Abstract
Objective:
Weight regain (WR) after Roux en-Y Gastric Bypass surgery (RYGB) starts to occur two years after surgery, ultimately affecting at least 25% of patients. A limited number of studies have evaluated the impact of anti-obesity medications (AOM) on this phenomenon.
Methods:
We reviewed the electronic medical records (EMR) of 1196 patients who had RYGB between 2004 and 2015. WR was evaluated by comparing each patient’s weight during subsequent post-operative office visits to nadir weight (lowest weight after RYGB, n=760), taking into consideration the interval during which WR occurred. Patients who were prescribed AOM and came to follow-up visits were classified as adherent users, while those who missed their follow-up visits were considered non-adherent. We used linear mixed model, Cox regression, and generalized equation estimator to determine the impact of AOM on WR trajectory, hazards ratio for time to event, and odds ratio for repeated event occurrence, respectively.
Results:
Despite lack of a unified protocol for using AOM, the three statistical models converged to show that phentermine and topiramate, used individually or in combination, can significantly reduce WR after RYGB.
Conclusions:
Phentermine and topiramate are effective in mitigating WR after RYGB. Further studies are needed to help ascertain optimal use of AOM after bariatric surgery.
Keywords: Weight Regain, Bariatric Surgery, Weight Loss Medications
Introduction
Bariatric surgery has evolved as a main treatment strategy for patients with severe obesity, especially in the presence of chronic comorbid conditions such as Type 2 diabetes (T2D) and obstructive sleep apnea (OSA). One attribute that has increased the popularity of surgical management of obesity is the expectation of durable weight loss, especially for patients with a history of weight-cycling. However, several studies have identified an increasing prevalence of weight regain (WR) and subsequent reemergence of obesity-related comorbidities after bariatric surgery (1, 2). The lack of a standard measure of WR after bariatric surgery has led to different methods of literature reporting such as percentage of pre-surgery weight, nadir weight, or maximum weight lost. It is estimated that 5 years following Roux-en-Y Gastric Bypass surgery (RYGB), approximately one-half of patients would have regained 15% of the nadir weight, and two-thirds would have regained more than 20% of the weight lost (2). Given the increasing number of bariatric surgery procedures being performed, the unfavorable impact of WR and the potential for recurrence of metabolic sequelae are likely to be substantial.
We recently showed that, in an 11-year cross-sectional analysis of our ethnically diverse patient population undergoing RYGB, mean WR relative to nadir was approximately 10%, with the highest quartile showing WR in excess of 14.3% of nadir weight. These estimates, which were based on the most recent office visit for each patient, represent a regain of more than 24% in one-half of the patients and more than 45% of the weight lost in the highest quartile. Significant factors affecting WR following RYGB were race, age, and pre-surgical body mass index (BMI).(3)
This retrospective study examined the utility of anti-obesity medications (AOM) in treating WR in the same multi-ethnic patient population after RYGB. We used linear mixed models to evaluate trajectories of weight change relative to the patients’ nadir weight. As in our previous study, we classified the rates of WR by quartiles and evaluated the impact of AOM using three different statistical modeling methodologies. The results of these analyses converge to show that AOM could play an important role in mitigating WR after RYGB. Although WR has been shown to occur after both sleeve gastrectomy and RYGB, our center is similar to others in that the RYGB population span a longer follow-up period since the sleeve gastrectomy became popular more recently. Therefore, we restricted our analysis to those patients who underwent RYGB during this time period.
Methods
Patients
This retrospective study used the electronic medical record (EMR) data of adult patients who had undergone bariatric surgery, specifically RYGB, at Boston Medical Center (BMC) between 2004 and 2015. This study was approved by the Boston Medical Center and Boston University Medical Campus Institutional Review Board under protocol H-26417.
Clinical Data
Race and ethnicity were self-identified as white, black/African-American, Hispanic, Asian, American Indian/Native American, Native Hawaiian/Pacific Islander, or not available/declined information. Data were extracted by the Boston University Clinical Data Warehouse including age at the time of surgery, date of enrollment in the bariatric program, date of the surgery, weight, height, and BMI.
We reviewed data from all patients who had bariatric surgery at BMC during this period (n=1516). We limited our study to patients who had RYGB from the three most common racial and ethnic groups in our population (n=1196): African American (AA), Hispanic (HA), and Caucasian (CA). Patients who became pregnant after surgery or underwent surgical revisions were excluded. Pre-surgical medical ICD-9 codes were used to identify comorbid conditions including T2D (250 and 790.29), osteoarthritis (OA) (715.9 and 716.9), hypertension (HTN) (401.1, 401.9 and 796.2), dyslipidemia (DLD) (272), and OSA (780.57, 780.53 and 327.23).
The zip code for each patient was used to estimate the socioeconomic status (SES) based on the zip code median income. Data on the median income by zip code in 2014 were obtained from the United States Census Bureau (4). Patients were classified into SES quartiles based on the zip code median income. Use of AOM was determined from the date of prescription of any of the following medications: phentermine, topiramate, lorcaserin (Belviq®), phentermine/topiramate (Qsymia®), and naltrexone/bupropion (Contrave®). Patients were classified as “adherent” if they showed up for an office visit within 60 days of the date of the prescription; those patients who did not return for an office visit within this interval were classified as “non-adherent”, due to the likelihood that prescriptions needed to be refilled at these missed visits.
Statistical analyses
The primary objective for this study was to determine the effect of AOM on long-term WR. A linear mixed model (treating subjects as a random effect) was used to examine AOM, race, sex, time, and presence of comorbid conditions as fixed effects on weight change. Age and pre-surgery BMI were included as covariates. Seven time-intervals were used in these analyses: pre-surgery, 1-2 years, 2-3 years, 3-4 years, 4-5 years, 5-6 years, and beyond 6 years post-surgery.
Patients who had not yet achieved nadir weight (n=436) were excluded from the following calculations. We calculated weight loss (WL) as the difference between the pre-surgical weight and the nadir weight for each subject (n=760). We defined WR as the difference between the weight obtained at each subsequent office visit following the nadir date and the nadir weight in kilograms. WR was evaluated relative to amount of weight lost (WR/WL, expressed as %) and relative to nadir (WR/nadir, expressed as %) for each patient based on the recorded weights at each office visit after nadir. Hence, WR/WL and WR/nadir are both repeated measures for each subject.
The rate of WR was calculated based on both WR/WL and WR/nadir at each subsequent weight measurement relative to the time elapsed since nadir (reported as % per 30-day interval). Differences in continuous variables between categorical groups were assessed by ANOVA. Categorical outcomes were assessed by cross tabulation and chi-squared distribution analysis. Multinomial logistic regressions were used to compare groups with more than two categorical outcomes across groups.
The 25%, 50% and 75% of the WR/nadir weight distribution based upon the last observed weight were used to define thresholds and group patients into quartiles of low, moderate, and high rates of WR. We performed a Cox regression model to determine the proportional hazard ratio (HR) of WR at each of these three thresholds. Hence, event occurrence was defined as the time to first occurrence for a patient that WR/nadir exceeded each specific cut-off threshold corresponding to 25th, 50th, and 75th WR/nadir distribution (WR/nadir of 1.49%, 6.25%, and 14.29%, respectively). Race, sex, SES, and each comorbid condition were entered individually into the Cox regression as categorical predictors; age and weight loss (WL) were entered as continuous predictors. The final multivariable Cox regression included all the factors with p values <0.05. Because AOM were prescribed intermittently, we further analyzed the rates of WR at each visit as “repeated exposures” using Generalized Equation Estimator (GEE) with an independent correlation structure to correct within-subject correlation. The odds ratio for occurrence of an event was determined by binomial logistic regression analysis. We used specific WR rates as “events” based on the quartile distribution of all WR rates for the study population, as described above. All data analyses were performed using SPSS version 25.
Results
A summary of the clinical characteristics of patients who achieved nadir weight and are at risk of WR post RYGB is presented in Table 1. Of the 760 in this cohort, 350 (46.1%) were documented users of AOM. Of the AOM users, 119 (34.0%) were prescribed phentermine alone, 74 used topiramate alone (21.1%), and 154 (44.0 %) were prescribed a combination of phentermine and topiramate. Only three patients (0.9%) were prescribed lorcaserin and none were prescribed bupropion/naltrexone generic or brand name or brand name phentermine/topiramate. Liraglutide 3.0 was not an available option at the time. There were no significant differences in pre-surgery BMI and prevalence of the comorbidities included in the analysis between the AOM users and non-users. There were more AOM users among HA and AA patients than among CA. Relative to CA, the odds ratio for AA to be prescribed AOM was 2.42 (CI 1.74-3.36, p<0.001) and 2.85 (CI 1.85-4.39, p<0.001) for HA. Patients in the lowest SES category were more likely to be prescribed AOM (OR 1.90, CI 1.26-2.87, P=0.002).
Table 1:
Characteristics of patients who achieved nadir weight stratified by AOM use
| None | Adherent | Non-adherent | Total | Sig | ||
|---|---|---|---|---|---|---|
| N | 410 | 157 | 193 | 760 | ||
| Age, y | 47.0±0.5 | 44.2±0.8 | 45.2±0.9 | 45.9±0.4 | 0.007 | |
| Female, % | 76.1 % | 91.1 % | 81.9 % | 80.7% | 0.000 | |
| Pre-surgical weight, kg | 92.4±2.6 | 100.3±3.9 | 90.5±4.0 | 93.6±1.9 | 0.18 | |
| Pre-surgical BMI, kg/m2 | 46.8±0.4 | 46.5±0.7 | 47.2±0.7 | 47.8±0.3 | 0.77 | |
| Race | ||||||
| Caucasian American (%) | 44.6 | 39.5 | 65.1 | 54.6 | 0.000 | |
| African American (%) | 24.4 | 40.1 | 36.8 | 30.8 | ||
| Hispanic American (%) | 10.5 | 20.4 | 18.7 | 14.6 | ||
| SES, % | 1 | 23.2 | 33.8 | 30.4 | 27.2 | 0.009 |
| 2 | 27.7 | 33.8 | 25.7 | 28.5 | ||
| 3 | 21.9 | 18.8 | 19.4 | 20.6 | ||
| 4 | 27.2 | 13.6 | 24.6 | 23.7 | NS | |
| T2D, % | 54.1 | 62.4 | 56.0 | 56.3 | NS | |
| Dyslipidemia, % | 46.3 | 48.4 | 28.0 | 42.1 | NS | |
| Hypertension, % | 69.5 | 70.7 | 64.8 | 68.6 | NS | |
| OSA, % | 36.1 | 35.0 | 37.3 | 36.2 | NS | |
| Osteoarthritis, % | 10.7 | 8.9 | 12.4 | 10.8 | NS |
Data are mean values ±SE
Socioeconomic status (SES): 1, < $47,297; 2, $47,297-$60,774; 3, $60,775-$76,924; 4, > $76,924;
T2D, type 2 diabetes; OSA, obstructive sleep apnea
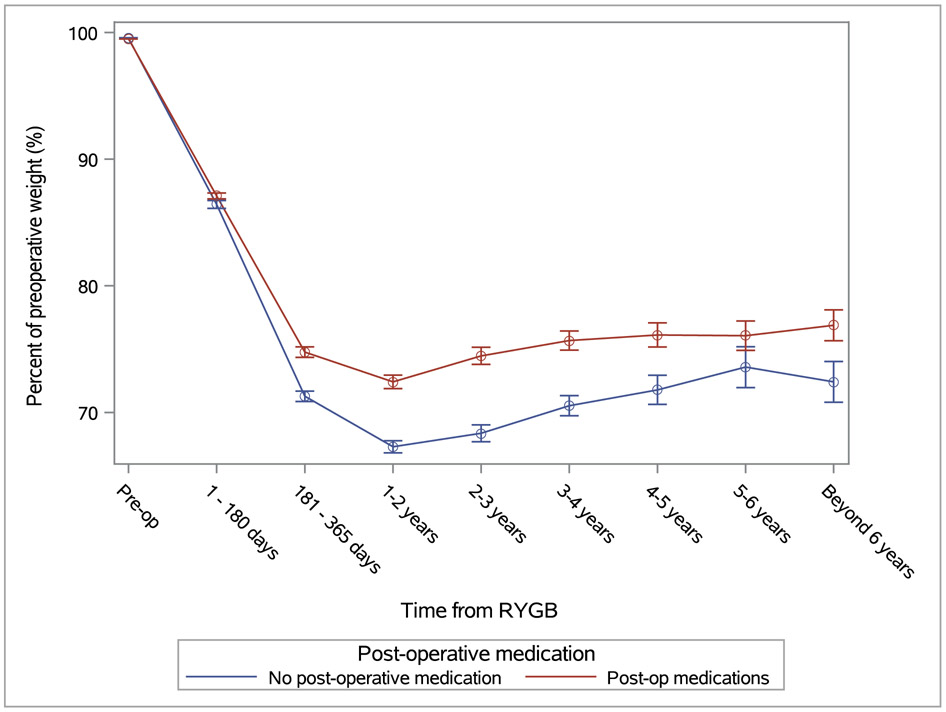
The weight trajectories for patients who used AOM are compared to non-AOM users in Figure 1. In the mixed model analysis, patients who used AOM had achieved less weight loss at nadir (p<0.0001) than patients who did not use AOM after bariatric surgery. Figure 1 also suggests that users of AOM had a smaller upward slope compared to non-users, implying slower WR in the former group during the observation period up to 11 years. However, the interaction of time x AOM use was not statistically significant, suggesting that the pattern of WR was similar in the two groups. Therefore, while AOM (phentermine and topiramate) were more likely to be prescribed in patients who had sub-optimal weight loss, their effectiveness in mitigating WR could not be determined solely from weight trajectory of the current cohort.
Figure 1:
Trajectory of changes in body weight by use of AOM. Weights are shown as percent of the immediate preoperative weight. Numbers of patients at pre-op, 1-180 days, 181 – 365 days, 1-2 years, 2-3 years, 3-4 years, 4-5 years, 5-6 years, beyond 6 years: overall=1196, 1183, 1090, 1078, 859, 763, 515, 373, 250; adherent=159, 158, 155, 155, 142, 136, 122, 105, 87; non-adherent=252, 250, 250, 259, 219, 203, 146, 98, 51; None=785, 775, 685, 673,498, 424, 247, 170, 112.
In order to further evaluate the effect of AOM on WR, we determined the increase in weight relative to nadir at the last available weight for each patient. These estimates of WR (percent of nadir weight) were then classified by quartiles. Cutoff points for the 25th, 50th and 75th percentiles of WR were 1.5%. 6.25%, and 14.3% relative to nadir, respectively. As a percentage of the amount of weight lost, WR was 1.8±0.2%, 17.2±0.8%, 30.3±1.4%, and 41.1±5.1%, respectively for the four quartiles. Thus, 50% of the patients (upper two quartiles) regained at least 1/3 of the weight they had previously lost. The clinical characteristics of these quartiles of WR were published previously (3). Briefly, the quartiles were similar in age and sex distribution; significant differences were noted in race, presence of T2D, and use of AOM. The upper two quartiles were more likely to include AA than CA (likelihood ratio, 1.553; CI, 1.023-2.358; p= 0.039, in the 3rd quartile; and 2.154; CI, 1.37-3.385, p = 0.001, in the 4th quartile). The quartile with highest WR was also more likely to include patients with T2D (likelihood ratio 1.689, CI 1.114-2.571, p=0.013) and patients who were prescribed AOM but were identified to be non-adherent (likelihood ratio relative to patients without AOM prescription 1.683, CI 1.041-2.557, p=0.033).
Since WR increases with time, differences between the quartiles could be explained by the duration of the observation period during which WR was assessed (the last office visit for each patient). Patients in the highest quartile of total WR were more likely to have had RYGB earlier (years post-procedure 4.3±0.2, 3.1±0.1, 4.3±0.2, and 5.5±0.2 for the lowest to highest quartile, respectively P<0.0001). Therefore, we normalized the WR/nadir as monthly rates. The corresponding cutoff WR rates (% per 30 days) were 0.18%, 0.58%, and 1.22%.
We used three statistical models to evaluate the impact of AOM on monthly WR rates for each of the quartile cutoff values shown. In the first model, a linear mixed model, adjusted for age, sex, race, and SES, was used to evaluate WR progression. Differences in WR between AOM users and non-users were statistically significant only in the highest (most rapid) WR quartile. The results of the subgroup of patients who regained weight relative to nadir at a rate equal or greater than 1.22% per month (highest WR quartile) are presented in Figure 2. Weight regain progression was smaller in the group who were prescribed AOM and were classified as adherent when compared to the non-adherent or non-users of AOM (p=0.012). By the end of the observation period, WR relative to nadir was approximately 10% lower among adherent AOM users in comparison to non-adherent users.
Figure 2:
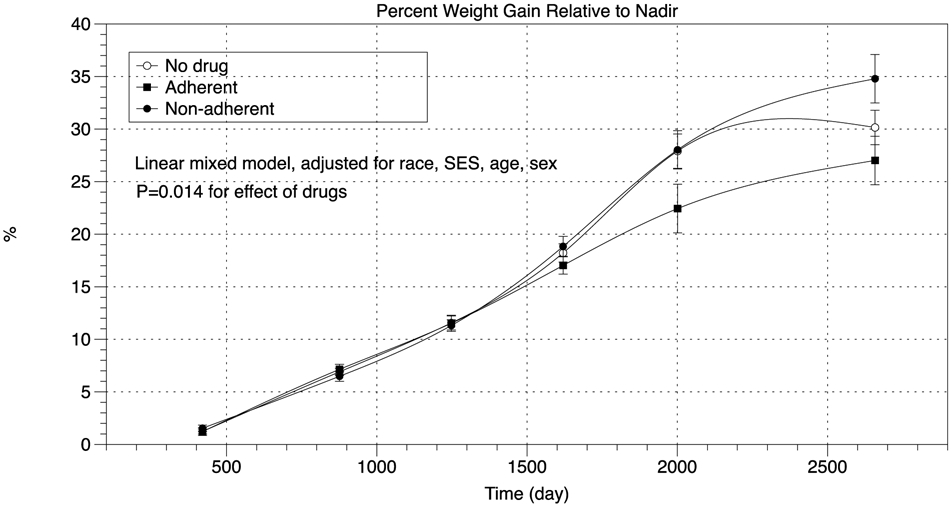
Trajectory of total weight regain in the highest quartile. Data are shown as percent of nadir weight. Highest quartile is defined by a rate of weight regain equivalent to 1.22% of nadir weight per 30-day-interval.
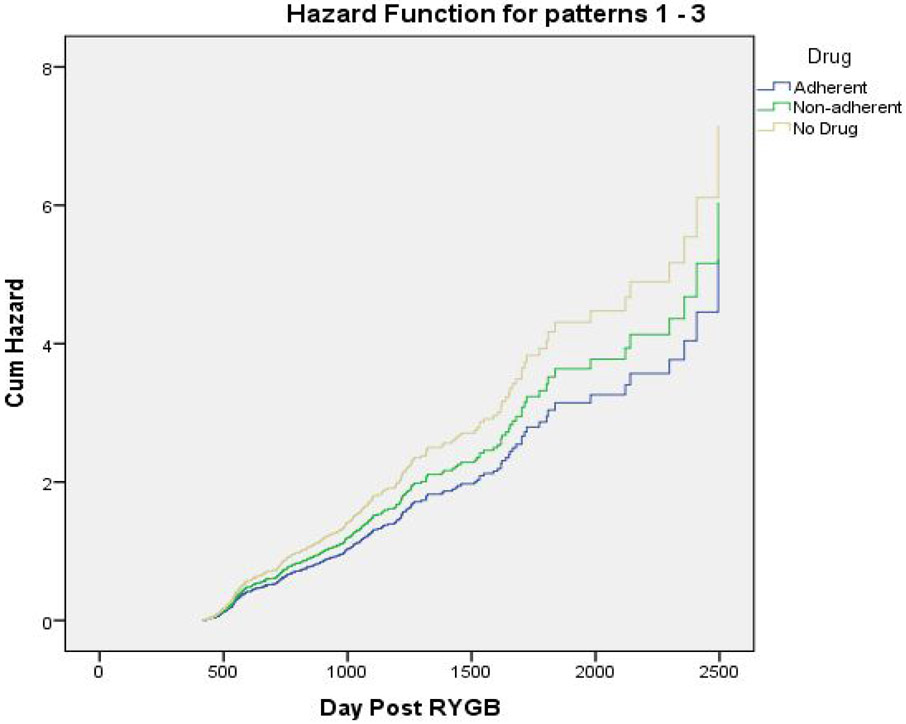
In the second model, we evaluated whether AOM can impact the occurrence of WR/nadir of at least 1.22% per month. The results of Cox regression analysis are presented in Figure 3 showing a proportional hazards ratio of 0.729 (CI 0.556-0.957, p=0.023) for the adherent AOM users compared to the non-users of AOM. Patients who were prescribed AOM but classified as non-adherent had a proportional hazards ratio of 0.844 (CI 0.659-1.081, P=0.18). Similar analyses for the lower three quartiles of WR did not reveal statistically significant differences.
Figure 3:
Cox regression for WR relative to nadir normalized for a 30-day interval stratified by AOM use. Data are for the top quartile (WR equal or greater than 1.22% of nadir weight).
It is important to note that use of AOM in this patient population was intermittent since patients were likely to discontinue the medications and resume it at subsequent times. The decision to prescribe and when to prescribe AOM were variable between providers and patients. Thus, multiple exposures to AOM occurred “randomly” per patient during the observation interval. Therefore, to further evaluate the effectiveness of AOM, we used the GEE approach with an independent correlation structure to correct within-subject correlation. In a binary logistic model, adjusted for age, sex, race, and SES, the odds ratio for occurrence of WR of 1.22% per month or greater relative to nadir was 0.579 (CI 0.371-0.877, P=0.01) in the adherent AOM group and 0.0.593-1.284, P=0.5) compared to the non-AOM users. These results imply that AOM effectively reduced the risk of recurrent bouts of rapid WR in our patient population.
Discussion
This large retrospective study of WR after RYGB suggests that the use of AOM and adherence to those medications is effective in mitigating WR after RYGB. Our results relate specifically to the use of phentermine and topiramate, either individually or in combination. We are unable to compare the effectiveness of other AOM due to the small numbers prescribed to this cohort at the time of analysis. The analytical approach in the current study focuses on the progression of body weight changes throughout an 11-year observation period in an ethnically diverse patient population. While it is conventional to evaluate the effectiveness of AOM by the amount of weight lost, this approach was not applicable in the current study. There were no specific clinical parameters that guided the providers’ prescriptions of AOM; and in most cases, the decision to take or withhold AOM was effectively a random event. Therefore, to overcome this limitation, we used three independent statistical models, which consistently demonstrated that AOM decrease cumulative WR by about 10% relative to nadir weight and reduce the odds of rapid WR after RYGB. As detailed elsewhere, rapid WR in our cohort is defined as a rate of increase in body weight of 1.22% per month relative to nadir weight (3). These results take into account that patients typically lost weight when taking AOM and regained weight during intervals when the medications were not prescribed; and that patients in the current study started AOM at different stages and used them over variable and intermittent periods. Therefore, controlled prospective clinical trials are needed to further evaluate the impact of AOM on weight loss parameters and long-term weight outcomes after bariatric surgery procedures.
Wider awareness of the clinical implications associated with obesity has increased the acceptance of surgical interventions. The beneficial effects of RYGB on T2D are clear and studies with only 1-2 years of follow-up have purported that RYGB is associated with long-term weight stability. However, recent reports of WR after RYGB highlight the need for adjunct treatment strategies to maximize the long-term benefit and reduce the recurrence risk of comorbid conditions. Estimated proportions of patients who have “significant” WR have varied from 17-30% two years from RYGB (5-8), to 59% in one small study with greater than 20% WR at least one year from RYGB (9) to 79% by self-report.(10). These estimates depend upon the definition of “significant” WR, the duration of the study, and the method of reporting WR (11-14). Without a standard definition of WR, it is difficult to compare data from different studies. For example, in the Swedish Obese Subjects (SOS) study, long-term weight outcomes are reported as weight changes relative to the pre-surgical weight. (15-18) This approach emphasizes the beneficial long-term outcomes of bariatric surgery since it continues to show significant “weight loss” 10 years following the surgical procedure. However, data presented as such do not provide clear and direct measures of the amount of WR. A discussion of the different ways of reporting WR and how they relate to clinical outcomes is presented by King et al (2). We have previously argued that since WR is the consequence of a specific physiological process characterized by a state of positive energy balance, it is better evaluated in terms of actual weight increase rather than by a decrease in previously achieved weight loss. Thus, we regard WR/nadir as a more clinically relevant parameter of the metabolic conditions associate with WR in the post RYGB phase. Because total WR increases with time, we determined WR/nadir as a rate per 30-days to normalize for differences in observation intervals between groups. Further details characterizing WR in our patient population are presented elsewhere.(3)
It is important to emphasize that the relapse of obesity is better defined by the recurrence of clinically significant co-morbid conditions rather than WR itself. Long-term observations, such as those from the SOS study consistently document the beneficial impact of bariatric surgery on mortality, diabetes, and vascular disease (16, 17, 19, 20). The relationship between WR and recurrence of specific co-morbidities remains unclear. While the SOS study has associated diabetes remission with the degree of weight loss at 2 and 10 years after surgery (21), it is difficult to evaluate the impact of WR from that study due to the specific way data are reported, as noted above. However, the potential for relapse is concerning and has prompted additional surgical procedures (“revisional surgery” (22)) aiming to increase malabsorption (“distalization”) (23), increase the restriction at the gastro-jejunal anastomosis (24), sclerotherapy (25) and to correct anatomical defects such as gastro-gastric fistula (26). Unfortunately, position statements and guidelines related to the implementation of surgical revisions continue to rely on expert opinion (27) rather than on controlled prospective studies. Thus, non-surgical interventions remain crucial for counteracting the growing problem of WR following bariatric surgery.
Interventions targeting disordered or maladaptive eating patterns such as night eating, grazing, and nibbling behaviors, which are widely reported among patients following bariatric surgery (6, 28, 29) have been effectively utilized to help mitigate WR (30). However, utilization of AOM has been limited. One possible reason is that many patients are more likely to be followed up by bariatric surgeons who have limited experience in the use of anorexigenic pharmacotherapy. Additionally, guidelines for use of AOM after bariatric surgery have not been established, thus leading to skepticism about their utility. Several possible physiological changes could potentially impact the effectiveness of AOM after bariatric surgery. For example, changes in the regulation of satiety and the pre-existing history of substantial weight loss after bariatric surgery could alter the effectiveness of AOM. The current study further confirms that AOM remain effective and are useful tools to help temper the brunt of WR among patients with history of RYGB, and potentially other bariatric surgery procedures. In our own experience, post-surgical AOM use in our clinic had been very limited prior to 2009 and had increased approximately four-fold by 2015 when more than 10% patients were prescribed phentermine, topiramate, or a combination of these two medications during post-surgery office visits.
Few other studies have demonstrated that AOM remain effective after bariatric surgery (31-36). Compared to the prior studies cited, ours is the largest retrospective study to date and one that features an ethnic underserved patient population. It is important to note the unique setting of this retrospective study. As previously described in more detail elsewhere (3), race is an important determinant of WR after RYGB with AA being at high risk. In our center, we typically find that AA and HA are more likely to be prescribed AOM than CA after RYGB, presumably because of their higher propensity to WR. Thus, it is possible that higher rates of WR among AA and HA could have influenced decisions to prescribe AOM in our patient population. However, these prescriptions were not based on pre-determined criteria or algorithms, but rather were initiated by individual patient-physician interactions during routine office visits. Differences in AOM prescription practices between providers could further contribute to the variance in the clinical outcomes in the current study. However, despite these drawbacks, we are able to show that AOM are important tools in the long-term management of patients with obesity and WR following RYGB and work as effectively as they do in medically managed patients with obesity. We also note that our previous findings of racial disparities of WR after RYGB may have been tempered by the higher rate of AOM prescribed to AA and HA in our patient population.
There are few prospective trials investigating “rescue” pharmacotherapy with AOM following bariatric surgery with only six published to date with small numbers of patients (37-43). One of the studies used an AOM which has since been withdrawn from the market, fenfluramine (40), and two other trials used AOM after the adjustable laparoscopic gastric band which is rarely used today (41, 42). The advent of larger randomized controlled clinical trials evaluating the use of single and combination AOM as rescue therapy for WR after bariatric surgery will be crucial to the development of treatment guidelines. The judicious use of multimodal medical and surgical treatment (43) for extreme obesity will further promote the advancement of the field of obesity medicine and mitigate the rising prevalence of this disease worldwide.
Strengths of this study are its large multi-ethnic patient population and its duration of 11 years. Details pertaining to factors that contribute to WR after RYGB have been already published. Its limitations include the retrospective nature of clinical observations, the lack of a unified protocol for use of AOM, that the data pertain only two AOM, and the randomness of decisions made by providers and patients to use or abstain from AOM.
Conclusion
Bariatric surgery is the most effective treatment strategy to combat obesity and achieve long-term resolution and/or improvement of most cardiometabolic dysfunction associated with it. However, as a chronic disease, obesity is subject to relapse years after bariatric surgery and could require further medical interventions. Here we show that phentermine and topiramate could play a role in the management of weight relapse after RYGB. However, the full potential of these agents and newer AOM to counter weight recidivism and prevent the recurrence of obesity-related comorbidities needs to be further explored in prospective clinical trials. Guidelines for initiating and monitoring the potential long-term use of AOM after bariatric surgery need to be established.
STUDY IMPORTANCE QUESTIONS.
What is already known about this subject?
The problem of weight regain after bariatric surgery is increasingly being recognized.
Tools to reduce the impact of weight regain after bariatric surgery are needed.
Anti-obesity medications have not been extensively utilized in patients with history of bariatric surgery.
What are the new findings in your manuscript?
Phentermine and topiramate alter the trajectory of weight regain after RYGB.
Phentermine and topiramate reduce the occurrence of rapid weight regain after RYGB.
How might your results change the direction of research or the focus of clinical practice?
Prospective clinical trials are needed to establish guidelines for use of anti-obesity medications in patients with history of bariatric surgery.
Acknowledgement:
The authors would like to thank Ashley McCarthy for her helpful feedback.
FUNDING: This work was supported in part by the National Institutes of Health (P30DK046200). The funder had no role in the collection, management, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
DISCLOSURE: CMA reports receiving personal fees from Nutrisystem, Zafgen, Sanofi-Aventis, Orexigen, EnteroMedics, GI Dynamics, Scientific Intake, Gelesis, Novo Nordisk, SetPoint Health, Xeno Biosciences, Rhythm Pharmaceuticals, Eisai, and Takeda outside of the funded work. CMA reports receiving grant funding from Aspire Bariatrics, GI Dynamics, Orexigen, Takeda, the Vela Foundation, Gelesis, Energesis, Coherence Lab and Novo Nordisk outside of the funded work. CMA reports past equity interest in ScienceSmart, LLC. No other disclosures were reported.
References
- 1.Yanos BR, Saules KK, Schuh LM, Sogg S. Predictors of Lowest Weight and Long-Term Weight Regain Among Roux-en-Y Gastric Bypass Patients. Obes Surg. 2015;25(8):1364–70. Epub 2014/12/19. doi: 10.1007/s11695-014-1536-z. PubMed PMID: 25519772. [DOI] [PubMed] [Google Scholar]
- 2.King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the Performance of Common Measures of Weight Regain After Bariatric Surgery for Association With Clinical Outcomes. JAMA. 2018;320(15):1560–9. Epub 2018/10/17. doi: 10.1001/jama.2018.14433. PubMed PMID: 30326125; PubMed Central PMCID: PMCPMC6233795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas DD, Anderson WA, Apovian CM, Hess DT, Yu L, Velazquez A, et al. Weight Recidivism After Roux-en-Y Gastric Bypass Surgery: An 11-Year Experience in a Multiethnic Medical Center. Obesity (Silver Spring). 2019;27(2):217–25. Epub 2018/11/14. doi: 10.1002/oby.22360. PubMed PMID: 30421862; PubMed Central PMCID: PMCPMC6345597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bureau USC. 2012-2016 American Community Survey 5-Year Estimates [cited 2018 June 22]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_16_5Y_R_S1903&prodType=table.
- 5.Bastos EC, Barbosa EM, Soriano GM, dos Santos EA, Vasconcelos SM. Determinants of weight regain after bariatric surgery. Arq Bras Cir Dig. 2013;26 Suppl 1:26–32. Epub 2014/01/28. PubMed PMID: 24463895. [DOI] [PubMed] [Google Scholar]
- 6.da Silva FB, Gomes DL, de Carvalho KM. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition. 2016;32(11-12):1250–3. Epub 2016/08/22. doi: 10.1016/j.nut.2016.01.018. PubMed PMID: 27544005. [DOI] [PubMed] [Google Scholar]
- 7.Shantavasinkul PC, Omotosho P, Corsino L, Portenier D, Torquati A. Predictors of weight regain in patients who underwent Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12(9):1640–5. Epub 2016/12/19. doi: 10.1016/j.soard.2016.08.028. PubMed PMID: 27989521. [DOI] [PubMed] [Google Scholar]
- 8.Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24(9):832–42. Epub 2008/08/30. doi: 10.1016/j.nut.2008.06.027. PubMed PMID: 18725080. [DOI] [PubMed] [Google Scholar]
- 9.Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2011;9(3):228–33. Epub 2010/11/26. doi: 10.1016/j.cgh.2010.11.004. PubMed PMID: 21092760; PubMed Central PMCID: PMCPMC3043151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20(3):349–56. Epub 2009/06/26. doi: 10.1007/s11695-009-9895-6. PubMed PMID: 19554382. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins RB, Mehaffey JH, McMurry TL, Kirby J, Malin SK, Schirmer B, et al. Clinical significance of failure to lose weight 10 years after roux-en-y gastric bypass. Surg Obes Relat Dis. 2017. Epub 2017/09/19. doi: 10.1016/j.soard.2017.08.004. PubMed PMID: 28919184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dakour Aridi HN, Wehbe MR, Shamseddine G, Alami RS, Safadi BY. Long-Term Outcomes of Roux-en-Y Gastric Bypass Conversion of Failed Laparoscopic Gastric Band. Obes Surg. 2017;27(6):1401–8. Epub 2017/01/22. doi: 10.1007/s11695-016-2529-x. PubMed PMID: 28108969. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032–40. Epub 2006/08/12. doi: 10.1381/096089206778026316. PubMed PMID: 16901357. [DOI] [PubMed] [Google Scholar]
- 14.Valezi AC, Mali Junior J, de Menezes MA, de Brito EM, de Souza SA. Weight loss outcome after silastic ring Roux-en-Y gastric bypass: 8 years of follow-up. Obes Surg. 2010;20(11):1491–5. Epub 2010/09/03. doi: 10.1007/s11695-010-0264-2. PubMed PMID: 20811958. [DOI] [PubMed] [Google Scholar]
- 15.Zentenius E, Andersson-Assarsson JC, Carlsson LMS, Svensson PA, Larsson I. Self-Reported Weight-Loss Methods and Weight Change: Ten-Year Analysis in the Swedish Obese Subjects Study Control Group. Obesity (Silver Spring). 2018;26(7):1137–43. Epub 2018/06/07. doi: 10.1002/oby.22200. PubMed PMID: 29873894. [DOI] [PubMed] [Google Scholar]
- 16.Kristensson FM, Andersson-Assarsson JC, Kanerva N, Peltonen M, Carlsson B, Carlsson LMS. Long-term effects of bariatric surgery in patients with obesity and chromosome 16 p11.2 microdeletion. Surg Obes Relat Dis. 2017. Epub 2017/06/11. doi: 10.1016/j.soard.2017.04.024. PubMed PMID: 28600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjoholm K, Pajunen P, Jacobson P, Karason K, Sjostrom CD, Torgerson J, et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia. 2015;58(7):1448–53. Epub 2015/05/01. doi: 10.1007/s00125-015-3591-y. PubMed PMID: 25924987. [DOI] [PubMed] [Google Scholar]
- 18.Sarzynski MA, Jacobson P, Rankinen T, Carlsson B, Sjostrom L, Bouchard C, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes (Lond). 2011;35(5):676–83. Epub 2010/08/25. doi: 10.1038/ijo.2010.166. PubMed PMID: 20733583. [DOI] [PubMed] [Google Scholar]
- 19.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. Epub 2007/08/24. doi: 10.1056/NEJMoa066254. PubMed PMID: 17715408. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson LMS, Sjoholm K, Karlsson C, Jacobson P, Andersson-Assarsson JC, Svensson PA, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5(4):271–9. Epub 2017/02/27. doi: 10.1016/S2213-8587(17)30061-X. PubMed PMID: 28237791; PubMed Central PMCID: PMCPMC5394228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjoholm K, Sjostrom E, Carlsson LM, Peltonen M. Weight Change-Adjusted Effects of Gastric Bypass Surgery on Glucose Metabolism: 2- and 10-Year Results From the Swedish Obese Subjects (SOS) Study. Diabetes Care. 2016;39(4):625–31. Epub 2015/12/19. doi: 10.2337/dc15-1407. PubMed PMID: 26681719. [DOI] [PubMed] [Google Scholar]
- 22.Hjorth S, Naslund I, Andersson-Assarsson JC, Svensson PA, Jacobson P, Peltonen M, et al. Reoperations After Bariatric Surgery in 26 Years of Follow-up of the Swedish Obese Subjects Study. JAMA Surg. 2019;154(4):319–26. Epub 2019/01/03. doi: 10.1001/jamasurg.2018.5084. PubMed PMID: 30601881; PubMed Central PMCID: PMCPMC6484798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himpens J, Coromina L, Verbrugghe A, Cadiere GB. Outcomes of revisional procedures for insufficient weight loss or weight regain after Roux-en-Y gastric bypass. Obes Surg. 2012;22(11):1746–54. Epub 2012/09/20. doi: 10.1007/s11695-012-0728-7. PubMed PMID: 22990874. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CC, Jacobsen GR, Schroder GL, Horgan S. Stoma size critical to 12-month outcomes in endoscopic suturing for gastric bypass repair. Surg Obes Relat Dis. 2012;8(3):282–7. Epub 2011/06/07. doi: 10.1016/j.soard.2011.03.014. PubMed PMID: 21640665. [DOI] [PubMed] [Google Scholar]
- 25.Abu Dayyeh BK, Jirapinyo P, Weitzner Z, Barker C, Flicker MS, Lautz DB, et al. Endoscopic sclerotherapy for the treatment of weight regain after Roux-en-Y gastric bypass: outcomes, complications, and predictors of response in 575 procedures. Gastrointest Endosc. 2012;76(2):275–82. Epub 2012/07/24. doi: 10.1016/j.gie.2012.03.1407. PubMed PMID: 22817783; PubMed Central PMCID: PMCPMC4428559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chahine E, Kassir R, Dirani M, Joumaa S, Debs T, Chouillard E. Surgical Management of Gastrogastric Fistula After Roux-en-Y Gastric Bypass: 10-Year Experience. Obes Surg. 2018;28(4):939–44. Epub 2017/10/07. doi: 10.1007/s11695-017-2949-2. PubMed PMID: 28983751. [DOI] [PubMed] [Google Scholar]
- 27.Mahawar KK, Himpens JM, Shikora SA, Ramos AC, Torres A, Somers S, et al. The first consensus statement on revisional bariatric surgery using a modified Delphi approach. Surg Endosc. 2019. Epub 2019/06/21. doi: 10.1007/s00464-019-06937-1. PubMed PMID: 31218425. [DOI] [PubMed] [Google Scholar]
- 28.Kruseman M, Leimgruber A, Zumbach F, Golay A. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc. 2010;110(4):527–34. Epub 2010/03/27. doi: 10.1016/j.jada.2009.12.028. PubMed PMID: 20338278. [DOI] [PubMed] [Google Scholar]
- 29.Conceicao E, Mitchell JE, Vaz AR, Bastos AP, Ramalho S, Silva C, et al. The presence of maladaptive eating behaviors after bariatric surgery in a cross sectional study: importance of picking or nibbling on weight regain. Eat Behav. 2014;15(4):558–62. Epub 2014/09/13. doi: 10.1016/j.eatbeh.2014.08.010. PubMed PMID: 25213792. [DOI] [PubMed] [Google Scholar]
- 30.Himes SM, Grothe KB, Clark MM, Swain JM, Collazo-Clavell ML, Sarr MG. Stop regain: a pilot psychological intervention for bariatric patients experiencing weight regain. Obes Surg. 2015;25(5):922–7. Epub 2015/03/10. doi: 10.1007/s11695-015-1611-0. PubMed PMID: 25750006. [DOI] [PubMed] [Google Scholar]
- 31.Toth AT, Gomez G, Shukla AP, Pratt JS, Cena H, Biino G, et al. Weight Loss Medications in Young Adults after Bariatric Surgery for Weight Regain or Inadequate Weight Loss: A Multi-Center Study. Children (Basel). 2018;5(9). Epub 2018/08/31. doi: 10.3390/children5090116. PubMed PMID: 30158481; PubMed Central PMCID: PMCPMC6162731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nor Hanipah Z, Nasr EC, Bucak E, Schauer PR, Aminian A, Brethauer SA, et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Relat Dis. 2018;14(1):93–8. Epub 2017/12/31. doi: 10.1016/j.soard.2017.10.002. PubMed PMID: 29287757. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz J, Chaudhry UI, Suzo A, Durkin N, Wehr AM, Foreman KS, et al. Pharmacotherapy in Conjunction with a Diet and Exercise Program for the Treatment of Weight Recidivism or Weight Loss Plateau Post-bariatric Surgery: a Retrospective Review. Obes Surg. 2016;26(2):452–8. Epub 2015/11/30. doi: 10.1007/s11695-015-1979-x. PubMed PMID: 26615406. [DOI] [PubMed] [Google Scholar]
- 34.Kalarchian M, Turk M, Elliott J, Gourash W. Lifestyle management for enhancing outcomes after bariatric surgery. Curr Diab Rep. 2014;14(10):540. Epub 2014/08/22. doi: 10.1007/s11892-014-0540-y. PubMed PMID: 25142721. [DOI] [PubMed] [Google Scholar]
- 35.Howland RH. Therapies for obesity and medication-associated weight gain. J Psychosoc Nurs Ment Health Serv. 2013;51(5):13–6. Epub 2013/04/18. doi: 10.3928/02793695-20130411-01. PubMed PMID: 23590816. [DOI] [PubMed] [Google Scholar]
- 36.Stanford FC, Alfaris N, Gomez G, Ricks ET, Shukla AP, Corey KE, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multicenter study. Surg Obes Relat Dis. 2017;13(3):491–500. Epub 2016/12/18. doi: 10.1016/j.soard.2016.10.018. PubMed PMID: 27986587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wharton S, Kuk JL, Luszczynski M, Kamran E, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9(4):e12323. Epub 2019/06/12. doi: 10.1111/cob.12323. PubMed PMID: 31183988; PubMed Central PMCID: PMCPMC6771702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suliman M, Buckley A, Al Tikriti A, Tan T, le Roux CW, Lessan N, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: Outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab. 2019;21(6):1498–501. Epub 2019/02/16. doi: 10.1111/dom.13672. PubMed PMID: 30768836. [DOI] [PubMed] [Google Scholar]
- 39.Miras AD, Perez-Pevida B, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–59. Epub 2019/06/09. doi: 10.1016/S2213-8587(19)30157-3. PubMed PMID: 31174993. [DOI] [PubMed] [Google Scholar]
- 40.Jester L, Wittgrove AC, Clark W. Adjunctive Use of Appetite Suppressant Medications for Improved Weight Management in Bariatric Surgical Patients. Obes Surg. 1996;6(5):412–5. Epub 1996/10/01. PubMed PMID: 10729886. [DOI] [PubMed] [Google Scholar]
- 41.Zilberstein B, Pajecki D, Garcia de Brito AC, Gallafrio ST, Eshkenazy R, Andrade CG. Topiramate after adjustable gastric banding in patients with binge eating and difficulty losing weight. Obes Surg. 2004;14(6):802–5. Epub 2004/08/21. doi: 10.1381/0960892041590926. PubMed PMID: 15318986. [DOI] [PubMed] [Google Scholar]
- 42.Zoss I, Piec G, Horber FF. Impact of orlistat therapy on weight reduction in morbidly obese patients after implantation of the Swedish adjustable gastric band. Obes Surg. 2002;12(1):113–7. Epub 2002/03/01. doi: 10.1381/096089202321144685. PubMed PMID: 11868286. [DOI] [PubMed] [Google Scholar]
- 43.Sudlow A, le Roux CW, Pournaras DJ. Review of multimodal treatment for type 2 diabetes: combining metabolic surgery and pharmacotherapy. Ther Adv Endocrinol Metab. 2019;10:2042018819875407. Epub 2019/10/04. doi: 10.1177/2042018819875407. PubMed PMID: 31579501; PubMed Central PMCID: PMCPMC6759694. [DOI] [PMC free article] [PubMed] [Google Scholar]