Abstract
Background
Accumulating evidence proposed Janus-associated kinase (JAK) inhibitors as therapeutic targets warranting rapid investigation.
Objective
This study evaluated the efficacy and safety of ruxolitinib, a JAK1/2 inhibitor, for coronavirus disease 2019.
Methods
We conducted a prospective, multicenter, single-blind, randomized controlled phase II trial involving patients with severe coronavirus disease 2019.
Results
Forty-three patients were randomly assigned (1:1) to receive ruxolitinib plus standard-of-care treatment (22 patients) or placebo based on standard-of-care treatment (21 patients). After exclusion of 2 patients (1 ineligible, 1 consent withdrawn) from the ruxolitinib group, 20 patients in the intervention group and 21 patients in the control group were included in the study. Treatment with ruxolitinib plus standard-of-care was not associated with significantly accelerated clinical improvement in severe patients with coronavirus disease 2019, although ruxolitinib recipients had a numerically faster clinical improvement. Eighteen (90%) patients from the ruxolitinib group showed computed tomography improvement at day 14 compared with 13 (61.9%) patients from the control group (P = .0495). Three patients in the control group died of respiratory failure, with 14.3% overall mortality at day 28; no patients died in the ruxolitinib group. Ruxolitinib was well tolerated with low toxicities and no new safety signals. Levels of 7 cytokines were significantly decreased in the ruxolitinib group in comparison to the control group.
Conclusions
Although no statistical difference was observed, ruxolitinib recipients had a numerically faster clinical improvement. Significant chest computed tomography improvement, a faster recovery from lymphopenia, and favorable side-effect profile in the ruxolitinib group were encouraging and informative to future trials to test efficacy of ruxolitinib in a larger population.
Key words: Ruxolitinib, COVID-19, cytokine storm, efficacy, safety, randomized controlled trial
Abbreviations used: COVID-19, Coronavirus disease 2019; CT, Computed tomography; D, Day; ICU, Intensive care unit; IQR, Interquartile range; JAK, Janus-associated kinase; MIP-1α, Macrophage inflammatory protein 1α; MIP-1β, Macrophage inflammatory protein 1β; SARS, Severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SoC, Standard-of-care; VEGF, Vascular endothelial growth factor
The end of 2019 witnessed an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its associated coronavirus disease 2019 (COVID-19) in Wuhan, China.1 , 2 The rapid global spread has been classified as a pandemic by the World Health Organization3 and now represent the most serious issue to public health globally. COVID-19 is a heterogeneous disease. Most patients are asymptomatic or exhibit mild to moderate symptoms,4, 5, 6 but around 15% progress to severe pneumonia and about 5% were eventually admitted to the intensive care unit (ICU) because of acute respiratory distress syndrome, septic shock, and/or multiple organ failure.7, 8, 9 Among 138 hospitalized patients with COVID-19, 26.1% were transferred to the ICU because their condition deteriorated or they developed complications, which mainly (61.1%) included acute respiratory distress syndrome.7 Patients who develop acute respiratory distress syndrome respond poorly to therapy and have an extremely dismal prognosis.8 , 10
So far, only remdesivir has been shown to accelerate recovery from advanced COVID-19 based on a preliminary data analysis,11 although supportive therapies still have a fundamental role in the treatment of COVID-19. Among the unmet medical needs related to COVID-19, one of the most urgent issues is to assess existing conventional drugs in the treatment of severe/critical COVID-19 to improve the unsatisfactory clinical outcomes. COVID-19, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome, is characterized by an exuberant cytokine storm.12, 13, 14, 15, 16 Upon virus infection, the body produces inflammatory cytokines to restrict the spread/replication of the virus and eliminate the virus. However, highly pathogenic coronaviruses often induce uncontrolled cytokine/chemokine response, known as a cytokine storm, which results in high morbidity and mortality due to immunopathology.17 Although virus-induced direct pathogenic effects have an essential role in disease severity, viral load is not correlated with the worsening of symptoms in SARS.18, 19, 20 Previous studies on SARS suggested that a dysregulated immune response results in an exuberant inflammation and lethal disease.21 In a recent study, which enrolled 41 cases with confirmed COVID-19, one-third of patients were admitted to ICUs, 10% patients required mechanical ventilation, and 6 eventually died (14.6%); among these patients, cytokine storm was found to be associated with disease severity.18 There is accumulating evidence on the key pathophysiological role of cytokines during the severe stage of COVID-19. In the context of lack of vaccine and specific antiviral agents, testing of immunomodulatory agents to reduce excessive or uncontrolled inflammation before it results in irreversible multiorgan dysfunction infection has received increasing research attention.
Ruxolitinib is a Janus-associated kinase (JAK)1/2 inhibitor approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of polycythemia vera and myelofibrosis.22 It is also a promising option in the treatment of steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation23 or secondary hemophagocytic lymphohistiocytosis24 by targeting the deleterious effects of aberrant host inflammatory response. Anemia was the most common adverse event in patients receiving ruxolitinib though most anemia events were mild to moderate in severity.25 Nonhematological adverse events were generally low with long-term ruxolitinib treatment.25 Accordingly, we hypothesized that ruxolitinib might be effective against the consequences of the elevated levels of cytokines in patients with COVID-19. Besides, the potential negative impact of ruxolitinib on virus clearance and SARS-CoV-2 antibody production need to be elucidated.
To evaluate the efficacy and safety of ruxolitinib for COVID-19, we conducted a randomized, multicenter, placebo-controlled, single-blind phase II trial in patients hospitalized with severe COVID-19.
Methods
Study design
This prospective, single-blind, randomized controlled phase II trial included participants with COVID-19 enrolled for screening between February 9 and February 28, 2020, across 3 hospitals including Tongji Hospital, No. 1 Hospital, and the Third Xiangya Hospital in China. The original protocol included secondary randomization in the treatment group for infusion of mesenchymal stem cells if the patient’s clinical response deteriorated at day 7 (D7) after the first randomization. Because no patients from the treatment group experienced deterioration at D7, secondary randomization was unnecessary after the first randomization, and the protocol was updated correspondingly. This study followed the principles of the Declaration of Helsinki and was approved by the medical ethics committees of Tongji Hospital, Wuhan No. 1 Hospital, and Third Xiangya Hospital.
Participants
Participants who met the following inclusion criteria were included in the study: (1) met the diagnostic criteria for COVID-19; (2) 18 years or older and younger than 75 years; (3) severe cases. The diagnosis and the illness severity of COVID-19 were defined according to the Chinese management guideline for COVID-19 (version 5.0),26 and the full translated edition of diagnostic criteria is available in this article’s Methods section in the Online Repository at www.jacionline.org. Exclusion criteria were as follows: (1) patients with concomitant malignant tumors; (2) patients with severe cardiovascular and metabolic disease that is not medically controlled; (3) patients with a mental or severe psychiatric disorder; (4) patients in need of invasive mechanic ventilation at recruitment; (5) patients who could not guarantee to complete all the scheduled treatment plans and follow-ups; (6) women of child-bearing age with positive pregnancy test results or those in the lactating period; and (7) patients whose condition was further complicated with other active infections. Written informed consent was obtained from all participants.
Randomization and masking
The enrolled patients were randomly allocated into 2 groups (1:1 allocation ratio) by an independent statistician using permuted blocks of 4 for all sites. The whole process of randomization was masked to all treating physicians. Patient unique identification number and treatment allocation codes were provided by a clinical research associate in sequentially numbered opaque envelopes. Treating physicians were aware of group allocations for safety concern, whereas the enrolled participants, the staff at trial sites, computed tomography (CT) radiologists, and laboratory personnel were masked to the trial group assignment.
Procedures
The first day of randomization was designated as D0. The second day and the fourth day after randomization were designated D1 and D3, respectively. Dend was the day before discharge. The enrolled patients were randomly separated into 2 groups: the treatment group (group B), which received oral intake of ruxolitinib 5 mg twice a day plus standard-of-care (SoC) treatment, and the control group (group A), which received placebo (100 mg vitamin C) twice a day with SoC treatment. The dose of 5 mg twice a day is a ruxolitinib dose frequently used for the treatment of autoimmune/inflammatory conditions and has shown effective inhibition of inflammation proteins in previous trials.27 All costs of ruxolitinib and vitamin C tablets were covered by the funding from principle investigators. The SoC treatment included antiviral therapy, supplemental oxygen, noninvasive and invasive ventilation, corticosteroid, antibiotic agents, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation. The safety profile was monitored daily by 2 senior physicians from the Safety Monitoring Committee of the trial center. Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.28 Non–contrast-enhanced chest CT examinations were performed on D0 and should be followed up at D14. Additional chest CT might be performed if the condition deteriorated. All CT images were reviewed using the Picture Archiving and Communication System. The CT features, which were blindly evaluated by 2 independent senior radiologists, were supposed to meet at least 1 of the following criteria to be considered as improved: the decreased presence of ground-glass opacities, decreased lung opacification, reduced density of consolidation, or decreased pleural effusion with existence of fibrous stripes.29 Disease progression is defined by comparing the scope, quantity, and density of lesions detected in 2 chest CT scans. Obviously, fusion of lesions, new lesions, and (or) increased lung density were considered progression.30 If independent senior radiologists did not observe a significant difference in the lesions between the 2 CT scans, the patient’s condition was considered stable.30 Peripheral blood was taken from patients on D1, D3, and Dend for the determination of viral load by RT-ddPCR, SARS-CoV-2–specific IgM, and IgG and 48 cytokines (see this article’s Methods section in the Online Repository). Data from each participant were filled in 1 case record form. All the case record form tables were input and saved by researchers into an electronic data capture system and validated by trial staff, including demographic characteristics, medical history, daily clinical findings, oximetric measurements, and laboratory data involving complete blood cell count, serum biochemical parameters, high-sensitivity C-reactive protein, and so forth.
Outcomes
The primary efficacy end point was the time to clinical improvement, defined as the time from randomization (D0) to an improvement of 2 points on a 7-category ordinal scale or live discharge from the hospital (Dend), and improvement rate of follow-up CT scans at D14. The 7-category ordinal scale has been used in other COVID-19 randomized controlled trials31 and also recommended by the World Health Organization R&D Blueprint expert group32 (see this article’s Methods section in the Online Repository). Other clinical outcomes included clinical improvement rate as assessed with the 7-category ordinal scale on D7, D14, D21, and D28, time from randomization to lymphocyte recovery and to invasive mechanical ventilation, the duration of hospitalization in survivors, and the time from treatment initiation to death and virus clearance time. The primary safety end point was the incidence of serious adverse events occurring up to 28 days. Safety outcomes included adverse events and serious adverse events that occurred during treatment. Particularly, the eventual negative impact of ruxolitinib on SARS-CoV-2 virus clearance and its specific IgM and/or IgG-antibody formation and/or lymphocyte recovery was also included in the safety profile. Lymphopenia was defined as peripheral absolute lymphocytes less than 1.0 × 109/L. Lymphocyte recovery time was defined as the first day at which lymphocytes returned to the normal levels within the observation period. The virus clearance time was defined as the time from randomization to the first day of at least 2 consecutive negative RT-PCR assays separated by 24 hours apart. The secondary end point is the overall mortality at D28. The investigational outcomes included the dynamic changes in the virus copies, cytokine profile, SARS-CoV-2–speicific antibody, and its correlation with clinical treatment response.
Statistical analysis
The trial was initiated in rapid response to COVID-19 public health emergency. Because limited information about clinical outcomes in hospitalized patients with COVID-19 was available at that time, we estimated the sample size in 2 different ways. We assumed that the median clinical improvement for the treatment group is 7 days, whereas that for the control group is about 15 days and the estimated sample size was set at 70 to provide the trial with 80% power (α = 0.05). We also hypothesized that there was 40% difference gap in terms of CT improvement at D14 between the 2 groups, with approximately 90% patients having CT improvement in the treatment group. Accordingly, the estimated sample size was set at 40 to provide the trial with 80% power (α = 0.05). We decided to set the final sample size to 70 cases to reduce the possibility of underpower of the trial. The number of cases in the experimental group and the control group was 1:1. The planned enrollment of 40 patients in the trial was accomplished quickly. Because of the slowing down of the pandemic in China and the fact that few newly diagnosed patients are available, recruitment to the trial was stopped earlier than expected.
Continuous variables were expressed as median (interquartile range [IQR]) and compared with the unpaired 2-sided student t test; categorical variables were expressed as number (%) and compared by chi-square test or Fisher exact test. A modified intention-to-treat analysis that excluded 2 patients (1 ineligible, 1 consent withdrawn) who did not take ruxolitinib in the ruxolitinib group was performed. For the primary end point, the time to clinical improvement was portrayed by Kaplan-Meier plot and compared using a log-rank test. Hazard ratios with 95% CIs were calculated using Mantel-Haenszel approach. The improvement rates of CT scan at D14 were compared using Wilcoxon rank sum test. The clinical improvement at D7, D14, and D21, time to clinical deterioration, clinical deterioration at D7 and D14, and mortality rate at D28 were compared using the Fisher exact tests. Time from randomization to discharge, to death, to lymphocyte recovery, and to virus clearance were portrayed by Kaplan-Meier plot and compared using a log-rank test. For comparing the serum level of cytokines, anti–SARS-CoV-2 specific antibody, and virus copy numbers, mean ± SEM is given for continuous variables and median and ranges are given for variables that were not normally distributed. Means were compared using t tests for normally distributed continuous variable. Otherwise, the Mann-Whitney U test was used. All statistical analyses were performed using SPSS (Statistical Package for the Social Science) version 13.0 software (SPSS Inc, Chicago, Ill). P value less than .05 (2-tailed) was considered statistically significant.
Results
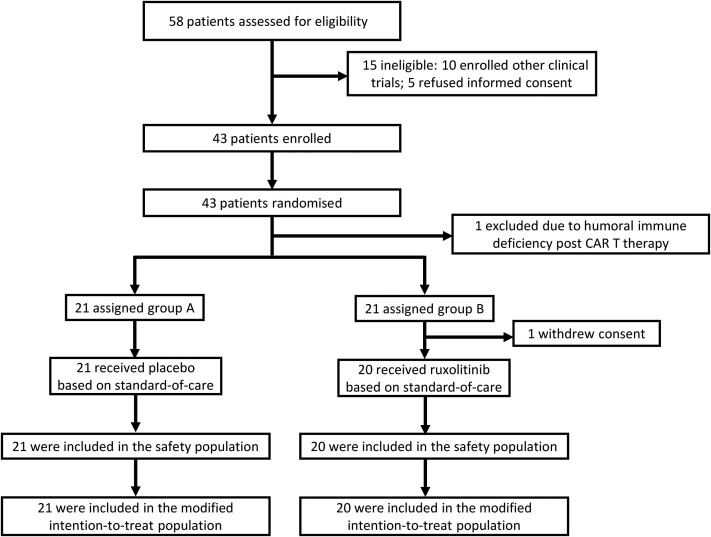
Among a total of 58 individuals who were screened for eligibility between February 9 and February 28, 2020, 43 patients were randomly assigned to receive ruxolitinib plus SoC treatment (22 patients, ruxolitinib group) or placebo based on SoC treatment (21 patients, control group). Fifteen patients were excluded from the study including 10 of them who participated in other clinical trials and 5 of them who refused to sign a written informed consent. After randomization, 2 patients were excluded from the ruxolitinib group because 1 was found to be ineligible because of persistent humoral immune deficiency after B-cell mature antigen–targeting chimeric antigen receptor T-cell therapy, while another withdrew the consent before treatment started (Fig 1 ). Their data were not included in the analyses. The demographic and clinical characteristics of the patients at baseline are outlined in Table I . At baseline, the median age of patients was 63 years (IQR, 58-68 years), ranging from 32 years to 75 years, and 58.5% of the patients were males. The median interval time from symptom onset to randomization was 20 days. There was no significant difference between the 2 groups in demographic characteristics, baseline laboratory test results, distribution of ordinal scale scores, or National Early Warning Score 2 scores at enrollment (Tables I and II ). During the study, the use of systemic corticosteroids was balanced between the ruxolitinib group (70.0%) and the control group (71.4%). The proportion of patients who received antivirals was balanced between the 2 groups (90.0% in the ruxolitinib group vs 90.5% in the control group, Table II).
Fig 1.
Randomization and trial profile. CAR, Chimeric antigen receptor.
Table I.
Demographic and clinical characteristic of the patients at baseline
| Characteristic | Total | Control group |
Ruxolitinib group |
P value |
|---|---|---|---|---|
| (N = 21) | (N = 20) | |||
| Age (y) | 63 (58-68) | 64 (59-71) | 63 (51-65) | .123 |
| Sex | >.999 | |||
| Female | 17 (41.5) | 9 (42.9) | 8 (40.0) | |
| Male | 24 (58.5) | 12 (57.1) | 12 (60.0) | |
| Comorbidity | 27 (65.9) | 13 (61.9) | 14 (70.0) | .744 |
| Hypertension | 16 (39.0) | 9 (42.9) | 7 (35.0) | .751 |
| Diabetes | 8 (19.5) | 3 (14.3) | 5 (25.0) | .454 |
| Coronary artery heart disease | 3 (7.3) | 1 (4.8) | 2 (10.0) | .606 |
| Smoking history | 4 (9.8) | 2 (9.5) | 2 (10.0) | >.999 |
| Respiratory rate >24 breaths/min | 8 (19.5) | 4 (19.0) | 4 (20.0) | >.999 |
| Pulse ≥125 beats/min | 7 (17.1) | 3 (14.3) | 4 (20.0) | .697 |
| Fever (temperature ≥37.3°C) | 5 (12.2) | 2 (9.5) | 3 (15.0) | .663 |
| White-cell count (×109/L) | 8.4 (6.1-11.0) | 8.3 (6.7-11.0) | 8.4 (5.6-11.0) | .652 |
| <4 | 2 (4.9) | 0 (0.0) | 2 (10.0) | .512 |
| 4-10 | 26 (63.4) | 14 (66.7) | 12 (60.0) | |
| >10 | 13 (31.7) | 7 (33.3) | 6 (30.0) | |
| Lymphocyte count (×109/L) | 1.1 (0.92-1.6) | 1.2 (0.97-2.0) | 1.0 (0.76-1.2) | .084 |
| ≥1.0 | 25 (61.0) | 15 (71.4) | 10 (50.0) | .208 |
| <1.0 | 16 (39.0) | 6 (28.6) | 10 (50.0) | |
| Platelet count | 264 (173-314) | 214 (175-285) | 297 (165-355) | .114 |
| ≥100 | 36 (87.8) | 19 (90.5) | 17 (85.0) | .663 |
| <100 | 5 (12.2) | 2 (9.5) | 3 (15.0) | |
| Serum creatinine (μmol/L) | 67 (56-75) | 66 (60-74) | 69 (52-75) | .872 |
| ≤133 | 38 (92.7) | 20 (95.2) | 18 (90.0) | .606 |
| >133 | 3 (7.3) | 1 (4.8) | 2 (10.0) | |
| Aspartate aminotransferase (U/L) | 36 (23-68) | 35 (19-88) | 39 (26-52) | .742 |
| ≤40 | 24 (58.5) | 13 (61.9) | 11 (55.0) | .758 |
| >40 | 17 (41.5) | 8 (38.1) | 9 (45.0) | |
| Alanine aminotransferase (U/L) | 25 (17-46) | 23 (18-50) | 26 (17-47) | .841 |
| ≤50 | 30 (73.2) | 15 (71.4) | 15 (75.0) | >.999 |
| >50 | 11 (26.8) | 6 (28.6) | 5 (25.0) | |
| Lactate dehydrogenase (U/L) | 275 (225-413) | 300 (226-438) | 262 (213-384) | .489 |
| ≤245 | 16 (39.0) | 8 (38.1) | 8 (40.0) | >.999 |
| >245 | 25 (61.0) | 13 (61.9) | 12 (60.0) | |
| Albumin (g/L) | 32.0 (30.0-34.0) | 32.0 (30.0-34.0) | 32.0 (30.0-35.0) | .821 |
| ≤35 | 32 (78.0) | 17 (81.0) | 15 (75.0) | .719 |
| >35 | 9 (22.0) | 4 (19.0) | 5 (25.0) | |
| D-Dimer (μg/mL) | 2.4 (0.65-7.5) | 2.5 (0.68-15.0) | 2.1 (0.62-3.5) | .248 |
| ≤1.0 | 14 (34.1) | 6 (28.6) | 8 (40.0) | >.999 |
| >1.0 | 23 (56.1) | 13 (61.9) | 10 (50.0) | |
| Missing data | 4 (9.8) | 2 (9.5) | 2 (10.0) | |
| High-sensitive cardiac troponin I (ng/mL) | 3.5 (2.0-6.3) | 3.0 (1.6-6.8) | 4.1 (2.2-6.5) | .483 |
| ≤28.0 | 33 (80.5) | 18 (85.7) | 15 (75.0) | .653 |
| >28.0 | 5 (12.2) | 2 (9.5) | 3 (15.0) | |
| Missing data | 3 (7.3) | 1 (4.8) | 2 (10.0) |
Data are median (IQR) or n (%).
Table II.
Patients’ status and treatments received at or after enrollment
| Characteristic | Total |
Control group |
Ruxolitinib group |
P value |
|---|---|---|---|---|
| (N = 41) | (N = 21) | (N = 20) | ||
| NEWS2 score at D1 | 5 (4-6) | 4 (4-5) | 5 (4-7) | .318 |
| Days from illness onset to randomization | 20 (17-28) | 22 (18-28) | 20 (16-27) | .457 |
| 7-category scale at day 1 | >.999 | |||
| 4: Hospitalization, requiring supplemental oxygen | 36 (87.8) | 18 (85.7) | 18 (90.0) | |
| 5: Hospitalization, requiring HFNC or noninvasive mechanical ventilation | 5 (12.2) | 3 (14.3) | 2 (10.0) | |
| Treatments during study period | ||||
| Vasopressor | 3 (7.3) | 3 (14.3) | 0 | .232 |
| Noninvasive mechanical ventilation | 6 (14.6) | 4 (19.0) | 2 (10.0) | .663 |
| Invasive mechanical ventilation | 3 (7.3) | 3 (14.3) | 0 | .232 |
| Glucocorticoid therapy | 29 (70.7) | 15 (71.4) | 14 (70.0) | >.999 |
| Renal-replacement therapy | 2 (4.9) | 2 (9.5) | 0 | .488 |
| Intravenous immunoglobin | 18 (43.9) | 11 (52.4) | 7 (35.0) | .350 |
| Antibiotic agent | 20 (48.8) | 12 (57.1) | 8 (40.0) | .354 |
| Antiviral agent∗ | 37 (90.2) | 19 (90.5) | 18 (90.0) | >.999 |
| Abidol | 27 (73.0) | 15 (78.9) | 12 (66.7) | .476 |
| Oseltamivir | 10 (27.0) | 4 (21.1) | 6 (33.3) |
HFNC, High-flow nasal cannula for oxygen therapy; NEWS2, National Early Warning Score 2.
Data are median (IQR) or n (%).
Antiviral agent included oral abidol or oseltamivir.
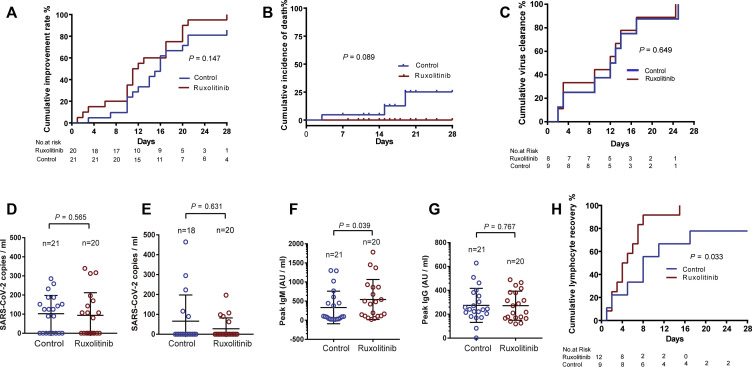
For primary efficacy end points, no statistical differences were detected in terms of clinical improvement between the 2 groups, although patients treated with ruxolitinib had a numerically shorter median time to clinical improvement (12 [IQR, 10-19] days vs 15 [IQR, 10-18] days; log-rank test P = .147; hazard ratio, 1.669; 95% CI, 0.836-3.335) compared with the control group (Table III and Fig 2 , A). Eighteen (90%) patients from the ruxolitinib group showed significant improvement on the follow-up chest CT scans at D14 compared with only 13 (61.9%) patients from the control group (P = .0495; Table III). No statistical differences were detected in terms of the percentage of improvement rates at D7, D14, and D21 between the 2 groups; however, the percentage was numerically higher at D7, D14, and D21 in the ruxolitinib group than in the control group. A total of 4 patients in control group experienced clinical deterioration. Three of 4 were transferred to the ICU and required invasive mechanical ventilation. The cumulative improvement rate was compared between the 2 groups (Fig 2, A).
Table III.
Outcomes in the modified intent-to-treat population
| Characteristic | Total |
Control group |
Ruxolitinib group |
P value |
|---|---|---|---|---|
| (N = 41) | (N = 21) | (N = 20) | ||
| Time to clinical improvement (d) | 14 (10-18) | 15 (10-18) | 12 (10-19) | .147 |
| Clinical improvement | ||||
| D7 | 6 (14.6) | 2 (9.5) | 4 (20.0) | .410 |
| D14 | 21 (51.2) | 9 (42.9) | 12 (60.0) | .354 |
| D21 | 36 (87.8) | 18 (85.7) | 18 (90.0) | >.999 |
| Time to clinical deterioration (d) | 6 (2-12) | 6 (2-12) | 0 | |
| Clinical deterioration | ||||
| D7 | 3 (7.3) | 3 (14.3) | 0 | .232 |
| D14 | 4 (9.8) | 4 (19.0) | 0 | .107 |
| CT scan follow-up at D14 | .0495 | |||
| Improvement | 31 (75.6) | 13 (61.9) | 18 (90.0) | |
| Stable | 7 (17.1) | 6 (28.6) | 1 (5.0) | |
| Progression | 3 (7.3) | 2 (9.5) | 1 (5.0) | |
| D28 mortality | 3 (7.3) | 3 (14.3) | 0 | .232 |
| Time from randomization to discharge (d) | 16 (11-20) | 16 (11-20) | 17 (11-21) | .941 |
| Duration of invasive mechanical ventilation (d) | 5 (2-8) | 5 (2-8) | 0 | |
| Time from randomization to death (d) | 15 (4-19) | 15 (4-19) | 0 | |
| Time to lymphocyte recovery (d) | 5 (2-8) | 8 (2-11) | 5 (2-7) | .033 |
| Virus clearance time (d) | 12 (3-16) | 12 (3-16) | 13 (5-16) | .649 |
Data are median (IQR) or n (%).
Fig 2.
Primary and secondary outcomes. A, The cumulative improvement rate in modified intention-to-treat analysis patients. B, Cumulative 28 days incidence of death. C, Cumulative incidence of virus clearance rate in analyzed patients. D, Comparison of blood viral loads of the control group and the ruxolitinib group at D1. E, Comparison of blood viral loads of the control group and the ruxolitinib group at discharge. F and G, The peak levels of SARS-CoV-2–specific IgM and IgG. H, Cumulative incidence of lymphocyte recovery rate in analyzed patients.
For secondary end point, 3 patients from the control group eventually died of respiratory failure. The 28-day overall mortality was 14.3% in the control group, whereas no patients died in the ruxolitinib group. The median time from randomization to death was 15 days (IQR, 4∼19) in the control group. There was no significant difference in the days from randomization to discharge between the 2 groups (Table III; P = .941). The cumulative incidence of death was compared between the 2 groups (Fig 2, B; P = .089).
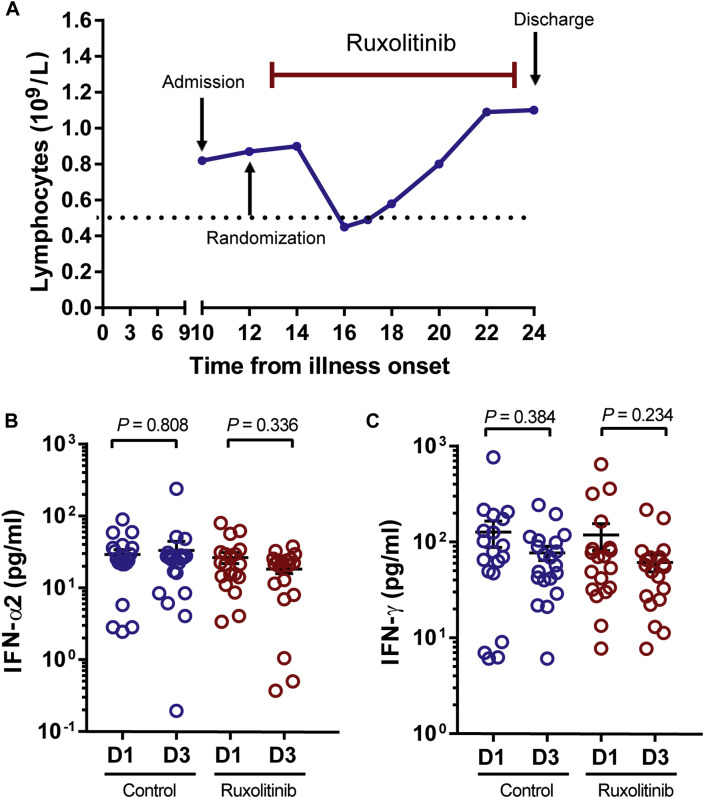
For primary safety end points, a total of 16 patients (80%) in the ruxolitinib group and 15 patients (71.4%) in the control group reported adverse events from randomization to D28. All adverse events are summarized in Table IV . There was no significant difference in the total number of adverse events of any grade in hematological, nonhematological toxicities and chemical laboratory abnormalities between the 2 groups (see Table E1 in this article’s Online Repository at www.jacionline.org). One patient in the ruxolitinib group developed grade 3 lymphocytopenia after taking ruxolitinib for 4 days, which improved in 2 days without interrupting ruxolitinib treatment (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). Grade 3 hypertension developed in 1 patient in the ruxolitinib group during the study and was judged by the investigators to be related to the trial medication and was transient and reversible. All serious adverse events, including secondary infection, sepsis, shock, and acute heart failure, occurred only in the control group. To address the concern that whether ruxolitinib has a negative influence on SARS-CoV-2 clearance, specific SARS-CoV-2 antibody production, and lymphocyte recovery, a total of 17 patient (8 patients in the ruxolitinib group and 9 patients in the control group) who had a positive RT-PCR result at D0 on the throat swab were serially followed up. Patients in the ruxolitinib group had similar median time of virus clearance (13 [IQR, 5-16] days vs 12 [IQR, 3-16] days; log-rank test P = .649; hazard ratio, 0.782; 95% CI, 0.271-2.257) compared with patients in the control group (Fig 2, C). One-step RT-ddPCR was used to further evaluate the clearance of SARS-CoV-2; the mean (± SEM) baseline blood viral RNA loads at D1 in the ruxolitinib group were comparable with those in the control group (Fig 2, D; 94 ± 26 copies/mL vs 102 ± 21 copies/mL; P = .565). The viral load at discharge did not differ between the ruxolitinib recipients and those receiving SoC treatment alone (Fig 2, E; P = .631). Interestingly, the peak level of anti-IgM of SARS-CoV-2 was profoundly higher in the ruxolitinib group than in the control group, whereas no significant difference was found in peak IgG between the 2 groups (Fig 2, F and G). A total of 21 patients (9 patients in the control group and 12 patients in the ruxolitinib group) were found to have lymphopenia at or after enrollment. Patients in the ruxolitinib group had a significantly shorter median time of recovery from lymphopenia compared with those in the control group (Fig 2, H; 5 [IQR, 2-7] days vs 8 [IQR, 2-11] days; log-rank test P = .033; hazard ratio, 3.307; 95% CI, 1.097-8.409).
Table IV.
Summary of adverse events∗
| Adverse event | Control group (N = 21) |
Ruxotlitinib group (N = 20) |
||||
|---|---|---|---|---|---|---|
| Any grade | Grades 1-2 | Grades 3-4 | Any grade | Grades 1-2 | Grades 3-4 | |
| Hematological adverse events | 12 (57.1) | 10 (47.6) | 2 (9.5) | 13 (65.0) | 12 (60.0) | 1 (5.0) |
| Neutrocytopenia | 1 (4.8) | 1 (4.8) | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Lymphocytopenia | 4 (19.0) | 3 (14.3) | 1 (4.8) | 2 (10.0) | 1 (5.0) | 1 (5.0) |
| Anemia | 9 (42.9) | 8 (38.1) | 1 (4.8) | 11 (55.0) | 11 (55.0) | 0 |
| Thrombocytopenia | 3 (14.3) | 2 (9.5) | 1 (4.8) | 4 (20.0) | 4 (20.0) | 0 |
| Chemical laboratory abnormalities | 7 (33.3) | 7 (33.3) | 0 | 10 (50.0) | 10 (50.0) | 0 |
| Alanine aminotransferase increase | 2 (9.5) | 2 (9.5) | 0 | 7 (35.0) | 7 (35.0) | 0 |
| Aspartate aminotransferase increase | 1 (4.8) | 1 (4.8) | 0 | 3 (15.0) | 3 (15.0) | 0 |
| Alkaline phosphatase increase | 1 (4.8) | 1 (4.8) | 0 | 2 (10.0) | 2 (10.0) | 0 |
| γ-GT increase | 2 (9.5) | 2 (9.5) | 0 | 2 (10.0) | 2 (10.0) | 0 |
| Hypoalbuminemia | 3 (14.3) | 3 (14.3) | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Hypercholesterolemia | 4 (19.0) | 4 (19.0) | 0 | 4 (20.0) | 4 (20.0) | 0 |
| Hypertriglyceridemia | 2 (9.5) | 2 (9.5) | 0 | 0 | 0 | 0 |
| Hypokalemia | 1 (4.8) | 1 (4.8) | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Hypochloremia | 2 (9.5) | 2 (9.5) | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Hypocalcemia | 2 (9.5) | 2 (9.5) | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Adverse events | 6 (28.6) | 6 (28.6) | 0 | 7 (35.0) | 7 (35.0) | 0 |
| Headache | 0 | 0 | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Dizziness | 1 (4.8) | 1 (4.8) | 0 | 2 (10.0) | 2 (10.0) | 0 |
| Rash | 1 (4.8) | 1 (4.8) | 0 | 2 (10.0) | 2 (10.0) | 0 |
| Nausea | 2 (9.5) | 2 (9.5) | 0 | 2 (10.0) | 2 (10.0) | 0 |
| Decreased appetite | 2 (9.5) | 2 (9.5) | 0 | 1 (5.0) | 1 (5.0) | 0 |
| Hypertension | 2 (9.5) | 2 (9.5) | 0 | 1 (5.0) | 0 | 1 (5.0) |
| Serious adverse events | 4 (19.0) | 0 | 4 (19.0) | 0 | 0 | 0 |
| Secondary infection | 2 (9.5) | 0 | 2 (9.5) | 0 | 0 | 0 |
| Acute heart failure | 2 (9.5) | 0 | 2 (9.5) | 0 | 0 | 0 |
| Shock | 2 (9.5) | 0 | 2 (9.5) | 0 | 0 | 0 |
| Sepsis | 1 (4.8) | 0 | 1 (4.8) | 0 | 0 | 0 |
Adverse events that occurred in more than 1 patient after randomization through D28 are shown. Some patients had more than 1 adverse event. The proportions of patients with values worse than baseline values are listed here. All deaths were due to respiratory failure.
Fig E1.
The dynamic change in lymphocytes, IFN-α2, and IFN-γ after treatment with ruxolitinib. A, Dynamic changes in lymphocytes in the patient who developed grade 3 lymphocytopenia after ruxolitinib treatment. The dotted line indicates absolute lymphocyte of 0.5 × 109/L. (B) IFN-α2 and (C) IFN-γ were assessed on D1 and D3 in the ruxolitinib group and the control group. Scatter plots represent the levels of IFN-α2 or IFN-γ.
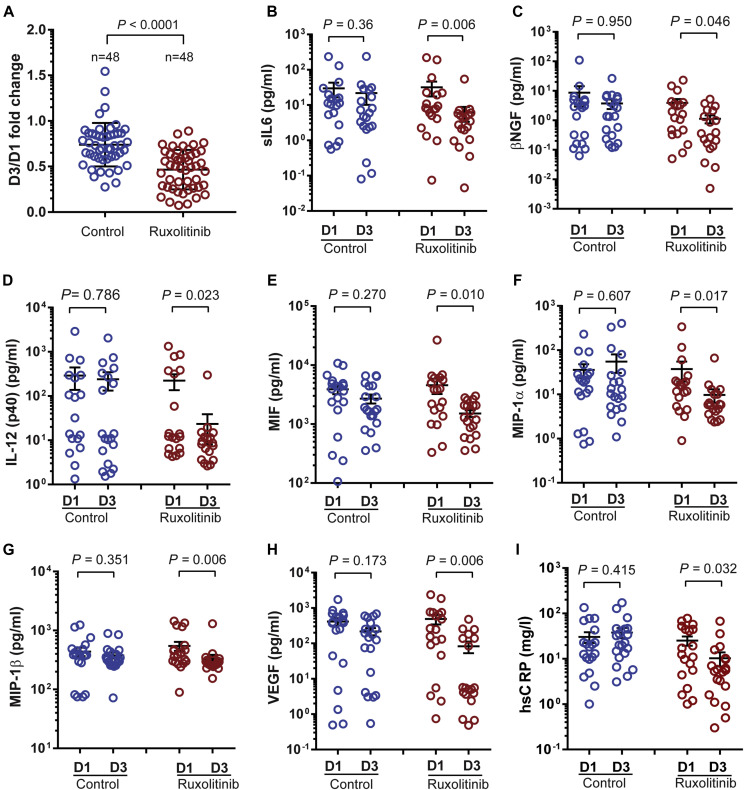
We investigated whether ruxolitinib could inhibit cytokines downstream of JAK by assessing the levels of 48 cytokines in the serum of patients who received ruxolitinib and controls. As shown in Fig 3 , A, in the control group, the patients’ average value of 44 cytokines decreased after SoC therapy, whereas the other 4—macrophage inflammatory protein 1α (MIP-1α), G-CSF, IFN-α2, and IL-1α—increased on D3. On the contrary, all average values from 48 cytokines decreased in patients from the ruxolitinib group on D3. Furthermore, the average fold-change in the ruxolitinib group was 0.466, whereas it was 0.739 in the control group. The ratios were significantly lower in the ruxolitinib group (P < .0001). Moreover, the levels of 7 cytokines—IL-6, nerve growth factor β, IL-12 (p40), macrophage migration inhibitory factor, MIP-1α, macrophage inflammatory protein 1β (MIP-1β), and vascular endothelial growth factor (VEGF)—were markedly decreased in the ruxolitinib group but not in the control group (Fig 3, B-H). In correlation with the reduction in these cytokines, a significant reduction in high-sensitivity C-reactive protein was observed in the ruxolitinib group but not in the control group (Fig 3, I). Although all the 3 ruxolitinib recipients with fever had rapid resolution of fever in 2 days, fever resolution took 4 or 5 days in 2 patients with fever in the control group. Furthermore, there was no significant difference in the levels of IFN-α2 and IFN-γ, 2 important cytokines protecting hosts against virus, between the ruxolitinib group and the control group (see Fig E1, B and C).
Fig 3.
Serial cytokine assessment of 48 cytokines and hsCRP was performed in the ruxolitinib group and the control group. A, The ratio of the mean value of each cytokine at D3 and D1 after randomization. B-H, Stacked scatter plots demonstrated cytokines, which were significantly decreased in the ruxolitinib group. I, hsCRP was significantly decreased in the ruxolitinib group. hsCRP, High-sensitivity C-reactive protein; MIF, migration inhibitory factor. All data represent mean ± SEM.
Discussion
This randomized trial found that ruxolitinib added to SoC treatment was not associated with significantly accelerated clinical improvement in severe patients with COVID-19, although ruxolitinib recipients had a numerically faster clinical improvement compared with the control group. Ruxolitinib recipients showed significantly faster improvement in the chest CT at D14 compared with the control group (18 [90%] vs 13 [61.9%]; P = .0495). The 28-day mortality was 14.3% in the comparison group. No death or deterioration occurred in ruxolitinib recipients. These data provide a rationale for further trials to determine whether ruxolitinib treatment can reduce the overall incidence of deterioration and death. Patients treated with ruxolitinib showed a significantly shorter lymphocyte recovery than those in the control group (5 [IQR, 2-7] days vs 8 [IQR, 2-11] days; P = .033). We assume that a faster recovery from lymphopenia is of clinical relevance because lymphopenia was associated with poor prognosis. A shorter duration of lymphopenia in ruxolitinib recipients was consistent with a higher mean peak level of IgM specific for SARS-CoV-2 in patients treated with ruxolitinib. Among 4 patients from the control group who experienced clinical deterioration, 3 were transferred to the ICU and required invasive mechanical ventilation. Three patients in the control group eventually died of respiratory failure. The demographic and clinical characteristics of the patients were balanced between the 2 groups at enrollment. The use of corticosteroids and antivirals was comparable between the control and ruxolitinib groups. Therefore, it was unlikely that the baseline characteristics and treatments of the 2 groups would affect the end points of our study.
The present randomized trial also found that ruxolitinib with SoC treatment was well tolerated with low hematological and nonhematological toxicities. All ruxolitinib recipients completed the full course of administration until discharge, whereas the control group needed more intensive supportive treatments after enrollment due to the deterioration in some cases. The addition of ruxolitinib based on SoC did not increase the risk of adverse events compared with the control group. The overall incidence of adverse events was similar between the 2 groups. Interestingly, although most of the adverse events occurred at grade 1 or 2, adverse events at grade 3 or 4 and serious adverse events were more common in the control group due to the progressive deterioration in patients with COVID-19 in this group. Among all ruxolitinib recipients, only 2 adverse events at grade 3 occurred and were transient and reversible. There were no unexpected adverse events and previously unknown events in ruxolitinib recipients. One of the major concerns related to the use of ruxolitinib in the treatment of COVID-19 is its therapeutic action in reducing systemic inflammation, and potential to unfavorably delay the clearance of viral loads and impair the generation of SARS-CoV-2–specific antibodies. In the current study, there was no significant difference in viral RNA loads or duration as well as IFN-α2 and IFN-γ levels between ruxolitinib recipients and the control group. Interestingly, the mean peak level of IgM specific for SARS-CoV-2 was profoundly higher in the ruxolitinib group compared with the control group, whereas no significant difference was found in the mean peak IgG against SARS-CoV-2 between the 2 groups. The favorable side-effect profile observed in the current trial provides a rationale for the initiation of a large-scale clinical trial at the same or higher ruxolitinib dose regimens in efforts to improve outcomes.
In the present study, we found that the addition of ruxolitinib to SoC treatment could significantly mitigate exuberant cytokine storm featured in severe COVID-19, which justified the use of ruxolitinib for reduction of systemic inflammation. In 2 published autopsy reports,33 , 34 it was identified that the severe immune injury was also involved in other organs without obvious viral inclusions, thus indicating the important role of cytokines storm instead of the direct viral damage to the whole body. The infiltrated immune cells in alveoli were mostly macrophages and monocytes, which was in accordance with our findings on cytokines changes. In particular, the levels of 7 cytokines—IL-6, nerve growth factor β, IL-12(p40), migration inhibitory factor, MIP-1α, MIP-1β, and VEGF—were markedly decreased in patients who received ruxolitinib in comparison to the control group. Among these cytokines, IL-6 has been reported as a critical cytokine driving proinflammatory activity in cytokine-mediated organ dysfunction and tissue damage35 and IL-6–directed therapy as the cornerstone of cytokine-based therapy after chimeric antigen receptor T-cell therapy.36 , 37 IL-12(p40), MIP-1α, and MIP-1β are critical chemokines for the recruitment of activated monocytes/macrophages and other cells to the site of infection.38, 39, 40 VEGF, which has been reported to recruit monocytes/macrophages, participates in increased capillary permeability syndrome that characterizes some types of viral pneumonia.41 These results indicate that ruxolitinib may exert its inhibitory effect by targeting multiple critical cytokines rather than any specific cytokine, and these cytokines could be used as surrogate biomarkers in future ruxolitinib trials.
The present study has several limitations. First, the sample size was small due to no eligible patients available at the end of the pandemic at our trial centers and the few end points reached statistical significance. The safety profile during this study was favorable, but further testing in larger patient cohorts with different ethnicity or disease status is required. Second, there are some limitations related to the ordinal scale that was used to evaluate primary end points. Because of the severity of the epidemic, even if the patients had a significant improvement in the CT scan as well as clinical symptoms, they still asked for nasal cannula oxygen (<2 L/min) until being discharged from hospital, which may contribute to the nonstatistically significant P value of clinical improvement. Finally, critically ill patients or patients with invasive ventilator dependence were not included in this study because of the lack of previous data and our concerns on the unknown safety profile of ruxolitinib treatment in pneumonia. Therefore, our conclusion is confined to patients with severe COVID-19. Nevertheless, this study is the first randomized controlled trial on the use of ruxolitinib in patients with severe COVID-19 based on a novel therapeutic rationale. These findings are hypothesis-generating and require additional larger controlled studies to confirm the possibility of a treatment benefit of ruxolitinib. However, these early data were promising and informative to future trials with ruxolitinib or other JAK1/2 inhibitors.
Clinical implications.
The favorable side-effect profile combined with a reduction in inflammation and significant chest CT improvements in the ruxolitinib plus SoC treatment group should inform future trials in a larger population to assess with ruxolitinib or other JAK1/2 inhibitors in patients with COVID-19.
Acknowledgments
We appreciate eStart Medical Technology Co, Ltd, Professor & Dr Jie Hou, Meng Chen, Zhongyan Zhang, Xu Ji, Chun Li, and so forth for discussion of study design, medical affairs, data management, and statistics. We also appreciate Professor Guangliang Shan and Wei Han, PhD, from the Department of Epidemiology and Biostatistics, School of Basic Medicine, Peking Union Medical College, for their kind assistance in statistical analysis.
Footnotes
This work was supported by the Emergency Research Project of Tongji Hospital (to J.Z.), Emergency Research Project of Tongji Hospital of Huazhong University of Science and Technology (grant no. 2020kfyXGYJ045 to J.Z.), and Emergency Research Project of Hubei province (grant no. 2020FCA006 to W.W.). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Methods
Clinical diagnosis and classification criteria of 2019-nCoV pneumonia (5th trial edition) Hubei Province Standard
Diagnostic criteria (Hubei province)
-
1.
Suspected cases
- Comprehensive analysis based on the following epidemiological history and clinical features:
-
1)Epidemiological history
-
(1)History of travel or residency in Wuhan and its surrounding areas or other communities with case reports within 14 days before the onset of illness;
-
(2)Exposure to people from Wuhan and its surrounding areas, or patients with fever or respiratory symptoms in communities with case reports within 14 days before the onset of illness;
-
(3)Clustered onset;
-
(4)History of exposure to patients infected with 2019-nCoV within 14 days, who were those with positive result of nucleic acid test.
-
(1)
-
2)Clinical features
-
(1)Fever and/or respiratory symptoms;
-
(2)The total white-cell count was normal or decreased, or the lymphocyte count was decreased in the early stage of disease.
-
(3)Having any of the epidemiological history or no epidemiological history, and meeting 2 of the clinical features at the same time.
-
(1)
-
1)
-
2.
Clinically diagnosed cases
Suspected cases with imaging features of pneumonia.
-
3.
Confirmed cases
- Clinically diagnosed or suspected cases with 1 of the following etiological evidence:
-
1)Real-time fluorescent RT-PCR of respiratory or blood specimens showed positive result of nucleic acid test for 2019-nCoV;
-
2)Result of gene sequencing of virus in respiratory or blood specimens was highly homologous to known 2019-nCoV.
- The severity of COVID-19 was classified as follows:
-
(1)Mild.
- The clinical symptoms were mild, and there were no imaging features of pneumonia.
-
(2)General.
- There was fever, respiratory symptoms, as well as imaging features of pneumonia.
-
(3)Severe.
- Meeting any of the following items:
-
•Respiratory distress, respiratory rate greater than or equal to 30 breaths/min;
-
•In the resting state, the oxygen saturation less than or equal to 93%;
-
•Partial pressure of oxygen in arterial blood/fraction of inspired oxygen less than or equal to 300 mm Hg (1 mm Hg = 0.133 kPa).
-
•
-
(4)Critically ill.
- Meeting any of the following items:
-
•Respiratory failure needing mechanical ventilation;
-
•Shock;
-
•Being complicated with other organ failures needing ICU monitoring and treatment.
-
•
-
(1)
-
1)
Sample collection
Serum samples were collected using a serum separator tube and allow samples to clot for 30 minutes at room temperature before centrifuging for 15 minutes at 1000g. Remove serum and assay immediately or aliquot and store samples at less than or equal to −20°C. Avoid repeated freeze-thaw cycles. Plasma samples were collected using EDTA or heparin as an anticoagulant. Centrifuge for 15 minutes at 1000g. Assay immediately or aliquot and store samples at less than or equal to −20°C. Avoid repeated freeze-thaw cycles.
Determination of SARS-CoV-2 copies number by 1-step RT-ddPCR
For quantitative detection of SARS-CoV-2 copies number, Viral RNA purification kit (QIAamp Viral RNA Mini Kit, Qiagen, Duesseldorf, Germany), 1-step RT-ddPCR advanced kit, QX200 droplet generator (BioRad, Hercules, Calif), and QX200 droplet reader (BioRad) were used following the manufacturer’s instructions. To increase sensitivity, the 4-well test was performed per sample in this study. The SARS-CoV-2–specific minor groove binder probe-primer set was designed for targeting the orf1ab region and the sequences were as follows: forward primer 5′TGA CCC TGT GGG TTT TAC ACT TAA3′; reverse primer 5′CAGCCATAACCTTTCCACATACC3′; probe 5′-FAM-AAC ACA GTC TGT ACC GTC T-MGB-3′.
SARS-CoV-2–specific IgM and IgG detection
The SARS-CoV-2–specific IgM and IgG were detected by paramagnetic particle chemiluminescent immunoassay using iFlash-SARS-CoV-2 IgM/IgG assay kit (Shenzhen Yhlo Biotech Co, Ltd, Shenzhen, China) and iFlash Immunoassay Analyzer (Shenzhen Yhlo Biotech Co, Ltd).
Cytokines measurements and analysis
The levels of serum cytokines, growth factors, and chemokines were assessed by Bio-Plex Pro Human Cytokines 48-Plex Screening assay (BioRad) using a Luminex FLEXMAP 3D system according to the manufacturer’s protocols. The 48-Plex Screening panel: basic fibroblast growth factor (Basic FGF), cutaneous T-cell attracting chemokine (CTACK), eotaxin, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth regulated oncogene (GRO)-α, human hepatocyte growth factor (HGF), intercellular cell adhesion molecule-1 (ICAM-1), IFN-α2, IFN-γ, IL-1α, IL-1rα, IL-2, IL-2Rα, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-16, IL-17A, IL-18, interferon-inducible protein-10 (IP-10), leukemia inhibitory factor (LIF), monocyte chemoattractant protein-1 (MCP-1), MCP-3, macrophage colony stimulating factor (M-CSF), migration inhibitory factor (MIF), monokine induced by gamma interferon (MIG), macrophage inflammation protein-1α (MIP-1α), MIP-1β, β-nerve growth factor (β-NGF), platelet derived growth factor BB (PDGF-BB), reduced upon activation mormal T expression and secreted (RANTES), stem cell factor (SCF), stem cell growth factor-β (SCGF-β), stromal cell-derived factor-1 (SDF-1α), tumor necrosis factor (TNF)-α, TNF-β, TNF-related apoptosis-inducing ligand (TRAIL), vascular cell adhesion molecule-1 (VCAM-1), vascular endothelial growth factor- A (VEGF-A).
The 7-category ordinal scale
The 7-category ordinal scale consists of the following criteria: (1) not hospitalized and able to resume normal activities; (2) not hospitalized, but unable to resume normal activities; (3) hospitalized, not requiring supplemental oxygen; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; (6) hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; and (7) death.
Table E1.
Summary of adverse events of any grade
| Adverse event | Control (N = 21) | Ruxotlitinib (N = 20) | P value |
|---|---|---|---|
| Hematological adverse events | 12 (57.1) | 13 (65.0) | .751 |
| Neutrocytopenia | 1 (4.8) | 1 (5.0) | >.999 |
| Lymphocytopenia | 4 (19.0) | 2 (10.0) | .663 |
| Anemia | 9 (42.9) | 11 (55.0) | .538 |
| Thrombocytopenia | 3 (14.3) | 4 (20.0) | .697 |
| Chemical laboratory abnormalities | 7 (33.3) | 10 (50.0) | .350 |
| Alanine aminotransferase increase | 2 (9.5) | 7 (35.0) | .067 |
| Aspartate aminotransferase increase | 1 (4.8) | 3 (15.0) | .343 |
| Alkaline phosphatase increase | 1 (4.8) | 2 (10.0) | .606 |
| γ-GT increase | 2 (9.5) | 2 (10.0) | >.999 |
| Hypoalbuminemia | 3 (14.3) | 1 (5.0) | .606 |
| Hypercholesterolemia | 4 (19.0) | 4 (20.0) | >.999 |
| Hypertriglyceridemia | 2 (9.5) | 0 | .488 |
| Hypokalemia | 1 (4.8) | 1 (5.0) | >.999 |
| Hypochloremia | 2 (9.5) | 1 (5.0) | >.999 |
| Hypocalcemia | 2 (9.5) | 1 (5.0) | >.999 |
| Adverse events | 6 (28.6) | 7 (35.0) | .744 |
| Headache | 0 | 1 (5.0) | .488 |
| Dizziness | 1 (4.8) | 2 (10.0) | .606 |
| Rash | 1 (4.8) | 2 (10.0) | .606 |
| Nausea | 2 (9.5) | 2 (10.0) | >.999 |
| Decreased appetite | 2 (9.5) | 1 (5.0) | >.999 |
| Hypertension | 2 (9.5) | 1 (5.0) | >.999 |
| Serious adverse events | 4 (19.0) | 0 | .107 |
| Secondary infection | 2 (9.5) | 0 | .488 |
| Acute heart failure | 2 (9.5) | 0 | .488 |
| Shock | 2 (9.5) | 0 | .488 |
| Sepsis | 1 (4.8) | 0 | >.999 |
γ-GT, γ-glutamyltraspeptidese.
Values are n (%).
Adverse events that occurred in more than 1 patient after randomization through D28 are shown. Some patients had more than 1 adverse event. The proportions of patients with values worse than baseline values are listed here. All deaths were due to respiratory failure.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 4.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths [published online ahead of print March 4, 2020]. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Liu Y., Liu L., Wang X., Luo N., Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-coronavirus-2 in Shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study [published online ahead of print April 3, 2020]. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis [published online ahead of print April 3, 2020]. J Med Virol. https://doi.org/10.1002/jmv.25822. [DOI] [PMC free article] [PubMed]
- 11.NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19 Available at:
- 12.Chien J.Y., Hsueh P.R., Cheng W.C., Yu C.J., Yang P.C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11:715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C.H., Liu C.Y., Wan Y.L., Chou C.L., Huang K.H., Lin H.C. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS. Respir Res. 2005;6:42. doi: 10.1186/1465-9921-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajayi S., Becker H., Reinhardt H., Engelhardt M., Zeiser R., von Bubnoff N. Ruxolitinib. Recent Results Cancer Res. 2018;212:119–132. doi: 10.1007/978-3-319-91439-8_6. [DOI] [PubMed] [Google Scholar]
- 23.Meng G., Wang J., Wang X., Wang Y., Wang Z. Ruxolitinib treatment for SR-aGVHD in patients with EBV-HLH undergoing allo-HSCT. Ann Hematol. 2020;99:343–349. doi: 10.1007/s00277-019-03864-y. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A., Merrill S.A., Alsawah F., Bockenstedt P., Campagnaro E., Devata S. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6:e630–e637. doi: 10.1016/S2352-3026(19)30156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiladjian J.J., Zachee P., Hino M., Pane F., Masszi T., Harrison C.N. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020;7:e226–e237. doi: 10.1016/S2352-3026(19)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.China National Health Commission . China National Health Commission; Beijing: 2020. Diagnosis and treatment of pneumonitis caused by new coronavirus (trial version 5)http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml Available at: [Google Scholar]
- 27.Jagasia M., Perales M.A., Schroeder M.A., Ali H., Shah N.N., Chen Y.B. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label, phase 2 trial. Blood. 2020;135:1739–1749. doi: 10.1182/blood.2020004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute Common terminology criteria for adverse events v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Available at: [DOI] [PMC free article] [PubMed]
- 29.Yan X.-h., Bao Y.-w., Zhu T., Ai T., Tang D. Evolution characteristics of thoracic lesions on CT of COVID-19 in recovery stage [in Chinese] Radiol Pract. 2020;35:428–432. [Google Scholar]
- 30.Li X., Zeng W., Li X., Chen H., Shi L., Li X. CT imaging changes of corona virus disease 2019 (COVID-19): a multi-center study in Southwest China. J Transl Med. 2020;18:154. doi: 10.1186/s12967-020-02324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coronavirus disease (COVID-2019) R&D Geneva: World Health Organization. http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/ Available at:
- 33.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. A pathological report of three COVID-19 cases by minimally invasive autopsies [in Chinese] Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 35.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H., Wang F., Zhang P., Zhang Y., Chen Y., Fan X. Management of cytokine release syndrome related to CAR-T cell therapy. Front Med. 2019;13:610–617. doi: 10.1007/s11684-019-0714-8. [DOI] [PubMed] [Google Scholar]
- 38.Schulz O., Hammerschmidt S.I., Moschovakis G.L., Forster R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol. 2016;34:203–242. doi: 10.1146/annurev-immunol-041015-055649. [DOI] [PubMed] [Google Scholar]
- 39.Cooper A.M., Khader S.A. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Menten P., Wuyts A., Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 41.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]