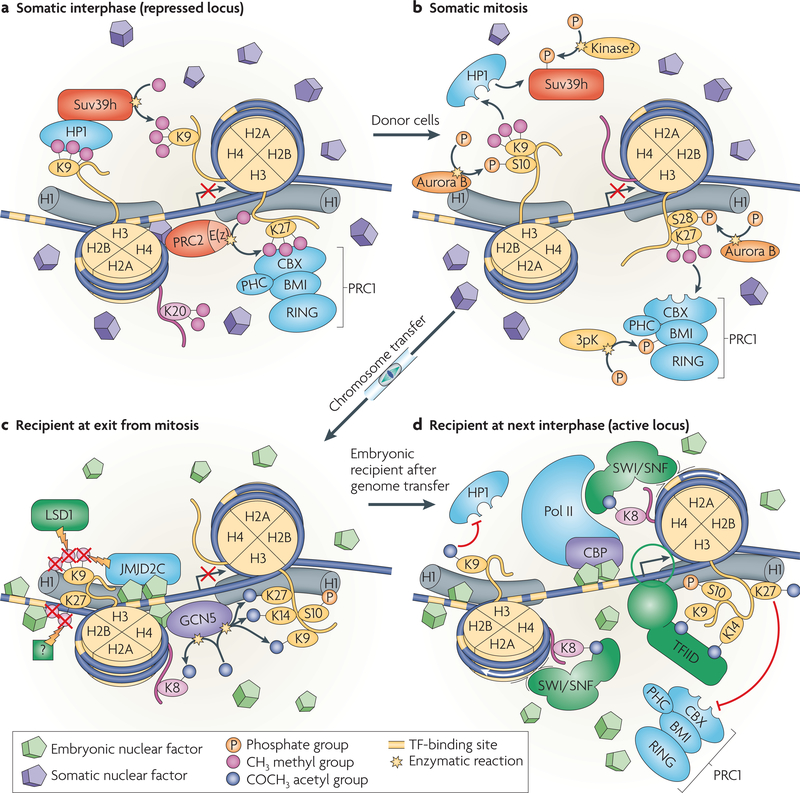
Figure 4 |. Model of reprogramming after transfer of a somatic cell genome into an oocyte or zygote in cell division.
a | A hypothetical locus in a repressed state, bound by polycomb repressive complex-1 (PRC1) and the heterochromatin protein-1 (HP1). The histone methyltransferase Suv39h methylates H3K9 to generate a binding site for HP1 and PRC2 methylates H3K27 to generate a binding site for PRC1. b | During mitosis, the phosphorylation of Ser10 on histone H3 by the mitotic kinase Aurora B results in the dissociation of HP1 from chromatin. Aurora B kinase also phosphorylates Ser28 on histone H3. PRC1 is phosphorylated during mitosis by 3pK (mitogen-activated protein kinase-activated protein kinase-3)161 and dissociates from methylated H3K27. An unknown kinase phosphorylates Suv39h, which also dissociates from chromatin. c | Following transfer of the somatic cell genome into a mitotic cell with an embryonic transcription factor environment, and following exit from mitosis, embryonic transcription factors bind to their target sites. Binding leads to the recruitment of chromatin-modifying activities and the establishment of a basal promoter complex. The recruitment of the histone demethylases jumonji domain-containing (JMJD) protein-2C (JMJD2C), JMJD-containing histone demethylation protein-2A (JHDM2A; not shown) and Lys-specific histone demethylase-1 (LSD1), which demethylate H3K9, can occur in a transcription-factor-dependent manner162,163. Unknown activities demethylate H3K27. The histone acetyl transferase general control of amino-acid-synthesis protein-5 (GCN5) acetylates Lys residues on the N-terminal tails of histone H3 and H4. d | The histone acetyl transferase CREB-binding protein (CBP)–RNA polymerase II (pol II) holoenzyme complex, is recruited to the promoter and nucleosome acetylation facilitates SWI/SNF recruitment to remodel chromatin and position nucleosomes. The transcription factor TFIID binds to the promoter and initiates transcriptional activation (see also ReF. 164). The active locus excludes the access of PRC1 or HP1 and prevents the formation of heterochromatin. CBX, chromobox homologue, a mammalian homologue of Drosophila melanogaster polycomb; E(z), enhancer of zeste; PHC, mammalian homologue of D. melanogaster polyhomeotic.
Epigenetic modifications persist through cell division.
Many but not all of the transcriptional regulators are removed from condensed chromatin, and for some, the dissociation is incomplete (Supplementary information S1, S2 (tables)). In contrast to most of the transcriptional regulators that have been studied, covalent modifications to histones and to the DNA itself persist through mitosis20,73,75. The methyl-DNA-binding proteins methyl-CpG-binding domain protein-1 (MBD1) and methyl-CpG- binding protein-2 (MeCP2) can also be found associated with both heterochromatic and euchromatic sites on mitotic chromosomes76,77; however, dissociation during cell division has also been observed48. In addition, in actively transcribed genes, histone H3.1 is replaced with the histone variant H3.3 (ReF. 78). The persistence of such histone variants and covalent histone modifications on chromosomes throughout mitosis might explain why the HSP70 promoter is accessible to DNase I during interphase as well as mitosis49,79.
It has been proposed that residual transcriptional regulators that are associated with chromatin in mitosis, and epigenetic marks on DNA and histones, form a transcriptional memory throughout cell division80,81. Support for this hypothesis comes from the inheritance of allele-specific gene expression of imprinted genes. For example, in mitotic trophectoderm stem cells, polycomb-group proteins dissociate from most of the genome, but are stably retained on the inactive X chromosome82,83, where they might be involved in stably maintaining the inactive state. By contrast, the association of some transcriptional regulators with mitotic chromosomes does not seem to be part of a transcriptional memory mechanism. During mitosis, HP1 is mostly dissociated from the chromosomal arms but is bound to the centromeric region of chromosomes and regulates chromosome segregation84,85. Also, the D. melanogaster GAGA transcription factor is displaced from euchromatin and binds to satellite repeats during mitosis, in which it also has a role in chromosome segregation86. Following chromosome decondensation, GAGA dissociates from the repeats and moves back to its high-affinity sites in euchromatin where it regulates gene expression during interphase87,88. It is unclear why an amount of TATA-binding protein remains associated with chromatin in mitosis89–91, as it is associated in a manner that is distinct from its normal binding to the TATA box51.
Mitosis disrupts nuclear organization.
The mammalian interphase nucleus is organized into structural and functional compartments92. Individual chromosomes occupy distinct positions93 and transcriptional activity is highly localized94. Gene-poor, transcriptionally inactive chromatin tends to localize beneath the nuclear envelope, whereas gene-dense, transcriptionally active chromatin is enriched in the nuclear interior. Nuclear architecture is stable during interphase95. During mitosis, nuclear-envelope breakdown and the condensation of chromosomes disrupts the organization of the nuclear architecture, and thereby allows repositioning of chromosomal regions96. Such repositioning can mediate a transition in transcriptional activity. The relocalization of a transcriptionally active locus from the interior of a mammalian nucleus to the nuclear membrane, by tethering it to the nuclear envelope, causes silencing. However, this repositioning and silencing could not occur during interphase and only occurred after passage through mitosis97.
During development, both gene activation and gene silencing can be associated with the repositioning of a locus, such as the immunoglobulin loci that re-localize from the nuclear periphery to the centre during B-cell development98. Such transitions can occur in response to transcriptional regulators following exit from cell division in early G1 (REF. 99). The physiological importance of nuclear structure in the regulation of gene expression is illustrated by Hutchinson-Gilford Progeria Syndrome, an early-onset ageing disorder. Affected patients express a mutant form of the nuclear-envelope protein lamin A. This defect leads to a loss of peripheral heterochromatin, transcriptional misregulation and defects in stem-cell differentiation100,101.
Transcriptional programmes are established de novo.
As the cell exits from mitosis, a new nuclear structure is formed, global transcriptional repression is relieved, transcriptional regulators associate with chromatin and origins of replication are established. These important events that occur during the early G1 phase are likely to determine the replicative behaviour and the transcriptional programme of a cell and, consequently, its cellular identity and developmental potential. The establishment of transcriptional programmes after cell division might be governed by mass action and determined by the concentrations and binding affinities of individual components that associate with chromatin at the entry into G1. Both stable inheritance and change of cellular phenotypes at all stages of differentiation would then primarily depend on the ability of a cell to establish gene-expression patterns on a genome-wide scale, according to the presence and stoichiometry of transcriptional regulators.
A key question for developmental biologists and those interested in reprogramming is: do the events that are discussed above have a role in facilitating the transition between the transcriptional programmes that generate new cell types?
Transitions between transcriptional programmes.
Asymmetric cell divisions provide an example of how mitosis mediates the transition between transcriptional states. The physical separation of regulators of gene expression from chromatin in mitosis is regularly used in development to segregate cell-fate determinants asymmetrically to daughter cells. Asymmetric cell divisions are used both to establish new cell lineages during development and to generate differentiated cells from stem cells102,103. For example, in dividing D. melanogaster neuroblasts, the transcription factor Prospero localizes asymmetrically to the cortex and segregates to only one of the two daughter cells. Prospero turns off neuroblast-specific genes and promotes neural differentiation, whereas the other daughter cell maintains neuroblast characteristics.
Mitosis might also aid the transition between cellular states in symmetrically dividing cells. experiments carried out in vitro reveal how the dissociation and reassociation of factors in mitosis could aid the transition from a repressed transcriptional state to an active one: SWI/SNF could not remodel nucleosomes following pre-incubation of nucleosomal arrays with the polycomb repressive complex-1 (PRC1). However, simultaneous addition of PRC1 or the addition of PRC1 after SWI/SNF resulted in nucleosome remodelling. PRC1 and SWI/SNF compete for access to chromatin and PRC1 must bind first at the template to block SWI/ SNF function104,105. The presence of polycomb on the target DNA inhibits the accessibility of a transactivator to its target DNA106. It seems that once a transcriptional state is established, it is more refractory to a change than when it needs to be newly established. The dissociation and association of transcriptional regulators, as well as the reorganization of nuclear structure in mitosis, might provide a window to relieve such constraints.
Reprogramming after nuclear transfer
The transfer of a somatic cell genome into an oocyte or a zygote in cell division transforms the transcriptional programme from somatic to embryonic. The main requirement for reprogramming to occur seems to be that the genetic information of the recipient cell is removed during mitosis or meiosis (when at least some transcriptional regulators localize to the cytoplasm) and not during interphase, when many of these regulators are associated with the genome. This cell-cycle-dependent localization of transcriptional regulators probably has an important role in reprogramming following the transfer of a new genome. The removal of the genetic information from a zygote or oocyte during mitosis and/or meiosis seems to result in a cytoplast with the main determinants that instruct cell-type-specific gene expression (FIG. 5a). Following transfer of a somatic genome into this cytoplast and entry into interphase, transcriptional regulators of the embryonic cell activate embryonic genes, and development can proceed. Importantly, the activation of embryonic genes occurs despite a mismatch between the somatic epigenome and the embryonic regulators of gene expression.
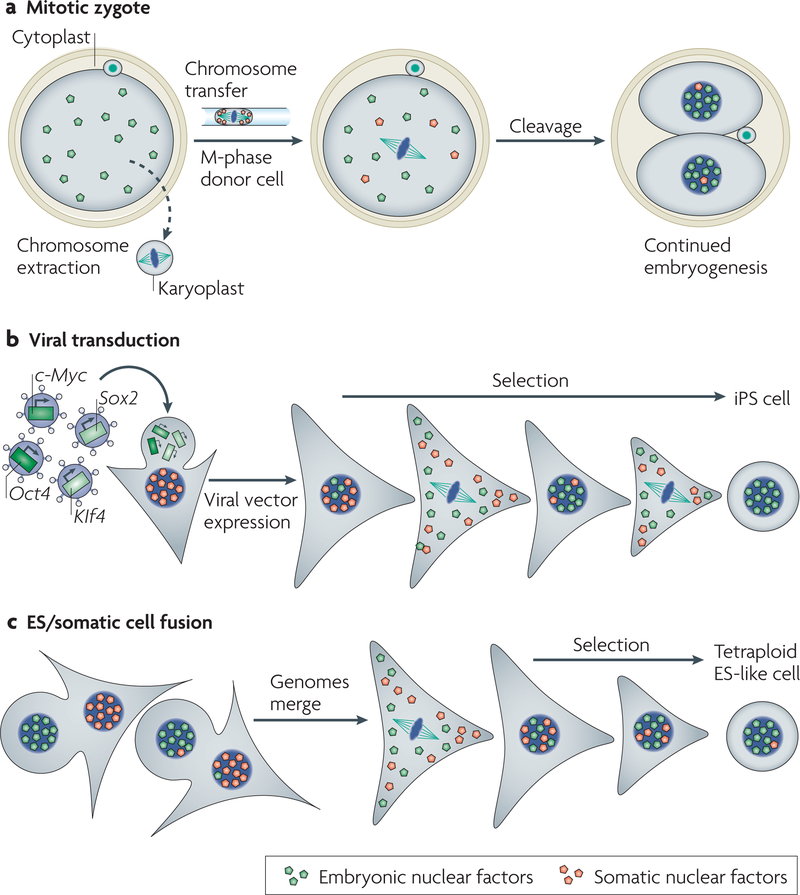
Figure 5 |. Different methods of reprogramming require transcriptional regulators and passage through cell division.
a | Removal of the condensed chromosomes from a zygote in mitosis (or an oocyte in meiosis) generates a karyoplast that lacks the factors that are required for the regulation of embryonic gene expression, and also generates a cytoplast that contains these factors. A new genome is transferred by microinjection. Following entry into interphase, nuclear factors localize to the new interphase nucleus and activate transcription, and thereby development occurs. b | Reprogramming by defined factors. Transgenes introduced with the help of viruses express the four transcription factors octamer-binding transcription factor-4 (Oct4), SRY-related high mobility group (HMG)-box protein-2 (Sox2), Kruppel-like factor 4 (Klf4) and c-Myc in a somatic cell. Following selection and multiple cell divisions, colonies arise that closely resemble embryonic stem (ES) cells. Reprogramming to induced-pluripotent stem (iPS) cells seems to be more efficient with rapidly dividing cell cultures120. The persistence of somatic nuclear factors might counteract the reprogramming process124. c | Fusion of a somatic cell (triangular shape) with an ES cell (round shape). A selection process results in the isolation of rare pluripotent stem cells with a tetraploid genome that contains both donor and recipient cell genomes.
The molecular mechanisms by which embryonic regulators of gene expression might reprogramme a somatic cell genome after nuclear transfer are not fully understood. We propose a model of how mitosis could help the transition from a silenced state in a somatic cell to an embryonic, transcriptionally active state for a hypothetical locus (FIG. 4). Chromosome condensation causes the dissociation of polycomb and HP1 from chromatin (FIG. 4a,b). on transfer of the genome of the somatic cell into the embryonic environment, and following exit from mitosis, embryonic transcription factors access their target sequence and recruit SWI/SNF and other remodelling activities to the previously inactive promoter (FIG. 4c,d), thereby relieving the silencing that is caused by polycomb and HP1.
Development after nuclear transfer typically fails with less than 5% of clones developing to adulthood, often with birth defects107 that are probably the result of faulty regulation of gene expression108,109. Development is slightly more efficient when the somatic donor genome is hypomethylated110 and following application of the histone deacetylase inhibitor TSA111,112. The mismatch between a somatic epigenome and embryonic transcriptional regulators therefore affects the efficiency of development and affects reprogramming to embryonic gene transcription, but does not represent an insurmountable obstacle.
Manipulating transcriptional programmes
Interest in the field of reprogramming grew with the realization that reprogramming human somatic cells to an embryonic state by nuclear transfer might have significant implications for medicine. embryonic cells that are genetically identical to a patient could then be differentiated in vitro into a desired cell type for study and possibly for cell-replacement therapy for the patient, a concept that is referred to as therapeutic cloning113,114. A limitation to this work, however, is a lack of material, principally human oocytes. The logistical difficulties associated with human embryonic stem (eS)-cell research have spurred the search for ways to reprogramme cells without the use of embryos.
Reprogramming by transcription factors.
Takahashi and Yamanaka recently discovered that the expression of four transcription factors, octamer-binding transcription factor-4 (Oct4), SRY-related high-mobility-group (HMG)-box protein-2 (Sox2) (ReFS 115,116), c-Myc and Kruppel-like factor-4 (Klf4), in differentiated mouse fibroblasts results in the reprogramming of these somatic cells to an eS-cell-like state117 (FIG. 5b). This method has also been successfully used to reprogramme human fibroblasts to an eS-cell-like state118–120. The transition from a somatic to an embryonic state is associated with extensive reprogramming of gene expression and the acquisition of DNA and histone epigenetic modifications that are almost identical to ES cells121–123. In the mouse, these induced-pluripotent stem cells (iPS cells) could differentiate into different cell types, giving rise to chimaeric mice with germ-line contribution121–123. The efficiency of iPS reprogramming is exceedingly low, but can be enhanced by interfering with the transcriptional programme of the somatic cell. The reprogramming of mature B lymphocytes to iPS cells was more efficient after specific knockdown of the transcription factor paired box protein-5 (PAX5) (REF. 124).The loss of PAX5 in mature B cells is sufficient to induce de-differentiation to uncommitted progenitors, suggesting that the differentiated state is actively propagated by PAX5 (REF. 125). Transcription factors of differentiated and embryonic cells, such as PAX5, OCT4 and SOX2, not only maintain a cellular identity, but some also have the ability to alter the identity of another cell type. Similarly, the expression of the muscle-specific transcription factor myoblast determination protein-1 (MYOD) in fibroblasts leads to the induction of muscle characteristics and the upregulation of muscle-specific gene products126. exposure of human fibroblasts to mouse muscle-cell cytoplasm after cell fusion also results in activation of human muscle genes127. Tissue-specific, transactivating factors — probably cell-type-specific transcription factors — therefore exist that perpetuate the differentiated state of a muscle cell and have the ability to reprogramme the genome of another cell type. Although these induced trans-differentiation events might be incomplete, it is nevertheless possible to generate an embryonic or differentiated cell type of interest by either cell fusion or ectopic expression of one or a combination of transcriptional regulators.
Is cell division required for reprogramming?
The above experiments argue for a central role of transcription factors in establishing cellular identity and in the reprogramming of gene expression; however, some do not absolutely require cell division for reprogramming. The activation of muscle genes in fibroblast–muscle-cell hybrids does not require replication or cell division, indicating that reprogramming in this system does not depend on the passage through S phase or M phase. Similarly, injection of somatic cell nuclei into interphase oocytes at the germinal vesicle stage without removing the oocyte nucleus resulted in low expression of the oocyte genes that were not previously expressed in the somatic cell. These genes were fully active after 7 days following injection of the nuclei128. These experiments demonstrate that transcriptional reprogramming can take place in the absence of cell division, which probably occurs by a mechanism that involves the exchange of DNA-bound proteins during interphase129. However, this reprogramming process is slow and incomparable to the speed and efficiency at which developmental processes and changes in gene expression normally occur, particularly in the frog. Within hours of fertilization, or after the transfer of a somatic nucleus into an unfertilized frog egg, the resulting embryos reach the blastula stage (this is the stage at which embryonic genome activation occurs), and within three days the somatic genome has supported the developmental steps leading to the formation of a tadpole with a beating heart and other complex organs130. An interpretation of the efficiency and functionality of reprogramming without cell division has been complicated by the fact that in most fusion experiments, and in the above-mentioned injections into interphase oocytes, the host-cell genome was not removed and probably ensured cell survival during the reprogramming period. Although a transition in the transcriptional state can occur without cell division, the events that occur during mitosis might nevertheless be essential for normal development. Carl Wu and colleagues49 proposed that “the mitotic displacement of transcription factors from chromatin might present a hitherto unrecognized window ... to be exploited for the resetting of some transcriptional programmes” during development (see also REFS 48,131).
Conclusions and future perspectives
Most cell types do not differ in their genetic information but in a multitude of other cellular components. The experiments described here provide a partial explanation for how cells establish and retain their identity, and how they can be manipulated to transit to another cell type. The number of these experiments is small, and conclusions drawn might only be applicable to the specific cell types or transcriptional regulators that have been studied. However, it might be that they are common for both programming and reprogramming developmental states. At all stages of differentiation, the main determinants of the transcriptional programme dissociate from the genome in cell division and re-associate in G1 to establish the entire transcriptional programme de novo. As a result it is reasonable to speculate that the epigenome is modified according to the presence of these transcriptional regulators and invigorates their action, thereby stabilizing the cellular state. exceptions to this model might be imprinted gene expression and the silencing of endogenous retroviral elements, in which the epigenome dominates the state of expression, a mechanism of gene regulation that might not apply to many developmentally regulated genes. our model incorporates the findings that the removal of the oocyte and zygote genome during cell division (but not during interphase) allows embryonic gene expression following somatic cell nuclear transfer. This suggests extensive dissociation of transcriptional regulators from condensed chromatin and the de novo establishment of the transcriptional programme in G1. Furthermore, they incorporate the findings that the transcription factors oCT4 and SoX2 can overrule the epigenetic state of a somatic cell and result in a stable transformation to an embryonic gene-expression programme. Finally, our model is supported by cell-fusion experiments between somatic and embryonic cells that can result in the activation of previously inactive genes and stable cell-fate changes132–134 (FIG. 5c). These latter experiments indicate that both pluripotent and differentiated cells contain transactivating transcriptional regulators that can programme, maintain and reprogramme a cellular identity.
The differences that are observed between cell fusion, nuclear transfer and reprogramming by ectopic expression of transcription factors might be mostly technical, with all three sharing the same underlying mechanism of reprogramming: the exposure of incoming chromatin to new transcriptional regulators, resulting in changes in gene expression (FIG. 5). Some important differences nevertheless do exist: cell fusion and nuclear transfer take advantage of the presence of all players in a complex network of transcriptional regulators, whereas the ectopic expression of transcriptional regulators is limited to a small number of factors that must presumably induce other genes to form a more complete regulatory circuitry135. This could be a reason why the induction of an embryonic gene-expression pattern by ectopic expression of transcriptional regulators requires more time than by cell fusion or nuclear transfer27,136–138.
There are several potential limitations to our model that need to be addressed. The most conclusive experiments to manipulate cell fate were done with pluripotent embryonic cell types and factors that were isolated from them. One could argue that these cells could differ in a fundamental way from a differentiated cell. It cannot currently be excluded that with progressing differentiation, certain cells lose cell-type-specific and sequence-specific transcriptional regulators, while they increasingly rely on autonomously propagating epigenetic marks that are stably associated with chromatin. A more detailed investigation of transcriptional regulators and their association with chromatin during the cell cycle could help to answer this question.
Further cell-fusion experiments would help address the question of whether somatic cells contain sufficient programming activities to reprogramme other cell types to their own identity. To date, fusion experiments between somatic cells and embryonic cells have only resulted in the recovery of cells with the embryonic transcriptional programme, suggesting that the embryonic state might be dominant. However, the reverse (programming of the embryonic stem-cell genome into a differentiated cell type) might simply be difficult to distinguish from spontaneous differentiation of the embryonic fusion partner. Cell-fusion experiments would yield more conclusive results if the genome of the recipient cell could be removed. It would also be desirable to develop methods of cell fusion that are more efficient and more controllable than those that are currently available. This advance would allow smaller cell numbers to be used and the fate of individual fusion events to be followed.
Our comprehension of how cells differ from one another and how they can be manipulated to convert to a different transcriptional state is not only central to our understanding of development, it is also of considerable medical interest. A sophisticated understanding of reprogramming will eventually allow the production of therapeutically relevant cell types, many of which are currently in short supply. In addition, many age and disease-related processes seem to be caused by malfunctions in the cellular circuitry regulating networksof gene expression139–141. It might eventually be possible to reprogramme these pathological malfunctions after they occur, ultimately reversing certain aspects of ageing and disease.
Supplementary Material
Acknowledgements
D.E. thanks I. Tabansky, A. J. Tanaka, C. Fitzgerald, A. Chen, K. Rodolfa, R. Jiao, K. Niakan, S. Sullivan, A. Tajonar, E. Son and R. Maehr for comments on the manuscript. D.E. is supported by a Harvard Stem Cell Institute (USA) seed grant funded by the Singer family foundation. K.E. is a fellow of the John D. and Catherine T. MacArthur Foundation. We apologize for the many exciting studies that could ot be included in this article because of space constraints.
Glossary
- Nuclear transfer
(NT). The transfer of a genome within an intact nucleus during interphase.
- Terminally differentiated cell
A cell that does not give rise to a cell type other than that of itself
- Oocyte
An unfertilized egg
- Reprogramming
An induced transition in cellular identity, usually meaning the reversal of differentiation
- Cellular state
A cellular phenotype that includes developmental potential, the state of differentiation and functional specialization, replicative life-span and whether a cell is transformed to display aspects of disease
- Zygote
A fertilized egg
- Blastocyst
The embryo before implantation that contains at least two distinct cell types: the trophectoderm and the inner cell mass
- Chromosome transfer
The transfer of a genome that is packaged in condensed chromosomes
- Replication origin
A site where replication is initiated during S phase. It is bound by the origin of replication complex
- M phase
Mitosis and meiosis
- Topoisomerase II
(topo II). A protein that decatenates DNA in an ATP-dependent manner. It is also required for chromosome condensation
- Cytoplast
A cell that does not contain a nuclear genome, but does contain mitochondria with genetic information
- Embryonic stem cell
(ES). A pluripotent cell that can be derived from the inner cell mass of the blastocyst-stage embryo
- Homeotic genes
Genes that encode homeodomain-containing transcription factors that are involved in the patterning of the body during development
- Heterochromatin
DNA packed into a transcriptionally repressive chromatin structure
- Pericentric heterochromatin
The heterochromatin of the chromosomal arms that is close to the centromeres
- Euchromatin
A form of chromatin that is lightly packed and often transcriptionally active during
- Karyoplast
A nucleus or mitotic genome without the cytoplasm
- Pluripotent stem cell
A cell that can give rise to cell types of the three germ layers — endoderm, mesoderm and ectoderm — and to germ cells
- Cell fusion
The fusion of two or more cells resulting in a single, fused cell. This can be done by the application of an electric field or chemicals, such as polyethylene glycol
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
c-Myc | Klf4 | Oct4 | Sox2 |
UniProtKB: http://ca.expasy.org/sprot
BMI1 | GCN5 | MBD1 | MYOD | OCT4 | ORC1 | PAX5 | PC2 | RING1 | SOX2
FURTHER INFORMATION
Kevin Eggan’s homepage: http://www.mcb.harvard.edu/Faculty/Eggan.html
Dieter Egli’s email address: degli@mcb.harvard.edu
SUPPLEMENTARY INFORMATION
References
- 1.Briggs R. & King TJ Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc. Natl Acad. Sci. USA 38, 455–463 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurdon JB, Elsdale TR & Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182, 64–65 (1958).Demonstrates that frogs can be cloned from somatic cells.
- 3.Wilmut I, Schnieke AE, McWhir J, Kinnotd AJ & Campbell KH Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813 (1997).Demonstrates the reversibility of mammalian differentiation.
- 4.Eggan K. et al. Mice cloned from olfactory sensory neurons. Nature 428, 44–49 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Hochedlingoteer K. & Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415, 1035–1038 (2002).Demonstrates that the genome of even the most differentiated cells can support development after nuclear transfer.
- 6.Inoue K. et al. Generation of cloned mice by direct nuclear transfer from natural killer T cells. Curr. Biol 15, 1114–1118 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Brambrink T, Hochedlinger K, Bell G. & Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc. Natl Acad. Sci. USA 103, 933–938 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyhan Z. et al. Transcriptional reprogramming of somatic cell nuclei during preimplantation development of cloned bovine embryos. Dev. Biol 305, 637–649 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Kouzarides T. Chromatin modifications and their function. Cell 128, 693–705 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Strahl BD & Allis CD The language of covalent histone modifications. Nature 403, 41–45 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Jaenisch R. & Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genet. 33 Suppl, 245–254 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Santos F, Hendrich B, Reik W. & Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol 241, 172–182 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Dean W. et al. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc. Natl Acad. Sci. USA 98, 13734–13738 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos F. et al. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr. Biol 13, 1116–1121 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Rideout WM, Eggan K. & Jaenisch R. Nuclear cloning and epigenetic reprogramming of the genome. Science 293, 1093–1098 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Morgan HD, Santos F, Green K, Dean W. & Reik W. Epigenetic reprogramming in mammals. Hum. Mol. Genet 14 R47–R58 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Simonsson S. & Gurdon JB Changing cell fate by nuclear reprogramming. Cell Cycle 4, 513–515 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447, 425–432 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Holliday R. The inheritance of epigenetic defects. Science 238, 163–170 (1987). [DOI] [PubMed] [Google Scholar]
- 21.Jenuwein T. & Allis CD Translating the histone code. Science 293, 1074–1080 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Chesne P. et al. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nature Biotechnol. 20, 366–369 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Wakayama T, Perry AC, Zuccotti M, Johnson KR & Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394, 369–374 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Mitalipov SM et al. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum. Reprod 22, 2232–2242 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Wakayama T, Tateno H, Mombaerts P. & Yanagimachi R. Nuclear transfer into mouse zygotes. Nature Genet. 24, 108–109 (2000). [DOI] [PubMed] [Google Scholar]
- 26.DiBerardino MA Nuclear and chromosomal behavior in amphibian nuclear transplants. Int. Rev. Cytol Suppl. 129–160 (1979). [DOI] [PubMed] [Google Scholar]
- 27.Egli D, Rosains J, Birkhoff G. & Eggan K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature 447, 679–685 (2007).Demonstrates that a genome can be exchanged during mitosis, resulting in developmental reprogramming.
- 28.Lemaitre JM, Danis E, Pasero P, Vassetzky Y. & Mechali M. Mitotic remodeling of the replicon and chromosome structure. Cell 123, 787–801 (2005).Shows that chromosome condensation is required to reprogramme the position of origins of replication.
- 29.Natale DA, Li CJ, Sun WH & DePamphilis ML Selective instability of Orc1 protein accounts for the absence of functional origin recognition complexes during the M–G1 transition in mammals. EMBO J. 19, 2728–2738 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu JR & Gilbert DM A distinct G1 step required to specify the Chinese hamster DHFR replication origin. Science 271, 1270–1272 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Cuvier O, Stanojcic S, Lemaitre JM & Mechali M. A topoisomerase II-dependent mechanism for resetting replicons at the S–M-phase transition. Genes Dev. 22, 860–865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romanowski P, Madine MA, Rowles A, Blow JJ & Laskey RA The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol 6, 1416–1425 (1996). [DOI] [PubMed] [Google Scholar]
- 33.McGrath J. & Solter D. Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science 220, 1300–1302 (1983). [DOI] [PubMed] [Google Scholar]
- 34.Modlinski JA & Smorag Z. Preimplantation development of rabbit embryos after transfer of embryonic nuclei into different cytoplasmic environment. Mol. Reprod. Dev 28, 361–372 (1991). [DOI] [PubMed] [Google Scholar]
- 35.Robl JM, Gilligan B, Critser ES & First NL Nuclear transplantation in mouse embryos: assessment of recipient cell stage. Biol. Reprod 34, 733–739 (1986). [DOI] [PubMed] [Google Scholar]
- 36.McGrath J. & Solter D. Inability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitro. Science 226, 1317–1319 (1984).Demonstrates that the exchange of nuclei between different cell types in interphase does not result in developmental reprogramming.
- 37.Cheong HT & Kanagawa H. Assessment of cytoplasmic effects on the development of mouse embryonic nuclei transferred to enucleated zygotes. Theriogenology 39, 451–461 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Tsunoda Y. et al. Full-term development of mouse blastomere nuclei transplanted into enucleated two-cell embryos. J. Exp. Zool 242, 147–151 (1987). [DOI] [PubMed] [Google Scholar]
- 39.Kono T. & Tsunoda Y. Development of single blastomeres from four- and eight-cell mouse embryos fused into the enucleated half of a two-cell embryo. Gamete Res. 22, 427–434 (1989). [DOI] [PubMed] [Google Scholar]
- 40.Willadsen SM Nuclear transplantation in sheep embryos. Nature 320, 63–65 (1986). [DOI] [PubMed] [Google Scholar]
- 41.Gao S. et al. Germinal vesicle material is essential for nucleus remodeling after nuclear transfer. Biol. Reprod 67, 928–934 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Prescott DM & Bender MA Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp. Cell Res 26, 260–268 (1962). [DOI] [PubMed] [Google Scholar]
- 43.Taylor JH Nucleic acid synthesis in relation to the cell division cycle. Ann. N. Y Acad. Sci 90, 409–421 (1960). [DOI] [PubMed] [Google Scholar]
- 44.Littau VC, Allfrey VG, Frenster JH & Mirsky AE Active and inactive regions of nuclear chromatin as revealed by electron microscope autoradiography. Proc. Natl Acad. Sci. USA 52, 93–100 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson TC & Holland JJ Ribonucleic acid and protein synthesis in mitotic HeLa cells. J. Cell Biol 27, 565–574 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouniol-Baly C. et al. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol. Reprod 60, 580–587 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Gottesfeld JM & Forbes DJ Mitotic repression of the transcriptional machinery. Trends Biochem. Sci 22, 197–202 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Sun F. et al. Nuclear reprogramming: the zygotic transcription program is established through an “erase-and-rebuild” strategy. Cell Res 17, 117–134 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Balbas MA, Dey A, Rabindran SK, Ozato K. & Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell 83, 29–38 (1995).Describes the extensive dissociation of transcriptional regulators from the DNA during mitosis.
- 50.Hershkovitz M. & Riggs AD Metaphase chromosome analysis by ligation-mediated PCR: heritable chromatin structure and a comparison of active and inactive X chromosomes. Proc. Natl Acad. Sci. USA 92, 2379–2383 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komura J. & Ono T. Disappearance of nucleosome positioning in mitotic chromatin in vivo. J. Biol. Chem 280, 14530–14535 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Boyd DC, Pombo A. & Murphy S. Interaction of proteins with promoter elements of the human U2 snRNA genes in vivo. Gene 315, 103–112 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Muchardt C, Reyes JC, Bourachot B, Leguoy E. & Yaniv M. The hbrm and BRG-1 proteins, components of the human SNF/SWI complex, are phosphorylated and excluded from the condensed chromosomes during mitosis. EMBO J 15, 3394–3402 (1996). [PMC free article] [PubMed] [Google Scholar]
- 54.Sif S, Stukenberg PT, Kirschner MW & Kingston RE Mitotic inactivation of a human SWI/SNF chromatin remodeling complex. Genes Dev. 12, 2842–2851 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schild C, Claret FX, Wahli W. & Wolffe AP A nucleosome-dependent static loop potentiates estrogen-regulated transcription from the Xenopus vitellogenin B1 promoter in vitro. EMBO J. 12, 423–433 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchenau P, Hodgson J, Strutt H. & Arndt-Jovin DJ The distribution of polycomb-group proteins during cell division and development in Drosophila embryos: impact on models for silencing. J. Cell Biol 141, 469–481 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voncken JW et al. Chromatin-association of the Polycomb group protein BMI1 is cell cycle-regulated and correlates with its phosphorylation status. J. Cell Sci 112, 4627–4639 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Miyagishima H. et al. Dissociation of mammalian Polycomb-group proteins, Ring1B and Rae28/Ph1, from the chromatin correlates with configuration changes of the chromatin in mitotic and meiotic prophase. Histochem. Cell Biol 120, 111–119 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Saurin AJ et al. The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J. Cell Biol 142, 887–898 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bannister AJ et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Lachner M, O’Carroll D, Rea S, Mechtler K. & Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Rea S. et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–599 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Kellum R, Raff JW & Alberts BM Heterochromatin protein 1 distribution during development and during the cell cycle in Drosophila embryos. J. Cell Sci 108, 1407–1418 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Minc E, Allory Y, Worman HJ, Courvalin JC & Buendia B. Localization and phosphorylation of HP1 proteins during the cell cycle in mammalian cells. Chromosoma 108, 220–234 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Terada Y. Aurora-B/AIM-1 regulates the dynamic behavior of HP1α at the G2–M transition. Mol. Biol. Cell 17, 3232–3241 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugimoto K, Tasaka H. & Dotsu M. Molecular behavior in living mitotic cells of human centromere heterochromatin protein HPLα ectopically expressed as a fusion to red fluorescent protein. Cell Struct. Funct 26, 705–718 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Murzina N, Verreault A, Laue E. & Stillman B. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4, 529–540 (1999). [DOI] [PubMed] [Google Scholar]
- 68.Hayakawa T, Haraguchi T, Masumoto H. & Hiraoka Y. Cell cycle behavior of human HP1 subtypes: distinct molecular domains of HP1 are required for their centromeric localization during interphase and metaphase. J. Cell Sci 116, 3327–3338 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Hsu JY et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102, 279–291 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Fischle W. et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438, 1116–1122 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Hirota T, Lipp JJ, Toh BH & Peters JM Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 438, 1176–1180 (2005). REFS 70 and 71 describe the mechanism that leads to the dissociation of HP1 from mitotic chromatin. [DOI] [PubMed] [Google Scholar]
- 72.Mateescu B, England P, Halgand F, Yaniv M. & Muchardt C. Tethering of HP1 proteins to chromatin is relieved by phosphoacetylation of histone H3. EMBO Rep. 5, 490–496 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters AH et al. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nature Genet. 30, 77–80 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Aagaard L, Schmid M, Warburton P. & Jenuwein T. Mitotic phosphorylation of SUV39H1, a novel component of active centromeres, coincides with transient accumulation at mammalian centromeres. J. Cell Sci 113, 817–829 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Stein R, Gruenbaum Y, Pollack Y, Razin A. & Cedar H. Clonal inheritance of the pattern of DNA methylation in mouse cells. Proc. Natl Acad. Sci. USA 79, 61–65 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nan X, Campoy FJ & Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997). [DOI] [PubMed] [Google Scholar]
- 77.Ng HH, Jeppesen P. & Bird A. Active repression of methylated genes by the chromosomal protein MBD1. Mol. Cell Biol 20, 1394–1406 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmad K. & Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Kuo MT, Iyer B. & Schwarz RJ Condensation of chromatin into chromosomes preserves an open configuration but alters the DNase I hypersensitive cleavage sites of the transcribed gene. Nucleic Acids Res. 10, 4565–4579 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarge KD & Park-Sarge OK Gene bookmarking: keeping the pages open. Trends Biochem. Sci 30, 605–610 (2005). [DOI] [PubMed] [Google Scholar]
- 81.John S. & Workman JL Bookmarking genes for activation in condensed mitotic chromosomes. Bioessays 20, 275–279 (1998). [DOI] [PubMed] [Google Scholar]
- 82.Plath K. et al. Developmentally regulated alterations in Polycomb repressive complex 1 proteins on the inactive X chromosome. J. Cell Biol 167, 1025–1035 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mak W. et al. Mitotically stable association of polycomb group proteins eed and enx1 with the inactive x chromosome in trophoblast stem cells. Curr. Biol 12, 1016–1020 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Allshire RC, Nimmo ER, Ekwall K, Javerzat JP & Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9, 218–233 (1995). [DOI] [PubMed] [Google Scholar]
- 85.Ekwall K. et al. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localisation of the chromo domain protein Swi6p and impair centromere function. J. Cell Sci 109, 2637–2648 (1996). [DOI] [PubMed] [Google Scholar]
- 86.Bhat KM et al. The GAGA factor is required in the early Drosophila embryo not only for transcriptional regulation but also for nuclear division. Development 122, 1113–1124 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Platero JS, Csink AK, Quintanilla A. & Henikoff S. Changes in chromosomal localization of heterochromatin-binding proteins during the cell cycle in Drosophila. J. Cell Biol 140, 1297–1306 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raff JW, Kellum R. & Alberts B. The Drosophila GAGA transcription factor is associated with specific regions of heterochromatin throughout the cell cycle. EMBO J. 13, 5977–5983 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen D, Hinkley CS, Henry RW & Huang S. TBP dynamics in living human cells: constitutive association of TBP with mitotic chromosomes. Mol. Biol. Cell 13, 276–284 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christova R. & Oelgeschlager T. Association of human TFIID-promoter complexes with silenced mitotic chromatin in vivo. Nature Cell Biol. 4, 79–82 (2002). [DOI] [PubMed] [Google Scholar]
- 91.Segil N, Guermah M, Hoffmann A, Roeder RG & Heintz N. Mitotic regulation of TFIID: inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev. 10, 2389–2400 (1996). [DOI] [PubMed] [Google Scholar]
- 92.Lanctot C, Cheutin T, Cremer M, Cavalli G. & Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nature Rev. Genet 8, 104–115 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Bolzer A. et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 3, e157 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iborra FJ, Pombo A, Jackson DA & Cook PR Active RNA polymerases are localized within discrete transcription ‘factories’ in human nuclei. J. Cell Sci 109, 1427–1436 (1996). [DOI] [PubMed] [Google Scholar]
- 95.Chubb JR, Boyle S, Perry P. & Bickmore WA Chromatin motion is constrained by association with nuclear compartments in human cells. Curr. Biol 12, 439–445 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Walter J, Schermelleh L, Cremer M, Tashiro S. & Cremer T. Chromosome order in HeLa cells changes during mitosis and early G1, but is stably maintained during subsequent interphase stages. J. Cell Biol. 160, 685–697 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddy KL, Zullo JM, Bertolino E. & Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 452, 243–247 (2008).Demonstrates that mitosis can mediate repositioning of a locus within the nucleus, resulting in a change in the state of gene expression.
- 98.Kosak ST et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296, 158–162 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Tumbar T. & Belmont AS Interphase movements of a DNA chromosome region modulated by VP16 transcriptional activator. Nature Cell Biol. 3, 134–139 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Goldman RD et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 101, 8963–8968 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scaffidi P. & Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nature Cell Biol. 10, 452–459 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knoblich JA Asymmetric cell division during animal development. Nature Rev. Mol. Cell Biol 2, 11–20 (2001). [DOI] [PubMed] [Google Scholar]
- 103.Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nature Rev. Mol. Cell Biol 9, 355–366 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Shao Z. et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37–46 (1999). [DOI] [PubMed] [Google Scholar]
- 105.King IF, Francis NJ & Kingston RE Native and recombinant polycomb group complexes establish a selective block to template accessibility to repress transcription in vitro. Mol. Cell Biol 22, 7919–7928 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zink D. & Paro R. Drosophila Polycomb-group regulated chromatin inhibits the accessibility of a trans-activator to its target DNA. EMBO J. 14, 5660–5671 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eggan K. et al. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl Acad. Sci. USA 98, 6209–6214 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Humpherys D. et al. Abnormal gene expression in cloned mice derived from embryonic stem cell and cumulus cell nuclei. Proc. Natl Acad. Sci. USA 99, 12889–12894 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boiani M, Eckardt S, Scholer HR & McLaughlin KJ Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 16, 1209–1219 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blelloch R. et al. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells 24, 2007–2013 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kishigami S. et al. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun 340, 183–189 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Rybouchkin A, Kato Y. & Tsunoda Y. Role of histone acetylation in reprogramming of somatic nuclei following nuclear transfer. Biol. Reprod 74, 1083–1089 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Gurdon JB & Colman A. The future of cloning. Nature 402, 743–746 (1999). [DOI] [PubMed] [Google Scholar]
- 114.Lanza RP, Cibelli JB & West MD Human therapeutic cloning. Nature Med. 5, 975–977 (1999). [DOI] [PubMed] [Google Scholar]
- 115.Niwa H, Miyazaki J. & Smith AG Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature Genet. 24, 372–376 (2000). [DOI] [PubMed] [Google Scholar]
- 116.Masui S. et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nature Cell Biol. 9, 625–635 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Takahashi K. & Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).The forced expression of embryonic transcription factors can change the transcriptional programme and the developmental potential from somatic to embryonic.
- 118.Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).A milestone in cellular reprogramming: human pluripotent stem cells can be generated just like in the mouse (see REF. 117).
- 119.Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). [DOI] [PubMed] [Google Scholar]
- 120.Park IH et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Wernig M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Okita K, Ichisaka T. & Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 160–162 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Maherali N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007). [DOI] [PubMed] [Google Scholar]
- 124.Hanna J. et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133, 250–264 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cobaleda C, Jochum W. & Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449, 473–477 (2007).Shows that the differentiated state of B cells requires the presence of the transcription factor PAX5.
- 126.Weintraub H. et al. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc. Natl Acad. Sci. USA 86, 5434–5438 (1989).Shows that the ectopic expression of a transcription factor of a differentiated cell can lead to trans-differentiation.
- 127.Blau HM et al. Plasticity of the differentiated state. Science 230, 758–766 (1985).Demonstrates the induction of muscle-gene expression after fusion of a non-muscle cell to a muscle cell.
- 128.De Robertis EM & Gurdon JB Gene activation in somatic nuclei after injection into amphibian oocytes. Proc. Natl Acad. Sci. USA 74, 2470–2474 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kikyo N, Wade PA, Guschin D, Ge H. & Wolffe AP Active remodeling of somatic nuclei in egg cytoplasm by the nucleosomal ATPase ISWI. Science 289, 2360–2362 (2000). [DOI] [PubMed] [Google Scholar]
- 130.Gurdon JB, Laskey RA & Reeves OR The developmental capacity of nuclei transplanted from keratinized skin cells of adult frogs. J. Embryol. Exp. Morphol 34, 93–112 (1975). [PubMed] [Google Scholar]
- 131.Holtzer H. et al. Lineages, quantal cell cycles, and the generation of cell diversity. Q. Rev. Biophys 8, 523–557 (1975). [DOI] [PubMed] [Google Scholar]
- 132.Pavlath GK & Blau HM Expression of muscle genes in heterokaryons depends on gene dosage. J. Cell Biol 102, 124–130 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cowan CA, Atienza J, Melton DA & Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 (2005).The first demonstration that the differentiated state of human cells can be reverted to an embryonic state.
- 134.Tada M, Takahama Y, Abe K, Nakatsuji N. & Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol 11, 1553–1558 (2001). [DOI] [PubMed] [Google Scholar]
- 135.Jaenisch R. & Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han DW et al. Pluripotential reprogramming of the somatic genome in hybrid cells occurs with the first cell cycle. Stem Cells 26, 445–454 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Stadtfeld M, Maherali N, Breault DT & Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230–240 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brambrink T. et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feinberg AP Phenotypic plasticity and the epigenetics of human disease. Nature 447, 433–440 (2007). [DOI] [PubMed] [Google Scholar]
- 140.Jones PA & Baylin SB The epigenomics of cancer. Cell 128, 683–692 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lanza RP et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 288, 665–669 (2000).Demonstrates that the process of ageing can be reverted after nuclear transfer into an unfertilized oocyte.
- 142.Bernstein BE, Meissner A. & Lander ES The mammalian epigenome. Cell 128, 669–681 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Henikoff S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nature Rev. Genet 9, 15–26 (2008). [DOI] [PubMed] [Google Scholar]
- 144.Costanzi C. & Pehrson JR Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393, 599–601 (1998). [DOI] [PubMed] [Google Scholar]
- 145.Bannister AJ & Kouzarides T. Reversing histone methylation. Nature 436, 1103–1106 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Matsuo K. et al. An embryonic demethylation mechanism involving binding of transcription factors to replicating DNA. EMBO J 17, 1446–1453 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kress C, Thomassin H. & Grange T. Active cytosine demethylation triggered by a nuclear receptor involves DNA strand breaks. Proc. Natl Acad. Sci. USA 103, 11112–11117 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kangaspeska S. et al. Transient cyclical methylation of promoter DNA. Nature 452, 112–115 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Metivier R. et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 452, 45–50 (2008). [DOI] [PubMed] [Google Scholar]
- 150.Ringrose L. & Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu. Rev. Genet 38, 413–443 (2004). [DOI] [PubMed] [Google Scholar]
- 151.Orlando V. Polycomb, epigenomes, and control of cell identity. Cell 112, 599–606 (2003). [DOI] [PubMed] [Google Scholar]
- 152.Schwartz YB & Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nature Rev. Genet 8, 9–22 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Simon JA & Tamkun JW Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes. Curr. Opin. Genet. Dev 12, 210–218 (2002). [DOI] [PubMed] [Google Scholar]
- 154.Francis NJ & Kingston RE Mechanisms of transcriptional memory. Nature Rev. Mol. Cell Biol 2, 409–421 (2001). [DOI] [PubMed] [Google Scholar]
- 155.Simon J, Chiang A. & Bender W. Ten different Polycomb group genes are required for spatial control of the AbdA and AbdB homeotic products. Development 114, 493–505 (1992). [DOI] [PubMed] [Google Scholar]
- 156.Juergens G. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature 316, 153–155 (1985). [Google Scholar]
- 157.Fischle W. et al. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 17, 1870–1881 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poux S, Horard B, Sigrist CJ & Pirrotta V. The Drosophila trithorax protein is a coactivator required to prevent re-establishment of polycomb silencing. Development 129, 2483–2493 (2002). [DOI] [PubMed] [Google Scholar]
- 159.Klymenko T. & Muller J. The histone methyltransferases Trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins. EMBO Rep. 5, 373–377 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wendt KS et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451, 796–801 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Voncken JW et al. MAPKAP kinase 3pK phosphorylates and regulates chromatin association of the polycomb group protein Bmi1. J. Biol. Chem 280, 5178–5187 (2005). [DOI] [PubMed] [Google Scholar]
- 162.Wissmann M. et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptordependent gene expression. Nature Cell Biol. 9, 347–353 (2007). [DOI] [PubMed] [Google Scholar]
- 163.Yamane K. et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125, 483–495 (2006). [DOI] [PubMed] [Google Scholar]
- 164.Agalioti T. et al. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell 103, 667–678 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.