Abstract
There are numerous methods of administering drugs to the body, both passive and active. Active methods include the use of penetration enhancers and assisted drug delivery. One of them is sonophoresis (phonophoresis). This term is used to describe the effects of ultrasound on the movement of drugs through intact living skin and into the soft tissues. Although the exact mechanism of sonophoresis is not known, drug absorption may involve a disruption of the stratum corneum lipids allowing the drug to pass through the skin.
In the future, drug release systems aided by ultrasound may be able to provide slow release of vaccines. Researchers are currently exploring the applications in various areas like cutaneous vaccination, transdermal heparin delivery, transdermal glucose monitoring, delivery of acetyl Cholinesterase inhibitors for the treatment of Alzheimer’s disease, treatment of bone diseases and Peyronie’s disease and dermal exposure assessment. The possibilities seem endless.
Drug administration through skin patches, with the advent and development of ultrasound-mediated transdermal transport, may soon become the name of the game. Besides, taking into account the varied possible applications of sonophoretic transdermal drug transport in the fields of biotechnology and genetic engineering, we can envision a whole gamut of newer technologies and products in the foreseeable future.
Keywords: sonophoresis, mechanism, application, ultrasound, cavitation, enhancement, transdermal, drug delivery
INTRODUCTION
Transdermal delivery of drugs offers several advantages over conventional delivery methods including oral and injection methods. Transdermal delivery that traditionally uses a patch containing drug substance pressed onto skin is non-invasive, convenient, and painless and can avoid gastrointestinal toxicity (e.g., peptic ulcer disease), degradation by gastrointestinal tract (e.g. polypeptides such as insulin) and the hepatic first pass metabolism.
Traditional transdermal delivery involves passive diffusion of a drug substance through the skin and subsequent absorption by the capillary system for systemic distribution. The main resistance to drug diffusion through the skin arises in the stratum corneum (SC) via a lipoidal pathway that consists of highly-ordered lipid bilayers located between disk-like dead cells called keratinocytes. The physiochemical nature of this pathway dictates that only lipophylic drugs will readily diffuse through stratum corneum. Few drugs can penetrate the skin at rates sufficient to yield therapeutic plasma levels by using tradì-tional transdermal delivery methods; such drugs are either of relatively low molecular weight (< 250 Da), such as nicotine and nitroglycerine, or of higher molecular weight (> 250 but < 500 Da), such as fentanyl, estradiol and testosterone, which have high therapeutic potency and thus are effective at low delivery rates.
Transdermal delivery of drugs of much higher molecular weight (> 500 Da) requires skin permeation enhancement mechanisms. Several methods have been proposed to facilitate and increase the rate of delivery of higher molecular weight drug through epidermis. These methods include the use of chemical enhancers (1), iontophoresis (2), electroporation (3), and sonophoresis (phonophoresis)- when ultrasound is used to drive molecules of a topically applied medication (4,5,6).
Physical properties of ultrasound
Ultrasound consists of inaudible, acoustic, high-frequency vibrations that may produce either thermal or non-thermal physiologic effects. Traditionally, it is used for the purpose of elevating tissue temperatures and is referred to, as a deep-heating modality. Ultrasound is unlike traditional electrical modalities because it involves the longitudinal waveform associated with sound and is not electromagnetic in nature. Sound waves represent a compression and refraction of a vibrating medium and require a medium for transmission, whereas electromagnetic waveforms can be transmitted across a vacuum. Sound waveforms obey rules of physics concerning reflection, absorption, refraction and dispersion.
Ultrasound uses a high-frequency generator that provides an electrical current through a coaxial cable to a transducer contained within an applicator wand or device. The ultrasonic waves are actually produced by a transducer composed of a piezoelectric crystal which converts elee-trie energy into mechanical energy in the form of oscillations which generate acoustic waves. These waves are partially reflected by the medium in which they are propagated, the other part penetrates and propagates into the medium. During its propagation, a wave is partially scattered and absorbed by the medium, resulting in attenuation of the emitted wave; the lost energy is converted into heat.
Ultrasound energy can travel through body tissues as a beam that is focused but non-uniform in intensity. The actual intensity delivered is dependent upon the quantity of energy delivered to the applicator or wand head. The energy (intensity) is expressed as the number of watts per square centimeter (W/cm2), ranging from about 0.1 to 3 W/cm2 in medicine (7).
The ultrasound emitted from the unit is actually sound waves that are outside the normal human hearing range. The ultrasonic unit sound transducer head is generally set to emit energy at 1 MHz at 0.5 to 1 W/cm2. As ultrasound waves, they can be reflected, refracted and absorbed by the medium, just like regular sound waves. Ultrasound can induce four basic physiologic effects in the body, including chemical reactions, biologic responses, mechanical responses and thermal effects.
Enhancement of chemical reactions by ultrasound can result in enhanced healing. Biologic responses are enhanced when ultrasound increases the transfer of fluids and nutrients into the tissues. Mechanical responses involve cavitation and tendon extensibility and the thermal effects include treatment referred to, as ultrasonic diathermy.
Ultrasound has been indicated in tissue healing, acute and chronic inflammation, joint contractures, muscle spasm, scar tissue, trigger points, myositis ossificans and plantar warts.
Ultrasound waves can be emitted continuously (continuous mode) or in a sequential mode, e.g. 0.1 s applied every second (discontinuous or pulsed mode). The rise in temperature is faster and more intense with the continuous mode. Pulsed mode is frequently used because it reduces the severity of adverse side effects such as thermal effects.
As it is mentioned earlier, due to potential heat build up, ultrasound can be administered in a pulsed mode, rather than continuous. The use of pulsed ultrasound results in a reduced average heating of the tissues. Interestingly enough, either pulsed or continuous low intensity ultrasound can produce nonthermal or mechanical effects that are associated with soft-tissue healing. Another modality should be mentioned for completeness; that of infrasonic therapy. Normal human hearing is in the range of 60 to 20 000 Hz; ultrasound uses the frequencies of 3 and 5 MHz; infrasound uses oscillations below the hearing range, at 8 to 14 Hz. Infrasound is used primarily as a therapeutic massage modality.
The frequency
The frequency of an emitted wave depends on the size of the crystal. It is by definition higher than 20 kHz. Attenuation of an acoustic wave is inversely proportional to its frequency, and thus the more the frequency increases, the less deeply the ultrasound penetrates into and under the skin. High frequencies (1-3 MHz) were first investigated as physical enhancers for transdermal delivery of drugs (8,9). These high frequencies are still used in current treatments. Because the outer layer of epidermis, the stratum corneum, is the main barrier to percutaneous penetration of drugs it initially seemed logical to concentrate the ultrasonic energy on this skin layer using very high frequency (10-20 MHz) (10,11). The use of low frequency ultrasound (20-150 kHz) was shown to be more effective in enhancing transdermal transport (5,12,13).
Intensity
Various ultrasound intensities in the range of 0.1 to 2 W/cm2 have been used for sonophoresis. In most cases, application of higher intensities is limited by thermal effects.
SONOPHORESIS
The term “sonophoresis” (“phonophoresis”) is used to describe the effects of ultrasound on the movement of drugs through intact living skin and into the soft tissues (14). Iontophoresis primarily involves delivery of “ions” (as well as some molecules through the process of electroendoosmosis) whereas sonophoresis involves the delivery of “molecules”. Although the exact mechanism is not known, drug absorption may involve a disruption of the stratum corneum lipids allowing the drug to pass through the skin.
Sonophoresis is actually a combination of ultrasound therapy with topical drug therapy to achieve therapeutic drug concentrations at selected sites in the skin. It is widely used by physiotherapists. Generally, it is said that sonophoresis will result in greater depth of penetration than iontophoresis; ultrasound waves have been reported to penetrate up to 4 to 6 cm into the tissues. Sonophoresis is commonly used in the treatment of muscle soreness, tendonitis and bursitis.
Although considerable attention has been given to the investigation of sonophoresis in the past years, its mechanisms were not clearly understood, reflecting the fact that several phenomena may occur in the skin upon ultrasound exposure. These include:
- Cavitation (generation and oscillation of gas bubbles).
- Thermal effects (temperature increase).
- Induction of convective transport.
- Mechanical effects (occurrence of stresses due to pressure variation induced by ultrasound).
Accordingly, if one identifies the dominant phenomena responsible for sonophoresis, a better selection of ultrasound parameters and surrounding physiochemical conditions can be made to selectively enhance the favourable phenomena, thereby broadening the types of drugs that can be administered transdermally (15).
MECHANISMS OF SONOPHORESIS
In order to understand the mechanisms of sonophoresis, it is important to identify various effects of ultrasound exposure on human tissue since one or more this effects may contribute to the mechanism of sonophoresis.
Cavitation
Cavitation involves the generation and oscillation of gaseous bubbles in a liquid medium and their subsequent collapse when such a medium is exposed to a sound wave, which may be an ultrasound. It can generate violent microstreams, which increase the bioavailability of the drugs (16).
Cavitation occurs due to the nucleation of small gaseous cavities during the negative pressure cycles of ultrasound, followed by the growth of these bubbles throughout subsequent pressure cycles. Whenever small gaseous nuclei already exist in a medium, cavitation takes place preferentially at those nuclei (15,17). This cavitation leads to the disordering of the lipid bilayers and formation of aqueous channels in the skin through which drugs can permeate (18,19,20).
The minimum ultrasound intensity required for the onset of cavitation, referred to as cavitation threshold, increases rapidly with ultrasound frequency (16,18). The most commonly used ultrasound conditions for sonophoresis (frequency 1-3 MHz, intensity 0-2 W/cm2) are called the therapeutic ultrasound conditions (21,22).
But as cavitational effects vary inversely with ultrasound frequency, it was found that any frequency lower than that corresponding to therapeutic ultrasound was more effective in enhancing transdermal transport. This is a direct consequence of reduced acoustic cavitation (formation, growth, and collapse of gas bubbles) at high ultrasound frequencies. Application of ultrasound generates oscillating pressures in liquids and nucleates cavitation bubbles.
At higher frequencies it becomes increasingly difficult to generate cavitation due to the fact that the time between the positive and negative acoustic pressures becomes too short, diminishing the ability of dissolved gas within the medium to diffuse into the cavitation nuclei. The number and size of cavitation bubbles is inversely correlated with application frequency (21, 23).
Cavitation inside the skin as a possible sonophoresis mechanism
Cavitation occurs in a variety of mammalian tissues, including muscle, brain and liver, upon exposure to ultrasound in different conditions. This occurrence of cavita-tion in biological tissue is attributed to the existence of a large number of gas nuclei. These nuclei are gas pockets trapped in either intracellular or intercellular structures. It has been shown that cavitation inside the skin plays a dominant role in enhancing transdermal transport upon ultrasound exposure (15). Cavitation inside the SC can potentially take place in the keratinocytes or in the lipid regions or in both. Since the effect of ultrasound on transdermal transport depends strongly on the dissolved air content in the surrounding buffer and because most of the water in the SC is present in the keratinocytes, it can be said that cavitation inside the SC takes place in the keratinocytes (Fig. 1). Oscillations of the ultrasound-induced cavitation bubbles near the keratinocyte-lipid bilayer interfaces may, in turn cause oscillations in the lipid bilayers, thereby causing structural disorder of the SC lipids.
Figure 1.
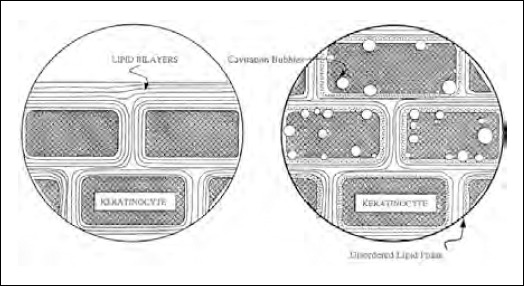
Schematic presentation of cavitation occurring in the keratinocytes. Cavitation occurs preferentially at the interface between the keratinocytes and the lipid bilayers (24).
Shock waves generated by the collapse of cavitation bubbles at the interfaces may also contribute to the structure disordering effect. Because the diffusion of permeants through a disordered bilayer phase can be significantly faster than that through a normal bilayer, transdermal transport in the presence of ultrasound is higher than passive transport. This, in essence, is the mechanism of sonophoresis.
Cavitation outside the skin as a possible sonophoresis mechanism
Cavitation in the saline surrounding the skin does occur after ultrasound exposure. These cavitation bubbles can potentially play a role in the observed transdermal transport enhancement. Firstly, these bubbles cause skin erosion, following their violent collapse on the skin surface, due to generation of shock waves, thereby enhancing transdermal transport. Secondly, the oscillations and collapse of cavitation bubbles also cause generation of velocity jets at the skin-donor solution interface, referred to as microstreaming. These induce convective transport across the skin, thereby enhancing the overall transdermal transport. Experimental findings suggest that cavita-tion outside the skin does not play that important a role in sonophoresis (11,15).
Thermal effects
The increase in the skin temperature resulting from the absorbance of ultrasound energy may increase the skin permeability coefficient because of an increase in the permeant diffusion coefficient. The absorption coefficient of a medium increases proportionally with the ultrasound frequency, indicating that the thermal effects of ultrasound are proportional to the ultrasound frequency. The increase in the temperature of a medium upon ultrasound exposure at a given frequency varies proportionally with the ultrasound intensity and exposure time. The thermal effects can be substantially reduced by pulsed application.
Role of convective transport in sonophoresis
Fluid velocities are generated in porous medium exposed to ultrasound due to interference of the incident and reflected ultrasound waves in the diffusion cell and oscillations of the cavitation bubbles. Fluid velocities generated in this way may affect transdermal transport by inducing convective transport of the permeant across the skin, especially through hair follicles and sweat ducts. Experimental findings suggest that convective transport does not play an important role in the observed transdermal enhancement (15).
Role of mechanical stresses in sonophoresis
Ultrasound is a longitudinal pressure wave inducing sinu-soldai pressure variations in the skin, which, in turn, induce sinusoidal density variation. At frequencies greater than 1 MHz, the density variations occur so rapidly that a small gaseous nucleus cannot grow and cavitational effects cease. But other effects due to density variations, such as generation of cyclic stresses because of density changes that ultimately lead to fatigue of the medium, may continue to occur. Lipid bilayers, being self-assembled structures, can easily be disordered by these stresses, which result in an increase in the bilayer permeability. This increase is, however, non-significant and hence mechanical effects do not play an important role in therapeutic sonophoresis. Thus cavitation induced lipid bilayer disordering is found to be the most important cause for ultrasonic enhancement of transdermal transport (15).
Drug delivery
Two different approaches are used for actual drug delivery. Originally, the drug-containing coupling agent was applied to the skin immediately followed by the ultrasound treatment. Today, generally the product is applied to the skin and a period of time allowed for the drug to begin absorption into the skin; then, the ultrasound is applied. Drug penetration is most likely in the 1 to 2 mm depth range (7).
Contraindications
Contraindications and/or precautions for the use of ultrasound and phonophoresis include:
infection,
cancer,
heart problems/pacemaker,
implants (metal, silicone, saline),
acute and post-acute injury, epiphyseal areas,
pregnancy,
thrombophlebitis,
impaired sensation and
around the eyes.
Coupling medium
The actual tissue penetration is also dependent upon the impedance or acoustic properties of the media or the efficiency of the coupling agent. A coupling medium is required to provide an air-tight contact between the skin and the ultrasound head. The coupling medium can also serve as the drug vehicle. The vehicle containing the drug must be formulated to be smooth and non-gritty as it will be rubbed into the skin by the head of the transducer. Agents used as coupling media include mineral oil, water-miscible creams and gels. In the immersion method, water is the best medium. The immersion method is best used for areas that are small or irregularly shaped. In this method, the drug is applied in a suitable vehicle prior to immersion into water and the application of the ultrasound.
Emulsions have been used but the oil/water interfaces in emulsions can disperse the ultrasonic waves, resulting in a reduction of the intensity of the energy reaching the skin. It may also cause some localized heating. Air should not be incorporated into the product as air bubbles may disperse the ultrasound waves resulting in heating at the liquid: air interface.
The pseudoplastic rheology flow exhibited by gels is advantageous as gels are “shear-thinning”, decreasing viscosity and friction when stress is applied. The product should be of relatively low viscosity for ease of application and ease of movement of the transducer head during the ultrasound process.
Gels work very well as a medium. The viscosity of the coupling agent and the quantity of gas dissolved in the medium may significantly affect transdermal transport (15). A decrease in sonophoretic transport of insulin, vasopressin and estradiol was reported in vitro when molecules were administered in a gel (15,25).
Similar findings were reported with lidocaine in vivo in hairless mice (26).
APPLICATION
For greater efficiency of both the ultrasound and sonophoresis, the skin should be will hydrated. Lack of moisture impedes sound transfer; the skin can be rehydrated using a warm, moist towel for about 10-15 minutes prior to the treatment. After treatment is completed, an occlusive dressing will help to maintain skin hydration, warmth and capillary dilatation; it will also keep the drug (residing on the skin surface) in intimate contact with the skin for further absorption.
There are numerous steps involved in successful treatment using sonophoresis, as follows:
- Determine if sonophoresis is indicated and that no contraindications are present,
- Clean and hydrate (if necessary) the body part,
- Determine the drug to be used,
- Determine the coupling method /direct (determine coupling agent) or water immersion/ to be used,
- Determine the frequency,
- Determine the intensity,
- Determine the time of treatment,
- Start treatment,
- If local, move the sound head at approximately 4 cm/sec, with an overlap one-half the width of the sound head,
- Determine the number of treatments per day,
- Record the necessary parameters,
- Clean the unit and the treated area,
- Apply an occlusive dressing if desired.
In summary, drug delivery can be maximized by controlling these variables. So, special attention should be to the following (9):
Both the drug and the coupler (or vehicle) should efficiently transmit ultrasound.
- Pretreat the skin using ultrasound, heat, moisture or by shaving.
- Position the patient to maximize circulation during treatment.
Use an occlusive dressing after treatment.
- Use an intensity of 1.5 W/cm2 to capture both the thermal and non-thermal effects of the ultrasound.
- Low-intensity ultrasound (0.5 W/cm2) should be used when treating open wounds or acute injuries.
DRUGS USED
Numerous drugs have been administered using sonophoresis. Hydrocortisone is the drug most often administered using sonophoresis in concentrations ranging from 1% to 10%. Other drugs administered include betamethasone dipropionate, oligodeoxynucleotides, chymotrypsin alpha, dexamethasone, fluocinonide, heparin, hyaluronidase, ibuprofen, indomethacin, insulin, iodine, ketoprofen, lidocaine, mecholyl, naproxen, piroxicam, sodium salicylate, trypsin, and zinc.
Stability of sonicated drugs
Eventual degradation of drugs to ultrasound was studied in vitro and showed absence of degradation for oligodeoxynucleotides (27), insulin (28), fentanyl and caffeine (29). The persistence of biological activity of insulin and low-molecular weight heparin in vivo is also in accordance with the absence of degradation in the conditions used (5, 30).
BIOLOGICAL EFFECT OF ULTRASOUND APPLICATION
Ultrasound over a wide frequency range has been used in medicine over the last century. For example, therapeutic ultrasound (1-3 MHz) has been used for massage, low frequency ultrasound in dentistry (23-40 kHz), and high frequency ultrasound (3-10 MHz) for diagnostic purposes. In view of this, significant attention has been given to the effects of ultrasound on biological tissues.
As described earlier, ultrasound affects biological tissues via three main effects: thermal effects, cavitational
effects, and acoustic streaming. Conditions under which these effects become critical are given as follows:
Thermal effects may be important when:
- The tissue has a high protein content
- A high intensity continuous wave ultrasound is used
- Bone is included in the heated volume
- Vascularization is poor Cavitation may be important when:
- Low frequency ultrasound is used
- Gassy fluids are exposed
- Small gas-filled spaces are exposed
- The tissue temperature is higher than normal Streaming may be important when:
The medium has an acoustic impedance different from its surroundings
The fluid in the biological medium is free to move Continuous wave application is used
Numerous investigators have reported histological studies of animal skin exposed to ultrasound under various conditions in order to assess its effect on living skin cells. Although these histological studies indicate no adverse effects of ultrasound, further research focusing on safety issues is required to evaluate limiting ultrasound parameters for safe exposure (24).
Some studies have also been carried out to determine the safety of low-frequency sonophoresis on human and rat skin by evaluating their structural modifications after ultrasound exposure. Skin samples were observed under optical and electron microscopy to detect any structural changes. The skin samples exposed to ultrasound intensities lower than 2.5 W/cm2 showed no modification. However it was found that high-intensity, low frequency (20 kHz) ultrasound equipment could cause severe skin lesions when used with inappropriate ultrasound conditions (31,32,33).
CONCLUSION
There is no doubt that ultrasound can markedly increase percutaneous absorption. Current published findings are encouraging, especially for diabetes. It is possible to decrease glucose blood levels with a non-invasive device in vivo in animals and moreover, measurement of blood glucose levels could be achieved in humans (34).
Sonophoretically enhanced transdermal drug transport promises to radically change the way in which we inject drugs in the near future. With further research, patients may soon possess small pocketsize sonicators used to “inject” drugs whenever required. Furthermore, these devices could be coupled with sensors that can monitor drug concentrations in the blood to formulate a self-controlled drug delivery method that can potentially eliminate patient compliance (34).
Sonophoretically enhanced transdermal drug transport may also have an application in tissue engineering and gene therapy. This method makes the engineering of a new organ, in situ, and culturing it outside the body seem within reach.
In the future, drug release systems aided by ultrasound may be able to provide slow release of vaccines such as that for tetanus, which need repeated booster shots; or for an AIDS vaccine. Researchers are currently exploring the applications in various areas like cutaneous vaccination, transdermal heparin delivery, transdermal glucose monitoring, delivery of acetyl Cholinesterase inhibitors for the treatment of Alzheimer’s disease, treatment of bone diseases and Peyronie’s disease and dermal exposure assessment. The possibilities seem endless (35).
In summary, even though today only few drugs are administered transdermally in general practice, with the advent and development of ultrasound-mediated transdermal transport, drug administration through skin patches may soon become the name of the game. Besides, taking into account the varied possible applications of sonophoretic transdermal drug transport in the fields of biotechnology and genetic engineering, we can envision a whole gamut of newer technologies and products in the foreseeable future.
References
- 1.Walters K.A. Skin penetration enhancers and their use. In: Hadgraft J, Guy R, editors. Transdermal Therapeutic Systems. New York: Marcel Dekker; 1989. pp. 197–245. [Google Scholar]
- 2.Burnette R.R. Iontophoresis. In: Hadgraft J, RGuy R, editors. Transdermal Therapeutic Systems. New York: Marcel Dekker; 1989. pp. 247–291. [Google Scholar]
- 3.Prausnitz M.R, Bose V.G, Langer R, Weaver J.C. Electroporation of mammolalian skin: a mechanism to enhance transdermal drag delivery. Proc Nat'l Acad Sci USA. 1993;90:10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy D, Kost J, Meshulam Y, Langer R. Effect of ultrasound on drag delivery to rates and guinea pigs. J Clin. Invest. 1989;83:2074–2078. doi: 10.1172/JCI114119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 6.Mitragotri s, Blankschtein D, Langer R. Transdermal drag delivery using low frequency sonophoresis. Pharm Res. 1996;13:411–420. doi: 10.1023/a:1016096626810. [DOI] [PubMed] [Google Scholar]
- 7.Kahn J. Principles and Practice of Electrotherapy. 4th Ed. Vol. 63. Philadelphia PA: Churchill Livingstone; 2000. [Google Scholar]
- 8.Pottenger F. J, Karalfa B.L. utilization of hydrocortisone phonophoresis in United States army physical therapy clinics. Military Medicine. 1989;154:355–358. [PubMed] [Google Scholar]
- 9.Byl N.B. The use of ultrasound as an enhancer for transcutaneous drag delivery: Phonophoresis. Phys Ther. 1995;75:539–553. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 10.Bommannan D, Okuyama H, Stauffer p, Guy R.H, Sonophoresis I. The use of high-frequency ultrasound to enhance transdermal drag delivery. Pharm. Res. 1992;9:559–564. doi: 10.1023/a:1015808917491. [DOI] [PubMed] [Google Scholar]
- 11.Bommannan D, Menon G.K, Okuyama H, Elias P.M, Guy R.H, Sonophoresis I. I Examination of the mechanism(s) of ultrasound-enhanced transdermal drag delivery. Pharm. Res. 1992;9:1043–1047. doi: 10.1023/a:1015806528336. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana K, Tachibana s. Transdermal delivery of insulin by ultrasonic vibration. J. Pharm. Pharmacol. 1991;43:270–271. doi: 10.1111/j.2042-7158.1991.tb06681.x. [DOI] [PubMed] [Google Scholar]
- 13.Tachibana K. Transdermal delivery of insulin to alloxan-diabetic rabbits by ultrasound exposure. Pharm. Res. 1992;9:952–954. doi: 10.1023/a:1015869420159. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi A.s, Dixit S.G. Skin electroporation for macro-molecular transdermal drag delivery. Bombay Technologist. 1998-1999;48:5–11. [Google Scholar]
- 15.Mitragotri s, Edwards D.A, Blankschtein D, Langer R. A mechanistic study of ultrasonically-enhanced transdermal drag delivery. J Pharm. Sci. 1995;84:697–706. doi: 10.1002/jps.2600840607. [DOI] [PubMed] [Google Scholar]
- 16.Tachibana K, Tachibana s. Application of ultrasound energy as a new drag delivery system. Jpn. J. Appi. Phys. 1999;38:3014–3019. [Google Scholar]
- 17.Mason T.J, Lorrimer J.p. Sonochemistry-theory, applications and uses of ultrasound in chemistry, Ellis Horwood. 1988 [Google Scholar]
- 18.Mitragotri s, Kost J. Low-frequency sonophoresis: a noninvasive method of drag delivery and diagnostics. Biotechnol. Prog. 2000;16:488–492. doi: 10.1021/bp000024+. [DOI] [PubMed] [Google Scholar]
- 19.Tezel A, Sens A, Mitragotri s. Investigation of the role of cavitation in low-frequency sonophoresis using acoustic spectroscopy. J Pharm. Sci. 1998;91:444–453. doi: 10.1002/jps.10024. [DOI] [PubMed] [Google Scholar]
- 20.Tang H, Mitragotri s, Blankschtein D, Langer R. Theoretical description of transdermal transport of hydrophilic permeante: Application to low frequency sonophoresis. J Pharm. Sci. 2001;90:543–566. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Tezel A, Sens A, Tuchscherer J, Mitragotri s. Frequency dependence of sonophoresis. Pharm. Res. 2001;18:1694–1700. doi: 10.1023/a:1013366328457. [DOI] [PubMed] [Google Scholar]
- 22.Mitragotri S, Farrell J, Tang H, Terahara T, Kost J, Langer R. Determination of threshold energy dose for ultrasound induced transdermal drag transport. J. Control. Release. 2000;63:41–52. doi: 10.1016/s0168-3659(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 23.Terahara T, Mitragotri s, Kost J, Langer R. Dependence of low-frequency sonophoresis ultrasound parameters, distance of the horn and intensity. Int J Pharm. 2002;235:35–42. doi: 10.1016/s0378-5173(01)00981-4. [DOI] [PubMed] [Google Scholar]
- 24.Mitragotri s, Blankschtein D, Langer R. Sonophoretic transdermal drag delivery. In: Swarbrick J, Boylan J.C, editors. Encyclopedia of Pharmaceutical Technology. Vol. 14. New York: Marcel Dekker; 1996. pp. 103–122. [Google Scholar]
- 25.Zhang I, Shung K.K, Edwards D.A. Hydrogels with enhanced mass transfer for transdermal drug delivery. J. Pharm. Sci. 1996;85:1312–1316. doi: 10.1021/js9601142. [DOI] [PubMed] [Google Scholar]
- 26.Tachibana K, Tachibana s. Use of ultrasound to enhance the local anesthetic effect of topically applied lidocaine. Anesthesiology. 1993;78:1091–1096. doi: 10.1097/00000542-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Meidan V.M, Walmsley A.D, Irwin W.J. 1995. Phonophoresisis it a reality? Int. J. Pharm. 1995;118:129–149. [Google Scholar]
- 28.Boucaud A, Garrigue M.Az, Machet L, Vaillant L, Patat F. Effect of sonication parameters on transdermal delivery of insulin to hairless rats. J. Control. Release. 2002;81:113–119. doi: 10.1016/s0168-3659(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 29.Boucaud A, Machet L, Arbeille B, Machet M.C, Sournac M, Mavon A, Patat F, Vaillant L. In vitro study of low-frequency ultrasound-enhanced transdermal transport of fentanyl and caffeine across human and hairless rat skin. Int. J. Pharm. 2001;228:69–77. doi: 10.1016/s0378-5173(01)00820-1. [DOI] [PubMed] [Google Scholar]
- 30.Mitragotri s, Kost J. Transdermal delivery of heparin and low-molecular weight heparin using low frequen-CYultrasound. Pharm. Res. 2001;18:1151–1156. doi: 10.1023/a:1010979010907. [DOI] [PubMed] [Google Scholar]
- 31.Monti D, Saettane M.F, Giannaccini B, Galli-Angeli D. Enhancement of transdermal penetration of dapiprazole through hairless mouse skin. J. Control. Release. 1995;33:71–77. [Google Scholar]
- 32.Levy D, Kost J, Meshulam Y, Langer R. Effect of ultrasound on transdermal drag delivery to rats and guinea pigs. J. Clin. Invest. 1989;83:2074–2078. doi: 10.1172/JCI114119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boucaud A, Monthara J.R.M, Machet L, Arbille B, Machet M.C, Patat F.D.R, Vaillant L. Clinical, histo-logical, and electronmicroscopy study of skin exposed to low frequency ultrasound. Anat. Rec. 2001;264:114–119. doi: 10.1002/ar.1122. [DOI] [PubMed] [Google Scholar]
- 34.Kost J, Mitragotri s, Gabbay R, Pisko M, Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nature. Med. 2000;6:347–350. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- 35.Joshi A, Raje J. Sonicated transdermal drag transport. J. Control. Release. 2002;83:13–22. doi: 10.1016/s0168-3659(02)00200-6. [DOI] [PubMed] [Google Scholar]


