Abstract
Cellular attachment and response to biomaterials are mediated by integrin receptor binding to extracellular matrix proteins adsorbed onto the material surface. Osteoblasts interact with their substrates via several integrin complexes including fibronectin-binding α5β1 and collagen-binding α1β1 and α2β1. Knockdown of α2 or β1 integrin subunits inhibits the production of factors that promote an osteogenic microenvironment, including osteocalcin, osteoprotegerin, and TGFβ1. Osteoblasts also secrete several angiogenic growth factors such as VEGF-A (VEGF165), FGF-2, and angiopoietin 1, which are regulated by titanium surface topography and surface energy. Here, we examined whether signaling through integrin receptor complexes regulates production and secretion of angiogenic factors during osteoblast differentiation on microtextured Ti surfaces. To do this, integrin subunits α1, α2, α5, and β1 were stably silenced in MG63 osteoblast-like cells cultured on grit-blasted/acid-etched hydrophobic Ti (SLA) or on hydrophilic SLA (modSLA). VEGF-A production increased in response to Ti surface topography and energy in integrin α2, α5, and β1 silenced cells but decreased in α1-silenced cells. FGF-2 decreased on modSLA substrates in both α1 and α2-silenced cells but was unchanged in response to silencing of either α5 or β1. In integrin α1, α2, and β1-silenced cells, Ang-1 increased on modSLA but α5-silencing did not affect Ang-1 production during surface mediated differentiation. These results suggest that signaling through specific integrin receptor complexes during osteoblast differentiation on microstructured Ti substrates, regulates the production of angiogenic factors by those cells, and this is differentially regulated by surface hydrophilicity.
Keywords: Titanium, Integrins, Osteoblast, Angiogenesis, Differentiation, Surface, Osteocalcin (OCN)
1. Introduction
The initial interaction of cells with a biomaterial surface plays a significant role in determining host tissue response to an implanted material. Upon implantation into the body, the surface of a biomaterial is conditioned with an adsorbed layer of proteins, ions, sugars and lipids present in the surrounding blood and interstitial fluid [1–4]. The surface properties of the implanted material determine which biological molecules adsorb. The orientation of the adsorbed biological molecules directly influences the attachment, proliferation, and differentiation of surrounding cells [5,6]. Adhesion of cells with a biomaterial surface involves several classes of proteins including extracellular matrix proteins, cytoskeletal proteins, cadherins, and integrin receptors [7].
Osteoblasts interact with their substrate primarily through integrin binding to extracellular matrix (ECM) proteins [8,9]. Integrins are heterodimeric transmembrane glycoprotein receptor complexes consisting of non-covalently associated α and β subunits. Osteoblasts express several integrin α and β subunits including αl, α2, α3, α4, α5, α6, αv, β1, and β3 [10–12]. Binding of integrin receptors to ECM proteins results in formation of signaling complexes inside the cell. The integrins cluster into focal adhesions, where they initiate intracellular signaling cascades to control proliferation and differentiation [13–20].
Expression of α and β integrin subunits in osteoblasts cultured on implant materials like titanium (Ti) and titanium-aluminum-vanadium (Ti6Al4V) is regulated by surface chemical composition, topography, and hydrophilicity [12,21,22]. In comparison to growth on tissue culture polystyrene (TCPS), expression of α2 and β1 integrin subunits is increased [21,23], suggesting that the surface roughness dependent differentiation of osteoblasts may be mediated specifically through α2β1 signaling. Knockdown of either the α2 or β1 integrin subunits in MG63 cells blocks surface roughness dependent differentiation of those cells [23,24] and affects the production of pro-angiogenic growth factors [25].
Integrins also play an important role in neovascularization. Vascular endothelial cells, similar to osteoblasts, express several integrin α and β subunits in a differential manner. In quiescent vessels, many integrins are either not expressed or are in an inactive state. During neovascularization, endothelial cells upregulate expression of integrin pairs that are also expressed during osteoblastic growth on Ti and Ti6Al4V, including α1β1, α2β1, and α5β1 [26,27]. Whereas α5β1 modulates osteoblast attachment and growth, α1β1 and α2β1 regulate osteoblast differentiation. Moreover, α1β1 primarily mediates effects of surface chemistry whereas α2β1 mediates the effects of surface microtopography [28].
Angiogenic growth factor receptors, including the vascular endothelial growth factor (VEGF) receptor Flk-1 and the platelet derived growth factor beta (PDGFβ) receptor interact with integrins on the surface of endothelial cells during neovascularization [29]. VEGF-A (VEGF165) has been shown to activate a cooperative pathway between the Flk-1 receptor and integrin αVβ3 complex in endothelial cells to regulate angiogenesis [30], and the pro-angiogenic effects of VEGF165b, a splice variant of VEGF-A, occurs via a β1/VEGFR autocrine loop [31]. While it is known that signaling through integrin receptors in osteoblasts affects the differentiation and expression of osteogenic markers in response to Ti surface roughness and energy, whether or not integrin signaling in osteoblasts affects the production of angiogenic growth factors in these cells is not known.
In this study, we investigated the role that signaling through specific integrin receptors has on the production of pro-angiogenic growth factors in osteoblast-like cells differentiating in response to substrate microtopography and surface free energy. In order to elucidate the roles of different integrin α and β subunits, we transduced shRNA specific for integrin subunits α1, α2 , α5, and β1 into an MG63 osteoblast-like cell line to knockdown expression of these integrin molecules and examined their response to Ti surface topography and energy.
2. Materials and methods
2.1. Titanium substrate preparation
Ti disks were prepared from 1 mm thick sheets of grade 2 unalloyed commercially pure Ti punched into 15 mm diameter disks and supplied by Institut Straumann AG (Basel, Switzerland). The production and characterization of smooth pretreatment (PT), grit-blasted, and acid-etched (SLA), and hydrophilic modified SLA (modSLA) surfaces have been described previously [22,32,33]. PT surfaces were degreased by washing Ti disks in acetone and processed in a 2% ammonium fluoride/2% hydrofluoric acid/10% nitric acid solution. SLA surfaces were made by coarse grit-blasting of the PT surfaces with 0.25–0.50 mm corundum grit followed by a dual acid etching procedure with hydrochloric acid and hydrofluoric acid. modSLA surfaces were made using the same procedure as SLA surfaces under nitrogen rinsing to prevent hydrocarbon deposition. 15 mm diameter discs were created with the expressed purpose to fit snuggly into the bottom of a 24 well cell culture plate.
2.2. Real-Time PCR for integrin expression
MG63 cells were plated at 10,000 cells/cm2 on TCPS, PT, SLA, or modSLA substrates in 24 well cell culture plates. Cells were cultured with Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin until cultures reached confluence on TCPS. At confluence, cells were incubated with fresh media for 12 h. RNA was isolated using TRIzol ® (Invitrogen, Carlsbad, CA). Briefly, 1 mL of TRIzol was added to each cell culture well and plates were agitated gently for 5 min to lyse cells. Samples were transferred to PCR clean 1.5 mL microcentrifuge tubes (Eppendorf North America, Hauppauge, NY). 200 μL of CHCl3 were added to the samples and shaken vigorously for 15 s and incubated at room temperature for 2–3 min. Samples were then spun at 12,000 g for 15 min at 4 degrees Celsius. The top aqueous phase of each sample was transferred to new microcentrifuge tubes. 500 μL of isopropanol was added and samples were incubated at −30 °C for 10 min and spun down again for at 12,000 g for 15 min at 4 degrees Celsius. Samples were decanted carefully to prevent the loss of the RNA pellet. The RNA pellet was washed with 1 mL of ice cold 75% ethanol and mixed by vortex and finally spin down at 10,000 g for 5 min at 4 degrees Celsius. Ethanol was decanted carefully into a waste container, the RNA pellet was briefly air-dried and resuspended in RNase free water.
RNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). 250 ng RNA was reverse transcribed (High Capacity Reverse Transcription cDNA kit, Applied Biosystems, Carlsbad, CA) to create a cDNA library. cDNA was used to quantify mRNA expression using real-time qPCR. Gene-specific primers and Power Sybr® Green Master Mix (Applied Biosystems) were used in the StepOnePlus Real-time PCR System (Applied Biosystems). Threshold cycles were quantified as starting mRNA quantities using known dilutions of MG63 cDNA. Gene expression is normalized to GAPDH. Primer sequences for GAPDH and integrins α1, α2, α5, and β1 were designed with Beacon designer software and synthesized by Eurofins MWG Operon (Huntsville, AL) and are listed in Table 1.
Table 1.
Human Primers Used in Real-Time PCR Analysis for Integrin Expression.
| Name | Abbreviation | Sequence | |
|---|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | F | GCTCTCCAGAACATCATCC |
| R | TGCTTCACCACCTTCTTG | ||
| Integrin α1 | ITGα1 | F | CACTCGTAAATGCCAAGAAAAG |
| R | TAGAACCCAACACAAAGATGC | ||
| Integrin α2 | ITGα2 | F | ACTGTTCAAGGAGGAGAC |
| R | GGTCAAAGGCTTGTTTAGG | ||
| Integrin α5 | ITGα5 | F | ATCTGTGTGCCTGACCTG |
| R | AAGTTCCCTGGGTGTCTG | ||
| Integrin β1 | ITGβ1 | F | ATTACTCAGATCCAACCAC |
| R | TCCTCCTCATTTCATTCATC | ||
2.3. Generation of integrin silenced cell lines
2.3.1. Integrin α2
MG63 osteoblast-like cells that were stably silenced for integrin α2 were generated by transfection with α2 integrin shRNA using a P-suppressor-neo vector system and shown to have a 70% reduction in α2 protein as described in detail previously [24,28]. Integrin α2-silenced MG63 cells were maintained in media containing geneticin (G418; Invitrogen) at a concentration of 600 μg/mL for the duration of cell culture.
2.3.2. Integrins α1, α5, and β1
MG63 osteoblast-like cells were transduced using Mission® lentiviral transduction particles (Sigma-Aldrich, St. Louis, MO) with shRNA specific for each target gene of interest following the manufacturer’s recommended protocol. Integrins α1 and β1 were described in detail previously [24,28]. Verification of silencing for integrin α5 subunit was done using western blot analysis and real-time qPCR (Supplemental Fig. 1).
2.4. Cell culture
Non-transduced MG63 cells and MG63 cells silenced for integrins α1, α2, α5, and β1 were plated in 24-well tissue culture plates on TCPS (used as a control for all studies), PT, SLA, and modSLA surfaces using DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were seeded at an initial density of 10,000 cells/cm2 and media were exchanged 24 h after seeding and every 48 h thereafter. When the cells were confluent on TCPS, media from all cultures were collected and examined for VEGF-A, fibroblast growth factor-2 (FGF-2), and angiopoietin (Ang-1) as described below. Osteocalcin levels in the conditioned media were determined as an indicator of osteoblast differentiation by radioimmunoassay (Biomedical Technologies, Inc., Stoughton, MA).
2.5. Cell response
2.5.1. Cell number
Cell number was determined for all cell types at the time of harvest, which for MG63 cells stably silenced for integrins α1, α2, α5, and β1 was after seven days in culture. At confluence, cells were released from TCPS and Ti surfaces using two sequential incubations with 0.25% trypsin for 10 min at 37 °C to ensure that no cells remained on the rough Ti surfaces and counted using an automated cell counter (Z1 Particle counter, Beckman Coulter, Fullerton, CA).
2.5.2. Osteocalcin
Osteocalcin levels in the conditioned media of MG63 cells grown on Ti surfaces were determined as a marker of osteoblast maturation using a commercially available radioimmunoassay (Biomedical Technologies, Inc., Stoughton, MA) following the manufacturer’s protocol.
2.5.3. VEGF-A (VEGF165), FGF-2, Ang-1
The levels of the angiogenic growth factors VEGF-A, FGF-2, and Ang-1 were determined in the conditioned media using commercially available sandwich ELISA assays (Duoset ELISA Development Systems, R&D Systems, Minneapolis, MN) following the manufacturer’s protocols.
2.6. Statistical analysis
The data presented here are from one of at least two separate sets of experiments. Both sets of experiments yielded comparable observations. For any given experiment, each data point represents the mean ± standard error of six individual cultures. Data were analyzed by ANOVA, and when statistical differences were detected, Student’s t-test for multiple comparisons using Bonferroni’s modification was used. p-values < 0.05 were considered significant.
3. Results
3.1. MG63 gene expression
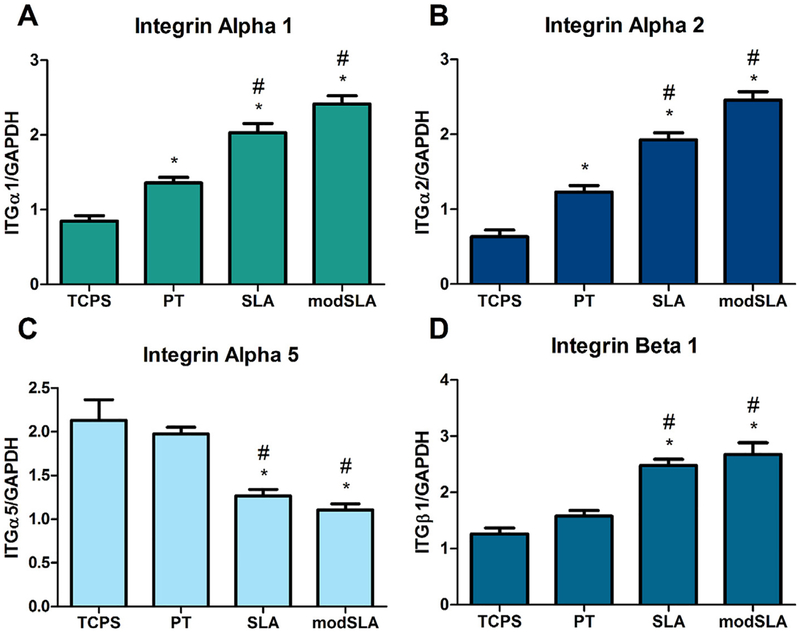
Realtime qPCR for integrins α1, α2, α5, and β1 showed that Ti substrate roughness and energy affected the expression of these integrins in MG63 osteoblast-like cells. Expression of α1 integrin was elevated on all three Ti surfaces compared to TCPS. Ti surface roughness further increased α1 expression while high surface free energy had no additional effect on integrin α1 expression (Fig. 1A). Similar to α1, expression of integrin α2 was increased on Ti surfaces and the addition of a rough surface microarchitecture further increased expression while increasing surface free energy did not significantly alter expression levels (Fig. 1B). Expression of integrin α5 was similar to control TCPS and smooth PT surfaces. In contrast to expression of both integrin α1 and integrin α2, α5 integrin expression was reduced on microrough SLA and modSLA Ti surfaces compared to TCPS and PT surfaces (Fig. 1C). β1 integrin levels were unchanged on smooth PT Ti surfaces compared to TCPS control surfaces. On SLA Ti surfaces, expression of β1 integrin was significantly increased compared to both TCPS and PT surfaces; however, the combination of a rough surface microtopography and high surface energy had no further effect on β1 levels (Fig. 1D).
Fig. 1.
Integrin gene expression in MG63 cells. MG63 cells cultured on control TCPS, PT, SLA and modSLA Ti substrates were examined for expression of specific integrin receptor subunits. (A) Integrin α1, (B) integrin α2, (C) integrin α5, and (D) integrin β1 gene expression levels. *p < 0.05 vs. TCPS; #p < 0.05 vs. PT.
3.2. MG63 cell response
Consistent with previously published results [25], for all experiments, MG63 cells exhibited a decrease in total cell number and an increase in secreted levels of osteocalcin, VEGF-A, and FGF-2 with increasing surface roughness and surface free energy, while secreted levels of Ang-1 were unaffected by either surface roughness or energy (Figs. 2–6).
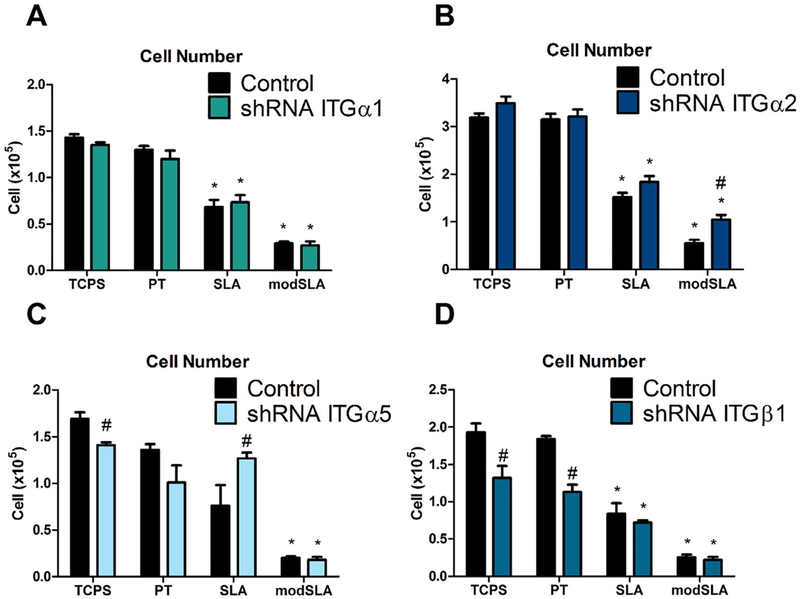
Fig. 2.
Total cell number in MG63 cells silenced for integrins α1, α2, α5, and β1. (A) Total cell number for integrin α1, (B) integrin α2, (C) integrin α5, and (D) integrin β1, as well as MG63 cells were determined. Values presented are mean ± SEM of six independent cultures. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni’s modification of Student’s t-test. *p < 0.05 vs. TCPS; #p < 0.05 vs. MG63 cultures on matching substrates.
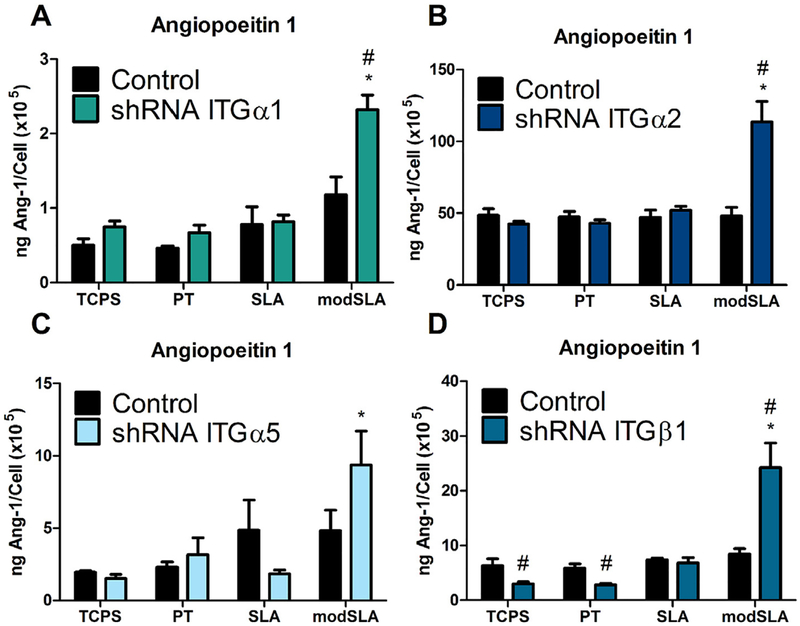
Fig. 6.
Ang-1 levels in the conditioned media in MG63 cells silenced for integrins α1, α2, α5, and β1. (A) Ang-1 levels in the conditioned media for integrin αl, (B) integrin α2, (C) integrin α5, and (D) integrin as well as MG63 cells were determined. Values presented are mean ±SEM of six independent cultures. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni’s modification of Student’s t-test. *p < 0.05 vs. TCPS; #p < 0.05 vs. MG63 cultures on matching substrates.
3.2.1. Cell number
Total cell number for integrin α1 silenced MG63 cells was reduced on microrough SLA and microrough, high surface energy modSLA Ti surfaces compared to TCPS and smooth PT Ti surfaces. Cell numbers for integrin α1 silenced cells were comparable to wild-type MG63 cells on all substrates (Fig. 2A). Similar to α1 silenced cells, cell number for integrin α2 silenced cells was reduced on SLA and modSLA surfaces compared to TCPS and PT surfaces. On modSLA surfaces, cell number in integrin α2 silenced cells was increased compared to wild-type MG63 cells, though on all other surfaces examined, cell numbers between α2 silenced cells and MG63 cells were comparable (Fig. 2B). Silencing of the α5 integrin subunit had a variable effect on cell number in response to Ti surface features. Cell number in α5 silenced cells was reduced on modSLA Ti substrates, but no reduction in cell number was observed in response to increasing surface roughness on SLA surfaces compared to TCPS and PT surfaces. On control TCPS surfaces, cell number in integrin α5 silenced cells was reduced compared to MG63 cells while cell numbers were similar on all other surfaces examined (Fig. 2C). β1 integrin silenced cell number was reduced on SLA and modSLA substrates, similar to MG63 cells and cells silenced for integrins α1 and α2. On control TCPS and smooth PT Ti surfaces, β1 silenced cell number was reduced compared to MG63 cell number (Fig. 2D).
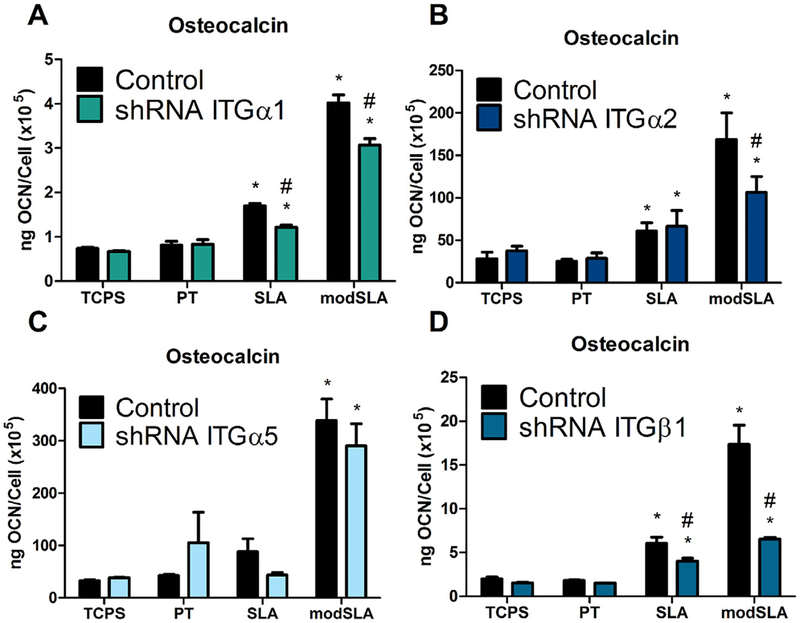
3.2.2. Osteocalcin
Targeted knockdown of specific integrin α and β subunits affected the production of osteocalcin, a late marker of osteoblast differentiation by MG63 cells in response to Ti surface microstructure and energy. Secreted levels of osteocalcin by MG63 cells stably silenced for integrins α1, α2, α5, and β1 displayed a surface roughness and energy dependent increase. However, this surface microstructure and energy dependent increase was significantly reduced in integrin α1, α2, and β1 silenced MG63 cells (Fig. 3A, B, D) while knockdown of the α5 integrin subunit had no effect on secreted levels of osteocalcin (Fig. 3C). In addition to the observed decrease in osteocalcin production on modSLA Ti substrates, in integrin α1 and β1 silenced cells, secreted levels of osteocalcin were also reduced in response to surface roughness alone compared to MG63 cells (Fig. 3A, D).
Fig. 3.
Osteocalcin levels in the conditioned media in MG63 cells silenced for integrins αl, α2, α5, and 31. (A) Osteocalcin levels in the conditioned media for integrin αl, (B) integrin α2, (C) integrin α5, and (D) integrin (β1, as well as MG63 cells were determined. Values presented are mean ± SEM of six independent cultures. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni’s modification of Student’s t-test. *p < 0.05 vs. TCPS; #p < 0.05 vs. MG63 cultures on matching substrates.
3.2.3. VEGF-A
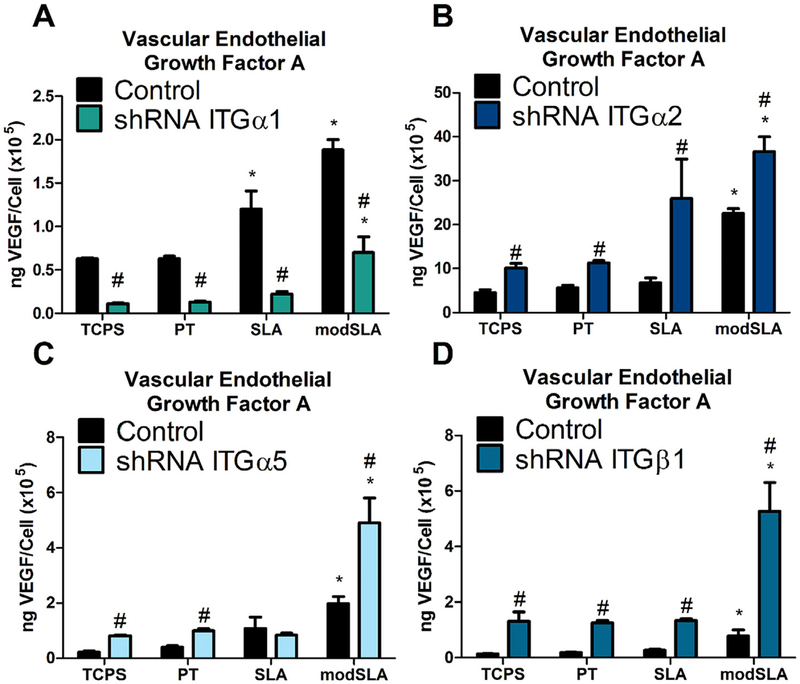
Production of VEGF-A was differentially regulated by integrin silencing. Integrin α1 silenced cells produced significantly less VEGF-A compared to wild-type MG63 cells on all substrates examined (Fig. 4A). In contrast, MG63 cells that had been stably silenced for expression of integrins α2, α5, and β1 exhibited significantly higher levels of VEGF-A in the conditioned media than wild-type MG63 cells regardless of Ti surface roughness or surface energy (Fig. 4B–D).
Fig. 4.
VEGF-A levels in the conditioned media in MG63 cells silenced for integrins αl, α2, α5, and 31. (A) VEGF-A levels in the conditioned media for integrin αl, (B) integrin α2, (C) integrin α5, and (D) integrin 31, as well as MG63 cells were determined. Values presented are mean ± SEM of six independent cultures. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni’s modification of Student’s t-test. *p < 0.05 vs. TCPS; #p < 0.05 vs. MG63 cultures on matching substrates.
3.2.4. FGF-2
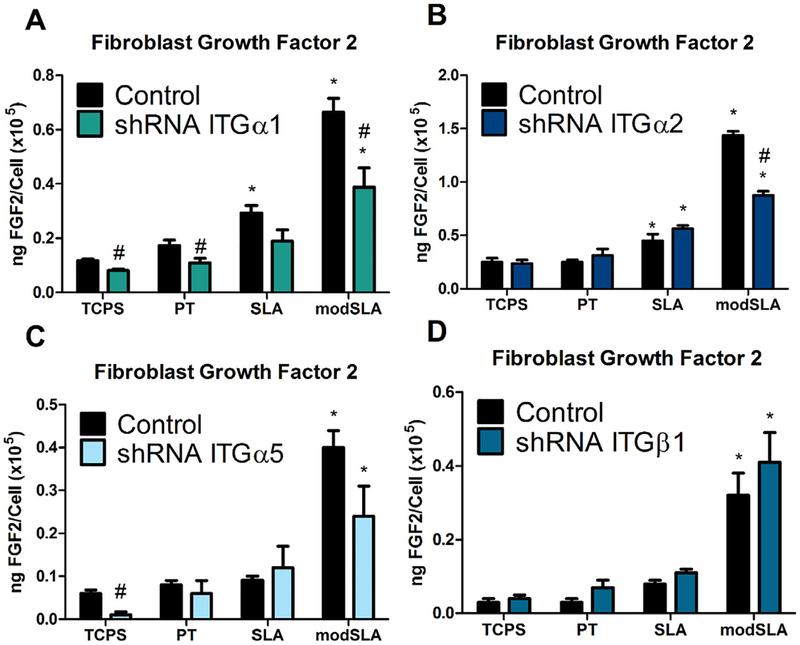
Ti surface roughness did not affect FGF-2 production by integrin α1 and α2 silenced MG63 cells, but the combination of a rough surface microtopography and high surface energy reduced production of FGF-2 by these cells significantly compared to wild-type MG63 cells (Fig. 5A, B). Targeted silencing of integrin subunits α5 and β1 in MG63 osteoblast-like cells did not affect the secreted levels of FGF-2 by these cells in response to either Ti surface microtopography or surface energy (Fig. 5C, D).
Fig. 5.
FGF-2 levels in the conditioned media in MG63 cells silenced for integrins α1, α2, α5, and β1. (A) FGF-2 levels in the conditioned media for integrin α1, (B) integrin α2, (C) integrin α5, and (D) integrin as well as MG63 cells were determined. Values presented are mean ±SEM of six independent cultures. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni’s modification of Student’s t-test. *p < 0.05 vs. TCPS; #p < 0.05 vs. MG63 cultures on matching substrates.
3.2.5. Angiopoietin-1
In response to targeted knockdown of integrin receptor subunits α1, α2, and β1, the secretion of this growth factor was significantly up-regulated in response to Ti surface microtopography and surface energy compared to MG63 cell cultures (Fig. 6A, B, D), while knockdown of the α5 integrin receptor subunit had no effect on production of Ang-1 in response to Ti surface features (Fig. 6C).
4. Discussion
Integrin binding to titanium substrates is important in triggering osteoblastic differentiation of cells in response to surface microstructure and energy. Many integrins have been identified as being involved in osteoblast attachment and differentiation [34,35]. Previous work in our lab demonstrated increased VEGF-A after silencing of integrin α2 but decreased OCN. This was particularly interesting as angiogenesis may be upregulated by more mature osteoblasts to recruit a vascular supply necessary for proper bone formation and that substrate properties are capable of regulating this process through specific integrins. In the present study, we found that knockdown of specific integrin subunits in an MG63 osteoblast-like cell line not only affects the differentiation of these cells in response to Ti surface topography and energy but also affects the production of pro-angiogenic growth factors.
The results indicate that the expression of integrin receptor subunits involved in osteoblastic differentiation is affected in response to Ti surface features. Our results are consistent with those found previously where expression of integrins α2 and β1 is increased on rough Ti surfaces and expression of integrin α5 is decreased [21,23,36]. Similar to integrins α2 and β1, we observed that expression of integrin α1 is increased on microrough Ti surfaces compared to smooth Ti and TCPS control surfaces. These data suggest that pre-osteoblastic MG63s are capable of maturing into osteoblast-like cells in response to surface properties like microroughness and hydrophilicity.
Hydrophilicity is also a contributor to overall cellular response. The osteoblast differentiation marker OCN was increased the greatest on hydrophilic surfaces and loss of key integrin signaling pathways inhibited this response. Alterations in integrin expression demonstrate that integrins mitigate the production of angiogenesis markers like VEGF-A and Ang-1. Specifically, loss of integrin signaling for each silenced integrin increased the production of Ang-1 only on hydrophilic substrates, suggesting that Ang-1 was directly regulated by surface free energy and was regulated by all integrins in the present study. Conversely, FGF2 exhibited an opposite effect when integrin α1 and α2 were silenced, showing that increased production of FGF2 on modSLA substrates was due to the increased surface free energy. Integrin α1 and α2 complex with β1 to bind collagen; therefore surface free energy most likely alters collagen binding sites [16,37].
Cell transfection is a powerful tool to investigate the role of specific signaling pathways during surface mediated osteoblastic differentiation or maturation. However, shRNA lentiviral particles do possess some off-target effects, which could alter cellular response. In the present study we controlled for these off-target effects by using TCPS as an optical culture control to exam cellular morphology of wild-type and silenced cell models. We observed minimal morphological differences during cell culture (data not shown), but cell proliferation was altered in integrin β1, α2, α5 silenced cell lines. Silencing integrin α5 or β1 decreased cell number on smooth substrates suggesting difficulty for cells to adhere to the implant surface but minimal differences were seen on microtextured surfaces. Therefore we controlled for local factor production by normalizing to total cell number. Cells cultured on Ti substrates presenting a rough surface morphology and high surface energy have a more differentiated phenotype displaying increased levels of osteocalcin, osteoprotegerin, and the local factor prostaglandin E2 [38]. Knockdown of either the α2 or β1 integrin subunits in these cells resulted in the loss of differentiation in response to Ti surface microstructure [23,39] whereas knockdown of the α5 subunit did not result in any significant changes in the differentiation of MG63 osteoblast-like cells [40]. Our results from this study further confirm these findings and show that targeted silencing of the integrin α1 subunit also inhibits the surface roughness and energy dependent differentiation of MG63 osteoblast-like cells.
The mechanism by which Ti surface microstructure and energy regulate the production of pro-angiogenic growth factors by osteoblasts is unclear. We found here that knockdown of several integrin receptor subunits differentially regulated the production of VEGF-A, FGF-2, and Ang-1, specifically during surface mediated differentiation of progenitor cells into osteoblasts. In particular, secreted levels of VEGF-A were increased in cells silenced for integrins α2, α5, and β1 whereas silencing of integrin α1 resulted in a decrease in VEGF-A production in response to Ti surface topography and energy. This effect is independent of integrin regulation of osteoblast differentiation, as demonstrated by decreased osteocalcin in silenced α1, α2 , and β1 cells.
VEGF-A produced by cells in response to surface topography mediated integrin signaling could participate as an au tocrine/paracrine regulator of osteoblast differentiation, thereby contributing to the overall osteogenic response observed on the microtextured substrates. VEGF-A signaling exhibits cross-talk down stream of production through autocrine/paracrine signaling to increase production of osteoblast markers such as osteocalcin and osteoprotegerin [41]. VEGF-A possesses Nrp1 binding domains in addition to the traditional VEGF receptor domain [42] and could increase osteoblastic differentiation by complexing with Nrp1 and activating semaphorin signaling [43]. In addition, VEGF165b acts on cells via interaction of its receptor with the b1 integrin [31].
Future work should examine if angiogenic factors generated by osteoblast lineage cells possess create an autocrine/paracrine loop, modulating osteoblast differentiation in addition to the paracrine effect altering endothelial cell recruitment and vessel formation. Previous work demonstrated that VEGF-A from conditioned media produced by MG63 cells cultured on microstructured Ti substrates are capable of increasing endothelial cell tube formation and length [41]. Both osteogenesis and vasculogenesis are critical for successful osseointegration.
Signaling by VEGFR2 and integrin αVβ3 may have served to regulate the alterations in integrin complexes in the present study. VEGF activation of Flk-1 has been shown to increase c-Src intracellularly in endothelial cells and in turn, phosphorylates integrin β3 changing binding affinity [30]. Supplemental data from previous studies show no differences in expression of integrin αV or β3 when integrin α2 is silenced [23], suggesting VEGF could be upregulated in integrin α2 silenced MG63s to mitigate the effect of α2 silencing to decrease osteoblast differentiation [16]. Expression of the other integrins found in bone could cause the differential effects seen in this study. Future studies will require a multi-modal approach to examine the co-localization of integrin complexes on the cell membrane of wild-type and altered integrin cell models for the full array of expressed integrins in bone.
Levels of FGF-2 were only affected in response to knockdown of either α1 or α2 and were decreased on modSLA Ti surfaces in these cells. In contrast, secretion of Ang-1 was increased on modSLA Ti surfaces in α1, α2, and β1 silenced cells compared to wild-type MG63 cells. Taken together, these interactions provide strong evidence that protein-surface interactions, influenced by biomaterial surface properties, can regulate multiple pathways during implant integration through differential expression of integrin receptors.
Integrin α5β1 binds fibronectin [44] and has been suggested to promote early osteoblast proliferation and differentiation events and shRNA targeting endogenous integrin α5 has been found to inhibit osteoblastic differentiation of mesenchymal stromal cells [39], potentially explaining why α5 silencing did not have a significant effect on the expression of osteogenic or angiogenic markers in MG63 cells. Both α1β1 and α2β1 bind collagen I in the extracellular matrix [13] and both integrin receptors recognize and bind the same GFOGER motif [10,45]. However, in our study, we found that silencing of the α1 integrin subunit resulted in a decrease in the production of VEGF-A by MG63 cells in response to Ti surface features while knockdown of the α2 integrin subunit increased VEGF-A production. These data suggest that activation of the α1β1 integrin is necessary for the production of VEGF-A in MG63 osteoblast-like cells whereas α2β1 integrin binding and activation serves to downregulate VEGF-A expression. This may indicate that downstream signaling events differ between α1β1 and α2β1 integrins, resulting in the different effects induced by knockdown of each integrin.
5. Conclusion
Overall, our results indicate that microroughened and hydrophilic implant surfaces alter integrin expression of pre osteoblast MG63s during surface mediated osteoblastic differentiation. Moreover, signaling through these integrin receptor complexes regulates the angiogenic response of osteoblasts to implant surface features. Our data demonstrate specific integrin regulation of angiogenic marker VEGF-A by integrin α1 during osteoblast maturation.
Supplementary Material
Statement of Significance.
Successful implantation of synthetic biomaterials into bone depends on the biological process known as osseointegration. Osseointegration is a highly regulated communication of cells that orchestrates the migration of progenitor cells towards the implant site and promotes the deposition and mineralization of extracellular matrix proteins within the implant microenvironment, to tightly join the implant to native bone. In this process, angiogenesis functions as the initiation site of progenitor cell migration and is necessary for matrix deposition by providing the necessary nutrients for bone formation. In the present study, we show a novel regulation of specific angiogenic growth factors by integrin receptor complexes. This research is important to develop biomaterials that promote and maintain osseointegration through proper vascularization and prevent implant failure in patients lacking sufficient angiogenesis.
Acknowledgments
NIH USPHS 2 R01 AR052102 and 1 R01 AR072500, Cell and Tissue Engineering (CTEng) NIH Biotechnology Training Grant (TG GM08433), ITI Foundation, and Children’s Healthcare of Atlanta provided funding for this study.
Footnotes
Disclosures
BDB is a consultant for Institut Straumann AG (Basel, Switzerland) and Titan Spine LLC (Mequon, WI). ZS is a consultant for AB Dental (Ashdod, Israel).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.actbio.2019.07.036.
References
- [1].Trindade R, Albrektsson T, Wennerberg A, Current concepts for the biological basis of dental implants: foreign body equilibrium and Osseointegration Dynamics, Oral Maxillofac. Surg. Clin. North Am 27 (2015) 175–183, doi: 10.1016/j.coms.2015.01.004. [DOI] [PubMed] [Google Scholar]
- [2].Boyan BD, Lotz EM, Schwartz Z, (*) Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties, Tissue Eng Part A 23 (2017) 1479–1489, doi: 10.1089/ten.TEA.2017.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thevenot L, Tang W, Hu P, Surface chemistry influence implant biocompatibility, Curr. Top. Med. Chem 811 (2008), doi: 10.1016/j.nano.2008.04.001.SURFACE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boyd AR, Burke GA, Duffy H, Holmberg M, O’Kane C, Meenan BJ, King-shott P, Sputter deposited bioceramic coatings: Surface characterisation and initial protein adsorption studies using surface-MALDl-MS, J. Mater. Sci. Mater. Med 22 (2011) 74–84, doi: 10.1007/s10856-010-4180-8. [DOI] [PubMed] [Google Scholar]
- [5].Rupp F, Gittens RA, Scheideler L, Marmur A, Boyan BD, Schwartz Z, Geis-Gerstorfer J, A review on the wettability of dental implant surfaces I: theoretical and experimental aspects, Acta Biomater. 10 (2014) 2894–2906, doi: 10.1016/j.actbio.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, Boyan BD , A review on the wettability of dental implant surfaces II: biological and clinical aspects, Acta Biomater. 10 (2014) 2907–2918, doi: 10.1016/j.actbio.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Anselme K, Osteoblast adhesion on biomaterials, Biomaterials 21 (2000) 667–681. [DOI] [PubMed] [Google Scholar]
- [8].Lai M, Hermann CD, Cheng A, Olivares-Navarrete R, Gittens RA, Bird MM, Walker M, Cai Y, Cai K, Sandhage KH, Schwartz Z, Boyan BD, Role of A2B1 integrins in mediating cell shape on microtextured titanium surfaces, J. Biomed. Mater. Res. Part A 103 (2015) 564–573, doi: 10.1002/jbm.a.35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z, Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage, Biomaterials 31 (2010) 2728–2735, doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barczyk M, Carracedo S, Gullberg D, lntegrins, Cell Tissue Res 339 (2010) 269–280, doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Siebers MC, Ter Brugge PJ, Walboomers XF, Jansen JA, Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review, Biomaterials 26 (2005) 137–146, doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- [12].Gronowicz G , Mccarthy MB, Response of human osteoblasts to implant materials : integrin-mediated adhesion, J. Orthop. Res 14 (1996) 878–887. [DOI] [PubMed] [Google Scholar]
- [13].Garcia AJ, Get a grip : integrins in cell - biomaterial interactions, Biomaterials 26 (2005) 7525–7529, doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- [14].Goriainov V, Cook R, Latham JM, Dunlop DG, Oreffo ROC, Bone and metal: an orthopaedic perspective on osseointegration of metals, Acta Biomater. 10 (2014) 4043–4057, doi: 10.1016/j.actbio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- [15].Lerner UH, Ohlsson C , The WNT system: background and its role in bone, J. lnt. Med 277 (2015) 630–649, doi: 10.1111/joim.12368. [DOI] [PubMed] [Google Scholar]
- [16].Marie PJ, Hay E, Saidak Z, Integrin and cadherin signaling in bone: role and potential therapeutic targets, Trends Endocrinol. Metab 25 (2014) 567–575, doi: 10.1016/j.tem.2014.06.009. [DOI] [PubMed] [Google Scholar]
- [17].Olivares-Navarrete R, Hyzy SL, Gittens RA, Schneider JM, Haithcock D, Ullrich P, Slosar PJ, Schwartz Z, Boyan BD, Rough titanium alloys regulate osteoblast production of angiogenic factors, Spine J. 13 (2013), doi: 10.1016/j.spinee.2013.03.047.10.1016/j.spinee.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Buser D, Schenk R, Steinemann S , Fiorellini J , Fox C , Stich H , Influence of surface characteristics on bone integration of titanium implants: a histomorphometric study in mini pigs, J. Biomed. Mater. Res 25 (1991) 889–902, [DOI] [PubMed] [Google Scholar]
- [19].Girasole G, Muro G, Mintz A, Chertoff J, Transforaminal lumbar interbody fusion rates in patients using a novel titanium implant and demineralized cancellous allograft bone sponge, Int. J. Spine Surg 7 (2013) e95–e100, doi: 10.1016/j.ijsp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lotz EM, Olivares-Navarrete R, Berner S, Boyan BD, Schwartz Z, Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces, J. Biomed. Mater. Res. Part A 104 (2016) 3137–3148, doi: 10.1002/jbm.a.35852. [DOI] [PubMed] [Google Scholar]
- [21].Raz P, Lohmann CH, Turner J, Wang L, Poythress N, Blanchard C, Boyan BD, Schwartz Z, 1α,25(OH) 2D3 Regulation of integrin expression is substrate dependent, J. Biomed. Mater. Res. Part A 71 (2004) 217–225, doi: 10.1002/jbm.a.30134. [DOI] [PubMed] [Google Scholar]
- [22].Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA,Wasilewski CE, Boyan BD, Schwartz Z, Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop, Biomaterials 32 (2011) 6399–6411, doi: 10.1016/j.biomaterials.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA,, Ornoy A, Boyan BD, Schwartz Z , Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 15767–15772, doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang L, Zhao G, Olivares-Navarrete R, Bell BF, Wieland M, Cochran DL, Schwartz Z, Boyan BD, Integrin β1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3, Biomaterials 27 (2006) 3716–3725, doi: 10.1016/j.biomaterials.2006.02.022. [DOI] [PubMed] [Google Scholar]
- [25].Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD, Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy, Biomaterials 31 (2010) 4909–4917, doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Langen UH, Pitulescu ME, Kim JM, Enriquez-gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B, Sorokin L, Vaquerizas JM, Adams RH, Cell - matrix signals specify bone endothelial cells during developmental osteogenesis, 19 (2017). doi: 10.1038/ncb3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Max R, Gerritsen RR, Nooigen P, Goodman S, Sutter A, Keilholz U, Ruiter D, De Waal R , Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas, Int. J. Cancer 71 (1997) 320–324. [DOI] [PubMed] [Google Scholar]
- [28].Olivares-navarrete R, Rodil SE, Hyzy SL, Dunn GR, Almaguerflores A, Schwartz Z, Boyan BD, Role of integrin subunits in mesenchymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructured surfaces, Biomaterials 51 (2015) 69–79, doi: 10.1016/j.biomaterials.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F, Role of α v β3 integrin in the activation of vascular endothelial growth factor receptor-2, EMBO J. 18 (1999) 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Somanath PR, Malinin NL, Byzova TV, Cooperation between integrin avf3 and VEGFR2 in angiogenesis, Angiogenesis 12 (2009) 177–185, doi: 10.1007/s10456-009-9141-9.Cooperation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boudria A, Faycal CA, Jia T, Gout S, Keramidas M, Didier C, Lemaître N, Manet S, Coll J, Toffart A, Moro-sibilot D, Albiges-rizo C, Josserand V, Fau-robert E, Brambilla C, Brambilla E, Gazzeri S, Eymin B, VEGF 165b, a splice variant of VEGF-A, promotes lung tumor progression and escape from anti-angiogenic therapies through a β1 integrin/VEGFR autocrine loop, Oncogene (2019) 1050–1066, doi: 10.1038/s41388-018-0486-7. [DOI] [PubMed] [Google Scholar]
- [32].Bang SM, Moon HJ, Kwon YD, Yoo JY, Pae A, Kwon IK, Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces, Clin. Oral Implants Res 25 (2014) 831–837, doi: 10.1111/clr.12146. [DOI] [PubMed] [Google Scholar]
- [33].Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R, Titanium surface characteristics, including topography and wettability, alter macrophage activation, Acta Biomater. 31 (2016) 425–434, doi: 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Saito T, Albelda SM, Brighton CT , Identification of integrin receptors on cultured human bone cells, J. Orthop. Res 12 (1994) 384–394. [DOI] [PubMed] [Google Scholar]
- [35].Brighton CT, Albelda SM, Identification of integrin cell-substratum adhesion receptors on cultured rat bone cells, J. Orthop. Res 10 (1992) 766–773. [DOI] [PubMed] [Google Scholar]
- [36].Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD, Integrin a 5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner, (2006). doi: 10.1002/jbm.a. [DOI] [PubMed] [Google Scholar]
- [37].Askari JA, Buckley PA, Mould AP, Humphries MJ, Linking integrin conformation to function, J. Cell Sci. 122 (2009) 165–170, doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD, High surface energy enhances cell response to titanium substrate microstructure, J. Biomed. Mater. Res. - Part A 74 (2005) 49–58, doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- [39].Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD, Integrin a5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner, J. Biomed. Mater. Res. Part A 80 (2007) 700–710, doi: 10.1002/jbm.a. [DOI] [PubMed] [Google Scholar]
- [40].Schwartz Z, Bell BF, Wang L, Zhao G, Olivares-Navarrete R, Boyan BD, Beta-1 integrins mediate substrate dependent effects of 1α,25(OH)2D3 on osteoblasts, J. Steroid Biochem. Mol. Biol 103 (2007) 606–609, doi: 10.1016/j.jsbmb.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raines AL, Berger MB, Patel N, Hyzy SL, Boyan BD, Schwartz Z, VEGF-A regulates angiogenesis during osseointegration of Ti implants via paracrine/autocrine regulation of osteoblast response to hierarchical microstructure of the surface, J. Biomed. Mater. Res. Part A 107 (2019) 423–433, doi: 10.1002/jbm.a.36559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Peach CJ, Mignone VW, Arruda MA, Hill SJ, Kilpatrick LE, Woolard J, Molecular pharmacology of VEGF-A isoforms : binding and signalling at VEGFR2, Int. J. Mol. Sci 19 (2018), doi: 10.3390/ijms19041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A,Takayanagi H, Osteoprotection by semaphorin 3A, Nature 485 (2012) 69–74, doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- [44].Moursi AM, Globus RK, Damsky CH, Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro, J. Cell Sci 110 (1997) 2187–2196. [DOI] [PubMed] [Google Scholar]
- [45].Bellahcene A, Castronovo V, Ogbureke KUE, Fisher LW, Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer, Nat. Rev. Cancer 8 (2008) 212–226, doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.