Abstract
Objectives:
To finalize and validate a disease-specific patient-reported outcome (PRO) measure: the ANCA-associated vasculitis patient-reported outcome (AAV PRO) questionnaire. Using a 35-item candidate questionnaire developed following 50 qualitative interviews in the UK, USA and Canada, a longitudinal survey was conducted to determine the final scale structure and validate the AAV-PRO.
Methods:
Participants were recruited via Vasculitis UK and the Vasculitis Patient-Powered Research Network. The 35-item candidate questionnaire was completed at baseline and three-months; UK participants completed the EuroQol-5D-5L (EQ-5D-5L), whilst US participants completed a test-retest exercise, three-to-five days after baseline. Scale structure was defined using exploratory factor analysis (EFA) and Rasch analysis. Convergent and known groups validity, test-retest reliability, and longitudinal construct validity were assessed.
Results:
There were 626 participants with AAV; >25% reporting “active disease”. EFA and Rasch analysis supported a 29-item profile measure comprising 6 domains: “Organ-Specific Symptoms”, “Systemic Symptoms”, “Treatment Side Effects”, “Social and Emotional Impact”, “Concerns about the Future”, and “Physical Function”. Mean domain scores were higher for participants with “active disease” versus “remission” (p<0.001). Construct validity was demonstrated by correlations between domain scores and the EQ-5D-5L (range r=−0.55 to 0.78), all p<0.0001. In participants reporting “no change” (n=97) during the test re-test, Intraclass Correlation Coefficient values were high (range 0.89–0.96) for each domain.
Conclusions:
The AAV-PRO, a new disease-specific PRO measure for AAV, has good face and construct validity, is reliable, feasible, and discriminates among disease states.
Keywords: Granulomatosis with polyangiitis, Systemic vasculitis, Patient perspective, Outcomes research, Corticosteroids, Glucocorticoids
Granulomatosis with polyangiitis (GPA, Wegener’s), eosinophilic granulomatosis with polyangiitis (EGPA, Churg-Strauss), and microscopic polyangiitis (MPA) are life- and organ-threatening disorders affecting the lungs, kidneys, ear, nose, throat, nerves, skin, and quality of life of affected patients and are collectively known as the ANCA-associated vasculitides (AAV)1,2. Despite improvement in mortality and morbidity with newer treatment regimens, the risk of relapse in AAV is 35% over five years3. Many patients experience persistent disease activity, long-term exposure to toxic therapies4, and the psychosocial impact of a serious illness5
Health related quality of life (HRQoL) is impaired in AAV6–8. A quarter of patients experience depression and more than 40% anxiety6. Work disability is high with a quarter unemployed due to AAV9, and 50% reported their careers had been hindered10. Fatigue and pain are important symptoms6,11. The opinions of patients and clinicians on the relative importance of outcomes often differ12,13.
The Outcome Measures in Rheumatology (OMERACT) core set of outcome measurements for use in clinical trials in AAV included the generic Short-Form 36 (SF-36) patient reported outcome (PRO) measuring HRQoL14–16. Generic PROs can lack specificity17 and the OMERACT Vasculitis Working Group identified the need for an AAV- specific PRO to fully capture the patient’s perspective18. An international steering committee comprising patient partners, methodologists, statisticians and clinicians from the UK, USA, and Canada have been developing a new disease-specific PRO, in line with guidance from the United States Food & Drug Administration (FDA)19. The project received critical scrutiny and feedback at three successive Vasculitis Workshop sessions at OMERACT conferences20,21.
A three-stage approach has been followed. Stage 1: Qualitative analysis, item production and testing in the UK, USA and Canada resulting in a 35-iten candidate AAV-PRO questionnaire (completed)22. Stage 2: Large-scale parallel survey of people with AAV in the UK and USA, to investigate the underlying scale structure of the AAV-PRO. Stage 3: Assessment and validation of the AAV-PRO’s measurement properties, including construct validity, reliability, discriminatory ability and ability to detect change. Stages 2 and 3 are reported here.
METHODS AND MATERIALS
An international steering committee, including four patient partners, had oversight of the patient survey materials, working with the patient groups Vasculitis UK and the Vasculitis Patient-Powered Research Network (the VPPRN).
Ethical approval was given by the Medical Sciences IDREC, University of Oxford, Oxford, Ref: MS-IDREC-C1–2015-087 for the UK and US survey. In the US, approval was given by the Institutional Review Boards at the University of Pennsylvania and the University of South Florida, Ref: Pro00018514. Patients were recruited between June and October 2015.
Inclusion / exclusion criteria:
Participants were required to have AAV, English speaking, aged ≥18 years and to fulfill the following:
1) Affirm that they had ANCA-associated vasculitis (AAV); and
2) Received either a positive test result for ANCA OR diagnostic biopsy OR angiogram; and
3) Currently or previously taken glucocorticoids or another immunosuppressant/s.
Participants with AAV were sent a pack by post (Vasculitis UK) or email (VPPRN in the US) containing a covering letter, information sheet, and forms for demographic (date of birth, location, sex, race, highest educational level, employment status) and disease-related data (type of AAV, date of diagnosis, positive ANCA test, current disease state, immunosuppressant medications), and the 35-item candidate AAV-PRO questionnaire. The first 12 items addressed symptom severity; the remaining 23 items addressing the impact of AAV, or its treatment, on HRQoL. Each item has five ordinal integer response options (three formats applying to different items: symptom severity, level of difficulty, frequency of experiencing a problem), scored 0 to 4; higher scores denoting greater severity or impact. UK participants were also sent an EQ-5D-5L questionnaire23 at baseline. This five-item generic measure assesses mobility, self-care, usual activities, pain/discomfort and anxiety/depression on a five-point scale. EQ-5D-5L index values were calculated using the cross-walk method24.
Three-to-five days after they provided baseline responses the US participants were sent a repeat 35-item AAV-PRO candidate questionnaire (test-retest), disease state questions and a transition question concerning change in disease state since baseline questionnaire completion: ‘Overall, how are you NOW (in terms of your vasculitis and any treatment side effects), compared with 5 days ago (when you first answered the questionnaire).
After three months, all UK and US participants were sent the same 35-item candidate AAV-PRO questionnaire and the transition item used for test retest, but with comparison made with ‘3 months ago’.
Sample size
Sample size for health status questionnaire development requires at least three respondents per questionnaire item tested25. The aim was to recruit at least 500 patients (250 from each country).
Statistical methods
Data were analysed using SPSS release 20 (PASW Statistics 20© 2015 SPSS Inc. SPSS (Hong Kong) Ltd). To minimize type-I error, the significance level for all analyses was set at two-sided p<0.01.
Criteria for questionnaire item reduction
1) Missing responses >3%26; 2) Distribution of responses exhibiting ceiling or floor effects (≥50% responses to an item taking either of the 2 most extreme response categories); 3) High inter-item correlation (≥0.80) or Cronbach’s alpha (≥ 0.93) suggesting redundancy; 4) Items poorly correlated with their overall domain/scale score (i.e. item-to-total correlations <0.3); 5) Cross-loading during factor analysis, 6) Particularly poor fit to the model (Item Trait Interaction p<0.01) on fitting to a Rasch unidimensional model to any identified domains.
Scale structure and dimensionality
Conceptual Framework
The AAV-PRO Conceptual Framework indicated that the PRO was likely to be multidimensional, i.e., containing items addressing symptom severity, and differing aspects of HRQoL (physical, psychological, social and global impact on health).
Factor structure
The formal process of item reduction and determination of scale structure was guided by Exploratory Factor Analysis27 (EFA), Rasch analysis28 (RUMM2010 software; RUMM Laboratory Pty Ltd; Western Australia 6023) and from insights from the Conceptual Framework26. EFA was conducted using FACTOR27, based on a polychoric correlation matrix, using Principal Axis Factoring extraction, with oblique rotation method. Items correlating with a factor of >0.4 were considered to significantly load and the item was assigned to that factor29.
Individual item functioning
The polytomous Rasch model (for items with >2 responses) is equivalent to a test of the theoretical construct validity and adequacy of a scale30,31, assessing the unidimensionality of items in a scale28,32,33.
Scale/domain properties
Internal consistency
Cronbach‟s alpha coefficients were calculated to assess the internal consistency of questionnaire domains. An alpha ≥0.70 is recommended to claim internal consistency34,35, alpha > 0.90 may suggest redundancies, requiring item reduction31, with 0.80 to 0.90 considered optimal36.
Convergent validity.
It was hypothesised that a large Pearson’s correlation (r≥0.5) would be obtained between the AAV-PRO domains and generic EQ-5D-5L index scores (UK baseline sample only). It was anticipated that negative correlations would be seen, as the two measures are scored in opposite directions.
Test retest reliability
Intraclass Correlation Coefficients (ICC) were used to compare baseline AAV-PRO domain scores, with scores obtained three-to-five days later (US sample only) in those individuals whose condition had remained stable. ICC values >0.60 are recommended37
Meaningful change
The Standard Error of the Mean (SEM) was the error estimated for a single use of the questionnaire and is directly related to the reliability of the scale. The Minimal Detectable Change (MDC) was defined as the smallest amount of change between two time points that indicated a real change in the patient‟s health status38. The MDC90 was set to indicate that 90% of stable patients demonstrated random variation of less than this magnitude when assessed on multiple occasions39–41.
Known groups validity26.
It was hypothesised that AAV-PRO domain scores would differ significantly between patients self-identifying at baseline as having ‘Active disease’ versus patients ‘In remission’.
Longitudinal construct validity: responsiveness
Responsiveness was assessed where respondents provided relevant outcomes data at baseline and 3-months. Change scores were calculated as the baseline score minus the 3-month follow-up score for each AAV-PRO domain. Effect sizes (ES) were calculated as the difference between the sample’s mean baseline score and mean 3-month follow-up score, divided by the standard deviation (SD) of baseline score. ES calculates the magnitude of change measured by an instrument in a standardised way allowing comparison between instruments42. Change scores and ES were compared with responses on a 3-month transition item regarding change in patients’ condition.
RESULTS
Study sample and characteristics
The baseline survey response rate was 74% (n=662/900). Of the 662 respondents, 626 were eligible for inclusion (95%). Demographic and clinical characteristics of participants shown in (Table 1 and online supplementary Table S1)). The mean age was 60.4 years (SD 13.2) and participants were predominantly female (397, 64%). The sample represented the UK (348/626) and the US (278/626) with 45% and 46% of the sample respectively; UK respondents were older (mean 63 vs 57 years, p<0.001), and more likely to be retired (59% versus 32%, p<0.001).
Table 1.
Demographic and clinical characteristics of survey participants.
| Demographic characteristics | UK N=348 (%) | USA N=278 (%) | All N=626 (%) | X2 | p= | |
|---|---|---|---|---|---|---|
| Sex (n=623) | Male | 135 (38.9) | 90 (32.7) | 225 (36.7) | 2.54 | 0.11 |
| Female | 212 (61.1) | 185 (67.3) | 397 (63.8) | |||
| Age group (years) (n=608) | ≤45 | 25 (7.3) | 51 (19.1) | 76 (12.5) | ||
| >45 ≤60 | 95 (27.9) | 90 (33.7) | 185 (30.4) | 40.64 | 0.00 | |
| >60 ≤75 | 166 (48.7) | 116 (43.4) | 282 (46.4) | |||
| >75 | 55 (16.1) | 10 (3.7) | 65 (10.7) | |||
| Ethnicity (n=624) | Asian | 5 (1.4) | 7 (2.5) | 12 (1.9) | ||
| Black or African/American | 1 (0.3) | 2 (0.7) | 3 (0.5) | 3.77 | 0.71 | |
| Black African or Caribbean British | 1 (0.3) | 0 (0) | 1 (0.2) | |||
| White | 333 (95.7) | 259 (93.8) | 592 (94.9) | |||
| American Indian or Alaska Native | 0 (0) | 1 (0.4) | 1 (0.2) | |||
| Multiple | 3 (0.9) | 3 (1.1) | 6 (1.0) | |||
| Other | 5 (1.4) | 4 (1.4) | 9 (1.4) | |||
| Qualifications (highest) (n=623) | Degree | 157 (45.4) | 204 (73.6) | 361 (57.9) | ||
| Vocational/employment related | 71 (20.5) | 26 (9.4) | 97 (15.6) | 59.46 | 0.00 | |
| School/high school qualifications | 90 (26.0) | 46 (16.6) | 136 (21.8) | |||
| None | 28 (8.1) | 1 (0.4) | 29 (4.7) | |||
| Employment status (n=623) | Disabled | 50 (14.5) | 48 (17.3) | 98 (15.7) | ||
| Employed with income | 78 (22.6) | 112 (40.3) | 190 (30.5) | |||
| Retired | 204 (59.1) | 88 (31.7) | 292 (46.9) | 56.68 | 0.00 | |
| Employed without income | 3 (0.9) | 3 (1.1) | 6 (1.0) | |||
| Homemaker/carer | 4 (1.2) | 9 (3.2) | 13 (2.1) | |||
| Unemployed | 6 (1.7) | 7 (2.5) | 13 (2.1) | |||
| Other (e.g. student, employed & student) | 0 (0.0) | 11 (4.0) | 11 (1.8) | |||
| Type of AAV | EGPA | 47 (13.5) | 48 (17.3) | 95 (15.2) | ||
| GPA | 251 (72.1) | 184 (66.2) | 435 (69.5) | 20.37 | 0.00 | |
| MPA | 28 (8.0) | 43 (15.5) | 71 (11.3) | |||
| Unspecified AAV | 22 (6.3 | 3 (1.1) | 25 (4.0) | |||
| Positive ANCA test | Yes | 270 (78.3) | 222 (79.9) | 492 (79.9) | ||
| No | 15 (4.3) | 31 (11.2) | 46 (7.4) | 17.66 | 0.00 | |
| Don’t know | 60 (17.4) | 25 (9.0) | 85 (13.6) | |||
| Current disease status | Active disease | 100 (29.8) | 75 (27.0) | 175 (28.5) | 0.58 | 0.45 |
| Remission | 236 (70.2) | 203 (73.0) | 439 (71.5) | |||
| Flare within the last 2 years | Yes | 135 (40.2) | 129 (46.4) | 264 (43.0) | ||
| No | 157 (46.7) | 112 (40.3) | 269 (43.8) | 5.09 | 0.17 | |
| Don’t know | 32 (9.5) | 21 (7.6) | 53 (8.6) | |||
| Never had a flare | 12 (3.6) | 16 (5.8) | 28 (4.6) | |||
| Organs affected by AAV | Lungs | 215 (61.8) | 205 (73.7) | 420 (67.1) | 10.01 | 0.00 |
| ENT | 249 (71.6) | 215 (77.3) | 464 (74.1) | 2.70 | 0.10 | |
| Eyes | 135 (38.8) | 124 (44.6) | 259 (41.4) | 2.15 | 0.14 | |
| Kidneys | 185 (53.2) | 153 (55.0) | 338 (54.6) | 0.22 | 0.64 | |
| Nerves | 139 (39.9) | 91 (32.7) | 230 (36.7) | 3.46 | 0.06 | |
| Skin | 128 (36.8) | 123 (44.2) | 251 (40.1) | 3.58 | 0.06 | |
| Joints | 192 (55.2) | 151 (53.6) | 341 (54.5) | 0.16 | 0.70 | |
| Time from diagnosis (yrs) | Mean (SD) [range] | 10.6 (7.5) [0.2–38.8] | 7.6 (7.4) [0.1–44.5] | 9.3 (7.5) [0.1 – 44.5] | t=4.89 | 0.00 |
Item response distribution - candidate AAV-PRO items
Candidate questionnaire items and baseline distribution of their responses are shown in Figure 1. Item response rates were high overall (maximum 1.6% missing data), supporting the feasibility of the questionnaire. One exception concerned „difficulties with sexual activity or desire’ (6.2% missing; 11.8% missing in age-group >65). Responses were generally evenly spread across responses, although >50% of respondents endorsed an extreme (‘No difficulty’) response on two items (‘Using hands for small careful movements’ and ‘Washing/drying/ dressing unaided’).
Figure 1.
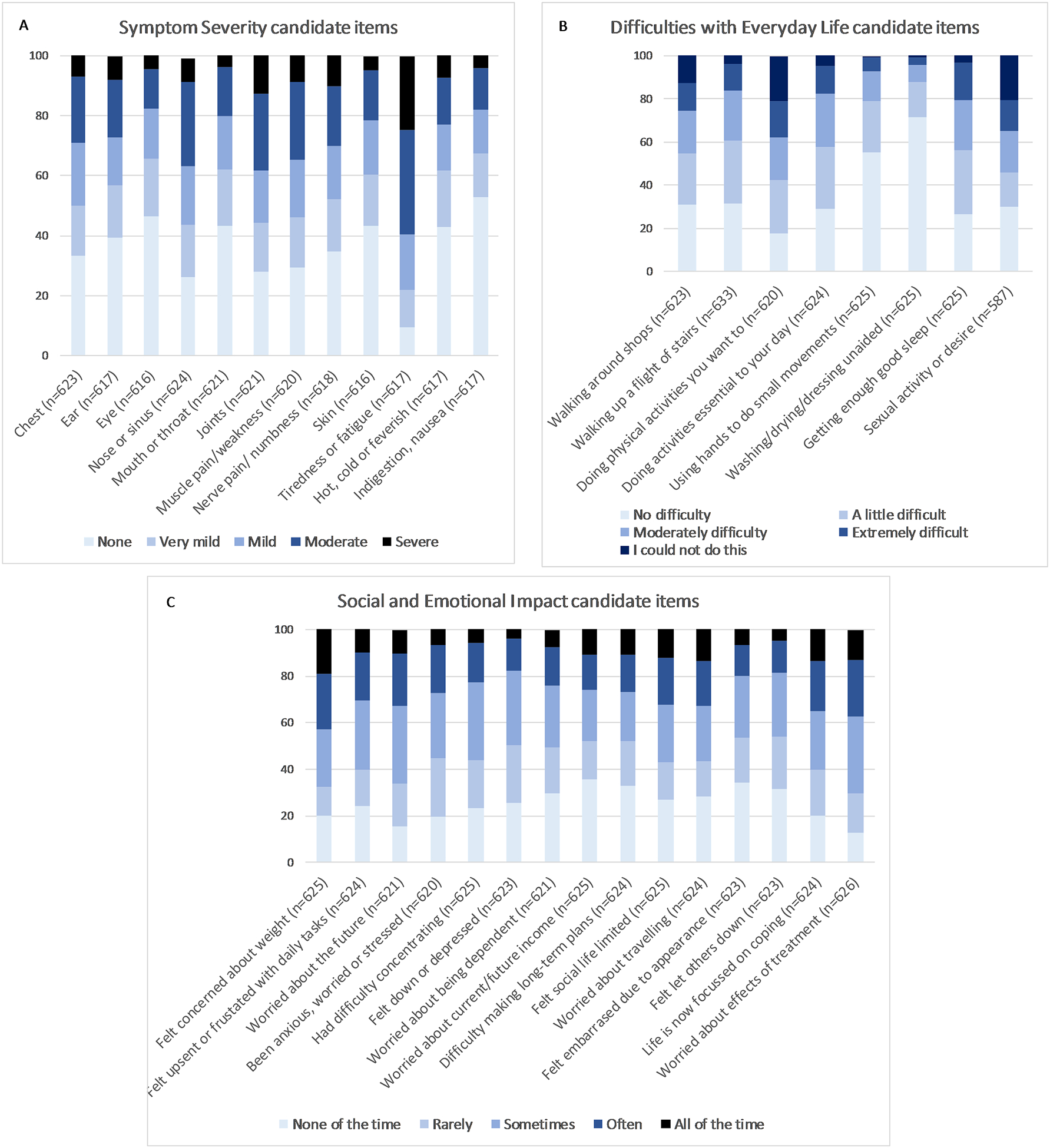
Survey responses at baseline of 35 candidate questionnaire items (N=626). A. Symptom severity; B. Difficulties with everyday life and C. Social and Emotional Impact. (n=individual response rate for each candidate item).
Final dimensionality and scale structure of the AAV-PRO
The final AAV-PRO including 29 individual questionnaire items is shown in Figure 2 Details of the Rasch and EFA analyses are given in online supplementary Figures S1–3 and Table S2. The AAV-PRO is a profile measure containing 6 different domains: ‘Organ Symptoms Severity (OSS)’, ‘Systemic Symptoms Severity (SSS)’, ‘Treatment Side-Effects (TSE)’, ‘Social and Emotional Impact (SEI)’, ‘Concerns About the Future (CAF)’ and ‘Physical Function (PF)’. The identified domains each fit the Rasch unidimensional model (Item Trait Interaction p>0.01) and had good internal consistency (Cronbach’s alphas 0.77–0.96) (Figure 2). Patient partners on the steering committee reviewed the items within each domain and developed the domain titles used above.
Figure 2. The AAV-PRO.
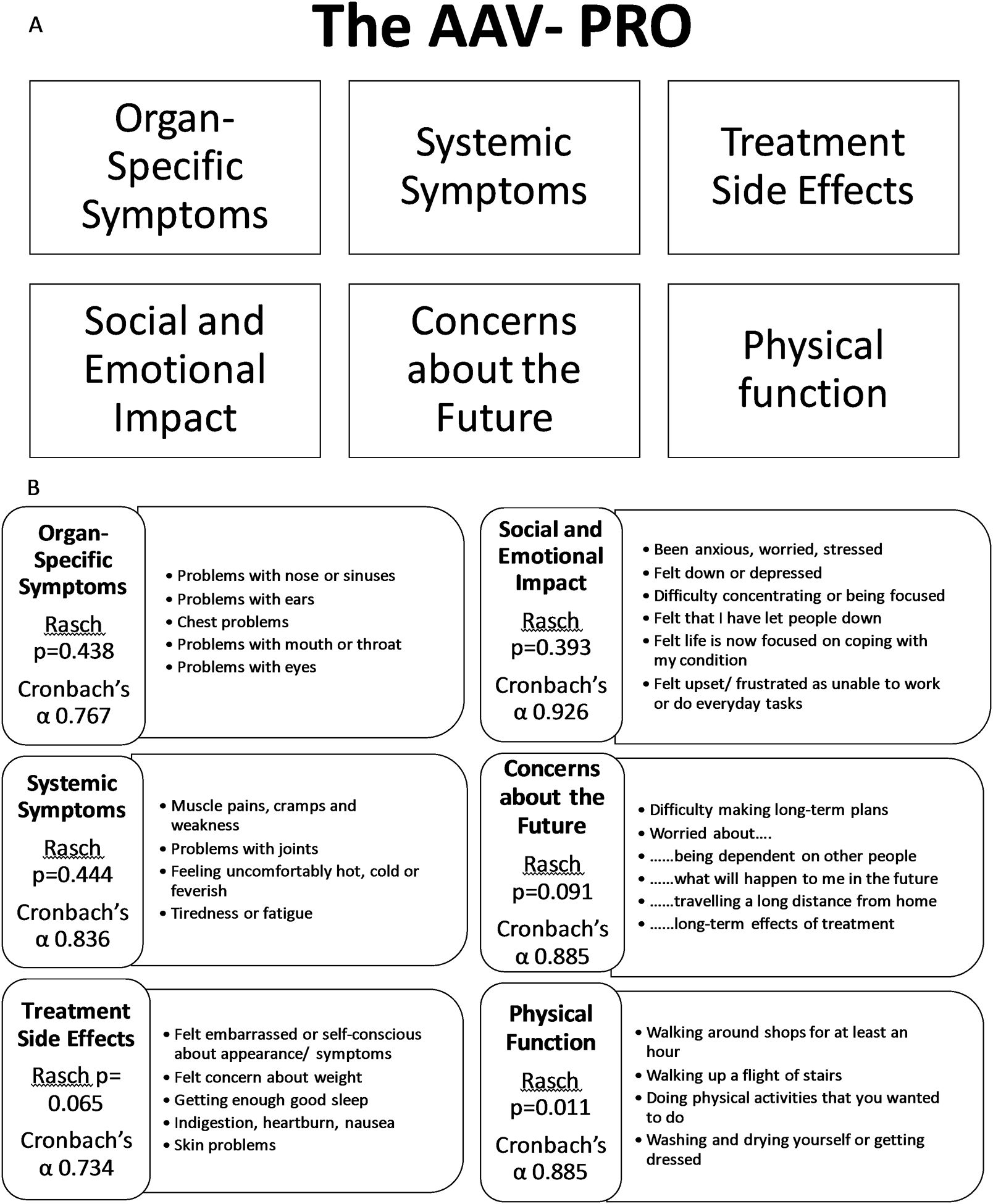
A profile measure containing 6 different domains which all individually fit the Rasch model and have good internal consistency. A. Domains of the AAV-PRO, B. Distribution of the 29 items of the AAV-PRO across the 6 domains.
Six questionnaire items were identified for rejection based on failure to fit within the Rasch model for a particular domain, plus insights from the Conceptual Framework, EFA and clinical input : “nerve pain or numbness” reflected damage and felt not suitable for a PRO as would not capture change; “sexual activity …” obtained poor response rate; “worried about income” was considered too contextual with responses influenced by differing healthcare; “using hands for small tasks” response distribution indicating a ceiling effect (skewed towards ‘no difficulty’); “… social life is limited” had strong overlap in responses with other better fitting items indicating redundancy; and “… activities essential to your day”, “walking around shops” and “walking up-stairs” were all highly correlated indicating redundancy (exact meaning of “essential” flagged as problematic by patient partners).
Scoring of the 29-item AAV-PRO profile measure
Scores for each domain are calculated as the sum of each individual item score, (online supplementary Figure S4). Examples of items with response categories are shown in supplementary Figure S5.
Measurement properties
Convergent validity
Correlations (Pearson) between baseline AAV-PRO domains and EQ-5D-5L index-scores (UK sample only) were all large (≥0.50): OSS r= −0.55, SSS r= −0.67, TSE r= −0.65, SEI r= −0.73, CAF r= −0.68, and PF r= −0.78 (all p<0.001).
Test-retest reliability
All Intraclass Correlation Coefficient (ICC) values between domain scores at baseline and three-to-five days later (US sample) were very good: OSS ICC = 0.89 (95%CI I 0.84 to 0.93); SSS ICC= 0.91 (95%CI 0.86 to 0.94); TSS= 0.95 (95%CI 0.93 to 0.97); SEI= 0.96 (95%CI 0.94 to 0.97); CAF= 0.95 (0.92 to 0.97); PF= 0.96 (0.94 to 0.97). (Table 2)
Table 2. Test-retest reliability and estimates of meaningful change.
Intraclass correlation coefficients (ICCs), standard error of the measure (SEM) and minimal detectable change (MDC90) for the 6 AAV-PRO scales/domains.
| AAV-PRO Scale (number of items) | ICCa | 95%CI | Baseline mean raw score (SD) | SEMb Raw score | SEMb 0–100 scale | MDC90c Raw score | MDC90c 0–100 scale |
|---|---|---|---|---|---|---|---|
| Organ Specific Symptoms (5) | 0.89 | 0.84 to 0.93 | 6.91 (4.70) | 1.56 | 7.80 | 3.64 | 18.20 |
| Systemic Symptoms (4) | 0.91 | 0.87 to 0.94 | 7.21 (4.40) | 1.32 | 8.25 | 3.08 | 22.75 |
| Treatment side effects (5) | 0.95 | 0.93 to 0.97 | 7.17 (4.43) | 0.99 | 4.95 | 2.31 | 11.55 |
| Social and Emotional Impact (6) | 0.96 | 0.94 to 0.97 | 9.88 (6.24) | 1.25 | 5.21 | 2.91 | 12.13 |
| Concerns about the Future (5) | 0.95 | 0.92 to 0.97 | 8.83 (5.35) | 1.20 | 6.00 | 2.79 | 13.95 |
| Physical Function (4) | 0.96 | 0.94 to 0.97 | 5.22 (4.17) | 0.83 | 5.19 | 1.94 | 12.13 |
ICC based on USA sample only, while SEM uses baseline scores from all USA/UK respondents combined.
SEM = SD × √1 – ICC. Computed using raw scores with scale converted to 0–100 metric at the end.
Example - for Organ Symptom Severity scale: SEM = 4.70 × √1−0.89 = 1.56 then Convert to 0–100 scale: 1.56 × 100/20 = 7.80
MDC90 = 1.65 × (√2) X SEM. Computed using raw scores with scale converted to 0–100 metric at the end.
Example - for Systemic Symptom Severity scale: MDC90 = 1.65 × 1.41 × 1.32 = 3.08 then Convert to 0–100 scale: 3.64 × 100/16 = 22.75
Meaningful change
The Standard Error of the Mean (SEM), Minimal Detectable Change (MDC90) estimate were calculated based on the ICC and the Standard Deviation of the baseline score (Table 2).
Known groups validity
AAV-PRO domain scores all differed significantly (p<0.001) between patients self-identifying as having ‘Active disease’ versus ‘In remission’ (see Table 3) as was also the case for the EQ-5D-5L.
Table 3.
Known groups validity. Comparison (using t-tests) of baseline AAV-PRO domain scores according to patient-reported current disease state “active” versus “in remission”.
| Current disease state | N | Mean | Std. Deviation | t | p | |
|---|---|---|---|---|---|---|
| ORGAN SPECIFIC SYMPTOMS | Active | 167 | 47.28 | 22.55 | 8.898 | <0.0001 |
| Remission | 425 | 29.35 | 21.86 | |||
| SYSTEMIC SYMPTOMS | Active | 168 | 60.75 | 25.37 | 9.525 | <0.0001 |
| Remission | 426 | 38.53 | 25.70 | |||
| TREATMENT SIDE-EFFECTS | Active | 171 | 48.54 | 22.12 | 9.565 | <0.0001 |
| Remission | 422 | 30.59 | 20.09 | |||
| SOCIAL & EMOTIONAL IMPACT | Active | 172 | 53.54 | 24.17 | 8.079 | <0.0001 |
| Remission | 430 | 35.65 | 24.69 | |||
| CONCERNS ABOUT THE FUTURE | Active | 170 | 56.76 | 24.39 | 7.999 | <0.0001 |
| Remission | 431 | 38.50 | 25.52 | |||
| PHYSICAL FUNCTION | Active | 172 | 44.08 | 25.76 | 7.370 | <0.0001 |
| Remission | 432 | 27.56 | 24.49 |
Longitudinal construct validity
Mean change scores and ES for the AAV-PRO domains were mapped to level of response to the 3-month transition item (Table 4). Results showed that respondents reporting ‘no change’ in their condition exhibited appropriate ES, close to zero, while positive ES range 0.21 to 0.28 were associated with the response ‘Much better’ for all domains. The response ‘slightly better’ had ES lying between zero and the value associated with ‘Much better’ responses. In general, responses indicating a worsening health state were associated with negative ES of a magnitude that mirrored results associated with positive responses/improvement. The exception here was the organ symptom severity domain with scores lacking a significant linear trend across the transition item responses (all other domains‟ linear trend p≤0.003).
Table 4. Longitudinal construct validity.
Mean changes (SD), (using 0–100 metric), and effect sizes for the AAV-PRO domains in relation to patients‟ responses to a transition item on 3-month follow-up survey.
| AAV-PRO DOMAINS | ||||||
|---|---|---|---|---|---|---|
| Responses: | ORGAN SYMPTOM SEVERITY Mean (SD) [ES] |
SYSTEMIC SYMPTOM SEVERITY Mean (SD) [ES] |
TREATMENT SIDE-EFFECTS Mean (SD) [ES] |
SOCIAL & EMOTIONAL IMPACT Mean (SD) [ES] |
CONCERNS ABOUT THE FUTURE Mean (SD) [ES] |
PHYSICAL FUNCTION Mean (SD) [ES] |
| Much better | n=38 3.68 (13.79) [0.22] |
n=40 6.09 (13.68) [0.21] |
n=39 4.62 (12.16) [0.28] |
n=40 5.31 (12.27) [0.21] |
n=40 6.13 (14.57) [0.23] |
n=41 5.79 (15.08) [0.24] |
| Slightly better | n=69 0.72 (14.86) [0.01] |
n=68 2.76 (11.85) [0.07] |
N=69 2.03 (12.44) [0.10] |
n=72 2.37 (13.36) [0.09] |
n=71 3.87 (16.52) [0.17] |
N=73 5.05 (10.66) [0.19] |
| No change/worse | n=186 0.54 (15.09) [0.01] |
n=186 0.44 (15.37) [0.00] |
n=185 1.24 (13.12) [0.09] |
n=190 1.58 (11.18) [0.06] |
n=187 1.66 (13.62) [0.05] |
n=193 0.32 (9.43) [0.01] |
| Slightly worse | n=64a −1.64 (16.67) [−0.07] |
n=63 −5.46 (17.84) [−0.19] |
n=62 −0.81 (15.92) [−0.01] |
n=65a 0.26 (16.02) [0.04] |
n=63 −0.87 (17.36) [−0.02] |
n=65 −3.08 (18.52) [−0.17] |
| Much worse | n=14 1.43 (11.17) [0.06] |
n=15 −6.25 (18.15) [−0.23] |
n=14 −10.71 (16.04) [−0.45] |
n=15a −7.78 (15.42) [−0.26] |
n=15 −13.67 (16.85) [−0.43] |
n=15 −9.58 (27.84) [−0.31] |
| P-value for linear trend | 0.185 | <0.001 | 0.001 | 0.003 | <0.001 | <0.001 |
| Total | n=374 0.64 (15.06) [0.02] |
n=377 0.22 (15.43) [−0.01] |
n=373 0.95 (13.71) [0.07] |
n=387 1.53 (12.90) [0.06] |
n=381 1.44 (15.41) [0.05] |
n=392 0.91 (13.69) [0.03] |
The ‘n’ in each cell varies slightly across the rows reflecting an occasional missing patient’s response to an item within the health status scale (hence the particular overall scale would not have been computed for that individual).
Comparison between the AAV-PRO domain scores and demographic and clinical features
There were no differences in mean scores between each of the three AAV (GPA, MPA, and EGPA) (p<0.01) and no correlation between length of time from diagnosis and any of the AAV-PRO scales (p<0.01). There were differences between i) USA and UK respondents, with UK scores higher (i.e. worse) (p≤0.001) on all scales, ii) Male and female mean scores, with women scoring higher on all scales (P<0.01), and iii) Younger and older respondents with higher mean scores on the Social and Emotional Impact Subscale in those in the ≤ 65 age-group compared with older participants (p<0.01) (see online supplements S3–7).
The final 29-item AAV-PRO is available from the corresponding author and is free for non-commercial academic and clinical use.
DISCUSSION
The AAV-PRO is a new 29-item, disease-specific PRO measure for use in ANCA-associated vasculitis. It has good face, content, and construct validity, is reliable, feasible, and discriminates among disease states. Patients have played a key role within every stage of development22. This manuscript describes the underlying structure of the final AAV-PRO and its validation in terms of reliability, feasibility, discrimination and construct validity.
The final 29-item questionnaire comprises six subscales/domains: “Organ-Specific Symptoms”, “Systemic Symptoms”, “Treatment Side Effects”, “Social and Emotional Impact”, “Concerns about the Future”, and “Physical Function”. The identified domains offer a comprehensive profile of the impact of AAV on patients’ everyday life and were felt by the patient partners to represent “what AAV was to them”. Each domain is unidimensional and has good measurement properties including good internal consistency (Cronbach’s alphas range 0.77 to 0.92) and test-retest reliability (Intraclass Correlation Coefficients (ICCs) range 0.89 to 0.96); plus evidence supporting concurrent validity, with moderate to high correlations (range r−0.55 to −0.78, all p<.0001) with EQ-5D-5L index scores, as hypothesised. All AAV-PRO domain scores distinguished between patients who self-reported having active disease versus disease in remission (p<0.0001), providing support for known groups validity. Length of time from diagnosis alone was not correlated with worse scores, indicating that disease activity, rather than duration of disease, is a key correlate to AAV-PRO scores. There were also no differences in mean scores between the different subtypes of AAV.
Characteristics of the UK and USA survey populations differed slightly, participants in the USA were on average younger, with shorter duration of disease, and higher educational level. This may reflect the different methods of data collection and may account for the differences seen in subscale scores between countries. Age, educational level, and socioeconomic status are associated with computer usage43,44.
Women scored higher (i.e. worse) on all six subscales of the AAV-PRO. Health- related quality of life is reduced in females in other conditions47 , and trends towards higher scores for women have been reported in AAV15. Younger people (<65) scored higher on the Social and Emotional Impact subscale of the AAV-PRO, and lower on mental health, a trend also seen in other chronic diseases in this age group47.
The design of the survey was to identify the scale structure and measurement properties of the AAV-PRO. As predicted, participants were generally stable regarding self-reported disease activity, with around 70% describing themselves as “in remission”. Follow up was 3-months. This somewhat limited the assessment of responsiveness and minimally important change, which are usually assessed over a longer time-period in participants expected to change in clinical state, e.g., within the context of a clinical trial19. The study produced evidence of longitudinal construct validity. Among participants who reported “no change” effect sizes were appropriately close to zero, and the few participants who reported their condition as “much better” demonstrated a small amount of change in AAV-PRO scores (ES range 0.21 to 0.28). Distribution-based estimates of minimal change (SEM and MDC90), which relate to the reliability (ICC) of each scale were all appropriate and will be useful for calculating sample sizes in future studies41 . Future studies will provide more robust estimates of minimal important differences (MID), further longitudinal construct validity48 and determine whether summary component scores can be derived.
Validated PROs are an important way of accurately measuring the impact and value of new drug treatments on HRQoL by measuring outcomes of importance to patients themselves17. PROs can be part of evidence submitted for new drug approvals and can also provide valuable information to clinicians and policymakers asked with making decisions about the use of new treatments49. The involvement of patients with AAV-PRO at every stage of development should ensure its face validity and relevance. In addition, it has also been shown that disease-specific instruments may be more responsive to change than generic instruments, which is a crucial characteristic for detecting treatment effect within randomized controlled trials50. The AAV-PRO is, therefore, presented as complementary to the SF-36 or EQ5D, which allow comparison with other conditions and population controls, but are not specific to AAV.
The AAV-PRO, a new disease-specific PRO measure for ANCA-associated vasculitis, has good face and construct validity, is reliable, feasible, and discriminates among disease states. The AAV-PRO is ready for inclusion within clinical trials and research studies as part of its ongoing validation and exploration of its measurement properties within different populations. The AAV-PRO provides the means to ensure patients‟ perspectives on their disease are represented in the study of AAV.
Supplementary Material
Acknowledgements
We would like to thank all of the patients who contributed their views and valuable time to assist us with this study. We also thank Vasculitis UK, our collaborators on the paper-based UK survey, for their assistance with identifying patients, and for organising and funding postage and packaging. We also thank the Vasculitis Patient-Powered Research Network for its collaboration on the US-based online survey, including identifying patients, building the online version of the questionnaire, and compiling a report on patient responses. Authors 1 and 2 contributed equally to this manuscript.
Grants and other financial support:
Sponsored by the University of Oxford and the Vasculitis Clinical Research Consortium (VCRC). Funding for the development of the PRO was received from the Medical Research Fund, Oxford, the Oxfordshire Health Services Research Committee Ref. 1098, and a Patient-Centered Outcomes Research Institute Pilot Project Grant. The VCRC has received support from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54 AR057319 and U01 AR51874), the National Center for Research Resources (U54 RR019497); and the Office of Rare Diseases Research. and the National Center for Advancing Translational Science. The VCRC is part of the Rare Diseases Clinical Research Network (RDCRN). Dr. Robson and Professor Luqmani were supported in part by the National Institute for Health Research Musculoskeletal Biomedical Research Unit, Oxford, UK. Dr Robson was supported by a National Institute for Health Research (NIHR) clinical lectureship. Dr. Milman was supported by a UCB/Canadian Rheumatology Association/Arthritis Society postgraduate rheumatology fellowship award and a research fellowship from the Department of Medicine at the Ottawa Hospital. Oxford University Innovation provided funding of translatability assessment.
Footnotes
Conflicts of interest
Nil declared.
Online supplementary Figures and Tables
Figure S1. Exploratory Factor Analysis of Symptom Specific Items
Figure S2. Exploratory Factor Analysis of Health-Related Quality of Life Items
Figure S3. Factor loading in final six subscales
Figure S4. Scoring of the AAV-PRO
Figure S5. AAV-PRO sample items
Table S1. Geographical distribution of participants
Table S2. Rasch analysis
Table S3. Comparison of baseline AAV-PRO scale scores among different types of AAV
Table S4. Comparison of baseline AAV-PRO subscales between male and female participants
Table S5. Comparison of baseline AAV-PRO scale scores by age groups (≤65 versus >65 years).
Table S6. Comparison of baseline AAV-PRO scale scores for UK versus USA participants.
Table S7. Relationship (Pearson correlation) between length of time since diagnosis and AAV-PRO subscale scores
Contributor Information
Joanna Robson, Consultant Senior Lecturer in Rheumatology, Faculty of Health and Applied Sciences, University of the West of England, Bristol, & Hon Senior Lecturer, School of Clinical Sciences, University of Bristol, & Hon Consultant in Rheumatology, University Hospitals Bristol NHS Trust. Bristol Royal Infirmary, Upper Maudlin Street, Bristol, BS2 8HW..
Jill Dawson, Nuffield Department of Population Health (HSRU), University of Oxford, Old Road Campus, Oxford, UK..
Helen Doll, ICON Clinical Outcomes Assessments, Abingdon, UK..
Peter F. Cronholm, Department of Family Medicine and Community Health, University of Pennsylvania, Philadelphia, PA, USA..
Nataliya Milman, Department of Rheumatology, University of Ottawa, Ottawa, Canada..
Katherine Kellom, PolicyLab, Children’s Hospital of Philadelphia, Philadelphia, US..
Susan Ashdown, Oxfordshire, UK..
Ebony Easley, Department of Family Medicine and Community Health, Mixed Methods Research Laboratory, University of Pennsylvania, Philadelphia, PA, USA..
Don Gebhart, Columbus, Ohio, USA..
Georgia Lanier, Boston, MA, USA..
John Mills, Derbyshire, Vasculitis UK, UK..
Jacqueline Peck, Oxfordshire, UK..
Raashid A. Luqmani, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK..
Judy A. Shea, School of Medicine, University of Pennsylvania, Philadelphia, PA, USA..
Gunnar Tomasson, Department of Public Health Sciences, University of Iceland, Reykjavik, Iceland..
Peter A. Merkel, Division of Rheumatology, Department of Medicine and Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania, Philadelphia, PA, USA..
REFERENCES
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis and rheumatism 2013;65(1):1–11. doi: 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 2.Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Annals of the rheumatic diseases 2011;70(3):488–94. doi: 10.1136/ard.2010.137778 [DOI] [PubMed] [Google Scholar]
- 3.Rhee RL, Hogan SL, Poulton CJ, et al. Trends in Long-Term Outcomes Among Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis With Renal Disease. Arthritis Rheumatol 2016;68(7):1711–20. doi: 10.1002/art.39614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robson J, Doll H, Suppiah R, et al. Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: long-term data from the European Vasculitis Study Group trials. Rheumatology 2015;54(3):471–81. doi: 10.1093/rheumatology/keu366 [DOI] [PubMed] [Google Scholar]
- 5.Mooney J, Poland F, Spalding N, et al. ‘In one ear and out the other - it’s a lot to take in’: a qualitative study exploring the informational needs of patients with ANCA-associated vasculitis. Musculoskeletal Care 2013;11(1):51–9. doi: 10.1002/msc.1030 [DOI] [PubMed] [Google Scholar]
- 6.Koutantji M, Harrold E, Lane SE, et al. Investigation of quality of life, mood, pain, disability, and disease status in primary systemic vasculitis. Arthritis and rheumatism 2003;49(6):826–37. doi: 10.1002/art.11471 [DOI] [PubMed] [Google Scholar]
- 7.Basu N, McClean A, Harper L, et al. The characterisation and determinants of quality of life in ANCA associated vasculitis. Annals of the rheumatic diseases 2014;73(1):207–11. doi: 10.1136/annrheumdis-2012-202750 [DOI] [PubMed] [Google Scholar]
- 8.Fardet L, Flahault A, Kettaneh A, et al. Corticosteroid-induced clinical adverse events: frequency, risk factors and patient’s opinion. Br J Dermatol 2007;157(1):142–8. doi: 10.1111/j.1365-2133.2007.07950.x [DOI] [PubMed] [Google Scholar]
- 9.Basu N, McClean A, Harper L, et al. Markers for work disability in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatology 2014;53(5):953–6. doi: 10.1093/rheumatology/ket483 [DOI] [PubMed] [Google Scholar]
- 10.Benarous L, Terrier B, Laborde-Casterot H, et al. Employment, work disability and quality of life in patients with ANCA-associated vasculitides. The EXPOVAS study. Clin Exp Rheumatol 2016 [PubMed] [Google Scholar]
- 11.Basu N, McClean A, Harper L, et al. Explaining fatigue in ANCA-associated vasculitis. Rheumatology 2013;52(9):1680–5. doi: 10.1093/rheumatology/ket191 [DOI] [PubMed] [Google Scholar]
- 12.Herlyn K, Hellmich B, Seo P, et al. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective Arthritis care & research 2010;62(11):1639–45. doi: 10.1002/acr.20276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo P, Jayne D, Luqmani R, et al. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology 2009;48(7):823–7. doi: 10.1093/rheumatology/kep103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkel PA, Aydin SZ, Boers M, et al. The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. The Journal of rheumatology 2011;38(7):1480–6. doi: 10.3899/jrheum.110276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh M, Mukhtyar C, Mahr A, et al. Health related quality of life in patients with newly diagnosed anti-neutrophil cytoplasm antibody associated vasculitis. Arthritis care & research 2011. doi: 10.1002/acr.20471 [published Online First: 2011/04/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasson G, Boers M, Walsh M, et al. Assessment of health-related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis care & research 2012;64(2):273–9. doi: 10.1002/acr.20649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick R, Davey C, Buxton MJ, et al. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2(14):i–iv, 1–74. [PubMed] [Google Scholar]
- 18.Robson JC, Milman N, Tomasson G, et al. Exploration, Development, and Validation of Patient-reported Outcomes in Antineutrophil Cytoplasmic Antibody-associated Vasculitis Using the OMERACT Process. The Journal of rheumatology 2015;42(11):2204–9. doi: 10.3899/jrheum.141143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value in Health 2007;10:S125–S37. doi: 10.1111/j.1524-4733.2007.00275.x [DOI] [PubMed] [Google Scholar]
- 20.Merkel PA, Aydin SZ, Boers M, et al. Current status of outcome measure development in vasculitis. The Journal of rheumatology 2014;41(3):593–8. doi: 10.3899/jrheum.131248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robson JC, Milman N, Tomasson G, et al. Exploration, Development, and Validation of Patient-reported Outcomes in Antineutrophil Cytoplasmic Antibody-associated Vasculitis Using the OMERACT Process. J Rheumatol 2015. doi: 10.3899/jrheum.141143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robson J, Dawson J, Cronholm PF, et al. Health related quality of life in ANCA associated vasculitis and item generation for a disease specific patient reported outcome measure. Patient Related Outcome Measures 2017;In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EuroQol G EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 24.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D -5L to EQ-5D-3L value sets. Value Health 2012;15(5):708–15. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 25.Barrett P, Kline P. The Observation to Variable Ratio in Factor Analysis. Personality Study and Group Behaviour 1981;1(1):23–33. [Google Scholar]
- 26.Fayers P, Machin D. Quality of Life- Assessment, Analysis and Interpretation. Chichester: John Wiley & Sons Ltd; 2000. [Google Scholar]
- 27.Lorenzo-Seva U, Ferrando PJ. FACTOR: a computer program to fit the exploratory factor analysis model. Behav Res Methods 2006;38(1):88–91. [DOI] [PubMed] [Google Scholar]
- 28.Andrich D Rasch Models for Measurement. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 29.Kaiser HF. The application of electronic computers to factor analysis Educ psychol measurement 1960;20:141–51. [Google Scholar]
- 30.Avlund K, Kreiner S, Schultz-Larsen K. Construct validation and the Rasch model: functional ability of healthy elderly people. Scand J Soc Med 1993;21(4):233–46. [DOI] [PubMed] [Google Scholar]
- 31.Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess 2003;80(1):99–103. doi: 10.1207/S15327752JPA8001_18 [DOI] [PubMed] [Google Scholar]
- 32.R.K H, Jones RW. Comparison of classical test theory and item response theory and their applications to test development. Educational Measurement Issues and Practice 1993;12:38–47. [Google Scholar]
- 33.Prieto L, Alonso J, Lamarca R. Classical Test Theory versus Rasch analysis for quality of life questionnaire reduction. Health Qual Life Outcomes 2003;1:27. doi: 10.1186/1477-7525-1-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunally JC, Bernstein IH. Psychometric theory. New York: Columbus OH; 1994. [Google Scholar]
- 35.Kline P A Handbook of Psychological Testing. London: Routledge; 1993. [Google Scholar]
- 36.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med 1993;118(8):622–9. [DOI] [PubMed] [Google Scholar]
- 37.Andrews F, ., Withey S. Social Indicators of Well-Being: American’s Perceptions of Life Quality. New York: Plenum; 1976. [Google Scholar]
- 38.Stratford PW, Binkley JM, Riddle DL. Health status measures: strategies and analytic methods for assessing change scores. Phys Ther 1996;76(10):1109–23. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10(4):407–15. [DOI] [PubMed] [Google Scholar]
- 40.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52(9):861–73. [DOI] [PubMed] [Google Scholar]
- 41.de Vet HC, Terwee CB, Ostelo RW, et al. Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual Life Outcomes 2006;4:54. doi: 10.1186/1477-7525-4-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care 1989;27(3 Suppl):S178–89. [DOI] [PubMed] [Google Scholar]
- 43.Nulty DD. The adequacy of response rates to online and paper surveys: what can be done? Assessment & Evaluation in Higher Education 2008;33(3):301–14. [Google Scholar]
- 44.File T, Ryan C. Computer and Internet Use in the United States: 2013 American community survey reports. In: Bureau USC, ed. www.census.go/comtent/dam/Census/library/publications/2014/acs/acs-28.pdf (last accessed 29/09/2016, 2014. [Google Scholar]
- 45.Muehlhausen W, Doll H, Quadri N, et al. Equivalence of electronic and paper administration of patient-reported outcome measures: a systematic review and meta-analysis of studies conducted between 2007 and 2013. Health Qual Life Outcomes 2015;13:167. doi: 10.1186/s12955-015-0362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gwaltney CJ, Shields AL, Shiffman S. Equivalence of electronic and paper-and-pencil administration of patient-reported outcome measures: a meta-analytic review. Value Health 2008;11(2):322–33. doi: 10.1111/j.1524-4733.2007.00231.x [DOI] [PubMed] [Google Scholar]
- 47.Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;44(2):123–30. doi: 10.1016/j.semarthrit.2014.05.001 [published Online First: 2014/06/30] [DOI] [PubMed] [Google Scholar]
- 48.Revicki DA, Cella D, Hays RD, et al. Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes 2006;4:70. doi: 10.1186/1477-7525-4-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willke RJ. Measuring the value of treatment to patients: patient-reported outcomes in drug development. Am Health Drug Benefits 2008;1(1):34–40. [PMC free article] [PubMed] [Google Scholar]
- 50.Wiebe S, Guyatt G, Weaver B, et al. Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol 2003;56(1):52–60. [published Online First: 2003/02/19] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


