Abstract
Obesity is becoming an epidemic in the United States and worldwide and increases risk for many diseases, particularly insulin resistance, type 2 diabetes, and cardiovascular disease. The mechanisms linking obesity with these diseases remain incompletely understood. Over the past 2–3 decades, it has been recognized that in obesity, inflammation, with increased accumulation and inflammatory polarization of immune cells, takes place in various tissues, including adipose tissue, skeletal muscle, liver, gut, pancreatic islet, and brain, and may contribute to obesity-linked metabolic dysfunctions, leading to insulin resistance and type 2 diabetes. Therapies targeting inflammation have shed light on certain obesity-linked diseases, including type 2 diabetes and atherosclerotic cardiovascular disease, but remain to be tested further and confirmed in clinical trials. This review focuses on inflammation in adipose tissue and its potential role in insulin resistance associated with obesity.
Keywords: obesity, adipose tissue, inflammation, insulin resistance, Metabolism
Introduction
Obesity is becoming a global epidemic and has nearly tripled worldwide since the 1970s. In 2016, more than 1.9 billion adults—39% of the world adult population—were overweight and more than 650 million were obese.1 Of note, 340 million children and adolescents aged 5–19 years and 41 million children under age 5 were overweight or obese worldwide.1 In the United States, more than 93 million adults—~40% of the adult population—and nearly 20% of children and adolescents aged 6–19 years were obese in 2015–2016.2
Obesity increases risks for many diseases, particularly insulin resistance and type 2 diabetes (T2DM). However, the underlying mechanisms remain incompletely understood. Over the last 2 decades, it has been recognized that obesity is associated with chronic low-grade inflammation in a variety of tissues, including adipose tissue (AT), skeletal muscle, liver, pancreas islet, intestine, and even brain.3–9 Both innate and adaptive immune cells can participate in obesity-linked inflammation, which, importantly, may serve as a causal link between obesity and insulin resistance and T2DM.3–9 In this review, we will summarize some advances in obesity-linked metabolic inflammation, AT inflammation in particular, and its potential role in development of insulin resistance, the basis for T2DM.
Type 1 and type 2 inflammation
Inflammation can be broadly classified as type 1 and type 2 inflammation, as initially described for adaptive immune response of CD4+ T lymphocytes, i.e., T helper 1 (Th1) and 2 (Th2) cells, respectively,10 depending on the stimuli/environments. Type 1 and type 2 inflammation involves different types of inflammatory cells, which express or produce distinct panels of inflammatory molecules via specific transcription factors and exert different functions (Table 1) (see reviews10–12). The term “proinflammation” or “inflammation” (including in this review) generally refers to type 1 inflammation. In contrast, in certain cases, type 2 inflammation is described or generalized as “anti-inflammation.”
Table 1.
Comparison of type 1 and type 2 inflammation
| Type 1 | Type 2 | |
|---|---|---|
| Stimuli | LPS, IFNγ, TNFα, IL-12 | IL-10, IL-4/IL-13, allergens |
| Major cells | Th1, CD8+ TEM/TE, M1 macrophages, neutrophils | Th2 cells, M2 macrophages, eosinophils, ILC2 |
| Secreted cytokines | IFNγ, TNFα, IL-1β, IL-6, IL-12 | IL-4, IL-5, IL-10, IL-13, TGFβ |
| Transcription factors | T-bet (Th1), STAT1, NF-κB, IRF5 | STAT3, STAT6, IRF4, GATA3, PPARγ |
| Major functions | Defense against intracellular pathogens | Immunoregulation, tissue remodeling, allergy, defense against extracellular parasites |
GATA3 indicates GATA binding protein 3; IFNγ, interferon-γ; IL, interleukin; ILC, innate lymphoid cells; IRF, interferon regulatory factor; LPS, lipopolysaccharide; NF-κB, nuclear factor–κB; PPARγ, peroxisome proliferator–activated receptor–γ; STAT, signal transducer and activator of transcription; TE, effector T cells; TEM, effector memory T cells; Th, T helper; and TNFα, tumor necrosis factor–α.
Inflammation in obesity
The first evidence for obesity-linked inflammation was from a report of elevated TNFα in AT in obesity.13 Since then, numerous studies have consistently shown increased inflammation in AT in obese animals and humans. Although inflammation takes place in a variety of tissues when obesity occurs, most of our knowledge on obesity-linked inflammation still derives from studies of AT.
Inflammation in visceral (VAT) and subcutaneous (SAT) AT:
The initial study attributed AT inflammation to adipocytes, the main resident cells in AT.13 Indeed, adipocytes can become inflamed and secrete a variety of inflammatory molecules.13–16 However, since the identification of macrophages in AT,17,18 macrophages and various other immune cells (Supplemental Table I) have been demonstrated to be the main inflammatory cells that release the majority of inflammatory molecules in AT of obese animals and humans.3–9
Macrophages:
The increase in macrophages is one of the hallmarks of obesity-linked inflammation in AT.3,17–22 Macrophages reside in AT, including VAT and SAT, in lean conditions and, with development of obesity, increase progressively and constitute the largest immune cell population in AT with established obesity.3,17,18,20,22 The initial study showed that the percentage of macrophages was positively correlated with adipocyte size and body mass in all AT depots examined, including VAT (perigonadal, perirenal, and mesenteric AT) and SAT in mice and SAT in humans.17 However, most studies of AT inflammation in mice have focused on perigonadal VAT.18,19,21 Histologically, in obesity, macrophages surround dead or dying adipocytes forming crown-like structures (CLS).17,21 Besides the change in numbers, AT macrophages can switch phenotypes from M0- or M2-like in lean conditions to M1-like in obesity, with increased expression of type 1 cytokines such as TNFα, IL-1β, and IL-6.21,23 In obesity, AT macrophages express high levels of CD11c,19–21 a β2 integrin that is commonly used to define dendritic cells in mice. Indeed, CD11c+ cells in obese AT express high levels of MHCII and include macrophages and dendritic cells, both of which contribute to AT inflammation.19,24,25 Compared to CD11c− macrophages, CD11c+ cells in obese AT express higher levels of inflammatory markers,21 contribute to obesity-linked AT inflammation,26 and therefore have been commonly considered “M1” macrophages in AT in obese mice. However, the phenotypic switch of AT macrophages may be more complex than the classical M1–M2 paradigm. Kratz et al showed that in obesity, macrophages in AT, including VAT in obese mice and VAT and SAT in obese humans, polarize into a “metabolically activated” phenotype, which is associated with elevated levels of type 1 inflammatory markers and also markers involved in lipid metabolism, such as CD36, ABCA1, and Plin2, that have been considered M2 markers.27 CD11c+ macrophages in AT of obese humans mostly coexpress CD206, a pattern recognition receptor also commonly considered an M2 marker,28 supporting a mixed phenotype rather than distinct M1 or M2 phenotype of AT macrophages.28 Indeed, CD11c+CD206+ macrophages from AT of obese humans express high levels of type 1 cytokines such as TNFα, IL-1β, and IL-6 and also IL-10, a type 2 cytokine.28 Of note, although most animal studies focus on VAT inflammation or indicate greater macrophage accumulation in VAT than SAT,18,19,21,27 SAT appears to contain more CD11c+CD206+ macrophages than VAT in obese women.28
Recent studies employing single-cell RNA-seq identified a CD9- and CD63-expressing macrophage population that expands in VAT of mice and humans with obesity.29,30 Compared to another macrophage population, which expresses Ly-6C and is also increased in AT of obese mice, CD9+ macrophages, which are Ly-6c−, are the predominant macrophages forming CLS, express high levels of type 1 cytokines such as MCP-1, IL-1, and TNFα, and can initiate inflammatory responses in lean AT, supporting an important role of CD9+ macrophages in AT inflammation.29 CD9+ macrophages also express Trem2, have a transcriptional signature of lipid metabolism and lysosomal pathways, are filled with intracellular lipids, and are therefore termed Trem2+ lipid-associated macrophages,29,30 perhaps similar to a metabolically activated phenotype.27 The relation of CD9+Trem2+ macrophages with CD11c+ macrophages remains to be determined. However, an earlier study indicated that obesity initiates lysosome biogenesis associated with lipid accumulation in AT macrophages, CD11c+ macrophages in particuar,31 suggesting that the newly identified CD9+ macrophages may share large similarities with the early described CD11c+ macrophages in obese AT.
T lymphocytes:
T cells are also increased in AT, with higher frequency in VAT than in SAT, of obese mice and humans14,32,33 and highly correlated with systemic inflammation in humans.32 Increases in AT T cells may precede and contribute to the increases in AT macrophages in mice fed high-fat diet (HFD).34–39 αβT cells as compared to γδT cells appear to have greater increases in obese AT.40 Within αβT cells, CD8+ T cells show greater increases than CD4+ T cells in AT of obese mice.36,38
CD8+ T cells:
In mice fed HFD, CD8+ T cells increase early in VAT, but not SAT, and, along with macrophages, participate in CLS formation.36 In obesity, VAT CD8+ T cells polarize into effector memory or effector phenotypes expressing high levels of IFNγ and activation markers such as CD69.36,38,41 In mice, depletion of CD8+ T cells attenuates and adoptive transfer of CD8+ T cells exacerbates obesity-associated macrophage accumulation and inflammation in VAT,36 supporting an important role of CD8+ T cells in AT macrophage accumulation and inflammation in obesity.
CD4+ T cells:
Within AT CD4+ T cells, the frequency of IFNγ-expressing Th1 cells is increased with obesity and is higher in VAT than SAT in both mice and humans.32,40,42 However, Th1 frequency in both AT depots correlates positively with inflammation in mice and humans.32,35,40,43–46 Ablation of the Th1 cytokine IFNγ attenuates AT inflammation, suggesting a crucial role of Th1 in AT inflammation.37,40 In contrast, regulatory T cells (Treg), usually a small portion of CD4+ T cells in lymphoid organs, are enriched in AT, particularly VAT, in lean conditions but dramatically decreased in VAT with obesity. Induction of AT Treg protects against obesity-associated AT inflammation.47,48 The proportion of Th2 cells in both VAT and SAT negatively correlates with systemic inflammation and insulin resistance in humans, indicating a protective role of Th2 cells in inflammation and metabolic dysfunction.32 Indeed, a study showed that with adoptive transfer into obese Rag1-null T cell–deficient mice, CD4+ T cells polarized into Th2, which was associated with reversal of enhanced weight gain and insulin resistance in recipient mice.42 However, another study indicated that transfer of naive CD4+ T cells in Rag1-null mice resulted in a Th1 response in AT.25 In addition, IL-17–producing Th17 cells and IL-22–expressing Th22 are increased in SAT in humans with obesity.25,32 Nevertheless, Th17 frequency is higher in VAT than SAT of obese humans.32
γδT cells:
γδT cells account for approximately 30% of total CD3+ T cells in VAT in lean conditions and may increase in AT with obesity.40,49,50 HFD-induced obesity in mice increased TNFα-expressing γδT cells in VAT.50 Deficiency of γδT cells or only Vγ4 and Vγ6 subsets reduced AT inflammation, with decreased accumulation of macrophages, CD11c+ M1-like macrophages in particular, in VAT of mice, supporting an essential role of γδT cells in obesity-linked inflammation.50 γδT cells may also be a major source of IL-17A in AT, and IL-17A–expressing γδT cells are increased in both VAT and SAT of mice with obesity induced by HFD or associated with aging.39,49,51
NKT cells:
NKT cells are a heterogeneous subset of T cells that express markers for αβT cells, αβTCR, and NK cells and can produce both Th1 and Th2 cytokines. Depending on the TCR repertoire, NKT cells can be classified into type I NKT cells, which express the invariant TCRα (Vα14-Jα18 in mice, Vα24-Jα18 in humans) and are therefore also called invariant NKT (iNKT), and type II NKT, which do not express this invariant TCRα chain.39 Studies of the change and role of NKT in AT inflammation have generated inconsistent data. Some studies indicated that HFD in mice increased or activated VAT NKT, including iNKT, and that deficiency of NKT or iNKT reduced and activation of iNKT cells enhanced macrophage infiltration and inflammation in VAT in mice, indicating a promoting role of NKT, including iNKT, cells in obesity-linked AT inflammation.52–54 In contrast, other studies showed that iNKT cells are enriched in lean VAT, decreased with obesity, and may play a protective role in obesity-linked AT inflammation, by producing type 2 cytokines such as IL-10 and IL-4.55–57
B lymphocytes:
B cells, particularly B2, are also elevated in VAT of mice on HFD and present in CLS in SAT of obese humans.33,58–60 Compared to those from lean mice, B cells from obese mice exhibit a type 1 inflammatory phenotype with increased production of type 1 cytokines such as IL-6 and pathogenic IgG antibodies.58,61 Deficiency or depletion of B cells decreases and adoptive transfer of B2 cells increases AT inflammation in obese mice.58,59,61,62 B cells, particularly B2 cells, may contribute to AT inflammation by producing type 1 cytokines and pathogenic IgG antibodies, activation of macrophages and T cells, and modulation of Treg.58,61 In contrast, B1 cells and regulatory B cells reside in VAT and SAT of mice and humans in normal conditions, are reduced with obesity, and may play a protective role in obesity-linked inflammation, possibly by producing IgM antibodies and IL-10 and suppressing CD8+ T cell activation.62–64 In lean mice, SAT appears to contain more B cells, with higher expression of IL-10, than VAT.64
Neutrophils:
Neutrophils are the most abundant leukocytes in peripheral blood in humans. HFD feeding in mice resulted in an early and sustained infiltration of neutrophils in AT, mainly VAT.65–68 Neutrophils may contribute to AT inflammation by secreting elastase, myeloperoxidase, and IL-1β and interacting with adipocytes recruiting monocytes/macrophages. Ablation of neutrophil elastase or myeloperoxidase reduces AT inflammation with decreased macrophage content and expression of inflammatory molecules.66,67,69 Neutrophils are also activated in peripheral blood and present in both SAT and VAT of humans with morbid obesity.33 Weight loss by bariatric surgery in humans reduced neutrophil content in both fat pads.33
Eosinophils:
Eosinophils, usually a small portion of leukocytes in peripheral blood, are enriched in both VAT and SAT in normal conditions but drastically reduced with obesity.70–72 In lean mice, VAT appears to contain more eosinophils than SAT.70,73 Caloric restriction, microbiota depletion, helminth infection, or CCR2 deficiency in obese mice elevates AT eosinophils, which positively correlate with M2-like macrophages and type 2 cytokines.70,73–75 Further studies show that AT eosinophils may play a role in inducing M2-like macrophages and suppressing type 1 inflammation via IL-4– and IL-13–mediated mechanisms.70,76–78 However, another study showed that elevating AT eosinophils in obese mice by administering recombinant IL-5 did not alter AT M2-like macrophages and other inflammatory markers, suggesting that eosinophils may not have a direct role in promoting macrophage M2-like phenotype and regulating inflammation in obese AT.71
Mast cells:
Mast cells are a type of immune cells that participate in allergic responses and inflammation by releasing a variety of inflammatory molecules, including cytokines/chemokines, histamine, serotonin, and proteases. Studies show that mast cells are also elevated and activated in VAT and SAT of mice and humans with obesity79–81 and may contribute to AT inflammation by producing various inflammatory mediators such as IL-6 and IFNγ.79,80 Genetic ablation or pharmacological stabilization of mast cells in mice decreases AT inflammation.79,80
Innate lymphoid cells (ILCs):
ILCs are a heterogeneous group of immune cells of the lymphoid lineage but lack the antigen-specific B or T cell receptors. Based on the cytokine and transcriptional signatures, ILCs can be classified into 3 subsets: group 1 ILCs (ILC1 and NK cells), group 2 ILCs (ILC2), and group 3 ILCs (ILC3), which simulate Th1, Th2, and Th17 and express Th1 cytokines, type 2 cytokines, and IL-17A, respectively. Obesity increases VAT, but not SAT, NK cell numbers and NK cell expression of type 1 cytokines, including IFNγ and TNFα. Genetic ablation or antibody depletion of NK cells reduces and expansion or adoptive transfer of NK cells exaggerates macrophage accumulation and inflammation in VAT with obesity.82–84 Obesity may also enhance ILC1 in SAT and VAT, which produces IFNγ and can therefore accelerate M1-like polarization of macrophages and contribute to AT inflammation.85–87 In contrast, ILC2 reside in VAT and SAT of both mice and humans in lean conditions, decrease with obesity, and may play an important role in normal AT homeostasis, including maintenance of eosinophils and M2-like macrophages.76,88,89
Inflammation in intermuscular/perimuscular AT:
In addition to expansion of VAT and SAT, obesity is also associated with expansion of AT in skeletal muscle between muscle fibers, so-called intermuscular/intermyocellular AT (IMAT), and AT surrounding skeletal muscle, so-called perimuscular AT (PMAT).6,17,20,22,26,45 IMAT and PMAT are extramyocellular fat but different from SAT, and expand with obesity and also aging.6,51,90–92 IMAT/PMAT are highly correlated with insulin resistance and may directly regulate muscle insulin sensitivity.90–93 A large number of studies indicated that inflammation also occurs in IMAT/PMAT in humans and mice with obesity. Similar to inflammation in VAT or SAT, IMAT/PMAT inflammation is mainly evidenced by increased immune cells, particularly macrophages and T cells,6,7,17,20,22,26,45,94–96 which can also form CLSs surrounding dead or dying adipocytes.45 Immune cells in IMAT/PMAT also show type 1 inflammatory phenotypes in obesity, with macrophages mostly expressing CD11c and exhibiting M1-like phenotypes, and T cells (CD4+) exhibiting Th1 phenotype with reduction in Treg.6,20,45,95 Consistently, type 1 cytokines such as TNFα and IFNγ are increased and type 2 cytokines such as IL-10 are decreased in skeletal muscle in obesity.6,20,45,94–96
Mechanisms for obesity-linked inflammation
The mechanisms underlying obesity-linked inflammation remain poorly understood and may vary depending on tissue types and location. Immune cell recruitment, interactions, and activation releasing inflammatory molecules may constitute the major component of AT inflammation; however, the causal factors for these processes are largely unknown. Below, we discuss potential mechanisms for obesity-linked inflammation in AT (Figure 1).
Figure 1. Regulation of inflammation in adipose tissue (AT).
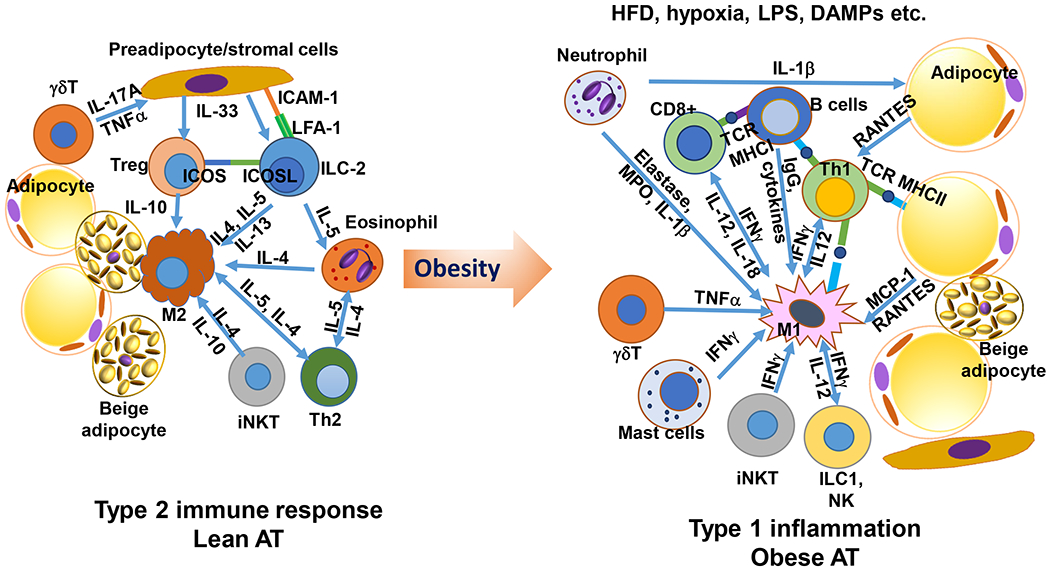
In lean conditions, AT is enriched with type 2 immune cells, including alternatively activated M2-like macrophages (M2), innate lymphoid type 2 cells (ILC2), regulatory T cells (Treg), T helper type 2 cells (Th2), eosinophils, and others, which interact with each other by producing type 2 cytokines such as interleukin-4 (IL-4), IL-5, and IL-13 and also by direct contact via interactions of cell surface molecules, to maintain a type 2 immune environment. With the development of obesity, particularly induced by high-fat diet (HFD), dietary saturated fatty acids, along with AT hypoxia, danger-associated molecular patterns (DAMPs), and “metabolic endotoxemia” with increased plasma levels of lipopolysaccharide (LPS) that develop in obesity, may promote type 1 inflammation in AT, with increased inflammation in adipocytes and elevated number and type 1 inflammatory polarization or “metabolically activated” activation of a variety of immune cells, including macrophages, T cells, B cells, neutrophils, and others, which produce a large number of type 1 inflammatory molecules and also interact directly with each other to induce a type 1 inflammatory environment in AT. ICAM-1 indicates intercellular adhesion molecule–1; ICOS, inducible costimulator; ICOSL, inducible costimulator ligand; IFNγ, interferon-γ; ILC, innate lymphoid cells; iNKT, invariant natural killer T cells; LFA-1, leukocyte function-associated antigen–1; MCP-1, monocyte chemoattractant protein–1; MHC, major histocompatibility complex; MPO, myeloperoxidase; NK, natural killer cells; RANTES, regulated on activation, normal T cell expressed and secreted; TCR, T-cell receptor; and TNFα, tumor necrosis factor–α. (Illustration Credit: Ben Smith).
Immune cell recruitment:
During obesity development, inflammation and recruitment of immune cells, mainly type 1 inflammatory cells, start early in AT.65,66,68,96–98 In particular, neutrophils start to increase in VAT of mice after 3 days on HFD.65,66 Increases in T cells appear to precede and contribute to the increases in macrophages in VAT of mice with diet-induced obesity.34–36 However, increases in macrophages occur earlier than increases in T cells in IMAT/PMAT during obesity development in mice.45 Macrophages that expand in obese AT appear to be mainly derived from recruited monocytes, including Ly-6Chigh, which are CD11c−, and Ly-6Clow, which are CD11c+,99,100 monocytes.19,21,23,29,30,101–103
For immune cell recruitment, interactions of chemokines with chemokine receptors on immune cells play crucial roles. Indeed, a large number of chemokines such as MCP-1, RANTES, fractalkine, KC, and MIP-2α are increased early and persistently in AT with obesity.14,65,68,96–98,104 Both immune cells and adipocytes/preadipoctyes can produce chemokines,13–16,45,69,96 which may play key roles in immune cell recruitment and inflammation in AT in obesity. MCP-1/CCR2 and RANTES/CCR5 pathways contribute to obesity-linked AT inflammation by recruiting immune cells such as macrophages and T cells14,45,104–107 whereas MIP-2α may be essential for neutrophil recruitment in AT in obesity.68 In addition, the arachidonic acid–derived leukotriene LTB4 is upregulated in VAT of obese mice and mediates macrophage recruitment in VAT in obesity.108 Interactions of adhesion molecules on immune cells and their receptors on endothelial cells also play pivotal roles in immune cell migration. Lymphocyte function-associated antigen–1 (LFA-1), a β2 integrin, plays an essential role in T cell infiltration and accumulation in VAT and IMAT/PMAT of obese mice, likely by interacting with ICAM-1 on endothelial cells or antigen-presenting cells.38,45 ICAM-1 may also play an important role in neutrophil recruitment in AT in obesity.68 α4 integrin and PSGL-1, a primary ligand on immune cells for P-selectin and E-selectin, may contribute to HFD-induced AT inflammation by mediating monocyte recruitment.109,110 In addition to recruitment, increased proliferation and impaired egress of macrophages and T cells may also contribute to accumulation of these immune cells in VAT in obesity.38,44,103,111,112
Immune cell interaction and activation:
Once recruited into AT, immune cells and also adipocytes can interact with each other through secreted molecules or direct cell–cell contact, leading to immune cell (and adipocyte) activation, polarization, and inflammation (Figure 1).
Cytokines/chemokines are important mediators for immune cell activation. A variety of type 1 cytokines such as TNFα, IFNγ, and IL-12 are increased in AT in obesity and are involved in obesity-linked AT immune cell activation and type 1 inflammation.13,37,45,94,113,114 TNFα, the signature cytokine of M1 macrophages, and its downstream intracellular IKK/NF-κB and JNK pathways, and IFNγ, the signature cytokine of Th1 that is also produced by ILC1 and mast cells, with its intracellular JAK/STAT1 pathway, are crucial for obesity-induced AT macrophage M1-like polarization and inflammation.37,45,79,83,115–117 IL-12, mainly produced by M1 macrophages/dendritic cells, and its downstream signaling molecule STAT4, play crucial roles in Th1 polarization and CD8+ T cell activation and also contribute to ILC1 accumulation in AT with obesity.38,85,114 In addition, elastase, myeloperoxidase, and cathelicidin produced by neutrophils may contribute to AT macrophage accumulation and activation in obesity.66,67,118
In contrast to AT type 1 inflammation in obesity, type 2 inflammation dominates in AT in lean healthy conditions, in which type 2 cytokines play essential roles in AT immune homeostasis. For example, IL-33, which is mainly produced by AT stromal cells, is important for maintenance of Treg and ILC2 in AT. Treg are protective against type 1 inflammation and autoimmunity, and ILC2 may play a role in accumulation and maintenance of eosinophils, Th2 cells, and M2 macrophages by producing type 2 cytokines such as IL-5, IL-4, and IL-13.47,70,76,89,119 In contrast, IFNγ may suppress AT ILC2 activation and Treg accumulation in obesity.89
Immune cells can also interact with each other and with adipocytes through direct contact. In mice with obesity, AT macrophages/dendritic cells and also adipocytes can activate T cells and induce T cell proliferation and Th1 polarization through the MHCII-TCR pathway.24,35,43,44 Further, costimulators/coinhibitors expressed on antigen-presenting cells and T cells are important for antigen-presenting cell–T cell interaction. CD40 and B7 molecules (CD80 and CD86) are induced in AT of obese mice.120–122 CD40 ligand may play a promoting role in T cell and M1-like macrophage accumulation and inflammation in AT of obese mice.123 However, CD40 may function as an inhibitor for AT inflammation in obesity.120,121 Similarly, CD80/CD86 may have a protective role in AT inflammation in mice with obesity.122 In contrast, OX40, a costimulatory molecule that is increased on AT CD4+ T cells in obesity, and CD11c expressed mainly on macrophages/dendritic cells may promote T cell activation and Th1 polarization in obese mice.19,124 In addition, ILC2 can interact with AT macrophages upon HFD feeding through the PD-1–PD-L1 pathway, leading to impaired ILC2 function,125 and with Treg via ICOSL-ICOS or with AT stromal cells via LFA-1–ICAM-1 in normal conditions sustaining ILC2 and Treg cells and type 2 inflammatory environment in AT.89,119
Triggers and sustainers:
Despite the above discussion on AT immune cell accumulation and activation in obesity, the signals that trigger and maintain these inflammatory changes are not well unknown and may include multiple factors, which are discussed below.
Dietary component and fatty acids (FAs):
In mice with diet-induced obesity, inflammation in AT starts early after initiating HFD and persists as long as HFD is maintained.65,66,96–98 On the other hand, AT inflammation is reduced quickly after switching from HFD to normal chow.126,127 These data suggest that diet may play an important role in regulation of AT inflammation. Indeed, the main HFD-derived dietary component, long-chain saturated FAs such as palmitic acid, induces inflammation including expression of chemokines and type 1 cytokines in a variety of cell types, including adipocytes and macrophages, possibly by interaction with pattern recognition receptors such as TLR4 and TLR2 and activation of NF-κB and JNK pathways and the NLRP3 inflammasome, which all indeed play essential roles in obesity-linked AT inflammation.6,15,20,128–131 Adipocyte-derived chemokines may play an important role in initiation of immune cell accumulation and inflammation in AT upon HFD feeding.14–16 With the progression of obesity, dietary FAs and other signals such as damage-associated molecular patterns, which are produced by stressed or damaged cells and bind to pattern recognition receptors, may play pivotal roles in maintenance of AT inflammation by inducing macrophage M1 polarization.6,20,21,128–131
Hypoxia:
During obesity development, rapid expansion of AT (adipocyte hypertrophy), with insufficient AT vascularization and also increased adipocyte oxygen consumption, results in AT hypoxia,132–134 which can activate hypoxia-inducible factors (HIFs), a family of transcription factors.135 Indeed, obesity in mice induces early and consistent upregulation of HIF1α in AT, associated with AT inflammation.132,133 Deletion of HIF1α in adipocytes reduces and overexpression of HIF1α in AT exacerbates HFD-induced AT inflammation in mice.132,134,136 By activating HIF-1α, hypoxia can induce adipocyte expression of chemokines such as MCP-1 and LTB4,132 which may contribute to AT inflammation by initiating immune cell accumulation and inflammation in AT. In addition, AT hypoxia may also contribute to AT inflammation by enhancing macrophage M1-like polarization, in which HIF1α and other signaling molecules such as JNK play essential roles.137–139 These studies indicate that similar to FAs, hypoxia may play a crucial role in initiation and maintenance of AT inflammation by inducing adipocyte expression of chemokines recruiting immune cells and by inducing macrophage M1-like polarization, mainly via HIF1α- and JNK-mediated mechanisms.
Intestinal microbiota and LPS:
Large numbers of studies show that obesity or HFD feeding is associated with alterations in intestinal microbiota, with increases in LPS-containing microbiota, and increased intestinal permeability,140,141 which can raise plasma levels of LPS leading to so-called metabolic endotoxemia.142,143 Experimental endotoxemia induced by low-dose injection of LPS induces AT inflammation in healthy humans and animals, supporting a direct role of metabolic endotoxemia in AT inflammation.142,144,145 LPS in metabolic endotoxemia may contribute to AT inflammation by eliciting type 1 inflammation including inducing expression of type 1 cytokines and chemokines in adipocytes and macrophages via interactions with TLR4 and CD14.142,146 In support of this hypothesis, germ-free mice or mice with antibiotic treatment to reduce metabolic endotoxemia or deletion of CD14, TLR4, or the related signaling molecule have ameliorated AT inflammation induced by HFD or obesity.128,146 Therefore, metabolic endotoxemia, resulting from alterations in intestinal microbiota and increased intestinal permeability, may also play a role in triggering and maintaining obesity-linked AT inflammation by impacting adipocytes, macrophages, and other immune cells.140,141
Inflammation and metabolism
Inflammation and insulin resistance:
As described above, in healthy conditions, normal AT homeostasis is associated with type 2 inflammatory environment (Figures 1 and 2). In contrast, obesity with insulin resistance is associated with type 1 inflammation in AT. While insulin resistance may precede and contribute to AT (type 1) inflammation,147 most studies still support that inflammation plays a causal role in the development of insulin resistance. Alternatively, inflammation may play differential roles at different conditions or stages of obesity. Once initiated, inflammation and insulin resistance may exacerbate each other. AT inflammation may contribute to local (AT) and systemic insulin resistance through autocrine effects of inflammatory cells/molecules on insulin signaling and metabolism in adipocytes and endocrine effects of inflammatory molecules secreted by AT (known as adipokines) on insulin sensitivity in other tissues, particularly skeletal muscle and liver. In addition, adverse effects of inflammation on preadipocyte/adipocyte metabolism can accelerate fat spillover from AT to skeletal muscle and liver, resulting in ectopic fat deposition and insulin resistance in these tissues, which play vital roles in systemic insulin resistance and T2DM.5,6,22,148,149
Figure 2. Impact of inflammation on AT metabolism and remodeling.
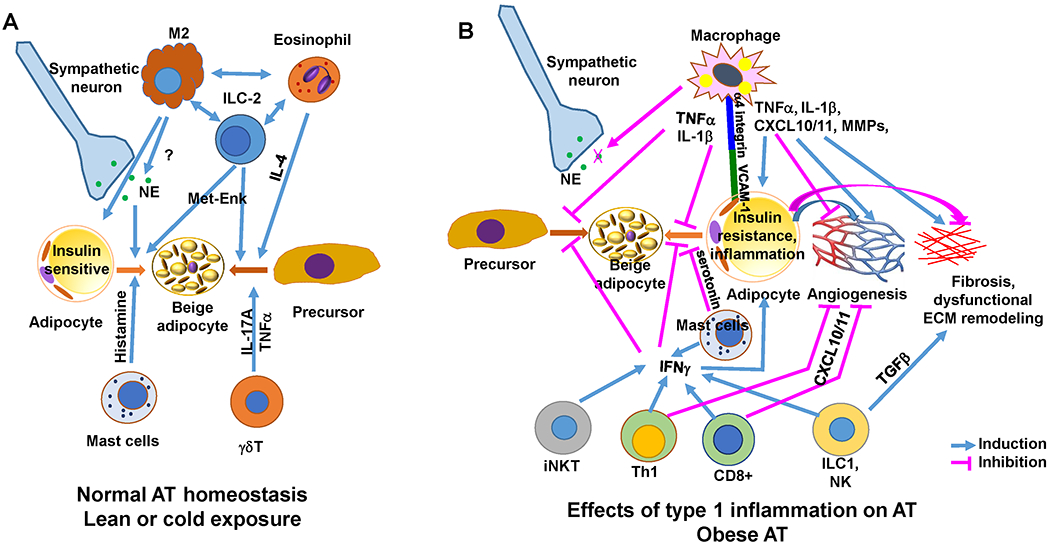
A. In lean conditions, with a type 2 immune environment, various immune cells through different mechanisms contribute to maintenance of normal AT functions including adipocyte insulin sensitivity and beige adipogenesis. B. In obesity, type 1 inflammatory or metabolically activated immune cells may contribute to AT dysfunctions by adversely regulating adipocyte metabolism and AT remodeling, including induction of insulin resistance in adipocytes, suppression of beige adipogenesis, and induction of dysfunctional AT remodeling. Studies also show that in obesity, adipocyte inflammation may be essential for healthy AT expansion and remodeling and that lipid storage within AT macrophages may protect against obesity-linked adipocyte dysfunctions. ECM indicates extracellular matrix; MET-ENK, methionine-enkephalin; MMPs, matrix metalloproteinases; NE, norepinephrine; TGFβ, transforming growth factor–β; and VCAM-1, vascular cell adhesion molecule–1. (Illustration Credit: Ben Smith).
Contributions of AT inflammation to insulin resistance:
Since the report of increased inflammation in AT in obesity, the association and potential contribution of AT inflammation to obesity-linked insulin resistance and T2DM have been revealed by numerous studies (Figure 2).
Earlier studies showed associations of increased AT TNFα and macrophages with insulin resistance in obese mice and humans.13,17,18 The increase in AT macrophages appears to precede insulin resistance in mice with HFD-induced obesity.18 Increasing AT macrophage content in mice by overexpressing MCP-1 was associated with exacerbated insulin resistance.106 Increasing AT inflammation by experimental endotoxemia also induces insulin resistance in healthy humans and mice.142,144,145 On the other hand, reductions in AT macrophages or TNFα by depletion of macrophages or TNFα or ablation of MCP-1 or its receptor in mice was associated with improved insulin resistance.26,106,107,113 All these data support a casual role of AT macrophages and TNFα in development of insulin resistance.
Increased AT T cells, particularly CD8+ effector/effector memory T cells and CD4+ Th1 cells, are associated with insulin resistance in both mice and humans.14,32,35,40,43–46 Genetic deficiency of CD8+ T cells or Th1 cells in αβT cell–null mice or ablation of IFNγ or T-bet, the master transcription factor for Th1 polarization, improves insulin resistance in obese mice, whereas adoptive transfer of Th1 or CD8+ T cells exacerbates insulin resistance in mice fed HFD,36,37,40,45,150 supporting contributions of Th1 cells and CD8+ T cells to development of obesity-linked insulin resistance. In contrast, adoptive transfer or expansion of Th2 cells or Treg prevents obese mice from developing insulin resistance,42,47,48 suggesting a protective role of type 2 immune cells in obesity-linked insulin resistance.
In addition, B2 cells, neutrophils, NK cells, and ILC1 have been shown to contribute to development of insulin resistance in obesity.58,59,61,66,67,82–87 In contrast, B1 cells, Breg cells, and ILC2 may protect against obesity-linked insulin resistance.62–64,76,88,89 A role of mast cells was demonstrated in development of obesity-linked insulin resistance in some,79 but not other,80,151 studies. Whereas some studies revealed a promoting role of NKT, including iNKT and type II NKT, in development of insulin resistance in obesity,52–54 other studies showed a protective role of iNKT cells against this effect.55–57 Eosinophils were initially demonstrated to protect against obesity-induced insulin resistance.70 However, elevation of AT eosinophils in obese mice by administering recombinant IL-5 did not improve insulin resistance.71
Molecular mechanisms:
The molecular mechanisms by which AT inflammation contributes to insulin resistance are not well understood and may be multifactorial. Most immune cells may contribute to obesity-linked insulin resistance through macrophages and T cells,53,58,61,82,84,87 which release type 1 cytokines such as TNFα, IL-1β, and IFNγ that can adversely regulate metabolism and cause insulin resistance in a variety of cell types, including adipocytes and skeletal muscle myocytes, by paracrine or endocrine effects. These cytokines act through interactions with their receptors on the cells, activating a series of intracellular signaling pathways (see below), which impair insulin signaling and induce insulin resistance in the cells (see the detailed review6).
IKK/NF-κB pathway:
The IKK/NF-κB pathway is mainly activated by cytokines such as TNFα and IL-1β and also by saturated FAs and damage-associated molecular patterns such as high-mobility group box 1 released from stressed or damaged cells. This pathway exhibits enhanced activation in various tissues including AT in obesity and may contribute to insulin resistance via IKK-mediated serine phosphorylation of insulin receptor substrate-1 (IRS-1) or insulin receptor (IR), with inhibition of insulin-induced tyrosine phosphorylation and downstream signaling.
JNKs and MAPKs:
JNKs and other MAPKs such as p38 MAPK can be activated by TNFα, IL-1β, ER stress, and saturated FAs and may induce insulin resistance by mediating serine and threonine phosphorylation of IRSs, leading to impairment in the interaction of IRSs with IR and downstream insulin signaling.
PKCs:
Both conventional PKCs and novel PKCs rely on diacylglycerol for full activation. Studies show that obesity or HFD feeding can activate PKCs, which may contribute to insulin resistance by inducing serine or threonine phosphorylation of IR or IRS-1, resulting in impairment of downstream insulin signaling.
JAK/STAT/SOCS pathways:
The main signaling for IFNγ, the Th1 signature cytokine, is the JAK1/JAK2/STAT1 pathway, whereas IL-6 mainly activates the STAT3 pathway. Both STAT1 and STAT3 pathways can become activated in obesity and may contribute to insulin resistance through their downstream molecules, suppressor of cytokines signaling (SOCS) proteins, particularly SOCS1 and SOCS3, which can repress IR tyrosine kinase activity, interrupt the interaction of IR with IRSs, and promote IRS degradation, thereby impairing insulin signaling.
“Noninflammatory role” of AT macrophages in insulin resistance:
In addition to their inflammatory role, AT macrophages may impact insulin sensitivity and glucose homeostasis via other pathways that are not directly related to inflammation. Obesity induces lysosome biogenesis associated with lipid catabolism and accumulation in AT macrophages, particularly CD11c+ macrophages31 or the recently identified Trem2+ macrophages.29,30 Deficiency of Trem2 in mice on HFD leads to reversal of the phenotype and reduction of lipid accumulation in AT Trem2+ macrophages, which are associated with exacerbated adipocyte hypertrophy, accelerated adiposity, and exaggerated glucose intolerance,30 indicating that lipid metabolism and accumulation in AT macrophages may play a role in control of HFD-induced adiposity and metabolic disease. Consistently, lipid storage in AT macrophages can regulate systemic glucose homeostasis and insulin sensitivity.152,153
Immunoregulation of AT browning (Figure 2):
Recent studies show that immune cells can regulate brown AT activity or white AT browning, thereby influencing adiposity and metabolism.154 An initial study showed that cold exposure increased M2-like macrophages in AT, which released catecholamines that activated brown AT and white AT browning.155 Eosinophils and type 2 cytokines, including IL-4 and IL-13, can promote white SAT browning and protect against obesity by inducing macrophage M2 polarization.78 Type 2 immune cells and cytokines may also mediate AT browning elicited by caloric restriction, microbiota depletion, or meteorin-like, a circulating factor that is induced in muscle by exercise and in AT with cold exposure.74,75,77 However, the studies on the role of eosinophils and M2 macrophages in promoting AT browning have been challenged by other studies that showed no or minimal role of M2 macrophages or eosinophils in AT browning.71,154,156 ILC2 can promote AT browning and protect against obesity by producing methionine-enkephalin peptides that can directly induce UCP1 expression in white AT88 or through type 2 signaling.157 iNKT cells may promote white AT browning by inducing FGF21 production.154 In addition, PLZF+ γδT cells, a subset of γδT cells, increase thermogenic activity in brown and white AT under cold exposure.49
In contrast to type 2 inflammation, type 1 inflammation has mostly been shown to suppress AT browning induced by cold or β3 agonist. M1-like macrophages and the cytokines TNFα and IL-1β inhibit UCP1 expression in AT/adipocytes in vitro and in vivo.158–160 Direct macrophage–adipocyte contact mediated by interaction of α4 integrin on macrophages and VCAM-1 on adipocytes also plays a role in M1-like macrophage-mediated suppression of AT browning.160 In addition, IFNγ inhibits adipocyte UCP1 expression and browning in tissue culture161,162 and may mediate the inhibitory effects of CD8+ T cells on AT browning in mice.163 Importantly, reduction in type 1 inflammation or anti-inflammatory (type 1 inflammation) drugs enhance (“derepress”) AT browning or brown AT activity.116,164 Of note, IL-10, usually functioning as an anti-inflammatory cytokine, inhibits AT browning by repressing thermogenic gene expression in adipoctyes.165 In addition, Pirzgalska et al described a type of macrophage in AT that is tightly associated with sympathetic neurons, so-called sympathetic neuron-associated macrophages, which are increased in obesity and inhibit thermogenesis by clearing noradrenaline.166 Mast cells may inhibit AT browning by releasing serotonin in obesity,167 but they promote AT browning by secreting histamine in cold.168
Inflammation and AT remodeling (Figure 2):
AT remodeling is a complex process by which AT is adapted to various stimuli including HFD feeding and obesity. During obesity development, adipocytes quickly become enlarged and hypertrophic. At same time, extracellular matrix remodeling and angiogenesis are initiated to accommodate AT expansion. Appropriate remodeling is essential for healthy AT expansion over excessive nutrients. Dysfunctional remodeling may contribute to development of obesity-linked metabolic disorders.
Inflammation plays important but complex roles in AT remodeling. Studies from Scherer’s group indicated that inflammation in adipocytes is essential for healthy AT expansion, extracellular matrix remodeling, and angiogenesis and that suppression of adipocyte inflammation results in AT dysfunction and systemic metabolic disease.169 Macrophages may contribute to AT remodeling in several different ways. At the tips of growing AT, macrophages may promote AT angiogenesis and extracellular matrix remodeling by producing VEGF, MMP1, and MMP12.170,171 Several cytokines such as TNFα and IL-8 secreted by macrophages have proangiogenic roles, thereby possibly contributing to healthy AT expansion and remodeling.171 M2-like macrophages may support adipogenesis during AT remodeling.170 In obesity, macrophages, by secreting cytokines such as TNFα and TGFβ or expressing macrophage-inducible C-type lectin, may suppress adipogenesis but promote AT fibrosis, which is generally considered to contribute to AT dysfunction in obesity.170–172 In mice with diet-induced obesity, macrophage inflammation is associated with widespread fibrosis and adipocyte death in VAT.173 Certain chemokines such as CXCL10 and CXCL11, which are upregulated in obese AT and possibly produced by T cells and macrophages, suppress angiogenesis,174 thereby possibly contributing to AT hypoxia in obesity. AT ILC1 may contribute to AT fibrosis by activating TGFβ signaling.86 Mast cells may play a role in fibrosis by releasing MCP-6 that induces collagen V expression in AT.175 T cells, particularly activated T cells, and the Th1 cytokine IFNγ adversely regulate adipogenesis.14,40 T cells may also regulate fibrosis in other tissues, but it remains to be determined whether T cells have the same effects in AT.
Targeting inflammation to treat obesity-linked metabolic disease
Because of the role of inflammation in obesity, targeting inflammation represents a new therapy for obesity-linked diseases, including insulin resistance and T2DM. Indeed, numerous animal studies have shown benefit of reduction or suppression of inflammation for obesity-linked insulin resistance and metabolic disease. Moreover, multiple antidiabetic drugs possess anti-inflammatory properties. Importantly, (pre)clinical trials testing the efficacy of anti-inflammatory drugs in treating insulin resistance and T2DM have generated some promising results. However, in general, the clinical approach of specifically targeting inflammation to treat metabolic disease remains challenging (see reviews176,177).
The classical broad spectrum anti-inflammatory drugs, salicylates, reduce inflammation and lower blood glucose levels in humans with obesity and/or insulin resistance and T2DM.176–180 Another generic anti-inflammatory drug, methotrexate, lowers HbA1c levels in patients with rheumatoid arthritis.181 However, the recent Cardiovascular Inflammation Reduction Trial (CIRT) revealed that low-dose methotrexate did not reduce IL-1β, IL-6, or CRP levels, failed to show benefits on cardiovascular effects, and had more adverse events in individuals with a history of myocardial infarction or multivessel coronary disease and with either T2DM or metabolic syndrome,182 making this drug unlikely to be used to treat cardiovascular disease and perhaps also T2DM. Colchicine, another anti-inflammatory drug, was recently shown to lower risk of cardiovascular disease in secondary prevention.183 Whether colchicine is effective in treating T2DM remains to be determined.
Several other anti-inflammatory drugs targeting specific pathways have also been tested for treatment or prevention of T2DM. Given the role of the NLRP3 inflammasome in obesity-linked AT inflammation,130,131 targeting the NLRP3 inflammasome or its downstream molecules including IL-1β and IL-18 may represent a potential therapeutic approach for obesity-related inflammation and metabolic dysfunctions. Indeed, IL-1β antagonists, including canakinumab, an anti-IL-1β antibody, and anakinra, an IL-1R antagonist, have been shown to reduce inflammation and lower blood levels of glucose or HbA1c in several trials.176,177,184–187 However, IL-1β inhibition by canakinumab in the CANTOS trial did not show long-term benefits in reducing HbA1c and reductions in incident T2DM.186 The potential of other therapies targeting the NLRP3 inflammasome pathway in treating T2DM remains to be assessed. Treatment with inhibitors for TNFα did not generate consistent results for glucose- or HbA1c-lowering effects.176,177 In addition, targeting immune cell infiltration represents another type of anti-inflammatory therapy. Indeed, antagonists for chemokine receptors such as CCR2 or CCR2 and CCR5 have been shown to reduce AT inflammation and improve insulin sensitivity in animal models of obesity188 and modestly improved glycemia in humans with T2DM in a small cohort.189 Clinical trials are also testing effects of anti-inflammatory drugs on diabetic complications. CCR2/CCR5 dual antagonists showed efficacy in treating diabetic nephropathy and nonalcoholic steatohepatitis with fibrosis in humans.190,191 A Jak1/Jak2 inhibitor, which inhibited inflammation and improved insulin resistance in obese mice,45 decreased inflammation, reduced albuminuria, and also lowered HbA1c in humans with T2DM and diabetic kidney disease.192
Additionally, caloric restriction or intermittent fasting has been shown to benefit health, including reducing obesity and improving metabolism.126,193–195 Caloric restriction or intermittent fasting also reduces AT inflammation,126,127,196 which may, at least partially, contribute to the beneficial effect of the dietary intervention on obesity-related metabolism.
Conclusion and Perspectives
A large body of evidence has shown increased inflammation in varieties of tissues in obesity, which is mainly characterized by increased accumulation and type 1 inflammatory polarization of various immune cells, including innate and adaptive immune cells. Diets, tissue microenvironment, and other factors such as gut microbiota may all contribute to initiation and maintenance of tissue inflammation. Inflammation, particularly long-term chronic inflammation, may play important roles in development and progression of obesity-linked insulin resistance and T2DM through multiple pathways regulating metabolism. Reduction or inhibition of inflammation is mostly associated with improvements in insulin resistance and metabolic functions in animal models of obesity and therefore holds promise as a new therapy for obesity-linked metabolic disease. To date, clinical studies testing the efficacy of therapies targeting inflammation for human metabolic disease have generated some promising results, but, in general, remain challenging and unsatisfactory. Inflammation is a very complex process involving a wide variety of inflammatory cells and pathways. Our current knowledge about the particular pathways for obesity-linked inflammation, especially related to human obesity, is still limited and hinders the development of novel, specific “obesity-targeted” anti-inflammatory approaches. Future studies will need to focus on identification of new pathways and related candidate targets specific for obesity-linked inflammation, which may lead to development of new therapies for obesity-related metabolic disease. Furthermore, obesity and most related diseases involving inflammation are a long-term, chronic process. The potential benefits versus side effects, impairment of host immunity in particular, of long-term systemic use of inflammation-targeting therapies will need to be carefully evaluated. Future studies will also need to explore the potential of targeting inflammation in specific organs/tissues to treat obesity-linked metabolic disease. Further advances in our knowledge of the role and mechanism of inflammation in metabolic disease and development of new technologies will create new opportunities to develop novel therapies for metabolic disease associated with obesity.
Supplementary Material
Acknowledgments
Acknowledgments
We thank Kerrie Jara for editorial assistance.
Sources of Funding
This work was supported by NIH grants to H.W. (R01HL098839, R01DK121348) and C.M.B (R01HL134320), an American Heart Association award to H.W. (AHA16GRNT30410012), and an American Diabetes Association award to H.W. (1-17-IBS-082).
Disclosures: H.W.: none; C.M.B.: grant/research support from and consultant for Novartis
Non-standard Abbreviations and Acronyms
- AT
adipose tissue
- CIRT
Cardiovascular Inflammation Reduction Trial
- CLS
crown-like structures
- FAs
fatty acids
- HFD
high-fat diet
- HIF
hypoxia-inducible factor
- ILC
innate lymphoid cells
- IMAT
intermuscular/intermyocellular adipose tissue
- iNKT
invariant NKT cells
- IR
insulin receptor
- IRS
insulin receptor substrate
- LFA-1
lymphocyte function-associated antigen–1
- PMAT
perimuscular adipose tissue
- SAT
subcutaneous adipose tissue
- T2DM
type 2 diabetes
- Th
T helper
- Treg
regulatory T cells
- VAT
visceral adipose tissue
References
- 1.World Health Organization. Obesity and overweight. Available at: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 25 November 2019.
- 2.Centers for Disease Control and Prevention. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed 25 November 2019.
- 3.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15–28. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. [DOI] [PubMed] [Google Scholar]
- 15.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes. 2009;58:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes Relat Metab Disord. 2005;29:146–150. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2010;30:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. [DOI] [PubMed] [Google Scholar]
- 21.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual Review of Physiology. 2010;72:219–246. [DOI] [PubMed] [Google Scholar]
- 23.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O’Rourke RW, Lumeng CN. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. J Immunol. 2016;197:3650–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumie A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, Vallabhajosyula P, Kambayashi T, Won KJ, Lazar MA. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–E5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E, Amit I. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178:686–698 e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, Hui Yen Chng M, Engleman E. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Rubio J, Leon J, Redruello-Romero A, Pavon E, Cozar A, Tamayo F, Caba-Molina M, Salmeron J, Carazo A. Cytometric analysis of adipose tissue reveals increments of adipocyte progenitor cells after weight loss induced by bariatric surgery. Sci Rep. 2018;8:15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. [DOI] [PubMed] [Google Scholar]
- 35.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, Sherman V, Wang HY, Phillips KJ, Webb P, Wong ST, Wang RF, Hsueh WA. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. [DOI] [PubMed] [Google Scholar]
- 37.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang E, Perrard XD, Yang D, Khan IM, Perrard JL, Smith CW, Ballantyne CM, Wu H. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb Vasc Biol. 2014;34:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Wu H. T Cells in Adipose Tissue: Critical Players in Immunometabolism. Front Immunol. 2018;9:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan IM, Dai Perrard XY, Perrard JL, Mansoori A, Smith CW, Wu H, Ballantyne CM. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis. 2014;233:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Travers RL, Motta AC, Betts JA, Bouloumie A, Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes (Lond). 2015;39:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho KW, Morris DL, DelProposto JL, Geletka L, Zamarron B, Martinez-Santibanez G, Meyer KA, Singer K, O’Rourke RW, Lumeng CN. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Rep. 2014;9:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan IM, Perrard XY, Brunner G, Lui H, Sparks LM, Smith SR, Wang X, Shi ZZ, Lewis DE, Wu H, Ballantyne CM. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond). 2015;39:1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Nguyen HN, Mina AI, Paras T, Tavakkoli A, von Andrian U, Banks AS, Shay T, Brenner MB, Lynch L. gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. 2018;19:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehta P, Nuotio-Antar AM, Smith CW. gammadelta T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. Journal of Leukocyte Biology. 2015;97:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalathookunnel A, Lian Z, Wu H. T Cells in Adipose Tissue in Aging. Front Immunol. 2018;9:2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoe K, Tsutsui H. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. [DOI] [PubMed] [Google Scholar]
- 53.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, Williams B, Wasserman DH, Van Kaer L. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012;109:E1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, Tsutsui H, Onoe K, Iwabuchi K. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7:e30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huh JY, Park J, Kim JI, Park YJ, Lee YK, Kim JB. Deletion of CD1d in adipocytes aggravates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2017;66:835–847. [DOI] [PubMed] [Google Scholar]
- 57.Huh JY, Park YJ, Kim JB. Adipocyte CD1d determines adipose inflammation and insulin resistance in obesity. Adipocyte. 2018;7:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, Oh DY, Li P, Osborn O, Olefsky JM. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J Clin Invest. 2017;127:1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q, Farb MG, Hess DT, Joseph L, Gokce N, Apovian CM. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity (Silver Spring). 2012;20:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harmon DB, Srikakulapu P, Kaplan JL, Oldham SN, McSkimming C, Garmey JC, Perry HM, Kirby JL, Prohaska TA, Gonen A, Hallowell P, Schirmer B, Tsimikas S, Taylor AM, Witztum JL, McNamara CA. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin Resistance. Arterioscler Thromb Vasc Biol. 2016;36:682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen L, Chng MH, Alonso MN, Yuan R, Winer DA, Engleman EG. B-1a lymphocytes attenuate insulin resistance. Diabetes. 2015;64:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, Kadowaki T, Nagai R. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 2013;18:759–766. [DOI] [PubMed] [Google Scholar]
- 65.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. [DOI] [PubMed] [Google Scholar]
- 66.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Q, Xie Z, Zhang W, Zhou J, Wu Y, Zhang M, Zhu H, Zou MH. Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes. 2014;63:4172–4185. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Hadad N, Burgazliev O, Elgazar-Carmon V, Solomonov Y, Wueest S, Item F, Konrad D, Rudich A, Levy R. Induction of cytosolic phospholipase a2alpha is required for adipose neutrophil infiltration and hepatic insulin resistance early in the course of high-fat feeding. Diabetes. 2013;62:3053–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe Y, Nagai Y, Honda H, Okamoto N, Yanagibashi T, Ogasawara M, Yamamoto S, Imamura R, Takasaki I, Hara H, Sasahara M, Arita M, Hida S, Taniguchi S, Suda T, Takatsu K. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 2019:fj201900477RR. [DOI] [PubMed] [Google Scholar]
- 70.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018;8:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolus WR, Hasty AH. Contributions of innate type 2 inflammation to adipose function. J Lipid Res. 2019;60:1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolus WR, Gutierrez DA, Kennedy AJ, Anderson-Baucum EK, Hasty AH. CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue. J Leukoc Biol. 2015;98:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fabbiano S, Suarez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, Trajkovski M. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab. 2016;24:434–446. [DOI] [PubMed] [Google Scholar]
- 75.Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, Ilievska M, Montet X, Seimbille Y, Hapfelmeier S, Trajkovski M. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Einwallner E, Kiefer FW, Di Caro G, Orthofer M, Witzeneder N, Hormann G, Itariu B, Zeyda M, Penninger JM, Stulnig TM, Esterbauer H, Todoric J. Mast cells are not associated with systemic insulin resistance. Eur J Clin Invest. 2016;46:911–919. [DOI] [PubMed] [Google Scholar]
- 81.Gurung P, Moussa K, Adams-Huet B, Devaraj S, Jialal I. Increased mast cell abundance in adipose tissue of metabolic syndrome: relevance to the proinflammatory state and increased adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2019;316:E504–E509. [DOI] [PubMed] [Google Scholar]
- 82.Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T, Kamei N, Park HS, Sasorith S, Woo JR, You J, Mosher W, Brady HJ, Shoelson SE, Lee J. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016;23:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Stimac D, Wunderlich FT, Bruning JC, Mandelboim O, Polic B. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–385. [DOI] [PubMed] [Google Scholar]
- 84.O’Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M, Zamarron B, Lumeng CN, Marks DL. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity (Silver Spring). 2014;22:2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ, Sun JC. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity. 2016;45:428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Shen L, Sun X, Liu F, Feng W, Jiang C, Chu X, Ye X, Jiang C, Wang Y, Zhang P, Zang M, Zhu D, Bi Y. Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nat Commun. 2019;10:3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan AE, Dold C, O’Connor D, Stutte S, Tavakkoli A, Winters D, Exley MA, O’Shea D, Brenner MB, von Andrian U, Lynch L. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity. 2017;46:273–286. [DOI] [PubMed] [Google Scholar]
- 88.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 91.Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, Fritsche A, Schick F. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. Journal of Magnetic Resonance Imaging. 2009;29:1340–1345. [DOI] [PubMed] [Google Scholar]
- 92.Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, Fantin F, Bosello O, Cominacini L, Harris TB, Zamboni M. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sachs S, Zarini S, Kahn DE, Harrison KA, Perreault L, Phang T, Newsom SA, Strauss A, Kerege A, Schoen JA, Bessesen DH, Schwarzmayr T, Graf E, Lutter D, Krumsiek J, Hofmann SM, Bergman BC. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am J Physiol Endocrinol Metab. 2019;316:E866–E879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Perrard XD, Perrard JL, Mukherjee A, Rosales C, Chen Y, Smith CW, Pownall HJ, Ballantyne CM, Wu H. ApoE and the role of very low density lipoproteins in adipose tissue inflammation. Atherosclerosis. 2012;223:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Bluher M, Olefsky JM, Sams A, Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring). 2014;22:747–757. [DOI] [PubMed] [Google Scholar]
- 96.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation Is Necessary for Long-Term but Not Short-Term High-Fat Diet-Induced Insulin Resistance. Diabetes. 2011;60:2474–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brake DK, Smith EO, Mersmann H, Smith CW, Robker RL. ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am J Physiol Cell Physiol. 2006;291:C1232–1239. [DOI] [PubMed] [Google Scholar]
- 98.Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, Mudgett J, Chen H, MacNeil DJ, Reitman ML, Qian S. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13:1311–1320. [DOI] [PubMed] [Google Scholar]
- 99.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu L, Dai Perrard X, Perrard JL, Yang D, Xiao X, Teng BB, Simon SI, Ballantyne CM, Wu H. Foamy Monocytes Form Early and Contribute to Nascent Atherosclerosis in Mice With Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gong Z, Zhang X, Su K, Jiang R, Sun Z, Chen W, Forno E, Goetzman ES, Wang J, Dong HH, Dutta P, Muzumdar R. Deficiency in AIM2 induces inflammation and adipogenesis in white adipose tissue leading to obesity and insulin resistance. Diabetologia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramkhelawon B, Hennessy EJ, Menager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner KJ, Moore KJ. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keophiphath M, Rouault C, Divoux A, Clement K, Lacasa D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:39–45. [DOI] [PubMed] [Google Scholar]
- 105.Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, Takamura T, Yamamoto H, Miyamoto K, Ginsberg HN, Mukaida N, Kaneko S, Ota T. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Maris M, Ofrecio JM, Taguchi S, Lu M, Olefsky JM. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feral CC, Neels JG, Kummer C, Slepak M, Olefsky JM, Ginsberg MH. Blockade of alpha4 integrin signaling ameliorates the metabolic consequences of high-fat diet-induced obesity. Diabetes. 2008;57:1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russo HM, Wickenheiser KJ, Luo W, Ohman MK, Franchi L, Wright AP, Bodary PF, Nunez G, Eitzman DT. P-selectin glycoprotein ligand-1 regulates adhesive properties of the endothelium and leukocyte trafficking into adipose tissue. Circ Res. 2010;107:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haase J, Weyer U, Immig K, Kloting N, Bluher M, Eilers J, Bechmann I, Gericke M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562–571. [DOI] [PubMed] [Google Scholar]
- 113.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. [DOI] [PubMed] [Google Scholar]
- 114.Dobrian AD, Galkina EV, Ma Q, Hatcher M, Aye SM, Butcher MJ, Ma K, Haynes BA, Kaplan MH, Nadler JL. STAT4 deficiency reduces obesity-induced insulin resistance and adipose tissue inflammation. Diabetes. 2013;62:4109–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. [DOI] [PubMed] [Google Scholar]
- 116.Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, Delproposto JB, Blackwell TS, Yull FE, Saltiel AR. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. [DOI] [PubMed] [Google Scholar]
- 118.Braster Q, Silvestre-Roig C, Hartwig H, Kusters P, Aarts S, den Toom M, Gallo RL, Weber C, Lutgens E, Soehnlein O. Cathelicidin regulates myeloid cell accumulation in adipose tissue and promotes insulin resistance during obesity. Thromb Haemost. 2016;115:1237–1239. [DOI] [PubMed] [Google Scholar]
- 119.Rana BMJ, Jou E, Barlow JL, Rodriguez-Rodriguez N, Walker JA, Knox C, Jolin HE, Hardman CS, Sivasubramaniam M, Szeto A, Cohen ES, Scott IC, Sleeman MA, Chidomere CI, Cruz Migoni S, Caamano J, Jorgensen HF, Carobbio S, Vidal-Puig A, McKenzie ANJ. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J Exp Med. 2019;216:1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wolf D, Jehle F, Michel NA, Bukosza EN, Rivera J, Chen YC, Hoppe N, Dufner B, Rodriguez AO, Colberg C, Nieto L, Rupprecht B, Wiedemann A, Schulte L, Peikert A, Bassler N, Lozhkin A, Hergeth SP, Stachon P, Hilgendorf I, Willecke F, von Zur Muhlen C, von Elverfeldt D, Binder CJ, Aichele P, Varo N, Febbraio MA, Libby P, Bode C, Peter K, Zirlik A. Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation. 2014;129:2414–2425. [DOI] [PubMed] [Google Scholar]
- 121.Chatzigeorgiou A, Seijkens T, Zarzycka B, Engel D, Poggi M, van den Berg S, van den Berg S, Soehnlein O, Winkels H, Beckers L, Lievens D, Driessen A, Kusters P, Biessen E, Garcia-Martin R, Klotzsche-von Ameln A, Gijbels M, Noelle R, Boon L, Hackeng T, Schulte KM, Xu A, Vriend G, Nabuurs S, Chung KJ, Willems van Dijk K, Rensen PC, Gerdes N, de Winther M, Block NL, Schally AV, Weber C, Bornstein SR, Nicolaes G, Chavakis T, Lutgens E. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proc Natl Acad Sci U S A. 2014;111:2686–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhong J, Rao X, Braunstein Z, Taylor A, Narula V, Hazey J, Mikami D, Needleman B, Rutsky J, Sun Q, Deiuliis JA, Satoskar AR, Rajagopalan S. T-cell costimulation protects obesity-induced adipose inflammation and insulin resistance. Diabetes. 2014;63:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poggi M, Engel D, Christ A, Beckers L, Wijnands E, Boon L, Driessen A, Cleutjens J, Weber C, Gerdes N, Lutgens E. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol. 2011;31:2251–2260. [DOI] [PubMed] [Google Scholar]
- 124.Liu B, Yu H, Sun G, Sun X, Jin H, Zhang C, Shi W, Tian D, Liu K, Xu H, Li X, Yin J, Hong X, Zhang D. OX40 promotes obesity-induced adipose inflammation and insulin resistance. Cell Mol Life Sci. 2017;74:3827–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oldenhove G, Boucquey E, Taquin A, Acolty V, Bonetti L, Ryffel B, Le Bert M, Englebert K, Boon L, Moser M. PD-1 Is Involved in the Dysregulation of Type 2 Innate Lymphoid Cells in a Murine Model of Obesity. Cell Rep. 2018;25:2053–2060 e2054. [DOI] [PubMed] [Google Scholar]
- 126.Wang Q, Perrard XD, Perrard JL, Mansoori A, Raya JL, Hoogeveen R, Smith CW, Ballantyne CM, Wu H. Differential effect of weight loss with low-fat diet or high-fat diet restriction on inflammation in the liver and adipose tissue of mice with diet-induced obesity. Atherosclerosis. 2011;219:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee YS, Kim JW, Osborne O, Oh da Y, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, Olefsky JM. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014;157:1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–1128. [DOI] [PubMed] [Google Scholar]
- 134.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia. 2013;56:1403–1412. [DOI] [PubMed] [Google Scholar]
- 138.Snodgrass RG, Boss M, Zezina E, Weigert A, Dehne N, Fleming I, Brune B, Namgaladze D. Hypoxia Potentiates Palmitate-induced Pro-inflammatory Activation of Primary Human Macrophages. J Biol Chem. 2016;291:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Cheang EC, Varlamov O, Corless CL, Roberts CT Jr., Marks DL. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 141.Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31:545–561. [DOI] [PubMed] [Google Scholar]
- 142.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 143.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101 e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Osto M, Zini E, Franchini M, Wolfrum C, Guscetti F, Hafner M, Ackermann M, Reusch CE, Lutz TA. Subacute endotoxemia induces adipose inflammation and changes in lipid and lipoprotein metabolism in cats. Endocrinology. 2011;152:804–815. [DOI] [PubMed] [Google Scholar]
- 146.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 147.Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jeno P, Beglinger C, Peterli R, Hall MN. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stolarczyk E, Vong CT, Perucha E, Jackson I, Cawthorne MA, Wargent ET, Powell N, Canavan JB, Lord GM, Howard JK. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab. 2013;17:520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR. Hematopoietic Kit Deficiency, rather than Lack of Mast Cells, Protects Mice from Obesity and Insulin Resistance. Cell Metab. 2015;21:678–691. [DOI] [PubMed] [Google Scholar]
- 152.Aouadi M, Vangala P, Yawe JC, Tencerova M, Nicoloro SM, Cohen JL, Shen Y, Czech MP. Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab. 2014;307:E374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese RV Jr. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Villarroya F, Cereijo R, Villarroya J, Gavalda-Navarro A, Giralt M. Toward an Understanding of How Immune Cells Control Brown and Beige Adipobiology. Cell Metab. 2018;27:954–961. [DOI] [PubMed] [Google Scholar]
- 155.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, Shin AC, Divanovic S, Brombacher F, Glasmacher E, Keipert S, Jastroch M, Nagler J, Schramm KW, Medrikova D, Collden G, Woods SC, Herzig S, Homann D, Jung S, Nedergaard J, Cannon B, Tschop MH, Muller TD, Buettner C. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sakamoto T, Nitta T, Maruno K, Yeh YS, Kuwata H, Tomita K, Goto T, Takahashi N, Kawada T. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am J Physiol Endocrinol Metab. 2016;310:E676–E687. [DOI] [PubMed] [Google Scholar]
- 159.Okla M, Zaher W, Alfayez M, Chung S. Inhibitory Effects of Toll-Like Receptor 4, NLRP3 Inflammasome, and Interleukin-1beta on White Adipocyte Browning. Inflammation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I, Nati M, Gebler J, Ziemssen T, Goelz SE, Phieler J, Lim JH, Karalis KP, Papayannopoulou T, Bluher M, Hajishengallis G, Chavakis T. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 2017;18:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moisan A, Lee YK, Zhang JD, Hudak CS, Meyer CA, Prummer M, Zoffmann S, Truong HH, Ebeling M, Kiialainen A, Gerard R, Xia F, Schinzel RT, Amrein KE, Cowan CA. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol. 2015;17:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koh EH, Chernis N, Saha PK, Xiao L, Bader DA, Zhu B, Rajapakshe K, Hamilton MP, Liu X, Perera D, Chen X, York B, Trauner M, Coarfa C, Bajaj M, Moore DD, Deng T, McGuire SE, Hartig SM. miR-30a Remodels Subcutaneous Adipose Tissue Inflammation to Improve Insulin Sensitivity in Obesity. Diabetes. 2018;67:2541–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moysidou M, Karaliota S, Kodela E, Salagianni M, Koutmani Y, Katsouda A, Kodella K, Tsakanikas P, Ourailidou S, Andreakos E, Kostomitsopoulos N, Skokos D, Chatzigeorgiou A, Chung KJ, Bornstein S, Sleeman MW, Chavakis T, Karalis KP. CD8+ T cells in beige adipogenesis and energy homeostasis. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov. 2016;15:405–424. [DOI] [PubMed] [Google Scholar]
- 165.Rajbhandari P, Thomas BJ, Feng AC, Hong C, Wang J, Vergnes L, Sallam T, Wang B, Sandhu J, Seldin MM, Lusis AJ, Fong LG, Katz M, Lee R, Young SG, Reue K, Smale ST, Tontonoz P. IL-10 Signaling Remodels Adipose Chromatin Architecture to Limit Thermogenesis and Energy Expenditure. Cell. 2018;172:218–233 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, Mendes R, Gres V, Kubasova N, Morris I, Arus BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X, Wang X, Yin H, Zhang L, Feng A, Zhang QX, Lin Y, Bao B, Hernandez LL, Shi GP, Liu J. Functional Inactivation of Mast Cells Enhances Subcutaneous Adipose Tissue Browning in Mice. Cell Rep. 2019;28:792–803 e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Finlin BS, Confides AL, Zhu B, Boulanger MC, Memetimin H, Taylor KW, Johnson ZR, Westgate PM, Dupont-Versteegden EE, Kern PA. Adipose Tissue Mast Cells Promote Human Adipose Beiging in Response to Cold. Sci Rep. 2019;9:8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martinez-Santibanez G, Lumeng CN. Macrophages and the regulation of adipose tissue remodeling. Annu Rev Nutr. 2014;34:57–76. [DOI] [PubMed] [Google Scholar]
- 171.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clement K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest. 2019;129:4032–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. [DOI] [PubMed] [Google Scholar]
- 174.Hueso L, Ortega R, Selles F, Wu-Xiong NY, Ortega J, Civera M, Ascaso JF, Sanz MJ, Real JT, Piqueras L. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int J Obes (Lond). 2018;42:1406–1417. [DOI] [PubMed] [Google Scholar]
- 175.Hirai S, Ohyane C, Kim YI, Lin S, Goto T, Takahashi N, Kim CS, Kang J, Yu R, Kawada T. Involvement of mast cells in adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2014;306:E247–255. [DOI] [PubMed] [Google Scholar]
- 176.Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 177.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest. 2017;127:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes. Endocr Pract. 2012;18:826–833. [DOI] [PubMed] [Google Scholar]
- 181.de Rotte MC, de Jong PH, den Boer E, Pluijm SM, Ozcan B, Weel AE, Lindemans J, Hazes JM, de Jonge R. Effect of methotrexate use and erythrocyte methotrexate polyglutamate on glycosylated hemoglobin in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2026–2036. [DOI] [PubMed] [Google Scholar]
- 182.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ, Investigators C. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, Lopez-Sendon J, Ostadal P, Koenig W, Angoulvant D, Gregoire JC, Lavoie MA, Dube MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin MC, Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019. [DOI] [PubMed] [Google Scholar]
- 184.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. [DOI] [PubMed] [Google Scholar]
- 185.Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, Solinger AM, Mandrup-Poulsen T, Dinarello CA, Donath MY. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35:1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71:2392–2401. [DOI] [PubMed] [Google Scholar]
- 187.Kataria Y, Ellervik C, Mandrup-Poulsen T. Treatment of type 2 diabetes by targeting interleukin-1: a meta-analysis of 2921 patients. Semin Immunopathol. 2019;41:413–425. [DOI] [PubMed] [Google Scholar]
- 188.Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR, Ko HJ, Chung CH. Dual CCR2/5 Antagonist Attenuates Obesity-Induced Insulin Resistance by Regulating Macrophage Recruitment and M1/M2 Status. Obesity (Silver Spring). 2018;26:378–386. [DOI] [PubMed] [Google Scholar]
- 189.Di Prospero NA, Artis E, Andrade-Gordon P, Johnson DL, Vaccaro N, Xi L, Rothenberg P. CCR2 antagonism in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Diabetes Obes Metab. 2014;16:1055–1064. [DOI] [PubMed] [Google Scholar]
- 190.Gale JD, Gilbert S, Blumenthal S, Elliott T, Pergola PE, Goteti K, Scheele W, Perros-Huguet C. Effect of PF-04634817, an Oral CCR2/5 Chemokine Receptor Antagonist, on Albuminuria in Adults with Overt Diabetic Nephropathy. Kidney Int Rep. 2018;3:1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tuttle KR, Brosius FC 3rd, , Adler SG, Kretzler M, Mehta RL, Tumlin JA, Tanaka Y, Haneda M, Liu J, Silk ME, Cardillo TE, Duffin KL, Haas JV, Macias WL, Nunes FP, Janes JM. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33:1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S, Tadic J, Eisenberg T, Magnes C, Stumpe M, Zuegner E, Bordag N, Riedl R, Schmidt A, Kolesnik E, Verheyen N, Springer A, Madl T, Sinner F, de Cabo R, Kroemer G, Obermayer-Pietsch B, Dengjel J, Sourij H, Pieber TR, Madeo F. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–476 e465. [DOI] [PubMed] [Google Scholar]
- 195.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31:92–104 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu B, Page AJ, Hatzinikolas G, Chen M, Wittert GA, Heilbronn LK. Intermittent fasting improves glucose tolerance and promotes adipose tissue remodeling in male mice fed a high-fat diet. Endocrinology. 2019;160:169–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


