Highlights
-
•
We report a case of transient existence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in the respiratory tract with the absence of an anti-SARS-CoV-2 antibody response.
-
•
SARS-CoV-2 RNA was absent in the six respiratory specimens that were subsequently tested.
-
•
Anti-SARS-CoV-2 IgM and IgG were absent in the acute and convalescent sera.
Keywords: COVID-19, SARS-CoV-2, Asymptomatic infection, Colonization, Contamination
Abstract
We report the case of a patient who had travelled to Japan and who presented mild respiratory symptoms during the COVID-19 outbreak period. There was transient existence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in his oropharynx. The RNA was absent in the six respiratory specimens that were subsequently tested. An anti-SARS-CoV-2 antibody response was absent in the acute and convalescent sera. The reported case indicates that transient colonization of SARS-CoV-2 in the upper respiratory tract is possible without inciting any antibody response against the virus.
Introduction
Early diagnosis of coronavirus disease 2019 (COVID-19) is essential for containing and mitigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections (Loeffelholz and Tang, 2020, Zhao et al., 2020, Liu et al., 2020). However, clinical diagnosis is difficult because of the variable clinical manifestations of SARS-CoV-2 infections, which can range from an asymptomatic infection or mild acute respiratory disease to severe pneumonia and acute respiratory distress syndrome (Lai et al., 2020). The detection of SARS-CoV-2 RNA in respiratory secretions and identification of anti-SARS-CoV-2 antibodies in serum are crucial for the diagnosis of COVID-19 (Loeffelholz and Tang, 2020, Zhao et al., 2020, Liu et al., 2020).
Case report
History and examination
A 30-year-old man, an engineer, presented to our hospital on February 27, 2020 with a mild cough since February 24, 2020 (day 1 of illness). He had joined a tour group to Japan, consisting of 22 people, between February 17 and 22, 2020. He denied any contact with suspected or confirmed COVID-19 patients. He had visited another hospital with the above-mentioned symptom on February 26 (day 3 of illness), where a throat swab sample was collected and sent to the Taiwan Centers for Diseases Control and Prevention (Taiwan CDC) for the detection of SARS-CoV-2 RNA by real-time reverse-transcription PCR (qRT-PCR) (Lee et al., 2020a). On the following day, the Taiwan CDC reported a positive qRT-PCR result based on positive findings for the E gene (cycle threshold (Ct) value of 31.9; a Ct value of ≤33 was considered a positive result) and RdRp2 gene (Ct value of 36.3 in S-shape); however, qRT-PCR was negative for the N and RdRp1 genes of SARS-CoV-2. The patient was then transferred to our hospital for isolation.
Hydroxychloroquine (200 mg every 12 h) was administered orally from day 7 to day 10 since the start of illness. During his hospitalization, the patient did not experience fever, rhinorrhea, headache, myalgia, arthralgia, dyspnea, abdominal pain, diarrhea, or dysuria. Laboratory data on day 4 of illness showed a white blood cell count of 4.85 × 109/l with 16.7% (0.810 × 109/l) lymphocytes. Follow-up lymphocyte counts, performed on day 9 and day 12 of illness, were normal (1.839 and 2.047 × 109/l, respectively). The C-reactive protein (CRP) level on day 4 of illness was 0.03 mg/l. Liver and renal function test results and coagulation study results were normal. Chest radiography (performed on days 4, 8, and 12 of illness) and chest computed tomography (performed on day 15 of illness) did not reveal any abnormal findings. The qRT-PCR tests for SARS-CoV-2 RNA, performed in triplicate on the oropharyngeal swabs and sputum samples collected on days 4, 6, and 8 of illness, gave negative results for all E/RdRp1/RdRp2/N genes.
The patient was discharged on day 14 since the start of illness when his condition was stable. The other 21 people who had accompanied him on the tour remained well and none of them was diagnosed with COVID-19. This reported case was listed as one of the 440 patients with confirmed COVID-19 in Taiwan (Taiwan CDC: https://www.cdc.gov.tw/en/Disease/SubIndex/, accessed on May 12, 2020).
Serological examination
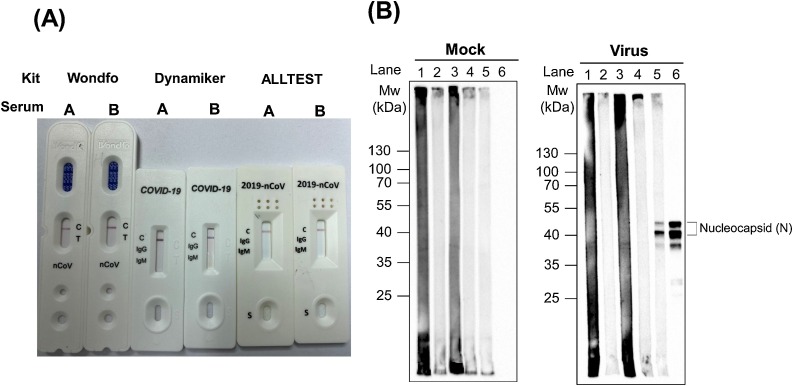
Serological tests were conducted using two serum samples (sera A and B) from the patient, obtained on days 8 and 17 of illness. Anti-SARS-CoV-2 IgM/IgG antibodies were detected using three commercially developed kits, including recombinant nucleocapsid protein-based lateral flow immunoassay (LFIA) kits: 2019-nCoV IgG/IgM Rapid Test Cassette (ALLTEST; Hangzhou ALLTEST Biotech Co., Ltd, China), Wondfo SARS-CoV-2 Antibody Test (Guangzhou Wondfo Biotech Co., Ltd, China), and 2019 nCoV IgG/IgM Rapid Test (Dynamiker Biotechnology (Tianjin) Co., Ltd., China) (Lee et al., 2020a, Lee et al., 2020b). All of these tests indicated the absence of anti-SARS-CoV-2 IgM and IgG in the two serum samples (Figure 1A). In addition, Western blots with lysates of mock and SARS-CoV-2-infected Vero E6 cells collected on day 6 of illness were performed to detect the anti-SARS-CoV-2 nucleocapsid protein antibody ( Liu et al., 2020). SARS-CoV-2 nucleocapsid protein bands were not found at 1:20 and 1:200 dilutions of the two serum samples (Figure 1B).
Figure 1.
Detection of anti-SARS-CoV-2 antibody in two serum samples. Sera A and B were collected on days 8 and 17 of illness, respectively. (A) Anti-SARS-CoV-2 total, IgM, and IgG antibodies were not detected by the three commercially developed lateral flow immunoassay kits: 2019-nCoV IgG/IgM Rapid Test Cassette (ALLTEST), Wondfo SARS-CoV-2 Antibody Test (Wondfo), and 2019 nCOV IgG/IgM Rapid Test (Dynamiker). (B) Cell lysates from mock and SARS-CoV-2-infected Vero E6 cells were used to conduct Western blotting analysis. Lanes 1 and 2, serum A with 1:20 and 1:200 dilutions, respectively. Lanes 3 and 4, serum B with 1:20 and 1:200 dilutions, respectively. Lane 5, a serum sample from a SARS-CoV-2-infected patient collected on day 9 of illness with 1:200 dilution. The presence of nucleocapsid protein was determined using anti-nucleocapsid protein (1:2000 dilution) and anti-rabbit (1:5000 dilution) antibodies (lane 6). SARS-CoV-2 nucleocapsid protein bands were present in the SARS-CoV-2-infected patient (lane 5), but were not visible for the two serum samples from the patient reported in this study (lanes 1–4).
Discussion
A recent study conducted by Zhao et al. evaluated the dynamic nature of the anti-SARS-CoV-2 antibody response among 173 patients with COVID-19 and found that the seroconversion rate (median seroconversion time) for total antibodies, IgM, and IgG was 93.1%, 82.7%, and 64.7% on days 11, 12, and 14 since the start of illness, respectively (Zhao et al., 2020). The presence of total antibodies, IgM, and IgG among the COVID-19 patients after day 15 of illness was 100.0%, 94.3%, and 79.8%, respectively. On the basis of these findings, the possibility of negative results for IgM, IgG, or total antibodies in the convalescent serum collected on day 17 of illness would be rare. However, the methods used to detect anti-SARS-CoV-2 antibodies in the two studies were different.
The performance of the three commercially available antibody detection kits using LFIA was evaluated with a limited number of serum/plasma/blood specimens from PCR-confirmed COVID-19 patients (package inserts). ALLTEST (IgM and IgG, respectively), Wondfo (all antibodies), and Dynamiker (mixed IgM and IgG) showed a sensitivity of 85.0% and 100%, 86.4%, and 93.2%, respectively, while the specificity values were 96% and 98%, 99.6%, and 95.3%, respectively. These results, obtained from limited data, indicate that <5% of COVID-19 patients who tested positive using these kits had no anti-SARS-CoV-2 antibody (IgM or IgG).
The patient who had travelled to Japan presented mild respiratory symptoms during the COVID-19 outbreak period. There was transient existence of SARS-CoV-2 RNA in his oropharynx. The RNA was absent in the six respiratory specimens that were subsequently tested, including the three lower respiratory tract secretion samples (sputum). Among the laboratory indices compatible with the diagnosis of COVID-19 (elevated CRP levels, lymphopenia, elevated levels of alanine aminotransferase, aspartate aminotransferase, creatine kinase, and D-dimer) (Guan et al., 2020, Lai et al., 2020), only lymphopenia (but transient) was found in our patient. Multiple mechanisms lead to lymphopenia among patients with or without COVID-19, and lymphopenia per se is a reliable predictor of disease severity and the prognosis in COVID-19 patients (Tan et al., 2020). Furthermore, the lack of anti-SARS-CoV-2 antibody response (IgM and IgG) in the acute and convalescent sera made the diagnosis of COVID-19 in this patient questionable. The viral load of SARS-CoV-2 RNA (high Ct value) was low in this patient. Additionally, the virus was probably eliminated by hydroxychloroquine treatment prior to induction of the immune response (Gautret et al., 2020). These factors may have contributed to the lack of specific anti-SARS-CoV-2 antibodies in the patient. However, other possibilities, such as transient viral colonization without induction of any antibody response, a false-positive qRT-PCR result, contamination during specimen processing, and cross-reaction with other etiologies cannot be ignored. Further studies to detect both anti-SARS-CoV-2 nucleoprotein and antibodies against the surface spike protein receptor binding domain are needed (To et al., 2020). Importantly, failure of the host immune response to the specific genotype of SARS-CoV-2 virus may also be responsible for this phenomenon.
In summary, the reported case indicates that transient colonization of SARS-CoV-2 in the upper respiratory tract is possible without inciting any antibody response against the virus.
Ethical approval
Informed consent was obtained from the patient for publication of this case report.
Funding
No specific funding was received for this work.
Conflict of interest
The authors declare no competing interests.
References
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Liu Y.H., Wang C.Y., Wang C.Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53 doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.Y., Lee C.W., Tai H.P., Chen P.L., Syue L.S., Li M.C. A case of COVID-19 and pneumonia returning from Macau in Taiwan: clinical course and anti-SARS-CoV-2 IgG dynamic. J Microbiol Immunol Infect. 2020;53 doi: 10.1016/j.jmii.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.L., Liao C.H., Liu P.Y., Cheng C.Y., Chung M.Y., Liu C.E. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020;(April) doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Wd, Chang Sy, Wang Jt, Tsai Mj, Hung Cc, Hsu Cl. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;(April) doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections-the state of the art. Emerg Microb Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D., Huang Q., Tang Y.Q., Wang Q. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.W., Tsang O.T.Y., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]