Highlights
-
•
Nafamostat, known as anticoagulant, has antiviral effect to COVID-19.
-
•
Nafamostat, a serine protease inhibitor, has antiinflammatory effect.
-
•
Nafamostat is a effective and safe drug to COVID-19 pneumonia.
Keywords: COVID-19, Nafamostat, Pneumonia, Oxygen treatment
Abstract
No effective treatment for COVID-19 has been well established yet. Nafamostat, known as anticoagulant, has potential anti-inflammatory and anti-viral activities against COVID-19. We report three cases of COVID-19 pneumonia who progressed while using antiviral drugs and needed supplementary oxygen therapy, improved after treatment with nafamostat. These preliminary findings show the possibility that Nafamostat can be considered to be used in elderly patients with COVID-19 pneumonia who need oxygen therapy. The effectiveness of nafamostat should be evaluated in further studies.
Introduction
Since the COVID-19 outbreak started in China, most deaths occur in the elderly or people with underlying diseases. The pathogenesis of COVID-19 is still not well understood why the viral infections lead to respiratory failure with a high mortality rate (Gao et al., 2020). An excessive immune response contributes to COVID-19 pathogenesis and lethality (Gao et al., 2020, Jin et al., 2020). Recently, complement suppression may represent a therapeutic approach to treat COVID-19 (Gao et al., 2020).
While COVID-19 was outbreak in Korea, no effective treatment for COVID-19 has been well established yet.
Recently, several journals mentioned the possibility of nafamostat(Guo et al., 2020, Hoffmann et al., 2020, Wang et al., 2020, Yamamoto et al., 2016). Nafamostat, serine protease inhibitor, can prevent the fusion of the envelope of the virus with host cell surface membranes, the first step in infection with the causative virus COVID-19 by inhibit TRPMSS2, a human cell surface serine protease (Guo et al., 2020, Hoffmann et al., 2020, Yamamoto et al., 2016). Nafamostat inhibits various enzyme systems, such as coagulation and fibrinolytic systems, the kallikrein–kinin system, the complement system, and activation of protease-activated receptors (Drugbank, 2020, March 01). It also inhibits lipopolysaccharide-induced nitric oxide production, apoptosis, and interleukin (IL)-6 and IL-8 levels in cultured human trophoblasts (Drugbank, 2020, March 01). It is shown to act as an antioxidant in TNF-α-induced ROS production (Drugbank, 2020, March 01). Therefore, nafamostat is thought to have anti-inflammatory and anti-viral properties to COVID-19 (Figure 1 ).
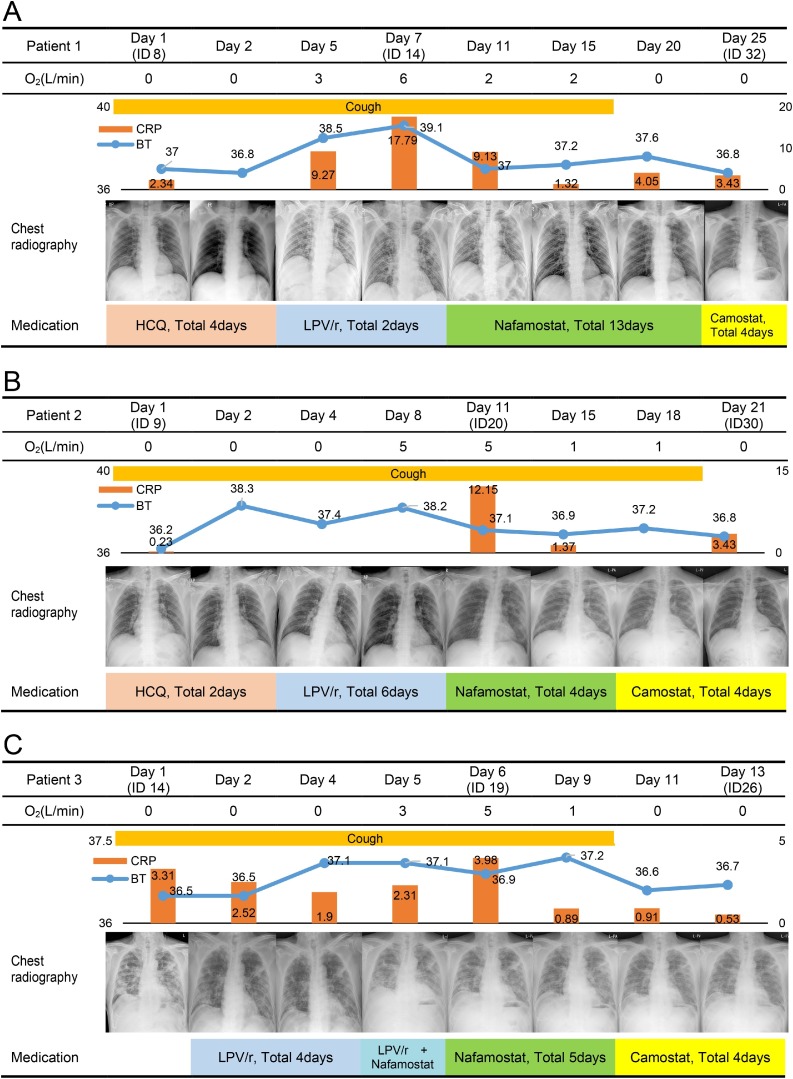
Figure 1.
Timeline of clinical symptoms, laboratory findings, and chest radiography findings. Abbreviations: ID, illness day; HCQ, hydroxychloroquine; LPV/r, lopinavir/ritonavir.
Case description
Three COVID-19 patients had the following conditions: pneumonia with progression despite antiviral treatment; were elderly, over 65 years; and had underlying diseases that were known as high risk factors. These three patients were continuously administered 200 mg of nafamostat for 24 h. This study was approved by the Institutional Review Board of Dankook University Hospital (DKUH 2020-03-031-001).
Patient 1: A 75-year-old man with hypertension and diabetes mellitus was diagnosed with COVID-19 on February 26, 2020. He had myalgia, and cough and sputum, which had begun the day before diagnosis. On admission, he was not dyspneic or cyanotic, with a body temperature of 37.0 °C, respiratory rate of 20 breaths per minute, heart rate of 75 beats per minute, and blood pressure of 140/90 mmHg. Initial laboratory results were as follows: white blood cell (WBC) count 5150/μL (65% segmented neutrophil), and C-reactive protein (CRP) 2.61 mg/dL. Initial chest radiography showed infiltrations in both lower lung fields, but high-resolution computed tomography (HRCT) on March 2, 2020, revealed multiple, ground-glass opacities located in both lungs. Oxygen was supplied beginning March 6 due to desaturation. On March 7, chest radiography showed increased infiltrations, and HRCT revealed that the existing lesions had transformed into consolidation. Laboratory results on March 8 showed an increased WBC count of 9510/μL (85% segmented neutrophil) and high CRP of 17.79 mg/dL. Beginning March 8, he was administered nafamostat. In addition, acetaminophen was administered regularly for fever control. After 2 days of nafamostat administration, his CRP level was decreased to 9.13 mg/dL and oxygen saturation was maintained above 98% in room air without oxygen supply. His CRP level was decreased to 1.32 mg/dL, 6 days after administration of nafamostat. On the 11th and 12th days after the nafamostat administration, a negative real-time reverse transcription-PCR (RT-PCR) results for severe acute respiratory syndrome coronavirus 2 was confirmed.
Patient 2: A 69-year-old man with hypertension experienced myalgia on February 28, and was confirmed to have COVID-19 on March 6, 2020. He had fever from the day of hospitalization, and cough began to develop. Physical examination revealed a respiratory rate of 20 breaths per minute, heart rate of 60 per minute, and blood pressure of 150/70 mmHg. Initial laboratory results were as follows: WBC count 6010/μL (74% segmented neutrophil), and CRP 0.23 mg/dL. Initial chest radiography, showed no infiltrations. However, HRCT revealed multifocal consolidations with peripheral ground glass opacities. Respiratory distress occurred on the 9th hospital day and oxygen was supplied. On March 14, chest radiography showed increased infiltrations, and laboratory results on March 15 revealed increased CRP of 13.0 mg/dL. Beginning March 14, he was administered nafamostat. After 4 days of nafamostat administration, his CRP level decreased to 5.27 mg/dL and oxygen saturation was maintained above 98% without oxygen supply. We changed the medication to camostat (600 mg/day) 4 days after nafamostat administration. On the 19th and 20th days after the camostat administration, a negative RT-PCR results for severe acute respiratory syndrome coronavirus 2 was confirmed.
Patient 3: A 66-year-old man with a history of poliomyelitis and hypertension who had experienced muscle pain since March 2 was diagnosed with COVID-19 on March 9, 2020, and was hospitalized on March 15. On admission, he was not dyspneic or cyanotic, with a body temperature of 36.5 °C, respiratory rate of 20 breaths per minute, heart rate of 81 per minute, and blood pressure of 132/84 mmHg. Initial blood tests revealed that his WBC count was 10,310/μL (75% segmented neutrophil), and CRP 3.31 mg/dL. Initial chest HRCT revealed multiple consolidations with peripheral ground-glass opacities located in both lungs. After hospitalization, oxygen therapy was initiated. On March 19, chest radiography showed increased infiltrations, and laboratory results revealed an increased WBC count of 15,210/μL (76% segmented neutrophil). Beginning March 19, he was administered 200 mg of nafamostat. After 5 days of nafamostat administration, his WBC count decreased to 7870/μL (64.1% segmented neutrophil) and oxygen saturation was maintained above 98% in ambient air. A Chest X-ray revealed further resolution of both lung infiltrates. He was discharged on day 15 of hospitalization because of improvement in symptoms and a negative RT-PCR test.
Discussion
The patients were all elderly people with underlying disease known as high risk group. At the time of transfer, both clinical and radiological deterioration were observed, and all patients were taking anti-virals including Lopinavir/ritonavir and hydroxychloroquine. There were no adverse events associated with nafamosta, and all patients improved and were discharged. According to the previous cases, four other patients with COVID-19 pneumonia are currently using nafamostat.
Nafamostat is a synthetic serine protease inhibitor, which prevents virus fusion and inhibits various enzyme systems to involve in inflammation. Recently, emerging evidence shows that COVID-19 can be complicated with coagulopathy namely disseminated intravascular coagulation (DIC) (Kollias et al., 2020, April 18). From the data, it is thought that nafamostat can prevent disease progression by controlling immune system such as the complement cascade, blocking DIC, and preventing virus invasion by inhibiting virus fusion on the cell membrane. It was used as a DIC dose in patient treatment (0.06∼0.2 mg/Kg/hour) to our patients. We experienced clinical and radiologic improvement in COVID-19 patients with pneumonia treated by nafamostat. Nafamostat is a relatively safe drug and can be considered to be used in patients with COVID-19 pneumonia.
In this preliminary uncontrolled cases of 3 elderly patients with exacerbated COVID-19 pneumonia, administration of nafamostat was followed by improvement in clinical status. These cases were treated with nafamostat in elderly patients with pneumonia who progressed while using antiviral drugs in a high-risk group with limited sample size and not through a randomized control study, and these observations require evaluation in clinical trials.
Author contributions
Professor J-Y. Rhee. and Professor S. Jang contributed equally.
Funding source
None.
Ethical approval
The study was approved by the Institutional Review Board of the Dankook University Hospital (DKUH 2020-03-031-001)
Disclosure
Authors have no potential conflicts of interest to disclose.
The names of authors should be same with those in the title page.
Acknowledgments
The authors appreciate the technical support of Dankook University Hospital for this study. We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.05.072.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Drugbank . 2020. Nafamostat. March 01. [Google Scholar]
- Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020 2020.03.29.20041962. [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol. 2020 doi: 10.1111/bjh.16727. Apr 18. doi: 10.1111/bjh.16727. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J. Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob Agents Chemother. 2016;60(11):6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.