Abstract
The serine/threonine kinase RIPK1 has numerous biological and pathological functions, mediating prosurvival as well as prodeath apoptotic and necroptotic signaling pathways downstream of various receptors, including death receptors and Toll-like receptors (TLRs). RIPK1 has been implicated in various diseases, including ischemia–reperfusion injury and inflammatory bowel disease (IBD). The recent generation of RIPK1 kinase inactive mice has enabled us to genetically interrogate the role of RIPK1 kinase-mediated necroptosis in disease models. Here, we describe procedures utilizing kinase inactive Ripk1D138N/D138N mice to analyze necroptosis induction in vitro in bone-marrow derived macrophages (BMDMs) and in vivo in a murine model of TNF-induced shock.
Keywords: Bone marrow-derived macrophages, Lipopolysaccharide, Caspase inhibitor, TNF shock, Cell death, Necroptosis
1. Introduction
Receptor interacting protein kinase 1 (RIPK1) is a serine/threonine kinase crucial in TNF-induced proinflammatory and prosurvival signaling through activation of the NF-κB and MAP kinase pathways. Additionally, RIPK1 can induce apoptotic death or caspase-independent necroptosis, which involves cell and organelle swelling, plasma membrane rupture, and release of damage-associated molecular patterns (DAMPs). RIPK1 and the related RIPK3 auto and transphosphorylate each other, allowing RIPK3 to phosphorylate and activate the pseudokinase MLKL, the downstream executioner of necroptosis and plasma membrane rupture. Necroptosis can be induced downstream of Toll-like receptors (TLR) 3 and 4, or death receptors such as TNFR1 and TRAIL [1, 2]. Although Ripk1−/− mice die at birth [3], the discovery of necrostatin-1 (Nec-1), a small molecule inhibitor of RIPK1 kinase activity and necroptosis [4, 5], has enabled the study of RIPK1 kinase-mediated signaling. However, Nec-1 has off-target effects and a short half-life in vivo [6]. Recently, viable RIPK1 kinase inactive mice were generated; Ripk1D138N/D138N targeting the aspartate residue in the activation loop and Ripk1K45A/K45A targeting the lysine of the catalytic triad [7–9]. Cells from these mice are resistant to various necroptotic stimuli in vitro and similar to Ripk3−/− mice, RIPK1 kinase inactive mice are protected from TNF-induced shock in vivo [7–10]. However, care should be taken in interpreting results from RIPK1 kinase inactive mice since RIPK1 kinase activity can trigger either apoptosis or necroptosis in a cell-type and context-dependent manner. Additionally, RIPK1-independent apoptosis and necroptosis can be triggered [1, 2], and both RIPK1 and RIPK3 kinases can mediate inflammatory signaling independently of necroptotic death, as evidenced in vivo by greater protection of Ripk1D138N/D138N and Ripk3−/− mice in certain mouse models compared to Mlkl−/− mice [11]. Here we describe the isolation and culture of primary bone marrow derived macrophages (BMDMs) isolated from WT, Ripk1D138N/D138N and Ripk3−/− mice. Macrophages can be induced to undergo RIPK1- and RIPK3-dependent necroptotic death in vitro by inhibiting caspase-8 via a pan-caspase inhibitor, zVAD-fmk, and stimulating TLR signaling by lipopolysaccharide (LPS) addition [12, 13] (Fig. 1). We describe the basics of setting up, optimizing and reading out cell death sensitivity in this in vitro BMDM necroptosis assay. Additionally, we describe using Ripk1D138N/D138N mice to analyze necroptotic death in vivo in a mouse model of TNF-induced shock (Fig. 2).
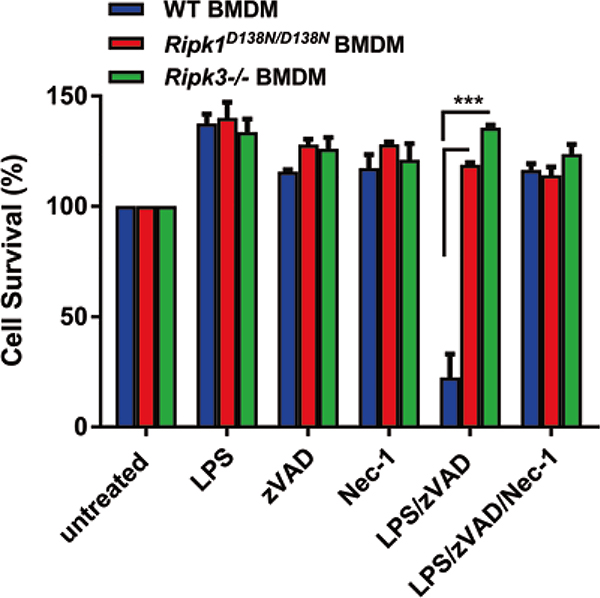
Fig. 1.
RIPK1 kinase inactive BMDMs are resistant to LPS/zVAD-induced necroptosis. Primary BMDMs isolated from WT, Ripk1D138N/D138N and Ripk3−/− mice were treated with zVAD-fmk and/or Nec-1 prior to treatment with LPS. Cell viability was measured with the MTS assay (n = 3 mice). Error bars represent SEM. (***p < 0.001)
Fig. 2.
RIPK1 kinase inactive mice are protected from TNF- and TNF/zVAD-induced shock. Body temperature and survival of WT and Ripk1D138N/D138N mice injected with TNF (a) or TNF/zVAD (b). Error bars represent SEM. (***p < 0.001, ****p < 0.0001). (Originally published in The Journal of Immunology. Polykratis A, Hermance N, Zelic M et al. 2014. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 193:1539–1543. Copyright 2014. The American Association of Immunologists, Inc.)
2. Materials
Prepare all solutions using ultrapure double distilled water (ddH2O). Store all commercially obtained reagents according to the manufacturer’s instructions. Prepare and store all reagents at room temperature, unless otherwise indicated.
2.1. Primary Cells and Culture Conditions (See Chapter 10)
Bone marrow derived macrophages (BMDMs) isolated from the bone marrow of WT, Ripk1D138N/D138N and Ripk3−/− mice.
BMDM cell culture media: DMEM, 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin, 1% L-glutamine, 20% L929 cell supernatant. From a 500 mL bottle of DMEM remove 160 mL, then add 50 mL FBS, 5 mL of Penicillin-Streptomycin, 5 mL l-glutamine, and 100 mL L929 supernatant. Mix well and store at 4 °C. Prior to using the media, warm to 37 °C using a water bath for 10–15 min.
10 cm tissue culture treated dishes.
2.2. Primary Cell Viability Assay
Clear flat bottom 96-well tissue culture treated plates.
1.5 mL microcentrifuge tubes, autoclaved.
15 mL conical tubes.
Trypan blue (0.4% solution in PBS).
Hemocytometer.
Dimethyl sulfoxide (DMSO).
0.25% trypsin–EDTA 1× (ThermoFisher).
Cell scraper.
Phosphate buffered saline (PBS; 10×): 14.4 g Na2HPO4, 80 g NaCl, 2 g KCl, 2.4 g KH2PO4, ddH2O to 1 L. Adjust pH to 7.4. Autoclave prior to use. Store at room temperature.
Lipopolysaccharide (LPS; 1 mg/mL) (Sigma-Aldrich): Prepare a 1 mg/mL stock solution in sterile-filtered water by weighing 5 mg LPS, then reconstituting in 5 mL water. Mix well, aliquot and store at −20 °C.
Necrostatin-1 (Nec-1; 30 mM) (Enzo Life Sciences): Prepare a 30 mM stock solution in DMSO by weighing 10 mg Nec-1 (MW: 259.3) and transfer it into a 1.5 mL microcentrifuge tube. Dissolve the powder in 1.29 mL DMSO to make a 30 mM stock. Mix well, aliquot and store at −20 °C.
Z-Val-Ala-Asp-fluoromethylketone (zVAD; 20 mM) (Enzo Life Sciences): Prepare a 20 mM stock solution in DMSO by reconstituting 1 mg zVAD (MW: 467.5) in 107 μL DMSO. Mix well, aliquot and store at −20 °C.
Sterile-filtered water (Sigma).
CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) kit (Promega).
Plate reader.
2.3. TNF and TNF/zVAD Shock in Mice
Recombinant mouse tumor necrosis factor alpha (rmTNFα; 250 μg/mL) (Cell Sciences): Prepare a 250 μg/mL stock solution in sterile-filtered PBS by reconstituting 1 mg TNFα in 4 mL PBS. Mix well, aliquot and store at −80 °C.
zVAD (Bachem; 20 mg/mL): Prepare a 20 mg/mL stock solution in sterile-filtered DMSO by reconstituting 5 mg zVAD in 250 μL DMSO. Mix well and store at −20 °C.
Sterile-filtered DMSO (Sigma-Aldrich).
Sterile-filtered PBS (Lonza Biowhittaker).
Scale.
Heat lamp.
Mouse restrainer.
Digital thermometer.
25G × 5/8 in. needle.
1 mL syringe.
WT and Ripk1D138N/D138N mice.
3. Methods
Perform all primary cell experiments and reagent reconstitutions and dilutions in an ultraviolet-sterilized vacuum hood. Incubate cells in a 5% CO2 incubator at 37 °C.
3.1. Primary BMDM Cell Culture
Isolation of hematopoietic progenitors for BMDM cultures is carried out as described in Chapter 6. Bone marrow progenitors are cultured in BMDM cell culture media for 7–8 days. Every 2–3 days aspirate all of the medium and loosely adherent cells, wash with sterile 1× PBS, and add 10 mL of fresh prewarmed BMDM cell culture media.
If macrophages start to reach confluence around day 4–5, split the cells 1:2. After aspirating the medium and washing with PBS, add 1 mL trypsin and put the plates in the 37 °C incubator for 5 min. Using a cell scraper, gently scrape the adhered macrophages off the bottom of the plate. Add 9 mL prewarmed BMDM cell media and after pipetting multiple times, disperse 5 mL of the cells to each of two new plates. Add 5 mL fresh BMDM cell media to each plate, swirl the plates well to make sure that the cells spread out evenly, and continue incubating at 37 °C.
3.2. Plating BMDMs for Viability Assay
On day 7 or 8, collect the BMDMs with trypsin and gentle scraping as described in Subheading 3.1, step 2.
Add prewarmed BMDM cell culture media, pipet multiple times to collect the cells, transfer to a 15 mL conical tube and centrifuge at 440 × g for 5 min to pellet the cells.
-
Aspirate the media and resuspend the BMDM cell pellet in 5 mL BMDM cell culture media to count the cells with a hemocytometer. Briefly, swirl the flask and take out 20 μL of the cells into a 1.5 mL microcentrifuge tube. Mix with 80 μL 0.4% trypan blue and load 10 μL onto the hemocytometer. Count the cells and determine the cell number per mL using the following formula (see Notes 1 and 2):
Number of cells per mL = [number of cells in four corner squares]/4 × dilution factor × 104
Plate 5 × 104 BMDMs in 100 μL per well in triplicate in 96-well plates for each genotype and treatment condition that will be used (see Note 3). Here there are three genotypes (WT, Ripk1D138N/D138N, and Ripk3−/−) and six treatment conditions (untreated, LPS, zVAD, Nec-1, LPS/zVAD, and LPS/zVAD/Nec-1) (see Note 4).
Plate the cells in BMDM cell culture media and incubate the plates at 37 °C for 3–4 h to let the BMDMs adhere.
3.3. Necroptotic Viability Assay In Vitro
Once the cells have adhered, aspirate the media and gently wash the wells with 1× PBS. Add 100 μL DMEM/10% FBS media to all the wells with cells, omitting the L929 supernatant (see Note 5).
Pretreat the cells for 1 h with zVAD (20 μM final concentration) and/or Nec-1 (30 μM final concentration) and incubate at 37 °C (see Note 6).
Add LPS to the cells (20 ng/mL final concentration) and incubate at 37 °C for 20 h (see Note 7).
Add 20 μL of CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) reagent to each well (see Note 8), mix by pipetting, and incubate at 37 °C for at least 1 h (see Note 9). Record the absorbance at 490 nm using a spectrophotometer (Fig. 1) (see Note 10).
To determine the relative cell viability in response to each treatment condition, first subtract the average of the blank well triplicate (media only) from each treatment absorbance reading, then average the triplicate values for each treatment condition. Utilize the following formula to obtain the relative percent cell viability of each individual treatment group compared to untreated control cells for the respective genotype (see Note 11):
Relative % viability = ([average A490 treated cell]/[average A490 untreated cell]) × 100
3.4. TNF and TNF/zVAD Shock In Vivo
For TNF-induced shock studies it is best to use age- and sex- matched mice, ideally littermates between the ages of 6–12 weeks (see Note 12).
To calculate an average weight use a scale to weigh the mice that will be used for the experiment (see Note 13).
Based on the mouse number and average weight, make up dilutions of rmTNFα and zVAD in sterile-filtered PBS (see Note 14). For TNF-induced shock inject 9 μg TNF per mouse in 200 μL PBS. For TNF/zVAD-induced shock inject 9 μg TNF and 16.7 mg/kg zVAD in 200 μL PBS. For example, a 25 g average weight would require 36 μL TNF, 20.9 μL zVAD, and 143.1 μL PBS per mouse (see Note 15).
Warm the mice up for 5 min by placing the cage next to a heat lamp (see Note 16).
Place a detachable 25G × 5/8 in. needle on a 1 mL syringe, making sure that the bevel faces up when aligned with the barrel measurements. Draw up 200 μL of the TNF or TNF–zVAD mixture in PBS for each mouse, making sure to get rid of any air bubbles in the syringe.
Place the prewarmed mouse in a restrainer, spray down the tail with 70% ethanol, and inject the TNF or TNF–zVAD mixture intravenously into the tail vein (see Note 17).
Once all the mice have been injected, monitor their temperature every few hours. Use the digital thermometer to record the temperature rectally (see Note 18).
Euthanize moribund mice and record time of sacrifice/death (Fig. 2) (see Note 19).
4. Notes
Count the live unstained cells, do not count blue stained cells as these are not viable.
In this case, the dilution factor is 5 (20 μL cells mixed with 80 μL trypan blue in a total volume of 100 μL) so input 5 into the equation to obtain the number of cells per mL.
With the MTS assay, MTS conversion results in a yellow to purple/dark brown color change, which is proportional to the number of living cells in culture. Cell growth rate and under-confluence or overconfluence can affect the accuracy of the results, thus it is best to optimize the cell number based on the cell type being used. With primary BMDMs, a good range to test would be 2 × 104, 5 × 104, and 1 × 105 cells per well in a 96-well plate. Cells can also be plated over-night, instead of for 3–4 h before starting treatment.
It is best to always take more cells than will be necessary, in this case 1 × 106 cells would be sufficient for 20 wells.
MTS assays can have a high background reading, so make sure to have three control wells with no cells and 100 μL media per well.
For both zVAD and Nec-1 the final dilution is 1:1000 from the stock concentration. Preferably, create a master mix of zVAD and/or Nec-1 in media at the final concentration, and add 100 μL to each well as needed, after having aspirated and washed the old media. Alternatively, dilute each compound 1:10 in media, then add 1 μL per well into 100 μL media.
Dilute the LPS 1:1000 in media to a concentration of 1 μg/ mL, then add 2 μL per well. Alternatively, make master mixes containing LPS, with or without zVAD and Nec-1, diluted to the final concentration and add 100 μL per well after aspirating and washing the old media.
The MTS reagent is light sensitive, so make sure to protect it from light. Thaw the reagent at room temperature. Open the bottle and add MTS to the plate in a sterilized vacuum hood with the light off, to prevent contamination and light damage.
With the MTS assay it is necessary to test a series of incubation times (e.g., 30 min, 1 h, 2 h, 3 h, etc.) after adding the MTS reagent. It is important to determine an optimal time so that the color signal is not saturated. The absorbance reading should be in the range from 0.0 to 2.0.
LPS, zVAD or Nec-1 should not induce death in BMDMs on their own. In a preliminary experiment, test a range of concentrations for each reagent to find the optimum tolerated concentration range. In combination, LPS and zVAD should induce death in WT BMDMs, which should be rescued with addition of Nec-1. Concentrations of reagents may also need to be optimized to see these effects.
RIPK1 kinase activity can mediate both apoptotic and necroptotic death, and RIPK1-independent necroptosis can occur [1, 2], so additional controls in addition to WT and Ripk1D138N/ D138N BMDMs should be utilized. Here, Nec-1 and Ripk3−/− BMDMs are added to test necroptotic death. Mlkl−/− BMDMs or RIPK3 kinase inhibitors can also be used. Furthermore, the MTS death assay can be utilized with other cell types isolated from RIPK1 kinase inactive mice, including mouse embryonic fibroblasts (MEFs) or bone marrow derived dendritic cells (BMDCs). These cells can be tested for necroptosis susceptibility with TNF/Cycloheximide/zVAD or TNF/Smac mimetic/zVAD treatment. Finally, other viability assays including CellTiter-Glo® and flow cytometry-based annexin V/propidium iodide staining can be used instead of the MTS assay.
-
Gender differences in susceptibility to shock and sepsis exist in both mouse models and patients. In general, females are thought to be more resistant due to decreased production of proinflammatory cytokines and increased production of immunosuppressive mediators [14, 15].
Additionally, differences in the gut microbiome can affect result interpretation in many mouse models [16]. Thus, it is important to use age- and sex-matched littermates in these TNF-induced shock experiments. If littermates are not available, it is best to wean mice together into the same cage or mix the bedding between cages for several weeks before performing the experiment.
If possible it is best to use mice of a similar weight, or a similar weight range for the different genotypes, in this case WT and Ripk1D138N/ D138N mice.
Thaw the TNF and zVAD on ice, and keep on ice once you have prepared the mixture.
Make a master mix based on the experimental mouse number. Remember to always make enough for a couple extra mice, as ~100 μL volume is lost in the shaft of the needle.
Warming up the mice helps to dilate the vein and makes it easier to visualize.
Inject at a shallow angle with the bevel of the needle pointed up, since the vein is close to the tail surface. Start injecting closer to the bottom half of the tail, so there is ample room to move up the tail if the initial injection angle is not successful. Push lightly on the plunger, if there is any resistance the needle is not in the vein. Press on the injection site briefly with a paper towel to stop the bleeding, and place the mouse back into the cage for recovery and monitoring.
Use sterile autoclaved Vaseline on the tip of the thermometer probe, and clean it between measurements on each mouse.
Before starting the shock experiments, obtain institutional approval from the Animal Care and Use Committee (IACUC) and follow the guidelines for monitoring and euthanasia as approved by the institution.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant AI075118.
References
- 1.Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517:311–320. 10.1038/nature14191 [DOI] [PubMed] [Google Scholar]
- 2.Weinlich R, Oberst A, Beere HM et al. (2017) Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18:127–136. 10.1038/nrm.2016.149 [DOI] [PubMed] [Google Scholar]
- 3.Kelliher MA, Grimm S, Ishida Y et al. (1998) The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297–303 [DOI] [PubMed] [Google Scholar]
- 4.Degterev A, Huang Z, Boyce M et al. (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119 [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, Hitomi J, Germscheid M et al. (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321. 10.1038/nchembio.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi N, Duprez L, Grootjans S et al. (2012) Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis 3:e437 10.1038/cddis.2012.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton K, Dugger DL, Wickliffe KE et al. (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343:1357–1360. 10.1126/science.1249361 [DOI] [PubMed] [Google Scholar]
- 8.Polykratis A, Hermance N, Zelic M et al. (2014) Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF- induced necroptosis in vivo. J Immunol 193:1539–1543. 10.4049/jimmunol.1400590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger SB, Kasparcova V, Hoffman S et al. (2014) Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPINdeficient mice. J Immunol 192:5476–5480. 10.4049/jimmunol.1400499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duprez L, Takahashi N, Van Hauwermeiren F et al. (2011) RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35:908–918. 10.1016/j.immuni.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 11.Newton K, Dugger DL, Maltzman A et al. (2016) RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23:1565–1576. 10.1038/cdd.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He S, Liang Y, Shao F et al. (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 108:20054–20059. 10.1073/pnas.1116302108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser WJ, Sridharan H, Huang C et al. (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288:31268–31279. 10.1074/jbc.M113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marriott I, Huet-Hudson YM (2006) Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res 34:177–192 [DOI] [PubMed] [Google Scholar]
- 15.Angele MK, Pratschke S, Hubbard WJ et al. (2014) Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5:12–19. 10.4161/viru.26982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laukens D, Brinkman BM, Raes J et al. (2016) Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev 40:117–132. 10.1093/femsre/fuv036 [DOI] [PMC free article] [PubMed] [Google Scholar]