Highlights
-
•
The characteristics of 200 patients with COVID-19 in Yichang were analyzed.
-
•
COVID-19 was of clustering onset, and can cause severe respiratory disease and death.
-
•
The mortality of ICU patients confirmed with COVID-19 was considerably high.
-
•
The lymphocytes might be the main target of SARS-CoV-2 and this may be related to the severity and mortality of the disease.
-
•
The cytokine storm may be associated with the worsening of the disease.
Keywords: COVID-19, Epidemiology, Clinical features, Pneumonia, SARS-CoV-2
Abstract
Background
The recent outbreak of coronavirus disease 2019 (COVID-19) has spread worldwide, with especially severe epidemics occurring in cities across China.
Objectives
To report the epidemiological and clinical futures of the 200 patients infected with COVID-19 in Yichang, Hubei Province, China.
Study design
200 patients confirmed with COVID-19 in a designated hospital in Yichang from Jan 30 to Feb 8, 2020 were investigated retrospectively. The epidemiological data and clinical characteristics were collected. The data between the ICU patients and non-ICU patients were compared. The patients were followed up till Feb 26, 2020.
Results
Of the 200 hospitalized patients with COVID-19, 98 (49.0 %) were male, and the mean age was 55 years. Eighty-seven (43.5 %) had no linkage to Wuhan or contact history. Familial clustering was found in 34 patients. Sixtyfive (32.5 %) suffered from chronic diseases. The common symptoms included fever (171[85.5 %]), cough (116[58.0 %]), and fatigue (64[32 %]). Most patients had lymphopenia. One hundred and seventy-two (86 %) patients showed typical imaging findings of viral pneumonia. Most patients received antiviral, antibiotic, and corticosteroid treatment. Compared with the non-ICU patients, 29 (14.5 %) patients in the ICU were older and more likely to show dyspnea and complications including ARDS. As of Feb 26, 15 (51.7 %) patients in the ICU had died.
Conclusions
The COVID-19 infection was of clustering onset and can cause severe respiratory disease and even death. The mortality of ICU patients with COVID-19 was considerably high.
1. Backgroud
In December 2019, a new pneumonia case of unknown etiology emerged and began to widely spread in Wuhan, Hubei Province, China. Most of the patients were epidemiologically linked to the Huanan Seafood Wholesale Market, indicating animal-to-human disease transmission mode, but soon there was considerable evidence to confirm the human-to-human transmission [1,2]. Within two weeks after the outbreak, the Chinese Center for Disease Control (CDC) isolated samples from the lower respiratory tracts of patients for deep sequencing analysis, suggesting the emergence of a novel coronavirus, which was officially named SARS-CoV-2 by WHO on Feb 11, 2020. Since the SARS-CoV-2 hascharacteristics typical of the coronavirus family, it is currently classified in the lineage B betacoronaviruses that also include severe acute respiratory syndrome (SARS) and middle eastern respiratory syndrome (MERS) [[3], [4], [5], [6]]. Although the source of the SARS-CoV-2 is still under investigation, genome sequencing and phylogenetic analysis proved that the sequence of RNA genome of the virus is similar to bat coronaviruses, indicating the bats maybe the primary source [7].
The disease caused by the SARS-CoV-2 was officially named Corona Virus Disease 2019 (COVID-19) by WHO [8]. Previous studies showed that the clinical manifestations of patients with COVID-19 in Wuhan mainly included fever, chill, cough, difficulty in breathing, muscle soreness, fatigue, and complications such as acute respiratory distress syndrome (ARDS), acute kidney injury, acute cardiac injury, etc [9]. Laboratory tests showed that the leukocytes were normal or decreased, and/or the lymphocytes decreased. Chest imaging revealed typical features of viral pneumonia [10]. Wang et al. reported that among the 138 investigated patients, 36 received ICU care, with a mortality of 4.3 %. It was found that COVID-19 was of clustering onset, and that older men with comorbidities were more likely to develop into critically ill patients [11].
Although the government adopted a series of measures to respond to this major public health emergency, such as prohibiting citizens from leaving or coming into Wuhan and group activities, in the following dozens of days, a large cluster of the pneumonia rapidly spread throughout China and beyond. And the epidemic was especially severe in cities around Wuhan in Hubei Province. As of Feb 26, 2020, there were 78,630 confirmed COVID-19 cases, 2358 suspected cases, 32,531 cured cases, and 2747 deaths in China, of which 65,596 were confirmed cases in Hubei Province and 929 were confirmed cases in Yichang City, which owns huge economic development potential in Hubei Province, second only to Wuhan [12,13].
So far, there have been several studies that have summarized and analyzed the epidemiological and clinical features of pneumonia caused by SARS-CoV-2 in Wuhan [[14], [15], [16]]. However, the information on the epidemiological and clinical features of COVID-19 in other areas is scarce.
2. Objectives
The main purpose of this study was to comprehensively analyze and compare the epidemiological, clinical, laboratory, radiological, treatment, and outcome data of confirmed patients with COVID-19 admitted in a designated hospital in Yichang, Hubei Province, China. We hope our findings can provide some references for understanding the epidemic and the clinical characteristics of this novel coronavirus outside Wuhan.
3. Study design
3.1. Participants
200 patients with confirmed COVID-19 were recruited from Jan 30 to Feb 08, 2020 at Yichang Central People's Hospital, a designated hospital in Yichang, Hubei Province, China. Patients throughout Yichang City were centrally admitted to the hospital without selectivity. All the enrolled COVID-19 patients in this study were diagnosed according to WHO interim guidance. The study was approved by the Ethics Committee of Yichang Central People's Hospital and written informed consent was obtained from the enrolled patients.
3.2. Procedures
The epidemiological, clinical, laboratory, treatment, and outcome data of the patients were obtained from electronic medical records. All the data were reviewed by two physicians (LH Yang and R Zhang). The information included demographic data, family clustering (≥2 infected family member), exposure history (living in or visiting Wuhan or contact with people from Wuhan ≤14 days before infection), comorbidities, clinical manifestations (symptoms and signs), laboratory tests, chest computed tomographic (CT) scans, treatment (i.e., medical treatment, respiratory support, renal replacement therapy), and outcomes (complications, including death). Throat-swab samples from the upper respiratory tract were collected during hospitalization. SARS−COV-2 was detected by real-time polymerase chain reaction (RT-PCR) following the recommended protocol. Infection was confirmed when there were at least 2 positive results.
3.3. Outcomes
We described and summarized demographic data, family clustering, exposure history, potential comorbidities, clinical manifestations, laboratory tests, chest CT scans, treatment measures, and prognosis.
3.4. Statistical analysis
All statistical analyses were performed using SPSS20.0 version software. Continuous variables were represented as mean, median, and interquartile range (IQR) values. Independent group t-test was used to compare the means of continuous variables when the data were normally distributed. Categorical variables were represented as frequency and percentage. The Mann-Whitney test was used when the data were non-normally distributed. Chi-square test was used to compare proportions of categorical variables, although Fisher's exact test was used in cases where data was limited. A two-sided α of less than 0·05 was considered statistically significant.
4. Results
A total of 200 patients with laboratory-confirmed COVID-19 were enrolled in the study. Among them, 98 (49.0 %) were male and 102 (51.0 %) were female, with the mean age of 55 years (SD 17.1 years). Three children and two pregnant women were infected. Of the 200 patients, 29 (14.5 %) were admitted to the ICU because of the requirement of high-level oxygen support measures or the development of organ dysfunction, and the remaining 171 (85.5 %) were admitted to isolation wards. The age of ICU patients (mean age 71 years, SD13.4) was higher than the non-ICU patients (mean age 52 years, SD 16.2, P = 0.000). About two thirds of the patients came from Xiling District of Yichang City. Of these patients, 24 (12.0 %) had a history of residing in or visiting Wuhan within 2 weeks before infection, 89 (44.5 %) had an intensive contact with visitors from Wuhan or confirmed COVID-19 patients, and the remaining 87 (43.5 %) had no linkage to Wuhan or contact history. Familial clustering was found in 34 patients, accounting for 17 %. Among the 200 patients, 65 (32.5 %) suffered from 1 or more comorbidities, with hypertension (45[22.5 %]), diabetes (21[10.5 %]), and chronic heart disease (11[5.5 %]) as the top three common coexisting conditions (Table 1 ).
Table 1.
Demographics and Epidemiological and Clinical Characteristics of Patients With Corona Virus Disease 2019 (COVID-19).
| Total (n = 200) | ICU (n = 29) | Non-ICU (n = 171) | P value | |
|---|---|---|---|---|
| Age, year | 55 (17.1) | 71 (13.4) | 52 (16.2) | 0.00 |
| Gender | ||||
| Male | 98 (49 %) | 16 (55.2 %) | 82 (48.0 %) | 0.47 |
| Female | 102 (51 %) | 13 (44.8 %) | 89 (52.0 %) | |
| District | ||||
| Xiling Distirct | 130 (65 %) | 21 (72.4 %) | 109 (63.8 %) | 0.37 |
| Non-Xiling District | 70 (35 %) | 8 (27.6 %) | 62 (36.3 %) | |
| Epidemiological history within 2 weeks before infection | ||||
| Wuhan residence or travelling | 24 (12 %) | 1 (3.4 %) | 23 (13.4 %) | 0.01 |
| Close contact history | 89 (44.5 %) | 8 (27.6 %) | 81 (47.4 %) | |
| Others | 87 (43.5 %) | 20 (69.0 %) | 67 (39.2 %) | |
| Familial clustering | 34 (17 %) | 4 (13.8 %) | 30 (17.5 %) | |
| Comorbidities | 65 (32.5 %) | 13 (44.8 %) | 52 (30.4 %) | 0.13 |
| Hypertension | 45 (22.5 %) | 9 (31.0 %) | 36 (21.1 %) | 0.23 |
| Chronic lung disease | 7 (3.5 %) | 4 (13.8 %) | 3 (1.8 %) | 0.01 |
| Diabetes | 21 (10.5 %) | 4 (13.8 %) | 17 (9.94 %) | 0.53 |
| Chronic heart disease | 11 (5.5 %) | 1 (3.4 %) | 10 (5.8 %) | 0.60 |
| Chronic kidney disease | 3 (1.5 %) | 2 (6.9 %) | 1 (0.6 %) | 0.01 |
| Chronic liver disease | 2 (1%) | 0 | 2 (1.2 %) | 0.56 |
| Malignancy | 4 (2%) | 1 (3.4 %) | 3 (1.8 %) | 0.55 |
| Smoking history | 9(4.5 %) | 1(3.4 %) | 8 (4.7 %) | 0.77 |
| Signs and symptoms | ||||
| Fever,℃ | 38.0 (37.5−38.5) | 38.1 (38.0−38.9) | 38.0 (37.5−38.5) | 0.06 |
| ≥37.3℃ | 171 (85.5 %) | 25 (86.2 %) | 146 (85.4 %) | 0.90 |
| <37.3℃ | 29 (14.5 %) | 4 (13.8 %) | 25 (14.6 %) | 0.90 |
| Cough | 116 (58.0 %) | 16 (55.2 %) | 100 (58.5 %) | 0.74 |
| Fatigue | 64 (32.0 %) | 13 (44.8 %) | 51 (29.8 %) | 0.23 |
| Chills or rigors | 34 (17.0 %) | 8 (27.6 %) | 26 (15.2 %) | 0.10 |
| Myalgia or malaise | 44 (22.0 %) | 5 (17.2 %) | 39 (22.8 %) | 0.50 |
| Sore throat | 26 (13.0 %) | 1 (3.4 %) | 25 (14.6 %) | 0.10 |
| Dyspnea | 29 (14.5 %) | 22 (75.9 %) | 7 (4.1 %) | 0.01 |
| Headache | 27 (13.5 %) | 2 (6.9 %) | 25 (14.6 %) | 0.26 |
| Diarrhea | 14 (7%) | 3 (10.3 %) | 11 (6.4 %) | 0.54 |
| Nausea and vomiting | 4 (2%) | 2 (6.9 %) | 2 (1.2 %) | 0.04 |
| Duration from illness onset to admission, d | 5.0 (2.25−7.0) | 4.0 (3.0−8.5) | 5.0 (2.0−6.0) | 0.73 |
Data are mean (SD), median (IQR), n (%). P values comparing patients with ICU and non-ICU are from t-test, χ² test, Fisher’s exact test, or Mann-Whitney U test NCP = novel coronavirus pneumonia. ICU = intensive care unit.
Most patients had fever (171[85.5 %]) and cough (116[58.0 %]) on admission. Some patients had fatigue (64[32 %]), myalgia and malaise (44[22 %]), dyspnea (29[14.5 %]), chills or rigors (34[17 %]), headache (27[13.5 %]), and sore throat (26[13 %]). Other symptoms included diarrhea (14[7%]), nausea, and vomiting (4[2%]). Compared with the patients admitted to the isolation wards, patients who received ICU care were more likely to show dyspnea, nausea, and vomiting. In addition, 6 patients with non-ICU care were asymptotic, all of whom found lung imaging changes after having epidemiological exposure history. The median time from illness onset to first admission was 5 days (IQR 2.25−7.0).
On admission, the leucocytes of most patients were below or within the normal range. Most patients had lymphopenia, with the median level of 0.91 × 109/L (IQR,0.69−1.22). There were significant differences in the median value of lymphocytes and the proportion of lymphopenia between patients with ICU care and those with non-ICU care (0.73 × 109/L [IQR,0.56−0.89] vs 0.94 × 109/L [IQR, 0.73−1.26], P = 0.000; 25 [86.2 %] vs 112 [65.5 %], P = 0.026). The C-reactive protein and d-dimer were higher in ICU patients than non-ICU patients(median C-reactive protein level 68.8 mg/L [IQR, 53.47–108.25] vs 18.7 mg/L [IQR,6.3−37.9], P = 0.000; median D-dimer level 1.1 mg/L[IQR, 0.6–1.8] vs 0.5 mg/L[0.3−0.7], P = 0.000). The incidences of ALT and AST above the normal range in ICU patients were higher than those in non-ICU patients (41.4 % vs 18.7 %, P = 0.006; 58.6 % vs 33.3 %, P = 0.009). Fifty-seven (28.5 %) patients had different degrees of kidney dysfunction, with serum creatinine above the normal range. Seven-seven (38.5 %) patients showed an elevation of lactate dehydrogenase, of whom 22 belonged to the 29 ICU-patients (81.5 %). Procalcitonin was increased in 25 (86.2 %) of ICU patients, higher than the non-ICU patients (23[13.5 %], P < 0.001). According to chest CT on admission, 176 (86 %) patients had bilateral involvement (Table 2 ).
Table 2.
Laboratory Findings of Patients With Corona Virus Disease 2019 (COVID-19).
| Patients(n = 200) | ICU(n = 29) | Non-ICU (n = 171) | P value | |
|---|---|---|---|---|
| Leucocytes, ×10⁹ /L | 4.4 (3.5−5.7) | 5.76 (4.06−7.82) | 4.37 (3.47−5.48) | 0.01 |
| <3.5 | 45 (22.5 %) | 2 (6.9 %) | 43 (25.1 %) | 0.04 |
| 3.5∼9.5 | 149 (74.5 %) | 25 (86.2 %) | 124 (72.5 %) | 0.11 |
| >9.5 | 6 (3%) | 2 (6.9 %) | 4 (2.3 %) | 0.18 |
| Neutrophils, × 10⁹ /L | 2.92 (2.15−4.07) | 4.8(2.8−6.39) | 2.78(2.1−3.79) | 0.00 |
| Lymphocytes, × 10⁹ /L | 0.91 (0.69−1.22) | 0.73(0.56−0.89) | 0.94 (0.73−1.26) | 0.00 |
| <1.1 | 137 (68.5 %) | 25 (86.2 %) | 112 (65.5 %) | 0.02 |
| Hemoglobin, g/L | 132.0 (17.5) | 123.72 (19.9) | 133.40 (16.7) | 0.00 |
| Platelets, × 10⁹ / L | 154.0 (124.2−189.0) | 145(120.5−177) | 155 (125−192) | 0.23 |
| <125 | 50 (25 %) | 9(31.0 %) | 41 (24.0 %) | 0.41 |
| Activated partial thromboplastin time, s |
34.0(30.3−37.3) | 36.7 (34.0−41.0) | 33.3 (30.1−36.2) | 0.00 |
| Prothrombin time, s | 11.0 (10.5−11.5) | 11.4 (10.6−12.0) | 11 (10.5−11.4) | 0.02 |
| <10.5 | 42/187 (22.5 %) | 5/29 (17.2 %) | 37/158 (23.4 %) | 0.55 |
| D-dimer, ng/mL | 532 (314−768) | 1100 (639−1824) | 497 (306−746) | 0.00 |
| >500 | 119/190(62.6 %) | 25/28 (89.7 %) | 94/162 (58.0 %) | 0.15 |
| Albumin, g/L | 37.88(34.61−40.22) | 32.49 (26.05−34.96) | 38.32 (35.48−40.70) | 0.00 |
| <40 | 144(72 %) | 28 (96.6 %) | 116 (67.8 %) | 0.00 |
| Alanine aminotransferase, U/L | 26 (19−75) | 30 (20.5−76.5) | 26 (19−36) | 0.04 |
| >40 | 44 (22 %) | 12 (41.4 %) | 32 (18.7 %) | 0.00 |
| Aspartate aminotransferase, U/L | 32 (26−39.75) | 41 (33.5−64.5) | 31 (25−39) | 0.00 |
| >35 | 74 (37 %) | 17 (58.6 %) | 57 (33.3 %) | 0.00 |
| Total bilirubin, mmol/L | 12.46 (10.5725−16.085) | 13.18 (10.155−18.285) | 12.45 (10.61−15.92) | 0.58 |
| Potassium, mmol/L | 3.73 (0.42) | 3.90 (0.54) | 3.7 (0.39) | 0.06 |
| Sodium, mmol/L | 137.7 (135.525−139.6) | 136.1 (133.25−139.3) | 137.8(136.2−139.6) | 0.05 |
| Serum creatinine,μmol/L | 64 (52.5−83.1) | 85.6 (65.875−23.275) | 61.8 (50.075−81.45) | 0.00 |
| >81 | 57(28.5 %) | 14 (48.3 %) | 43 (25.1 %) | 0.01 |
| Creatine kinase,IU/L | 92 (61.25−151.75) | 113 (72−192.5) | 88 (61−144) | 0.09 |
| >200 | 31/189 (16.2 %) | 6/27 (22.2 %) | 25/162 (15.4 %) | 0.45 |
| Lactate dehydrogenase, U/L | 229 (187−298) | 342(260.5−468.5) | 217(183−274) | 0.00 |
| >250 | 74/189 (38.5 %) | 22/27(81.5 %) | 52/162(32.1 %) | 0.00 |
| CKMB,IU/L | 11 (9−14) | 12.5 (10−19) | 11 (9−14) | 0.03 |
| >25 | 5/189 (2.6 %) | 3/27 (11.1 %) | 2/162 (1.2 %) | 0.00 |
| Procalcitonin > 0.05 ng/mL | 44/182 (24.2 %) | 23/27 (85.2 %) | 21/155 (13.5 %) | 0.00 |
| C-reactive protein, mg/L | 22.95 (7.45−54.085) | 68.8 (53.47−108.25) | 18.7 (6.3−37.9) | 0.00 |
| >10 | 141/200 (70.5 %) | 29/29 (100 %) | 112/171 (65.5 %) | 0.00 |
| Bilateral involvement of chest CT | 172/200 (86 %) | 28/29 (96.6 %) | 144/171 (84.2 %) | 0.07 |
Data are median (IQR), mean (SD), n (%), or n/N (%), where N is the total number of patients with available data. P values comparing patients with ICU and non-ICU are from t-test, χ² test, Fisher’s exact test, or Mann-Whitney U test NCP = novel coronavirus pneumonia. ICU = intensive care unit.
Many of the patients presented with complications, including 32 (16 %) with acute respiratory distress syndrome (ARDS), 10(%) with acute cardiac injury, 24 (12 %) with acute renal injury, 12 (6.0 %) with secondary infection, and 4 (2%) with shock (Table 3 ).
Table 3.
Complications, Treatments, and Prognosis of Patients with Corona Virus Disease 2019 (COVID-19).
| Patients (n = 200) | ICU(n = 29) | Non-ICU(n = 171) | P value | |
|---|---|---|---|---|
| Complications | ||||
| ARDS | 32 (16 %) | 21 (72.4 %) | 11 (6.4 %) | 0.00 |
| Acute cardiac injury | 20 (10 %) | 15 (51.7 %) | 5 (2.9 %) | 0.00 |
| Acute kidney injury | 24 (12 %) | 12 (41.3 %) | 12 (7.0 %) | 0.00 |
| Secondary infection | 12 (6.0) | 5 (17.2 %) | 7 (4.1 %) | 0.00 |
| Shock | 4 (2%) | 4 (13.8 %) | 0 | 0.00 |
| Treatment | ||||
| Antiviral therapy | 199 (99.5 %) | 28 (96.6 %) | 171 (100 %) | 0.01 |
| Antibiotic therapy | 141 (70.5 %) | 29 (100 %) | 112 (65.5 %) | 0.00 |
| Use of corticosteroid | 112 (56 %) | 20 (69.0 %) | 92 (53.8 %) | 0.12 |
| CRRT | 2 (1%) | 2 (6.9 %) | 0 | 0.00 |
| Oxygen support | ||||
| Nasal cannula | 158 (79 %) | 5(17.2 %) | 153 (89.5 %) | 0.00 |
| Non-invasive ventilation or high-flow nasal cannula | 35 (17.5 %) | 24 (82.8 %) | 11 (6.4 %) | 0.00 |
| Invasive mechanical ventilation |
16 (8.0 %) | 14 (48.3 %) | 2 (1.2 %) | 0.00 |
| Prognosis | ||||
| Hospitalization | 143 (71.5 %) | 11 (37.9 %) | 132 (77.2 %) | 0.00 |
| Discharge | 42 (21 %) | 4 (13.8 %) | 38 (22.2 %) | 0.94 |
| Death | 15 (7.5 %) | 14 (48.3 %) | 1 (0.6 %) | 0.00 |
And it was found that the complications were more common in the ICU patients than in the non-ICU patients. One hundred and ninety-nine (99.5 %) of the 200 patients were treated with antiviral therapy (Abidol, Oseltamivir, Lopinavir/ritonavir), and 141 (70.5 %) received antibiotic treatment (Moxifloxacin, Ceftriaxone). More than half of the patients (112[56 %]) received corticosteroid therapy. Two patients (2[1 %]) were treated with continuous renal replacement therapy (CRRT). All patients received oxygen support on admission. Most patients (24[82.8 %]) in the ICU received high-flow oxygen or noninvasive ventilation. Fourteen patients (48.3 %) required invasive mechanical ventilation to relieve hypoxemia. In the non-ICU wards, high-flow oxygen or noninvasive ventilation was required in 11 patients (9.9 %), two of whom received invasive mechanical ventilation with the worsening of the disease. As of Feb 26, 2020, 42 (21 %) of 200 patients had been discharged including two children and 16 (8.0 %) patients had died. Of the 29 patients in the ICU, 12 were still hospitalized, 3 had been discharged and15 had died. Through analyzing and tracking laboratory results of the patients who had died, we found that the neutrophils, PCT, IL-6, and CRP were 14.2 × 109/L(SD 8.4), 4.49 ng/mL (IQR 1.57–25.8), 248.85 pg/mL (IQR,77.54–1671.3), and 105.7 mg/L(IQR 82.7–220.1) before they died, which were significantly higher than the normal range.
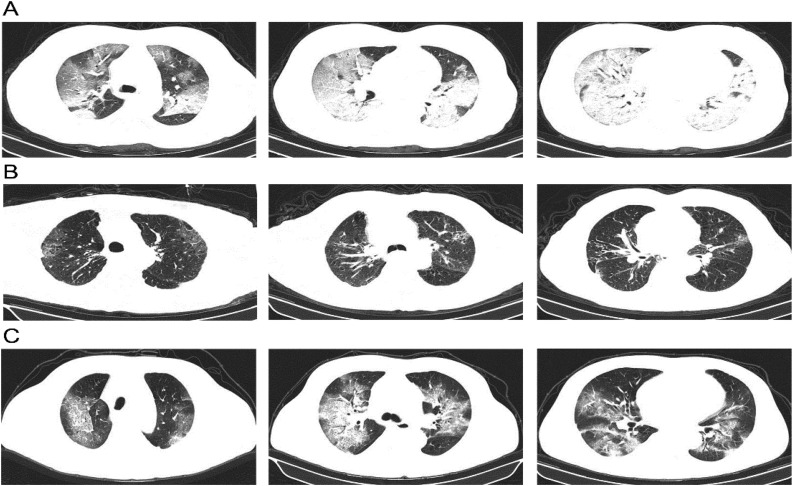
The main imaging findings included bilateral mottling and ground glass opacity, multiple lobular or subsegmental areas of consolidation (Fig. 1 ). All the 200 cases of SARS-CoV-2 infection were confirmed by RT-PCR.
Fig. 1.
Chest Computed Tomographic Images.
A. Chest CT of a 51-year-old female taken on Feb 06, 2020 showed diffuse patchy shadows and ground glass opacity, with lobular and subsegmental consolidation. Chest CT images from an 83-year-old male who had COPD showed multiple mottling and ground-glass opacity on Feb 1, 2020 (B). However, the lesions in both lungs were larger with patchy and nodular increased-density shadow on Feb 6, 2020 (C).
5. Discussion
Herein, we reported a total of 200 patients with confirmed COVID-19. The 200 patients were admitted to the designated hospital in just 10 days from Jan 30, 2020 to Feb 8, 2020, indicating the outbreak of the COVID-19 in Yichang, China. Meanwhile, the local government actively adopted public health outbreak response tactics, including quarantine, isolation, school closure, social distancing, community containment, etc. As of Feb 23, 2020 in Yichang, 924 cases were confirmed, 304 cases were cured, and 29 cases were dead, with the case fatality rate of 3.14 %. While according to the latest study by Xb Yang and his colleagues, the case fatality rate of critically ill patients with COVID-19 at 28 days was 61.5 %, which was very considerable [17]. Similarly, during our 28-day follow-up after admission in our study, 15 of the 29 ICU patients had died, and the case fatality rate in the ICU was as high as 51.7 %. Our study also found that the population was generally susceptible to the virus and that older patients with comorbid conditions usually developed into critically ill cases with many complications and required ICU admission.
As mentioned in the previous section, many patients came from Xiling District of Yichang City, which may be related to the fact that some residents of the area worked in Wuhan. Through the epidemiological survey, only 24 (12.0 %) were founded to have a history of Wuhan residence or travel within 14 days before onset of illness. Almost half of the patients had no linkage to Wuhan or contact history. This suggests human-to-human transmission and high contagiousness of the SARS−COV-2. At present, the main transmission routes of SARS−COV-2 are proved to be droplet transmission and contact transmission, with the possibility of aerosol transmission in prolonged exposure to high concentrations of aerosol in a relatively closed environment. Finding out the transmission routes can help to prevent the spread of the SARS−COV-2 more effectively [18].
Similar to those infected with betacoronaviruses such as SARS-CoV and MERS-CoV, most patients with SARS-CoV-2 infection presented with fever, cough, fatigue, dyspnoea, and bilateral ground-glass opacities and patchy shadows in chest computed tomography. Sore throat, headache, diarrhea, nausea, and vomiting occurred in a few patients infected with SARS-CoV-2 [19]. In addition, 6 people were asymptomatic. As for the laboratory tests, the lymphocytes of most patients were below the normal range, and the lymphocytes in ICU patients were less than those in non-ICU patients. This result suggests that lymphocytes, especially T lymphocytes might be the main target of SARS-CoV-2 and this may be related to the severity and mortality of disease. Z Xu and his colleagues showed, by autopsy of a patient with COVID-19, that the T cells were excessively activated while the counts were substantially reduced [20]. Moreover, our study also found that the neutrophils, PCT, IL-6, and CRP of the 14 patients who had died were significantly higher than the normal range, suggesting that the cytokine storm maybe associated with the disease. Virus particles penetrate the respiratory mucosa and activate lymphocytesand then forms a cytokine storm, which causes severe immune damage to the lungs and other organs [21,22]. However, the specific pathophysiology of COVID-19 remains to be further explored.
At present, some effective treatment methods for COVID-19 have been summarized based on clinical practice [[23], [24], [25]]. The antiviral drugs for COVID-19 currently used in clinic mainly includeα-interferon aerosol inhalation, Lopinavir / ritonavir, Ribavirin, Chloroquine phosphate, and Abidol [26]. Antibiotics should be used cautiously [27]. If the patient has dyspnea and chest imaging progress, glucocorticoids can be used within a short period of time (3–5 days) with the dose not exceeding 2 mg / kg / day of methylprednisolone [24]. Other treatments include respiratory support, traditional Chinese medicine treatment, the plasma treatment of rehabilitation patients, extracorporeal blood purification technologies such as plasma exchange, adsorption, perfusion, and hemofiltration. A vaccine against SARS-CoV-2 is under development.
There are some limitations in this study. First of all, this study only included patients admitted to Yichang Central People's Hospital, which might not fully represent all the patients in the city. Multi-center collaborative studies with larger sample size in the future are required. Secondly, throat swab specimens were used to diagnose COVID-19 by RT-PCR. However, specimens collected from the upper respiratory tract may lead to false negatives, thus leading to missed diagnosis and pseudo-cure cases. The viral load and viral antigen in blood can be detected to provide a better assessment of the condition. Thirdly, many patients are still hospitalized at the time of manuscript submission, and the outcomes at present may not fully reflect the prognosis of all patients with COVID-19.
In conclusion, of the 200 patients infected with COVID-19 in Yichang, China, 14.5 % of patients were admitted to the ICU, who were mainly older patients with comorbidities and ARDS. The mortality of confirmed patients in the ICU was high.
CRediT authorship contribution statement
Luhuan Yang: Conceptualization, Methodology, Writing - original draft. Jinglan Liu: Visualization, Investigation. Rong Zhang: Data curation, Formal analysis. Mingwu Li: Project administration, Methodology. Zifeng Li: Methodology, Investigation. Xiaojing Zhou: Investigation, Supervision. Chuanjun Hu: Software, Validation. Fei Tian: Formal analysis. Fating Zhou: Investigation. Yunhong Lei: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
None declared.
Acknowledgments
We thank all participating patients and their families, as well as staffs of all the institutions involved in this study.
References
- 1.Michaels M.G., La Hoz R.M., Danziger Isakov L. Coronavirus disease 2019: implications of emerging infections for transplantation. Am. J. Transplant. 2020:1–5. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao S., Lin Q., Ran J. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazanji N., Klein R.E., Lohani S., Mertens A.N., Le J. A case of hypermucoviscous Klebsiella pneumoniae liver abscess syndrome in an Iraqi male. J. QJM. 2016;109(7):493–494. doi: 10.1093/qjmed/hcw049. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y., Fujii K., Yoshida A., Morisaki H., Kohno Y., Morisaki T. Artery tortuosity syndrome exhibiting early-onset emphysema with novel compound heterozygous SLC2A10 mutations. Am. J. Med. Genet. A. 2013;161A(4):856–859. doi: 10.1002/ajmg.a.35776. [DOI] [PubMed] [Google Scholar]
- 5.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Tawfiq J.A., Zumla A., Gautret P. Surveillance for emerging respiratory viruses. Lancet Infect. Dis. 2014;14(10):992–1000. doi: 10.1016/S1473-3099(14)70840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee A. Wuhan novel coronavirus (COVID-19): why global control is challenging? Public Health. 2020;179:A1–A2. doi: 10.1016/j.puhe.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo H., Oh J., Park C. Characteristics of fever and response to antipyretic therapy in military personnel with adenovirus-positive community-acquired pneumonia. Mil. Med. Res. 2020;7(1):6. doi: 10.1186/s40779-020-00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jernigan D.B. Team CC-R. update: public health response to the coronavirus disease 2019 outbreak - United States, February 24, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(8):216–219. doi: 10.15585/mmwr.mm6908e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youmans A.J., Harwood J. Gross and histopathological findings in the first reported vaping-induced lung injury death in the United States. Am. J. Forensic Med. Pathol. 2020;41(1):1–4. doi: 10.1097/PAF.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 14.Albarello F., Pianura E., Di Stefano F. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int. J. Infect. Dis. 2020;93(11):192–197. doi: 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carinci F. Covid-19: preparedness, decentralisation, and the hunt for patient zero. BMJ. 2020;368 doi: 10.1136/bmj.m799. bmj m799. [DOI] [PubMed] [Google Scholar]
- 16.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohrabi C., Alsafi Z., O’Neill N. World Health Organization declares global emergency: a review of the 2019 Novel Coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Zhao S., Teng T. Systematic comparison of two animal-to-Human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):13–17. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;01(09):112–114. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y.H., Dong J.H., An W.M. Clinical and computed tomographic imaging features of Novel Coronavirus Pneumonia caused by SARS-CoV-2. J. Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Liu J., Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X.L., Chen Z., Zhu J.J. Management strategies of neonatal jaundice during the coronavirus disease 2019 outbreak. World J. Pediatr. 2020:1–4. doi: 10.1007/s12519-020-00347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can. J. Anaesth. 2020;67(5):568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeri N.C., Shrestha N., Rahman M.S. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020 doi: 10.1093/ije/dyaa033. dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]